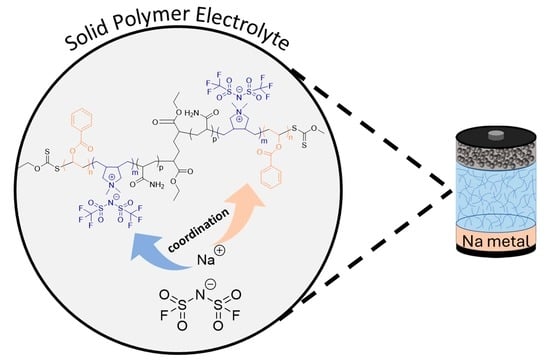
Poly(vinyl benzoate)-b-poly(diallyldimethyl ammonium TFSI)-b-poly(vinyl benzoate) Triblock Copolymer Electrolytes for Sodium Batteries
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Poly(vinyl benzoate)-b-poly(diallyldimethyl ammonium TFSI) PVB-b-PDADMTFSI-b-PVB Triblock Copolymers
Solid Polymer Electrolytes Based on Blends of NaFSI and PVB-b-PDADMATFSI-b-PVB Triblock Copolymers
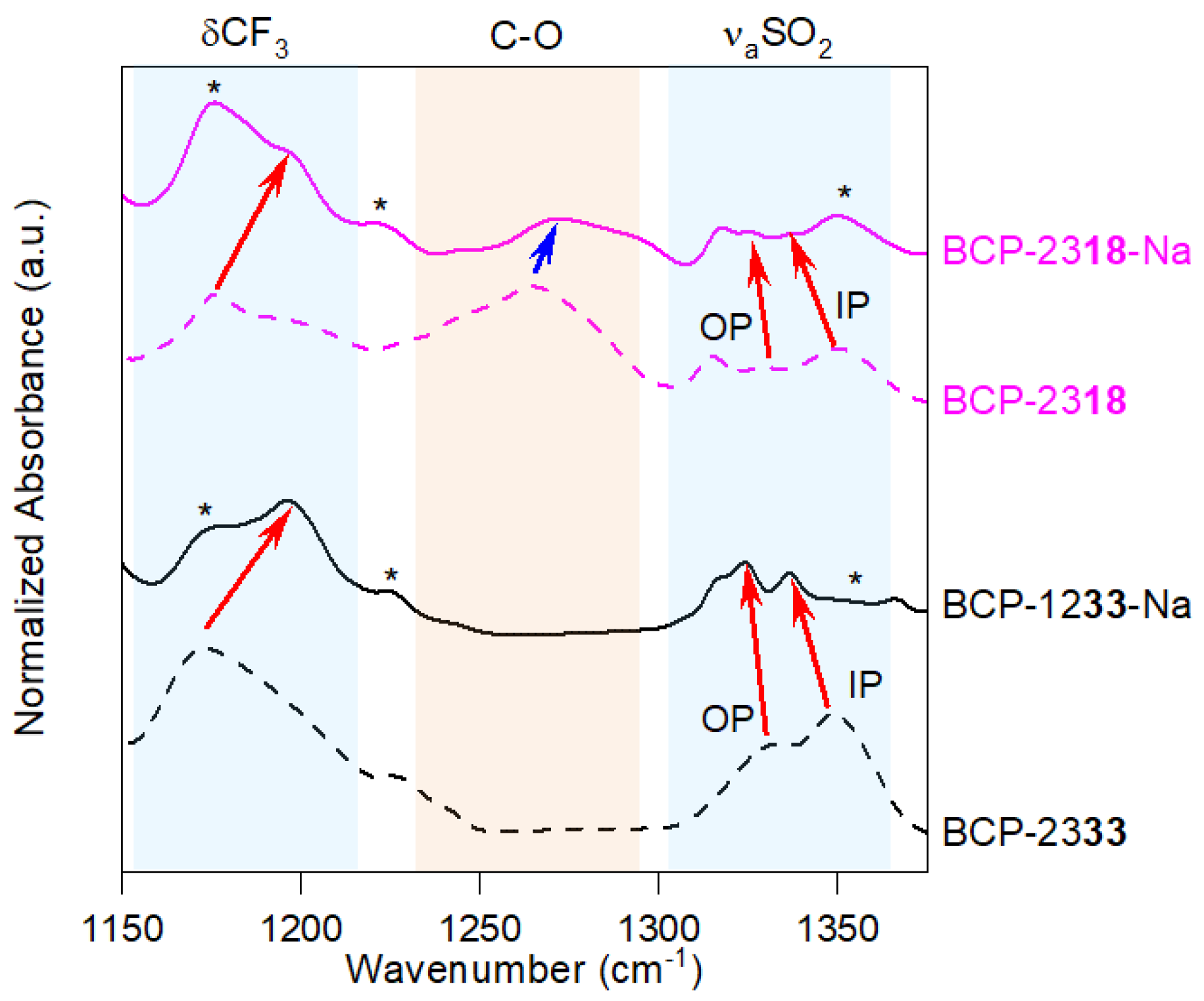
2.2. Electrochemical Properties and Applicability of Triblock Polymer Electrolytes in a Na–O2 Battery
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, F.; Maier, J.; Yu, Y. Guidelines and Trends for Next-Generation Rechargeable Lithium and Lithium-Ion Batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef] [PubMed]
- Masias, A.; Marcicki, J.; Paxton, W.A. Opportunities and Challenges of Lithium Ion Batteries in Automotive Applications. ACS Energy Lett. 2021, 6, 621–630. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M.; Paolella, A.; Armand, M.; Zaghib, K. Building Better Batteries in the Solid State: A Review. Materials 2019, 12, 3892. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, H.; Zhou, Q.; Qu, H.; Dong, T.; Zhang, M.; Tang, B.; Zhang, J.; Cui, G. Safety-Enhanced Polymer Electrolytes for Sodium Batteries: Recent Progress and Perspectives. ACS Appl. Mater. Interfaces 2019, 11, 17109–17127. [Google Scholar] [CrossRef] [PubMed]
- Skundin, A.M.; Kulova, T.L.; Yaroslavtsev, A.B. Sodium-Ion Batteries (a Review). Russ. J. Electrochem. 2018, 54, 113–152. [Google Scholar] [CrossRef]
- Kulova, T.L.; Fateev, V.N.; Seregina, E.A.; Grigoriev, A.S. A Brief Review of Post-Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2020, 15, 7242–7259. [Google Scholar] [CrossRef]
- Yin, H.; Han, C.; Liu, Q.; Wu, F.; Zhang, F.; Tang, Y.; Yin, H.; Wu, F.Y.; Tang, Y.B.; Han, C.J.; et al. Recent Advances and Perspectives on the Polymer Electrolytes for Sodium/Potassium-Ion Batteries. Small 2021, 17, 2006627. [Google Scholar] [CrossRef] [PubMed]
- Gebert, F.; Knott, J.; Gorkin, R.; Chou, S.L.; Dou, S.X. Polymer Electrolytes for Sodium-Ion Batteries. Energy Storage Mater. 2021, 36, 10–30. [Google Scholar] [CrossRef]
- Delmas, C. Sodium and Sodium-Ion Batteries: 50 Years of Research. Adv. Energy Mater. 2018, 8, 1703137. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, L.; Qi, X.; Lu, Y.; Wu, F.; Zhao, J.; Yu, Y.; Hu, Y.S.; Chen, L. Solid-State Sodium Batteries. Adv. Energy Mater. 2018, 8, 1703012. [Google Scholar] [CrossRef]
- Chen, F.; Wang, X.; Armand, M.; Forsyth, M. Cationic Polymer-in-Salt Electrolytes for Fast Metal Ion Conduction and Solid-State Battery Applications. Nat. Mater. 2022, 21, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Saito, T. Synthesis and Properties of Polymerized Ionic Liquids. Eur. Polym. J. 2017, 90, 245–272. [Google Scholar] [CrossRef]
- Forsyth, M.; Porcarelli, L.; Wang, X.; Goujon, N.; Mecerreyes, D. Innovative Electrolytes Based on Ionic Liquids and Polymers for Next-Generation Solid-State Batteries. Acc. Chem. Res. 2019, 52, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Li, Z.; Fu, J.L.; Guo, X. Sodium-Ion Conducting Polymer Electrolytes. Rare Met. 2023, 42, 1–16. [Google Scholar] [CrossRef]
- An, Y.; Han, X.; Liu, Y.; Azhar, A.; Na, J.; Nanjundan, A.K.; Wang, S.; Yu, J.; Yamauchi, Y. Progress in Solid Polymer Electrolytes for Lithium-Ion Batteries and Beyond. Small 2022, 18, 2103617. [Google Scholar] [CrossRef]
- Wang, H.; Sheng, L.; Yasin, G.; Wang, L.; Xu, H.; He, X. Reviewing the Current Status and Development of Polymer Electrolytes for Solid-State Lithium Batteries. Energy Storage Mater. 2020, 33, 188–215. [Google Scholar] [CrossRef]
- Rollo-Walker, G.; Malic, N.; Wang, X.; Chiefari, J.; Forsyth, M.; Lufrano, F.; Aricò, A.S.; Baglio, V.; Chiefari@csiro, J.; Au, J.C. Development and Progression of Polymer Electrolytes for Batteries: Influence of Structure and Chemistry. Polymers 2021, 13, 4127. [Google Scholar] [CrossRef]
- Loo, W.S.; Galluzzo, M.D.; Li, X.; Maslyn, J.A.; Oh, H.J.; Mongcopa, K.I.; Zhu, C.; Wang, A.A.; Wang, X.; Garetz, B.A.; et al. Phase Behavior of Mixtures of Block Copolymers and a Lithium Salt. J. Phys. Chem. B 2018, 122, 8065–8074. [Google Scholar] [CrossRef] [PubMed]
- Young, W.S.; Kuan, W.F.; Epps, T.H. Block Copolymer Electrolytes for Rechargeable Lithium Batteries. J. Polym. Sci. B Polym. Phys. 2014, 52, 1–16. [Google Scholar] [CrossRef]
- Ding, P.; Lin, Z.; Guo, X.; Wu, L.; Wang, Y.; Guo, H.; Li, L.; Yu, H. Polymer Electrolytes and Interfaces in Solid-State Lithium Metal Batteries. Mater. Today 2021, 51, 449–474. [Google Scholar] [CrossRef]
