New Insights to Characterize Paint Varnishes and to Study Water in Paintings by Nuclear Magnetic Resonance Spectroscopy (NMR)
Abstract
1. Introduction
2. Results
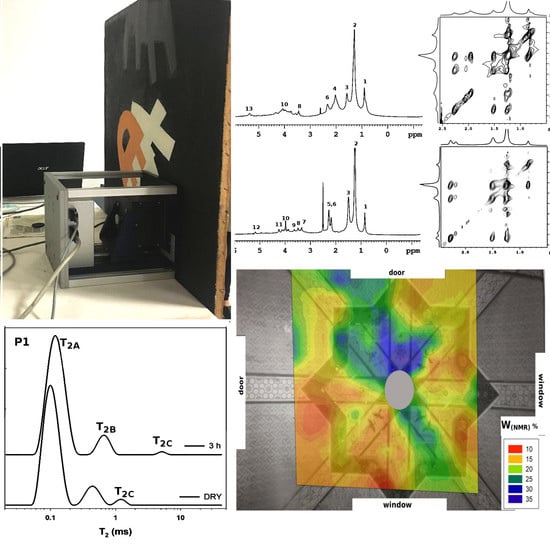
2.1. Unilateral NMR to Analyze the Water Content in Wall Paintings
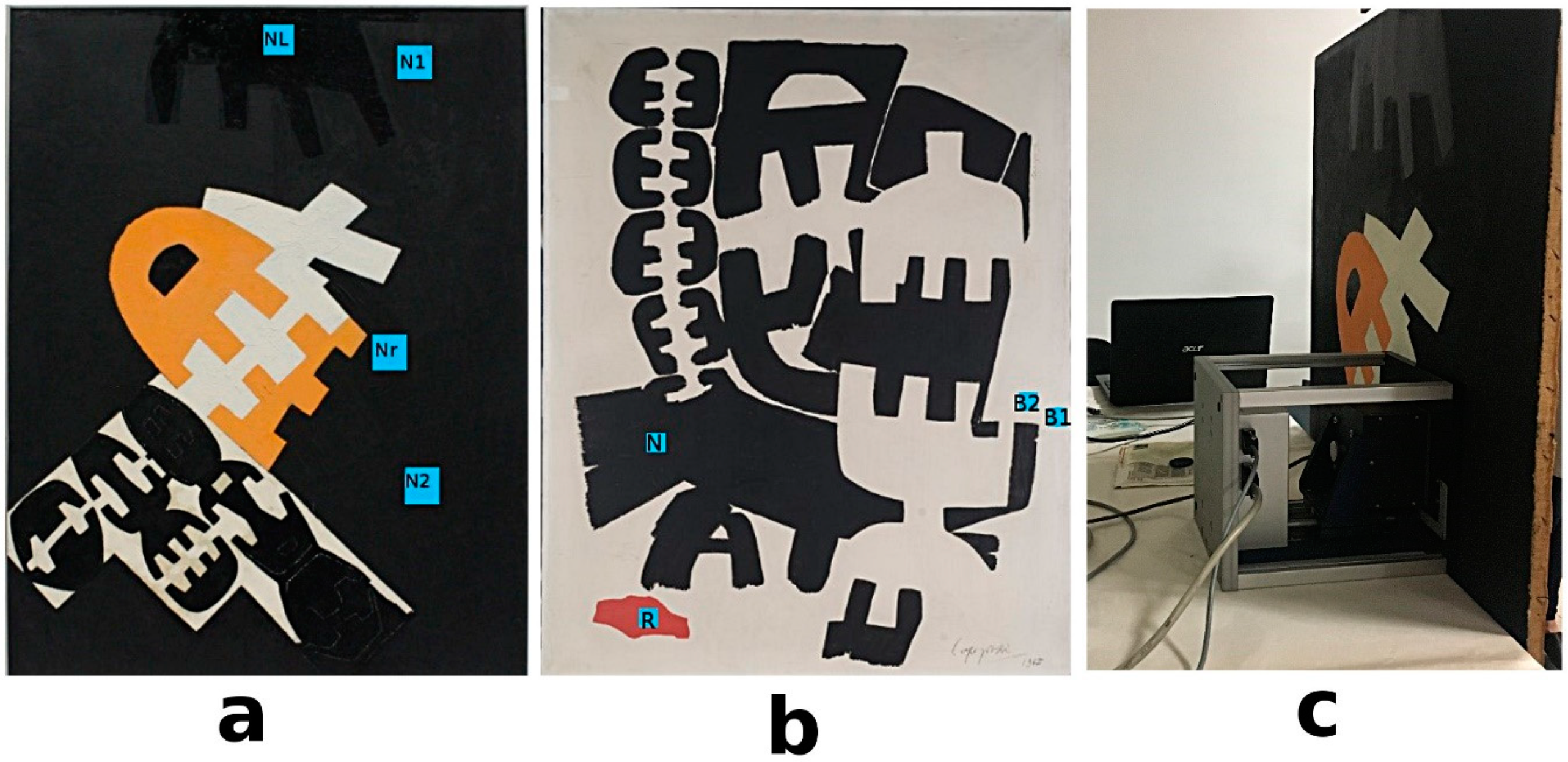
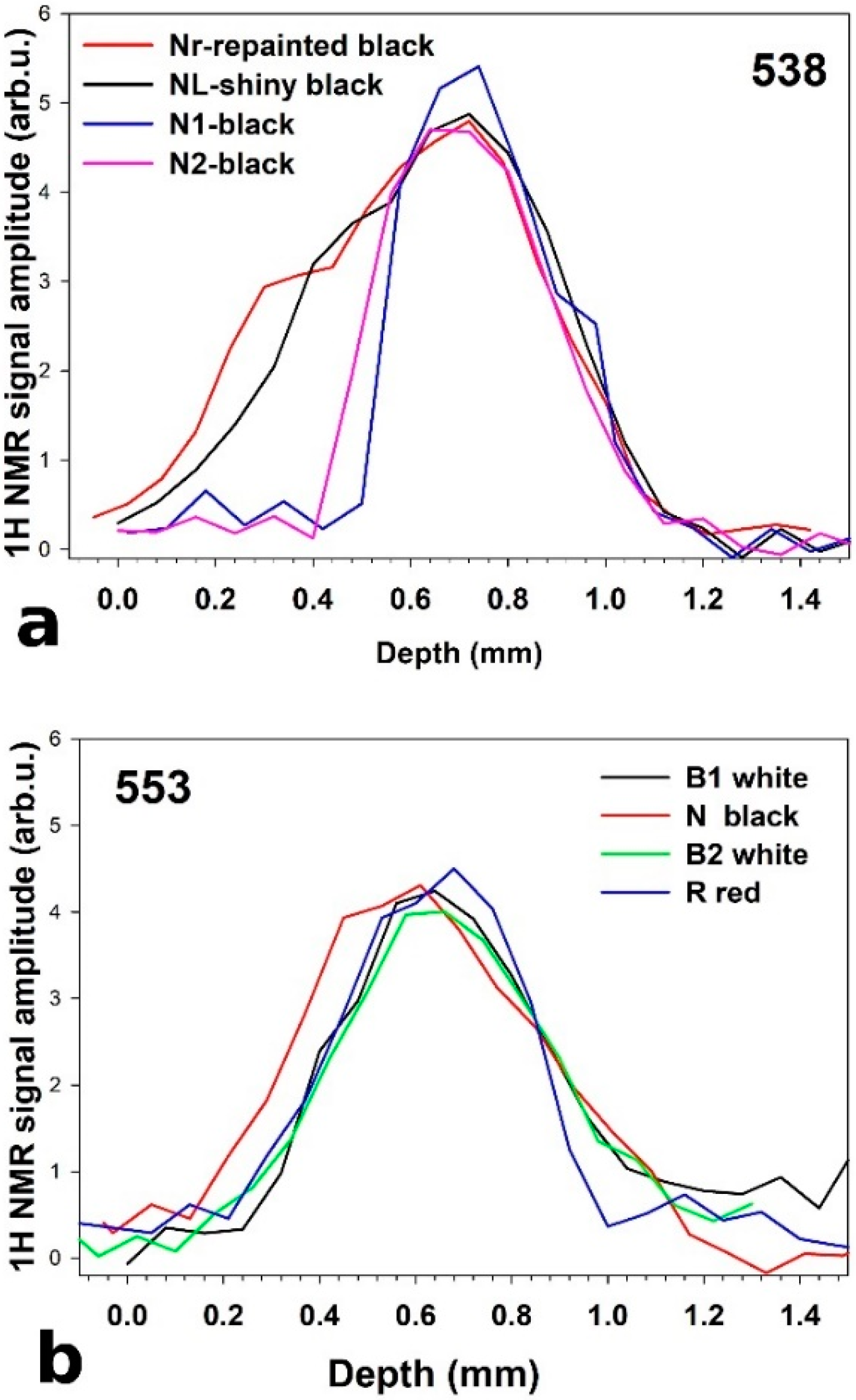
2.2. Unilateral NMR to Evaluate Water-Based Cleaning Systems Applied on Oil Paintings
2.3. HR-MAS to Characterize Organic Materials in Paintings: Linseed Oil, Shellac, Colophony, and Sandarac
3. Materials and Methods
3.1. Cases Studies and Model Oil Paints
3.2. Unilateral NMR
3.3. High Resolution NMR Spectroscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Casadio, F.; Giangualano, I.; Piqué, F. Organic materials in wall paintings: The historical and analytical literature. Stud. Conserv. 2004, 49, 63–80. [Google Scholar] [CrossRef]
- Casoli, A.; Santoro, S. Organic materials in the wall paintings in Pompei: A case study of Insula del Centenario. Chem. Cent. J. 2012, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Di Tullio, V.; Sciutto, G.; Proietti, N.; Prati, S.; Mazzeo, R.; Colombo, C.; Cantisani, E.; Romè, V.; Rigaglia, D.; Capitani, D. 1H NMR depth profiles combined with portable and micro-analytical techniques for evaluating cleaning methods and identifying original, non-original, and degraded materials of a 16th century Italian wall painting. Microchem. J. 2018, 141, 40–50. [Google Scholar] [CrossRef]
- Hagan, E.; Murray, A. Effects of Water Exposure on the Mechanical Properties of Early Artists’ Acrylic Paints. MRS Online Proc. Libr. Arch. 2004, 852, OO2.9. [Google Scholar] [CrossRef]
- van Loon, A. Color Changes and Chemical Reactivity in Seventeenth-Century Oil Paintings. Ph.D. Thesis, University of Amsterdam, Amsterdam, The Netherlands, 2008. [Google Scholar]
- Keune, K.; Boon, J.J. Analytical Imaging Studies of CrossSections of Paintings Affected by Lead Soap Aggregate Formation. Stud. Conserv. 2007, 52, 161–176. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Brett, R.A. Chemical reactions in paint films. J. Oil Colour Chem. Assoc. 1969, 52, 1054–1074. [Google Scholar]
- Rehorn, C.; Blümich, B. Cultural Heritage Studies with Mobile NMR. Angew. Chem. Int. Ed. 2018, 57, 7304. [Google Scholar] [CrossRef]
- Baias, M.; Blümich, B. Nondestructive Testing of Objects from Cultural Heritage with NMR. In Modern Magnetic Resonance; Webb, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 293–304. [Google Scholar]
- Blümich, B.; Casanova, F.; Perlo, J.; Presciutti, F.; Anselmi, C.; Doherty, B. Noninvasive Testing of Art and Cultural Heritage by Mobile NMR. Acc. Chem. Res. 2010, 43, 6, 761–770. [Google Scholar] [CrossRef]
- Kobayashi, T.; Perras, F.A.; Murphy, A.; