Scrub Typhus: Historic Perspective and Current Status of the Worldwide Presence of Orientia Species
Abstract
1. Early History of Scrub Typhus and the Etiologic Agents of the Tsutsugamushi Triangle
1.1. Scrub Typhus Disease Presentation and Diagnosis
1.2. Early History of Scrub Typhus
China
Japan
The Etiology of Scrub Typhus in Japan
Indonesia
Taiwan
The Philippines
Australia
Vietnam
Korea
Malaysia
India, Burma, Ceylon, and the Maldives
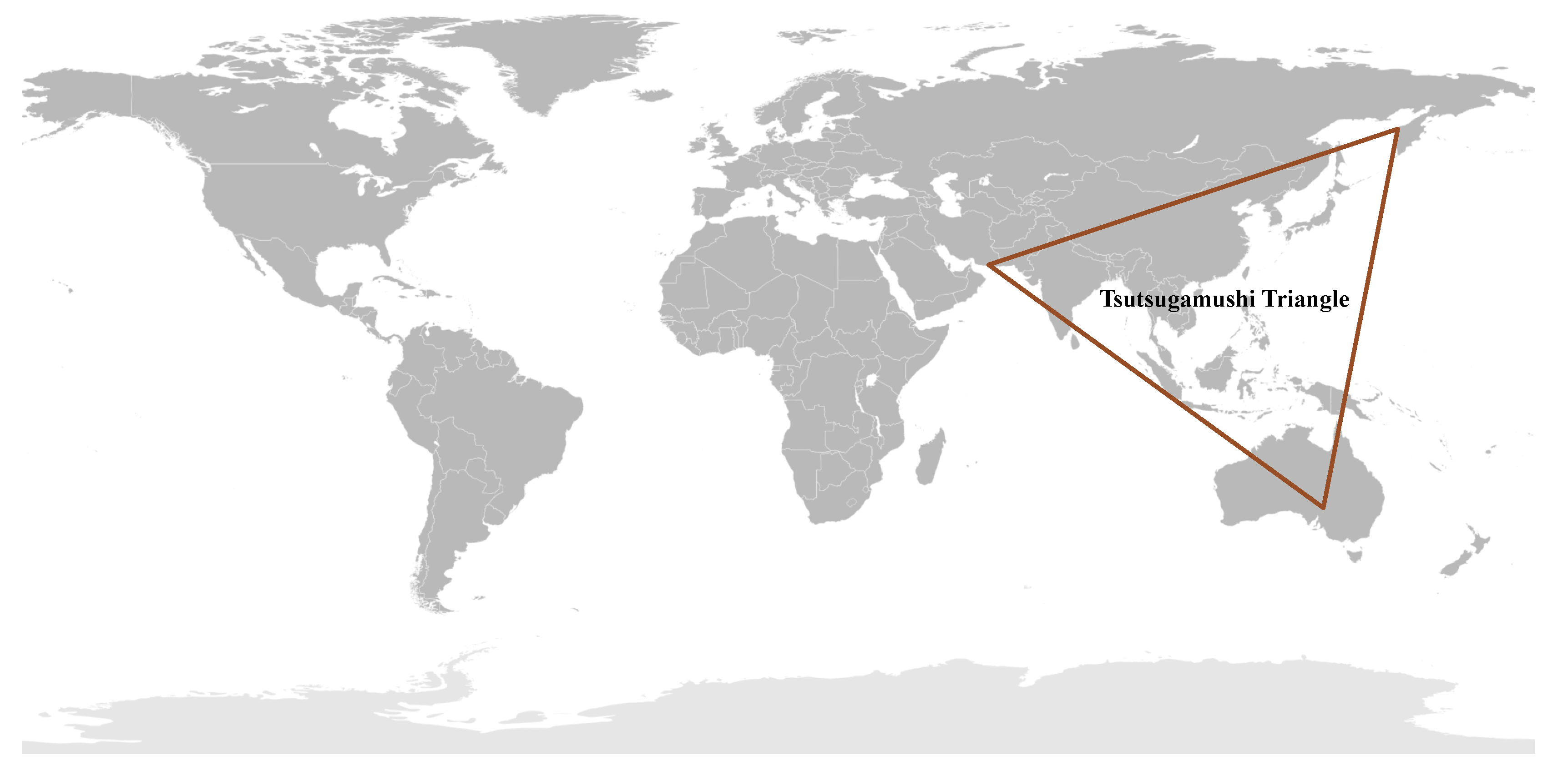
1.3. The Tsutsugamushi Triangle
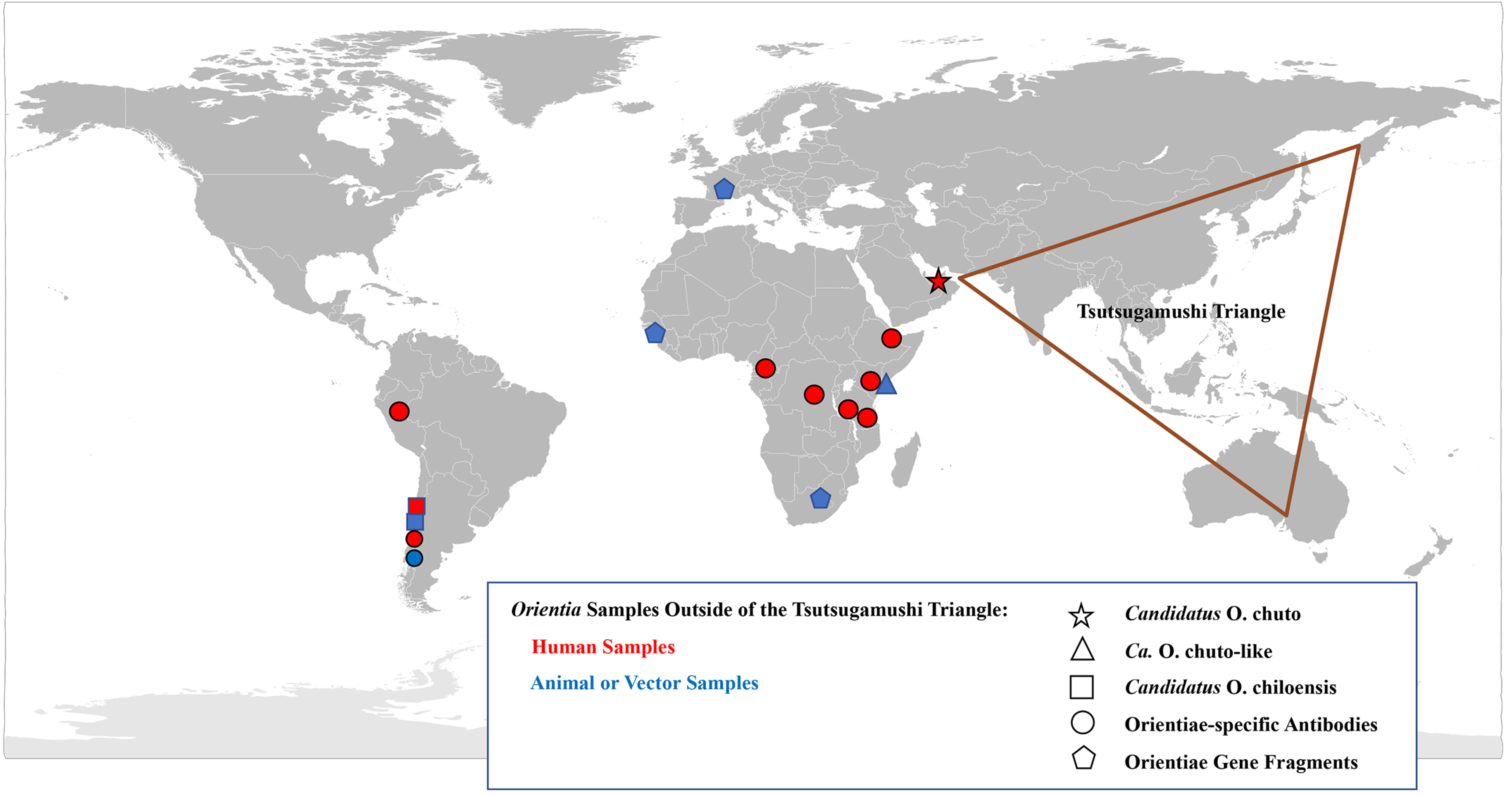
2. Scrub Typhus Outside the Tsutsugamushi Triangle
2.1. Case Investigations
Africa
United Arab Emirates
Chile
2.2. Serological Evidence of Scrub Typhus Outside the Tsutsugamushi Triangle
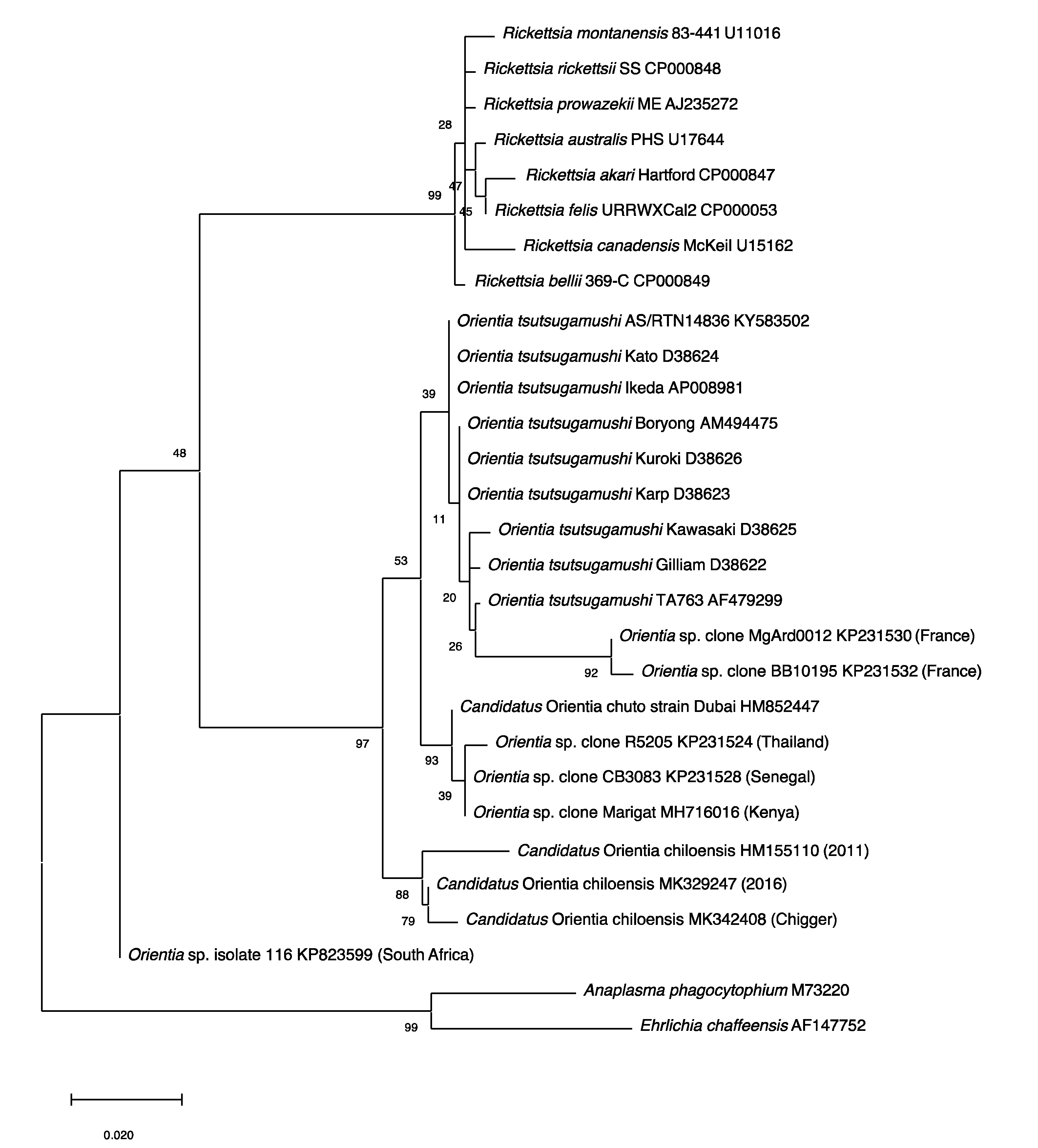
2.3. Molecular Evidence of Scrub Typhus Outside the Tsutsugamushi Triangle
2.4. Endemic Scrub Typhus in South America
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ashburn, P.M.; Craig, C.F. A comparative study of tsutsugamushi disease and spotted or tick fever of Montana. Boston Med. Surg. J. 1908, 158, 749–761. [Google Scholar] [CrossRef]
- Watt, G.; Kantipong, P. Orientia tsutsugamushi and scrub typhus. In Rickettsial Diseases; Raoult, D., Parola, P., Eds.; Informa Healthcare USA, Inc.: New York, NY, USA, 2007; pp. 237–256. [Google Scholar]
- Paris, D.H.; Shelite, T.R.; Day, N.P.; Walker, D. Unresolved Problems Related to Scrub Typhus: A Seriously Neglected Life-Threatening Disease. Am. J. Trop. Med. Hyg. 2013, 89, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.J.; Foley, D.; Richards, A.L. A spatiotemporal database to track human scrub typhus using the VectorMap application. PLoS Neglected Trop. Dis. 2015, 9, e0004161. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, S.; Weeratunga, P.; Sivayoganathan, S.; Fernando, S.D. Clinical manifestations of scrub typhus. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Abdad, M.Y.; Abdallah, R.A.; Fournier, P.-E.; Stenos, J.; Vasoo, S. A Concise Review of the Epidemiology and Diagnostics of Rickettsioses: Rickettsia and Orientia spp. J. Clin. Microbiol. 2018, 56, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Luce-Fedrow, A.; Mullins, K.; Kostik, A.P.; John, H.K.S.; Jiang, J.; Richards, A.L. Strategies for detecting rickettsiae and diagnosing rickettsial diseases. Future Microbiol. 2015, 10, 537–564. [Google Scholar] [CrossRef]
- Kawamura, A., Jr.; Tanaka, H.; Tamura, A. Tsutsugamushi Disease; Univ. Tokyo Press: Tokyo, Japan, 1995. [Google Scholar]
- Yoshimoto, T.; Yoshimoto, T. Scrub typhus in Japan. Am. J. Clin. Microbiol. Antimicrobiol. 2019, 2, 1042. [Google Scholar]
- Palm, T.A. Some account of a disease called Shima Mushi or Island Insect Diseases by the natives of Japan. Edin. Med. J. 1878, 24, 128. [Google Scholar]
- Bälz, E.; Kawakami. Die Japanische Fluss-oder Ueber-schwemmungsfieber. Virchow’s Arch. 1879, 78, 373. [Google Scholar] [CrossRef]
- Hayashi, N. Etiology of Tsutsugamushi disease. J. Parasitol. 1920, 7, 53–68. [Google Scholar] [CrossRef]
- Hayashi, N. On Ricketts’ corpuscles. Trans. Japan. Pathol. Soc. 1924, 22, 569–576. (In Japanese) [Google Scholar]
- Nagayo, M.; Miyagawa, Y.; Mitamura, T.; Imamura, A.; Tamiya, T.; Sato, K. On the experimental Tsutsugamushi disease in monkeys by intracutaneous inoculation of the virus. Sci. Rep. Gov. Inst. Infect. Dis. 1923, 2, 371–373. (In Japanese) [Google Scholar]
- Ogata, N.; Unno, Y. On the transfer of causative agent of Tsutsugamushi disease to the rabbit testis and microorgansims appearing in the testis. J. Chiba Med. Soc. 1929, 7, 1215–1222. (In Japanese) [Google Scholar]
- Nagayo, M.; Tamiya, T.; Mitamura, T.; Sato, K. On the virus of Tsutsugamushi disease and its demonstration by a new method. Trans. Japan Pathol. Soc. 1930, 20, 556–566. [Google Scholar]
- Ogata, N. Aetiology der Tsutsugamushikrankheit: Rickettsia tsutsugamushi. Zbl f Bakt 1931, 122, 249–253. [Google Scholar]
- Kawamura, R.; Imagawa, Y. Die Feststellung des Erregers bei der Tsutsugamushikrankheit. Zbl f Bakt 1931, 122, 261. [Google Scholar]
- Hayashi, N. On Tsutsugamushi disease. Trans. Japan Pathol. Soc. 1932, 22, 689–690. (In Japanese) [Google Scholar]
- Ogata, N.; Nakajima, G.; Kajima, S. Animals employed for experimental infection by pathogenic rickettsiae in laboratory—Especially recommendation to use mouse for the detection of Rickettsia tsutsugamushi. Tokyo Med. J. 1932, 2760, 155–160. [Google Scholar]
- Luce-Fedrow, A.; Lehman, M.L.; Kelly, D.J.; Mullins, K.; Maina, A.N.; Stewart, R.L.; Ge, H.; John, H.S.; Jiang, J.; Richards, A.L. A Review of Scrub Typhus (Orientia tsutsugamushi and Related Organisms): Then, Now, and Tomorrow. Trop. Med. Infect. Dis. 2018, 3, 8. [Google Scholar] [CrossRef]
- Tamura, A.; Ohashi, N.; Urakami, H.; Miyamura, S. Classification of Rickettsia tsutsugamushi in a New Genus, Orientia gen. nov., as Orientia tsutsugamushi comb. nov. Int. J. Syst. Bacteriol. 1995, 45, 589–591. [Google Scholar] [CrossRef]
- Schüffner, W. Geneesk. Tijdschr. Med. Indie 1909, 49, 64. [Google Scholar]
- Schüffner, W. Pseudotyphoid fever in Deli, Sumatra (a variety of Kedani fever). Philipp. J. Sci. 1915, 10, 345. [Google Scholar]
- Logue, J.B. Scrub typhus. Report of epidemic in the Southwest Pacific. US Nav. Med. Bull. 1944, 43, 645–649. [Google Scholar]
- Hadi, T.R.; Nalim, S.; Sukaeri, S.; Dennis, D.T. Scrub typhus survey of Biak and Owi islands: ectoparasites of small mammals and rickettsial isolations. Southeast Asian J. Trop. Med. Public Health 1980, 11, 220–226. [Google Scholar] [PubMed]
- Dennis, D.T.; Hadi, T.R.; Brown, R.J.; Sukaeri, S.; Leksana, B.; Cholid, R. A survey of scrub and murine typhus in the Ancol section of Jakarta, Indonesia. Southeast Asian J. Trop. Med. Public Health 1981, 12, 574–580. [Google Scholar]
- Richards, A.L.; Soeatmandji, D.W.; Widodo, M.A.; Sardjono, T.W.; Yanuwiadi, B.; Hernowati, T.E.; Baskoro, A.D.; Roebiyoso, E.; Hakim, L.; Soendoro, M. Seroepidemiological evidence for murine and scrub typhus in Malang, Indonesia. Am. J. Trop. Med. Hyg. 1997, 57, 91–95. [Google Scholar] [CrossRef]
- Peterson, R.K.D. The Real Enemy: Scrub Typhus and the Invasion of Sansapor. Am. Entomol. 2009, 55, 91–94. [Google Scholar] [CrossRef][Green Version]
- Widjaja, S.; Williams, M.; Winoto, I.; Farzeli, A.; Stoops, C.A.; Barbara, K.A.; Richards, A.L.; Blair, P.J. Geographical Assessment of Rickettsioses in Indonesia. Vector-Borne Zoonotic Dis. 2016, 16, 20–25. [Google Scholar] [CrossRef]
- Hatori, J. On the endemic tsutsugamushi disease in Formosa. Ann. Trop. Med. Parasit. 1919, 13, 233–258. [Google Scholar] [CrossRef]
- Gale, J.L.; Irving, G.S.; Wang, H.C.; Lien, J.C.; Chen, W.F.; Cross, J.H. Scrub typhus in Eastern Taiwan, 1970. Am. J. Trop. Med. Hyg. 1974, 23, 679–684. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Kuo, K.-C.; Sun, W.; Lin, J.-N.; Lai, C.-H.; Lee, C.-H. Clinicoepidemiologic characteristics of scrub typhus and murine typhus: A multi-center study in southern Taiwan. J. Microbiol. Immunol. Infect. 2019, 52, 769–778. [Google Scholar] [CrossRef]
- Yamamiya, C.; Honda, S. Observations on tsutsugamushi disease of the Pescadores. J. Formosan Med. Assoc. 1933, 32, 1803–1804. [Google Scholar]
- Cooper, W.C.; Chen, W.F.; Lien, J.C.; Hsu, S.H. Scrub Typhus in the Pescadores Islands: An Epidemiologic and Clinical Study. Am. J. Trop. Med. Hyg. 1964, 13, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.; Ho, C.; Van Peenen, P.; Santana, F. Isolation of Rickettsia tsutsugamushi from mammals and chiggers (Fam. Trombiculidae) in the Pescadores Islands, Taiwan. Trans. R. Soc. Trop. Med. Hyg. 1978, 72, 192–194. [Google Scholar] [CrossRef]
