Abstract
The demand for organ transplantation continues to rise worldwide, intensifying the gap between supply and demand and driving research in tissue engineering (TE). Bioprinting, particularly light-based vat photopolymerization (VP) methods such as digital light processing (DLP), has emerged as a promising strategy to fabricate complex, cell-compatible tissue constructs with high precision. In this study, we developed an open-source, bottom-up DLP bioprinter designed to provide a cost-effective and modular alternative to commercial systems. The device was built from commercially available components and custom-fabricated parts, with tolerance allocation and deviation analyses applied to ensure structural reliability. Mechanical and optical subsystems were modeled and validated, and the control architecture was implemented on the Arduino platform with a custom Python-based graphical interface. The system achieved a theoretical Z-axis resolution of 1 m and a vertical travel range of 50 mm, with accuracy and repeatability comparable to research-grade bioprinters. Initial printing trials using polyethylene glycol diacrylate (PEGDA) hydrogels demonstrated high-fidelity microfluidic constructs with adequate dimensional precision. Collectively, these results validate the functionality of the proposed system and highlight its potential as a flexible, precise, and cost-effective platform that is also easy to customize to advance the democratization of biofabrication in TE.
1. Introduction
Worldwide, the number of patients in need of organ transplantation continues to grow, driven by longer life expectancy and expanding population size []. Research findings suggest that donor shortages have resulted in an ongoing disequilibrium between organ supply and demand []. Consequently, tissue engineering (TE) is emerging as a rapidly growing discipline dedicated to repairing, replacing, or regenerating tissues and organs through the convergence of cell biology, materials science, and biomedical engineering []. In recent years, a new approach has rapidly advanced, shifting from a relatively niche technology to a central component of TE, three-dimensional (3D) bioprinting. Bioprinting is a manufacturing process that utilizes bioinks, formulated from living cells and/or biodegradable biomaterials, often in combination with bioactive components, to create engineered constructs with biological functionality []. The transition towards a main component in TE reflects the growing recognition of its potential to fabricate 3D constructs using biocompatible materials that ultimately become functional tissues, offering potential solutions for the replacement of damaged or diseased organs [,,,]. Beyond this, bioprinting also plays an essential role in the development of engineered in vitro biomimetic constructs that mimic human physiological responses. These constructs serve as advanced platforms for drug testing, addressing the limitations of traditional in vitro models and reducing the need for animal experimentation [].
The main advances in 3D bioprinting have occurred through the development of novel bioinks and the modification of conventional 3D printers [,]. Nowadays, there are four primary bioprinting technologies: extrusion-based, jetting-based, laser-assisted, and light-based vat photopolymerization (VP) bioprinting [,,]. Extrusion-based bioprinting has probably emerged as the most commonly employed technique, largely due to its affordability and its capability to process and print a wide range of cells and biomaterials simultaneously []. Jetting-based bioprinting operates on the same working principle as conventional inkjet printers, wherein droplets of bioink are selectively deposited to form successive layers on a hydrogel substrate or Petri dish. Laser-assisted bioprinting employs a focused laser beam to deposit biomaterials with cells onto a target surface, but typically yields a low cell viability, due to the heat generated by the laser []. Light-based VP bioprinting involves the use of photosensitive materials that undergo curing and solidification upon exposure to light []. The technology is one of the most precise of the bioprinting techniques. Early implementations of light-based VP bioprinting [] were limited by a lack of suitable photosensitive hydrogels and the cytotoxic effects of the ultraviolet (UV) light employed. Recent advancements in the field have addressed these initial issues through research on new visible-light-sensitive materials, which significantly enhance biocompatibility and facilitate the use of this technique in TE [].
Light-based VP bioprinting encompasses all methods that employ photosensitive materials and rely on light-induced polymerization of bioinks [,]. These techniques are generally classified into single-photon and multi-photon polymerization []. Single-photon polymerization primarily includes stereolithography apparatus (SLA), digital light processing (DLP), and volumetric printing []. Among the various VP-based techniques, DLP has probably emerged as one of the most promising methods for biofabrication [,]. DLP bioprinting employs projected digital photomasks to induce localized photopolymerization of a liquid bioink, thereby assembling constructs in a layer-by-layer fashion. This technique uses a digital micromirror device (DMD) as a light modulator, enabling precise digital control of high-resolution, pixel-based light patterns that preserve structural fidelity []. The integration of the DMD considerably accelerates the bioprinting process []. Compared to SLA, DLP significantly accelerates the printing process by curing entire layers simultaneously rather than tracing each feature point-by-point with a laser. Unlike volumetric bioprinting, DLP can produce constructs of larger volume while maintaining a spatial resolution that remains comparable to that of volumetric techniques []. Therefore, the key advantages of this approach include rapid printing speed, sufficient resolution for biofabrication applications, and comparatively low manufacturing cost.
DLP has attracted increasing interest in both commercial [] and academic environments. Commercial systems such as the Lumen X platform by Cellink (Gothenburg, Sweden) exemplify the growing adoption of DLP in biomedical research. Simultaneously, various research teams have proposed custom-built DLP bioprinters to expand functionality and reduce fabrication costs by embracing the do-it-yourself (DIY) philosophy []. Owing to these benefits, research groups worldwide have proposed novel developments and design modifications, mostly aimed at broadening the applicability and performance of this technique within their laboratories. Several recent studies have worked on expanding the versatility and growing sophistication of DLP-based bioprinting systems. For example, Grigoryan et al. (2019) developed a DLP system capable of fabricating complex, vascularized tissue constructs, including functional alveolar lung units that exhibited effective blood oxygenation []. Building upon this foundation, the same research group later introduced a multi-material DLP platform incorporating a layer-cleaning mechanism, thereby enabling the fabrication of intricate, multi-material tissue architectures []. In parallel, Bhusal et al. (2022) presented a distinct multi-material DLP bioprinter that employed a rotary base system for material switching, further advancing the modularity of bioprinting hardware []. Complementing these hardware innovations, Yang et al. (2024) [] addressed a key challenge in the field—thermal sensitivity of biological materials—by developing a low-temperature DLP printing process using collagen methacryloyl (ColMA) hydrogels. Their approach yielded mechanically stable constructs with high cell viability, highlighting the clinical potential of DLP platforms for patient-specific scaffold fabrication. Further analyses of different VP bioprinting technologies, available photocurable materials, and future developments in the area of these technologies can be found in several comprehensive reviews of existing literature [,,,,,]. Collectively, these advances underscore the rapid evolution of DLP bioprinting into a versatile and clinically promising platform, with the potential to transform the field of TE.
There is a need to develop flexible DLP bioprinters under open-source licenses and DIY designs. DIY bioprinters are typically developed for targeted applications, and their design is strongly influenced by the characteristics of the bioinks utilized []. This work presents the development of an open-source bottom-up DLP bioprinter designed to offer a cost-effective alternative to commercial systems. The bioprinter is easy to personalize and customize to best fit researchers’ needs. The DLP projector can be easily exchanged with other systems. The system achieved superior Z-axis resolution and precision relative to other research-grade bioprinters after carrying out a thorough assessment of design tolerances often overlooked in other studies. Moreover, it operates entirely with open-source and free software, eliminating the need for proprietary applications. Initial print trials using polyethylene glycol diacrylate (PEGDA) hydrogels—without cells—demonstrated the system’s functionality. Taken together, these features established a robust, accessible, and highly precise DLP bioprinter that advances the democratization of biofabrication.
2. Materials and Methods
2.1. Materials
2.1.1. Mechanical Components
Custom-designed parts were manufactured using aluminum, steel, polylactic acid (PLA), and photo-polymer resin. Steel S275JR EN 10025-2 in sheet and 6061 aluminium blocks (Randrade S.L., Pontevedra, Spain) were machined to produce the required parts. PLA filament (1 spool, Ø ) (Eolas Prints, S.L., Reocin, Spain) and Tough 2000 standard photopolymer resin from Formlabs (Formlabs Inc., Somerville, MA, USA) were used for the production of the small printed parts by both 3D printing technologies.
