Abstract
Brewery’s spent grain (BSG) consists of the largest by-product by volume in the beer production sector and offers potential for both bio-composite material production, high-added-value molecular extraction and bioenergy recovery. Aiming at exploring the ideal biorefinery approach for this agro-industrial residual, the present study experimentally investigated several methodologies to enhance the reuse of BSG and proposed a scheme of biorefinery focused on it. According to it, BSGs were firstly tested to produce high-added-value byproducts, such as protein hydrolysates and for the extraction of lignin via ionic liquids-based methods. The residuals were then used for biogas/biomethane production via anaerobic codigestion. The different matrices were rearranged in varying mixtures, aiming at ensuring high availability of nutrients for methanogens, thus achieving higher energy production than what achievable with untreated BSG. For the scope, further agro-industrial wastes were considered. The resulted digestate was finally composted. Untreated BSGs were also directly tested as fillers for bio-composite material production (in a mixture with PHB). Different concentrations were tested and the mechanical properties of each sample were compared with those of pure PHB. Disintegration tests were finally carried out to measure the improved biodegradability of the produced bio-composite material.
1. Introduction
1.1. Brewery’s Spent Grain Production
The beer industry is big and well-established worldwide, experiencing continuous global growth. In 2023, the largest beer producer worldwide was China, with a production volume of 359.1 million hL, followed by the United States with 193 million hL and Brazil with 148.9 million hL [1].
Beer production relies on four primary ingredients: cereal malt, water, hops, and yeast. This process generates three major by-products: Brewer’s Spent Grain (BSG), Brewer’s Spent Yeast, Brewer’s Spent Hops and wastewater [2,3]. Among these, BSG is the most abundant, accounting for approximately 85% of the produced by-products and is commonly derived from malted cereals, most commonly barley. Before malting, barley undergoes cleaning and grinding to achieve the desired particle size. The malting process comprises three main steps: steeping, germination, and drying [4].
In the steeping phase, which lasts approximately two days, barley grains are placed in tanks and hydrated at 8–15 °C. This phase initiates germination. After steeping, the grains are transferred to germination vessels for the germination phase, which lasts 6–7 days and exposed to a humid air stream to maintain a temperature of 15–21 °C. This environment activates enzymes such as amylases and β-glucanases, which convert starches into fermentable sugars.
The final step of the malting process is named the drying phase, during which germinated grains are dried at 40–60 °C until their moisture content is reduced to 4–5% [4,5].
After 3–4 weeks of storage, the malted barley is ready for the brewing process and undergoes milling, mashing and filtration. During milling, the malt is crushed to facilitate the subsequent mashing phase, where it is mixed with water, and the temperature is gradually increased to approximately 38 °C. This latter temperature value promotes the enzymatic hydrolysis of starch into fermentable sugars, such as maltose and maltotriose, and non-fermentable sugars, like dextrins. In addition, proteins are hydrolyzed by proteases into peptides and amino acids. These biochemical reactions result in the formation of a sweet liquid known as wort.
The wort is then filtered to remove solid particles. This process separates the liquid wort, which continues in the brewing process, from the solid fraction known as Brewer’s Spent Grain, the primary by-product of brewing. It is estimated that BSG accounts for approximately 31% of the initial malt weight, which translates to about 20 kg of BSG generated for every 100 L of beer produced [2].
1.2. Brewery’s Spent Grain Applications
Brewer’s Spent Grain is characterized by its high protein and fiber content, making it a valuable biomass for reuse. Numerous studies have focused on the sustainable valorization of BSG across various applications. One of the main uses of BSG is as animal feed [6,7,8]. This biomass has increasingly become a key component in feed mixtures, replacing soybeans for livestock such as cattle, pigs, goats, and poultry. A significant advantage of this application is that, in many cases, the raw biomass does not require extensive processing. However, BSG has high moisture content and fermentable sugars, posing challenges in its preservation, as they promote rapid microbial growth [9].
BSG has also found applications in human food products. It has been incorporated into bread, cookies, cakes and snacks [10,11,12]. Furthermore, BSG can serve as a source of food additives, such as phenolic compounds, which act as antioxidants [13] by inhibiting microbial growth and extending the product’s shelf life [14].
Other applications of Brewer’s Spent Grain include biochar production [15], which can be used in catalysis as adsorbent, fuel (bio-oil and biogas) and soil amendments [16].
BSG has also been investigated for its potential role in soil remediation. For instance, Martinkosky et al. utilized BSG to cultivate earthworms involved in soil remediation processes [17]. Additionally, BSG has been used in phytoremediation, serving as a soil amendment that improves the growth of Jatropha curcas involved in the remediation of contaminated soils [18].
Brewer’s Spent Grain has been used as a filler in developing composite materials, due to its high fiber content, as it can enhance certain mechanical properties of the polymeric matrix, or reduce the amount of polymer required, thereby lowering the cost of the final composite material [19,20,21]. Given the global demand, the resulting composite must be biodegradable [22].
In addition, BSG has applications in biorefinery processes. In this context, BSG serves as a feedstock for producing various bio-based products, including value-added molecules, bioenergy and biopolymers [15]. Recent studies have demonstrated that BSG can be used to produce enzymes, fatty acids, alcohols, carotenoids, protein hydrolysates, cellulose-enriched materials, lignin and fermentable sugars [23,24,25,26]. Moreover, BSG is employed in anaerobic digestion (AD) for biogas production [27].
In light of the above, this research aimed to develop an integrated biorefinery approach to intelligently and sustainably valorize BSG, fully complying with the principles of the Circular economy.
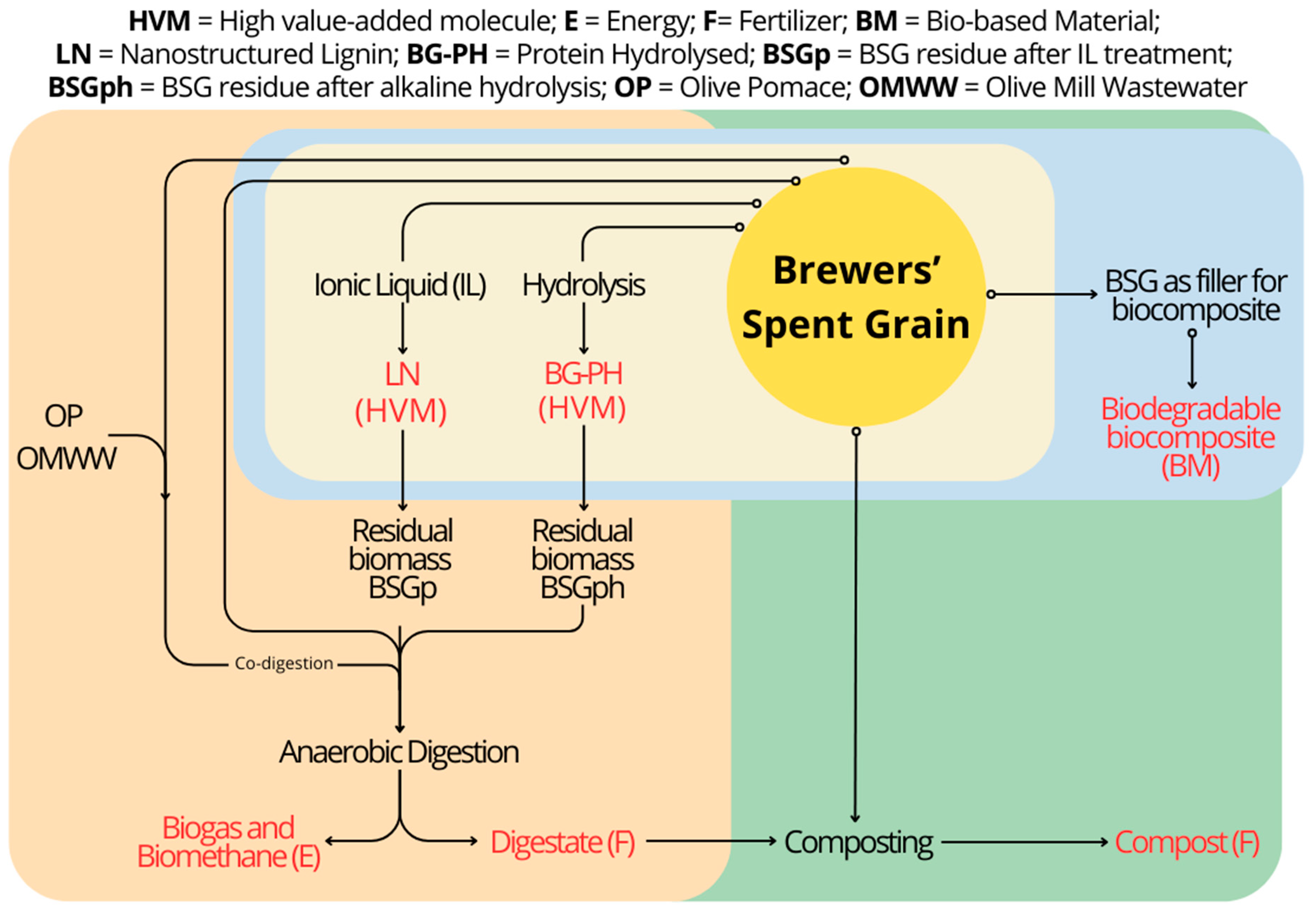
The procedure followed in this study is summarized in Figure 1.
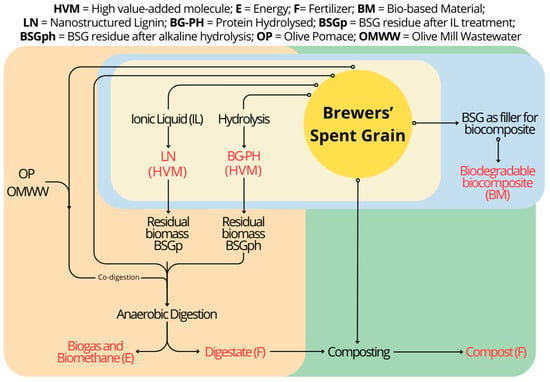
Figure 1.
Schematic representation of the process involved in BSG valorization, including the extraction of high-added-value molecules, biocomposite production, as well as bioenergy and fertilizer generation.
To this end, BSG was exploited through the extraction of protein hydrolysates (PH), a fibrous fraction enriched with cellulose (pulp), and the production of bioenergy and biocomposites. In particular, the obtainment of protein hydrolysates (PH) was investigated by applying BSG milder thermal hydrolysis to obtain peptides and amino acids. The cellulose-enriched pulp was obtained by treatment of BSG with an ionic liquid composed of triethylamine sulphuric acid. The choice to use this ionic liquid was driven by the fact that it is suitable for biomass treatment, is inexpensive, and shows high thermal stability and recyclability [28,29]. Subsequently, this research investigated the potential of BSG for the production of bioenergy by anaerobic digestion (AD), thus evaluating the yield of untreated BSG, of the residual biomass after PH extraction, and BSG in combination with the pulp and other agro-industrial wastes (olive pomace—OP—and olive mill wastewater—OMWW). Biocomposites were also developed by exploiting raw BSG as a reinforcing filler for polyhydroxybutyrate (PHB), then evaluating some of the properties of the materials obtained and testing their biodegradability in compost.
