Abstract
Volatile fatty acids (VFA) are valuable intermediates with growing demand in chemical, pharmaceutical, and environmental applications. Their sustainable production from organic waste is increasingly explored in the context of circular economy and biorefinery models. This study investigates the co-fermentation of waste-activated sludge (WAS) and the organic fraction of municipal solid waste (OFMSW) as a strategy for integrated VFA and biogas production. Semi-continuous experiments were carried out to assess the effect of the substrates ratio (WAS:OFMSW = 90:10 and 30:70), hydraulic retention time (HRT), and pH control (5, 9, no control) on VFA yield and composition. Results showed that higher OFMSW content and alkaline conditions favoured VFA production, with a maximum yield of 144.9 mgHAc·gVS−1 at pH 9 and 70:30 ratio. Acetate dominated, while butyrate production peaked at 114.1 mgHBu·gVS−1 under high sludge conditions. However, the addition of alkali required for pH control may lead to excessive accumulation of alkaline-earth metal ions, which can disrupt biological processes due to their potential toxicity. Anaerobic digestion of fermentation residues enhanced biomethane yields significantly (0.27 NL·gVS−1 vs. 0.05 NL·gVS−1 from raw sludge). The proposed process demonstrates potential for converting wastewater treatment plants into biorefineries, maximising resource recovery while reducing environmental impact.
1. Introduction
Volatile fatty acids (VFAs) are chemical building blocks experiencing a significant surge in market demand. Recently, the recovery of value-added products from waste materials has been a significant focus for researchers and engineers worldwide, particularly those working on United Nations Sustainable Development Goals 6 and 11. From this perspective, VFAs are mainly regarded as noteworthy products due to their diverse potential applications [1].
Acetic, propionic, and butyric acids, the most used types of VFA, with a global market demand of 18.5 Mt for 2020, are extensively used in various industries, including pharmaceuticals, food, and chemicals [2,3].
However, their traditional production from fossil resources is unsustainable due to the environmental impact and high energy intensity of petrochemical processes, such as oxidation and carboxylation [2,4]. For this reason, an increase in their use as industrial chemicals is expected, supported by the development of more sustainable production methods [4]. To address these challenges, alternative bio-based production routes are gaining traction [5]. Biological fermentation of organic waste—including waste-activated sludge (WAS) and the organic fraction of municipal solid waste (OFMSW)—offers a promising pathway for sustainable VFA production [6,7,8]. Since mono-fermentation has some limitations, co-fermentation of multiple substrates is considered a more efficient alternative [9,10].
Co-fermentation enhances the process by (i) increasing the organic loading rate (OLR), (ii) providing additional buffering capacity to prevent pH drops and reduce alkaline consumption, (iii) altering the composition of organic matter, (iv) balancing macro- and micro-nutrients, (v) diluting potentially inhibitory or toxic compounds, and (vi) fostering a more active and diverse fermentative microbial community [9,10,11].
The co-fermentation strategy aligns with the evolving role of wastewater treatment plants (WWTPs) as biorefinery facilities, not only for waste treatment, but also for energy and resource recovery. Although AD has been widely adopted in WWTPs for sludge stabilisation and methane production, its performance is increasingly constrained. Changes in influent composition and stricter effluent standards have led to process line modifications—especially the introduction of biological nutrient removal (BNR) and extended solids retention times (SRTs)—that reduce the biodegradability of sludge and its methane potential [12,13].
In this context, a paradigm shift is necessary; WWTPs should transition from energy-consuming facilities to integrated biorefineries capable of recovering multiple high-value products, such as VFA and biomethane. This strategy supports both environmental sustainability and economic feasibility, in line with circular economy principles and future regulatory frameworks.
This study proposes a biorefinery-oriented model for WWTPs, aiming to integrate the production of VFA and biogas through co-fermentation and anaerobic co-digestion. While the co-digestion of OFMSW and excess sludge for biogas production has been extensively studied, their co-fermentation for the targeted production of VFA has received comparatively limited attention, especially in configurations where excess sludge is the primary substrate [14]. In particular, the impact of the OFMSW:sludge ratio on co-fermentation performance is still underexplored, especially in continuous systems, despite being a key design parameter [14]. This research focuses on evaluating the effect of OFMSW-to-sludge ratio on VFA production and composition through semi-continuous co-fermentation, followed by downstream biogas production from the fermentation residues.
VFA derived from waste can be used in wastewater treatment as a carbon source to enhance BNR, providing a sustainable alternative to methanol [15]. Due to their small molecular size, VFA promote the growth of bacteria responsible for nitrogen and phosphorus removal, reducing the need for larger biological reactors and decreasing the use of external chemicals, thus increasing the self-sufficiency of treatment plants [14,16,17,18,19,20].
The co-fermentation of WAS and OFMSW has been explored in the literature, particularly in batch assays, focusing on the effects of pH and temperature [21,22,23,24]. However, only a few studies have investigated semi-continuous and continuous co-fermentation processes [23,24,25]. These considerations underscore co-fermentation as an emerging and worthwhile research topic, particularly for the full development of WWTPs into resource recovery facilities.
Furthermore, VFA also have potential applications as renewable feedstocks, particularly for producing polyhydroxyalkanoates (PHA), biodegradable polymers with high added value [26]. Mannina et al. 2020 expressly stated that the co-fermenter’s VFA-rich stream serves as a feedstock for PHA production [27].
Moretto et al. (2020) [25] developed a biorefinery process for the production of PHA and biogas from OFMSW and WAS, using a process that combines batch fermentation, solid-liquid separation, and anaerobic co-digestion (AcoD). Their results suggest that co-fermentation could improve yield and profitability in future WWTPs.
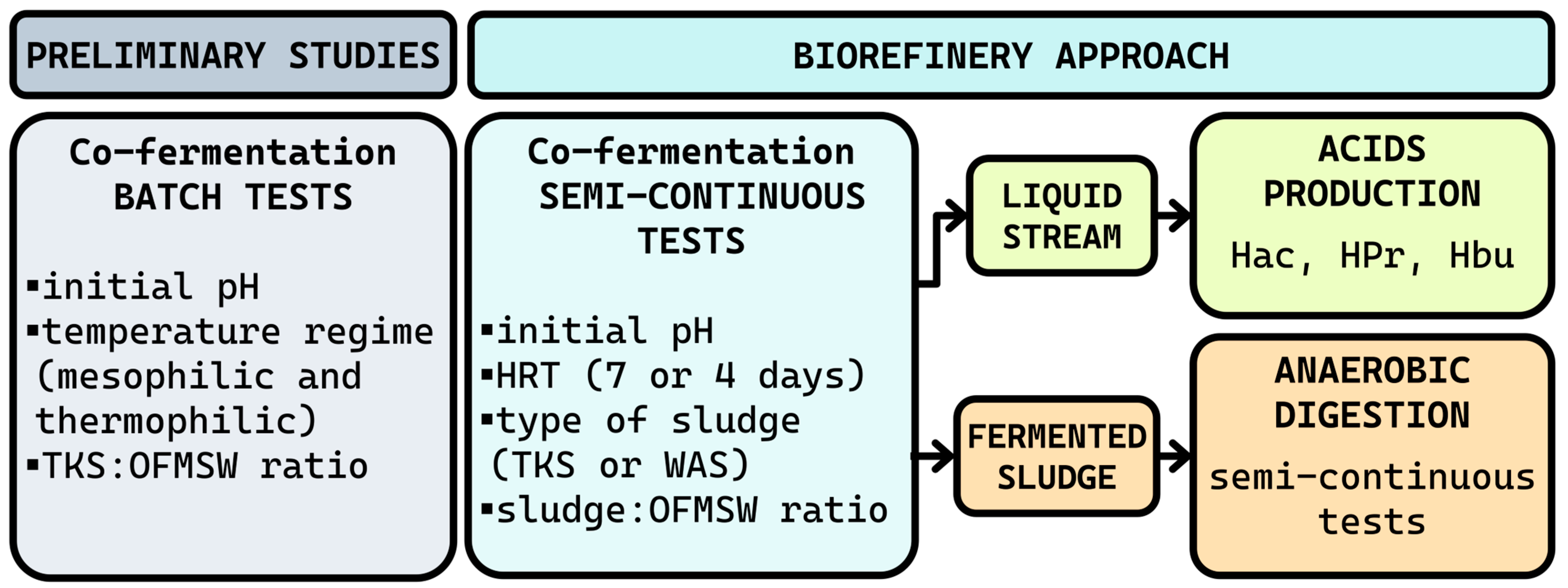
The experimental approach was structured into three interconnected phases to reflect a bio-refinery perspective for co-treating sludge and OFMSW. First, batch co-fermentation tests were conducted to identify suitable substrate ratios and initial pH conditions for VFA production. These insights informed the design of semi-continuous fermentation experiments, which aimed to evaluate the yield and composition of VFA under steady-state conditions. Finally, the anaerobic co-digestion phase assessed the biomethane potential of the residual solids to evaluate the feasibility of integrating VFA production and AD in a circular treatment scheme. This integrated design offers a comprehensive assessment of the co-fermentation/co-digestion system. A schematic overview of the experimental workflow is provided in Figure 1.