All the mechanical elements were commercially available. The vertical motion of the stage was solved using a KR1501A-0075-P0-00A0 (THK Co., Yokohama, Japan) linear guide providing a positioning repeatability and accuracy of and , respectively. Motion from the stepper motor was transmitted via a D16L23 flexible coupling (Zhejiang Longwei Tech Co., Shenzhen, China) with Ø3 mm and Ø5 mm bores for the leadscrew and motor shafts. The structural frame was constructed using 30 × 30 extruded aluminum profiles, coupled with 8 mm T-slot hammer head nuts (Bosch Rexroth, Lohr am Main, Germany). The structure was assembled using A2 stainless steel fasteners (ISO 4762) obtained from Rational World S.L., Zaragoza, Spain.
2.1.2. Electronic and Optical Systems
The electronic system was powered by a 24 VDC, 15 A power supply (Mouser Electronics, Inc., Barcelona, Spain), operating within an input voltage range of 110–220 VAC. A NEMA 11 closed-loop bipolar stepper motor model ESS11-01 (StepperOnline, Nanjing, China) was employed to drive the vertical axis. The control architecture was based on the Arduino platform. Specifically, an Arduino Mega 2560 microcontroller board (Arduino, Turin, Italy) served as the central processing unit (CPU) and was interfaced with a RAMPS 1.4 shield (RepRap Project, Bath, UK). An auxiliary Arduino Nano board was integrated to synchronize motion system components.
The optical setup consisted of a LuxBeam 4KAc digital light projector (Visitech, Drammen, Norway), equipped with a DMD DLP660TE (Texas Instruments, Dallas, TX, USA) with a resolution of 2716 × 1528 pixels and a micromirror pitch of . Two types of projection lenses were considered: a standard LRS-20 UV lens (Visitech, Drammen, Norway) with a nominal working distance of 90 and a magnification factor of 2.0×, and an LRS-WQm 3.6× lens (Visitech, Drammen, Norway) with a working distance of and a magnification factor of 3.7×. The LRS-20 UV configuration provided a projected pixel size of over an image area of 29.3 × , whereas the LRS-WQm 3.6× configuration produced a pixel size of 20 across an image area of 54.3 × . Illumination was provided by a light-emitting diode (LED) array with a central wavelength of 405 and an output power of .
2.2. Mechanical Design
The bioprinter was designed with a bottom-up configuration, in which the DLP projector was positioned beneath the stage []. Mechanical modeling followed a bottom-up methodology, with each component designed a priori and independently. A modular architecture was employed to limit interdependence between its modules []. All components were modeled using Autodesk® Inventor (v2024, Autodesk, San Francisco, CA, USA).
The theoretical positioning resolution along the Z-axis, , was determined as follows:
where P is the lead of the screw in mm/rev, and N is the number of full microsteps per revolution of the stepper motor measured in microsteps/rev.
2.3. Tolerance Allocation and Deviation Analysis
Geometrical and dimensional tolerances were assigned according to the functional relevance of each component []. Structural elements, such as the bottom base or FDM-printed electronic supports, were limited by the manufacturing precision of their respective processes. By contrast, elements directly influencing optical accuracy and mechanical alignment—including the projector assembly, vat, and Z-axis platform—were subject to stricter dimensional and geometrical tolerances.
The analysis was performed under worst-case conditions to guarantee robust assembly and reliable performance. A top-to-bottom approach was applied for the base-projector assembly, including (i) assessment of base parallelism deviations, (ii) evaluation of dimensional and perpendicularity tolerances of the spacers, and (iii) estimation of angular deviations in the projector supporting frames. The tolerances were distributed under the assumption that the upper surface of the base was perfectly horizontal, owing to the three-foot calibration system and the use of a circular bubble level. For the Z-axis system, potential inclinations around the X- and Y-axes were evaluated and converted into linear displacement errors over the full actuator stroke, to verify that these remained negligible compared with the theoretical repeatability of the motion mechanism.
Tolerance stack-up was analyzed through trigonometric relations, propagating local deviations into global angular errors. Deviation angle due to parallelism and perpendicularity errors was calculated as follows:
where is the parallelism or perpendicularity tolerance and is the horizontal distance considered.
For the spacer, the worst-case scenario occurred when two adjacent spacers reached the minimum and maximum limits of the dimensional tolerance, while the perpendicularity deviation attained its maximum value on opposite faces. The minimum admissible length was calculated as follows:
where d is the nominal length of the spacer, is the dimensional tolerance, and is obtained from Equation (2) for the perpendicularity tolerance. Conversely, the maximum length was calculated as follows:
where D is the spacer diameter. The angular deviation due to spacer tolerances, , was expressed as follows:
with denoting the distance between spacers.
The total angular deviation was obtained as the sum of all contributions. The regulation capacity r required for the adjustment screws was modeled as follows:
where is the distance between one screw and the line joining the other two.
Residual misalignment was estimated by comparing the maximum error with the design regulation range, and its effect on optical performance was quantified through the image translation at the focal plane, , and the pixel deformation, :
where is the distance between the projector reference surface and the focal plane, is the uncompensated angular deviation, and p is the original pixel dimension.
Finally, for the Z-axis linear guide, potential inclinations were converted into linear displacements errors using
where s is the stroke of the linear guide, and and are the total angular deviations about the X- and Y-axes, respectively, obtained from Equation (2) for each geometrical tolerance and accumulated.
2.4. Fabrication Techniques
The fabrication techniques employed for prototype construction were high-speed machining for aluminum components and 3D printing for polymeric parts. Plasma cutting and turning were also used for the production of specific parts. Aluminum elements were produced using a Datron NEO 2 machining center (DATRON AG, Ober-Ramstadt, Germany). Steel sheets were cut using an CNC plasma router model 20.10 Hellraiser (Agri-cutter, Salamanca, Spain). Two technologies were employed for 3D printing: fused deposition modelling (FDM), using a Prusa i3 MK3S+ (Prusa Research®, Prague, Czech Republic), and stereolithography (SLA), using a Form 3 printer (Formlabs, Somerville, MA, USA). All machines were provided by the Escuela Técnica Superior de Ingeniería Industrial de Béjar, Universidad de Salamanca, Spain.
2.5. Dimensional and Geometrical Verification Protocols
Dimensional and geometrical verifications were conducted using calibrated metrology instruments. Measurements of linear dimensions, hole diameters, and geometrical tolerances—such as parallelism and perpendicularity—were performed.
Dimensional verification was carried out using Vernier calipers and micrometers (Insize Ltd., Suzhou, China), depending on the required precision and measurement range. Hole diameters were measured using internal micrometers and bore gauges, where applicable (Mitutoyo Corporation, Tokyo, Japan). For the assessment of geometrical tolerances—flatness, perpendicularity, and parallelism—a granite surface plate was used as the reference plane, along with dial indicators (Insize Ltd., Suzhou, China) mounted on appropriate stands to detect deviations.
All instruments were verified for calibration validity prior to use and operated under controlled environmental conditions to minimize measurement uncertainty. Multiple measurements were taken for each dimension, and the arithmetic mean was calculated. The standard deviation (SD) was maintained below one-tenth of the specified dimensional tolerance. A similar protocol was followed for geometrical verification: three repeated measurements were performed, and the mean and standard deviation were computed. Here as well, the standard deviation was required to be equal to or less than one-tenth of the corresponding geometrical tolerance, ensuring consistency and repeatability in the verification process.
2.6. System Control and Programming
A modified version of an already published Marlin 3D printer firmware version 1.0.0 [] was used as the program on the ATmega2560 microcontroller. The firmware is deposited into the open data repository for this work. An Arduino Nano served as an intermediary communication interface between the computer and the motor. Communication with the computer was established via a serial port for the bioprinter and through a Video Graphics Array (VGA) connection for the projector. The system integrates a stepper motor with an internal driver, a Z-axis limit switch, and a 24 V fan for cooling the motor driver. The stepper motor is controlled by the ATmega2560 through the RAMPS 1.4 shield, while the Arduino Nano monitors the state of the motor enable signal pin to determine whether the motor is active. Based on this status, the computer software projects the corresponding layer image. The electronic schematic and wiring diagram of the system are presented in Figure 1.