2. Results and Discussion
2.1. Biochemical Characterization of BSG Protein Hydrolysate
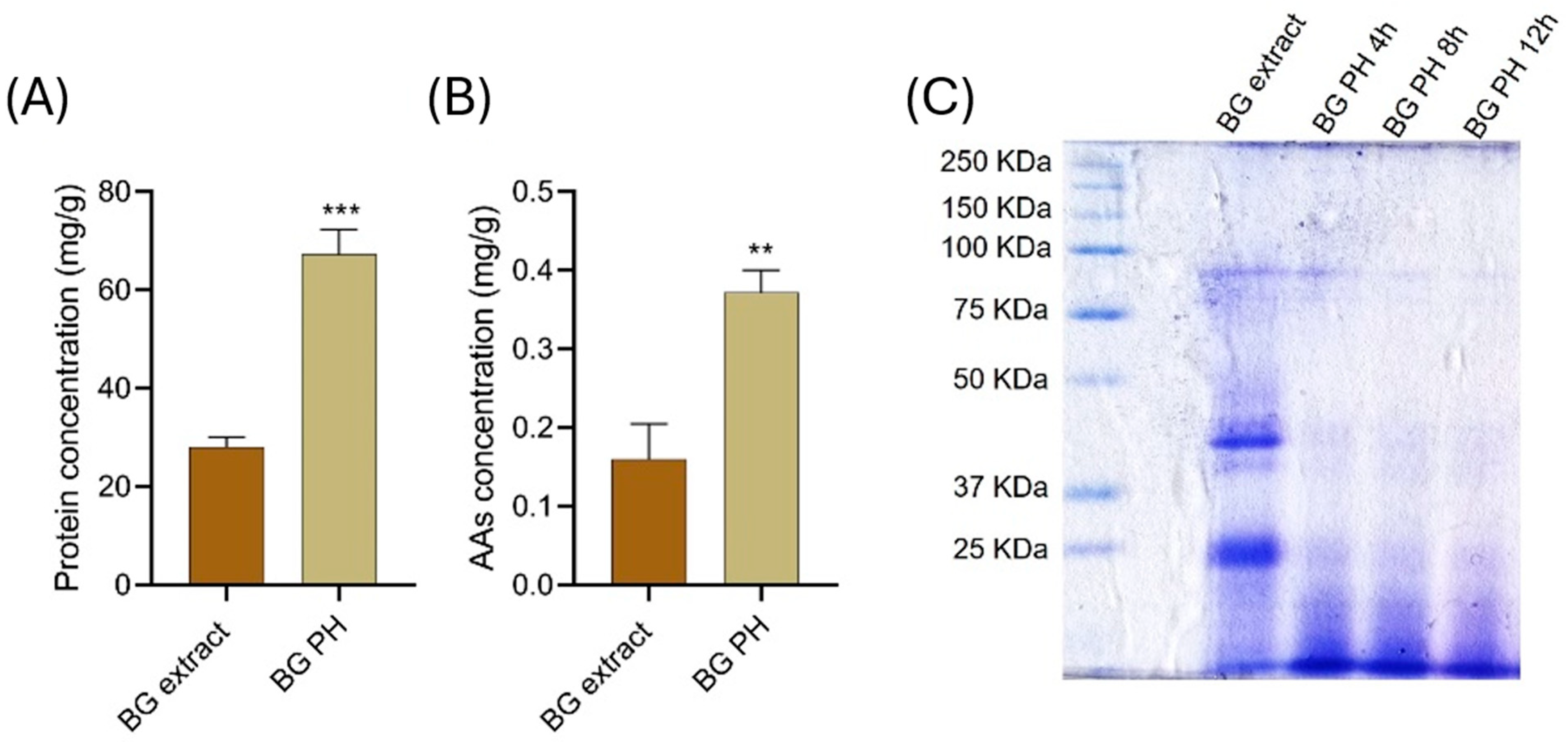
The protein concentration of the BG extract and BG-PH was determined using the Bradford protocol. As shown in Figure 2A, BG-PH exhibited a significantly higher protein concentration (approximately 70 mg/g of dry weight) compared to the crude BG extract (~25 mg/g of dry weight). Similarly, the amino acids (AAs) content (Figure 2B), determined by the Ninhydrin assay, was significantly higher in BG-PH than in the crude BG extract (~0.4 mg/g vs. ~0.18 mg/g). The enhanced release of free amino acids suggests that the mild thermal hydrolysis process efficiently cleaves peptide bonds, increasing the bioavailability of smaller peptides and free amino acids. These results confirm the effectiveness of the hydrolysis process in solubilizing proteins from the recalcitrant raw material.
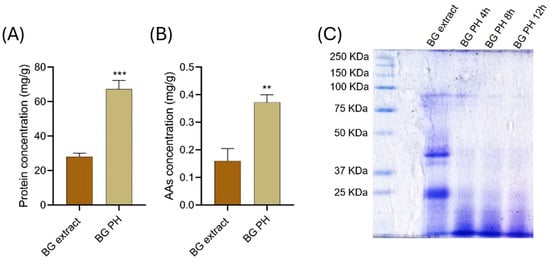
Figure 2.
Protein and amino acid concentration, and electrophoretic profile of brewery spent grain (BG) extracts and hydrolysates (BG PH). (A) Protein concentration expressed as mg/g of dry weight determined by the Bradford assay, *** p < 0.001. (B) AAs concentration expressed as mg/g of dry weight measured by the Ninhydrin assay, ** p < 0.01. (C) SDS-PAGE analysis of BG extract and BG-PH collected after 4, 8, and 12 h of hydrolysis.
The SDS-PAGE analysis (Figure 2C) provides further insights into the protein hydrolytic process. The BG extract lane exhibits distinct protein bands, suggesting the presence of intact high-molecular-weight proteins. Conversely, the hydrolyzed biomass (BG PH) shows progressive fragmentation after 4, 8, and 12 h of hydrolysis, with an increased presence of low-molecular-weight peptides and a reduction in intact protein bands. This confirms the breakdown of complex protein structures into smaller peptides and free amino acids over time [30]. The observed degradation pattern is consistent with the process cleavage mechanism, where prolonged incubation facilitates further peptide bond hydrolysis.
Protein hydrolysates (PHs) obtained from Brewery’s spent grain are characterized by a high content of low molecular weight peptides, making them valuable bio-based products with significant potential for different applications. PHs are predominantly utilized in agriculture as biostimulants and biofertilizers, as well as in animal nutrition as feed additives for livestock and pets. Furthermore, the described production process enables a zero-waste approach, as the minimal residues generated are further processed through aerobic and/or anaerobic digestion and subsequently used in agriculture as biofertilizers, thus allowing a total recovery of the starting biomass [31].
2.2. BSG Pulp After IL Treatment
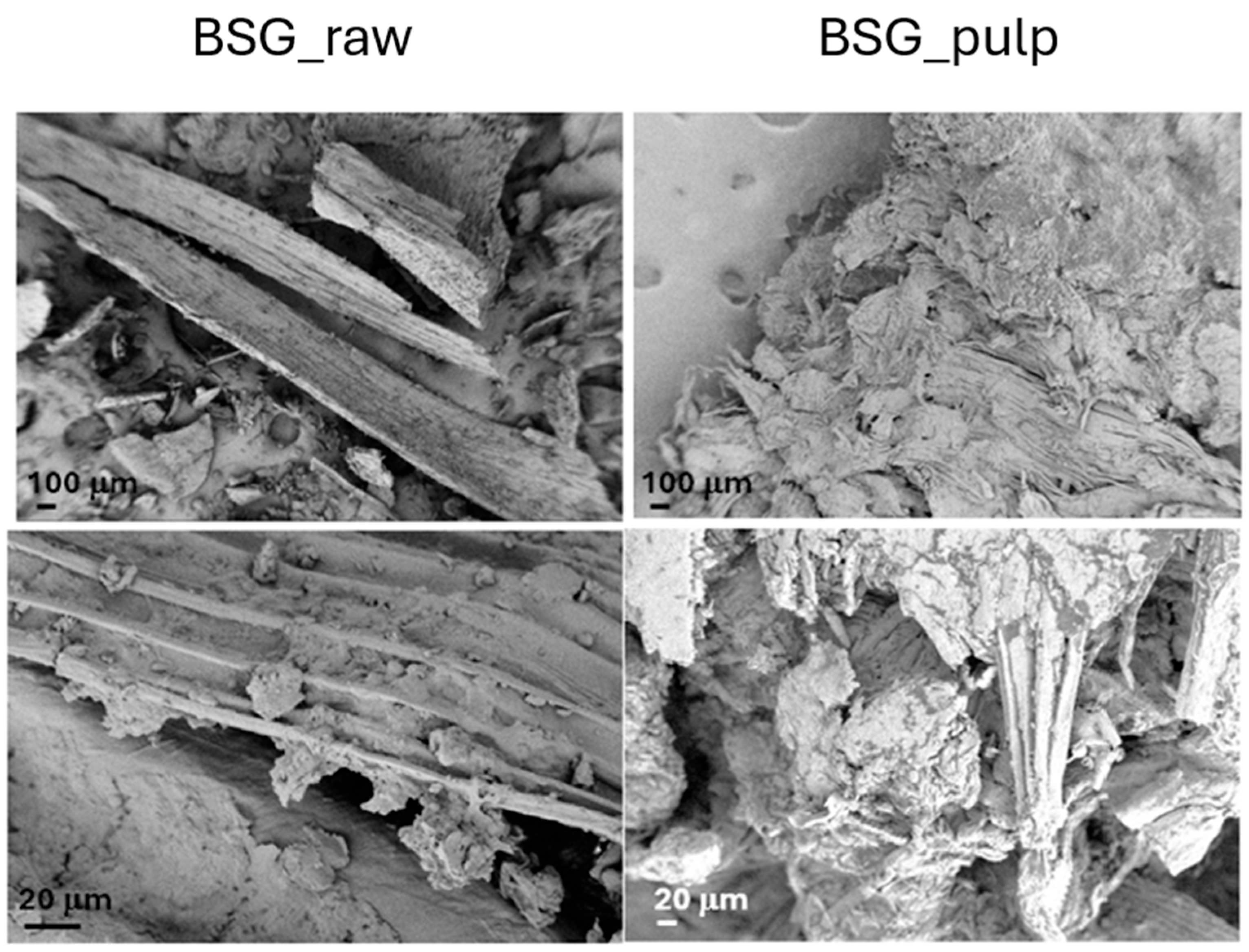
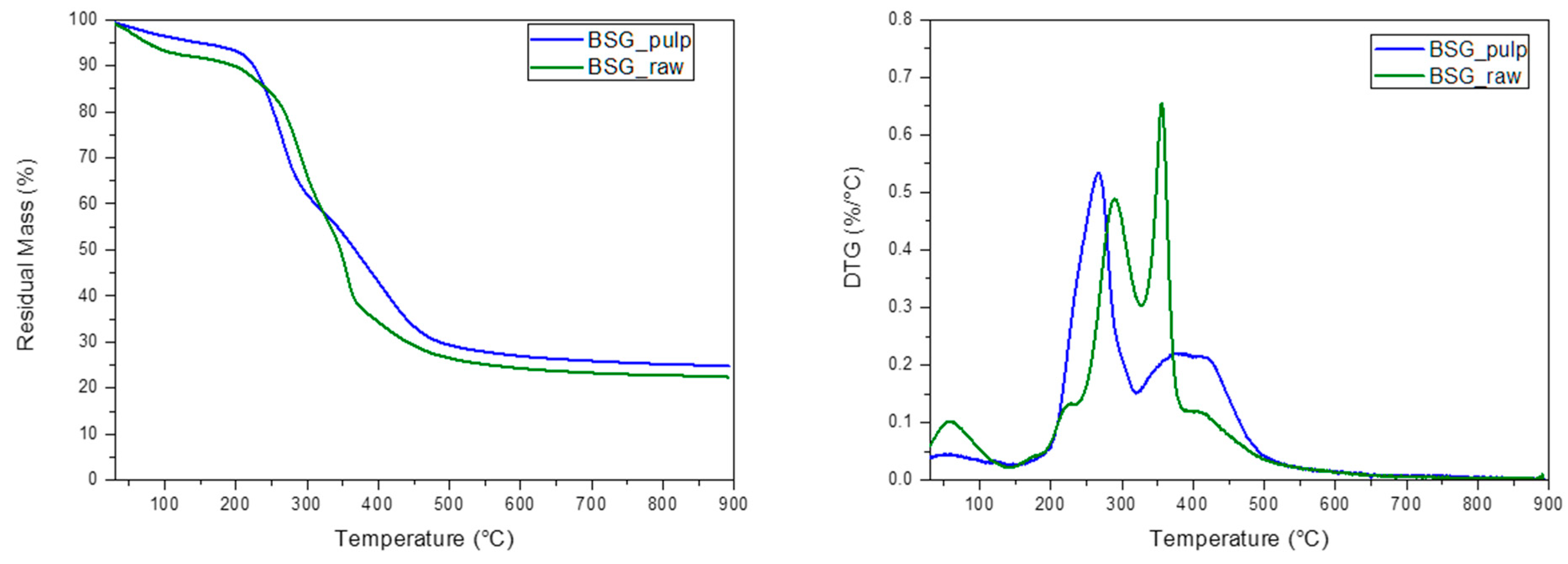
The BSG_raw and BSG_pulp were characterized in terms of morphological (FESEM) and thermal (TGA) behaviour before and after IL treatment (Figure 3 and Figure 4). The FESEM reveals significant morphological differences between BSG_raw and BSG_pulp, which point out that the treatment removed some structural components, thus resulting in a more fragmented and porous structure. This suggests that IL destructured the fibrous matrix, and this can, in turn, lead to biomass degradation in a shorter time than the BSG_raw by AD. The TGA results align with the differences evidenced by FESEM. The thermal degradation process of BSG_raw and BSG_pulp exhibited a first weight loss of up to 150 °C, which can be attributed to moisture evaporation [32,33]. However, BSG_pulp showed a more pronounced loss of water, which can be associated with the deconstruction of the lignocellulosic matrix. Such an effect can be attributed to IL treatment, which resulted in lignin removal, higher porosity of the treated biomass, and an increased content of cellulose and hemicellulose, both of which are hydrophilic biopolymers [34]. Subsequently, BSG_raw showed a second degradative pattern between 200 and 260 °C, related to the start of cellulose degradation, which was followed by an endothermic depolymerization process ranging from about 310 to 500 °C [34]. BSG-raw also showed a degradation peak, which can be ascribed to the hemicellulose degradation, with a maximum at about 300 °C [35,36]. Lignin in BSG_raw was associated with a thermal decomposition at temperatures between 300 and above 500 °C [37]. In the case of BSG_pulp, the TGA pointed out that IL deconstructed the fiber organization as the number of signals decreased, and, generally, they evidenced a wider shape and lower thermal stability with a reduced amount of lignin. Due to delignification, the increased cellulose accessibility in BSG_pulp could result in better digestibility and faster consumption of the material by AD. On the other hand, it should be noted that the treatment with IL decreased the thermal stability of BSG_pulp, limiting its use for applications operating at temperatures above 250 °C.
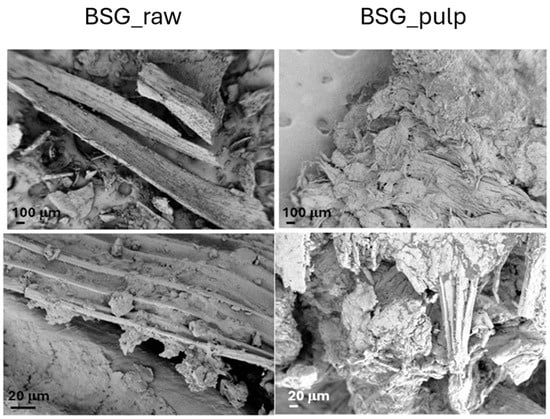
Figure 3.
FESEM images of the brewer spent grain (BSG_raw) and the pulp derived from its treatment with IL 80% (BSG_pulp).
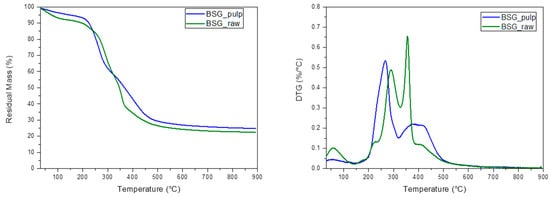
Figure 4.
DTG and TG curves of the brewer spent grain (BSG_raw) and the pulp derived from its treatment with IL 80% (BSG_pulp).
2.3. Biogas and Biomethane Production
Five different matrices were investigated for biogas/biomethane production via AD. Firstly, the potential of untreated BSG was tested. The same process was repeated for treated BSG and for BSG mixed with other agro-industrial wastes, such as olive mill wastewater (OMWW) and olive pomace (OP). Tests carried out with treated BSG allow us to define the feasibility of optimizing the recovery of by-products from agro-industrial wastes, firstly producing high-added-value products, such as lignin or protein hydrolysates and then producing energy from the residual biomasses. The amounts of biogas/biomethane produced from the above samples, together with the energy efficiency of the whole process, were previously characterized in Di Mario et al. [23], where a more detailed description of the anaerobic digestion process can be found.
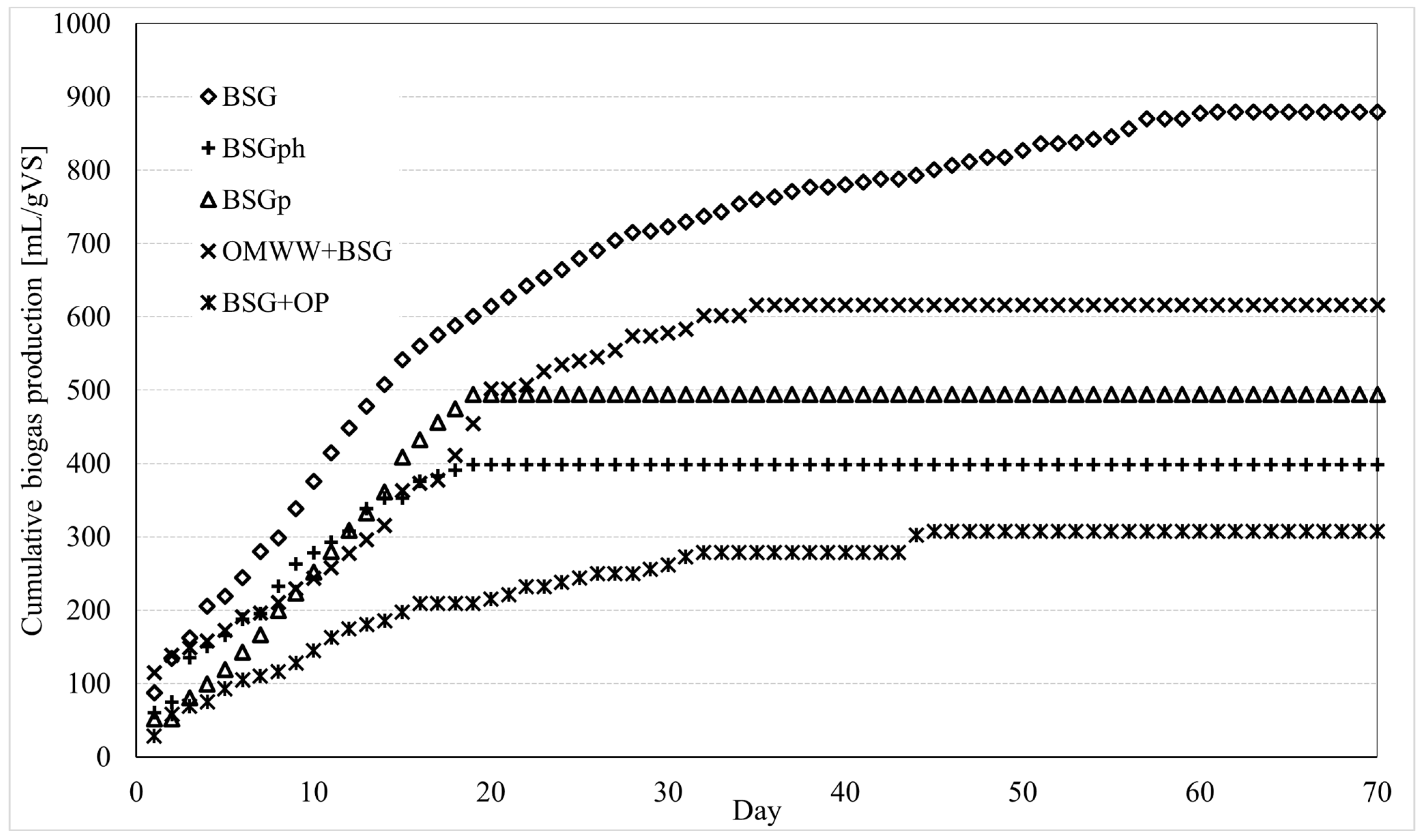
The process was carried out according to the experimental procedure described in Section 2.3. Experiments were carried out in triplicate. For each different sample tested, the mean values between the three experiments were used to produce the cumulative curve shown in this section and to define the total production of biogas/biomethane and, consequently, to carry out the energy evaluations. For each sample, the deviation of the various experiments from the mean values reported here remained below 10 mL/gVS. Figure 5 shows the cumulative biomethane production for the various samples; the total amount of biomethane, normalized per mass unit of VS, and the production period are indicated in Table 1.
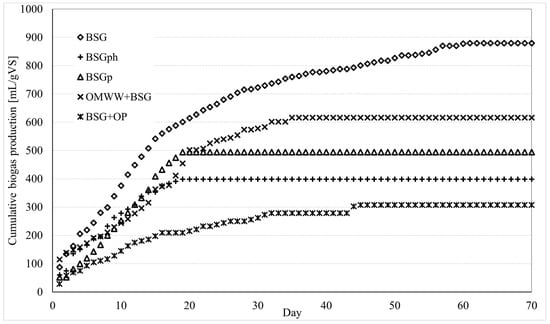
Figure 5.
Cumulative biomethane production from the various matrices tested in this study: BSG: Brewery’s spent grain; BSGph: BSG residue after alkaline hydrolysis; BSGp: BSG residue after IL treatment; BSG + OMWW: BSG mixed with olive mill wastewater; BSG + OP: BSG mixed with olive pomace. Each sample was tested in triplicate; the mean values are shown here. The deviation of each test from the corresponding mean values remained below ±10 mL/gVS.

Table 1.
Total quantity of biomethane produced and days passed before reaching the process completion.
Untreated brewers’ spent grain led to the most abundant production of biomethane and also the longest production period: approximately 870 mL of biogas were obtained during 60 days of production. The addition of OMWW made the overall production lower, but at the same time, significantly reduced the production period. With the BSG + OMWW sample, 616 mL of biomethane were produced during 35 days, meaning that the quantity of biomethane produced per day was greater than in the previous case, where only untreated BSG was used. By itself, OMWW alone cannot be considered for biogas production, since its richness in polyphenols, together with its high humidity content, makes it unsuitable for this scope. However, several studies revealed that the addition of OMWW to other matrices often allows us to enhance the anaerobic digestion process [37]. In this sense, the present research (together with [23]) represents a further validation of that thesis.
Interesting results were also obtained when using BSGph and BSGp: the overall production was lower, respectively, equal to 398 and 494 mL; however, the process required only 19 days to be completed. The cumulative curves clearly proved that, for these latter samples, the production of biogas/biomethane started with intensity and defined a trend close to the one observed for BSG + OMWW, albeit the process finished in advance (after 19 days), thus leading to widely different results in terms of total amount of biomethane produced. Such a particular trend, regarding the nitrogen amount in the samples, can be discussed. Biomasses like BSG or bran have high protein content; nitrogen plays a crucial role during anaerobic digestion, being one of the most essential nutrients for microbial communities operating during the methanogenic activity [38]. The processes were soon considered, especially the protein hydrolysis, which produced, as a residual, BSGph. It can be assumed that the low nitrogen content in BSGph acted as a limiting factor and caused the premature interruption of anaerobic digestion. Finally, the sample consisting of BSG + OP showed the worst results: 308 mL of biomethane were obtained during 45 days of production.
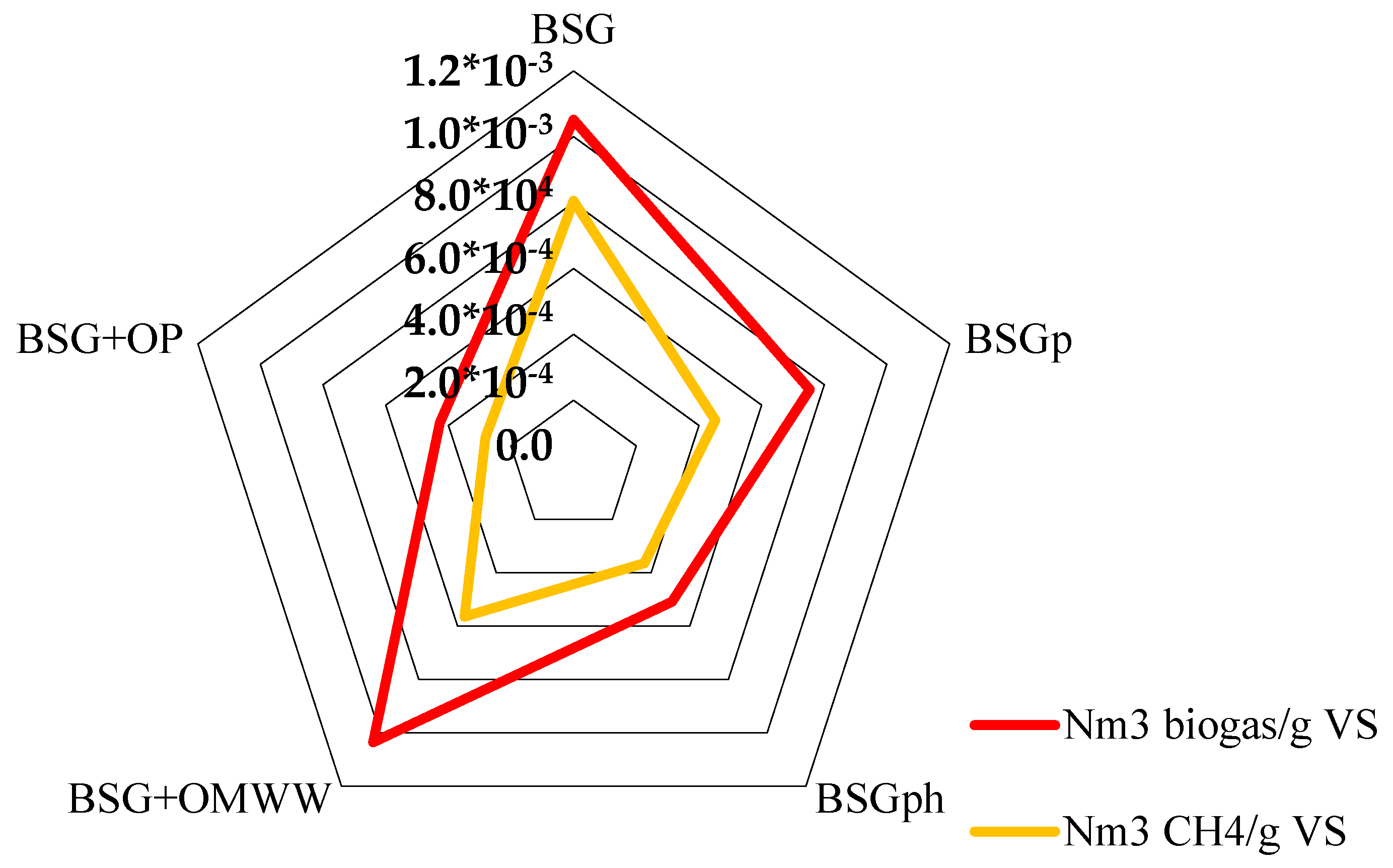
Figure 6 shows the effective quantity of biomethane produced per unit mass of biomass tested. In particular, the diagram shows the quantity of biogas and biomethane obtained (in Nm3) per gram of volatile solids (VS) of the matrices used during the process. The values plotted in the figure are summarized in Table 2.
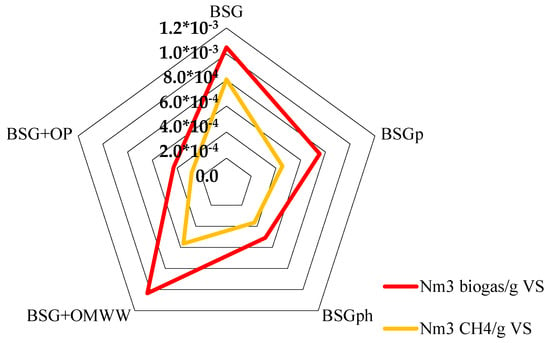
Figure 6.
For each sample, the quantity of biogas and biomethane produced (in Nm3) per unit mass of volatile solids (g) has been calculated.

Table 2.
Quantity of biogas/biomethane produced per unit mass of volatile solids, for each sample.
In terms of biogas produced per unit mass of VS, BSG and BSG + OMWW showed the same result; in detail, 1.05 × 10−3 and 1.04 × 10−3 Nm3/g vs., respectively. Conversely, the quantity of biomethane produced differed significantly between the two species: 0.81 × 10−3 Nm3/g vs. were obtained with BSG, while only 0.57 × 10−3 Nm3/g vs. were achieved with BSG + OMWW. The other samples led to a lower production, as visible in Figure 7 and in Table 2. However, the quantitative analysis of biomethane revealed that BSGp and BSG + OMWW produced similar quantities, respectively, 0.46 × 10−3 and 0.57 × 10−3 Nm3/g VS. In agreement with the results shown in Figure 6 and discussed in Table 3, the lowest production, per mass unit of VS, was measured for BSG + OP: only 0.28 × 10−3 Nm3/g vs. was obtained with this raw material.
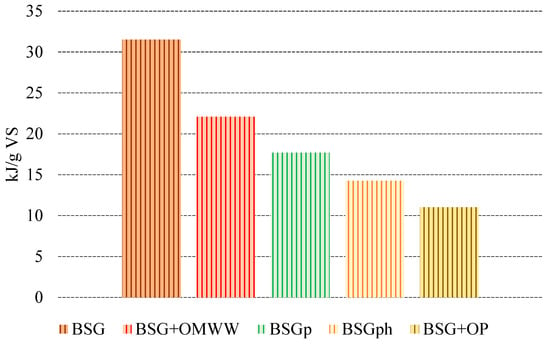
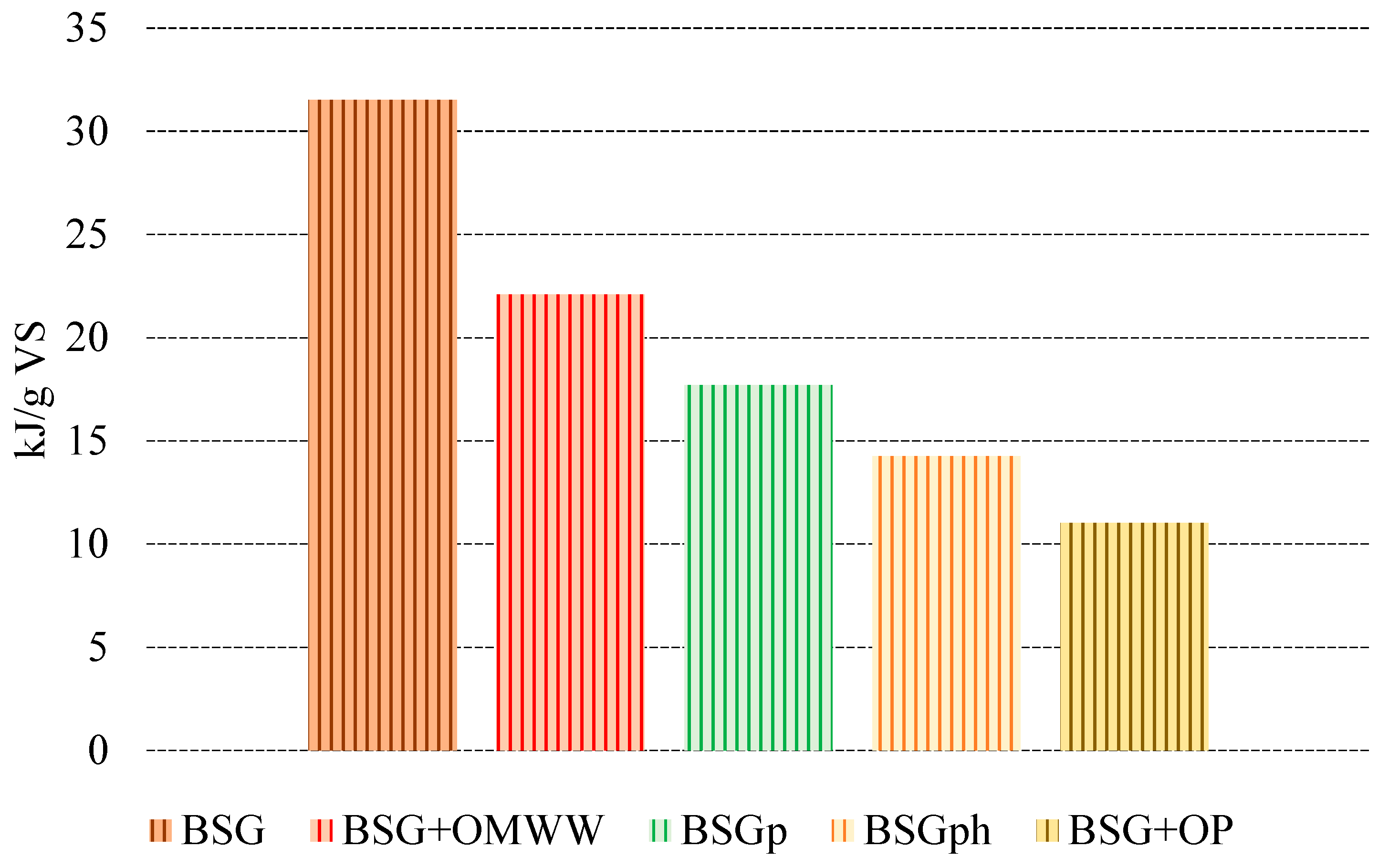
Figure 7.
Quantification of energy produced (kJ) per unit mass of volatile solids (g) for each species.

Table 3.
Characterization of the obtained compost. TVS = Total Volatile Solids; TOC = Total Organic Carbon; TKN = Total Kjeldahl Nitrogen; DW = Dry Weight.
Given the quantity of biomethane produced and considering its LHV (52 MJ/kg), Figure 7 shows the quantity of energy produced per unit mass of VS for each sample. The different biomasses were positioned in descending order, based on the quantity produced.
As expected, the best performance was registered for BSG (31.53 kJ/g VS), followed by BSG + OMWW (22.10 kJ/g VS) and BSGp (17.69 kJ/g VS). The energy gap between these two latter species and BSG is acceptable if considering that the usage of BSG + OMWW allows for recycling and treating a highly impactful agro-industrial waste (OMWW), while in the case of BSGp, the additional value generated via lignin extraction must be considered.
The lowest quantities of energy produced were observed for BSGph (14.27 kJ/g VS) and BSG + OP (11.03 kJ/g VS).
2.4. Characterization of Brewery’s Spent Grain Compost
The obtained compost was characterized to assess its suitability for use in accordance with the Italian regulation D.lgs 75/2010 [39]. The characterization results are presented in Table 3. All measured parameters fall within the limits established by the legislative framework, confirming that the compost can be used as an amendment. The germination test results indicate that this material does not exhibit any phytotoxic activity. Moreover, the data suggest a priming and stimulatory effect on seeds, as the germination index for the 30% dilution exceeds 100.
The resulting compost proves to be a viable amendment due to its characteristics. As demonstrated in this study and previously reported by Alessandri D. (2021) [40], the incorporation of lignocellulosic materials into BSG is essential for improving the final compost properties. Previous research [40,41,42] has investigated BSG composting, highlighting its positive effects on plant growth. In this study, our primary goal was to produce a suitable BSG-based compost and assess its compliance with legislative parameters. Therefore, rather than evaluating the entire composting process, we focused solely on characterizing the final product.
