Abstract
The aim of this study is to investigate new materials that can be employed as cathode hosts in Li-S batteries, which would be able to overcome the effect of the shuttling of soluble polysulfides and maximize the battery capacity and energy density. Density functional theory (DFT) simulations are used to determine the adsorption energy of lithium sulfides in two types of cathode hosts: lithiated 1T-MoS2 (1T-LixMoS2) and hybrid 1T-LixMoS2/graphene. Initial simulations of lithiated 1T-MoS2 structures led to the selection of an optimized 1T-Li0.75MoS2 structure, which was utilized for the formation of an optimized 1T-Li0.75MoS2 bilayer and a hybrid 1T-Li0.75MoS2/graphene bilayer structure. It was found that all sulfides exhibited super-high adsorption energies in the interlayer inside the 1T-Li0.75MoS2 bilayer and very good adsorption energy values in the interlayer inside the hybrid 1T-Li0.75MoS2/graphene bilayer. The placement of sulfides outside each type of bilayer, over the 1T-Li0.75MoS2 surface, yielded good adsorption energies in the range of −2 to −3.8 eV, which are higher than those over a 1T-MoS2 substrate.
1. Introduction
Despite the great expectations of high energy density from lithium–sulfur (Li-S) batteries at least in terms of their theoretical capacity, their commercialization is still behind initial predictions due to the fact that these batteries cannot realize their full theoretical capacity and have a limited lifetime [1,2,3]. The main reason for this is the problem of migration of the soluble sulfur and polysulfides to the anode during discharge and further shuttling between the anode and cathode during charge [4,5,6,7]. This occurs for liquid electrolytes in which sulfides Li2Sx, x = 4–8, are highly soluble and Li2S2 is partially soluble [8,9]. Hence, different schemes to trap the soluble polysulfides have been devised [10], including electrolyte-lean cells [11,12,13,14], sparingly solvating electrolytes that induce a quasi-solid state reaction mechanism [15,16], hollow conductive particles [17,18,19,20,21,22] or high tortuosity conductive platelets [17] as cathode hosts which delay the transport of polysulfides away from the cathode. Layered graphene platelets have the possibility of high tortuosity [23], but they tend to curl and form large pockets in which sulfur is impregnated and unfortunately from which any soluble sulfides can easily migrate [17,24,25]. Ionic liquid polymer binders that attract polysulfides may also act as trapping means and may be used in meso- and microporous cathode coatings to prevent the migration of polysulfides [26,27]. Alternatively, 1T-MoS2 is another conductive 2D material the nanoplatelets of which might also curl [28,29] but can also form layered, large, conductive platelets [30,31,32,33] that can reduce the migration of soluble sulfides via the high tortuosity and high adsorption energy of the 1T-MoS2 which also acts as an excellent electrocatalyst in the electrochemical reactions of Li-S batteries [31].
Functionalizing graphene platelets [34] or other conductive hosts, such as oriented or random carbon nanotubes [35,36], with groups that have high adsorption energy towards sulfides, such as single-atom catalysts [34,36,37,38,39,40] or amines [41], may not have the intended results if the cathode host materials have large mesopores or macropores where the adsorption energy falls as the distance of the sulfide ion increases from the pore wall [42]. As a result, 1T-MoS2-based MXenes still seem a very attractive cathode host due to their long-range lamellar structure with interlayer distances of the order of 1 nm [43,44].
Initial experimental studies [45,46,47] and molecular modeling in the form of DFT (density functional theory) simulations [48] focused on hexagonal 2H-MoS2 [45,46] or hybrid 2H-MoS2/graphene [49] or reduced graphene oxide (rGO) [50] as cathode host materials for Li-S batteries. It was soon realized that triangular 1T-MoS2 has higher electrical conductivity than 2H-MoS2 which makes it a more preferable cathode host in Li-S batteries on its own [51,52] or anchored on carbon [53,54] in hybrids with graphene [55,56,57] and also mixed with conductive polymers such as polypyrrole [58]. DFT simulations conducted by our group [31] provided data for the adsorption energies with different lithium sulfides [31,48], preferred conversion pathways from Li2S4 to Li2S and related activation energies for cathode hosts of 1T-MoS2, in pristine form or with one or two sulfur vacancy defects, demonstrating that 1T-MoS2 is very good at trapping liquid polysulfides and is an excellent electrocatalyst for the electrochemical reactions in Li-S batteries [31,59]. This pioneered the development of Li-S batteries with an optimized design of the 1T-MoS2 cathode host in terms of the number of sulfur vacancies in its structure [31] which offered distinct benefits to Li-S technology [31] and sister chemistries [60,61,62]. It also paved the way for further developments concerning the formation of 1T-LixMoS2 and its use as a novel cathode host, which yielded a high energy density Li-S battery of 440 Wh/kg of cell for a monolayer pouch cell, with about a 20% higher capacity of cells with the LixMoS2 cathode host compared to the equivalent cells with the 1T-MoS2 cathode host [63].
Before this most recent development [63], MxMoS2 was proposed as an electrode for monovalent [64,65] or multivalent metal (M)-ion batteries [43,66], on its own [43,64,65,66] or in the form of carbon or graphene or rGO hybrids [67,68]. The first question has been, of course, the intercalation of metal ions which, in this case, concerns Li+ ions in the MoS2 structure. Different intercalation methods have been studied which produced different interlayer distances, such as electrochemical intercalation [69,70,71], a solvothermal reaction type of intercalation [72,73,74,75] and chemical solution intercalation [76,77], also used in the most successful study for Li-S batteries [63]. Furthermore, for some compositions, a polytype phase transition has been observed upon the intercalation of Li+ ions, forming a stable allotrope of 1T-LixMoS2 from the 2H phase, where x was varied in the range of 0 to 0.8 [69,77,78]. This observation was confirmed by DFT studies, where the critical content of lithium for the initialization of the 2H to 1T phase transition was reported be x = 0.4 [79,80], with the intercalation energy approaching a plateau for x > 0.6 and reaching a minimum at x = 1 [79]. A benefit of such intercalated structures reported by the DFT simulations is that the expanded interlayer spacing can lower the intercalation energy of further intercalating ions [81,82].
In an attempt to model and simulate the successful Li-S cell [63] to unravel the effect of special features of the LixMoS2-based cell on the targeted high performance compared to other cathode hosts [17,83,84,85] and help researchers extend such beneficial features to other cathode materials [86,87,88], DFT simulations are conducted in this study to provide data for the adsorption energy and reaction activation energy of the high-performance host material with lithium sulfides. The lithium content is specified as x = 0.75, i.e., Li0.75MoS2, which ensures the presence of a high proportion of metallic 1T-Li0.75MoS2 [89] as demonstrated in the experimental study [63] which is the basis of the present investigation. The investigation starts with a first step of determining the optimum distribution of Li+ ions in a 1T-Li0.75MoS2 bilayer structure. It follows with DFT simulations of Li2Sx sulfides, x = 1,2,4,6,8, encountered in Li-S batteries with typical liquid electrolytes [90,91,92,93], in optimized structures with the 1T-Li0.75MoS2 bilayer, in the interlayer spacing or over an outer surface of the bilayer. Finally, such DFT simulations are repeated with a hybrid 1T-Li0.75MoS2/graphene bilayer to evaluate this lower mass density structure as a potential cathode host for Li-S batteries.
2. Materials and Methods
All structures required to be inputted for the DFT simulations were created, visualized and tested in VESTA and subjected to an initial geometry optimization in Avogadro. The lattice parameters were optimized by allowing the supercell lattice vectors and ionic positions to change in a simulation box with the vacuum space of 35 Å in the Z-direction. Each 1T-MoS2 monolayer was a (4 × 4) 1T-MoS2 supercell of 48 atoms, comprising 16 Mo and 32 S atoms [31]. The graphene layer was a graphene lattice of 50 C atoms.
The Li0.75MoS2 bilayer was created from two layers of 1T-MoS2 at an interlayer spacing of 1.14 nm and was geometrically optimized using CASTEP v19.1.1. Starting from a single 1T-MoS2 layer, 12 Li atoms were distributed around the 1T-MoS2 layer to create the 1T-Li0.75MoS2 structure. DFT simulations using CASTEP v19.1.1 were employed to identify the optimum structure with the lowest energy, corresponding to the optimum distribution of the Li atoms.
Proceeding to create the Li0.75MoS2 bilayer, the interlayer spacing in the 1T-Li0.75MoS2 bilayer was 1.14 nm as determined from XRD data in [63], with the MoS2 material exfoliated and lithiated as described in [63,94,95,96]. In these data (Cu source of wavelength of 0.15418 nm), the single wide peak of MoS2 at 2θ = 14.05° (interlayer spacing of 0.63 nm), upon intercalation with 75 at% Li, was translated into two peaks at an intensity ratio of 4:1. Of these, the major sharp peak at 2θ = 7.75° corresponds to an interlayer spacing of 1.14 nm and represents widened interlayer spacing after Li+ ion intercalation, whereas the low-intensity peak at 2θ = 15.46° corresponds to an interlayer spacing of 0.57 nm which represents non-intercalated MoS2 material which has been compressed by the Li+ intercalation in neighboring interlayers.
Finally, the hybrid bilayer structure of 1T-Li0.75MoS2/graphene was created by assembling a layer of 1T-Li0.75MoS2 and a layer of graphene. In the absence of further experimental data, the interlayer spacing in the hybrid 1T-Li0.75MoS2/graphene bilayer was maintained the same as for the 1T-Li0.75MoS2 bilayer, at 1.14 nm, for consistency in the DFT investigation.
CASTEP v19.1.1 was employed to perform all spin-polarized DFT simulations [31,97,98,99] with a spin polarization of 2. The exchange–correlation potential was calculated using the generalized gradient approximation (GGA) exchange–correlation function as described by Perdew−Burke−Ernzerhof (PBE) [100]. The Brillouin zone was sampled with the Monkhorst–Pack grid using a 2 × 2 × 1 K-point mesh, as optimized for the convergence of the 1T-MoS2 structures in [31] and the structures of the present study. The valence electrons identified on a plane wave basis were used with a cut-off energy of 500 eV. Tight convergence criteria of energy and force tolerance of 10−5 eV and 10−4 eV/A°, respectively, were set to ensure the precision of calculations. The van der Waals (vdW) dispersion correction as described by Grimme’s empirical method was further included in all the simulations [101].
Considering a Li-S battery with a typical liquid electrolyte leading to the production of sulfides Li2Sx, x = 1, 2, 4, 6, 8, in the cathode [90,91,92,93], the adsorption energy of each of these sulfides was determined via DFT simulations for (a) a 1T-Li0.75MoS2 bilayer cathode host and (b) a hybrid 1T-Li0.75MoS2/graphene bilayer cathode host. Each sulfide was placed either in the interlayer between the two layers or on one side of the bilayer. The adsorption energy, Eads:sulfide-substrate, was calculated via the equation from the difference between the energy of the combined structure, Esulfide-substrate, and the sum of energies of each individual structure, i.e., Esulfide and Esubstrate:
Eads:sulfide-substrate = Esulfide-substrate − (Esulfide + Esubstrate)
To evaluate the catalytic effect of the tested cathode hosts, two alternative disproportionation reaction pathways were investigated for the slow conversion of Li2S4 to Li2S:
- (i)
- A two-step process: Li2S4 → Li2S2 + S2 followed by Li2S2 → Li2S + S;
- (ii)
- A one-step process: Li2S4 → Li2S + S3.
For each of the above reactions, the activation energy (activation barrier) was calculated from the results of the transition state search (TSS) task of a DFT simulation in CASTEP v19.1.1 based on the transition states between the optimized structures of the reactant sulfide in the cathode host and the products in the cathode host. The complete linear synchronous transit and quadratic synchronous transit (LST/QST) methods were used for the TSS task, where the root mean square forces per atom convergence criterion was set to be 10−3 eV/Å.
An initial grid parametric study considered DFT and TSS simulations with different sizes of the K-point mesh: 6 × 6 × 1, 4 × 4 × 1 and 2 × 2 × 1. In all simulations, the cut-off energy was 500 eV, the convergence criterion of energy tolerance was 10−5 eV and the convergence criterion of force tolerance was 10−4 eV/A°. The 2 × 2 × 1 K-point mesh was the only mesh size at which the DFT and TSS simulations of all structures converged, so it was the grid size selected for all structures for consistency to be able to compare the results of adsorption energies and activation energies.
3. Results
3.1. Optimizing the Li Distribution in the 1T-Li0.75MoS2 Structure
Starting from the optimized fully relaxed 48-atom 1T-MoS2 monolayer of an energy E1T-MoS2 = −39,651.307 eV, 12 Li atoms were added gradually. First of all, four sites were considered for the adsorption of a Li atom as shown in Figure 1a: (1) the top site of a S atom in the upper plane, (2) the top site of a Mo atom, (3) the top site of a S atom in the lower plane, and (4) the bridge site above a S–Mo bond, in alignment with first-principles calculations reports [102]. The calculated total energies by the DFT simulations showed that the preferred adsorption site for a Li atom was over the top of a Mo atom (site 2 in Figure 1a) with a little deviation from the structure with the Li over a Mo–S bond.

Figure 1.
Optimized structures of LixMoS2 with different number of Li atoms adsorbed over one side of 48-atom 1T-MoS2 monolayer: (a) one Li atom, (b) four Li atoms, (c) eight Li atoms.
The further addition of three Li atoms favored the bridge site above a S–Mo bond of next nearest neighboring Mo atoms, with a minimum Li-Li distance of 5.7 Å and Li-S distance of 2.7 Å as seen in Figure 1b. The addition of the fifth, sixth, seventh and eighth Li atoms followed similar trends, with the Li atoms close to sites over the nearest neighboring Mo atoms, with Li-S distance of 2.7 Å. Figure 1c depicts the structure with eight Li atoms on the same side with an obvious distortion of the structure of the 1T-MoS2 monolayer. It was noticed that increasing the number of the adsorbed Li atoms on one side of the 1T-MoS2 monolayer resulted in a lower structure energy. The rest of the four Li atoms were added on the other side of the 1T-MoS2 monolayer following a similar optimization procedure, resulting in the structure displayed in Figure 2.
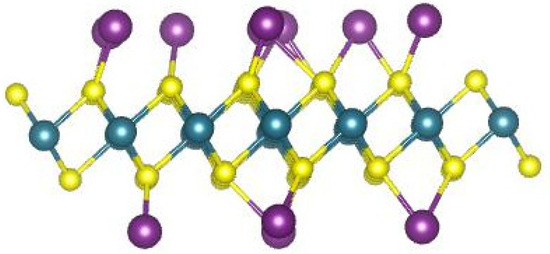
Figure 2.
Optimized structure of 1T-Li0.75MoS2 monolayer.
3.2. DFT Similations of the 1T-Li0.75MoS2 Bilayer as Cathode Host in Li-S Batteries
Combining two monolayer structures as in Figure 2 with an interlayer spacing of 1.14 nm, as determined from XRD data [63], the optimized 1T-Li0.75MoS2 bilayer structure is presented in Figure 3a (same as Figure 4a), with a total calculated energy of E1T-Li0.75MoS2 bilayer = −84,196.343 eV. The sulfides Li2Sx, x = 1, 2, 4, 6, 8, were placed either inside or outside the 1T-Li0.75MoS2 bilayer structure, and the optimized structures are presented in Figure 3b–f and Figure 4b–f, respectively. Table 1 depicts the resulted adsorption energies for each sulfide inside or outside the 1T-Li0.75MoS2 bilayer structure and compares them with the corresponding adsorption energy values with respect to the 1T-MoS2 cathode host. It is clear that the 1T-Li0.75MoS2 bilayer structure traps the soluble sulfides much better than the 1T-MoS2 host, especially in the interlayer, inside the 1T-Li0.75MoS2 bilayer structure.
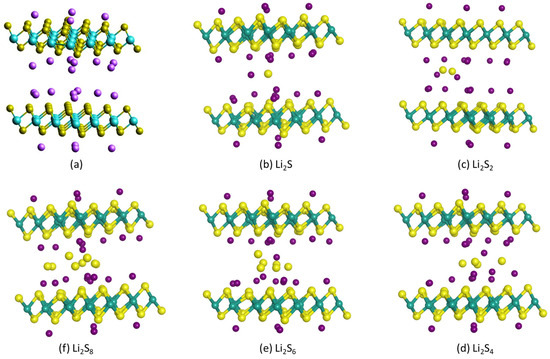
Figure 3.
Optimized structures of (a) 1T-Li0.75MoS2 bilayer and (b–f) 1T-Li0.75MoS2 bilayer with a Li2Sx sulfide between the two 1T-Li0.75MoS2 layers.
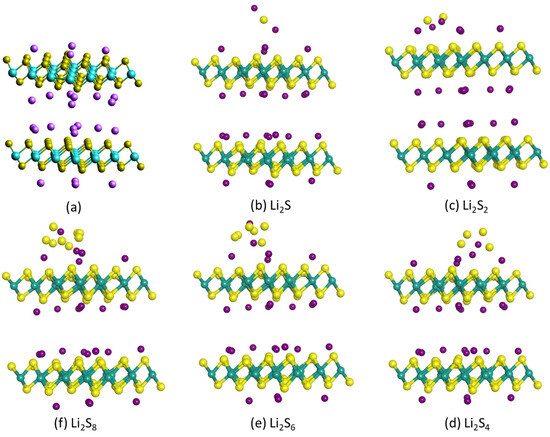
Figure 4.
Optimized structures of (a) 1T-Li0.75MoS2 bilayer and (b–f) 1T-Li0.75MoS2 bilayer with a Li2Sx sulfide outside the 1T-Li0.75MoS2 bilayer.

Table 1.
Adsorption energies of each lithium sulfide inside or outside the 1T-Li0.75MoS2 bilayer compared to the corresponding adsorption energies of each lithium sulfide over a 1T-MoS2 monolayer [31].
The obtained reaction activation energies, Era, for the reactions of the two alternative pathways for the conversion from Li2S4 to Li2S indicated that the preferred pathway with the lowest Era value was, in all cases, the one-step process:
with the Era values presented in Table 2, where it can be seen that the pure reaction activation energy either inside or outside the 1T-Li0.75MoS2 bilayer is higher than that on the 1T-MoS2 cathode host. However, one may consider this process as a combination of a first adsorption step in which Li2S4 is adsorbed at the catalytic site of the cathode host and a second reaction step [103]. Therefore, the whole activation energy, Ea, is considered as the sum of Era and Eads. The Ea values presented in Table 2 indicate that, overall, the adsorption and reaction process has the lowest energy in the interlayer of 1T-Li0.75MoS2.
Li2S4 → Li2S + S3

Table 2.
Activation energies of the one-step process: Li2S4 → Li2S + S3 inside or outside the 1T-Li0.75MoS2 bilayer compared to the corresponding energies over a 1T-MoS2 monolayer [31].
3.3. DFT Similations of the Hybrid 1T-Li0.75MoS2/Graphene Bilayer as Cathode Host in Li-S Batteries
Figure 5a depicts the hybrid 1T-Li0.75MoS2/graphene bilayer which has been formed by keeping the same interlayer distance of 1.14 nm as in the 1T-Li0.75MoS2 bilayer in Figure 3a and Figure 4a. The sulfides Li2Sx, x = 1, 2, 4, 6, 8, were placed either inside the hybrid structure in the interlayer between the 1T-Li0.75MoS2 and graphene layers, as in Figure 5b–f, or outside over the 1T-Li0.75MoS2 layer, as in Figure 6b–f, or outside over the graphene layer, as in Figure 7b–f. Table 3 presents the adsorption energies of each of these lithium sulfides for each configuration and the adsorption energies of each corresponding sulfide over a 1T-MoS2 layer and over a graphene layer. It seems that the adsorption energies of soluble sulfides inside the hybrid bilayer are lower than those for the corresponding sulfides inside the 1T-Li0.75MoS2 bilayer but higher than those outside the 1T-MoS2 layer or the graphene layer. The adsorption energies of the soluble sulfides outside the 1T-Li0.75MoS2 layer are similar to those of the corresponding sulfides outside the 1T-Li0.75MoS2 bilayer in Table 1 but higher than those outside the 1T-MoS2 layer. The adsorption energies of the soluble sulfides outside the graphene layer of the hybrid 1T-Li0.75MoS2/graphene bilayer in Table 3 are similar to those of the corresponding sulfides outside the pure graphene layer in Table 3.
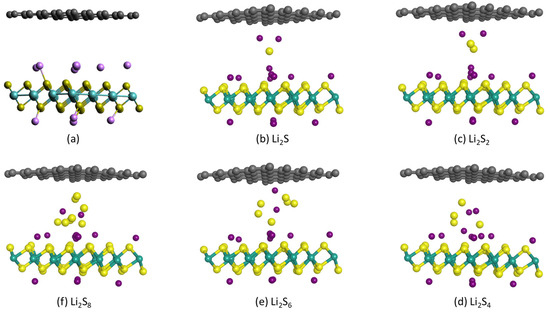
Figure 5.
Optimized structures of (a) hybrid 1T-Li0.75MoS2/graphene bilayer and (b–f) 1T-Li0.75MoS2/graphene bilayer with a Li2Sx sulfide between the 1T-Li0.75MoS2 and the graphene layers.
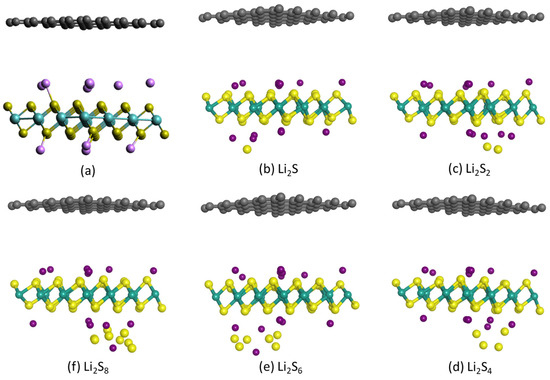
Figure 6.
Optimized structures of (a) hybrid 1T-Li0.75MoS2/graphene bilayer and (b–f) 1T-Li0.75MoS2/graphene bilayer with a Li2Sx sulfide outside the 1T-Li0.75MoS2 layer.
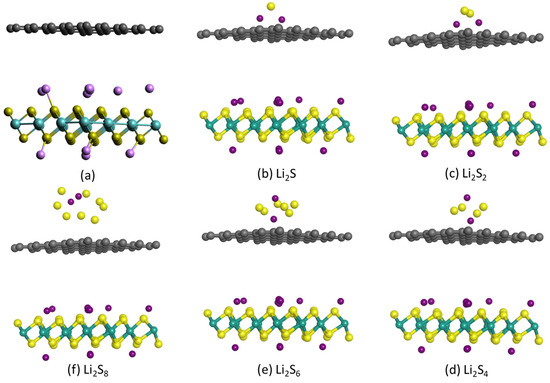
Figure 7.
Optimized structures of (a) hybrid 1T-Li0.75MoS2/graphene bilayer and (b–f) 1T-Li0.75MoS2/graphene bilayer with a Li2Sx sulfide outside the graphene layer.

Table 3.
Adsorption energies of each lithium sulfide inside or outside the hybrid 1T-Li0.75MoS2/graphene bilayer compared to the corresponding adsorption energies of each lithium sulfide over a 1T-MoS2 monolayer [31] and a graphene monolayer [34].
4. Discussion and Conclusions
The Li intercalation step in Section 3.1 offers valuable novel contributions in the literature, not only for Li-S batteries but also for Li-ion batteries. The finally optimized structure of the 1T-Li0.75MoS2 monolayer in Figure 2 is different from symmetrically distributed Li atoms on either side of 1T-MoS2 in the literature ([79], for example), with eight Li atoms on one side (inner side in the bilayer) and four Li atoms on the other side (outer side of the bilayer) in this study. The selection of this structure in this study was based on the process of introducing the Li atoms sequentially and structure optimization after each step of introduction, which resulted in lower energy each time as up to eight Li atoms were introduced on one side. Furthermore, the interlayer distance of 1.14 nm in the 1T-Li0.75MoS2 bilayer in this study was specified on the basis of experimental XRD data [63].
The DFT investigations of the 1T-Li0.75MoS2 bilayer in relation to lithium sulfides and their reactions have provided novel data and useful insights for 1T-Li0.75MoS2 as the cathode host in Li-S batteries. The focus on the challenge of trapping the sulfides is on the highly soluble sulfides in liquid electrolytes, which include Li2S4, Li2S6 and Li2S8 [8,9] and how the cathode hosts investigated in this study compare with other cathode hosts for Li-S batteries. For sulfides over the outer surface of the 1T-Li0.75MoS2 bilayer, the adsorption energies presented in Table 1 for the soluble sulfides Li2S8 and Li2S6 are higher than their adsorption energies caused by 1T-MoS2. Additionally, the adsorption energies of all soluble lithium sulfides over the outer surface of the 1T-Li0.75MoS2 bilayer in Table 1 are higher than those caused by single-atom catalysts Fe-N4-C and Co-N4-C reported in [34] but not by V-N4-C and W-N4-C [34]. However, the DFT simulation results reported in Table 1 and Table 3 demonstrate the super-high adsorption energies of all soluble sulfides in the interlayer inside the 1T-Li0.75MoS2 bilayer compared to all the other structures examined in this study, to 1T-MoS2 [31] and also to many single-atom catalysts such as Fe-N4-C, Co-N4-C, V-N4-C and W-N4-C [34]. This has been confirmed in an experimental study of Li2S4 adsorption from electrolyte solution for different cathode hosts, including 1T-Li0.75MoS2 [63], and the high Li-S battery performance with this cathode host [63].
One of the reasons for the higher adsorption energy of the lithium sulfides in the interlayer inside the 1T-Li0.75MoS2 bilayer is the additive effect of the superimposed adsorption energies caused by the two 1T-Li0.75MoS2 monolayers on either side. However, it is noticed that the adsorption energy of the lithium sulfides in the interlayer is 3–4 times the adsorption energy of these sulfides on the outer layer, i.e., well more than double. We believe that the reason for such a high adsorption energy is that the S atoms of the sulfides are bonded not only with the Li atoms of Li2Sx but are also shared by the intercalated Li atoms in the 1T-Li0.75MoS2 bilayer that are at a higher density in the interlayer than on the outer surface (only four Li atoms on each outer surface). This is also the reason for the higher adsorption energies in the 1T-Li0.75MoS2 bilayer than over 1T-MoS2.
The DFT-aided evaluation of the hybrid 1T-Li0.75MoS2/graphene bilayer is another novel feature of this study, where the advantage of graphene is its low density compared to MoS2, the density of which is more than double the density of graphene. Table 1 and Table 3 reveal that the hybrid 1T-Li0.75MoS2/graphene bilayer has very good but not so high adsorption energies of the soluble sulfides inside the bilayer which may still lead to the trapping of soluble sulfides. The high adsorption of lithium sulfides caused by 1T-Li0.75MoS2 compared to graphene can be attributed to two reasons: (a) the sharing of the Li atoms of the sulfides by the S atoms of MoS2, which are the lithiophilic (as well as hydrophilic [55]) sites of MoS2; and (b) the sharing of the S atoms of the sulfides by the intercalated Li in 1T-Li0.75MoS2.
The other important issue is the catalytic role of the cathode host. The high adsorption energy of a reactant caused by a catalyst may favor the attraction of the reactant to the catalytic site and ultimately lower the reaction activation energy [104]. However, there is an optimum adsorption energy value for species under reduction [105,106], such as sulfur, sulfides or oxygen, above which the species is too strongly adsorbed on the substrate site to participate in further reduction reactions, in which case the reaction activation energy is not reduced. This explains how the super-high adsorption energy of Li2S4 inside the 1T-Li0.75MoS2 bilayer (Eads = −8.25 eV) in Table 1 yields a rather high reaction activation energy of Era = 6.5 eV in Table 3 for the conversion of Li2S4 to Li2S. Li et al. [63] derived activation energies for the lithium sulfide conversion chain in Li-S batteries from the variation in the charge transfer resistance with temperature at different battery cell voltages. From their experimental data [63], it can be concluded that the maximum activation energy for the low-voltage plateau reaction(s) (conversion of Li2S4 to Li2S) is the lowest at 0.1 eV for the 1T-Li0.75MoS2 cathode host compared to 0.15 eV for the 1T-MoS2 cathode host. Hence, it was thought, in the present study, that the sum of adsorption and reaction activation energies would be more representative of the total activation energy [103]. This resulted in the Eads + Era values reported in Table 2, which confirms the trend that, overall, the 1T-Li0.75MoS2 cathode host has a lower total activation energy than the 1T-MoS2 host. The improved electrocatalyst properties of 1T-Li0.75MoS2 are expected to reduce the overpotential of the Li-S battery cell, which would enable the cell to operate at higher C rates.
The contribution of the DFT simulations in this study is providing data of adsorption energies, the optimum reaction pathway and the reaction activation energy regarding the 1T-Li0.75MoS2 and the hybrid 1T-Li0.75MoS2/graphene structure as cathode hosts in Li-S batteries. In terms of trapping the soluble polysulfides, the DFT simulations concluded that the 1T-Li0.75MoS2 bilayer has a higher adsorption energy than the hybrid 1T-Li0.75MoS2/graphene structure, which, however, has better adsorption energies than mere graphene. However, although these values of adsorption energies are encouraging, they do not tell the full story demonstrating the high performance and cyclability of the corresponding Li-S batteries.
The microstructure of the porous cathode host is equally important in Li-S batteries. While micropores and hollow particles hold the sulfides and sulfur and prevent their migration to the anode, open meso- and macropores let the sulfides escape to the anode [17]. Hence, large-size pores need the high adsorption energy and would most benefit from 1T-Li0.75MoS2. The adsorption energy fades with the distance away from the pore walls, where the adsorption energies of 1T-Li0.75MoS2 fall to the level of a graphene wall for 1T-Li0.75MoS2 mesopore widths as follows: a 9 nm pore has Eads = −0.7 eV for Li2S8, a 4 nm pore has Eads = −0.85 eV for Li2S6 and Eads = −0.9 eV for Li2S2 and a 6 nm pore has Eads = −0.6 eV for Li2S4; larger pores will have even lower adsorption energies towards the soluble sulfides. The electrocatalytic effect can of course be considered only if the sulfides are in close distance with the pore walls of the 1T-Li0.75MoS2 materials.
Therefore, further experimental studies or continuum model-based simulations, taking into account the pore size distribution and tortuosity of the structure [17], are needed to assess the 1T-Li0.75MoS2 cathode host and the hybrid 1T-Li0.75MoS2/graphene cathode host in Li-S batteries, with regards to the trapping of polysulfides and the battery performance. The benefit of the present study is that it will contribute the data of the adsorption energies and reaction activation energies to be used as input data in the continuum simulations, taking into account multiple factors, including the microstructure of cathodes, adsorption energies and electrocatalytic effects of pore walls. The adsorption energies of the hybrid 1T-Li0.75MoS2/graphene structure will also be useful in continuum simulations and in providing insights beyond graphene monolayers, such as 1T-Li0.75MoS2-coated carbon fabric [107,108].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/batteries10040124/s1, LixMoS2_graphene_SI1.xyz: xyz.datafile of optimized structure Li0.75MoS2/graphene.