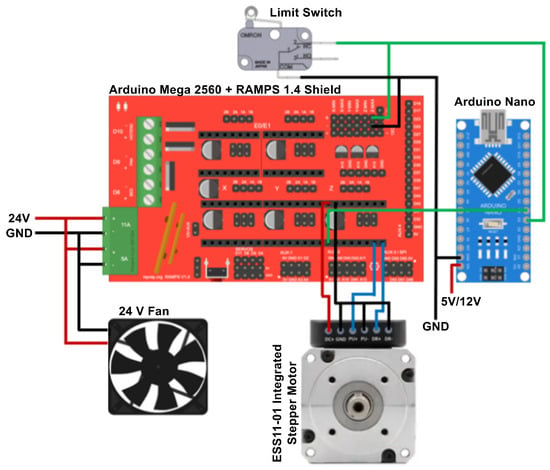
Figure 1.
Overview of the system wiring diagram for the DLP bioprinter control electronics.
Fabrication and manual control of the bioprinter was managed with new graphical user interface (GUI) application. The GUI was developed in Python (version 3.12) with the core functionality relying only packages like Pygame [] and CustomTkinter []. The GUI controls the systems by sending G-Code commands for the vertical movement of the stage, and a sequence of photomasks (images) to the projector, which represent the digital model. The STL (Standard Tessellation Language) files of the bioprinting models were prepared for 3D printing using the freely available Chitubox Basic V2.2.0 software (Chitubox, Shenzhen, China), generating a projection image or photomask for each layer.
2.7. Mechanical Assembly and System Calibration
The bioprinter assembly began with construction of the structural frame to ensure stable connections and precise component fitting. The linear guide was then calibrated relative to the frame, maintaining perpendicularity to the upper base and parallelism to the structure. Fine adjustments were achieved by loosening the guide screws before final tightening.
The projection module was installed after alignment of the frame and the linear guide. The upper base was leveled using a bubble level and the calibration system. The projector was subsequently aligned using its three adjustable screws and the bubble level, and its focusing mechanism was adjusted symmetrically to maintain horizontality. Once sharpness was optimized, the fixing screws were tightened to prevent shifts due to vibrations.
Stage alignment was performed to ensure homogeneous layer thickness. The build platform was manually lowered through the GUI until it nearly touched the resin bucket. The ball-joint screws were loosened, and the vertical adjustment was used to bring the platform into contact with the Polydimethylsiloxane (PDMS)-coated bottom surface. The screws were then re-tightened and the adjustment nut was fixed. The Z-axis stroke was calibrated by running the homing function in the GUI, defining the reference position of the stage.
2.8. Analysis of Accuracy and Repeatability
The accuracy and repeatability of the machine were evaluated using an adaptation of the ISO 9283:1998 standard, which is intended for characterizing these parameters in numerically controlled axes []. The machine was tested at the same location where it had previously been calibrated. The test was conducted while maintaining the room temperature within the range of 18–22 °C.
The positive direction of the linear guide was defined as upward. The load applied during testing corresponded to the maximum printable volume and was equal to . The machine was operated at 100% of its rated speed during the procedure.
The test consisted of 30 motion cycles between two positions, and , under the maximum load and speed conditions of the machine. Positions and were located 5 from the upper and lower travel limits of the machine, respectively. The motion began at position and proceeded to position , where data recording started. Data acquisition was carried out using dial indicators—with a resolution of —placed at the initial positions, and , which were assumed to be theoretically correct reference points. Upon completion of the 30 motion cycles, the recorded position data were used to calculate the accuracy and repeatability values. These parameters were calculated to evaluate the system’s capacity to achieve a specific position and to travel a given distance.
The accuracy of position, , was calculated according to the following expressions:
where is the theoretical coordinate of the programmed point, is the coordinate at cycle i, and n is the total number of cycles.
The repeatability of position, , was determined using the following expressions:
where
The accuracy and repeatability of position were calculated for both points and .
On the other hand, the accuracy of distance, , was calculated according to the following expressions:
where is the coordinate at the achieved point , is the coordinate at the departure point , and and are the theoretical coordinates of points and .
The repeatability of distance, , was determined using the following expression:
2.9. Bioprinting of DLP-Based Constructs
The custom-designed DLP bioprinter was employed to fabricate high-resolution constructs and demonstrate system functionality. Polyethylene glycol diacrylate (PEGDA) bioink was used to validate the design. PEGDA500 PhotoInk was purchased from CELLINK (San Diego, CA, USA) and used as received, following the manufacturer’s protocol.
In brief, the bioprinting process was performed in a 50 mm PDMS-coated Petri dish. A 405 nm light source was projected through the PEGDA bioink, inducing layer-by-layer photopolymerization. After the exposure of each layer, the stage was raised by 100 , enabling adhesion of successive layers upon subsequent illumination.
To evaluate the reproducibility of the printing process, the same 3D model was printed four times () under identical conditions. In addition, a second, more complex construct—based on the axial vessel and helix design reported by Grigoryan et al. []—was produced to further demonstrate the capabilities of the bioprinting system.
After printing, the constructs were gently detached from the stage and washed three times with sterile water. The printed microfluidic models were then soaked in water for 15–20 min to remove residual unpolymerized bioink. The constructs were transferred to 50 mL Falcon tubes containing sterile water for preparation for subsequent experiments or microscopy.
2.10. Inverted Optical Microscopy
An inverted optical microscope (Leica DMi8, University of Valencia, Spain) was used to analyze the final geometry of the printed parts and to identify potential fabrication defects. The dimensions of the microfluidic chips were measured using ImageJ (version 1.54i) software (National Institutes of Health, Rockville, MD, USA).
2.11. Statistical Analysis
Statistical analysis was performed using MATLAB © (version R2024b). For each printed part, each selected dimension was measured four times, and the average value of these repeated measurements was calculated. These average values, one per part, were then used to evaluate the reproducibility of the process. The mean and standard deviation (SD) of these values were calculated to characterize the dimensional consistency between the printed parts.
3. Results and Discussion
3.1. 3D Bioprinter Design and Prototyping
3.1.1. Detailed CAD Modeling and Final Prototype
The design of DLP bioprinters must take into consideration a wide range of specifications to be capable of creating highly complex tissue structures with high surface quality. These specifications include printing dimensions, the position and wavelength of the light source, and the type of bioink to be employed []. Herein, we proposed a modular design that potentially avoids restricting these specifications to a predefined set []. This approach allowed the development of a system that can be readily adapted to parameter changes, thereby enhancing the overall versatility of the machine. An initial set of parameter values was established, after which the system was divided into modules, resulting in five primary modules (Figure 2). The initial parameter values are summarized in Table 1.
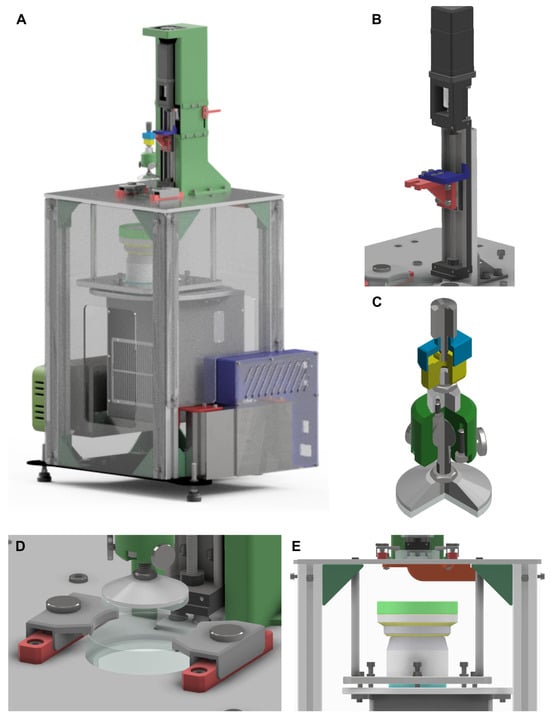
Figure 2.
Design of the light-based VP bioprinter. (A) General CAD model of the fully assembled system. (B) Z-axis module, (C) main stage, (D) vat-fixation mechanism, and (E) light source system.

Table 1.
Operational parameters and main specifications of the bioprinting platform proposed in the design.
Figure 2A shows the CAD assembly of the prototype, while Figure 2B–E illustrate its five main modules. Figure 2B depicts the Z-axis module, which provides vertical motion of the stage. This module comprises a linear guide coupled to a stepper motor and an aluminum frame that supports the entire Z-axis. The theoretical resolution of this axis, determined by Equation (1), was 1 . This resolution was deemed sufficient for operating a DLP bioprinter in terms of bioink properties and cell culture requirements [,]. In addition, it should be noted that the nominal positioning accuracy and repeatability reported by the manufacturer of the linear guide are and , respectively. Overall, the theoretical resolution and manufacturer-reported tolerances confirmed that the Z-axis module provided the precision necessary for reliable and reproducible operation of the DLP bioprinter.
The stage module is mounted on the Z-axis module as shown in Figure 2C. The printing platform was designed to maintain parallelism with the vat, which consists of a Petri dish containing a cured PDMS film. To achieve the requirements in terms of parallelism, a circular glass platform with a ball joint was incorporated into the design. This systems allows a precise parallel alignment during the calibration phase. In addition, a vertical adjustment mechanism was integrated into the system, enabling a fine control of the contact between the glass and the vat. Both mechanisms ensure homogeneity of the layer height across the whole are of the glass. This configuration, together with the specifications of the selected linear guide, provided a usable vertical travel range of approximately 50 . To secure the vat, the fixation mechanism shown in Figure 2D employs a screw–spring jaw system that facilitates both easy handling and rapid replacement.
Another crucial element of the system is the light source module (Figure 2E), which was designed to accommodate different projection lenses or microscope objectives, as well as alternative DLP projectors. This adaptability enables researchers to work with a broader range of bioinks than with commercially available equipment. The module consists of a supporting frame equipped with a three-screw tilt mechanism that ensures proper alignment of the projected image at the focal plane. The design incorporates custom-made spacers for each optical lens, allowing straightforward compensation for differences in focal length. Two optical lenses were evaluated in the system: an LRS-20 model, with a nominal working distance of 90 and a spacer length of , and an LRS-WQm 3.6× lens, with a nominal working distance of 125 and a spacer length of . Considering that the DMD generates an image of 2716 × 1528 pixels with a micromirror pitch of , the use of these lenses allowed the projection of images with different resolutions and field sizes. The LRS-20 lens, providing a magnification factor of 2.0×, yielded a projected image area of 29.3 × with an effective pixel size of . In contrast, the LRS-WQm 3.6× lens, with a magnification factor of 3.7×, produced a larger image area of 54.3 × but with a lower spatial resolution, corresponding to a pixel size of 20 . If different resolutions are required, other optical configurations, such as the LRS-10 lens (1.0× magnification), can also be implemented simply by adapting the spacer length, further demonstrating the versatility of the module design. Once the appropriate spacers were installed, fine alignment was achieved using the three-screw tilt mechanism. A second set of three screws was used to maintain a constant distance between the two frames, preventing any defocus during operation. The system design deliberately avoids the use of mirrors for light redirection, resulting in a less compact bioprinter but eliminating issues associated with the limited reflectance wavelength range of mirrors. The adopted vertical arrangement provides a flexible optical platform that facilitates the integration of various lenses and projection systems by modifying the supporting frame and spacer dimensions.
All modules were assembled onto the structural module, which can be identified in Figure 2A. This metallic frame was designed to provide sufficient rigidity to minimize vibrations during operation while also supporting overall system calibration. A three-leg calibration system with anti-vibration feet was implemented to ensure horizontal alignment of the upper platform. The lower platform was planned to be fabricated from plasma-cut steel, whereas for the upper platform the technology assigned was High-Speed Machining (HSM) to meet the required geometrical and dimensional tolerances. The upper platform also included precisely designed pockets for the accurate positioning of the Z-axis frame and the Petri dish. The results of the final prototype are shown in Figure 3.

Figure 3.
Detail of the final prototype. (A) General view, (B) Z-axis system, (C) DLP supporting structure and (D) linear guide.
Dividing the design into independent modules not only facilitates assembly but also enables future upgrades and modifications. For instance, the Z-axis module can be modified with a longer linear guide, along with corresponding adaptations to the stage and upper frame, if larger printing volumes are required. In the current configuration, the printing volume was considered sufficient for the intended applications, since microfluidic devices for tissue engineering are generally small in size. These devices typically include microscale channels and chambers designed to mimic cellular microenvironments; therefore, their fabrication does not require large amounts of material. The estimated Z-axis resolution was comparable to that of commercial printers; however, the actual accuracy and repeatability of the device are discussed in later sections. The optical configuration also allows adjustment of the XY resolution and projected image size by replacing the projection lens. The LRS-20 lens provides a pixel size of , while the LRS-10 lens can achieve a finer pixel size of , although this comes at the cost of a reduced printing area due to the smaller pixel dimensions imposed by the collimation system. Alternatively, projection lenses with larger pixel sizes—such as the LRS-WQm 13×, which yields a pixel size of 70 —can also be installed to fabricate larger parts. This, however, would require structural modifications to the frame and platform, owing to the increased working distance and image size. Furthermore, the projector design allows modification of the illumination wavelength by replacing the LED matrix. In the present configuration, both UV (405 ) and visible (465 ) monochromatic light sources can be employed, enabling the use of different photoinitiators and improving compatibility with a wide range of bioinks. The use of visible light is particularly advantageous for maintaining cell viability []. In addition, the projector was mounted vertically, eliminating optical aberrations, distortions, and power losses typically associated with the use of 45° mirrors for image redirection.
The cost of the device, excluding the projection system, was EUR 2045, which is reasonable for research applications. The DLP projector and the two optical lenses had a combined cost of EUR 11,210–EUR 6060 for the projector and approximately EUR 2550 per lens—resulting in a total system cost of EUR 13,255. Notably, the modular architecture allows the projection module to be replaced independently, enabling cost optimization by selecting alternative projectors. For instance, using the DLPDLCR4710EVM-G2 from Texas Instruments, currently priced at approximately EUR 1800 in Europe, in combination with a standard 2.0× magnification optical lens (EUR 2500), would reduce the overall cost to around EUR 6345, representing a decrease of more than 50% without compromising system functionality. This modular approach allows the total expenditure to be adjusted to the available budget while remaining substantially lower than that of commercial DLP bioprinters. Furthermore, it provides exceptional flexibility, as both the light source and the XY resolution can be easily modified—capabilities that are typically absent in standard commercial systems. Overall, the platform constitutes a scalable and adaptable framework that satisfies current research requirements and ensures compatibility with future developments in bioprinting technology.
3.1.2. Tolerance Allocation and Maximum Deviation Analysis
The tolerance allocation guaranteed the mechanical stability, optical accuracy, and overall reliability of the developed bioprinter. The analysis was carried out separately for the optical system and the Z-axis motion system, as illustrated in Figure 4.
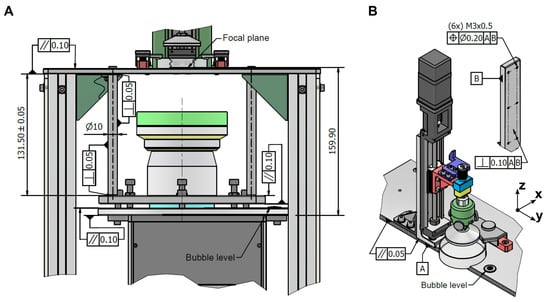
Figure 4.
Tolerance allocation on critical areas for the resolution of printing: (A) Projector assembly module and (B) Z-axis module.