- Philip, C.B.; Woodward, T.E.; Sullivan, R.R. Tsutsugamushi Disease (Scrub or Mite-Borne Typhus) in the Philippine Islands during American Re-Occupation in 1944–45. Am. J. Trop. Med. Hyg. 1946, 26, 229–242. [Google Scholar] [CrossRef]
- Kelly, D.J.; Richards, A.L.; Temenak, J.; Strickman, D.; Dasch, G. The Past and Present Threat of Rickettsial Diseases to Military Medicine and International Public Health. Clin. Infect. Dis. 2002, 34 (Suppl. 4), s145–s169. [Google Scholar] [CrossRef]
- Berge, T.O.; Gauld, R.L.; Kitaoka, M. A field trial of a vaccine prepared from the Volner strain of Rickettsia tsutsugamushi. Am. J. Epidemiol. 1949, 50, 337–342. [Google Scholar] [CrossRef]
- Cross, J.H.; Basaca-Sevilla, V. Seroepidemiology of scrub typhus and murine typhus in the Philippines. Philipp. J. Microbiol. Infect. Dis. 1981, 10, 25–34. [Google Scholar]
- Smithson, O. Mossman Fever. J. Trop. Med. 1910, 13, 351. [Google Scholar]
- Breinl, A.; Priestley, H.; Fielding, J.W. On the occurrence and pathology of endemic glandular fever, a specific fever, occurring in the Mossman District of North Queensland. Med. J. Aust. 1914, 1, 391–395. [Google Scholar] [CrossRef]
- Breinl, A.; Priestley, H.; Fielding, J.W. On the occurrence and pathology of endemic glandular fever, a specific fever, occurring in the Mossman District of North Queensland. J. Trop. Med. Hyg. 1915, 30–33, Abstracted from the Med. J. Aust. 1914, 1, 391–395.. [Google Scholar]
- Langan, A.M.; Mathew, R.Y. The establishment of “Mossman,” “coastal” and other previously unclassified fevers of north Queensland as endemic typhus. Med. J. Aust. 1935, 2, 145–148. [Google Scholar] [CrossRef]
- Carley, J.G.; Doherty, R.L.; Derric, E.H.; Pope, J.H.; Emanuel, M.L.; Ross, C.H. The investigation of fevers in North Queensland by mouse inoculation, with particular reference to scrub typhus. Aust. Ann. Med. 1955, 4, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Faa, A.G.; McBride, W.; Garstone, G.; Thompson, R.E.; Holt, P. Scrub Typhus in the Torres Strait Islands of North Queensland, Australia. Emerg. Infect. Dis. 2003, 9, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Odorico, D.M.; Graves, S.R.; Currie, B.; Catmull, J.; Nack, Z.; Ellis, S.; Wang, L.; Miller, D.J. New Orientia tsutsugamushii strain from scrub typhus in Australia. Emerg. Infect. Dis. 1998, 4, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, M.L.; Chappell, T.; Golledge, C.L. Scrub typhus in Western Australia. Commun. Dis. Intel. 1993, 17, 570–571. [Google Scholar]
- Graves, S.; Wang, L.; Nack, Z.; Jones, S. Rickettsia serosurvey in Kimberley, Western Australia. Am. J. Trop. Med. Hyg. 1999, 60, 786–789. [Google Scholar] [CrossRef]
- Noc, F.; Gautron, P. Deux cas de fièvre indéterminée rappelant le pseudo-typhus de Delhi observés à Saigon. Bull. Soc. Med. Chir d’ Indoch. 1915, 6, 108. [Google Scholar]
- Beytout, D. Rickettsioses Diagnostiquées Par Microagglutination De Janvier 1962 A Juin 1963 A Saigon. Bull. Société Pathol. Exot. 1964, 57, 257–263. [Google Scholar]
- Duong, V.; May, T.T.X.; Blasdell, K.; Lo, L.V.; Morvan, C.; Lay, S.; Anukool, W.; Wongprompitak, P.; Suputtamongkol, Y.; Laurent, D.; et al. Molecular epidemiology of Orientia tsutsugamushi in Cambodia and Central Vietnam reveals a broad region-wide genetic diversity. Infect. Genet. Evol. 2013, 15, 35–42. [Google Scholar] [CrossRef]