- Alipoori, S.; Mazinani, S.; Aboutalebi, S.H.; Sharif, F. Review of PVA-Based Gel Polymer Electrolytes in Flexible Solid-State Supercapacitors: Opportunities and Challenges. J. Energy Storage 2020, 27, 101072. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, K.; Liu, Y.; Gao, H.; Bai, Y.; Wang, X.; Wu, C. Polymer Electrolytes and Interfaces toward Solid-State Batteries: Recent Advances and Prospects. Energy Storage Mater. 2020, 33, 26–54. [Google Scholar] [CrossRef]
- Arefin, A.M.E.; Khatri, N.R.; Kulkarni, N.; Egan, P.F. Polymer 3D Printing Review: Materials, Process, and Design Strategies for Medical Applications. Polymers 2021, 13, 1499. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Lu, X.; Wang, W.; Kang, N.G.; Mays, J.W. Block Copolymers: Synthesis, Self-Assembly, and Applications. Polymers 2017, 9, 494. [Google Scholar] [CrossRef]
- Dau, H.; Jones, G.R.; Tsogtgerel, E.; Nguyen, D.; Keyes, A.; Liu, Y.S.; Rauf, H.; Ordonez, E.; Puchelle, V.; Basbug Alhan, H.; et al. Linear Block Copolymer Synthesis. Chem. Rev. 2022, 122, 14471–14553. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Ramsey, J.D.; Samadi, A.; Atoufi, Z.; Yazdi, M.K.; Ganjali, M.R.; Amirabad, L.M.; Zangene, E.; Farokhi, M.; Formela, K.; et al. Poloxamer: A Versatile Tri-Block Copolymer for Biomedical Applications. Acta Biomater. 2020, 110, 37–67. [Google Scholar] [CrossRef]
- Huang, C.; Zhu, Y.; Man, X. Block Copolymer Thin Films. Phys. Rep. 2021, 932, 1–36. [Google Scholar] [CrossRef]
- Karayianni, M.; Pispas, S. Block Copolymer Solution Self-Assembly: Recent Advances, Emerging Trends, and Applications. J. Polym. Sci. 2021, 59, 1874–1898. [Google Scholar] [CrossRef]
- Malunavar, S.; Gallastegui, A.; Wang, X.; Makhlooghiazad, F.; Mecerreyes, D.; Armand, M.; Galceran, M.; Howlett, P.C.; Forsyth, M. Formulation and Characterization of PS-Poly(Ionic Liquid) Triblock Electrolytes for Sodium Batteries. ACS Appl. Polym. Mater. 2022, 4, 8977–8986. [Google Scholar] [CrossRef]
- Liu, C.; Hong, C.Y.; Pan, C.Y. Polymerization Techniques in Polymerization-Induced Self-Assembly (PISA). Polym. Chem. 2020, 11, 3673–3689. [Google Scholar] [CrossRef]
- Cornel, E.J.; Jiang, J.; Chen, S.; Du, J. Principles and Characteristics of Polymerization-Induced Self-Assembly with Various Polymerization Techniques. CCS Chem. 2021, 3, 2104–2125. [Google Scholar] [CrossRef]