For the optical system (Figure 4A), the evaluation of tolerances was conducted in three stages. In the first stage, the parallelism of the upper base was analyzed, revealing a maximum angular deviation of . This value was obtained with Equation (2), assuming that the tolerance interval reached its maximum between two joints with the spacers. In the second stage, the dimensional variability of the spacer was assessed. Considering the LRS-20 lens with a nominal length of , and applying Equations (3) and (4), the spacer length was found to range between 131.44 and . This variation resulted, according to Equation (5), in an angular deviation of . In the third stage, the projector supporting frame was examined, contributing an additional deviation of .
The combination of these three contributions led to a total angular deviation of . Based on this value, the regulation capacity required for the adjustment screws was estimated using Equation (6), yielding . Since the system design incorporated a regulation range of 4 , the projector calibration mechanism was adequately dimensioned to compensate for the accumulated deviation, ensuring that the system maintained the required alignment. After this regulation, the only residual misalignment originated from the projector supporting frame. In practice, alignment was performed with a bubble level placed on the frame itself, which meant that small variations in the projector mount were not entirely corrected. The residual angular deviation was therefore . Assuming this deviation originated at the projector surface ( ), Equation (7) predicted an image translation of , a value that is fully absorbed by the optical system. Additionally, the pixel deformation introduced by the optical lens is negligible, corresponding to for a pixel size of .
For the Z-axis system (Figure 4B), the tolerance analysis showed maximum angular deviations of around the X-axis and around the Y-axis under worst-case conditions. Over the 50 stroke of the linear guide, these angular deviations corresponded, according to Equation (9), to a linear positioning error of . This error is negligible compared with the repeatability specified by the manufacturer ( ), thus confirming that the linear guide performance was not compromised by tolerance-induced deviations.
Based on the deviation analysis, the results confirm that compliance with the tolerance specifications of the components ensures that the deviations introduced by these elements are either negligible or effectively corrected by the projector’s calibration system. Consequently, the machine maintains high precision on the XY-plane—both in terms of pixel deformation and focus—as well as in the Z-axis translation controlled by the linear guide.
3.1.3. Prototype Fabrication and Verification of Compliance with Design Specifications
The prototype was fabricated using the technologies described in Section 2.4. Verification of compliance with design specifications focused on dimensions and geometrical features that significantly influence assembly performance. Measurements were evaluated to determine whether they fell within the specified tolerance limits. The results for geometrical and dimension are presented in Table 2 and Table 3, respectively.

Table 2.
Geometrical tolerances and measurement results of critical components. Units in mm.

Table 3.
Dimensional tolerance and measurement results of the Spacer braces. Units in mm.
The results in Table 2 and Table 3 show that the critical components were fabricated within the specified tolerance limits. The average intervals of the measured features were well below the maximum values of the corresponding Interval Tolerance (IT), indicating that the assigned tolerances could have been more stringent, allowing higher precision without compromising manufacturability. These relatively small intervals also reduce error accumulation during assembly, ensuring that the initial assembly presents deviations lower than those predicted by the tolerance allocation strategy and thereby achieving superior alignment from the outset. Most components exhibited highly consistent dimensions, with standard deviations below one-tenth of the corresponding IT, confirming both the reliability and the repeatability of the measurements. Among them, the upper frame, the lower DLP frame, and the Z-axis frame showed slightly larger average intervals. For the upper base, this variation corresponds to the part’s overall dimensions, as its longest length is 300 . For the other two, the greater deviations are attributable to their fabrication via 3D printing rather than high-speed machining, since this technique imposes stricter limitations on manufacturing tolerances. Nevertheless, in all these components, the IT was comfortably satisfied. Regarding dimensional tolerances, only the length of the spacers was measured, and the obtained values were very close to the nominal length, demonstrating excellent dimensional fidelity.
Overall, these results validate that the combination of the selected fabrication technologies and the applied tolerance allocation strategy yields a prototype that not only meets the design specifications but also provides robustness in assembly, minimizing cumulative errors and supporting high-quality functional performance.
3.2. Graphical User Interface and Device Operation
Devices intended for industrial or biomedical applications are often restricted to proprietary control software, which increases acquisition and operating costs and creates reliance on the manufacturer for technical support, usually subject to additional fees. To address these limitations, a custom open-source control package was developed in Python, compatible with any DLP-based device. This solution eliminates licensing costs, improves accessibility, and provides users with complete control. The program employs the Pygame library for image projection and CustomTkinter for the graphical interface. Printing is initiated by importing sliced images—generated with free software such as Chitubox—into a designated folder, from which the program automatically produces and executes the corresponding G-code.
The GUI comprises three menus: Auto Print, Calibration, and Manual Mode. Auto Print (Figure 5A) allows users to define parameters (layer thickness, exposure time, lifting distance, etc.) and initiate fabrication with a single command. A “Bed Temperature” option was included as a placeholder for future upgrades of the vat system for fine tune of hydrogel temperature. Calibration (Figure 5B) guides the alignment of the printing carriage; this step is recommended before each bioprinting process. Manual Mode (Figure 5C) provides direct control of the bioprinter and the DLP projector through G-code commands or dedicated buttoss, enabling relative platform movement and on-demand image projection. To facilitate use, each menu incorporates a console that reports system status, warnings, and errors. Collectively, these menus provide users with the basic controls required for bioprinting. The open-source nature of the GUI allows the future integration of additional features such as motion visualization, temperature monitoring, or custom slicing algorithms.
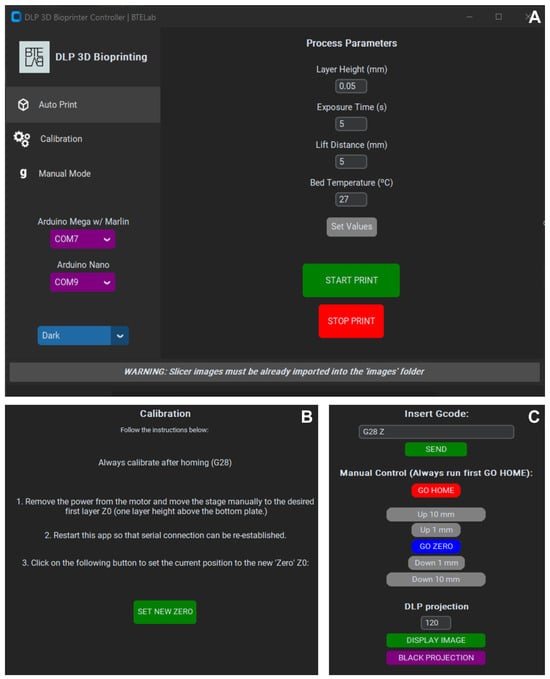
Figure 5.
Main menus of the open-source GUI software: (A) Auto Print, (B) Calibration, and (C) Manual Mode.
To execute these functions, the GUI version 0.1 software communicates with the system through two channels: (i) two serial ports for motion control and (ii) a VGA connector for transmitting images to the DLP projector. This lightweight architecture enables reproducible operation, reduces setup time, and minimizes operator error, making the system accessible even to users with limited knowledge of 3D printing. In addition, basic error handling has been implemented in the form of a warning that alerts the user if the input images are not located in the designated folder prior to starting a print.
Synchronization between platform motion and image projection is handled sequentially: the Z-axis motor receives a movement command, and the software monitors the motor’s enable signal via the Arduino Nano. Once the enable signal indicates that the platform has reached the commanded height, image projection begins. After exposure, the platform returns to its retracted position, with the software similarly detecting the completion of the movement. Given that both layer movement and exposure times are on the order of several seconds, communication latency—typically below a few tens of milliseconds—is negligible. Consequently, dedicated latency testing was not required, and this sequential procedure ensures correct alignment between platform motion and image projection without the need for high-speed synchronization. Overall, we believe that our system provides the end user with tools that make 3D bioprinting possible with even more flexibility and adaptability than other proprietary solutions, which indeed are sometimes restricted to specific hardware.
