Abstract
The demand for jellyfish is growing worldwide, especially due to their high nutraceutical value. In this study, the extraction and characterization of crude gelatin from the brown cannonball jellyfish (Stomolophus meleagris), which is periodically found in large volumes on the American Pacific coasts, were carried out. The crude gelatin obtained by alkaline treatment, with subsequent heat and dialysis treatment, showed an ability to quench free radicals (via ABTS and ORAC methods), and protect human cells against oxidative damage (through inhibition of hemolysis by AAPH), and they protected against mutations caused by aflatoxin B1 in the Salmonella enterica Typhimurium TA100 strain. Furthermore, it was established that these extracts were innocuous for eukaryotic cells (genotoxicity assay). The amino acid profiles indicate a high concentration of glycine and proline, as well as charged amino acids. Electrophoretic, FT-IR, and 1H-NMR studies indicated that one of the main proteins present in this crude gelatin is collagen. The presence of collagen and other proteins was identified by proteomic studies. Alkaline crude gelatin from brown jellyfish could be considered as potential candidates to be evaluated as antioxidant agents in foods in future research.
Key Contribution:
This study shows that jellyfish Stomolophus meleagris proteins are a promising alternative source of antioxidant compounds. Furthermore, detecting different proteins with antioxidant capacities, such as collagen, tubulins, and histones, would further clarify, at least in part, jellyfish-related health benefits. This obtained information can be utilized for new protein findings or subsequent comparative investigations to enhance our comprehension of marine organisms’ biological properties.
1. Introduction
The demand for jellyfish suitable for human consumption is increasing worldwide, with uses as food that provide beneficial compounds for the consumer [1]. Some species of said edible jellyfish have been captured around the American continent, such as Stomolophus sp. [2] These marine species represent a low-cost, raw material for obtaining nutraceutical products [1]. Therefore, the identification and characterization of one of their main chemical components, proteins, are necessary.
Jellyfish are marine organisms that are found on the coasts of many countries, and some products have been obtained from them, but with a low commercial value. Given their reproduction rate, jellyfish are often regarded as plagues, mainly in coastal cities. One of the causes to which this massive increase in jellyfish has been attributed to is climate change. Previous research in various countries have been performed assessing the potential changes generated in the ecosystems by jellyfish [3].
Among the most common jellyfish species identified are the moon and cannonball jellyfish. The latter belongs to the genus Stomolophus spp. and is abundant on the coasts of the Pacific Ocean and the Gulf of Mexico. Different species of jellyfish can be found between the northern part of Sonora, in the Gulf of California, and Ecuador in South America [4]. Their high-water content and proportion of stromal proteins makes jellyfish a highly perishable species, so a conservation process must be performed immediately after capture [3]. The large amounts of jellyfish processed leads to the generation of waste, particularly comprising anatomical areas that are not used. These residues can be recovered and used efficiently, which can only reduce the negative impact on the environment, but also generate products with high commercial value, such as proteins.
It has been previously reported that in jellyfish species, in addition to their biochemical composition, their proteins have a high antioxidant activity [5,6,7,8,9,10,11,12,13], which makes these organisms a wholesome food and antioxidant compound supply.
In addition to the potential use of jellyfish for their bioactive compounds, these organisms have a high collagen content [14]. The collagen extracted from jellyfish via heat treatment has shown good functional properties, making it amenable to appliances in the food industry [13,15,16,17]. However, the information available on using crude gelatin samples from S. meleagris jellyfish in the foodstuff sector still needs to be improved.
The main aim of this work was to obtain a crude gelatin with bioactive properties using alkaline conditions from the brown cannonball jellyfish (Stomolophus meleagris). However, it has been widely documented that crude gelatin obtained under alkaline conditions represent a complex system of proteins with different structures and molecular masses. Therefore, to deepen our knowledge of this crude gelatin, proteomics can be utilized, which allows for the analysis of proteins on a large scale in a particular biological system [18]. Based on our information, no other reports exist whereby proteins obtained from the brown cannonball jellyfish under alkaline conditions have been successfully identified by proteomic analysis. In this investigation, the antioxidant and antimutagenic activities of crude gelatin obtained from the jellyfish under alkaline conditions and after heat treatment were established, and chemical-structural analyzes and further identification of the obtained crude gelatin using proteomic techniques were performed.
2. Materials and Methods
2.1. Sample Preparation
The jellyfish (Stomolophus meleagris) were obtained from the Gulf of California by local fishermen from Puerto Peñasco (31°18′24″ N 113°32′24″ W). The jellyfish were kept on ice during handling and transferred cut to the laboratory. They were rinsed and then stored at −20 °C inside polyethylene bags.
2.2. Protein Extraction and Gelatin Production
Protein extraction and gelatin production were executed, as explained in previous research [19], with certain adjustments. The jellyfish was cut into small pieces, soaked in alkali (0.1 N NaOH, 1:5 w/v for 24 h), and rinsed until the pH dropped to 7. After that, a thermic treatment was applied (water bath at 60 °C for 12 h). The protein solution was then filtered through the gauze and dialyzed at 4 °C in water, employing a 10 kDa molecular weight cut-off cellulose membrane. The gathered samples were frozen (−25 °C) and lyophilized.
2.3. Biological Activity
2.3.1. Antioxidant Activity
The in vitro antioxidant activities for the crude gelatin sample were evaluated by four spectrophotometric assays: ABTS [2,2′-Azino-Bis (3-ethylbenzothiazoline-6-sulfonic acid)], FRAP (ferric reducing-antioxidant power), ORAC (oxygen radical absorbance capacity), and the protective effect on human erythrocyte against AAPH [2,2′-azobis(2-amidinopropane) dihydrochloride].
The ABTS radical scavenging activity was evaluated as described previously [20]. Potassium persulfate (2.45 mM) was employed to dissolve ABTS. The 7 mM ABTS solution was incubated for 12 h in dark at room temperature to generate ABTS radicals. The ABTS solution was diluted (distilled water) until its absorbance (734 nm) reached 0.7 ± 0.02. A crude gelatin sample dissolved in water (20 μL) was taken and mixed with ABTS solution (270 μL) and kept in the dark at 25 °C (30 min). Finally, the reduction in absorbance (734 nm) was determined. The sample concentration required to inhibit 50% ABTS (IC50) was used to express ABTS findings. A curve standard, considering X-axis as the gelatin sample concentration and Y-axis as the % inhibition, was employed to establish the IC50 value.
A FRAP assay was then carried out [21]. The method used was based on reducing a compound consisting of TPTZ (2,4,6-tripyridyl-s-triazine) and Fe+3 (ferric iron). The presence of an antioxidant in an acid medium induces the formation of a colorless complex, in contrast to Fe+2, which exhibits a greenish-blue color [21]. For this determination, the sample (20 mL) was mixed with a solution (280 mL) containing acetic acid–sodium acetate (pH 3.4), TPTZ, and FeCl3 (10:1:1). The mixture was placed on a microplate reader, and after 30 min incubation, the absorbance at 630 nm was read. The results were indicated as Trolox equivalents.
The ORAC assay was executed as described previously [22]. In brief, fluorescence loss in the presence of AAPH was assessed at 37 °C for 90 min. The reaction was executed into 75 mM sodium phosphate buffer (pH 7.4), with the reached total volume of 2000 μL containing: 1700 μL buffer, 100 μL fluorescein, 100 μL AAPH, and 100 μL of the sample. The results were shown as Trolox equivalents.
The protective effect on human erythrocytes was determined via the method established previously [23]. A pure suspension of erythrocytes was achieved by washing them thrice with ten mM phosphate-buffered saline at pH 7.4. For the hemolysis assay, a 2% solution was produced by resuspending the erythrocytes in PBS (phosphate-buffered saline). The suspended erythrocytes solution (100 μL) was mixed with crude gelatin sample (100 μL) and AAPH (100 μL); the mixture was then incubated at 37 °C and shaken at 30 rpm in the dark (3 h). Then, PBS (1 mL) was added to the solution and centrifuged at 1500× g (10 min). The supernatants were then transferred to 96-well microplates using a spectrophotometric microplate reader (Multiskan Go, Thermo Scientific, Waltham, MA, USA). The percentage inhibition of hemolysis was established by measuring the absorbance (540 nm).
2.3.2. Ames Assay
The antimutagenic potential of the jellyfish crude gelatin sample was determined by the Ames assay [24]. The bioactivated Salmonella enterica Typhimurium T100 strain (using the S9 enzymatic mix) was taken as the biological model. Briefly, the bacterial strain was mixed with the jellyfish crude gelatin sample with a top agar containing L-histidine and D-biotin, as well as an S9 enzyme mixture. The mixtures were then quickly disposed of over minimal glucose agar plates and incubated at 37 °C (48 h).
2.3.3. Genotoxicity Test
Onions (Allium cepa) were allowed to germinate by immersion in distilled water and were then stored in the dark at room temperature (25 ± 2 °C). When the roots were about 5 cm long, the onions were ready for testing. The roots of the onions were treated with the jellyfish crude gelatin sample in two concentrations of 50 and 100 ppm for 24 h. The control group was treated with distilled water. The root tips were dehydrated for 45 min in a 3:1 (v/v) ethanol–acetic acid solution, and then fixed in 1N hydrochloric acid for 2 min at 60 °C, after which they were stained with orcein for 1 min, and finally squashed and observed with a microscope to count the mitotic cells [25].
2.4. Protein Analysis
2.4.1. Chemical Analysis
Moisture (oven-dried), crude protein (with the Kjeldahl method), and ash (muffle furnace) contents were determined using the official methods [26]. The nitrogen-free material was estimated by difference [100 − (ash + moisture + protein)].
2.4.2. Amino Acid Analysis
The amino acid analysis was performed by reverse-phase high-performance liquid chromatography (Model GmbH Hewlett-Packard RP- HPLC, Agilent Technologies Inc., Santa Clara, CA, USA) [27]. The crude gelatin sample (100 mg) was homogenized with performic acid (100 mL). Performic acid was prepared before being used by mixing 30% hydrogen peroxide (1 mL) with 97% formic acid (19 mL), which was maintained in a closed container at room temperature (2 h). Then, the prepared performic acid was cooled (0 °C) and added to the gelatin sample. After keeping the mixture at 0 °C for 2.5 h, cold water was added (0.9 mL). An aliquot of this mixture (200 mL) was lyophilized. After that, the crude gelatin sample was hydrolyzed at 110 °C (18 h) under pressure in a 6 M HCL and sodium thioglycolate (1:1, v/v). The hydrolyzed sample was neutralized with 4 N NaOH. An aliquot was carried out and mixed with an equal volume of 10 mg/mL of s l-α-amine n-butyric acid as an internal standard. Then, an aliquot was acquired and vortexed (1 min), with four parts of potassium borate buffer (pH 10.4) and O-phthaldialdehyde (1:1, v/v). Immediately, 20 mL was injected into the reverse phase column (C18 octadecyl dimethylsilane, 100 mm × 4.6 mm), coupled to a pre-column (30 mm × 4.6 mm) packed with the same material. Amino acids were analyzed with a fluorescence detector, and the peaks produced were evaluated (Chem Station program, Agilent Technologies Inc., Santa Clara, CA, USA). Elution involved two buffers at a flow of 1.0 mL/min during 25 min. Peak areas and retention times were contrasted with the commercial amino acid’s standards mixture.
2.4.3. Electrophoretic Profile (SDS-PAGE)
The electrophoretic profile was determined using sodium dodecyl sulfate (SDS)-polyacrylamide (PAGE) gels of 4% and 8% concentrations; separation systems were respectively prepared and injected into the sample (20 µg). Coomassie R–250 blue was used to stain the gels, and a mixture of methanol, water, and acetic acid (5:4:1, v/v/v) was employed to decolorize [28].
2.4.4. Fourier Transformed–Infrared Spectroscopy (FT-IR)
The FT-IR spectrum of the jellyfish crude gelatin sample was recorded (PerkinElmer FT-IR/FIT spectrometer, Waltham, MA, USA) from 4000 to 500 cm−1 with a resolution of 4 cm−1. The crude gelatin sample (1 mg) was mixed with dry potassium bromide (100 mg, KBr) pellet [29].