- Yagci, Y.; Tasdelen, M.A. Mechanistic Transformations Involving Living and Controlled/Living Polymerization Methods. Prog. Polym. Sci. 2006, 31, 1133–1170. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. RAFT Polymerization and Some of Its Applications. Chem. Asian J. 2013, 8, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Dadashi-Silab, S.; Galluzzo, M.D.; Chakraborty, S.; Loo, W.S.; Matyjaszewski, K.; Balsara, N.P. Effect of Added Salt on Disordered Poly(Ethylene Oxide)-Block-Poly(Methyl Methacrylate) Copolymer Electrolytes. Macromolecules 2021, 54, 1414–1424. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Doerk, G.S.; Saavedra, H.M.; Mullen, T.J.; Zhang, P.; Wu, S.-L.; Javier, A.E. Block Copolymers as (Single-Ion Conducting) Lithium Battery Electrolytes. Nanotechnology 2021, 33, 062002. [Google Scholar] [CrossRef]
- Coote, J.P.; Adotey, S.K.J.; Sangoro, J.R.; Stein, G.E. Interfacial Effects in Conductivity Measurements of Block Copolymer Electrolytes. ACS Polym. Au 2023, 3, 331–343. [Google Scholar] [CrossRef]
- Lingua, G.; Grysan, P.; Vlasov, P.S.; Verge, P.; Shaplov, A.S.; Gerbaldi, C. Unique Carbonate-Based Single Ion Conducting Block Copolymers Enabling High-Voltage, All-Solid-State Lithium Metal Batteries. Macromolecules 2021, 54, 6911–6924. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, F.; Girard, G.M.A.; Zhu, H.; MacFarlane, D.R.; Mecerreyes, D.; Armand, M.; Howlett, P.C.; Forsyth, M. Poly(Ionic Liquid)s-in-Salt Electrolytes with Co-Coordination-Assisted Lithium-Ion Transport for Safe Batteries. Joule 2019, 3, 2687–2702. [Google Scholar] [CrossRef]
- Demarteau, J.; Fernandez De Anãstro, A.; Shaplov, A.S.; Mecerreyes, D. Poly(Diallyldimethylammonium) Based Poly(Ionic Liquid) Di- and Triblock Copolymers by PISA as Matrices for Ionogel Membranes. Polym. Chem. 2020, 11, 1481–1488. [Google Scholar] [CrossRef]
- Wang, X.; Girard, G.M.A.; Zhu, H.; Yunis, R.; Macfarlane, D.R.; Mecerreyes, D.; Bhattacharyya, A.J.; Howlett, P.C.; Forsyth, M. Poly(Ionic Liquid)s/Electrospun Nanofiber Composite Polymer Electrolytes for High Energy Density and Safe Li Metal Batteries. ACS Appl. Energy Mater. 2019, 2, 6237–6245. [Google Scholar] [CrossRef]
- Bhandary, R.; Schönhoff, M. Polymer Effect on Lithium Ion Dynamics in Gel Polymer Electrolytes: Cationic versus Acrylate Polymer. Electrochim. Acta 2015, 174, 753–761. [Google Scholar] [CrossRef]
- Brinkkötter, M.; Lozinskaya, E.I.; Ponkratov, D.O.; Vlasov, P.S.; Rosenwinkel, M.P.; Malyshkina, I.A.; Vygodskii, Y.; Shaplov, A.S.; Schönhoff, M. Influence of Anion Structure on Ion Dynamics in Polymer Gel Electrolytes Composed of Poly(Ionic Liquid), Ionic Liquid and Li Salt. Electrochim. Acta 2017, 237, 237–247. [Google Scholar] [CrossRef]
- Makhlooghiazad, F.; Mejía, L.M.G.; Rollo-Walker, G.; Kourati, D.; Galceran, M.; Chen, F.; Deschamps, M.; Howlett, P.; O’Dell, L.A.; Forsyth, M. Understanding Polymerized Ionic Liquids as Solid Polymer Electrolytes for Sodium Batteries. J. Am. Chem. Soc. 2024, 146, 1992–2004. [Google Scholar] [CrossRef] [PubMed]
- Young, W.S.; Epps, T.H. Ionic Conductivities of Block Copolymer Electrolytes with Various Conducting Pathways: Sample Preparation and Processing Considerations. Macromolecules 2012, 45, 4689–4697. [Google Scholar] [CrossRef]
- Cao, X.H.; Li, J.H.; Yang, M.J.; Yang, J.L.; Wang, R.Y.; Zhang, X.H.; Xu, J.T. Simultaneous Improvement of Ionic Conductivity and Mechanical Strength in Block Copolymer Electrolytes with Double Conductive Nanophases. Macromol. Rapid Commun. 2020, 41, 1900622. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, M.; Hilder, M.; Zhang, Y.; Chen, F.; Carre, L.; Rakov, D.A.; Armand, M.; Macfarlane, D.R.; Pozo-Gonzalo, C.; Howlett, P.C. Tuning Sodium Interfacial Chemistry with Mixed-Anion Ionic Liquid Electrolytes. ACS Appl. Mater. Interfaces 2019, 11, 43093–43106. [Google Scholar] [CrossRef] [PubMed]
- Hilder, M.; Gras, M.; Pope, C.R.; Kar, M.; Macfarlane, D.R.; Forsyth, M.; O’Dell, L.A. Effect of Mixed Anions on the Physicochemical Properties of a Sodium Containing Alkoxyammonium Ionic Liquid Electrolyte. Phys. Chem. Chem. Phys. 2017, 19, 17461–17468. [Google Scholar] [CrossRef] [PubMed]
- Kerner, M.; Plylahan, N.; Scheers, J.; Johansson, P. Ionic Liquid Based Lithium Battery Electrolytes: Fundamental Benefits of Utilising Both TFSI and FSI Anions? Phys. Chem. Chem. Phys. 2015, 17, 19569–19581. [Google Scholar] [CrossRef]
- Kim, S.J.; Yoon, S.G.; Kim, I.Y.; Kim, S.I. Swelling Characterization of the Semiinterpenetrating Polymer Network Hydrogels Composed of Chitosan and Poly(Diallyldimethylammonium Chloride). J. Appl. Polym. Sci. 2004, 91, 2876–2880. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Z.; Yang, K.; Yang, L. Polymeric Ionic Liquid-Poly(Ethylene Glycol) Composite Polymer Electrolytes for High-Temperature Lithium-Ion Batteries. ChemElectroChem 2018, 5, 328–334. [Google Scholar] [CrossRef]