Figure 1.
Experimental design flowchart.
This study provides an additional contribution to the scientific community in the research related to VFA production from co-fermentation processes, reporting the results of a comprehensive analysis based on semi-continuous co-fermentation. A biorefinery strategy was implemented through several phases: batch co-fermentation to identify optimal conditions, semi-continuous co-fermentation to assess VFA yields, solid–liquid separation to analyse VFA in the liquid phase for potential BNR applications, and the use of fermentation sludge residues in semi-continuous AD to evaluate methane production.
The novelty of this work lies in the integrated biorefinery approach—combining semi-continuous co-fermentation and anaerobic co-digestion—and in addressing the underexplored influence of OFMSW:sludge ratio on VFA production in a continuous system.
2. Results
2.1. Batch Tests
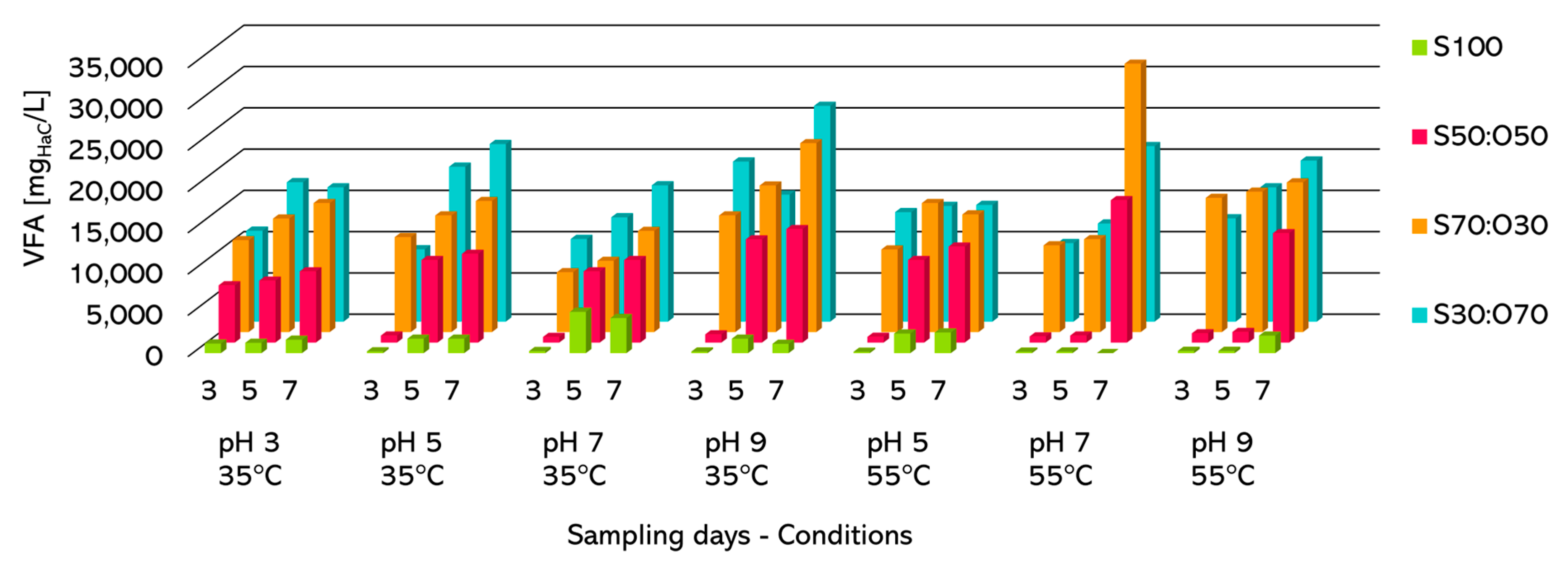
Figure 2 presents the total VFA obtained from the batch reactors under the different tested conditions. The results indicate that the best performance was achieved with a TKS: OFMSW volumetric ratio of 70:30 and an alkaline initial pH, regardless of whether the process was conducted under mesophilic or thermophilic conditions.

Figure 2.
VFA production in batch tests (S—Sludge: O—OFMSW).
In Table S1, the results are presented explicitly in descending order (1st quartile) based on the normalised concentration of VFA, calculated by dividing the measured VFA concentration by the amount of fed OFMSW. Both absolute and normalised values indicate that the highest concentrations were observed at pH 9 and with longer hydraulic retention times, regardless of temperature. There is no evident benefit in conducting the process under thermophilic conditions, which are more energy-intensive and technologically complex.
Batch Test: Statistical Analysis
The results (Table S2) indicate that, for samplings carried out after 3 days of test, there are no significant differences related to the initial pH or the process temperature. However, it was observed that the results from tests conducted with VTKS:VOFMSW of 30:70 are statistically similar to those with 70:30, and those with 50:50 are statistically similar to those carried out with TKS (100:0).
The only difference for samplings carried out after 5 days (Table S2) is that experiments with VTKS:VOFMSW of 50:50 and 100:0 are statistically different, while those with VTKS:VOFMSW equal to 30:70 or 70:30, respectively, remain statistically similar.
The results differ for samplings (Table S3) after 7 days. An influence of the initial pH is observed, with the results of tests carried out at pH 5 and 9 being statistically similar. Those conducted at pH 3, 5, and 7 are also statistically similar. Additionally, the process temperature has a significant impact on the results. However, similarly to a sampling equal to 5 days, the tests with a VTKS:VOFMSW of 50:50 and 100:0 yield statistically different results from each other and the couple 30:70/70:30.
2.2. Semi-Continuous Tests
As previously highlighted, the semi-continuous fermentation tests aimed to evaluate the influence of initial pH, HRT, sludge type (TKS or WAS), and the sludge: OFMSW ratio on VFA production of the selected acids (acetic, propionic, and butyric).