Author Contributions
Conceptualization, Q.C. and C.L.; methodology, S.B., E.H., Q.C. and C.L.; software, S.B., E.H. and C.L.; validation, S.B., E.H. and C.L.; formal analysis, S.B., E.H. and C.L.; investigation, S.B., E.H. and C.L.; resources, Q.C. and C.L.; data curation, Q.C. and C.L.; writing—original draft preparation, S.B., E.H. and C.L.; writing—review and editing, C.L. and Q.C.; visualization, S.B., E.H. and C.L.; supervision, Q.C. and C.L.; project administration, C.L.; funding acquisition, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Faraday Institution LiSTAR program (EPSRC EP/S003053/1, Grant FIRG014). Via our membership in the UK’s HEC Materials Chemistry Consortium, which is funded by EPSRC (EP/L000202), this work used the UK Materials and Molecular Modelling Hub for computational resources, MMM Hub, which is partially funded by EPSRC (EP/R029431, EP/P020194 and EP/T022213).
Data Availability Statement
All data are made available in this study. Additionally, the data of the optimized structure of the hybrid 1T-Li0.75MoS2-graphene are given as xyz file dataset in the Supplementary Materials File. Any further data will become available upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhu, K.; Wang, C.; Chi, Z.; Ke, F.; Yang, Y.; Wang, A.; Wang, W.; Miao, L. How far away are lithium-sulfur batteries from commercialization? Front. Energy Res. 2019, 7, 00123. [Google Scholar] [CrossRef]
- Tokur, M. A Promising Approach towards the commercialization of lithium sulfur batteries: Prelithiated graphene. ChemistrySelect 2023, 8, e202302576. [Google Scholar] [CrossRef]
- Kumar, R.; Liu, J.; Hwang, J.-Y.; Sun, Y.-K. Recent research trends in Li–S batteries. J. Mater. Chem. A 2018, 6, 11582–11605. [Google Scholar] [CrossRef]
- Mistry, A.N.; Mukherjee, P.P. “Shuttle” in polysulfide shuttle: Friend or foe? J. Phys. Chem. C 2018, 122, 23845–23851. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, L.; Zhang, C.; Liu, L.; Li, Y.; Qiao, Z.; Lin, J.; Wei, Q.; Wang, L.; Xie, Q.; et al. Recent advances and strategies toward polysulfides shuttle inhibition for high-performance Li–S batteries. Adv. Sci. 2022, 9, 2106004. [Google Scholar] [CrossRef]
- Song, Y.-X.; Shi, Y.; Wan, J.; Lang, S.-Y.; Hu, X.-C.; Yan, H.-J.; Liu, B.; Guo, Y.-G.; Wen, R.; Wan, L.-J. Direct tracking of the polysulfide shuttling and interfacial evolution in all-solid-state lithium–sulfur batteries: A degradation mechanism study. Energy Environ. Sci. 2019, 12, 2496–2506. [Google Scholar] [CrossRef]
- Moy, D.; Manivannan, A.; Narayanan, S.R. Direct measurement of polysulfide shuttle current: A window into understanding the performance of lithium-sulfur cells. J. Electrochem. Soc. 2015, 162, A1. [Google Scholar] [CrossRef]
- Dent, M.; Jakubczyk, E.; Zhang, T.; Lekakou, C. Kinetics of sulphur dissolution in lithium–sulphur batteries. J. Phys. Energy 2022, 4, 024001. [Google Scholar] [CrossRef]
- Adeoye, H.A.; Dent, M.; Watts, J.F.; Tennison, S.; Lekakou, C. Solubility and dissolution kinetics of sulfur and sulfides in electrolyte solvents for lithium–sulfur and sodium–sulfur batteries. J. Chem. Phys. 2023, 158, 064702. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Z.; Chen, H.; Fu, X.; Awuye, D.E.; Yin, X.; Zhao, Y. Breaking the barrier: Strategies for mitigating shuttle effect in lithium–sulfur batteries using advanced separators. Polymers 2023, 15, 3955. [Google Scholar] [CrossRef]
- Dent, M.; Grabe, S.; Lekakou, C. The challenge of electrolyte impregnation in the fabrication and operation of Li-ion and Li-S batteries. Batter. Supercaps 2023, 7, e202300327. [Google Scholar] [CrossRef]
- Ye, H.; Li, Y. Towards practical lean-electrolyte Li–S batteries: Highly solvating electrolytes or sparingly solvating electrolytes? Nano Res. Energy 2022, 1, 9120012. [Google Scholar] [CrossRef]
- Zhao, M.; Li, B.-Q.; Peng, H.-J.; Yuan, H.; Wei, J.-Y.; Huang, J.-Q. Lithium–sulfur batteries under lean electrolyte conditions: Challenges and opportunities. Angew. Chem. 2020, 59, 12636–12652. [Google Scholar] [CrossRef] [PubMed]
- Jeoun, Y.; Kim, M.-S.; Lee, S.-H.; Um, J.H.; Sung, Y.-E.; Yu, S.-H. Lean-electrolyte lithium-sulfur batteries: Recent advances in the design of cell components. Chem. Eng. J. 2022, 450, 138209. [Google Scholar] [CrossRef]
- Castillo, J.; Soria-Fernández, A.; Rodriguez-Peña, S.; Rikarte, J.; Robles-Fernández, A.; Aldalur, I.; Cid, R.; González-Marcos, J.A.; Carrasco, J.; Armand, M.; et al. Graphene-based sulfur cathodes and dual salt-based sparingly solvating electrolytes: A perfect marriage for high performing, safe, and long cycle life lithium-sulfur prototype batteries. Adv. Energy Mater. 2024, 14, 2302378. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Nomura, N.; Ueno, K.; Dokko, K.; Watanabe, M. Enhancing Li–S battery performance with limiting Li[N(SO2F)2] content in a sulfolane-based sparingly solvating electrolyte. ACS Appl. Mater. Interfaces 2024, 16, 8570–8579. [Google Scholar] [CrossRef] [PubMed]
- Grabe, S.; Dent, M.; Zhang, T.; Tennison, S.; Lekakou, C. A physicochemical model-based digital twin of Li–S batteries to elucidate the effects of cathode microstructure and evaluate different microstructures. J. Power Sources 2023, 580, 233470. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Shen, J.; Moganty, S.S.; Corona, A.; Archer, L.A. Porous hollow carbon-sulfur composites for high-power lithium–sulfur batteries. Angew. Chem. Int. Ed. 2011, 50, 5904–5908. [Google Scholar] [CrossRef]
- He, G.; Evers, S.; Liang, X.; Cuisinier, M.; Garsuch, A.; Nazar, L.F. Tailoring porosity in carbon nanospheres for lithium-sulfur battery cathodes. ACS Nano 2013, 7, 10920–10930. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.; Wang, X.; Ahn, W.; Jiang, G.; Feng, K.; Lui, G.; Chen, Z. Gas pickering emulsion templated hollow carbon for high rate performance lithium sulfur batteries. Adv. Funct. Mater. 2016, 26, 8408–8417. [Google Scholar] [CrossRef]
- Chen, M.; Su, Z.; Jiang, K.; Pan, Y.; Zhang, Y.; Long, L. Promoting sulfur immobilization by a hierarchical morphology of hollow carbon nanosphere clusters for high-stability Li–S battery. J. Mater. Chem. A 2019, 7, 6250–6258. [Google Scholar] [CrossRef]
- Ding, X.; Jin, J.; Huang, X.; Zhou, S.; Xiao, A.; Chen, Y.; Zuo, C. An in situ template for the synthesis of tunable hollow carbon particles for high-performance lithium−sulfur batteries. ACS Omega 2019, 4, 16088–16094. [Google Scholar] [CrossRef]
- Baboo, J.P.; Babar, S.; Kale, D.; Lekakou, C.; Laudone, G.M. Designing a graphene coating-based supercapacitor with lithium ion electrolyte: An experimental and computational study via multiscale modeling. Nanomaterials 2021, 11, 2899. [Google Scholar] [CrossRef]
- Reece, R.; Lekakou, C.; Smith, P.A.; Grilli, R.; Trapalis, C. Sulphur-linked graphitic and graphene oxide platelet-based electrodes for electrochemical double layer capacitors. J. Alloys Compd. 2019, 792, 582–593. [Google Scholar] [CrossRef]
- Vermisoglou, E.C.; Giannakopoulou, T.; Romanos, G.; Boukos, N.; Psycharis, V.; Lei, C.; Lekakou, C.; Petridis, D.; Trapalis, C. Graphene-based materials via benzidine-assisted exfoliation and reduction of graphite oxide and their electrochemical properties. Appl. Surf. Sci. 2017, 392, 244–255. [Google Scholar] [CrossRef]
- Santiago, A.; Robles-Fernández, A.; Soria-Fernández, A.; Lopez-Morales, J.L.; Castillo, J.; Fraile-Insagurbe, D.; Casado, N.; Armand, M.; Garcia-Suarez, E.J.; Carriazo, D. Polymeric ionic liquid as binder: A promising strategy for enhancing Li–S battery performance. J. Energy Storage 2024, 80, 110285. [Google Scholar] [CrossRef]
- Vizintin, A.; Guterman, R.; Schmidt, J.; Antonietti, M.; Dominko, R. Linear and cross-linked ionic liquid polymers as binders in lithium–sulfur batteries. Chem. Mater. 2018, 30, 5444–5450. [Google Scholar] [CrossRef]
- Tian, L.; Wu, R.; Liu, H.Y. Synthesis of Au-nanoparticle-loaded 1T@2H-MoS2 nanosheets with high photocatalytic performance. Energy Mater. 2019, 54, 9656–9665. [Google Scholar] [CrossRef]
- Li, L.; Chen, J.; Wu, K.; Cao, C.; Shi, S.; Cui, J. The stability of metallic MoS2 nanosheets and their property change by annealing. Nanomaterials 2019, 9, 1366. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Xu, Y.; Li, C.; Liu, W.; Yi, S.; Wang, K.; Sun, X.; Wu, Z.-S.; Ma, Y. Tetrabutylammonium-intercalated 1T-MoS2 nanosheets with expanded interlayer spacing vertically coupled on 2D delaminated MXene for high-performance lithium-ion capacitors. Adv. Funct. Mat. 2021, 31, 2104286. [Google Scholar] [CrossRef]
- Hojaji, E.; Andritsos, E.I.; Li, Z.; Chhowalla, M.; Lekakou, C.; Cai, Q. DFT Simulation-based design of 1T-MoS2 cathode hosts for Li-S batteries and experimental evaluation. Int. J. Mol. Sci. 2022, 23, 15608. [Google Scholar] [CrossRef]
- Guo, Y.; Dun, C.; Xu, J.; Li, P.; Huang, W.; Mu, J.; Hou, C.; Hewitt, C.A.; Zhang, Q.; Li, Y.; et al. Wearable thermoelectric devices based on Au-decorated two-dimensional MoS2. ACS Appl. Mater. Interfaces 2018, 10, 33316–33321. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhou, H.; Huang, Z.; Zhai, B.; Liu, L.; Hu, L. Three-dimensionally layers nanosheets of MoS2 with enhanced electrochemical performance using as free-standing anodes of lithium ion batteries. J. Mater. Sci. Mater. Electron. 2018, 29, 3110–3119. [Google Scholar] [CrossRef]
- Andritsos, E.I.; Lekakou, C.; Cai, Q. Single-atom catalysts as promising cathode materials for lithium–sulfur batteries. J. Phys. Chem. C 2021, 125, 18108–18118. [Google Scholar] [CrossRef]
- Murugesh, A.K.; Uthayanan, A.; Lekakou, C. Electrophoresis and orientation of multiple wall carbon nanotubes in polymer solution. Appl. Phys. A 2010, 100, 135–144. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Wang, J.; Zhao, Y.; Luo, D.; Yu, A.; Wang, X.; Chen, Z. Engineering oversaturated Fe-N5 multifunctional catalytic sites for durable lithium-sulfur batteries. Angew. Chem. Int. Ed. 2021, 60, 26622–26629. [Google Scholar] [CrossRef]
- Zhao, H.; Tian, B.; Su, C.; Li, Y. Single-atom iron and doped sulfur improve the catalysis of polysulfide conversion for obtaining high-performance lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2021, 13, 7171–7177. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, W.; Li, G.; Liu, J.; Luo, D.; Zhang, Y.; Zhao, Y.; Zhou, G.; Shui, L.; Wang, X.; et al. Coordinatively deficient single-atom Fe-N-C electrocatalyst with optimized electronic structure for high-performance lithium-sulfur batteries. Energy Storage Mater. 2022, 46, 269–277. [Google Scholar] [CrossRef]
- Sun, X.; Qiu, Y.; Jiang, B.; Chen, Z.; Zhao, C.; Zhou, H.; Yang, L.; Fan, L.; Zhang, Y.; Zhang, N. Isolated Fe-Co heteronuclear diatomic sites as efficient bifunctional catalysts for high-performance lithium-sulfur batteries. Nat. Commun. 2023, 14, 291. [Google Scholar] [CrossRef]
- Li, S.; Lin, J.; Chang, B.; Yang, D.; Wu, D.-Y.; Wang, J.; Zhou, W.; Liu, H.; Sun, S.; Zhang, L. Implanting single-atom N2-Fe-B2 catalytic sites in carbon hosts to stabilize high-loading and lean-electrolyte lithium-sulfur batteries. Energy Storage Mater. 2023, 55, 94–104. [Google Scholar] [CrossRef]
- Ma, L.; Zhuang, H.L.; Wei, S.; Hendrickson, K.E.; Kim, M.S.; Cohn, G.; Hennig, R.G.; Archer, L.A. Enhanced Li–S batteries using amine-functionalized carbon nanotubes in the cathode. ACS Nano 2016, 10, 1050–1059. [Google Scholar] [CrossRef]
- Wasalathilake, K.C.; Roknuzzaman, M.; Ostrikov, K.; Ayoko, G.A.; Yan, C. Interaction between functionalized graphene and sulfur compounds in a lithium–sulfur battery–A density functional theory investigation. RSC Adv. 2018, 8, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Tao, Z.; Zhu, Y.; Tan, Y.; Wang, A.; Zhou, H.; Yang, Y.-Y. A nano interlayer spacing and rich defect 1T-MoS2 as cathode for superior performance aqueous zinc-ion batteries. Nanoscale Adv. 2021, 3, 3780–3787. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, X.; Jiang, S.; Zhang, L.; Ma, W.; Ma, R.; Zhou, Z. Controllable fabrication and structure evolution of hierarchical 1T-MoS2 nanospheres for efficient hydrogen evolution. Green Energy Environ. 2022, 7, 314–323. [Google Scholar] [CrossRef]
- Kızılaslan, A.; Çetinkaya, T.; Akbulut, H. 2H-MoS2 as an artificial solid electrolyte interface in all-solid-state lithium–sulfur batteries. Adv. Mat. Interfaces 2020, 7, 2001020. [Google Scholar] [CrossRef]
- Versaci, D.; Canale, I.; Goswami, S.; Amici, J.; Francia, C.; Fortunato, E.; Martins, R.; Pereira, L.; Bodoardo, S. Molybdenum disulfide/polyaniline interlayer for lithium polysulphide trapping in lithium-sulphur batteries. J. Power Sources 2022, 521, 230945. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, C.; Liu, Y.; Liu, W.; Wei, J. Application of MoS2 in the cathode of lithium sulfur batteries. RSC Adv. 2020, 10, 7384–7395. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Sun, X.; Wang, Z. Trapping polysulfide on two-dimensional molybdenum disulfide for Li–S batteries through phase selection with optimized binding. Beilstein J. Nanotechnol. 2019, 10, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Zhao, H.; Zhang, Z.; Li, Z.; Xia, Q.; Zhang, Y.; Zhao, L.; Du, X.; Du, Z.; Lv, P.; et al. MoS2 Nanosheets vertically grown on graphene sheets for lithium-ion battery anodes. ACS Nano 2016, 10, 8526–8535. [Google Scholar] [CrossRef]
- You, Y.; Ye, Y.; Wei, M.; Sun, W.; Tang, Q.; Zhang, J.; Chen, X.; Li, H.; Xu, J. Three-dimensional MoS2/rGO foams as efficient sulfur hosts for high-performance lithium-sulfur batteries. Chem. Eng. J. 2019, 355, 671–678. [Google Scholar] [CrossRef]
- Du, Z.; Guo, Y.; Wang, H.; Gu, J.; Zhang, Y.; Cheng, Z.; Li, B.; Li, S.; Yang, S. High-Throughput Production of 1T MoS2 Monolayers Based on Controllable Conversion of Mo-Based MXenes. ACS Nano 2021, 15, 19275–19283. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-L.; Onofrio, N.; Wang, J. Boosting the anchoring and catalytic capability of MoS2 for high-loading lithium sulfur batteries. J. Mater. Chem. A 2020, 8, 17646–17656. [Google Scholar] [CrossRef]
- Chen, D.; Zhan, W.; Fu, X.; Zhu, M.; Lan, J.; Sui, G.; Yang, X. High-conductivity 1T-MoS2 catalysts anchored on a carbon fiber cloth for high-performance lithium–sulfur batteries. Mater. Chem. Front. 2021, 5, 6941–6950. [Google Scholar] [CrossRef]
- Moon, S.-H.; Kim, M.-C.; Choi, J.-H.; Kim, Y.-S.; Kim, H.; Park, K.-W. 1T-MoS2/carbon nanofiber composite as an interlayer fabricated by an in situ electrochemical fabrication method for lithium-sulfur batteries. J. Alloys Compd. 2021, 857, 158236. [Google Scholar] [CrossRef]
- He, J.; Hartmann, G.; Lee, M.; Hwang, G.S.; Chen, Y.; Manthiram, A. Freestanding 1T MoS2/graphene heterostructures as a highly efficient electrocatalyst for lithium polysulfides in Li–S batteries. Energy Environ. Sci. 2019, 12, 344–350. [Google Scholar] [CrossRef]
- Yu, B.; Chen, Y.; Wang, Z.; Chen, D.; Wang, X.; Zhang, W.; He, J.; He, W. 1T-MoS2 nanotubes wrapped with N-doped graphene as highly-efficient absorbent and electrocatalyst for Li–S batteries. J. Power Sources 2020, 447, 227364. [Google Scholar] [CrossRef]
- Wang, H.; Tran, D.; Qian, J.; Ding, F.; Losic, D. MoS2/Graphene composites as promising materials for energy storage and conversion applications. Adv. Mater. Interfaces 2019, 6, 1900915. [Google Scholar] [CrossRef]
- Wen, G.; Zhang, X.; Sui, Y.; Rao, K.; Liu, J.; Zhong, S.; Wu, L. PPy-encapsulated hydrangea-type 1T MoS2 microspheres as catalytic sulfur hosts for long-life and high-rate lithium-sulfur batteries. Chem. Eng. J. 2022, 430 Pt 3, 133041. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Li, Z.; Bi, J.; Shi, Q.; Song, H. Recent advances of metal groups and their heterostructures as catalytic materials for lithium-sulfur battery cathodes. J. Electron. Mater. 2023, 52, 3526–3548. [Google Scholar] [CrossRef]
- Guo, X.; Song, E.; Zhao, W.; Xu, S.; Zhao, W.; Lei, Y.; Fang, Y.; Liu, J.; Huang, F. Charge self-regulation in 1T‴-MoS2 structure with rich S vacancies for enhanced hydrogen evolution activity. Nat. Commun. 2022, 13, 5954. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, M.T.; Wu, S.; Geng, J.; Han, Z.; Chan, K.; Gao, P.; Li, H. Rational design of stable sulfur vacancies in molybdenum disulfide for hydrogen evolution. J. Catal. 2020, 382, 320–328. [Google Scholar] [CrossRef]
- Liu, B.; Ma, C.; Liu, D.; Yan, S. Sulfur-vacancy defective MoS2 as a promising electrocatalyst for nitrogen reduction reaction under mild conditions. ChemElectroChem 2021, 8, 3030–3039. [Google Scholar] [CrossRef]
- Li, M.; Sami, I.; Yang, J.; Li, J.; Kumar, R.V.; Chhowalla, M. Lithiated metallic molybdenum disulfide nanosheets for high-performance lithium–sulfur batteries. Nat. Energy 2023, 8, 84–93. [Google Scholar] [CrossRef]
- George, C.; Morris, A.J.; Modarres, M.H.; De Volder, M. Structural evolution of electrochemically lithiated MoS2 nanosheets and the role of carbon additive in li-ion batteries. Chem. Mater. 2016, 28, 7304–7310. [Google Scholar] [CrossRef]
- Lane, C.; Cao, D.; Li, H.; Jiao, Y.; Barbiellini, B.; Bansil, A.; Zhu, H. Understanding phase stability of metallic 1T-MoS2 anodes for sodium-ion batteries. Condens. Matter 2019, 4, 53. [Google Scholar] [CrossRef]
- Lee, W.S.V.; Xiong, T.; Wang, X.; Junmin Xue, J. Unraveling MoS2 and transition metal dichalcogenides as functional zinc-ion battery cathode: A perspective. Small Methods 2021, 5, 2000815. [Google Scholar] [CrossRef] [PubMed]
- Larson, D.T.; Fampiou, I.; Kim, G.; Kaxiras, E. Lithium intercalation in graphene–MoS2 heterostructures. J. Phys. Chem. C 2018, 122, 24535–24541. [Google Scholar] [CrossRef]
- Yang, F.; Feng, X.; Glans, P.-A.; Guo, J. MoS2 for beyond lithium-ion batteries. APL Mater. 2021, 9, 050903. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Z.; Xu, S.; Kong, D.; Cha, J.J.; Zheng, G.; Hsu, P.-C.; Yan, K.; Bradshaw, D.; Prinz, F.B. Electrochemical tuning of vertically aligned MoS2 nanofilms and its application in improving hydrogen evolution reaction. Proc. Natl. Acad. Sci. USA 2013, 110, 19701–19706. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, A.; Wu, X.; van de Groep, J.; Tang, P.; Li, S.; Liu, B.; Shi, F.; Wan, J.; Li, Q.; et al. Reversible and selective ion intercalation through the top surface of few-layer MoS2. Nat. Commun. 2018, 9, 5289. [Google Scholar] [CrossRef]
- El Garah, M.; Bertolazzi, S.; Ippolito, S.; Eredia, M.; Janica, I.; Melinte, G.; Ersen, O.; Marletta, G.; Ciesielski, A.; Samorì, P. MoS2 nanosheets via electrochemical lithium-ion intercalation under ambient conditions. FlatChem 2018, 9, 33–39. [Google Scholar] [CrossRef]
- Wang, X.; Guan, Z.; Li, Y.; Wang, Z.; Chen, L. Guest–host interactions and their impacts on structure and performance of nano-MoS2. Nanoscale 2015, 7, 637–641. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, C.; Cao, M. Three-Dimensional MoS2 hierarchical nanoarchitectures anchored into a carbon layer as graphene analogues with improved lithium ion storage performance. Chem. Asian J. 2013, 8, 2701–2707. [Google Scholar] [CrossRef]
- Palencia-Ruiz, S.; Uzio, D.; Legens, C.; Laurenti, D.; Afanasiev, P. Stability and catalytic properties of 1T-MoS2 obtained via solvothermal synthesis. Appl. Catal. A Gen. 2021, 626, 118355. [Google Scholar] [CrossRef]
- Ali, L.; Bang, S.; Lee, Y.J.; Byeon, C.C. Ion-intercalation assisted solvothermal synthesis and optical characterization of MoS2 quantum dots. J. Korean Phys. Soc. 2019, 74, 191–195. [Google Scholar] [CrossRef]
- Fan, X.; Xu, P.; Zhou, D.; Sun, Y.; Li, Y.C.; Nguyen, M.A.T.; Terrones, M.; Mallouk, T.E. Fast and efficient preparation of exfoliated 2H MoS2 nanosheets by sonication-assisted lithium intercalation and infrared laser-induced 1T to 2H phase reversion. Nano Lett. 2015, 15, 5956–5960. [Google Scholar] [CrossRef] [PubMed]
- Haddadi, S.A.; Amini, M.; Ghaderi, S.; Ramazani, A.S.A. Synthesis and cation-exchange behavior of expanded MoS2 nanosheets for anticorrosion applications. Mater. Proc. 2018, 5, 15573–15579. [Google Scholar]
- Wang, L.; Xu, Z.; Wang, W.; Bai, X. Atomic mechanism of dynamic electrochemical lithiation processes of MoS2 nanosheets. J. Am. Chem. Soc. 2014, 136, 6693–6697. [Google Scholar] [CrossRef] [PubMed]
- Enyashin, A.N.; Seifert, G. Density-functional study of LixMoS2 intercalates (0 ≤ x ≤ 1). Comput. Theor. Chem. 2012, 999, 13–20. [Google Scholar] [CrossRef]
- Wu, L.; Dzade, N.Y.; Yu, M.; Mezari, B.; van Hoof, A.J.F.; Friedrich, H.; de Leeuw, N.H.; Hensen, E.J.M.; Hofmann, J.P. Unraveling the role of lithium in enhancing the hydrogen evolution activity of MoS2: Intercalation versus adsorption. ACS Energy Lett. 2019, 4, 1733–1740. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, Y.; Shu, H.; Zhou, X.; Ding, J.; Chen, X.; Lu, W. The effect of lithium adsorption on the formation of 1T-MoS2 phase based on first-principles calculation. Phys. Lett. A 2016, 380, 1767–1771. [Google Scholar] [CrossRef]
- Chen, S.; Wang, L.; Shao, R.; Zou, J.; Cai, R.; Lin, J.; Zhu, C.; Zhang, J.; Xu, F.; Cao, J.; et al. Atomic structure and migration dynamics of MoS2/LixMoS2 interface. Nano Energy 2018, 48, 560–568. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Zhang, L. Designing principles of advanced sulfur cathodes toward practical lithium-sulfur batteries. SusMat 2022, 2, 34–64. [Google Scholar] [CrossRef]
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. Advances in lithium–sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 2016, 1, 16132. [Google Scholar] [CrossRef]
- Mori, R. Cathode materials for lithium-sulfur battery: A review. J. Solid State Electrochem. 2023, 27, 813–839. [Google Scholar] [CrossRef]
- Shuai, J.; Yoo, H.D.; Liang, Y.; Li, Y.; Yao, Y.; Grabow, L.C. Density functional theory study of Li, Na, and Mg intercalation and diffusion in MoS2 with controlled interlayer spacing. Mater. Res. Express 2016, 3, 64001. [Google Scholar] [CrossRef]
- Attanayake, N.H.; Thenuwara, A.C.; Patra, A.; Aulin, Y.V.; Tran, T.; Chakraborty, H.; Borguet, E.; Klein, M.L.; Perdew, J.P.; Strongin, D.R. Effect of intercalated metals on the electrocatalytic activity of 1T-MoS2 for the hydrogen evolution reaction. ACS Energy Lett. 2018, 3, 7–13. [Google Scholar] [CrossRef]
- Sun, D.; Huang, D.; Wang, H.; Xu, G.-L.; Zhang, X.; Zhang, R.; Tang, Y.; EI-Hady, D.A.; Alshitari, W.; AL-Bogami, A.S.; et al. 1T MoS2 nanosheets with extraordinary sodium storage properties via thermal-driven ion intercalation assisted exfoliation of bulky MoS2. Nano Energy 2019, 61, 361–369. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Zhang, W.; Lee, C.-S. Interlayer nanoarchitectonics of two-dimensional transition-metal dichalcogenides nanosheets for energy storage and conversion applications. Adv. Energy Mater. 2017, 7, 1700571. [Google Scholar] [CrossRef]
- Grabe, S.; Dent, M.; Babar, S.; Zhang, T.; Tennison, S.; Watts, J.F.; Lekakou, C. Investigation and determination of electrochemical reaction kinetics in lithium-sulfur batteries with electrolyte LiTFSI in DOL/DME. J. Electrochem. Soc. 2023, 170, 020527. [Google Scholar] [CrossRef]
- Liu, Y.; Elias, Y.; Meng, J.; Aurbach, D.; Zou, R.; Xia, D.; Pang, Q. Electrolyte solutions design for lithium-sulfur batteries. Joule 2021, 5, 2323–2364. [Google Scholar] [CrossRef]
- Lu, Y.-C.; He, Q.; Gasteiger, H.A. Probing the lithium−sulfur redox reactions: A rotating-ring disk electrode study. J. Phys. Chem. C 2014, 118, 5733. [Google Scholar] [CrossRef]
- Ponnada, S.; Kiai, M.S.; Gorle, D.B.; Nowduri, A. History and recent developments in divergent electrolytes towards high-efficiency lithium–sulfur batteries–A review. Mater. Adv. 2021, 2, 4115–4139. [Google Scholar] [CrossRef]
- Acerce, M.; Akdoan, E.K.; Chhowalla, M. Metallic molybdenum disulfide nanosheet-based electrochemical actuators. Nature 2017, 549, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Acerce, M.; Voiry, D.; Chhowalla, M. Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials. Nat. Nanotechnol. 2015, 10, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Xu, G.-L.; Yu, Z.; Zhang, L.; Hwang, I.; Mo, Y.-X.; Ren, Y.; Cheng, L.; Sun, C.-J.; Ren, Y.; et al. A high-energy and long-cycling lithium–sulfur pouch cell via a macroporous catalytic cathode with double-end binding sites. Nat. Nanotechnol. 2021, 16, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Roch, J.G.; Froehlicher, G.; Leisgang, N.; Makk, P.; Watanabe, K.; Taniguchi, T.; Warburton, R.J. Spin-polarized electrons in monolayer MoS2. Nat. Nanotechnol. 2019, 14, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Bouarissa, A.; Gueddim, A.; Bouarissa, N.; Maghraoui-Meherzi, H. Optical spectra of monolayer MoS2 from spin-polarized all electrons density-functional calculations. Optik 2020, 222, 165477. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Xu, B.; Wang, L.; Chen, H.J.; Zhao, J.; Liu, G.; Wu, M.S. Adsorption and diffusion of lithium on 1T-MoS2 monolayer. Comput. Mater. Sci. 2014, 93, 86–90. [Google Scholar] [CrossRef]
- Zeradjanin, A.R.; Narangoda, P.; Masa, J.; Schlögl, R. What controls activity trends of electrocatalytic hydrogen evolution reaction?—Activation energy versus frequency factor. ACS Catal. 2022, 12, 11597–11605. [Google Scholar] [CrossRef]
- Roduner, E. Understanding catalysis. Chem. Soc. Rev. 2014, 43, 8226–8239. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiu, W.; Song, E.; Gu, F.; Zheng, Z.; Zhao, X.; Zhao, Y.; Liu, J.; Zhang, W. Adsorption-energy-based activity descriptors for electrocatalysts in energy storage applications. Natl. Sci. Rev. 2018, 5, 327–341. [Google Scholar] [CrossRef]
- Darby, M.T.; Reocreux, R.; Sykes, E.C.H.; Michaelides, A.; Stamatakis, M. Elucidating the stability and reactivity of surface intermediates on single atom alloy catalysts. ACS Catal. 2018, 8, 5038–5050. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Z.; Ni, J.; Li, L. Freestanding nanosheets of 1T-2H hybrid MoS2 as electrodes for efficient sodium storage. J. Mater. Sci. Technol. 2021, 67, 237–242. [Google Scholar] [CrossRef]
- Yu, H.; Jiang, G.; Ni, J.; Li, L. Architecting core-shell nanosheets of MoS2-polypyrrole on carbon cloth as a robust sodium anode. Sustain. Mater. Technol. 2021, 28, e00255. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).