- Coussirat, V.; Amarilla, F.; Peruzzo, P.J.; Cortizo, M.S. Dioctyl Fumarate-Co-Vinyl Benzoate Copolymers Preparation and Their Performance as Flow Improvers in Waxy Crude Oils. J. Pet. Sci. Eng. 2019, 182, 106290. [Google Scholar] [CrossRef]
- Jebrane, M.; Sèbe, G. A New Process for the Esterification of Wood by Reaction with Vinyl Esters. Carbohydr. Polym. 2008, 72, 657–663. [Google Scholar] [CrossRef]
- Yunis, R.; Girard, G.M.A.; Wang, X.; Zhu, H.; Bhattacharyya, A.J.; Howlett, P.; MacFarlane, D.R.; Forsyth, M. The Anion Effect in Ternary Electrolyte Systems Using Poly(Diallyldimethylammonium) and Phosphonium-Based Ionic Liquid with High Lithium Salt Concentration. Solid State Ion. 2018, 327, 83–92. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Kim, G.T.; Shi, J.; Paillard, E.; Judeinstein, P.; Lyonnard, S.; Bresser, D.; Iojoiu, C. Nanostructured Multi-Block Copolymer Single-Ion Conductors for Safer High-Performance Lithium Batteries. Energy Environ. Sci. 2018, 11, 3298–3309. [Google Scholar] [CrossRef]
- Chintapalli, M.; Le, T.N.P.; Venkatesan, N.R.; Mackay, N.G.; Rojas, A.A.; Thelen, J.L.; Chen, X.C.; Devaux, D.; Balsara, N.P. Structure and Ionic Conductivity of Polystyrene-Block-Poly(Ethylene Oxide) Electrolytes in the High Salt Concentration Limit. Macromolecules 2016, 49, 1770–1780. [Google Scholar] [CrossRef]
- Mohan, V.M.; Raja, V.; Sharma, A.K.; Rao, V.V.R.N. Ionic Conductivity and Discharge Characteristics of Solid-State Battery Based on Novel Polymer Electrolyte (PEO + NaBiF4). Mater. Chem. Phys. 2005, 94, 177–181. [Google Scholar] [CrossRef]
- Yoon, H.; Zhu, H.; Hervault, A.; Armand, M.; MacFarlane, D.R.; Forsyth, M. Physicochemical Properties of N-Propyl-N-Methylpyrrolidinium Bis(Fluorosulfonyl)Imide for Sodium Metal Battery Applications. Phys. Chem. Chem. Phys. 2014, 16, 12350–12355. [Google Scholar] [CrossRef]
- Herstedt, M.; Smirnov, M.; Johansson, P.; Chami, M.; Grondin, J.; Servant, L.; Lassègues, J.C. Spectroscopic Characterization of the Conformational States of the Bis(Trifluoromethanesulfonyl)Imide Anion (TFSI-). J. Raman Spectrosc. 2005, 36, 762–770. [Google Scholar] [CrossRef]