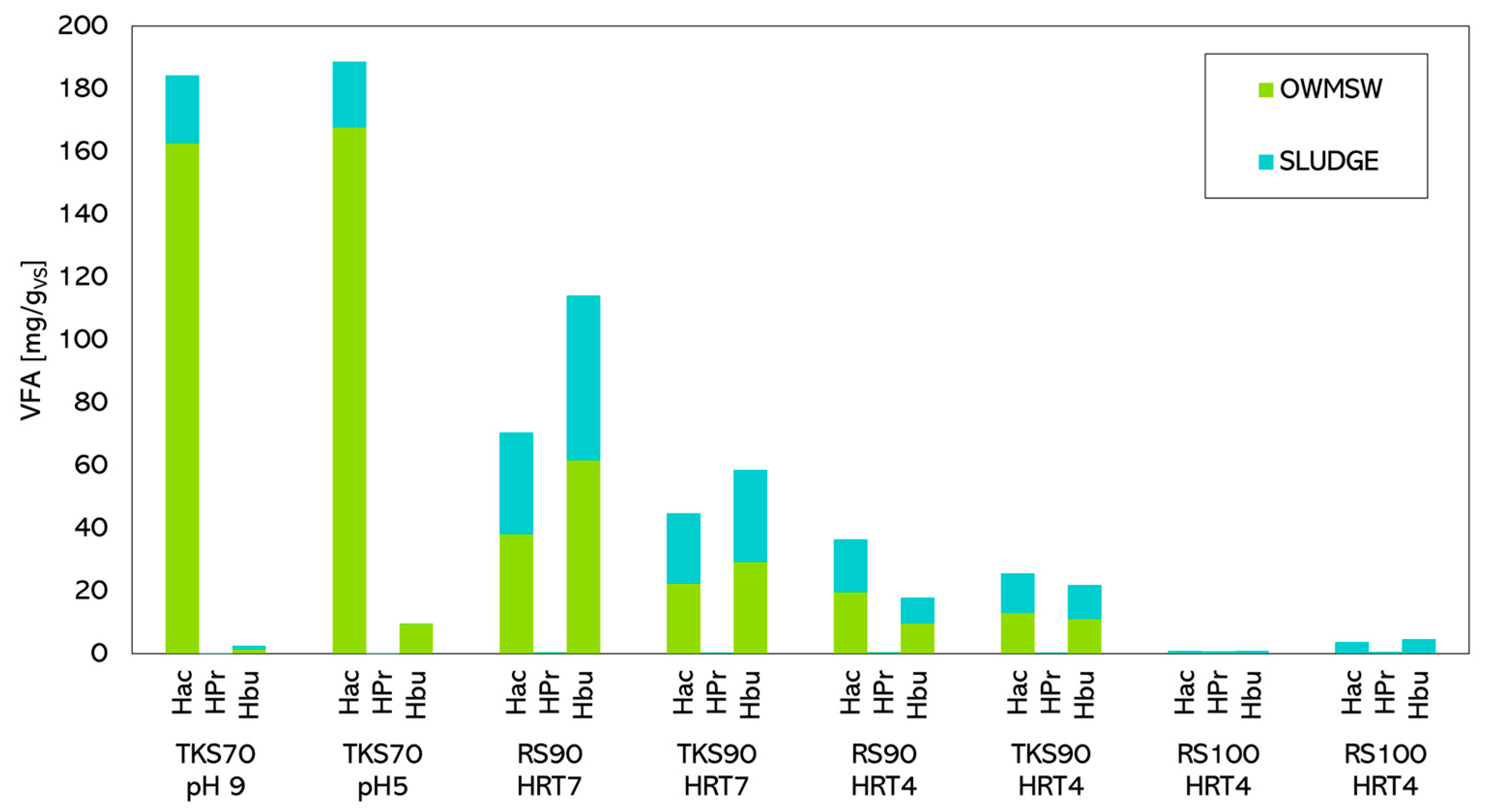
Figure 3 graphically represents the data, illustrating the production indices of acetate, propionate, and butyrate under various operating conditions.

Figure 3.
VFA specific production.
A higher HRT favours acid production, while initial pH control has a limited effect; continuous control would undoubtedly be expensive in terms of chemicals. HRT should be long enough to solubilise complex organic matter and promote subsequent acidogenic fermentation of the hydrolysates. Moreover, the system’s low pH inhibits methanogenic fermentation [28].
It is evident that when the sludge is digested alone, fermentation does not start, and the process intensity is negligible, as shown by the final pH and specific production. When higher proportions of OFMSW are fed, production increases, confirming previous findings [21,22].
However, even a minimum addition of organics to the sludge significantly increases acid production and makes butyrate production predominant, reaching 114.1 mgHBu⋅VStot−1. In this configuration, of the analysed VFA, 61.6% is butyrate, followed by acetate at 38.1% and propionate at only 0.3%. The use of TKS seems more promising than untreated WAS.
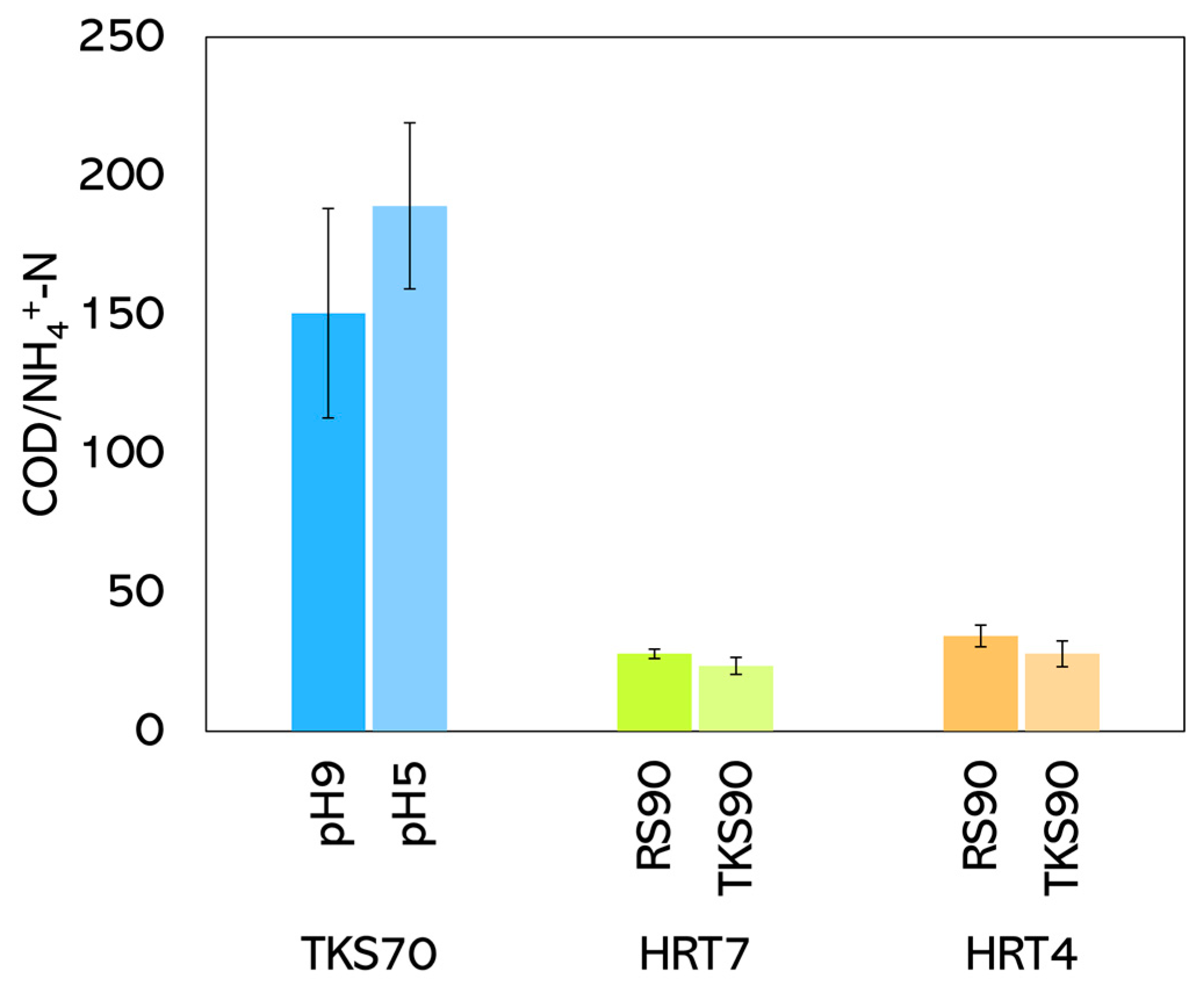
The average COD-to-ammonium ratio of the liquid stream produced by fermentate centrifugation during the semi-continuous experiments is presented in Figure 4. The COD/NH4+-N ratio ranged from 21 to 225, reflecting a wide range of organic carbon availability relative to ammonium nitrogen linked to the different amounts of OFMSW used as co-substrate. The minimum COD value (3270 mg·L−1was measured for the experiment using a 90:10 VTKS: VOFMSW mix.

Figure 4.
COD/NH4+-N ratio.
The highest COD/NH4+-N ratio (225) was observed using a 30:70 VTKS: VOFMSW mixture with an HRT of 7 days, where the higher proportion of organics (maximum COD measured: 53,300 mg·L−1) provided more carbon for biological processes. Conversely, the lowest COD/NH4+-N ratio (21) was recorded using a 90:10 VTKS:VOFMSW.