2.5. Mechanical and Morphological Characterization of PHB-BSG Composites and Their Biodegradability
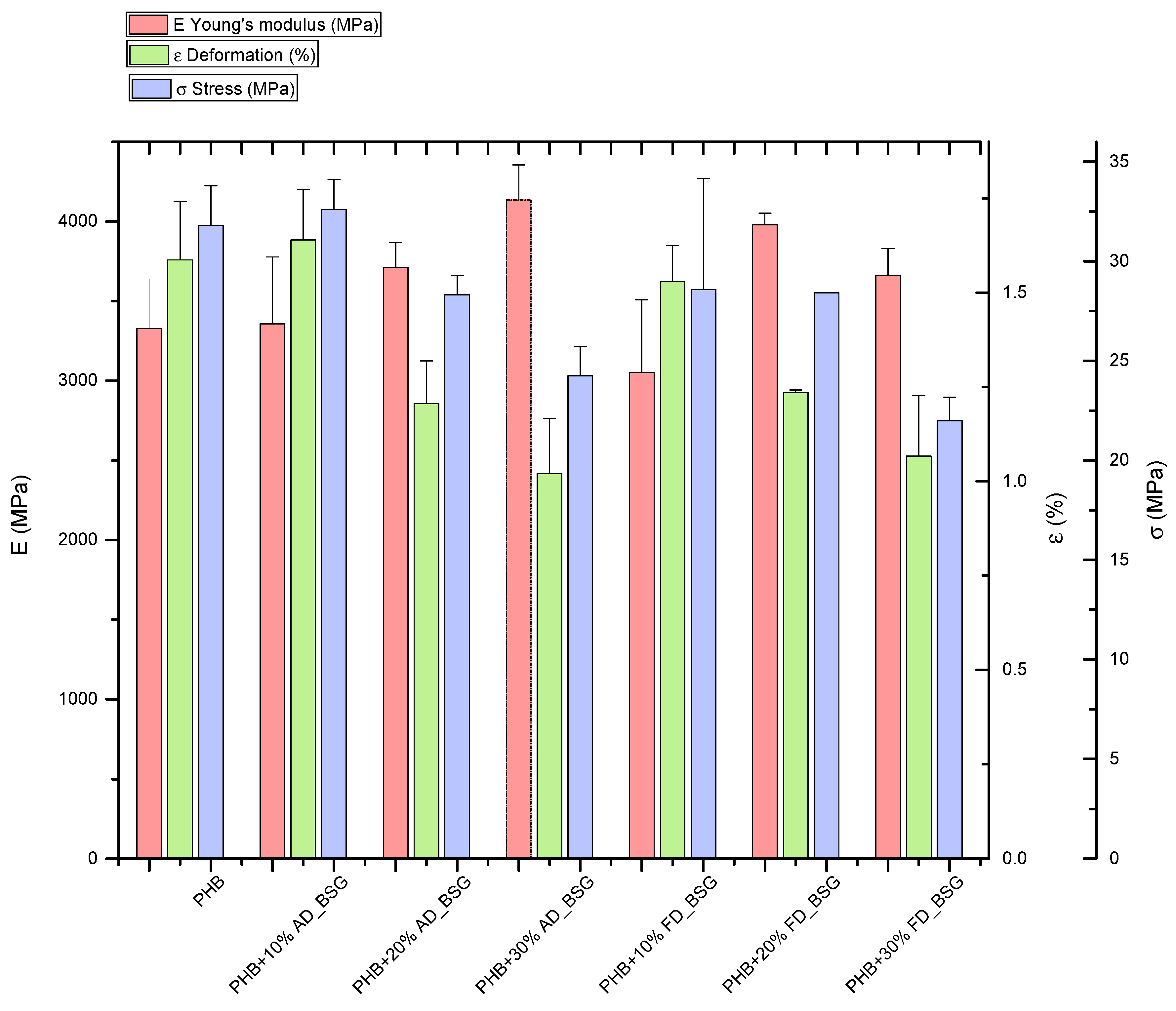
Results of mechanical characterization of PHB-BSG composites (Tensile strength and Elongation at break) indicate that BSG, as expected, was able to improve stiffness, while strength decreased when the higher amount of BSG (30% wt.) was added, limiting at the same time also the deformability of the composite systems (Figure 8). Similar trends were observed for air dried (AD) and lyophilized (FD) particles, even if an overall decrease was found for composites containing lyophilized fibers: as reported in [43], oven drying of BSG gave rise to small decreases in protein and fat contents in comparison with the wet (frozen) sample, influencing the reinforcing role of BSG in the produced samples. Oven drying is indeed the method with lowest economic cost, which makes it more suitable for preserving the BSG for exploitation in different processes. In both cases, extrusion process can both eliminate the agglomeration of particles due to moisture removal, but also generate melanoidins during the extrusion treatment, especially at elevated temperatures, degrading BSG to give colored samples [44,45,46].
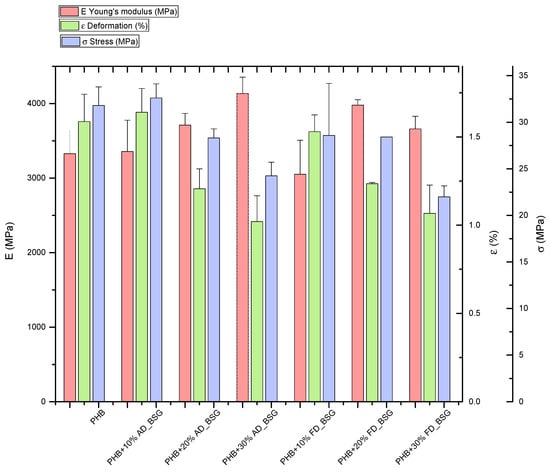
Figure 8.
Results of tensile characterization of PHB-BSG composites.
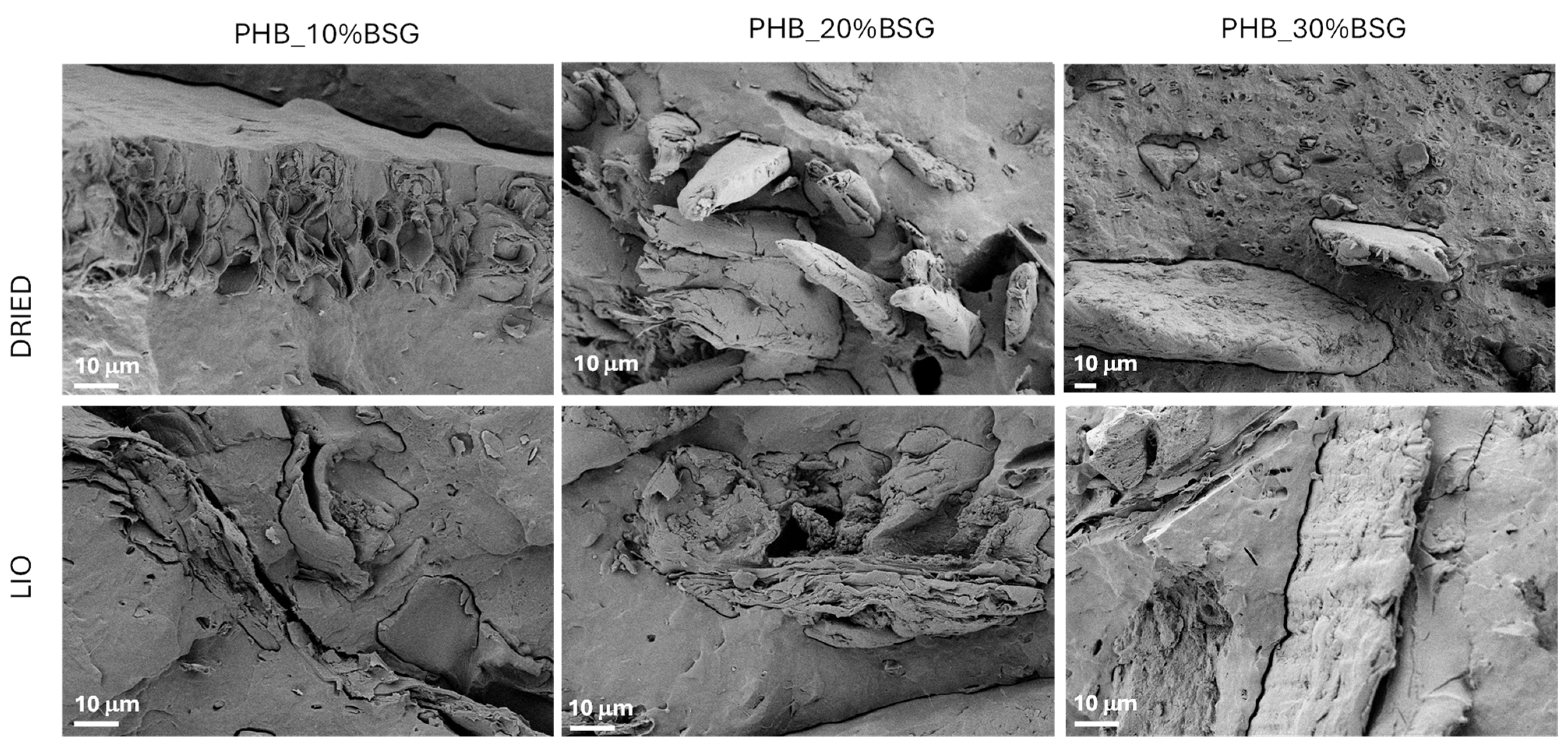
The results of mechanical characterization can also be justified by the analysis of fractured surface morphology for the produced composite after the tensile test: Figure 9 presents the surfaces of the composite bulk PHB-based samples containing 10, 20 and 30 wt% BSG. Poor adhesion between filler and matrix is evident, with holes resulting from the pull-out of BSG during tensile testing in the case of lyophilized samples.
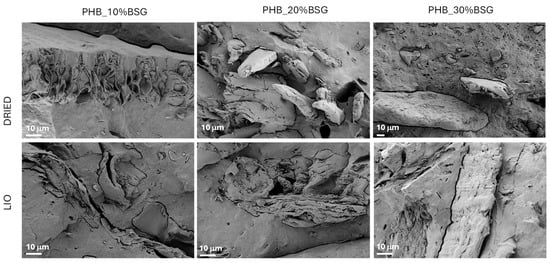
Figure 9.
FESEM images of fractured surfaces for PHB-BSG composites.
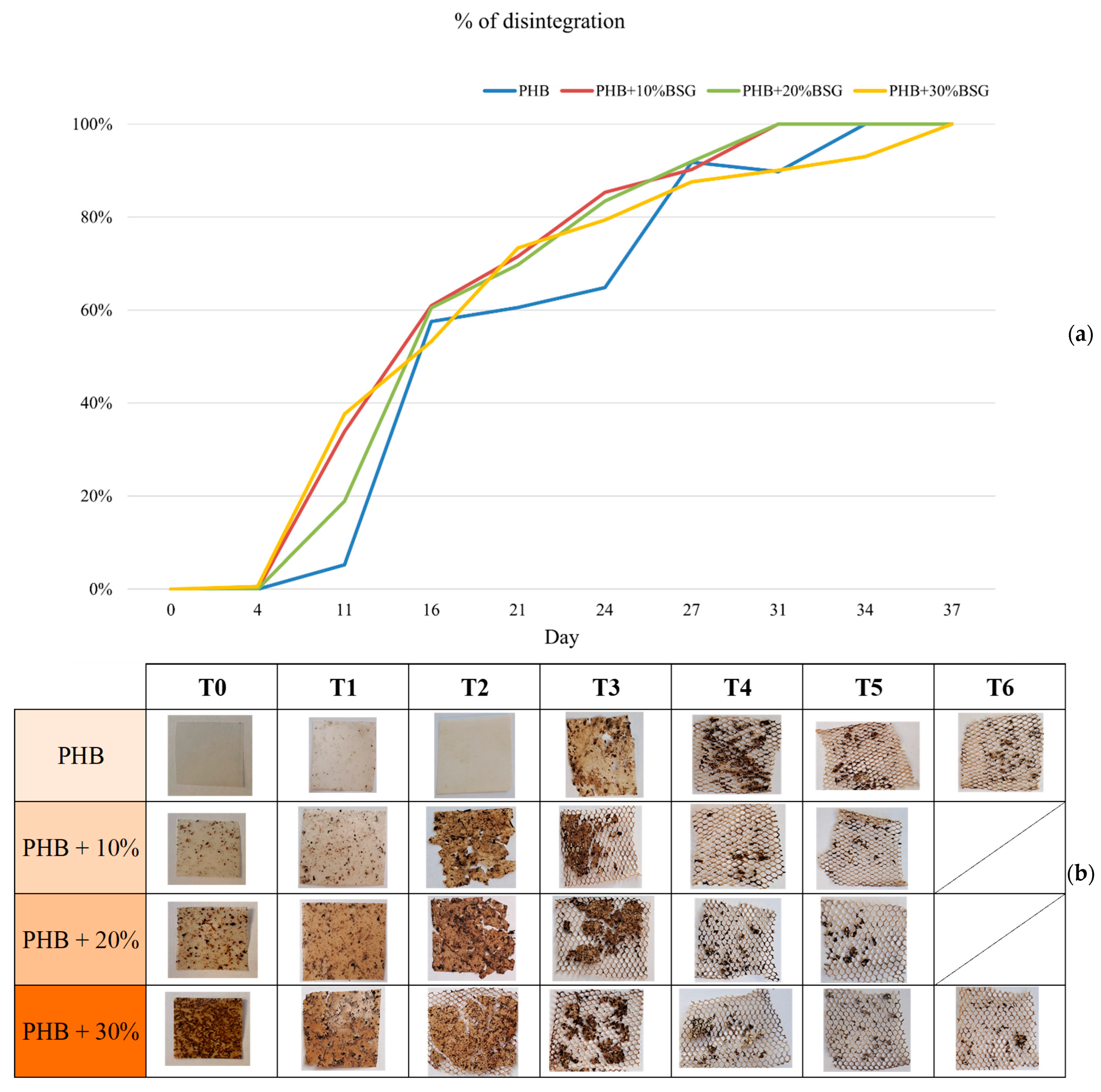
Bio-degradation of polyhydroxyalkanoates happens by two overlapped steps, mainly mediated by microorganisms (bacteria, fungi, etc.): the first one is the heterogeneous enzymatic hydrolysis of polymer chains to give small and generally soluble organic molecules and oligomers (disintegration). Subsequently, the complete oxidative degradation of these compounds takes place, thus producing CO2 [47]. Disintegration (D%) was determined by monitoring weight loss as a function of composting (Figure 10). No remarkable weight loss was appreciated until day 4 for any sample (induction time). From this point, the disintegration rate abruptly increased, while a roughening of the surface of all the tested samples was clearly observed. Moreover, regardless of the rate, all samples disappeared at 37 days. The biodegradation rate of PHB depends on several factors, including the environmental conditions (temperature, moisture content, aeration) and the microbial population present in the compost. On the other hand, BSG is primarily composed of lignocellulosic materials and other compounds, which are rich in carbon and essential nutrients for microbial growth during composting.
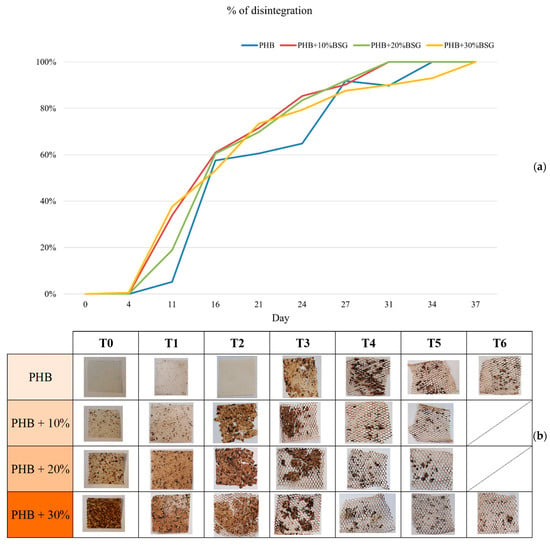
Figure 10.
Gravimetric weight loss % (a) and visual appearance (b) for PHB based materials at different stages of incubation in compost.
It was observed that observed that the rate of PHB degradation was slightly increased by the presence of BSG, as it can exert a priming effect on the compost microbial community or favor the interactions with the PHB matrix, thus achieving a more effective polymer chain breaking [48]. The rate of degradation of the PHB-BSG composite also depends on the ratios of PHB to BSG: while PHB may degrade relatively quickly, BSG’s lignocellulosic materials (especially the recalcitrant lignin) will require more time and specialized microbes to break down fully [49], so times for disintegration of 30% wt BSG containing samples was slightly slower than other composites at the longer times.
3. Materials and Methods
3.1. Materials and Procedure
Raw Brewers’ spent grain (BSG) utilized in this study was generously provided by the brewery “La Gramigna” (Perugia, PG, Italy). BSG characterization is reported in Table 4. BSG was employed in parallel processes to produce Protein Hydrolysates (PH), BSG pulp, BSG compost, and BSG digestate. After the extraction of PH and ionic liquid treatment, the residual biomass undergoes anaerobic digestion to assess the biogas production potential. BSG was also used in co-digestion with other biomass, Olive Pomace (OP) and Olive Mill Wastewater (OMWW), to assess its effect on biogas production. OP and OMWW were provided by “Frantoio Centumbrie” (Agello, PG, Italy).

Table 4.
Chemical composition of BSG. vs. = Volatile Solid; TOC = Total Organic Carbon; TKN = Total Kjeldahl Nitrogen; DM = Dry Matter.
Additionally, BSG served as a filler in the development of biocomposites with polyhydroxybutyrate (PHB) as matrix. The PHB polymer (grade ENMAT Y3000P, MFI 5-18, g/10 min) was purchased from Helian Polymers BV (Bremweg, The Netherlands).
Moisture Content and Volatile Solids Determination
The humidity of the samples was assessed according to the official protocol by weighing two grams of each sample and drying them at 105 °C until constant weight was achieved [50]. For the determination of Volatile Solids (VS), the dried samples were placed in ceramic crucibles and heated in a muffle furnace at an increasing temperature until reaching 550 °C, where they were maintained for 24 h, in accordance with the official protocol [23].
3.2. Molecule Extraction
3.2.1. Production of Protein Hydrolisates (PHs) from Brewery’s Spent Grain
Protein Hydrolysates from the brewers’ spent grain (BG-PH) were obtained from a mild thermal hydrolysis process. A total of 2.0 g of dry biomass was pulverized by grinding and resuspended with a 1 M of KOH solution, and placed in Multi-Therm Shaker (Benchmark Scientific, Sayreville, NJ, USA), at 600 rpm and 60 °C for 12 h. After the thermal incubation, the hydrolysate was neutralized with an acidic solution to achieve a neutral pH. The hydrolysates were then centrifuged at 10,000× g for 10 min at 4 °C to remove the non-hydrolyzed fibrous component.
Determination of Protein Content and Amino Acids Content
To compare the protein and amino acid content of BG-PH with non-hydrolyzed biomass, a crude extract (BG-extract) was obtained by adding 20 mL of 0.2 M NaOH to 1 g of dry biomass. The mixture was incubated in Multi-Therm Shaker, at 500 rpm and 50 °C for 1 h and then centrifuged at 2000× g for 5 min. The concentration of protein content was determined using the Bradford assay [51], while the amino acid content was evaluated using the Ninhydrin assay [52].
Evaluation of Hydrolysis Degree by SDS-PAGE and Coomassie Blue Staining
The electrophoretic profile of BG-PH and the starting biomass were analyzed using SDS-PAGE following the Laemmli method [53]. Each sample (40 µL) was combined with 5X sample buffer (0.5 M Tris-HCl, pH 6.8, 10% (w/v) SDS, 50% (v/v) glycerol, 0.01% (w/v) bromophenol blue, and 125 mM dithiothreitol in a 4:1 (v/v) ratio). The mixtures were then boiled for 5 min and loaded onto a 12% acrylamide gel for electrophoresis at 40 mA. Finally, gels were stained with Coomassie Blue R-250.
3.2.2. BSG Treatment for Cellulose Extraction
The isolation of cellulosic fractions (pulp) from raw brewers’ spent grain to extract the cellulose component was performed using a procedure based on the use of triethylamine and sulfuric acid (IL: [Et3N] [HSO4]). The IL was synthesized according to [28]. Specifically, triethylamine (0.009 moles) was added to a round-bottomed flask with a volume of 250 mL and brought to a temperature of 60 °C, and then added dropwise of sulfuric acid in a stoichiometric ratio of 1:1. The mixture was thus allowed to react at 70 °C for 1.5 h. For biomass treatment, the raw BSG, dried and sieved to 0.25 mm, was introduced into a Teflon reactor and added with a solution of IL and water in a ratio (4:1—w:w). The suspension was kept at 120 °C for 4 h to destroy the fibrous matrix. After treatment, the system was allowed to cool and reach room temperature, and ethanol was added to solubilize and remove both lignin and hemicellulose by vacuum filtration. This liquid phase, obtained from the above, was concentrated by filtration under reduced pressure using a rotary evaporator. Lignin was subsequently recovered and removed from the hemicellulosic fraction by precipitation, due to the addition of water [53]. The cellulose-enriched pulp (solid fraction) was separated by vacuum filtration, washed thoroughly with ethanol and deionized water to remove any residual traces of IL, and then dried at 50–60 °C to constant weight.
3.3. Anaerobic Digestion
The energetic valorization of BSG was evaluated by assessing the biogas production through Anaerobic Digestion (AD) process, conducted using a batch lab-scale bioreactor at 37 °C (mesophilic conditions). Biomethane production was measured by introducing an alkaline trap containing 0.5 M NaOH and thymolphthalein as a pH indicator. A detailed schematization of bioreactors used in this study can be found elsewhere in the literature [23,29]. The inoculum and biomass were mixed in a specific ratio of 3:1 based on volatile solids weight. The composition of each bioreactor is detailed in Table 5.

Table 5.
Description of samples composition for anaerobic digestion. DW = dry weight; BSG = Brewer’s Spent Grain; OMWW = Olive Mill Wastewater; OP = Olive Pomace.
3.4. Lab-Scale Composting System
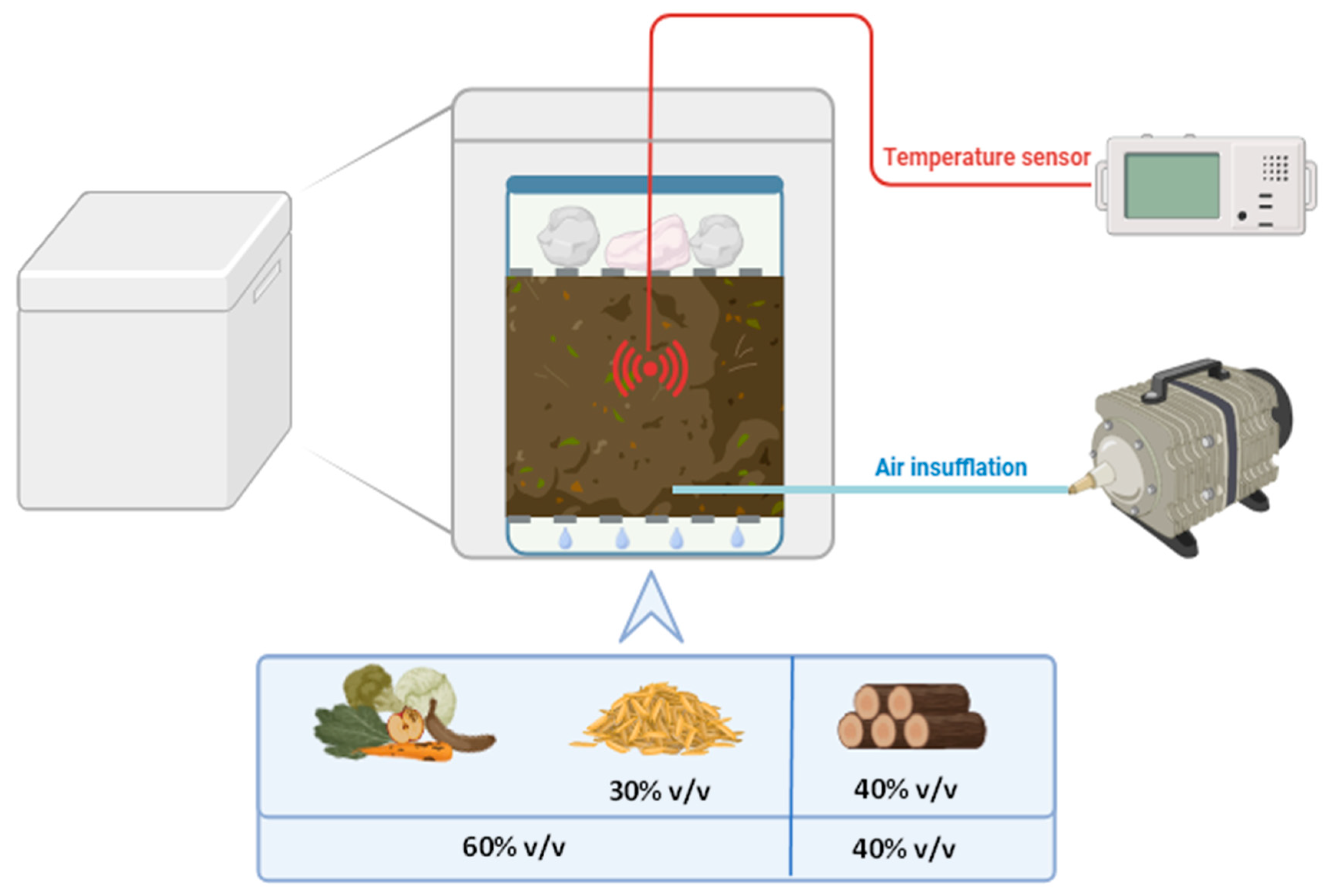
Brewers’ Spent Grain (BSG) composting was conducted using a batch lab-scale composter (Figure 11) with controlled airflow and temperature. Airflow was maintained at 0.6 L/min for the first 10 days, then reduced to 0.1–0.3 L/min for the remaining period. The temperature was monitored using a sensor placed in the center of the composting mixture inside the box. The matrix was also manually mixed every three days. The temperature was monitored to ensure that it reached the appropriate ranges for composting in a lab-scale reactor during the three main phases: mesophilic (>20 °C), thermophilic (>40 °C), and maturation (>20 °C).