- Umebayashi, Y.; Mitsugi, T.; Fukuda, S.; Fujimori, T.; Fujii, K.; Kanzaki, R.; Takeuchi, M.; Ishiguro, S.I. Lithium Ion Solvation in Room-Temperature Ionic Liquids Involving Bis(Trifluoromethanesulfonyl) Imide Anion Studied by Raman Spectroscopy and DFT Calculations. J. Phys. Chem. B 2007, 111, 13028–13032. [Google Scholar] [CrossRef]
- Sanchez-Cupido, L.; Pringle, J.M.; Siriwardana, A.I.; Hilder, M.; Forsyth, M.; Pozo-Gonzalo, C. Correlating Electrochemical Behavior and Speciation in Neodymium Ionic Liquid Electrolyte Mixtures in the Presence of Water. ACS Sustain. Chem. Eng. 2020, 8, 14047–14057. [Google Scholar] [CrossRef]
- Pont, A.L.; Marcilla, R.; De Meatza, I.; Grande, H.; Mecerreyes, D. Pyrrolidinium-Based Polymeric Ionic Liquids as Mechanically and Electrochemically Stable Polymer Electrolytes. J. Power Sources 2009, 188, 558–563. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, J.; Qi, X.; Rong, X.; Shao, Y.; Feng, W.; Nie, J.; Hu, Y.S.; Li, H.; Huang, X.; et al. A New Na[(FSO2)(n-C4F9SO2)N]-Based Polymer Electrolyte for Solid-State Sodium Batteries. J. Mater. Chem. A Mater. 2017, 5, 7738–7743. [Google Scholar] [CrossRef]
- Ortiz-Vitoriano, N.; Monterrubio, I.; Garcia-Quintana, L.; López Del Amo, J.M.; Chen, F.; Rojo, T.; Howlett, P.C.; Forsyth, M.; Pozo-Gonzalo, C. Highly Homogeneous Sodium Superoxide Growth in Na-O2 Batteries Enabled by a Hybrid Electrolyte. ACS Energy Lett. 2020, 5, 903–909. [Google Scholar] [CrossRef]
- Ortiz Vitoriano, N.; Ruiz de Larramendi, I.; Sacci, R.L.; Lozano, I.; Bridges, C.A.; Arcelus, O.; Enterría, M.; Carrasco, J.; Rojo, T.; Veith, G.M. Goldilocks and the Three Glymes: How Na+ Solvation Controls Na–O2 Battery Cycling. Energy Storage Mater. 2020, 29, 235–245. [Google Scholar] [CrossRef]
- Wang, J.; Ni, Y.; Liu, J.; Lu, Y.; Zhang, K.; Niu, Z.; Chen, J. Room-Temperature Flexible Quasi-Solid-State Rechargeable Na-O2 Batteries. ACS Cent. Sci. 2020, 6, 1955–1963. [Google Scholar] [CrossRef]
- Ha, T.A.; Fdz De Anastro, A.; Ortiz-Vitoriano, N.; Fang, J.; MacFarlane, D.R.; Forsyth, M.; Mecerreyes, D.; Howlett, P.C.; Pozo-Gonzalo, C. High Coulombic Efficiency Na-O2 Batteries Enabled by a Bilayer Ionogel/Ionic Liquid. J. Phys. Chem. Lett. 2019, 10, 7050–7055. [Google Scholar] [CrossRef]
- Chang, S.; Hou, M.; Xu, B.; Liang, F.; Qiu, X.; Yao, Y.; Qu, T.; Ma, W.; Yang, B.; Dai, Y.; et al. High-Performance Quasi-Solid-State Na-Air Battery via Gel Cathode by Confining Moisture. Adv. Funct. Mater. 2021, 31, 2011151. [Google Scholar] [CrossRef]
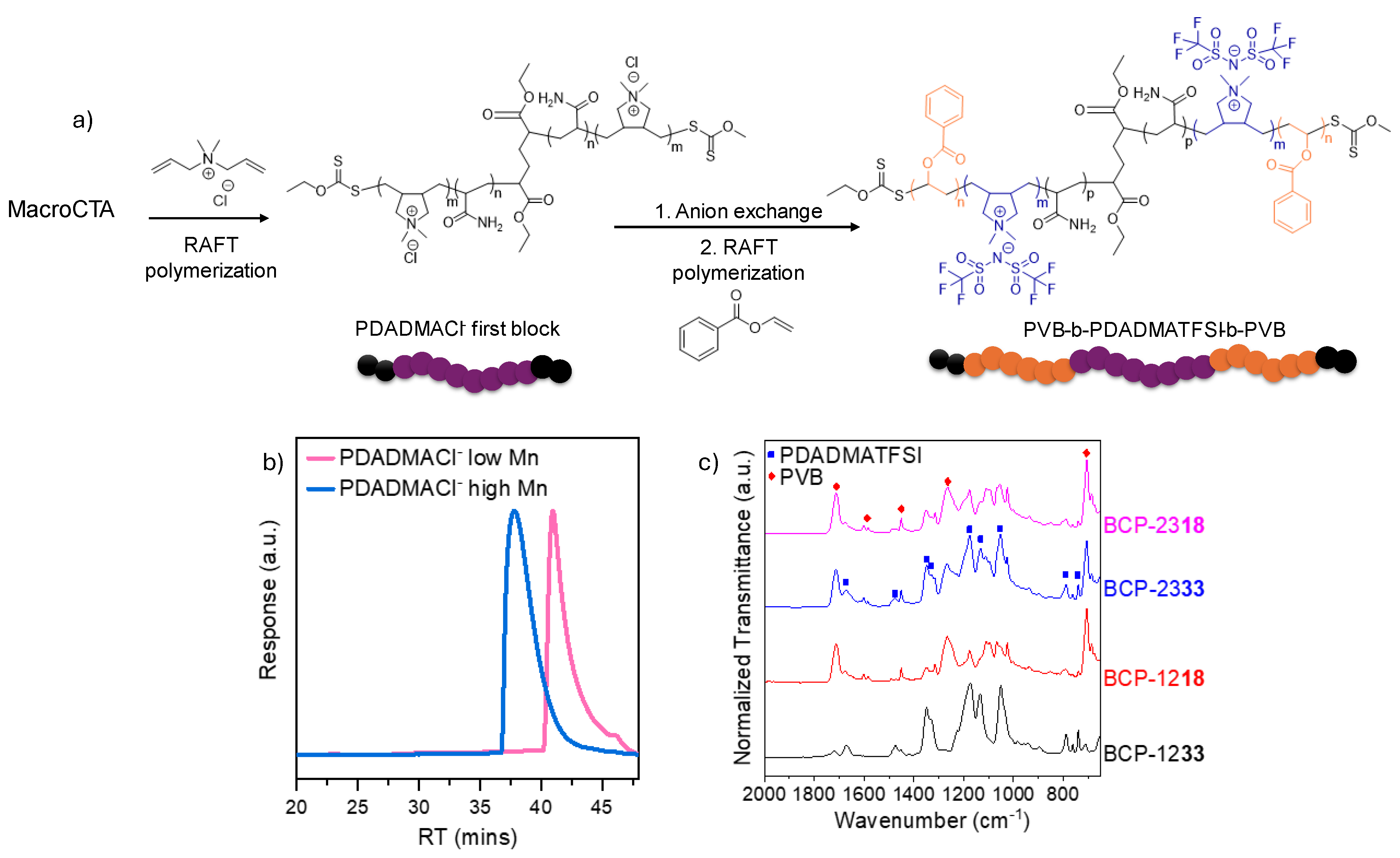




| Sample | Conversion a (%) | Mn, th (kg mol−1) | Mn, exp (kg mol−1) | PI (Mn/Mw) | Name |
|---|---|---|---|---|---|
| X-PAm-DiEst-PAm-X | 66 | 2.8 | 1.9 b | - | - |
| PDADMACl-high Mn | 50 | 21.5 | 17.7 c | 1.7 | - |
| PDADMACl-low Mn | 50 | 11.4 | 5.4 c | 1.9 | - |
| PDADMATFSI-high Mn | 90 | 36.8 | 33.1 | - | - |
| PDADMATFSI-low Mn | 95 | 18.4 | 17.5 | - | - |
| PVB11.5K—b—PDADMATFSI33K—b—PVB11.5K | 80 | 13 | 10.4 a | 2.1 | BCP-1233 |
| PVB11.5K—b—PDADMATFSI17.5K—b—PVB11.5K | 99 | 13 | 12.8 a | 2.5 | BCP-1218 |
| PVB22.5K—b—PDADMATFSI33K—b—PVB22.5K | 87 | 26 | 20.8 a | 2.2 | BCP-2333 |
| VB22.5K—b—PDADMATFSI17.5K—b—PVB22.5K | 94 | 26 | 24.4 a | 2.2 | BCP-2318 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stigliano, P.L.; Gallastegui, A.; Villacis-Segovia, C.; Amores, M.; Kumar, A.; O’Dell, L.A.; Fang, J.; Mecerreyes, D.; Pozo-Gonzalo, C.; Forsyth, M. Poly(vinyl benzoate)-b-poly(diallyldimethyl ammonium TFSI)-b-poly(vinyl benzoate) Triblock Copolymer Electrolytes for Sodium Batteries. Batteries 2024, 10, 125. https://doi.org/10.3390/batteries10040125
Stigliano PL, Gallastegui A, Villacis-Segovia C, Amores M, Kumar A, O’Dell LA, Fang J, Mecerreyes D, Pozo-Gonzalo C, Forsyth M. Poly(vinyl benzoate)-b-poly(diallyldimethyl ammonium TFSI)-b-poly(vinyl benzoate) Triblock Copolymer Electrolytes for Sodium Batteries. Batteries. 2024; 10(4):125. https://doi.org/10.3390/batteries10040125
Chicago/Turabian StyleStigliano, Pierre L., Antonela Gallastegui, Carlos Villacis-Segovia, Marco Amores, Ajit Kumar, Luke A. O’Dell, Jian Fang, David Mecerreyes, Cristina Pozo-Gonzalo, and Maria Forsyth. 2024. "Poly(vinyl benzoate)-b-poly(diallyldimethyl ammonium TFSI)-b-poly(vinyl benzoate) Triblock Copolymer Electrolytes for Sodium Batteries" Batteries 10, no. 4: 125. https://doi.org/10.3390/batteries10040125
APA StyleStigliano, P. L., Gallastegui, A., Villacis-Segovia, C., Amores, M., Kumar, A., O’Dell, L. A., Fang, J., Mecerreyes, D., Pozo-Gonzalo, C., & Forsyth, M. (2024). Poly(vinyl benzoate)-b-poly(diallyldimethyl ammonium TFSI)-b-poly(vinyl benzoate) Triblock Copolymer Electrolytes for Sodium Batteries. Batteries, 10(4), 125. https://doi.org/10.3390/batteries10040125