- Hamaguchi, S.; Cuong, N.C.; Tra, D.T.; Doan, Y.H.; Shimizu, K.; Tuan, N.Q.; Yoshida, L.M.; Mai, L.Q.; Duc-Anh, D.; Ando, S.; et al. Clinical and epidemiological characteristics of scrub typhus and murine typhus among hospitalized patients with acute undifferentiated fever in Northern Vietnam. Am. J. Trop. Med. Hyg. 2015, 92, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.L.; Pham, H.T.; Nguyen, T.V.; Hoang, P.V.; Le, M.T.; Takemura, T.; Hasebe, F.; Hayasaka, D.; Yamada, A.; Miura, K. The genotypes of Orientia tsutsugamushi, identified in scrub typhus patients in northern Vietnam. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Le Viet, N.; Laroche, M.; Pham, H.L.T.; Viet, N.L.; Mediannikov, O.; Raoult, D.; Parola, P. Use of eschar swabbing for the molecular diagnosis and genotyping of Orientia tsutsugamushi causing scrub typhus in Quang Nam province, Vietnam. PLoS Negl. Trop. Dis. 2017, 11, e0005397. [Google Scholar] [CrossRef]
- Trung, N.V.; Hoi, L.T.; Toan, T.K.; Hoa, T.M.; Fox, A.; Van Kinh, N.; Van Doorn, H.R.; Wertheim, H.F.L.; Bryant, J.E.; Nadjm, B.; et al. Seroprevalence of Scrub Typhus, Typhus, and Spotted Fever Among Rural and Urban Populations of Northern Vietnam. Am. J. Trop. Med. Hyg. 2017, 96, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Trung, N.V.; Hoi, L.T.; Cuong, D.D.; Ha, D.T.; Hoa, T.M.; Lien, V.N.; Hoa, N.T.; Hoa, L.N.M.; Huong, D.T.; Bich, V.T.N.; et al. Analysis of the 56-kDa type specific antigen gene of Orientia tsutsugamushi from northern Vietnam. PLoS ONE 2019, 14, e0221588. [Google Scholar] [CrossRef] [PubMed]
- Weir, H.H. A continued fever of Korea. China Med. J. 1915, 29, 307–315. [Google Scholar]
- Chung, M.H.; Kang, J.S. History of tsutsugamushi disease in Korea. Infect. Chemother. 2019, 51, 196–209. [Google Scholar] [CrossRef]
- Lee, I.Y.; Kim, H.C.; Lee, Y.S.; Seo, J.H.; Lim, J.W.; Yong, T.S.; Klein, T.A.; Lee, W.J. Geographical distribution and relative abundance of vectors of scrub typhus in the Republic of Korea. Korean J. Parasitol. 2009, 47, 381–386. [Google Scholar] [CrossRef]
- O’Guinn, M.L.; Klein, T.A.; Lee, J.S.; Richards, A.L.; Kim, H.-C.; Ha, S.J.; Shim, S.H.; Baek, L.J.; Song, K.-J.; Chong, S.-T.; et al. Serological Surveillance of Scrub Typhus, Murine Typhus, and Leptospirosis in Small Mammals Captured at Firing Points 10 and 60, Gyeonggi Province, Republic of Korea, 2001–2005. Vector-Borne Zoonotic Dis. 2010, 10, 125–133. [Google Scholar] [CrossRef]
- Chang, W.H.; Kang, J.S. Isolation of Rickettsia tsutsugamushi from Korean patients. J. Korean Med. Assoc. 1987, 30, 999–1008. [Google Scholar]
- Jiang, J.; Myers, T.E.; Rozmajzl, P.J.; Graf, P.C.; Chretien, J.-P.; Gaydos, J.C.; Richards, A.L. Seroconversions to Rickettsiae in US Military Personnel in South Korea. Emerg. Infect. Dis. 2015, 21, 1073–1074. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-W.; Cho, P.Y.; Moon, S.-U.; Na, B.-K.; Kang, Y.-J.; Sohn, Y.; Youn, S.-K.; Hong, Y.; Kim, T.-S. Current situation of scrub typhus in South Korea from 2001−2013. Parasites Vectors 2015, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, W.; Lesslar, J.E. “Tropical typhus” in the Federated Malay States; Bulletin Institute of Medical Research: Kuala Lumpur, Malaysia, 1925. [Google Scholar]
- Cruikshank, R. The Weil-Felix reaction in typhus fever. J. Hyg. (Lond.) 1927, 27, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.J.; A Fuerst, P.; Richards, A.L. Origins, Importance and Genetic Stability of the Prototype Strains Gilliam, Karp and Kato of Orientia tsutsugamushi. Trop. Med. Infect. Dis. 2019, 4, 75. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, W.; Lessar, J.E. Tropical typhus in the Federated Malay States; Bulletin Institute of Medical Research: Kuala Lumpur, Malaysia, 1926. [Google Scholar]
- Fletcher, W.; Field, J.W. The Tsutsugamushi Disease in the Federated Malay States; Bulletin Institute Medical Research: Kuala Lumpur, Malaysia, 1927. [Google Scholar]
- Fletcher, W.; Lessar, J.E.; Lewthwaite, R. The aetiology of tsutsugamushi disease and tropical typhus in Federated Malay States: A preliminary note. Part, I. Trans. R. Soc. Trop. Med. Hyg. 1928, 22, 161–174. [Google Scholar] [CrossRef]
- Fletcher, W.; Lessar, J.E.; Lewthwaite, R. The aetiology of the tsutsugamushi disease and tropical typhus in Federated Malay States. Part II. Trans. R. Soc. Trop. Med. Hyg. 1929, 23, 57–70. [Google Scholar] [CrossRef]
- Fletcher, W. Tropical Typhus. BMJ 1932, 2, 1140–1141. [Google Scholar] [CrossRef]
- Tee, T.S.; Devi, S.; A Suan, K.; Chun, S.S.; Ming, H.T.; Yasin, R.M.; Kamalanathan, M. Seroepidemiologic survey of Orientia tsutsugamushi, Rickettsia typhi, and TT118 spotted fever group rickettsiae in rubber estate workers in Malaysia. Am. J. Trop. Med. Hyg. 1999, 61, 73–77. [Google Scholar] [CrossRef][Green Version]
- Tay, S.T.; Ho, T.M.; Rohani, M.Y.; Shamala, D. Antibody prevalence of Orientia tsutsugamushi, Rickettsia typhi and TT118 spotted fever group rickettsiae among febrile patients in rural areas of Malaysia. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 280–284. [Google Scholar] [CrossRef]
- Kwa, B.H. Environmental change, development and vector borne disease: Malaysia’s experience with filariasis, scrub typhus and dengue. Environ. Dev. Sustain. 2006, 10, 209–217. [Google Scholar] [CrossRef]
- Tay, S.T.; Mohamed Zan, H.A.; Lim, Y.A.L.; Ngui, R. Antibody prevalence and factors associated with exposure to Orientia tsutsugamushi in different aboriginal subgroups in West Malaysia. PLoS Negl. Trop. Dis. 2013, 7, e2341. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Zan, H.A.; Ponnampalavanar, S.; Faridah, S.O.S.; Savithiri, P.D.; Lim, Y.A.L.; Tay, S.T. Genetic variants of Orientia tsutsugamushi identified from scrub typhus cases in Malaysia. Trop. Biomed. 2016, 33, 203–208. [Google Scholar]
- Low, V.L.; Tan, T.K.; Khoo, J.J.; Lim, F.S.; Abubakar, S. An overview of rickettsiae in Southeast Asia: Vector-animal-human interface. Acta Trop. 2020, 202, 105282. [Google Scholar] [CrossRef] [PubMed]
- Christian, C.R. A case of typhus due to tick bite. J. R. Army Med. 1932, 59, 445–450. [Google Scholar]
- MacNamara, C.V. An epidemic of typhus (vector unknown) in the Simla Hills. J. R. Army Med. Corps 1934, 64, 174–186. [Google Scholar]
- Mehta, D.R. Studies on typhus in Simla Hills. VIII. Ectoparasites of rats and shrews with special reference to their possible role in the transmission of typhus. Indian J. Med. Res. 1936, 25, 353–365. [Google Scholar]
- Boyd, J.S.K. Fevers of typhus group in Inda: Analysis of 110 cases reported in 1934. J. R. Army Med. Corps 1935, 65, 289–305. [Google Scholar]
- Farner, D.S.; Katsampes, C.P. Tsutsugamushi disease. US Naval Med. Bull. 1944, 43, 800–836. [Google Scholar]
- Bardhan, P.N. Typhus in the United Provinces of India. Being a contribution to the study of typhus fever. Indian Med. Gaz. 1944, 79, 150–154. [Google Scholar]
- Maitra, G.C.; Gupta, P.N.S. A Note on Cases of Typhus Fever in Burma and Their Distribution. Indian Med. Gaz. 1936, 71, 572–574. [Google Scholar]
- Nicholls, L. A case of tsutsugamushi (rural typhus) in Ceylon. Br. Med. J. 1940, 2, 490. [Google Scholar]
- Audy, J.R. A summary topographical account of scrub typhus 1908–1946. In Bulletins from the Institute for Medical Research; Federation of Malaya, No.1 of 1949; Government Press: Kuala Lumpur, Malaysia, 1949. [Google Scholar]
- Lewis, M.D.; Yousuf, A.A.; Lerdthusnee, K.; Razee, A.; Chandranoi, K.; Jones, J.W. Scrub Typhus Reemergence in the Maldives. Emerg. Infect. Dis. 2003, 9, 1638–1641. [Google Scholar] [CrossRef]
- Varghese, G.M.; Janardhanan, J.; Mahajan, S.K.; Tariang, D.; Trowbridge, P.; Prakash, J.A.J.; David, T.; Sathendra, S.; Abraham, O.C. Molecular epidemiology and genetic diversity of Orientia tsutsugamushi from patients with scrub typhus in 3 regions of India. Emerg. Infect. Dis. 2015, 21, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Rahi, M.; Gupte, M.; Bhargava, A.; Varghese, G.M.; Arora, R. DHR-ICMR Guidelines for Diagnosis & Management of Rickettsial Diseases in India. Indian J. Med Res. 2015, 141, 417–422. [Google Scholar]
- Khan, S.A.; Bora, T.; Chattopadhyay, S.; Jiang, J.; Richards, A.L.; Dutta, P. Seroepidemiology of rickettsial infections in Northeast India. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Biswal, M.; Kumar, A.; Zaman, K.; Jain, S.; Bhalla, A. Scrub Typhus in a Tertiary Care Hospital in North India. Am. J. Trop. Med. Hyg. 2016, 95, 447–451. [Google Scholar] [CrossRef]
- Chaudhry, R.; Thakur, C.K.; Gupta, N.; Sagar, T.; Bahadur, T.; Wig, N.; Sood, R.; Misra, M.C. Mortality due to scrub typhus—Report of five cases. Indian J. Med. Res. 2019, 149, 790–794. [Google Scholar] [CrossRef]
- Kelly, D.J.; Fuerst, P.A.; Ching, W.M.; Richards, A.L. Scrub typhus: The geographical distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin. Infect. Dis. 2009, 48, S203–S230. [Google Scholar] [CrossRef]
- Bonell, A.; Lubell, Y.; Newton, P.N.; Crumb, J.A.; Paris, D.H. Estimating the burden of scrub typhus: A systematic review. PLoS Negl. Trop. Dis. 2017, 11, e0005838. [Google Scholar] [CrossRef]
- Xu, G.; Walker, D.H.; Jupiter, D.; Melby, P.C.; Arcari, C.M. A review of the global epidemiology of scrub typhus. PLoS Negl. Trop. Dis. 2017, 11, e0006062. [Google Scholar] [CrossRef]
- Biswal, M.; Zaman, K.; Suri, V.; Rao, H.; Kumar, A.; Kapur, G.; Sharma, N.; Bhalla, A.; Jayashree, M. Use of eschar for the molecular diagnosis and genotypic characterisation of Orientia tsutsugamushi causing scrub typhus. Indian J. Med Microbiol. 2018, 36, 422–425. [Google Scholar] [CrossRef]
- Elliott, I.; Pearson, I.; Dahal, P.; Thomas, N.V.; Roberts, T.; Newton, P.N. Scrub typhus ecology: a systematic review of Orientia in vectors and hosts. Parasites Vectors 2019, 12, 513–536. [Google Scholar] [CrossRef]
- Batty, E.M.; Chaemchuen, S.; Blacksell, S.D.; Richards, A.L.; Paris, D.; Bowden, R.; Chan, C.; Lachumanan, R.; Day, N.; Donnelly, P.; et al. Long-read whole genome sequencing and comparative analysis of six strains of the human pathogen Orientia tsutsugamushi. PLoS Negl. Trop. Dis. 2018, 12, e0006566. [Google Scholar] [CrossRef] [PubMed]
- Fleshman, A.C.; Mullins, K.E.; Sahl, J.W.; Hepp, C.M.; Nieto, N.C.; Wiggins, K.B.; Hornstra, H.; Kelly, D.J.; Chan, T.C.; Phetsouvanh, R.; et al. Comparative pan-genomic analyses of Orientia tsutsugamushi reveal an exceptional model of bacterial evolution driving genomic diversity. Microb. Genom. 2018, 4, 4. [Google Scholar] [CrossRef]
- Giroud, P.; Jadin, J. Presence des anticorps vis-a-vis de Rickettsia orientalis chez les indigenes et des Asiatiques vivant au Ruanda-urundi (Congo Belge). Bull. Société Pathol. Exotique 1951, 44, 50–51. [Google Scholar]
- Osuga, K.; Kimura, M.; Goto, H.; Shimada, K.; Suto, T. A case of tsutsugamushi disease probably contracted in Africa. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 95–96. [Google Scholar] [CrossRef]
- Ghorbani, R.P.; Ghorbani, A.J.; Jain, M.K.; Walker, D.H. A case of scrub typhus probably acquired in Africa. Clin. Infect. Dis. 1997, 25, 1473–1474. [Google Scholar] [CrossRef]
- Groen, J.; Nur, Y.A.; Osterhaus, M.E. Scrub and murine typhus among Dutch travellers. Infection 1999, 27, 291–292. [Google Scholar]
- Izzard, L.; Fuller, A.; Blacksell, S.D.; Paris, D.H.; Richards, A.L.; Aukkanit, N.; Nguyen, C.; Jiang, J.; Fenwick, S.; Day, N.P.J.; et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. Clin. Microbiol. 2010, 48, 4404–4409. [Google Scholar] [CrossRef]
- Balcells, M.E.; Rabagliati, R.; García, P.; Poggi, H.; Oddo, D.; Concha, M.; Abarca, K.; Jiang, J.; Kelly, D.J.; Richards, A.L.; et al. Endemic scrub typhus-like illness, Chile. Emerg. Infect. Dis. 2011, 17, 1659–1663. [Google Scholar] [CrossRef]
- Thiga, J.W.; Mutai, B.; Eyako, W.; Ng’ang’a, Z.; Jiang, J.; Richards, A.L.; Waitumbi, J.N. High sero-prevalence and IgG titers for spotted fever and scrub typhus in patients with febrile illness in Kenya. Emerg. Infect. Dis. 2015, 21, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Maina, A.N.; Farris, C.M.; Odhiambo, A.; Jiang, J.; Laktabai, J.; Armstrong, J.; Holland, T.; Richards, A.L.; O’Meara, W.P. Q fever, scrub typhus, and rickettsial diseases in children, 2011–2012 Kenya. Emerg. Infect. Dis. 2016, 22, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Horton, K.C.; Jiang, J.; Maina, A.; Dueger, E.; Zayed, A.; Ahmed, A.A.; Pimentel, G.; Richards, A.L. Evidence of Rickettsia and Orientia infections among abattoir workers in Djibouti. Am. J. Trop. Med. Hyg. 2016, 95, 462–465. [Google Scholar] [CrossRef]
- Cosson, J.F.; Galan, M.; Bard, E.; Razzauti, M.; Bernard, M.; Morand, S.; Brouat, C.; Dalecky, A.; Bâ, K.; Charbonnel, N.; et al. Detection of Orientia sp. DNA in rodents from Asia, West Africa and Europe. Parasites Vectors 2015, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Kolo, A.O.; Sibeko-Matjila, K.P.; Maina, A.N.; Richards, A.L.; Knobel, D.L.; Matjila, P.T. Molecular detection of zoonotic rickettsiae and Anaplasma spp. in domestic dogs and their ectoparasites in Bushbuckridge, South Africa. Vector-Borne Zoonotic Dis. 2016, 16, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Masakhwe, C.; Linsuwanon, P.; Kimita, G.; Mutai, B.; Leepitakrat, S.; Yalwala, S.; Abuom, D.; Auysawasi, N.; Gilbreath, T.; Wanja, E.; et al. Identification and characterization of Orientia chuto in trombiculid chigger mites collected from wild rodents in Kenya. J. Clin. Microbiol. 2018, 56, e01124-18. [Google Scholar] [CrossRef]
- Kocher, C.; Jiang, J.; Morrison, A.C.; Castillo, R.; Leguia, M.; Loyola, S.; Ampuero, S.; Bausch, D.G.; Richards, A.L. Scrub typhus in the Peruvian Amazon Basin. Emerg. Infect. Dis. 2017, 23, 1389–1391. [Google Scholar] [CrossRef]
- Weitzel, T.; Dittrich, S.; López, J.; Phuklia, W.; Martinez-Valdebenito, C.; Velásquez, K.; Blacksell, S.D.; Paris, D.H.; Abarca, K. Endemic scrub typhus in South America. N. Engl. J. Med. 2016, 375, 954–961. [Google Scholar] [CrossRef]
- Weitzel, T.; Jiang, J.; Acosta-Jamett, G.; Martinez-Valdebenito, C.; Lopez, J.; Richards, A.L.; Abarca, K. Canine seroprevalence to Orientia species in southern Chile: A cross–sectional survey on the Chiloé Island. PLoS ONE 2018, 13, e0200362. [Google Scholar] [CrossRef]
- Abarca, K.; Weitzel, T.; Martínez-Valdebenito, C.; Acosta-Jamett, G. Scrub typhus, an emerging infectious disease in Chile. Rev. Chil. Infectol. 2018, 35, 696–699. [Google Scholar] [CrossRef]
- Weitzel, T.; Acosta-Jamett, G.; Martínez-Valdebenito, C.; Richards, A.L.; Grobusch, M.P.; Abarca, K. Scrub typhus risk in travelers to southern Chile. Travel Med. Infect. Dis. 2019, 29, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, T.; Martínez-Valdebenito, C.; Acosta-Jamett, G.; Jiang, J.; Richards, A.L.; Abarca, K. Scrub typhus in continental Chile, 2016–2018. Emerg. Infect. Dis. 2019, 25, 1214–1217. [Google Scholar] [CrossRef] [PubMed]
- Abarca, K.; Martinez-Valdebenito, C.; Angulo, J.; Jiang, J.; Farris, C.M.; Richards, A.L.; Acosta-Jamett, G.; Weitzel, T. Molecular description of a novel Orientia species causing scrub typhus in South America. Emerg. Infect. Dis. 2020, submitted. [Google Scholar]
- Weitzel, T.; Aylwin, M.; Martinez-Valdebenito, C.; Jiang, J.; Munita, J.M.; Thompson, L.; Abarca, K.; Richards, A.L. Imported scrub typhus: First case in South America and review of the literature. Trop. Dis. Travel Med. Vaccines 2018, 4, 10. [Google Scholar] [CrossRef]