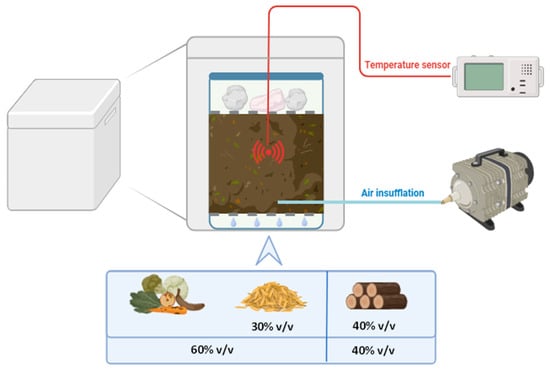
Figure 11.
Scheme of the lab-scale composting system used in this study. The system consisted of a plastic box placed within a polystyrene box, which provided thermal insulation. A metal mesh was positioned at the bottom of the box to facilitate water drainage. Another metal mesh was placed on top of the composting mixture to support approximately 1 kg of stones, ensuring proper compression. Air injection was introduced at the bottom of the box and controlled by an air pump equipped with a flowmeter. Temperature was monitored using a probe inserted into the center of the composting mixture.
To ensure proper compression of the composting mixture, 1 kg of stones was placed on top. The compost was separated from the bottom of the box by a metal mesh, creating 1.5 cm free space at the bottom to facilitate water drainage. The initial composting mixture consisted of 60% v/v organic fraction and 40% v/v lignocellulosic fraction for a total volume of 2 L. The organic fraction comprised 50% v/v municipal solid waste organic fraction and 50% v/v raw BSG. The lignocellulosic fraction consisted of grape pruning, chopped into small pieces with an average size of 1 cm.
Germination Test
The germination test was conducted following the UNI EN 16086-2:2012 standard [54], which provides a method for evaluating the potential phytotoxicity of compost using Lepidium sativum L. seeds. The compost extract was prepared by adjusting its moisture content to 85%, after which a dilution of 30% was carried out. Filter papers were placed in 9 cm Petri dishes, and 5 mL of the compost extract was added to each dish. Ten seeds were placed in each dish and incubated in darkness at 25 ± 2 °C for 48 h. A control treatment using only distilled water was included. Each treatment was performed in triplicate. After incubation, the number of germinated seeds and the corresponding root lengths were recorded. The germination index (GI) was calculated using the formula (1) [54]:
where Gc and Gt are the average number of germinated seeds in the control and treatment; Lc and Lt are the average length of the roots of the germinated seeds in the control and treatment.
3.5. Biocomposites Development and Characterization
To produce the biocomposites, BSG was first dried using two different methods to assess their impact on the final material characteristics: air drying and freeze drying. Air drying was performed by placing the Brewers’ spent grain in the oven at fixed temperature of 30 °C to prevent any changes in its properties. Freeze drying was carried out using ScanVac CoolSafe by LaboGene.
After drying, the biomass was ground into a powder. The composite was then made by mixing PHB pellets, previously dried at 80 °C for 3 h in an oven, with different concentrations of BSG: 10%, 20%, and 30% wt. The samples (dumbbells shape) were plasticized in the extruder Xplore 5&15 Micro Compounder (DSM, Sittard NL) with a temperature profile of 170–180–185 °C in the feeding-compression-metering zones mixing for 90 s at 60 rpm; then, the melt mixed biocomposites were transferred into the hot chamber of the Xplore Micro Injection Moulding Machine 10cc (DSM, Sittard NL) set at 200 °C. The injection pressure–time profile was set at 5-0.1; 6-0.5; 6-12.0 bar-s during the injection–compression–holding cycle, keeping the mold at 45 °C. Thin plates were obtained by Campana P7/91 hot plates vertical press (DGTS, Veduggio—IT), setting a pressure–time profile of 7-4; 70-2; 90-14 bar-minutes in melting–compressing–cooling cycles, keeping the plates temperature at 190 °C during melting–compressing steps and then cooling to room temperature. These thin plates were used to carry out the disintegration test under composting conditions. Then, the cast samples were molded with a press micro 10 cm3 injection molding machine (DSM, Sittard NL) coupled to the extruder. The described manufacture processes were the same for all BSG concentrations and led to the production of samples in the shape of dumbbells for tensile characterization and thin plates for disintegration tests.
For the tensile tests were used samples type 1BA, in agreement with ISO 527-2 Standard—Annex A [55] for small specimens. Dumbbell Xplore mold, 1BA test specimens and geometrical details of PHB-BSG samples, are provided as Supplementary Materials.
Biocomposite Disintegration Test
To assess the compostability of the obtained biocomposites and to evaluate the effect of BSG on PHB degradation kinetics, the disintegration test was carried out under composting conditions according to ISO 20200:2023 [56]. A specific quantity of compost, supplied by BYS Ambiente Impianti (Foligno, Italy), was mixed together with the synthetic biowaste, prepared with certain amount of sawdust, rabbit food, starch, sugar, oil and urea. The water content of the substrate was around 50 wt.% and the aerobic conditions were guaranteed by mixing it softly. The samples were buried at 4–6 cm depth in perforated boxes, containing the prepared mix, and incubated at 58 °C [56].
For the test, a film was produced by pressing the injected sample and small samples of the dimension of 25 mm × 25 mm and a thickness of less than 0.5 mm were prepared. The disintegration percentage was calculated as in Equation (2), where %D is the percentage of degradation; mi is the initial dry mass of the biocomposite; mr is the residual dry mass of the biocomposite at the selected sampling times (4, 11, 16, 21, 24, 27, 31 and 34 days) [56]:
The degraded samples were recovered at different disintegration steps, washed with distilled water, dried in an oven at 37 °C for 24 h, and weighed.
4. Conclusions
In the present study, a multipurpose approach, specifically aimed at the valorization of brewers’ spent grain, was developed. Historically, this biomass has been widely utilized, primarily in the feed and food industries. Traditional methods, such as composting and anaerobic digestion of raw BSG, represent common reuse strategies that valorize by-products, although they do not fully exploit the intrinsic value of the biomass. Therefore, alternative valorization pathways were explored. The main goal consisted of identifying innovative ways to repurpose this by-product, enhancing its added value and developing a system aligned with the principles of the circular economy.
Based on the results achieved in this study, the following conclusions were stated:
- -
- The protein hydrolysates obtained are rich in low-molecular-weight proteins, resulting from an efficient hydrolysis process. The residual biomass from this process has potential applications as a plant biostimulant. Additionally, the residue can be anaerobically digested to produce biogas. Although the biogas yield is lower compared to raw BSG, it is important to consider that the primary process yields a high-value product with commercial potential, followed by energy generation through biogas production.
- -
- The treatment with IL improves the morphology of the biomass by removing lignin and hemicellulosic fractions. Moreover, the extracted lignin can be recovered and repurposed for various applications, adding further value to the process. The residual pulp can be used for biogas generation.
- -
- The use of BSG as a filler is a promising alternative. It is a low-cost option, particularly when oven-dried, and contributes to the development of biodegradable biocomposites. The presence of BSG as a filler increases the stiffness of the material, compared to the neat PHB, and accelerates the biodegradability of the PHB matrix used in our biocomposites.
- -
- BSG can also be used in co-digestion with recalcitrant biomass, such as OMWW, which is typically an environmental challenge due to its complex disposal requirements.
This study demonstrates how a multidisciplinary approach enables the complete utilization of BSG. Future research will be carried out for scaling up the process to assess its feasibility on a field-scale, also focusing attention on the economic perspective.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/recycling10040124/s1, Figure S1: Dumbbell PHB_BSG injection molded samples; Figure S2: Electrophoretic profile of brewery spent grain (BG) extracts and hydrolysates (BG PH) obtained by SDS-PAGE with 12% acrylamide gel electrophoresis and stained with Coomassie Blue R-250.
Author Contributions
Conceptualization, A.B. and E.C.; methodology, J.D.M.; software, D.D.B.; validation, C.E., D.P. (Dario Priolo), D.P. (Debora Puglia) and G.G.; formal analysis, A.M.G. and F.D.; investigation, M.R.; resources, C.E. and G.G.; data curation, D.P. (Dario Priolo), E.C., F.D. and M.R.; writing—original draft preparation, J.D.M.; writing—review and editing, A.M.G., D.D.B., D.P. (Debora Puglia) and E.C.; supervision, G.G.; project administration, D.D.B., C.E., D.P. (Debora Puglia) and G.G.; funding acquisition, C.E. and G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the European Union—NextGenerationEU— as part of the National Innovation Ecosystem (grant ECS00000041—VITALITY (to Prof. Giovanni Gigliotti and Prof. Carla Emiliani)) promoted by Ministero dell’Università e della Ricerca (MUR).
Data Availability Statement
Data used for this study are already included in the text. Further data and information will be made available upon request.
Acknowledgments
We thank the University of Perugia and MUR for their support within the VITALITY project.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BSG | Brewery’s Spent Grain |
| PHB | Polyhydroxybutyrate |
| AD | Anaerobic Digestion |
| HVM | High-value-added Molecule |
| E | Energy |
| F | Fertilizer |
| BM | Bio-based material |
| LN | Nanostructured Lignin |
| IL | Ionic Liquid |
| PH | Protein Hydrolysates |
| BSGp | BSG residue after ILs treatment |
| BSGph | BSG residue after alkaline hydrolysis |
| OP | Olive Pomace |
| OMWW | Olive Mill Wastewater |
| VS | Volatile Solids |
| DM | Dry Matter |
| TOC | Total Organic Carbon |
| TKN | Total Kjendahl Nitrogen |
| GI | Germination Index |
References
- Barth-Haas Group BARTHHAAS REPORT 2023/24. Available online: https://www.barthhaas.com/company/news/news-article/bh/barthhaas-report-2023-2024 (accessed on 1 December 2024).