2.4.5. Nuclear Magnetic Resonance of Proton (1H-NMR)
1H-NMR spectrum of the jellyfish crude gelatin sample was recorded at 400 MHZ on a Bruker Advance 400 NMR spectrometer (Billerica, MA, USA, EE. UU.). The crude gelatin sample (1 mg) was dissolved in 0.5 mL of a 1% (v/v) deuterated potassium hydroxide solution (KOD 40% in D2O) with D2O water. The reference employed was dimethylsilapentane-5-sulfonic acid (DSS). The spectral frame was 20 ppm at 24 ± 1 °C [29].
2.4.6. Protein Identification
The crude gelatin sample was dissolved, and then identified and quantified with the nano LCMS/MS platform (Ultimate 3000 nano UHPLC system, attached to an Obitrap Q Exactive HF mass spectrometer with Nanospray Flex Ion Source, Thermo Scientific).
Proteins were precipitated from the protein solution using methanol and chloroform. Then, the protein pellet was dissolved in a 2 M urea aqueous solution. After that, the crude gelatin sample was denatured under the following conditions: 10 mM dithiothreitol and incubation for 60 min at 56 °C. The denatured sample was then alkylated with 50 mM indole-3-acetic acid. Then, the sample was incubated at room temperature in the dark (60 min). Afterward, 0.1% trifluoracetic acid (TFA) was added into the solution to make a final concentration of 0.1%TFA with a pH of 1.0. Pepsin was used as the specific protease. The salt from the peptides produced was performed with a C18 SPE column (Thermo Scientific). Prior to LC-MS/MS analysis, the obtained peptides were resuspended in a solution of 20 mL of 0.1% formic acid. The crude gelatin sample (1 mg) was then loaded onto a Nanoflow UPLC by using two buffers as the mobile phase: 0.1% formic acid in water (buffer A), and 0.1% formic acid in acetonitrile (buffer B). The total flow rate was 250 nL/min. Buffer B was employed to obtain the LC linear gradient: 5 min from 2% to 8%, 60 min from 8% to 20%, 33 min from 20% to 40%, and 4 min from 40% to 90%. The full scan (300–1650 m/z) was acquired using a single high resolution of 60,000 (at 200 m/z), and the automatic gain control target was set to 3 × 106. The MS/MS Top 20 mode scan resolution was 15,000 at 200 m/z, and the normalized collision energy was 28%. The automatic gain control target was then established to 1 × 105, and the maximum injection time was 19 ms. The dynamic exclusion parameters were time window (1.4 Th) of 30 s, charge state of 1, and unassigned were rejected, while charge states >6 were not dismissed. All the MS/MS spectra were analyzed using Peaks Study 8.5 software (Bioinformatics Solutions Inc., Waterloo, ON, Canada) against the jellyfish protein database. The following restrictions were used: pepsin cleavage with up to 2 missed cleavage sites, tolerance of 10 ppm as the precursor, and 0.5 Da as the fragment ion mass. Carbamidomethylation of cysteine was accounted for as a fixed modification. The permissible variable was methionine oxidation.
2.5. Statistical Analyses
A randomized entirely design was employed in this study. Each trial was replicated at least threefold. A one-way analysis of variance was applied to analyze the obtained data from the antioxidant activity, Ames assay, and genotoxicity tests. The Tukey–Kramer method was used to carry out the comparison of the means. A significance level of 95% (p < 0.05) was applied. The obtained results were analyzed using the INFOSTAT statistical software. Data from electrophoretic analysis, FTIR, NMR, and proteomics were evaluated by descriptive statistics.
3. Results and Discussion
3.1. Biological Activity
The biological activity of the jellyfish crude gelatin sample was measured by different assays. The ABTS scavenging ability was used to establish antioxidant capacity. ABTS can become a stable molecule after accepting an electron atom from the antioxidants. The IC50 values were 0.42 ± 0.10 mg/mL. The IC50 value of the crude gelatin sample was lower than those reported for the amaranth proteins (A. mantegazzianus; IC50 of 10.2 ± 0.8), suggesting that the jellyfish crude gelatin sample exhibits higher antioxidant effects than amaranth, but have comparable hydrolysates, with values of 1.16 ± 0.09 mg/mL [30]. These can be compared with the hydrolysate values from other sources, such as mutton ham or bacteria (Spirulina platensis), of 0.71 ± 0.23 and 1.5 ± 0.1, respectively [31,32].
The crude gelatin sample’s ability to quench the peroxyl radicals generated by the azo compound AAPH was evaluated by the ORAC assay. The hydrogen-donating antioxidant obstructs the peroxyl radical chain reactions. The antioxidant activity of the crude gelatin sample was assessed by comparing its fluorescence decay curve against a blank [33]. The ORAC value for the jellyfish crude gelatin sample was measured at 479 ± 7.17 mmol TE/g; these values can be compared to those of other marine organisms, such as the lipid extracts from seaweeds, with values of 461.84 and 362.53 mmol TE/g for M. pyrifera and E. radiate, respectively, and krill protein (Euphausia superba), with values between 274 and 537 mmol TE/g [34,35].
Although the jellyfish crude gelatin samples showed the ability to quench radicals, they did not show the ability to reduce ferric-tripyridyltriazine (FeIII-TPTZ) and form ferrous-tripyridyltriazine (FeII-TPTZ).
The radical AAPH induces oxidation, causing the hemolysis of erythrocytes. Radical degradation can be inhibited by antioxidant agents. The jellyfish crude gelatin sample presented an IC50 of 6 mg/mL. IC50 values of 0.12 ± 0.01 mg/mL have been reported in peptides from dairy products [36]; however, an IC50 of 10 mg/mL is considered to yield high protection on the part of erythrocytes against radical damage [37]. Therefore, the jellyfish crude gelatin sample appears to be protective against the AAPH radical, preventing oxidative damage occurring in the erythrocytes.