3.3. Evaluation of Accuracy and Repeatability
The application of Equation (1), with the parameters of the linear guide and the stepper motor employed in the design, yielded a theoretical Z-axis resolution of 1 . This value represents an ideal estimation that assumes a perfectly rigid system, neglecting practical factors such as mechanical backlash between the leadscrew and the ball nut, the inclination of the linear guide, and slight deviations in motor shaft rotation. To evaluate the actual performance of the machine under operating conditions, an experimental assessment of accuracy and repeatability was performed.
Figure 6A illustrates the experimental setup used to measure accuracy and repeatability, as described in Section 2.8. Two dial indicators were positioned at the test points. The resulting measurements and their differences are presented in Figure 6B. A total of 30 motion cycles were executed, with a travel distance of 40 . From these results, positioning accuracy and repeatability were and in , and and at , respectively. The distance accuracy and repeatability were and , respectively. As shown in Figure 6B, the evolution of the real position of the actuator in the cycles exhibited minimal variation. For , the variation was restricted to an interval of 2 , with a minimum value of 3 and a maximum of 5 . In contrast, showed a wider interval, consistent with its higher repeatability value. The deviation from the theoretical position ranged between 1 and 5 . These results indicate that, over 30 cycles, the position reached in each cycle remained stable, demonstrating that the accumulation of backlash was effectively counteracted during software development. Similarly, distance accuracy and repeatability exhibited a comparable trend: the difference between the theoretical and actual travel distance was limited to an interval of approximately −3 to 2 across all cycles.
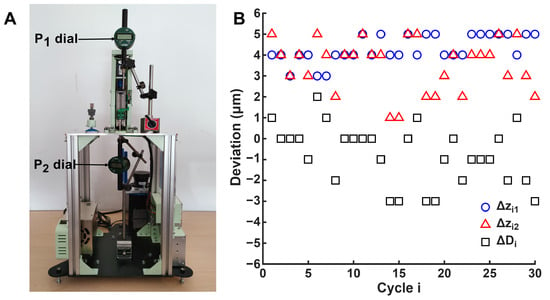
Figure 6.
Accuracy and repeatability tests. (A) Experimental setup. (B) Differences between theoretical and actual positions for () and (), and between the theoretical and actual displacement of the linear actuator ().
The accuracy and repeatability values obtained fall within the expected range according to the manufacturer’s specifications for the linear guide (20 nominal positioning accuracy and 3 repeatability) and, in some cases, improve them. The manufacturer’s positioning accuracy refers to the maximum error between the commanded and the actual travel distance, which corresponds to the distance accuracy defined in Equation (16). Meanwhile, positioning repeatability refers to the difference between the theoretical and actual position of a given point, comparable to the repeatability expressed in Equation (12). As shown, distance accuracy improved significantly relative to the manufacturer’s data (from 20 to ). This improvement can be attributed to the use of a high-precision motor with a theoretical resolution of 1000 microsteps per revolution, enabling a minimum displacement of 1 . Additional factors, such as optimized microstep configuration in Marlin and backlash compensation, further underscore the importance of motor control and actuation in enhancing performance. In contrast, positioning repeatability results were comparable to those reported by the manufacturer. Our measurements at and were and , respectively, compared with the manufacturer’s reported 3 . This outcome indicates that system control and calibration were correctly managed and did not limit the precision of the fabrication. Minor deviations between experimental and reference values can be attributed to test conditions, such as orientation of the axis (horizontal vs. vertical), calculation methods, load variations, and environmental factors. A potential improvement for future work is the implementation of a closed-loop control system using the incremental encoder integrated into the motor. However, as Marlin currently operates in open loop, this integration would need to be managed at the software level.
From an application standpoint, the measured positioning and distance accuracy (50 –75 ) remained significantly higher—by more than an order of magnitude—than the typical layer thickness in bioprinting, which is largely constrained by cell dimensions. Although the actuator showed a tendency to slightly overshoot during the first travel, this affected only the first layer, while subsequent layers were unaffected. For a typical layer height of 50 , the impact corresponds to about 10% of the layer thickness, which can be absorbed by the fabrication process. Consequently, the risk of print failure due to positioning errors was minimal, and the machine provided sufficient accuracy for biofabrication. With respect to repeatability, the values obtained for position and distance were more than adequate for this application. The maximum repeatability error was for the travel distance, which is less than 10% of the layer thickness. Therefore, these effects can be considered negligible compared with other critical parameters, such as the curing capacity of the bioink or the parallelism between the stage and the printing plate.
Overall, the results indicate that the machine achieves consistent and reliable Z-axis displacements, ensuring that successive layers are deposited with adequate precision. Nevertheless, further refinements could still be pursued, particularly through improved motor control algorithms or optimization of environmental conditions.
3.4. Initial Printing Tests with PEGDA Hydrogel Models
To demonstrate the possibility of complex printing constructs using different bioinks, a series of initial printing tests were conducted using PEGDA hydrogels to evaluate the basic functionality of the bioprinter. For this purpose, the representative microfluidic chip with a serpentine micro-channel shown in Figure 7A was fabricated under controlled conditions. The LRS-WQm 3.6× optical lens was used, which provides a projected image area of 54.3 mm × with a pixel size of 20 . For the layer thickness considered of 100 , we found that the best printing parameters for PEGDA were 5 exposure time and 5 retraction distance. These values balanced the crosslinking of the hydrogels with minimal overexposure and were in agreement with other publications in the field []. These values, together with the resin vat employed, seemed to promote suitable layer separation from the PDMS film. Other additional mechanisms could be proposed to minimize suction forces during the printing process; however, they should be compatible with the use of cells in the bioink [].
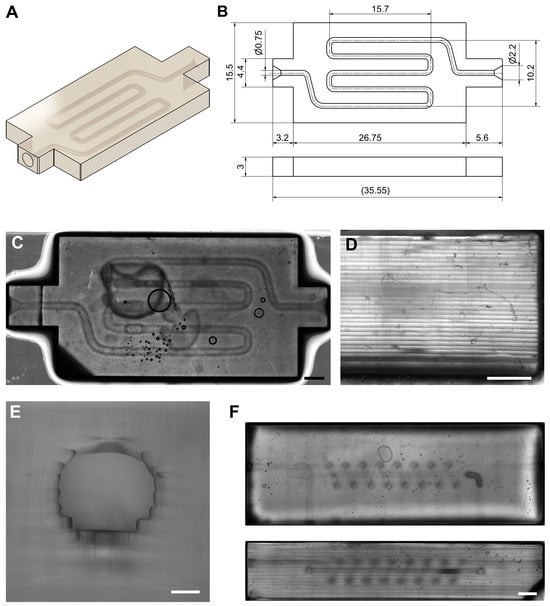
Figure 7.
Representative images of the microfluidic serpentine micro-channel model printed with the DLP: (A) overview of the three-dimensional model; (B) main dimensions of the model; (C) top view of a printed model (Scale bar: 2 mm); (D) details of the printed layers in the lateral view (Scale bar: 1 mm); (E) detail of the circumferential ducts (Scale bar: 200); (F) top (up) and lateral (down) views of another printed microfluidic serpentine model (Scale bar: 2 mm).
The model was printed four times () under identical conditions to assess the reproducibility of the bioprinter. The consistent fabrication of the replicas confirmed the stability of the projection system, uniformity of the bioink photopolymerization, and precision on the layer alignment. These factors would assess the reliability of the mechanical and optical setup proposed. The main dimensions of the digital model are shown in Figure 7B, while Figure 7C–E present representative optical images of the fabricated constructs. Figure 7C,D illustrate the morphological fidelity of the printed parts. Figure 7C shows the complete serpentine structure, where the macroscopic geometry of the fabricated model closely resembles the CAD design, while picture D displays a detailed view of the stacked layers, where the homogeneity and planarity of each layer can be appreciated. It is important to note that the planarity of these hydrogel surfaces is highly dependent on their hydration state. After removal from the aqueous solution, PEGDA constructs begin to lose water and gradually deform, which can slightly affect the dimensional accuracy if they are not measured under hydrated conditions.
A comparison between theoretical CAD dimensions and the experimentally obtained measurements confirmed the high degree of fidelity achieved by the bioprinting process. The following main dimensions were analyzed: overall chip length () , overall chip width () , total height () , serpentine channel diameter () , and mean layer height () . A notable observation is that while the layer thickness is close to the theoretical value, the total height of the printed parts is approximately higher than expected. This discrepancy is attributed to the fabrication of the first layer, which is manually calibrated by the user. During this calibration, the platform was likely adjusted in a way that resulted in a larger initial layer thickness. This is corroborated by Figure 7D, where the lower region shows a layer that is almost three times thicker than the others. Despite this deviation—which does not affect the functionality of the chip—the measured dimensions remained within acceptable limits for biofabrication applications, demonstrating both dimensional precision and reproducibility.
One of the main achievements of this system is the successful fabrication of internal microchannels and conduits (Figure 7E). The printed conduits retained the designed geometry with minimal collapse or occlusion, confirming adequate hydrogel drainage and controlled curing depth. Nevertheless, as expected in the layer-by-layer additive process, the circularity of conduits tends to be partially lost due to the discretization inherent to the printing layers. The thinner the layer thickness, the better the representation of circular geometries. For this initial validation test, layer thickness of 100 was considered sufficient, ensuring fast printing while maintaining acceptable resolution. However, based on the observed reproducibility and mechanical stability and precision of the system, it is feasible to achieve significantly thinner layers, potentially down to approximately 30 , without compromising adhesion or optical curing uniformity. Such refinement will allow future work to reach substantially higher geometric accuracy, especially in curved and internal features. Additionally, the orientation of the model during printing has a significant influence on the accuracy of conduits and overhangs. By reorienting the piece, it is possible to reduce the stair-stepping effect and improve the roundness and continuity of the internal channels—an important consideration for microfluidic and perfusion-based constructs.
Figure 7F presents another example of a microfluidic chip printed model with increased geometric complexity based on a model previously published []. This microfluidic system is composed of an axial vessel and helix around the prior one. The model has enough complexity to demonstrate the capacity of the system to fabricate intricated 3D architectures. The images shows a multilayered serpentine channel with overlapping features of acceptable print resolution. These printed model confirmed that the developed printer can produce high-resolution hydrogel constructs suitable for advanced bioprinting and tissue engineering applications.
Overall, these initial tests confirm that the custom DLP bioprinter provides excellent repeatability, high structural integrity, and good dimensional fidelity when printing PEGDA hydrogels. These results represent a proof of concept demonstrating the feasibility of the device for the fabrication of hydrogel-based microfluidic models. In this study, PEGDA was selected as the printing material because it is commercially available and relatively easy to process compared to other biomaterials. The use of more complex bioinks, such as Gelatin-methacrylate (GelMA) or ColMA, would require stricter temperature control and heating systems. These features are already implemented in the software but have not yet integrated into the physical device. In addition, the constructions fabricated in this work did not include cells, since the main objective was to validate the device’s capacity to produce complex structures characteristic of microfluidic models. Therefore, biological validation was beyond the scope of the present study.
Building on these encouraging results, future efforts will focus on extending the validation of the device for 3D bioprinting applications. This includes printing with diverse bioink formulations, fabricating cell-laden constructs with different cell types, and performing systematic assessments of cell viability. Further studies will also explore a wider range of printing parameters (e.g., exposure time, light intensity, retraction speed), as well as different XY resolutions and light wavelengths and smaller layer thicknesses. Finally, more complex architectures—such as multilayered and branched channel networks will be tested to further evaluate the reliability, versatility, and potential of the system in advanced biofabrication applications. The proposed platform could be potentially used to explore another adaptation to substitute the DLP projector by a LCD-based system; an approach that could be beneficial in terms of cost, precision, and applicability to complex tissue fabrication.
4. Conclusions
This work presents the design, prototyping, and experimental validation of an open-source, bottom-up DLP bioprinter tailored for the fabrication of hydrogel-based microfluidic and TE models. The modular design ensures easy adaptation to future bioprinting requirements and provides flexibility for integrating different light sources and optics. The system also facilitates straightforward calibration. Tolerance allocation and deviation analyses confirmed that the design provides satisfactory mechanical stability and reliable optical alignment, with deviations well within the limits required for standard operation. Fabrication of the prototype and subsequent verification of dimensional and geometrical tolerances demonstrated excellent compliance with the initial specifications. The prototype achieved sufficient accuracy and repeatability to support layer-based bioprinting. The accumulation of errors due to mechanical backlash was successfully mitigated by software compensation, which increased the precision of the system.
The developed open-source Python-based GUI enhanced system accessibility by reducing costs, eliminating licensing barriers, and providing transparent device control. Initial printing trials with PEGDA hydrogel validated the feasibility of the platform for generating microscale microfluidic systems with good fidelity to the digital design. These results confirmed that the system can achieve consistent layer homogeneity, a critical requirement for applications in microfluidics and TE. Overall, the system demonstrates that a cost-effective, modular, and adaptable bioprinter can be fabricated using widely accessible technologies while still delivering performance metrics comparable to commercial equipment. The platform is a promising foundation for further advancements in bioprinting research. Future work will focus on expanding the range of compatible bioinks, the integration of closed-loop calibration, and exploring more complex tissue constructs to fully exploit the potential of this modular architecture in biomedical applications.
Author Contributions
Conceptualization, D.S.-G., A.G.-E.-A., A.G.-M. and A.S.-G.; methodology, D.S.-G., A.G.-E.-A., A.G.-M. and A.S.-G.; software, D.S.-G., A.G.-E.-A., A.G.-M. and A.S.-G.; validation, D.S.-G., A.G.-E.-A., A.G.-M. and A.S.-G.; formal analysis, D.S.-G., A.G.-E.-A., A.G.-M. and A.S.-G.; investigation, D.S.-G., A.G.-E.-A., A.G.-M. and A.S.-G.; resources, D.S.-G., A.G.-E.-A., A.G.-M. and A.S.-G.; data curation; writing—original draft preparation, D.S.-G., A.G.-E.-A., A.G.-M. and A.S.-G.; writing—review and editing, D.S.-G., A.G.-E.-A., A.G.-M. and A.S.-G.; visualization, D.S.-G., A.G.-E.-A., A.G.-M. and A.S.-G.; supervision, D.S.-G., A.G.-E.-A., A.G.-M. and A.S.-G.; project administration, D.S.-G., A.G.-E.-A., A.G.-M. and A.S.-G.; funding acquisition, D.S.-G., A.G.-E.-A., A.G.-M. and A.S.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Consejería de Educación, Junta de Castilla y León (SA108P24); Ministerio de Ciencia, Innovación y Universidades y Agencia Estatal de Investigación (PID2023-149836NB, PLEC2022-009392 and FPU22/03616); Fundación General de la Universidad de Salamanca (PC_TCUE1820P_034); Conselleria de Sanitat Conselleria de Sanidad (CDEI-02/20-A); Agencia Valenciana de la Innovación (CAICO/2023/282).
Data Availability Statement
All data is available upon request and is on the Open Source Framework: https://osf.io/nzfer/ (accessed on 1 September 2025).
Acknowledgments
D.S.-G. would like to thank the Ministerio de Ciencia, Innovación y Universidades for the personal grant and FPU22/03616. The authors also acknowledge the use of QuillBot for style, and spelling purposes; and ChatGPT-5 for English grammar correction. All substantive content and interpretations were developed by the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TE | Tissue engineering |
| 3D | Three-dimensional |
| VP | Vat photopolymerization |
| UV | Ultraviolet |
| SLA | Stereolithography apparatus |
| DLP | Digital light processing |
| DMD | Digital micromirror device |
| DIY | Do-it-Yourself |
| ColMA | Collagen methacryloyl |
| PEGDA | Polyethylene glycol diacrylate |
| PLA | Polylactic acid |
| CPU | Central processing unit |
| LED | Light-emitting diode |
| FDM | Fused deposition modelling |
| SD | Standard deviation |
| VGA | Video graphics array |
| GUI | Graphical user interface |
| STL | Standard tessellation language |
| PDMS | Polydimethylsiloxane |
| CAD | Computer-aided design |
| HSM | High-speed machining |
| IT | Interval tolerance |
| GelMA | Gelatin methacrylate |
| LCD | Liquid crystal display |
References
- Israni, A.K.; Zaun, D.; Rosendale, J.D.; Schaffhausen, C.; Snyder, J.J.; Kasiske, B.L. OPTN/SRTR 2017 Annual Data Report: Deceased Organ Donation. Am. J. Transplant. 2019, 19, 485–516. [Google Scholar] [CrossRef]
- Lewis, A.; Koukoura, A.; Tsianos, G.I.; Gargavanis, A.A.; Nielsen, A.A.; Vassiliadis, E. Organ donation in the US and Europe: The supply vs demand imbalance. Transplant. Rev. 2021, 35, 100585. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, C.A. The history of tissue engineering. J. Cell. Mol. Med. 2006, 10, 569–576. [Google Scholar] [CrossRef]
- Groll, J.; Burdick, J.A.; Cho, D.W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Jüngst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A definition of bioinks and their distinction from biomaterial inks. Biofabrication 2018, 11, 013001. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef]
- Garciamendez-Mijares, C.E.; Agrawal, P.; García Martínez, G.; Cervantes Juarez, E.; Zhang, Y.S. State-of-art affordable bioprinters: A guide for the DiY community. Appl. Phys. Rev. 2021, 8, 031312. [Google Scholar] [CrossRef]
- Vanderburgh, J.; Sterling, J.A.; Guelcher, S.A. 3D Printing of Tissue Engineered Constructs for In Vitro Modeling of Disease Progression and Drug Screening. Ann. Biomed. Eng. 2017, 45, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi Hosseinabadi, H.; Dogan, E.; Miri, A.K.; Ionov, L. Digital Light Processing Bioprinting Advances for Microtissue Models. ACS Biomater. Sci. Eng. 2022, 8, 1381–1395. [Google Scholar] [CrossRef]
- Garciamendez-Mijares, C.E.; Aguilar, F.J.; Hernandez, P.; Kuang, X.; Gonzalez, M.; Ortiz, V.; Riesgo, R.A.; Ruiz, D.S.R.; Rivera, V.A.M.; Rodriguez, J.C.; et al. Design considerations for digital light processing bioprinters. Appl. Phys. Rev. 2024, 11, 031314. [Google Scholar] [CrossRef]
- Cui, X.; Li, J.; Hartanto, Y.; Durham, M.; Tang, J.; Zhang, H.; Hooper, G.; Lim, K.; Woodfield, T. Advances in Extrusion 3D Bioprinting: A Focus on Multicomponent Hydrogel-Based Bioinks. Adv. Healthc. Mater. 2020, 9, 1901648. [Google Scholar] [CrossRef]
- Vanaei, S.; Parizi, M.; Vanaei, S.; Salemizadehparizi, F.; Vanaei, H. An Overview on Materials and Techniques in 3D Bioprinting Toward Biomedical Application. Eng. Regen. 2021, 2, 1–18. [Google Scholar] [CrossRef]
- Wu, Y.; Su, H.; Li, M.; Xing, H. Digital light processing-based multi-material bioprinting: Processes, applications, and perspectives. J. Biomed. Mater. Res. Part A 2023, 111, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H.; et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Kopyeva, I.; Brady, R.P.; DeForest, C.A. Light-based fabrication and 4D customization of hydrogel biomaterials. Nat. Rev. Bioeng. 2025, 3, 159–180. [Google Scholar] [CrossRef]
- Levato, R.; Dudaryeva, O.; Garciamendez-Mijares, C.E.; Kirkpatrick, B.E.; Rizzo, R.; Schimelman, J.; Anseth, K.S.; Chen, S.; Zenobi-Wong, M.; Zhang, Y.S. Light-based vat-polymerization bioprinting. Nat. Rev. Methods Prim. 2023, 3, 47. [Google Scholar] [CrossRef]
- Li, W.; Wang, M.; Ma, H.; Chapa-Villarreal, F.A.; Lobo, A.O.; Zhang, Y.S. Stereolithography apparatus and digital light processing-based 3D bioprinting for tissue fabrication. iScience 2023, 26, 106039. [Google Scholar] [CrossRef]
- Ke, D.; Niu, C.; Yang, X. Evolution of 3D bioprinting-from the perspectives of bioprinting companies. Bioprinting 2022, 25, e00193. [Google Scholar] [CrossRef]
- Grigoryan, B.; Sazer, D.W.; Avila, A.; Albritton, J.L.; Padhye, A.; Ta, A.H.; Greenfield, P.T.; Gibbons, D.L.; Miller, J.S. Development, characterization, and applications of multi-material stereolithography bioprinting. Sci. Rep. 2021, 11, 3171. [Google Scholar] [CrossRef]
- Bhusal, A.; Dogan, E.; Nguyen, H.A.; Labutina, O.; Nieto, D.; Khademhosseini, A.; Miri, A.K. Multi-material digital light processing bioprinting of hydrogel-based microfluidic chips. Biofabrication 2021, 14, 014103. [Google Scholar] [CrossRef]
- Yang, X.; Yao, L.; Sun, X.; Wang, L.; Xiao, J. Low-temperature DLP 3D printing of low-concentration collagen methacryloyl for the fabrication of durable and bioactive personalized scaffolds. Chem. Eng. J. 2024, 497, 155650. [Google Scholar] [CrossRef]
- Pagac, M.; Hajnys, J.; Ma, Q.P.; Jancar, L.; Jansa, J.; Stefek, P.; Mesicek, J. A Review of Vat Photopolymerization Technology: Materials, Applications, Challenges, and Future Trends of 3D Printing. Polymers 2021, 13, 598. [Google Scholar] [CrossRef] [PubMed]
- Alparslan, C.; Bayraktar, S. Advances in Digital Light Processing (DLP) Bioprinting: A Review of Biomaterials and Its Applications, Innovations, Challenges, and Future Perspectives. Polymers 2025, 17, 1287. [Google Scholar] [CrossRef] [PubMed]
- Al Rashid, A.; Ahmed, W.; Khalid, M.Y.; Koç, M. Vat photopolymerization of polymers and polymer composites: Processes and applications. Addit. Manuf. 2021, 47, 102279. [Google Scholar] [CrossRef]
- Nieto, D.; Jorge de Mora, A.; Kalogeropoulou, M.; Bhusal, A.; Miri, A.K.; Moroni, L. Bottom-up and top-down VAT photopolymerization bioprinting for rapid fabrication of multi-material microtissues. Int. J. Bioprint. 2024, 10, 1017. [Google Scholar] [CrossRef]
- Pahl, G.; Beitz, W.; Feldhusen, J.; Grote, K.H. Engineering Design: A Systematic Approach, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Green, P. (Ed.) The Geometrical Tolerancing Desk Reference; Newnes: Oxford, UK, 2005. [Google Scholar] [CrossRef]
- Community, P. Pygame-Python Game Development. Version 2.5.2. 2024. Available online: https://www.pygame.org (accessed on 7 August 2025).
- Schimansky, T. CustomTkinter: A Modern Themed Tkinter UI Library. Version 5.2.1. 2025. Available online: https://github.com/TomSchimansky/CustomTkinter (accessed on 7 August 2025).
- ISO 9283:1998; Manipulating Industrial Robots—Perfomance Criteria and Related Test Methods. International Organization for Standarization (ISO): Geneva, Switzerland, 1998.
- Krause, D.; Eilmus, S. A methodical approach for developing modular product families. In DS 68-4, Proceedings of the 18th International Conference on Engineering Design (ICED 11), Impacting Society Through Engineering Design, Lyngby/Copenhagen, Denmark, 15–19 August 2011; The Design Society: Glasgow, UK, 2011; Volume 4: Product and Systems Design; pp. 299–308. [Google Scholar]
- Wu, L.; Dong, Z.; Du, H.; Li, C.; Fang, N.X.; Song, Y. Bioinspired Ultra-Low Adhesive Energy Interface for Continuous 3D Printing: Reducing Curing Induced Adhesion. Research 2018, 2018, 4795604. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).