- Acosta-Jamett, G.; Martínez-Valdebenito, C.; Beltrami, E.; Silva-de La Fuente, M.C.; Jiang, J.; Richards, A.L.; Weitzel, T.; Abarca, K. Identification of trombiculid mites (Acari: Trombiculidae) on rodents from Chiloé Island and molecular evidence of infection with Orientia species. PLoS Negl. Trop. Dis. 2020, 14, e0007619. [Google Scholar] [CrossRef]
| Year | Location | Book or Individual |
|---|---|---|
| 313 | China | Zhouhofang by Hong Ge |
| 610 | China | Yuan-Fang Chao |
| 1596 | China | Ben Cao Gang Mu by Shi-Zhen Li |
| 1810 | Japan | Hakuju Hashimoto |
| 1902 | Indonesia | Wilhelm Schüffner |
| 1908 | Philippines | P.M. Ashburn and C.F. Craig |
| 1908 | Taiwan | J. Hatori |
| 1910 | Australia | O. Smithson |
| 1915 | Vietnam | F. Noc and P. Gautron |
| 1915 | Malaysia | A. Kawamura Jr., H. Tanaka, A. Tamura |
| 1932 | India | C.R. Christian |
| Synonyms | Country |
|---|---|
| Shashitsu | China |
| Tsutsugamushi | Japan |
| Kedani disease | Japan |
| Japanese river fever | Japan |
| Flood fever | Japan |
| Island fever | Japan |
| Akamushi disease | Japan |
| Shimamushi disease | Japan |
| Pseudotyphoid | Indonesia |
| Chigger-borne rickettsiosis | Ubiquitous |
| Mite-borne typhus | Ubiquitous |
| Mite fever | Ubiquitous |
| Rural or “K” form of tropical typhus | Malaysia |
| Fiévre exanthématique avec ulcére primaire | French Indo-China |
| Indian mite typhus | India |
| Mijtekoorts | Indonesia |
| Mossman fever | Australia |
| Sarina fever | Australia |
| Tropical typhus | Malaysia |
| Rural typhus | French Indo-China |
| Previous Agent Names | Country | Reference |
|---|---|---|
| Theileria tsutsugamushi | Japan | [12] |
| Rickettsia orientalis | Japan | [16] |
| Rickettsia tsutsugamushi | Japan | [17] |
| Rickettsia akamushi | Japan | [18] |
| Orientia tsutsugamushi | Japan | [22] |
| Locations | Evidence |
|---|---|
| Belgian Congo, Africa | Outbreak investigation |
| Republic of Congo, Africa | Case report |
| Cameroon, Africa | Case report |
| Tanzania, Africa | Case report |
| Dubai, United Arab Emirates | Case report |
| Chiloé Island, Chile, South America | Chiloé cases; continental cases; dog serosurveillance; molecular characterization of cases; detection of agent in mites and rodent tissues |
| Kenya | Seroprevalence study; seroconversion cases; and molecular surveillance among mites and rodents |
| Europe and West Africa | Rodents tissue surveillance |
| Djibouti, Africa | Seroprevalence/conversion among abattoir workers |
| South Africa | Dog blood |
| Iquitos, Peru, South America | Seroprevalence/conversion among fever patients |
| Agent | Location |
|---|---|
| Orientia tsutsugamushi | Tsutsugamushi Triangle |
| Candidatus O. chuto | United Arab Emirates |
| Ca. O. chuto-like | Kenya |
| Candidatus O. chiloensis | Chile |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richards, A.L.; Jiang, J. Scrub Typhus: Historic Perspective and Current Status of the Worldwide Presence of Orientia Species. Trop. Med. Infect. Dis. 2020, 5, 49. https://doi.org/10.3390/tropicalmed5020049
Richards AL, Jiang J. Scrub Typhus: Historic Perspective and Current Status of the Worldwide Presence of Orientia Species. Tropical Medicine and Infectious Disease. 2020; 5(2):49. https://doi.org/10.3390/tropicalmed5020049
Chicago/Turabian StyleRichards, Allen L., and Ju Jiang. 2020. "Scrub Typhus: Historic Perspective and Current Status of the Worldwide Presence of Orientia Species" Tropical Medicine and Infectious Disease 5, no. 2: 49. https://doi.org/10.3390/tropicalmed5020049
APA StyleRichards, A. L., & Jiang, J. (2020). Scrub Typhus: Historic Perspective and Current Status of the Worldwide Presence of Orientia Species. Tropical Medicine and Infectious Disease, 5(2), 49. https://doi.org/10.3390/tropicalmed5020049