The antioxidant activities yielded by all the utilized methods in this study were different since they all have other mechanisms of action. The ABTS evaluated the ability of antioxidants to scavenge the performed radicals by donating an electron. In contrast, the ORAC and antihemolytic tests measured the ability of antioxidants to act as hydrogen donors and thus inhibit radical initiation, including the scavenging of formed radicals in biological systems [33,37]. In addition to the mechanism of each methodology, the antioxidant activity of the proteins depends on the amino acid sequence, the structure, and the size of the peptides. The authors of [31,38] report that aromatic amino acid residues including Tyr, Trp, and Phe might have to repress the unpaired electron activity by forming a conjugated electron system that inhibits the reaction process of peroxidation mediated by the free radicals [39]. Nucleophilic sulfur-containing amino acids such as Met, and other amino acids such as Pro and Leu have been confirmed [40]. Peroxyl radicals are stabilized by adding hydrogen that can be donated by several amino acids including His, Tyr, Leu, and Met. Zheng L. et al. [41] suggested that Tyr- and Trp-Gly protect the erythrocytes against AAPH-induced hemolysis primarily by acting as direct scavengers of ROO. Therefore, in the present study, it was considered that the presence of Gly, Leu, Phe, Pro, and Tyr, and the absence of His in the protein (as will be shown in the amino acid results section) are amenable to the antioxidant activity detected.
Concerning the antimutagenic activity, the jellyfish crude gelatin sample showed the capacity to inhibit mutation induced by AFB1 in the S. enterica Typhimurium TA100 strain (Figure 1). The percentage of inhibition was greater than 50% when 0.5 mg/plate was tested, representing moderate inhibition [42], whereas with 4.0 mg/plate, the inhibition was greater than 60%, representing strong inhibition [42]. A similar antimutagenic activity (40–50%) was derived when 0.5 mg/plate of ethanolic extracts from salmon (Oncorhynchus masou) was tested [43], but this activity was higher than that of the protein hydrolysate derived from giant squid skin, which showed 50% inhibition when 5 mg/plate was evaluated [44].
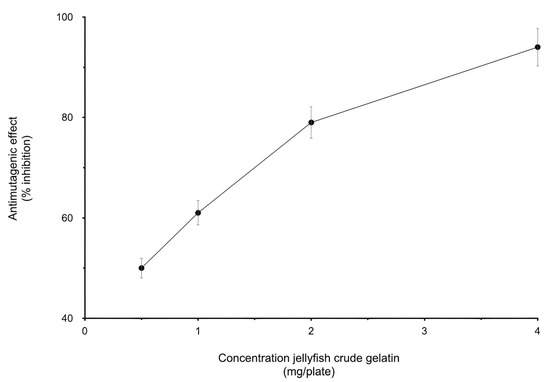
Figure 1.
Effect of the gelatinous extract obtained from the cannonball jellyfish on mutagenicity induced by AFB1, based on the Salmonella enterica Typhimurium TA100 assay.
3.2. Genotoxicity Test
Different phases of normal and abnormal mitosis are shown in Figure 2. Based on these images, we determined whether the crude gelatin sample had an adverse effect on the chromosomes. Table 1 shows the percentages of clastogenic cells; cells exposed to jellyfish crude gelatin sample at 50 ppm reached 3.44%, and those exposed to 100 ppm exceeded 5.17%. These values are low compared to those of other food additives, such as butylated hydrotoluene and butylated hydroxyanisole, at 28.67% and 28.60%, respectively [45].
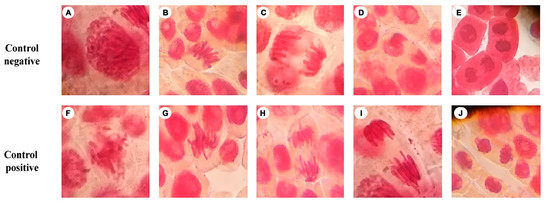
Figure 2.
Phases of normal and abnormal mitoses exposed to water and sodium azide. Chromosomal aberrations observed in Allium cepa root tip cells: (A) normal prophase; (B) normal metaphase; (C) normal anaphase; (D) normal late anaphase; (E) normal telophase; (F) abnormal metaphase; (G) abnormal metaphase; (H) abnormal anaphase with lagging; (I) abnormal anaphase with lag; and (J) abnormal telophase.

Table 1.
Clastogenic effects of the jellyfish crude gelatin sample (JCG) on the mitotic cells of Allium cepa *.
3.3. Protein Analysis
3.3.1. Yield
The yield of crude gelatin sample, expressed on a dry basis, and utilizing NaOH for extraction, was 10.49 ± 0.183 g lyophilized from 100 g of dry weight. This result is higher than other previously reported values, such as in S. meleagris (7.5 g/100 g) [17] and for Cyanea nozakii Kishinouye (5.5 g/100 g) [9].
3.3.2. Chemical Composition
The chemical composition of the jellyfish crude gelatin sample exhibited a moisture content of 10.67 ± 0.74%, which is comparable to other marine organisms, such as red tilapia (gelatin—9.59%) [46] or commercial bovine (gelatin—9.7%) [47]. The crude protein result was 76.38 ± 0.91%, which is higher than in dry-salted jellyfish (29.54%) [17], but comparable to commercial gelatin (87.52%) [47]. The ash result was 5.83 ± 0.16%, which is low compared to dry-salted jellyfish gelatin (56.61 ± 0.13%). However, the value was much higher than commercial gelatin (0.9 ± 0.2%) [47]. This may be due to the marine nature of the crude gelatin sample, possibly containing high concentrations of salts [17]. A more effective method for salt removal should be developed since ash contents up to 2.5% are accepted for food products. The value of nitrogen-free material was high when compared to commercial gelatin (1.8%) [47].
3.3.3. Amino Acid Analysis
The amino acid composition of the jellyfish crude gelatin sample was determined (as shown in Table 2). The most abundant amino acid found was Gly. High concentrations of Pro and charged amino acids such as Arg, Asp, Glu, and Lys, and aromatic Phe and Tyr were all observed. This result was expected since Gly is the main constituent of collagen. It is typical to find repeating triplet Gly-X-Y sequence, X and Y, in most likely any amino acid. However, Pro and Hyp residues were the most frequent triplet in collagen [48]. The amino acid composition trends (high concentration of Gly, Arg, and Pro) are comparable to another marine organism, deep water rose shrimps [49].

Table 2.
Amino acid composition of the crude gelatin sample obtained from cannonball jellyfish (Stomolophus meleagris) *.
Interestingly, histidine (His), considered a potent antioxidant amino acid, was not detected in the obtained crude gelatin sample. This result differs from other reports, such as those developed in Rhizostoma pulmo [50]. The absence of His could limit the crude gelatin sample’s capacity to trap metallic ions, as was detected in this study. However, more studies will be required to confirm this.
3.3.4. Electrophoretic Profile
The jellyfish crude gelatin sample’s electrophoretic profile displayed two bands in continuous positions, with molecular weights of approximately 200 kDa and 97 kDa (Figure 3, Lane B). This profile suggests that the crude gelatin sample molecules comprise at least b and a collagen chains. Three polypeptide chains, named a chain, coiled around one another in a triple-helical shape, are present in all collagen molecules. Typical collagen gelatin displays one β band (200–210 kDa) and two α bands (around 100 kDa for α1 and α2), which in the triple helix are the unfolding chains [48,51]. The results were deemed to be similar to those of other marine organisms [52,53], wherein thermic treatment degrades the triple helix into the β and α chains.
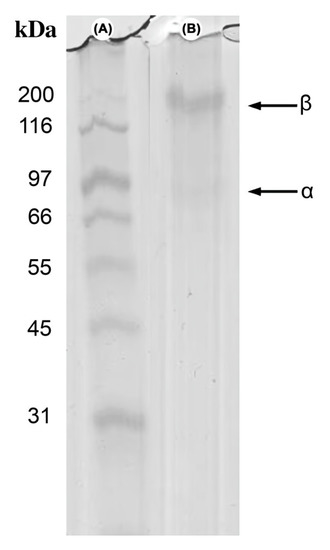
Figure 3.
SDS–polyacrylamide protein pattern of the jellyfish (Stomolophus meleagris) crude gelatin sample. (A): molecular weight marker; (B): jellyfish crude gelatin sample.
3.3.5. Fourier Transformed–Infrared Spectroscopy (FT-IR)
The jellyfish crude yielded an FT-IR spectrum (Figure 4) similar to other jellyfish species, such as Rhopilema esculentum and Rhizostoma pulmo [50,54]. The FT-IR spectra exhibited peaks characteristic of amides A and B, and amides I, II, and III. Amide A was observed at 3290 cm−1; the NH stretching vibrations determined its position. The position of the amide B band was detected at 2920 cm−1 due to asymmetric CH stretching. At 1660 cm−1 the detection of the amide I band is due to C=O stretching. The amide II band was observed at 1585 cm−1, resulting from N-H and C-N torsional vibrations. Amide I and II bands’ amplitudes are linked to the collagen structural spiral form, and linkages of a-helices, respectively [55,56]. The amide III band was observed at 1220 cm−1 due to the combined peaks of C-N stretching vibrations, and deformation of N-H from the amide linkages, and wagging vibration of the CH2 groups of the Gly-backbone and Pro-side chains [57].
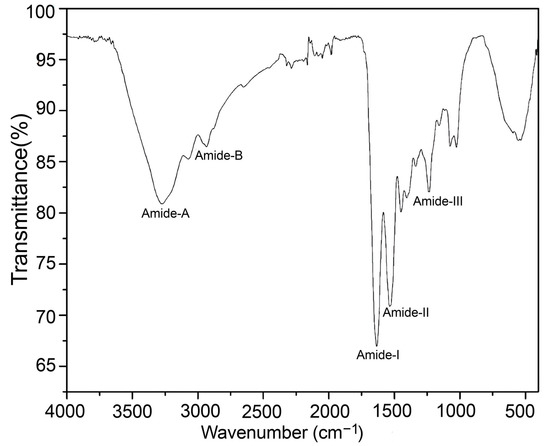
Figure 4.
FT-IR spectra of the jellyfish crude gelatin sample.
3.3.6. Nuclear Magnetic Resonance of Proton (1H-NMR)
The 1H-NMR spectra of the jellyfish crude gelatin sample (Figure 5) were compared to those from previous studies and spectrum databases. Figure 5 indicates the signals corresponding to each amino acid, as well as the integration of the most relevant amino acids. The MestReNova software 8 (MestReNova v9.0.1-13254, Mestrelab Research S.L., Santiago de Compostela, Spain) was employed to analyze the 1H-NMR spectra, and the areas of glycine, proline, and hydroxyproline were obtained by the integration of the different chemical shifts. The signal centered at 4.19 ppm, corresponding to the glycine signal (CH2), while the signals at 1.19, 2.19, and 3.79 correspond to proline and CH2, and the signals at positions 3.28 and 3.39 ppm correspond to hydroxyproline. These integrals suggest a 1:1 ratio of glycine to proline, while hydroxyproline is present at a smaller proportion. These observations are consistent with the amino acid content profiles discussed previously [13,58].
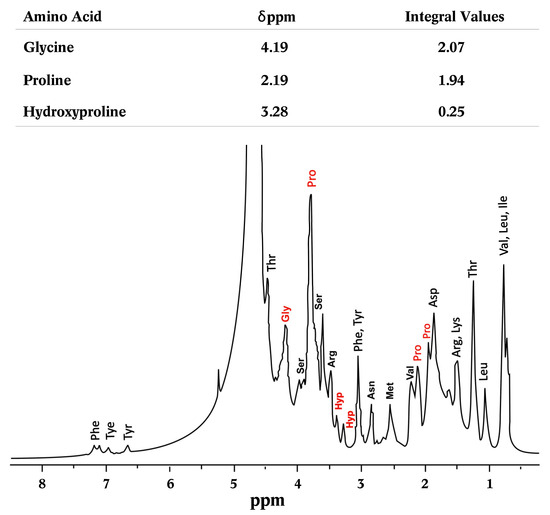
Figure 5.
1H-NMR spectra of the jellyfish crude gelatin sample. Amino acids are indicated by their corresponding peaks.