- Kunze, W. Technology Brewing & Malting, 5th ed.; Hendel, O., Ed.; Versuchs- und Lehranstalt für Brauerei (VLB): Berlin, Germany, 2004. [Google Scholar]
- Maria, M.P.; Torres, N.H.; Nascimento, V.R.S.; Chagas, T.S.A.; Saratale, G.D.; Mulla, S.I.; Bharagava, R.N.; Cavalcanti, E.B.; Romanholo Ferreira, L.F. Current Advances in the Brewery Wastewater Treatment from Anaerobic Digestion for Biogas Production: A Systematic Review. Environ. Adv. 2023, 13, 100394. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ Spent Grain: Generation, Characteristics and Potential Applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Cook, A.H. Barley and Malt: Biology, Biochemistry, Technology; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Reis, J.M.L.; Menezes, E.M. Barley Residue Reinforced Polymer Mortars: Fracture Mechanics Approach. Compos. Struct. 2017, 173, 53–57. [Google Scholar] [CrossRef]
- Mahmood, A.S.N.; Brammer, J.G.; Hornung, A.; Steele, A.; Poulston, S. The Intermediate Pyrolysis and Catalytic Steam Reforming of Brewers Spent Grain. J. Anal. Appl. Pyrolysis 2013, 103, 328–342. [Google Scholar] [CrossRef]
- Gebremedhn, B.; Niguse, M.; Hagos, B.; Tesfamariam, T.; Kidane, T.; Berhe, A.; Gebresilassie, L.; Gebreegziabher, L.; Gebremariam, T.; Gebremeskel, Y. Effects of Dietary Brewery Spent Grain Inclusion on Egg Laying Performance and Quality Parameters of Bovans Brown Chickens. J. Poult. Sci. 2019, 21, eRBCA-2018. [Google Scholar] [CrossRef]
- Nigam, P.S. An Overview: Recycling of Solid Barley Waste Generated as a by-Product in Distillery and Brewery. Waste Manag. 2017, 62, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Waters, D.M.; Jacob, F.; Titze, J.; Arendt, E.K.; Zannini, E. Fibre, Protein and Mineral Fortification of Wheat Bread through Milled and Fermented Brewer’s Spent Grain Enrichment. Eur. Food Res. Technol. 2012, 235, 767–778. [Google Scholar] [CrossRef]
- Ajanaku, K.O.; Dawodu, F.A.; Ajanaku, C.O.; Nwinyi, O.C. Functional and Nutritional Properties of Spent Grain Enhanced Cookies. Am. J. Food Technol. 2011, 6, 763–771. [Google Scholar] [CrossRef]
- Chiş, M.S.; Pop, A.; Păucean, A.; Socaci, S.A.; Alexa, E.; Man, S.M.; Bota, M.; Muste, S. Fatty Acids, Volatile and Sensory Profile of Multigrain Biscuits Enriched with Spent Malt Rootles. Molecules 2020, 25, 442. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Franco, D.; Trindade, M.A.; Lorenzo, J.M. Characterization of Phenolic Composition in Chestnut Leaves and Beer Residue by LC-DAD-ESI-MS. LWT Food Sci. Technol. 2016, 68, 52–58. [Google Scholar] [CrossRef]
- Kitrytė, V.; Šaduikis, A.; Venskutonis, P.R. Assessment of Antioxidant Capacity of Brewer’s Spent Grain and Its Supercritical Carbon Dioxide Extract as Sources of Valuable Dietary Ingredients. J. Food Eng. 2015, 167, 18–24. [Google Scholar] [CrossRef]
- Mussatto, S.I. Brewer’s Spent Grain: A Valuable Feedstock for Industrial Applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, S.A.L.; Calvete, T.; Féris, L.A. Potential Applications of Brewery Spent Grain: Critical an Overview. J. Environ. Chem. Eng. 2022, 10, 106951. [Google Scholar] [CrossRef]
- Martinkosky, L.; Barkley, J.; Sabadell, G.; Gough, H.; Davidson, S. Earthworms (Eisenia Fetida) Demonstrate Potential for Use in Soil Bioremediation by Increasing the Degradation Rates of Heavy Crude Oil Hydrocarbons. Sci. Total Environ. 2017, 580, 734–743. [Google Scholar] [CrossRef]
- Agamuthu, P.; Abioye, O.P.; Aziz, A.A. Phytoremediation of Soil Contaminated with Used Lubricating Oil Using Jatropha Curcas. J. Hazard. Mater. 2010, 179, 891–894. [Google Scholar] [CrossRef]
- Patricio, J.; Axelsson, L.; Blomé, S.; Rosado, L. Enabling Industrial Symbiosis Collaborations between SMEs from a Regional Perspective. J. Clean. Prod. 2018, 202, 1120–1130. [Google Scholar] [CrossRef]
- Mello, L.R.P.F.; Mali, S. Use of Malt Bagasse to Produce Biodegradable Baked Foams Made from Cassava Starch. Ind. Crops Prod. 2014, 55, 187–193. [Google Scholar] [CrossRef]
- Vercelheze, A.E.S.; Oliveira, A.L.M.; Rezende, M.I.; Muller, C.M.O.; Yamashita, F.; Mali, S. Physical Properties, Photo- and Bio-Degradation of Baked Foams Based on Cassava Starch, Sugarcane Bagasse Fibers and Montmorillonite. J. Polym. Environ. 2013, 21, 266–274. [Google Scholar] [CrossRef]
- Fazeli, M.; Florez, J.P.; Simão, R.A. Improvement in Adhesion of Cellulose Fibers to the Thermoplastic Starch Matrix by Plasma Treatment Modification. Compos. B Eng. 2019, 163, 207–216. [Google Scholar] [CrossRef]
- Di Mario, J.; Gambelli, A.M.; Gigliotti, G. Biomethane Production from Untreated and Treated Brewery’s Spent Grain: Feasibility of Anaerobic Digestion After Pretreatments According to Biogas Yield and Energy Efficiency. Agronomy 2024, 14, 2980. [Google Scholar] [CrossRef]
- Klempová, T.; Slaný, O.; Šišmiš, M.; Marcinčák, S.; Čertík, M. Dual Production of Polyunsaturated Fatty Acids and Beta-Carotene with Mucor Wosnessenskii by the Process of Solid-State Fermentation Using Agro-Industrial Waste. J. Biotechnol. 2020, 311, 1–11. [Google Scholar] [CrossRef] [PubMed]
- da Silva Menezes, B.; Rossi, D.M.; Ayub, M.A.Z. Screening of Filamentous Fungi to Produce Xylanase and Xylooligosaccharides in Submerged and Solid-State Cultivations on Rice Husk, Soybean Hull, and Spent Malt as Substrates. World J. Microbiol. Biotechnol. 2017, 33, 58. [Google Scholar] [CrossRef]
- Gullón, P.; González-Muñoz, M.J.; Parajó, J.C. Manufacture and Prebiotic Potential of Oligosaccharides Derived from Industrial Solid Wastes. Bioresour. Technol. 2011, 102, 6112–6119. [Google Scholar] [CrossRef] [PubMed]
- Panjičko, M.; Zupančič, G.D.; Fanedl, L.; Logar, R.M.; Tišma, M.; Zelić, B. Biogas Production from Brewery Spent Grain as a Mono-Substrate in a Two-Stage Process Composed of Solid-State Anaerobic Digestion and Granular Biomass Reactors. J. Clean. Prod. 2017, 166, 519–529. [Google Scholar] [CrossRef]
- Tolisano, C.; Luzi, F.; Regni, L.; Proietti, P.; Puglia, D.; Gigliotti, G.; Di Michele, A.; Priolo, D.; Del Buono, D. A way to valorize pomace from olive oil production: Lignin nanoparticles to biostimulate maize plants. Environ. Technol. Innov. 2023, 31, 103216. [Google Scholar] [CrossRef]
- Brandt-Talbot, A.; Gschwend, F.J.V.; Fennell, P.S.; Lammens, T.M.; Tan, B.; Weale, J.; Hallett, J.P. An economically viable ionic liquid for the fractionation of lignocellulosic biomass. Green Chem. 2017, 19, 3078–3102. [Google Scholar] [CrossRef]
- Calzoni, E.; Cesaretti, A.; Tacchi, S.; Caponi, S.; Pellegrino, R.M.; Luzi, F.; Cottone, F.; Fioretto, D.; Emiliani, C.; Di Michele, A. Covalent immobilization of proteases on polylactic acid for proteins hydrolysis and waste biomass protein content valorization. Catalysts 2021, 11, 167. [Google Scholar] [CrossRef]
- Cesaretti, A.; Montegiove, N.; Calzoni, E.; Leonardi, L.; Emiliani, C. Protein hydrolysates: From agricultural waste biomasses to high added-value products (minireview). AgroLife Sci. J. 2020, 9, 79–87. [Google Scholar]
- Neto, W.P.F.; Silvério, H.A.; Dantas, N.O.; Pasquini, D. Extraction and characterization of cellulose nanocrystals from agro-industrial residue-Soy hulls. Ind. Crops Prod. 2013, 42, 480–488. [Google Scholar] [CrossRef]
- dos Santos, D.M.; Bukzem, A.L.; Ascheri, D.P.R.; Signini, R.; de Aquino, G.L.B. Microwave-assisted carboxymethylation of cellulose extracted from brewer’s spent grain. Carbohydr. Polym. 2015, 131, 125–133. [Google Scholar] [CrossRef]
- Chen, W.H.; Kuo, P.C. Isothermal torrefaction kinetics of hemicellulose, cellulose, lignin and xylan using thermogravimetric analysis. Energy 2011, 36, 6451–6460. [Google Scholar] [CrossRef]
- Kumar, S.; Prasad, L.; Biijwan, P.P.; Yadav, A. Thermogravimetric analysis of lignocellulosic leaf-based fiber-reinforced thermosets polymer composites: An overview. Biomass Convers. Biorefinery 2024, 14, 12673–12698. [Google Scholar] [CrossRef]
- Nguyen, T.; Zavarin, E.; Barrall II, E.M. Thermal Analysis of Lignocellulosic Materials. J. Macromol. Sci. C 1981, 20, 1–65. [Google Scholar] [CrossRef]
- Siciliano, A.; Stillitano, M.A.; De Rosa, S. Biogas production from wet olive mill wastes pretreated with hydrogen peroxide in alkaline conditions. Renew. Energy 2016, 85, 903–916. [Google Scholar]
- Morozova, I.; Nikulina, N.; Oechsner, H.; Krumpel, J.; Lemmer, A. Effects of increasing nitrogen content on process stability and reactor performance in anaerobic digestion. Energies 2020, 13, 1139. [Google Scholar] [CrossRef]
- D.lgs 75/2010–Riordino e revisione della disciplina in materia di fertilizzanti, a norma dell’articolo 13 della legge 7 luglio 2009, n. 88. Available online: https://www.masaf.gov.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/10087 (accessed on 1 January 2025).
- Assandri, D.; Pampuro, N.; Zara, G.; Cavallo, E.; Budroni, M. Suitability of Composting Process for the Disposal and Valorization of Brewer’s Spent Grain. Agriculture 2021, 11, 2. [Google Scholar] [CrossRef]
- Assandri, D.; Pampuro, N.; Zara, G.; Bianco, A.; Cavallo, E.; Budroni, M. Co-Composting of Brewers’ Spent Grain with Animal Manures and Wheat Straw: Influence of Two Composting Strategies on Compost Quality. Agronomy 2021, 11, 1349. [Google Scholar] [CrossRef]
- Bianco, A.; Melito, S.; Garau, M.; Giannini, V.; Zara, G.; Assandri, D.; Oufensou, S.; Coronas, R.; Pampuro, N.; Budroni, M. The potential use of brewers’ spent grain-based substrates as horticultural bio-fertilizers. Front. Sustain. Food Syst. 2024, 8, 1404914. [Google Scholar] [CrossRef]
- Santos, M.; Jiménez, J.J.; Bartolomé, B.; Gómez-Cordovés, C.; del Nozal, M.J. Variability of brewer’s spent grain within a brewery. Food Chem. 2003, 80, 17–21. [Google Scholar] [CrossRef]
- Hejna, A.; Marć, M.; Kowalkowska-Zedler, D.; Pladzyk, A.; Barczewski, M. Insights into the Thermo-Mechanical Treatment of Brewers’ Spent Grain as a Potential Filler for Polymer Composites. Polymers 2021, 13, 879. [Google Scholar] [CrossRef]
- Lin, L.; Mirkin, S.; Park, H.E. Biodegradable Composite Film of Brewers’ Spent Grain and Poly(Vinyl Alcohol). Processes 2023, 11, 2400. [Google Scholar] [CrossRef]
- Hejna, A.; Barczewski, M.; Skórczewska, K.; Szulc, J.; Chmielnicki, B.; Korol, J.; Formela, K. Sustainable upcycling of brewers’ spent grain by thermo-mechanical treatment in twin-screw extruder. J. Clean. Prod. 2021, 285, 124839. [Google Scholar] [CrossRef]
- Feijoo, P.; Marín, A.; Samaniego-Aguilar, K.; Sánchez-Safont, E.; Lagarón, J.M.; Gámez-Pérez, J.; Cabedo, L. Effect of the Presence of Lignin from Woodflour on the Compostability of PHA-Based Biocomposites: Disintegration, Biodegradation and Microbial Dynamics. Polymers 2023, 15, 2481. [Google Scholar] [CrossRef] [PubMed]
- Hejna, A.; Barczewski, M.; Kosmela, P.; Mysiukiewicz, O.; Aniśko, J.; Sulima, P.; Przyborowski, J.A.; Saeb, M.R. The impact of thermomechanical and chemical treatment of waste Brewers’ spent grain and soil biodegradation of sustainable Mater-Bi-Based biocomposites. Waste Manag. 2022, 154, 260–271. [Google Scholar] [CrossRef]
- Qazanfarzadeh, Z.; Masek, A.; Chakraborty, S.; Kumaravel, V. Development of brewer’s spent grain-derived bio nanocomposites through a multiproduct biorefinery approach for food packaging. Ind. Crops Prod. 2024, 220, 119226. [Google Scholar] [CrossRef]
- Montegiove, N.; Gambelli, A.M.; Calzoni, E.; Bertoldi, A.; Puglia, D.; Zadra, C.; Emiliani, C.; Gigliotti, G. Biogas Production with Residuals Deriving from Olive Mill Wastewater and Olive Pomace Wastes: Quantification of Produced Energy, Spent Energy, and Process Efficiency. Agronomy 2024, 14, 531. [Google Scholar] [CrossRef]
- Brandford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Applications of the ninhydrin reaction for analysis of amino acids, peptides, and proteins to agricultural and biomedical sciences. J. Agric. Food Chem. 2004, 52, 385–406. [Google Scholar] [CrossRef]
- Laemmli, U.K. SDS-page Laemmli method. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- UNI EN 16086-2:2012; Soil Improvers and Growing Media—Determination of Plant Response—Part 2: Petri Dish Test Using Cress. UNI: Milan, Italy, 2012.
- ISO 527-2:2025; Plastics —Determination of tensile properties, Part 2: Test conditions for moulding and extrusion plastics. International Organization for Standardization: Geneva, Switzerland, 2025.
- ISO 20200:2023; Plastics—Determination of the Degree of Disintegration of Plastic Materials under Composting Conditions in a Laboratory-Scale Test. International Organization for Standardization: Geneva, Switzerland, 2023.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).