2.3. AD Semi-Continuous Tests
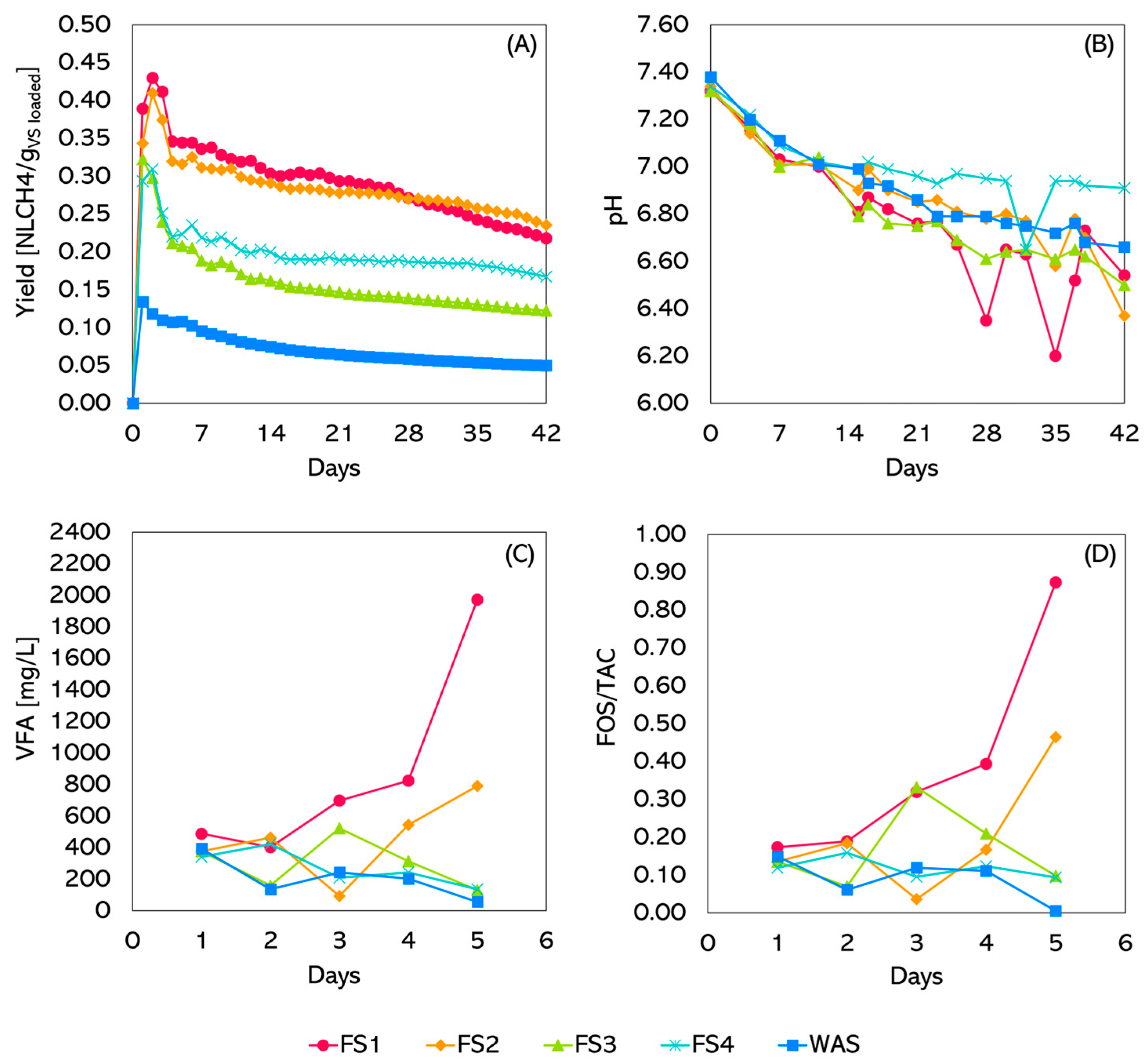
Figure 5A reports the AD tests’ methane yield. Cumulative methane production (Figure 5A) varied significantly across the reactors, reflecting the impact of FS pre-fermentation composition and pH conditions. Notably, FS4 and TKS showed a stable yield, while FS1 and FS2 had a continuously decreasing production.

Figure 5.
Semicontinuous results: (A) yield, (B) pH, (C) VFA, (D) FOS/TAC.
FS2 achieved the highest methane yield (0.27 NL⋅gVS−1 in the period with more stable values in the second part of the experiment), followed closely by FS1 (0.26 NL⋅gVS−1). FS3 and FS4 showed intermediate yields (0.13 and 0.18 NL⋅gVS−1, respectively), while the lowest methane production was observed for TKS (about 0.05 NL⋅gVS−1), indicating suboptimal conditions.
The pH values of all reactors (Figure 5B) showed a gradual decline over time due to acid production. Initially, all reactors started within the optimal range for methanogenesis. As digestion progressed, acid accumulation decreased pH, particularly in the FS1-fed reactor, which dropped below 6.6 by day 28, confirming the possible negative impact of an excess of OFMSW used as a co-substrate for fermentation. FS4 demonstrated the most stable pH, likely due to its higher sludge content, which provides better buffering capacity. FS3 exhibited a smoother trend, while TKS fluctuated near the critical threshold in the last part of the experiment, suggesting potential instability. This behaviour signals a possible problem with the syntropy between acidogenic and methanogenic microorganisms regulating the AD process in experiments with TKS alone and with higher amounts of OFMSW [29,30,31,32]. These issues are likely, at least partially, linked to the low HRT set during the experiments’ design, which severely stressed the systems. Increasing HRT to 20–30 days could improve process stability, but requires a larger reactor volume to treat the same amount of substrate.
The TS and VS content decreased over time in all reactors, but the extent of reduction varied significantly depending on the feedstock composition. The TKS-fed reactor kept higher TS and VS values, confirming a lower degradation rate. Reactors fed with FS1 and FS2 showed a steeper decline, indicating intense organic matter breakdown. This was justified by the higher OFMSW content in the substrate of the co-fermentation test and, thus, of a readily biodegradable substrate promptly consumed during AD. For specular reasons, FS3- and FS4-fed reactors exhibited a more stable trend for both TS and VS. The decline of TS and VS also implies the wash-out of the microbial community responsible for AD and, thus, a reduction in the overall process rate. This confirms that the decrease in methane yield, especially for the FS4-fed reactor, showing a stable operation, could be linked to an excessively short HRT.
VFA concentration and FOS/TAC ratio are key process stability indicators (Figure 5C). FS1 showed an early fluctuation followed by a sharp VFA increase on day 5, indicating acid accumulation and potential process imbalance. FS2-fed reactor displayed a more gradual increase, suggesting better process control under alkaline conditions. FS3- and FS4-fed reactors confirmed more stable operation. FOS/TAC trends confirmed these observations: FS1- and FS2-fed reactors exhibited a progressive increase, which, for FS1, led to unacceptable process conditions. In the other cases, also with some significant fluctuations for the FS3-fed reactor, FOS/TAC values confirmed process stability.
3. Discussion
3.1. Batch Tests
Batch tests results align with previous studies. Feng et al. (2011, 2009) [33,34] investigated the co-fermentation of WAS and OFMSW at varying pH levels (ranging from 4 to 11 in unit increments) in batch assays at room temperature (~20 °C). Both studies achieved the highest co-fermentation yield at pH 8 and 9 [33,34].
Moretto et al. (2019) [23] also examined the impact of pH on WAS and OFMSW co-fermentation in batch assays conducted at 37 and 55 °C [23]. Similar to the current experiment, pH was adjusted only at the beginning. Their findings were consistent with the results of this study and those reported by Feng et al. (2011, 2009) [33,34], as the highest fermentation yields were observed at an initial pH of 7 and 9.
When the volumetric ratio between excess sludge and OFMSW is considered, analysing the normalised VFA production (Figure 1), it is clear that the 70:30 ratio predominates. This is further supported by Moretto et al. (2019) [23], who found that pH had a more significant influence than temperature on fermentation yield.
Compared to neutral and acidic pH, the increased organic matter solubilisation at alkaline pH is well documented in the literature [35,36]. However, due to the naturally acidic nature of fermentation, witnessed by the final pH, maintaining alkaline conditions in the fermenter would require large quantities of chemicals. Another important aspect needs to be considered. pH control is a crucial aspect in anaerobic biological processes, but its regulation can be challenging. Significantly altering the pH often requires the addition of large amounts of alkaline substances. This can lead to a risk of disruption of the biological process due to the toxicity associated with excessive concentrations of alkaline earth metal ions (e.g., Na+, K+, Ca2+, Mg2+). Given the natural tendency of the system to remain within an acidic pH range, it is essential to carefully consider both the quantity and the type of corrective agents used. According to the literature, the toxicity thresholds for inorganic compounds containing alkali and alkaline earth metal ions typically range between 4000 and 8000 mg/L, beyond which adverse effects on microbial activity may occur [37].
However, considering the added complexity associated with pH correction and thermophilic processes, as well as the fact that the results of the experiments carried out at pH 5 in mesophilic conditions are also promising, adopting mesophilic conditions without pH adjustment appears to be the most promising alternative. Therefore, this approach has been tested in semi-continuous experiments.
3.2. Semi-Continuous Tests
Semi-continuous tests highlight that when OFMSW content is high, the VFA mixture is predominantly composed of acetic acid. Performance is slightly better under initial alkaline pH conditions (144.9 compared to ⋅gVStot−1 at pH 5) in line with previous studies on semi-continuous [38] and continuous co-fermentation [23] where the highest VFA yield was achieved at pH 9. Moreover, pH 9 enhances butyric acid production, although its concentration remains generally low under the operating conditions applied during the experiments. Specifically, butyric acid production reached 8.2 ⋅ at pH 9, compared to 1.1 ⋅ at pH 5.
Butyric acid is primarily produced by Clostridium butyricum through the fermentation of carbohydrates. Its production is favoured under limited glucose conditions and inhibited by excessive glucose due to osmotic stress [39]. Therefore, in this specific case, combining various factors may have promoted butyric acid production during the co-digestion at low OFMSW levels, limiting readily biodegradable substrate availability.
In mixed microbial cultures, several bacterial strains can contribute to butyric acid production through the fermentation of various organic macromolecules, highlighting the metabolic versatility and complexity of the co-fermentation process.
The COD/NH4+-N ratio values are particularly relevant, as the addition of the liquid stream in the anaerobic (to enhance P and N removal) or anoxic tank (to enhance N removal) of the WWTP would significantly contribute to the mass balance of readily biodegradable carbon, thereby improving the efficiency of BNR. As previously mentioned, utilising the VFA produced to support mainstream BNR is one of the most practical ways to integrate co-fermentation into WWTPs, thereby reducing reliance on external carbon sources, such as methanol or ethanol [40,41]. Some full-scale WWTPs have already implemented sludge mono-fermentation for this purpose [17,42,43], but it has been proven that the current approach based on co-fermentation, even with a limited addition of OFMSW, is significantly more efficient.
Among the few studies investigating co-fermentation in WWTPs to support BNR, Long et al. (2014) [41] reported that the solubilisation of nitrogen and phosphorus during fermentation may influence effluent quality and overall nutrient balance. The wide range observed in this study suggests the potential for process optimisation, mainly by adjusting the feed composition to maintain a ratio within the optimal range for efficient nitrogen and phosphorus removal.
3.3. AD Semi-Continuous Tests
Literature studies generally report lower yields for the mono-digestions of OFMSW and excess sludge. Pangallo et al. 2023 [12] highlighted that AD of excess sludge and OFMSW alone is problematic for opposite reasons, typically yielding about 0.1 NL⋅gVS−1. However, co-digestion nearly doubled methane production (yield of about 0.18–0.19 NL⋅gVS−1) and guaranteed higher process stability.
The high biodegradability of OFMSW makes it a promising organic substrate for AD. Still, mono-digestion of food waste often leads to digester instability or even failure at higher OLR > 2.5 gVS⋅L−1⋅d−1 due to the accumulation of VFA and ammonia [44]. Several studies have reported lower methane yields in OFMSW mono-digestion if compared to co-digestion with excess sludge, with an increase of up to 40% [45,46], and in some cases, even higher improvements [47,48].
Similarly, the mono-digestion of WAS and TKS is often constrained by limiting factors such as an imbalanced C/N ratio, low biogas yield, inefficient VS removal, and ammonia accumulation [49]. To address these limitations, anaerobic co-digestion with OFMSW has been widely applied in recent years, leading to a methane yield increase compared to sludge mono-digestion [50,51].
This means that co-fermentation of TKS, even with a minimum addition of OFMSW (10%), can lead to not only producing a VFA-rich liquid stream to be used for chemicals recovery, PHA production, or BRS intensification but also to a more than tripled bio-methane production with respect to WAS mono-digestion. Moreover, this processing, which requires ordinary equipment widely available on the market, increases the TS content of the FS destined for AD, with the possibility of augmenting existing plant potential and reducing the volume required for new installations.
Specifically, we highlight that a pre-fermentation reactor operating under limited HRT could be practically implemented using existing volumes within conventional wastewater treatment plants. This would not require additional plant footprint or major infrastructural changes. By introducing only a small amount of OFMSW, the existing anaerobic digesters can still accommodate the increased load without exceeding their volumetric capacity.
The primary benefit of this integrated approach is the substantial increase in biomethane production compared to the traditional anaerobic digestion of waste-activated sludge alone. This aligns with the goals of the new Urban Wastewater Treatment Directive (EU) 2024/3019, which sets a target to achieve energy neutrality in plants exceeding 10,000 PE by 2045 [52].
Moreover, the fermented liquid, rich in VFA, can serve as an internal carbon source for BNR, reducing the need for external carbon additions and thus improving economic and environmental sustainability. This integrated strategy contributes to a circular economy model within WWTPs and supports compliance with upcoming European regulations.
4. Materials and Methods
4.1. Substrates for Fermentation Experiments
The substrates used for fermentation experiments were OFMSW (synthetic and real, as explained below), thickened WAS (TKS), and raw WAS.
For batch tests, synthetic OFMSW was prepared according to a consolidated recipe mimicking the composition of Italian source-separated OFMSW [12,13]. The prepared synthetic substrate was dried for storage and handling reasons, shredded (mechanical pre-treatment with a pulper would undoubtedly be used in a full-scale plant), stored at −20 °C, and thawed the day before the start of the experiment.
The OFMSW for semi-continuous experiments was real (source-separated and collected from households in the Municipality of Reggio Calabria). Immediately after sampling, it was shredded to facilitate handling and then characterised in terms of pH, Total solids (TS), and Volatile Solids (VS). It was stored at −20 °C and then thawed the day before its use at 4 °C.
During all experimental phases, pH was measured directly using a digital pH meter (XS Instruments). TS and VS were determined following standard methods [53].
Synthetic OFMSW ensures higher replicability, so it was used for preliminary batch tests. However, for semi-continuous experiments, the objective is to reproduce the actual process condition as much as possible, so real OFMSW was preferred.
TKS and WAS were sampled from a full-scale WWTP (30,000 people equivalent capacity) located in Reggio Calabria (Italy), where BNR (nitrogen only) is applied. They were characterised for pH, TS and VS and stored at 4 °C until use.
Table 1 shows the characteristics of the substrates used during the batch and semi-continuous fermentation tests.

Table 1.
Batch and semi-continuous fermentation tests on substrates.
4.2. Co-Fermentation Experiments Set-Up: Batch Tests
Co-fermentation batch experiments were carried out in glass reactors (Bioprocess Control Bioreactor, BPC Instruments, Lund, Sweden) with an operating volume of 1.9 L under anaerobic conditions. A sampling port was available, allowing for the sampling of the fermentate. The glass reactors were immersed in a thermostatic bath that kept the temperature at 35 °C (mesophilic conditions) or 55 °C (thermophilic conditions).
Table S4 summarises in detail the batch tests that were performed. Specifically, during the experiment, 16 mesophilic tests and 12 thermophilic tests were carried out to investigate the influence of the initial pH (ranging from strongly acidic to strongly alkaline), the temperature regime (mesophilic and thermophilic) and the TKS:OFMSW ratio. Each batch test lasted 7 days, and the fermentate was analysed in terms of pH and VFA concentration.