3.3.7. Proteomic Identification
The protein identification of the crude gelatin sample from jellyfish is summarized in Table 3. These results confirm the presence of collagen, alongside other proteins, such as tubulins and histones. Tubulins carry out various cellular functions, such as in providing structural support and have been used as a drug for multiple diseases, including cancer [59]. Histone proteins, which were also identified in this work, are DNA-binding proteins present in many animal species, including the jellyfish Stomolophus meleagris [60]. Histones, such as peptides of the H3 class, are present in the obtained crude gelatin sample. Histones can act as antimicrobials against viruses, bacteria, fungi, and parasites [61]. The collagen in this study was characterized as belonging to type IV. Collagen extracted from jellyfish has been shown to reduce different human cancer lines [1]. Therefore, the bioactive properties identified in the present study could be associated with other proteins in the crude gelatin extracts alongside collagen. Still, these proteins need to be validated using further bioactivity assays.

Table 3.
Proteins identified in the brown cannonball jellyfish crude gelatin sample with nanoLCMS/MS and bioinformatic analysis.
4. Conclusions
Under the conditions of this study, the alkaline-extracted proteins obtained from the cannonball jellyfish showed in vitro antioxidant and antimutagenic capacities. Moreover, the extracts are not clastogenic. The bioactivities detected in this study can be attributed to the presence of proteins such as collagen, tubulins, and histones. All of these can contribute to free radical scavenging and antimutagenic activities. This information suggests that cannonball jellyfish proteins have potential uses in the food industry as antioxidant agents. Currently, work is in progress on applying jellyfish gelatin in the preservation of food products, and the results of this study further demonstrate the bioactivity of jellyfish proteins.
Author Contributions
Conceptualization, D.M.E.-E., H.d.C.S.-O. and J.M.E.-B.; data curation, D.M.E.-E., M.P.-J., S.P.A., F.R.-F. and J.A.S.-L.; formal analysis, D.M.E.-E. and H.d.C.S.-O.; funding acquisition, J.M.E.-B.; investigation, D.M.E.-E., J.A.S.-L. and J.M.E.-B.; methodology, D.M.E.-E., S.P.A. and M.P.-J.; project administration, J.M.E.-B.; resources, J.M.E.-B.; supervision, H.d.C.S.-O., F.R.-F. and J.M.E.-B.; writing—original draft preparation, D.M.E.-E.; writing—review and editing, H.d.C.S.-O., M.P.-J., S.P.A. and J.M.E.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by a grant from the Aquaculture Institute of the State of Sonora, Mexico, funding number CV-IAS-006-2018.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Our study did not involve humans. The human blood employed on this work were donated by an accredited commercial laboratory (ISO-IEC 17,025 [NMX-EC-17025] and ISO 15,189, technical committee ISO/TC 212 [Clinical Laboratory Testing and In Vitro Diagnostic Systems]).
Data Availability Statement
The data presented in this study are available in the article. Further information is available upon request from the corresponding author.
Acknowledgments
Dania Marisol Esparza-Espinoza acknowledges to National Research and Technology Council (CONACyT) via the Mexican Government for the Ph.D. scholarship number 919695. All the authors would like to thank Jesús Enrique Chan-Higuera for his technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Riccio, G.; Martinez, A.K.; Martín, J.; Reyes, F.; D’Ambra, I.; Lauritiano, C. Jellyfish as an alternative source of bioactive antiproliferative compounds. Mar. Drugs 2022, 20, 350. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, J.; Álvarez-Tello, J. The jellyfish fishery in Mexico. Agri. Sci. 2013, 4, 57. [Google Scholar] [CrossRef]
- Bleve, G.L.; Ramires, F.A.; Gallo, A.; Leone, A. Identification of safety and quality parameters for preparation of jellyfish based novel food products. Foods 2019, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Balamurugan, E.; Menon, V. In Vitro radical scavanging activities of Chrysaora quinquecirrha nematocyst venom. Drug Discov. Ther. 2009, 3, 56–61. [Google Scholar] [PubMed]
- Harada, K.; Maeda, T.; Hasegawa, Y.; Tokunaga, T.; Ogawa, S.; Fukuda, K.; Nagatsuka, N.; Nagao, K.; Ueno, S. Antioxidant activity of the giant jellyfish Nemopilema nomurai measured by the oxygen radical absorbance capacity and hydroxyl radical averting capacity methods. Mol. Med. Rep. 2011, 4, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, H.; Xing, R.; Liu, S.; Qing, Y.; Li, K.; Li, B.; Meng, X.; Cui, J.; Li, P. Isolation, identification and characterization of a novel antioxidant protein from the nematocyst of the jellyfish Stomolophus meleagris. Int. J. Biol. Macromol. 2012, 51, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Barzideh, Z.; Latiff, A.A.; Gan, C.Y.; Abedin, M.Z.; Alias, A.K. ACE inhibitory and antioxidant activities of collagen hydrolysates from the ribbon jellyfish (Chrysaora sp.). Food Technol. Biotechnol. 2014, 52, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Khong, N.M.H.; Yusoff, F.M.; Jamilah, B.; Basri, M.; Maznah, I.; Chan, K.W.; Nishikawa, J. Nutritional composition and total collagen content of three commercially important edible jellyfish. Food Chem. 2016, 196, 953–960. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, C.; Zhao, J.; Shi, X.; Sun, J.; Liu, J.; Fu, Y.; Jin, W.; Zhu, B. Separation and characterization of antioxidative and angiotensin converting enzyme inhibitory peptide from jellyfish gonad hydrolysate. Molecules 2018, 23, 94. [Google Scholar] [CrossRef]
- Leone, A.; Lecci, R.M.; Durante, M.; Meli, F.; Piraino, S. The bright side of gelatinous blooms: Nutraceutical value and antioxidant properties of three Mediterranean jellyfish (Scyphozoa). Mar. Drugs 2015, 13, 4654–4681. [Google Scholar] [CrossRef] [PubMed]
- Upata, M.; Siriwoharn, T.; Makkun, S.; Yarnpakdee, S.; Regenstein, J.M.; Wangtueai, S. Tyrosinase inhibitory and antioxidant activity of enzymatic protein hydrolysate from jellyfish (Lobonema smithii). Foods 2022, 11, 615. [Google Scholar] [CrossRef] [PubMed]
- Villalba-Urquidy, B.S.; Velazquez-Valdez, L.P.; Bracamontes-Picos, S.J.; Del Toro-Sánchez, C.L.; Chan-Higuera, J.E.; Ezquerra-Brauer, J.M. Conversion of dry-salted cannonball jellyfish (Stomolophus meleagris) umbrella and oral arms to cornmeal snacks and gelatin with antioxidant properties. Fishes 2022, 7, 277. [Google Scholar] [CrossRef]
- Addad, S.; Exposito, J.Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, characterization and biological evaluation of jellyfish collagen for use in biomedical applications. Mar. Drugs 2011, 9, 967–983. [Google Scholar] [CrossRef]
- Cho, S.; Ahn, J.R.; Koo, J.S.; Kim, S.B. Physicochemical properties of gelatin from jellyfish Rhopilema hispidum. Fish. Aquatic Sci. 2014, 17, 299–304. [Google Scholar] [CrossRef]
- Rodsuwan, U.; Thumthanaruk, B.; Kerdchoechuen, O.; Laohakunjit, N. Functional properties of type A gelatin from jellyfish (Lobonema smithii). Int. Food Res. J. 2016, 23, 507–514. Available online: http://www.ifrj.upm.edu.my/23%20(02)%202016/(8).pdf (accessed on 6 November 2022).
- Chiarelli, P.G.; Pegg, R.B.; Kumar, D.; Sloval, K.M. Exploring the feasibility of developing novel gelatine powders from salted, dried cannonball jellyfish (Stomolophus meleagris). Food Biosci. 2021, 44, 101397. [Google Scholar] [CrossRef]
- Carrera, M.; Cañas, B.; Gallardo, J.M. Proteomics for the assessment of quality and safety of fishery products. Food Res. Int. 2013, 54, 972–979. [Google Scholar] [CrossRef]
- Cuevas-Acuña, D.A.; Placencia-Jatomea, M.; Santacruz-Ortega, H.C.; Torres-Arreola, W.; Ezquerra-Brauer, J.M. Development of chitosan/squid skin gelatin hydrolysate films: Structural, physical, antioxidant, and antifungal properties. Coatings 2021, 11, 1088. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Protoggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 5, 3273–3327. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Jin, Y.; Liu, G. Flavonoids from the leaves of Actinidia kolomikta. Chem. Nat. Compd. 2010, 46, 205–208. [Google Scholar] [CrossRef]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mut. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef] [PubMed]
- Türkoğlu, S. Genotoxicity of five food preservatives tested on root tips of Allium cepa L. Mut. Res. 2007, 626, 4–14. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Vazquez-Ortiz, F.; Moron-Fuenmayor, O.; Gonzalez-Mendez, N. Hydroxyproline measurement by HPLC: Improved method of total collagen determination in meat samples. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 2771–2780. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Sarabia-Sainz, H.M.; Torres-Arreola, W.; Márquez-Ríos, E.; Santacruz-Ortega, H.C.; Rouzaud-Sández, O.; Valenzuela-Soto, E.M.; Burgara-Estrella, A.J.; Ezquerra-Brauer, J.M. Interrelation of collagen chemical structure and nanostructure with firmness of three body regions of jumbo squid (Dosidicus gigas). Food Biophys. 2017, 12, 491–499. [Google Scholar] [CrossRef]
- Orsini, D.; Valeria, A.; Tironi, M.; Añón, C. Antioxidant activity of amaranth protein or their hydrolysates under simulated gastrointestinal digestion. LWT-Food Sci. Technol. 2011, 44, 1752–1760. [Google Scholar] [CrossRef]
- Wang, J.; Guo, M.; Wang, Q.; Dong, J.; Lu, S.; Lyu, B.; Ma, X. Antioxidant activities of peptides derived from mutton ham, Xuanwei ham and Jinhua ham. Int. Food Res. J. 2021, 142, 110195. [Google Scholar] [CrossRef]
- Mohammadi, M.; Soltanzadeh, M.; Ebrahimi, A.R.; Hamishehkar, H. Spirulina platensis protein hydrolysates: Techno-functional, nutritional and antioxidant properties. Algal Res. 2022, 65, 2211–9264. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Mildenberger, J.; Bruheim, I.; Solibakke, P.; Atanassova, M. Development of a protein concentrate for human consumption by direct enzymatic hydrolysis of antarctic krill (Euphausia superba). LWT-Food Sci. Technol. 2023, 173, 114254. [Google Scholar] [CrossRef]
- Kindleysides, S.; Quek, S.; Miller, M.R. Inhibition of fish oil oxidation and the radical scavenging activity of New Zealand seaweed extracts. Food Chem. 2012, 133, 1624–1631. [Google Scholar] [CrossRef]
- Tenore, G.C.; Ritieni, A.; Campiglia, P.; Stiuso, P.; Di-Maro, S.; Sommella, E.; Pepe, G.; D’Urso, E.; Novellino, E. Antioxidant peptides from “Mozzarella di Bufala Campana DOP” after simulated gastrointestinal digestion: In Vitro intestinal protection, bioavailability, and anti-haemolytic capacity. JFF 2015, 15, 365–375. [Google Scholar] [CrossRef]
- Del-Toro-Sánchez, C.L.; Rodríguez-Félix, F.; Cinco-Moroyoqui, F.J. Recovery of phytochemical from three safflower (Carthamus tinctorius L.) by-products: Antioxidant properties, protective effect of human erythrocytes and profile by UPLC-DAD-MS. J. Food Process. Preserv. 2021, 45, e15765. [Google Scholar] [CrossRef]
- Heydari, S.; Hosseini, S.; Mortazavian, A.M.; Taheri, S. Extraction of bioactive peptides produced in probiotic yoghurt and determination of their biological activities. Int. Dairy J. 2023, 139, 105544. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, M.; Lin, S.; Cheng, S. Contribution of specific amino acid and secondary structure to the antioxidant property of corn gluten proteins. Int. Food Res. J. 2018, 105, 836–844. [Google Scholar] [CrossRef]
- Mudd, N.; Martin-Gonzalez, F.; Ferruzzi, M.; Liceaga, A.M. In vivo antioxidant effect of edible cricket (Gryllodes sigillatus) peptides using a Caenorhabditis elegans model. FHFH 2022, 2, 100083. [Google Scholar] [CrossRef]
- Zheng, L.; Dong, H.; Su, G.; Zhao, Q.; Zhao, M. Radical scavenging activities of Tyr-, Trp-, Cys-and Met-Gly and their protective effects against AAPH-induced oxidative damage in human erythrocytes. Food Chem. 2016, 197, 807–813. [Google Scholar] [CrossRef]
- Ikken, Y.; Morales, P.; Martinez, A.; Marin, M.L.; Haza, A.I.; Cambero, M.I. Antimutagenic effect of fruit and vegetable ethanolic extracts against N-nitrosamines evaluated by the Ames test. J. Agric. Food Chem. 1999, 47, 3257–3264. [Google Scholar] [CrossRef] [PubMed]
- Hyun-Taek, O.; Soo-Hyun, K.; Hyun-Jin, C.; Mi-Ja, C.; Seung-Shi, H. Antioxidative and antimutagenic activities of 70% ethanol extract from masou salmon (Oncorhynchus masou). Toxicol. In Vitro 2008, 22, 1484–1488. [Google Scholar] [CrossRef]
- Suárez-Jiménez, G.M.; Burgos-Hernández, A.; Torres-Arreola, W.; López-Saiz, C.M.; Velázquez Contreras, C.A.; Ezquerra-Brauer, J.M. Bioactive peptides from collagen hydrolysates from squid (Dosidicus gigas) by-products fractionated by ultrafiltration. Int. J. Food Sci. Technol. 2019, 54, 1054–1061. [Google Scholar] [CrossRef]
- Pandey, H.; Kumar, V.; Roy, B.K. Assessment of genotoxicity of some common food preservatives using Allium cepa L. as a test plant. Toxicol. Rep. 2014, 1, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Nurilmala, M.; Suryamarevita, H.; Hizbullah, H.; Jacoeb, M.; Ochiai, Y. Fish skin as a biomaterial for halal collagen and gelatin. Saudi J. Biol. Sci. 2022, 29, 1100–1110. [Google Scholar] [CrossRef]
- Rahman, M.S.; Al-Saidi, G.S.; Guizani, N. Thermal characterisation of gelatin extracted from yellowfin tuna skin and commercial mammalian gelatin. Food Chem. 2008, 108, 472–481. [Google Scholar] [CrossRef]
- Sionkowska, A. Collagen blended with natural polymers: Recent advances and trends. Prog. Polym. Sci. 2021, 122, 101452. [Google Scholar] [CrossRef]
- Rusanova, P.; Bono, G.; Dara, M.; Falco, F.; Gancitano, V.; Lo Brutto, S.; Okpala, C.O.R.; Nirmal, N.P.; Quattrocchi, F.; Sardo, G.; et al. Effect of different packaging methods on the free amino acid profiles of the deep-water rose shrimp (Parapenaeus longirostris) during frozen storage. Front. Nutr. 2022, 9, 955216. [Google Scholar] [CrossRef]
- Derkus, B.; Arslan, Y.; Tahir, A.; Kantarcioglu, I.; Emregul, K.; Emregul, E. Development of a novel aptasensor using jellyfish collagen as matrix and thrombin detection in blood samples obtained from patients with various neurodisease. Sens. Actuators B Chem. 2016, 228, 725–736. [Google Scholar] [CrossRef]
- Giacomelli, C.; Rodrigues, A.; Lima, A.; Mello, R.; Morisso, F.; Prestes-Dornelles, R.C.; Kubota, H.E. Gelatin extracted from jundiá skin (Rhamdia quelen): An alternative to the discarded by-product. Int. Food Res. J. 2022, 161, 111829. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhao, Y.Q.; Wang, Y.M.; Yang, X.R.; Chi, C.F.; Wang, B. Gelatins and antioxidant peptides from Skipjack tuna (Katsuwonus pelamis) skins: Purification, characterization, and cytoprotection on ultraviolet-A injured human skin fibroblasts. Food Biosci. 2022, 50 Pt B, 102138. [Google Scholar] [CrossRef]
- Yang, L.; Yang, m.; Xu, J.; Nie, Y.; Wu, W.; Zhang, T.; Wang, X.; Zhong, J. Structural and emulsion stabilization comparison of four gelatins from two freshwater and two marine fish skins. Food Chem. 2022, 371, 131129. [Google Scholar] [CrossRef] [PubMed]
- Felix-Felician, F.; Rui-He, Y.; Meng-Zhen, L.; Chun-Jie, L.; Hui-Qin, C.; Ying, J.; Tao, T.; Wei-Yan, Q.; Han-Mei, X. The wound healing potential of collagen peptides derived from the jellyfish Rhopilema esculentum. CJT 2019, 22, 12–20. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–333. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S. Characteristics of gelatin from the skin of unicorn leatherjacket (Aluterus monoceros) as influenced by acid pretreatment and extraction time. Food Hydrocoll. 2011, 25, 381–388. [Google Scholar] [CrossRef]
- Pamungkas, B.F.; Murdiati, A.S.; Indrati, R. Characterization of the acid- and pepsin-soluble collagens from haruan (Channa striatus) scales. Pak. J. Nutr. 2019, 18, 324–332. [Google Scholar] [CrossRef]
- Angilè, F.; Del Coco, L.; Girelli, C.R.; Basso, L.; Rizzo, L.; Piraino, S.; Stabili, L.; Fanizzi, F.P. 1H NMR Metabolic Profile of scyphomedusa Rhizostoma pulmo (Scyphozoa, Cnidaria) in female gonads and somatic tissues: Preliminary results. Molecules 2020, 25, 806. [Google Scholar] [CrossRef]
- Binarová, P.; Tuszynski, J. Tubulin: Structure, functions and roles in disease. Cells 2019, 8, 1294. [Google Scholar] [CrossRef]
- Li, R.; Yu, H.; Xing, R.; Liu, S.; Qing, Y.; Li, K.; Li, B.; Meng, X.; Cui, J.; Li, P. Application of nanoLC–MS/MS to the shotgun proteomic analysis of the nematocyst proteins from jellyfish Stomolophus meleagris. J. Chromatogr. B 2012, 899, 86–95. [Google Scholar] [CrossRef]
- Hoeksema, M.; Van-Eijk, M.; Haagsman, H.P.; Hartshorn, K.L. Histones as mediators of host defense, inflammation and thrombosis. Future Microbiol. 2016, 11, 441–453. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).