4.3. Co-Fermentation Experiments Set-Up: Semicontinuous Test
The experimental activity was carried out using the same reactors used for batch tests, but in semi-continuous mode. The substrate was manually fed through the inlet port three times a week, and the fermented material was discharged through the outlet port simultaneously.
Fermentation tests were conducted to determine the influence of the initial pH (with and without pH control), the hydraulic retention time (HRT), the type of sludge used (TKS or WAS), and the OFMSW:sludge ratio on VFA production. Predetermined amounts of sludge and OFMSW were combined directly in the reactor and gently stirred. Subsequently, the pH was measured and adjusted with analytical-grade H2SO4 or NaOH (Sigma-Aldrich, St. Louis, MO, USA) or left at its natural value.
Table S2 summarises the design of the experiments. Each fermentation experiment lasted 2.5 HRT. In particular, tests A, B, C, and D were run for 17 days and monitored through 7 sampling events; tests E and F were run for 10 days and monitored through 4 sampling events, and tests G and H (controls) were run for 24 days and monitored through 10 sampling events.
4.4. Analytical Procedures
Throughout the experiments, pH was measured at each sampling and analyses were performed to measure the VFA concentration.
VFA concentration in the fermented liquid extracted from batch experiments was assessed using a rapid quantitative analysis based on a titration technique. This method proved effective, even at extremely low initial pH levels, such as those recorded in the analysed supernatant [54,55]. For the semi-continuous experiments, a detailed analysis of the concentration of the individual VFA (acetic acid, propionic acid, butyric acid) was carried out by using gas chromatography-mass spectrometry (GC-MS), following the APAT CNR IRSA 29/2003 Met. 4020 methodology, the Italian Environmental Protection Agency (APAT) and the Water Research Institute of the National Research Council (IRSA-CNR) developed this Italian standard procedure.
During the semi-continuous experiments, COD and NH4-N values were also measured in the liquid fraction, which was separated from the fermentative samples by centrifugation at 9000 rpm for 10 min. COD and ammonium were determined by using pre-dosed cuvettes (Merck Millipore COD Cell Test 114555 and Ammonium Cell Test 114559, respectively).
To make data analysis more straightforward and, despite varying reactor conditions in terms of co-substrates, the results were expressed in terms of specific production, PS-VFA (mgVFA·gVS−1), by dividing the output of the individual volatile fatty acid (acetic, propionic or butyric), PVFA (mgVFA), by the amount of volatile solids (gVS) supplied with the substrates. Moreover, the specific contribution of the OFMSW and the sludge was calculated proportionally to the VS contribution in the substrates’ mixture. This operation was carried out to ensure direct comparability of results. It allowed the evaluation of experiments conducted under different operating conditions and identified optimal process conditions for initial pH, HRT, substrate ratio, and sludge type to maximise VFA production.
4.5. Substrates for Semi-Continuous AD Test
The substrates used in the semi-continuous AD tests consist of the fermentation sludge (FS), separated from the fermentate of the previous semi-continuous co-fermentation experiments using centrifugation at 9000 rpm for 10 min. It was stored at −20 °C and then thawed the day before use at 4 °C. The solid fraction was characterised in terms of TS, VS and pH. WAS was used as a control substrate during AD tests (Table 2).

Table 2.
Anaerobic digestion semi-continuous tests substrates.
The inoculum used for AD tests was liquid digestate sourced from a full-scale anaerobic digestion plant in the Reggio Calabria province (Italy), which processes manure and agro-waste. Upon collection, the digestate was sieved and pre-incubated at 35 °C before the experiment commenced.
4.6. AD Semi-Continuous Tests: Experiment Set-Up
The experiments utilised the identical reactors described above (Bioprocess Control Bioreactor, BPC Instruments), which were fully immersed in a thermostatic water bath maintained at 35 °C and equipped with the available internal stirrer to ensure complete mixing. Substrates were fed into the reactors through glass funnels, while digestate was simultaneously discharged; feeding was typically performed five times per week. Biomethane production was automatically measured using a patented water/gas displacement system.
Five reactors (A fed with FS1, B with FS2, C with FS3, D with FS4, and E with TKS) were set up to assess the biomethane production from the semicontinuous co-fermentation test. In addition, the direct production from TKS, as this is the most used traditional option for the proposed process, was also evaluated. The reactors were operated with an OLR of 1 gVS⋅(L⋅d)−1 and HRT of 14 days for a total experimental duration of 3 HRTs (42 days). The digestion process was monitored by measuring methane production, pH variations, and VS.
4.7. Statistical Analysis
The batch tests enabled a robust statistical analysis using the specialised software XLSTAT. A three-way analysis of variance (ANOVA) with repeated measures (3, 5, and 7 days) was applied to VFA concentration (response variable), assuming the initial pH, temperature, and VTKS:VOFMSW as factors. Tukey’s tests (at p-level < 0.05) were used to identify differences in the response variables between pairs of tests. Before ANOVA, the homogeneity of variance and normality of data were checked using Levene’s and Shapiro-Wilk’s tests, respectively.
Semi-continuous tests (fermentation and AD) often lack replicates due to their labour-intensive nature, long duration, and high costs [11,25,56,57]. For these reasons, statistical analysis was not performed on the semi-continuous AD and co-fermentation tests. The reliability of the results from the same tests is guaranteed by the reactor volume (about 2 L in this case) and by the duration of the experiments (usually 2–3 HRT).
5. Conclusions
This study highlights the potential of VFA as key biorefinery products derived from the co-fermentation of OFMSW and excess sludge. The results of the semi-continuous tests indicate that co-substrate composition and HRT significantly influence VFA production, with a 70:30 sludge-to-OFMSW ratio achieving the highest yield of 144.9 mgHAc·gVS−1 at pH 9. Higher OFMSW ratios enhanced acid production and shifted the product distribution towards acetate, while increased sludge content led to a more balanced mix of acetate and butyrate, with butyrate reaching 114.1 mgHBu·gVS−1. Due to economic and process constraints, pH control and thermophilic conditions do not appear viable for full-scale applications. The high COD/NH4+-N ratio highlighted a significant presence of available organic carbon (with a maximum COD value of 53,300 mg·L−1). This result demonstrates an additional potential for valorising the liquid fraction obtained from the fermentation process, which can be used in WWTPs to enhance phosphorus and nitrogen removal in the anaerobic tank and optimise nitrogen removal in the anoxic tank. This contributes significantly to the mass balance of readily biodegradable carbon, thereby improving the efficiency of BNR processes.
Additionally, AD of fermentation sludge demonstrated that co-fermentation not only supports VFA recovery but also, even with a limited (10%) addition of OFMSW to TKS, significantly boosts biomethane production, with yields of 0.18 NL·gVS−1—more than triple than the 0.05 NL·gVS−1 obtained from direct WAS digestion. The proposed approach holds promise for enhancing WWTP sustainability by reducing external chemical inputs for BNR and maximising resource recovery. Future research should focus on optimising continuous fermentation processes and scaling up biorefinery applications to further improve the economic and environmental viability of VFA production from waste-derived sources.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/recycling10040125/s1, Table S1: Highest normalized VFAs production (1st quartile), Table S2: ANOVA results for HRT equal to 3, 5 and 7 days, Table S3: Design of the semi-continuous co-fermentation tests, Table S4: Design of the batch tests.
Author Contributions
Conceptualisation, P.S.C.; methodology, P.S.C., D.P. and A.P.; validation, P.S.C., investigation, D.P., M.F., A.P. and D.A.Z.; data curation, D.A.Z.; writing—original draft preparation, P.S.C., D.P., A.P. and D.A.Z.; writing—review and editing, P.S.C., D.P. and A.P.; visualisation, D.P. and A.P.; supervision, P.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fondazione AMGA, Genova, Italy through the grant Project 4.0.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to a specific Laboratory policy.
Acknowledgments
This work was financially supported by Fondazione AMGA—Genova, through the call “Project 4.0—third edition”. Financed project “Joint valorisation of the organic fraction of municipal solid waste from separated collection and of excess sludge for the production of biogas and volatile fatty acids”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vázquez-Fernández, A.; Suárez-Ojeda, M.E.; Carrera, J. Review about Bioproduction of Volatile Fatty Acids from Wastes and Wastewaters: Influence of Operating Conditions and Organic Composition of the Substrate. J. Environ. Chem. Eng. 2022, 10, 107917. [Google Scholar] [CrossRef]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-Based Volatile Fatty Acid Production and Recovery from Waste Streams: Current Status and Future Challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef]
- Valentino, F.; Munarin, G.; Biasiolo, M.; Cavinato, C.; Bolzonella, D.; Pavan, P. Enhancing Volatile Fatty Acids (VFA) Production from Food Waste in a Two-Phases Pilot-Scale Anaerobic Digestion Process. J. Environ. Chem. Eng. 2021, 9, 106062. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A Review of the Production and Applications of Waste-Derived Volatile Fatty Acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Agnihotri, S.; Yin, D.-M.; Mahboubi, A.; Sapmaz, T.; Varjani, S.; Qiao, W.; Koseoglu-Imer, D.Y.; Taherzadeh, M.J. A Glimpse of the World of Volatile Fatty Acids Production and Application: A Review. Bioengineered 2022, 13, 1249–1275. [Google Scholar] [CrossRef]
- Dai, K.; Wen, J.-L.; Zhang, F.; Zeng, R.J. Valuable Biochemical Production in Mixed Culture Fermentation: Fundamentals and Process Coupling. Appl. Microbiol. Biotechnol. 2017, 101, 6575–6586. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, J.; Yan, Y.; Feng, L. Enhanced Production of Short-Chain Fatty Acid by Co-Fermentation of Waste Activated Sludge and Kitchen Waste under Alkaline Conditions and Its Application to Microbial Fuel Cells. Appl. Energy 2013, 102, 1197–1204. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile Fatty Acids Production from Food Waste: Effects of pH, Temperature, and Organic Loading Rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, X.; Zhang, P.; Wan, J.; Guo, H.; Ghasimi, D.S.; Morera, X.C.; Zhang, T. Overview of Key Operation Factors and Strategies for Improving Fermentative Volatile Fatty Acid Production and Product Regulation from Sewage Sludge. J. Environ. Sci. 2020, 87, 93–111. [Google Scholar] [CrossRef]
- Peces, M.; Pozo, G.; Koch, K.; Dosta, J.; Astals, S. Exploring the Potential of Co-Fermenting Sewage Sludge and Lipids in a Resource Recovery Scenario. Bioresour. Technol. 2020, 300, 122561. [Google Scholar] [CrossRef]
- Wu, Q.-L.; Guo, W.-Q.; Zheng, H.-S.; Luo, H.-C.; Feng, X.-C.; Yin, R.-L.; Ren, N.-Q. Enhancement of Volatile Fatty Acid Production by Co-Fermentation of Food Waste and Excess Sludge without pH Control: The Mechanism and Microbial Community Analyses. Bioresour. Technol. 2016, 216, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Pangallo, D.; Gelsomino, A.; Fazzino, F.; Pedullà, A.; Calabrò, P.S. The Fate of Biodegradable Plastic during the Anaerobic Co-Digestion of Excess Sludge and Organic Fraction of Municipal Solid Waste. Waste Manag. 2023, 168, 98–106. [Google Scholar] [CrossRef]
- Pangallo, D.; Pedullà, A.; Zema, D.A.; Calabrò, P.S. Influence of the Preliminary Storage on Methane Yield of Anaerobic Digestion of the Organic Fraction of Municipal Solid Waste. Processes 2021, 9, 2017. [Google Scholar] [CrossRef]
- Perez-Esteban, N.; Vinardell, S.; Vidal-Antich, C.; Peña-Picola, S.; Chimenos, J.M.; Peces, M.; Dosta, J.; Astals, S. Potential of Anaerobic Co-Fermentation in Wastewater Treatments Plants: A Review. Sci. Total Environ. 2022, 813, 152498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, J.; Li, M.; Yan, F.; Gong, C.; Wang, Q. Biological Nitrate Removal Using a Food Waste-Derived Carbon Source in Synthetic Wastewater and Real Sewage. J. Environ. Manag. 2016, 166, 407–413. [Google Scholar] [CrossRef]
- Elefsiniotis, P.; Li, D. The Effect of Temperature and Carbon Source on Denitrification Using Volatile Fatty Acids. Biochem. Eng. J. 2006, 28, 148–155. [Google Scholar] [CrossRef]
- Liu, H.; Han, P.; Liu, H.; Zhou, G.; Fu, B.; Zheng, Z. Full-Scale Production of VFAs from Sewage Sludge by Anaerobic Alkaline Fermentation to Improve Biological Nutrients Removal in Domestic Wastewater. Bioresour. Technol. 2018, 260, 105–114. [Google Scholar] [CrossRef]
- Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Production of Volatile Fatty Acids through Co-Digestion of Sewage Sludge and External Organic Waste: Effect of Substrate Proportions and Long-Term Operation. Waste Manag. 2020, 112, 30–39. [Google Scholar] [CrossRef]
- Satira, A.; Paone, E.; Bressi, V.; Iannazzo, D.; Marra, F.; Calabrò, P.S.; Mauriello, F.; Espro, C. Hydrothermal Carbonization as Sustainable Process for the Complete Upgrading of Orange Peel Waste into Value-Added Chemicals and Bio-Carbon Materials. Appl. Sci. 2021, 11, 10983. [Google Scholar] [CrossRef]
- Fazzino, F.; Pedullà, A.; Calabrò, P.S. Boosting the Circularity of Waste Management: Pretreated Mature Landfill Leachate Enhances the Anaerobic Digestion of Market Waste. Biofuel Res. J. 2023, 10, 1764–1773. [Google Scholar] [CrossRef]
- Cerdán, J.M.A.; Tejido-Nuñez, Y.; Aymerich, E.; de GoñiGoñi, J.G.-M.; Garcia-Aguirre, J. A Comprehensive Comparison of Methane and Bio-Based Volatile Fatty Acids Production from Urban and Agro-Industrial Sources. Waste Biomass Valorization 2021, 12, 1357–1369. [Google Scholar] [CrossRef]
- Ma, H.; Liu, H.; Zhang, L.; Yang, M.; Fu, B.; Liu, H. Novel Insight into the Relationship between Organic Substrate Composition and Volatile Fatty Acids Distribution in Acidogenic Co-Fermentation. Biotechnol. Biofuels 2017, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Moretto, G.; Valentino, F.; Pavan, P.; Majone, M.; Bolzonella, D. Optimization of Urban Waste Fermentation for Volatile Fatty Acids Production. Waste Manag. 2019, 92, 21–29. [Google Scholar] [CrossRef]
- Vidal-Antich, C.; Perez-Esteban, N.; Astals, S.; Peces, M.; Mata-Alvarez, J.; Dosta, J. Assessing the Potential of Waste Activated Sludge and Food Waste Co-Fermentation for Carboxylic Acids Production. Sci. Total Environ. 2021, 757, 143763. [Google Scholar] [CrossRef] [PubMed]
- Moretto, G.; Russo, I.; Bolzonella, D.; Pavan, P.; Majone, M.; Valentino, F. An Urban Biorefinery for Food Waste and Biological Sludge Conversion into Polyhydroxyalkanoates and Biogas. Water Res. 2020, 170, 115371. [Google Scholar] [CrossRef]
- Bengtsson, S.; Karlsson, A.; Alexandersson, T.; Quadri, L.; Hjort, M.; Johansson, P.; Morgan-Sagastume, F.; Anterrieu, S.; Arcos-Hernandez, M.; Karabegovic, L.; et al. A Process for Polyhydroxyalkanoate (PHA) Production from Municipal Wastewater Treatment with Biological Carbon and Nitrogen Removal Demonstrated at Pilot-Scale. New Biotechnol. 2017, 35, 42–53. [Google Scholar] [CrossRef]
- Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Carrera, J.; Suárez-Ojeda, M.E. Recovery of Polyhydroxyalkanoates (PHAs) from Wastewater: A Review. Bioresour. Technol. 2020, 297, 122478. [Google Scholar] [CrossRef]
- Strazzera, G.; Battista, F.; Garcia, N.H.; Frison, N.; Bolzonella, D. Volatile Fatty Acids Production from Food Wastes for Biorefinery Platforms: A Review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Sanders, W.T.M.; Siegrist, H.; Vavilin, V.A. The IWA Anaerobic Digestion Model No 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. The Roles of Acetotrophic and Hydrogenotrophic Methanogens during Anaerobic Conversion of Biomass to Methane: A Review. Rev. Environ. Sci. Biotechnol. 2008, 7, 173–190. [Google Scholar] [CrossRef]
- Schink, B. Energetics of Syntrophic Cooperation in Methanogenic Degradation. Microbiol. Mol. Biol. Rev. 1997, 61, 262–280. [Google Scholar] [CrossRef]
- Stams, A.J.M.; Plugge, C.M. Electron Transfer in Syntrophic Communities of Anaerobic Bacteria and Archaea. Nat. Rev. Microbiol. 2009, 7, 568–577. [Google Scholar] [CrossRef]
- Feng, L.; Chen, Y.; Zheng, X. Enhancement of Waste Activated Sludge Protein Conversion and Volatile Fatty Acids Accumulation during Waste Activated Sludge Anaerobic Fermentation by Carbohydrate Substrate Addition: The Effect of pH. Environ. Sci. Technol. 2009, 43, 4373–4380. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yan, Y.; Chen, Y. Co-Fermentation of Waste Activated Sludge with Food Waste for Short-Chain Fatty Acids Production: Effect of pH at Ambient Temperature. Front. Environ. Sci. Eng. China 2011, 5, 623–632. [Google Scholar] [CrossRef]
- Garcia-Aguirre, J.; Esteban-Gutiérrez, M.; Irizar, I.; González-Mtnez de Goñi, J.; Aymerich, E. Continuous Acidogenic Fermentation: Narrowing the Gap between Laboratory Testing and Industrial Application. Bioresour. Technol. 2019, 282, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Hu, H.; Wang, J.; Liao, K.; Ma, H.; Ren, H. The Characterization of Dissolved Organic Matter in Alkaline Fermentation of Sewage Sludge with Different pH for Volatile Fatty Acids Production. Water Res. 2019, 164, 114924. [Google Scholar] [CrossRef]
- Romero-Güiza, M.S.; Vila, J.; Mata-Alvarez, J.; Chimenos, J.M.; Astals, S. The Role of Additives on Anaerobic Digestion: A Review. Renew. Sustain. Energy Rev. 2016, 58, 1486–1499. [Google Scholar] [CrossRef]
- Chen, H.; Meng, H.; Nie, Z.; Zhang, M. Polyhydroxyalkanoate Production from Fermented Volatile Fatty Acids: Effect of pH and Feeding Regimes. Bioresour. Technol. 2013, 128, 533–538. [Google Scholar] [CrossRef]
- Yang, X.; Liu, X.; Chen, S.; Liu, G.; Wu, S.; Wan, C. Volatile Fatty Acids Production from Codigestion of Food Waste and Sewage Sludge Based on β-Cyclodextrins and Alkaline Treatments. Archaea 2016, 2016, 1698163. [Google Scholar] [CrossRef]
- Gao, Y.; Peng, Y.; Zhang, J.; Wang, S.; Guo, J.; Ye, L. Biological Sludge Reduction and Enhanced Nutrient Removal in a Pilot-Scale System with 2-Step Sludge Alkaline Fermentation and A2O Process. Bioresour. Technol. 2011, 102, 4091–4097. [Google Scholar] [CrossRef]
- Long, H.; Latimer, R.; Khunjar, W.; Bilyk, K.; Bott, C.; Chiesa, S.; Balzer, B.; Nicholson, J.; DeArmond, J. FOG, Not Just for Co-Digestion: An Evaluation of Primary Sludge and Grease Trap Waste Fermentation for a Nutrient Removal Carbon Source. Proc. Water Environ. Fed. 2014, 2014, 799–807. [Google Scholar] [CrossRef]
- Jensen, T.R.; Lastra Milone, T.; Petersen, G.; Andersen, H.R. Accelerated Anaerobic Hydrolysis Rates under a Combination of Intermittent Aeration and Anaerobic Conditions. Water Sci. Technol. 2017, 75, 1944–1951. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tooker, N.B.; Srinivasan, V.; Li, G.; Fernandez, L.A.; Schauer, P.; Menniti, A.; Maher, C.; Bott, C.B.; Dombrowski, P.; et al. Side-Stream Enhanced Biological Phosphorus Removal (S2EBPR) Process Improves System Performance—A Full-Scale Comparative Study. Water Res. 2019, 167, 115109. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Fdez-Güelfo, L.A.; Zhou, Y.; Álvarez-Gallego, C.J.; Garcia, L.I.R.; Ng, W.J. Anaerobic Co-Digestion of Organic Fraction of Municipal Solid Waste (OFMSW): Progress and Challenges. Renew. Sustain. Energy Rev. 2018, 93, 380–399. [Google Scholar] [CrossRef]
- Sosnowski, P.; Klepacz-Smolka, A.; Kaczorek, K.; Ledakowicz, S. Kinetic Investigations of Methane Co-Fermentation of Sewage Sludge and Organic Fraction of Municipal Solid Wastes. Bioresour. Technol. 2008, 99, 5731–5737. [Google Scholar] [CrossRef] [PubMed]
- Borowski, S. Co-Digestion of the Hydromechanically Separated Organic Fraction of Municipal Solid Waste with Sewage Sludge. J. Environ. Manag. 2015, 147, 87–94. [Google Scholar] [CrossRef]
- Ara, E.; Sartaj, M.; Kennedy, K. Enhanced Biogas Production by Anaerobic Co-Digestion from a Trinary Mix Substrate over a Binary Mix Substrate. Waste Manag. Res. 2015, 33, 578–587. [Google Scholar] [CrossRef]
- Bawiec, A.; Pawęska, K.; Jarząb, A. Changes in the Microbial Composition of Municipal Wastewater Treated in Biological Processes. J. Ecol. Eng. 2016, 17, 41–46. [Google Scholar] [CrossRef]
- Creamer, K.S.; Chen, Y.; Williams, C.M.; Cheng, J.J. Stable Thermophilic Anaerobic Digestion of Dissolved Air Flotation (DAF) Sludge by Co-Digestion with Swine Manure. Bioresour. Technol. 2010, 101, 3020–3024. [Google Scholar] [CrossRef]
- Borowski, S.; Boniecki, P.; Kubacki, P.; Czyżowska, A. Food Waste Co-Digestion with Slaughterhouse Waste and Sewage Sludge: Digestate Conditioning and Supernatant Quality. Waste Manag. 2018, 74, 158–167. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Pangallo, D.; Zema, D.A. Wastewater Treatment in Lagoons: A Systematic Review and a Meta-Analysis. J. Environ. Manag. 2024, 359, 120974. [Google Scholar] [CrossRef] [PubMed]
- Directive-EU-2024/3019-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/dir/2024/3019/oj/eng (accessed on 7 June 2025).
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; Rice, E.W., Baird, R.B., Eaton, A.D., Clesceri, L.S., Eds.; American Public Health Association, American Water Works Association, Water Environment Federatio: Washington, DC, USA, 2012; ISBN 978-0-87553-013-0. [Google Scholar]
- Baxter, T.E. Approximate Volatile Acids by Titration (Standard Operating Procedure AMBL-101-A); Northern Arizona University: Flagstaff, AZ, USA, 2014. [Google Scholar]
- DiLallo, R.; Albertson, O.E. Volatile Acids by Direct Titration. J. Water Pollut. Control Fed. 1961, 33, 356–365. [Google Scholar]
- Cavinato, C.; Bolzonella, D.; Pavan, P.; Fatone, F.; Cecchi, F. Mesophilic and Thermophilic Anaerobic Co-Digestion of Waste Activated Sludge and Source Sorted Biowaste in Pilot- and Full-Scale Reactors. Renew. Energy 2013, 55, 260–265. [Google Scholar] [CrossRef]
- Silvestre, G.; Bonmatí, A.; Fernández, B. Optimisation of Sewage Sludge Anaerobic Digestion through Co-Digestion with OFMSW: Effect of Collection System and Particle Size. Waste Manag. 2015, 43, 137–143. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).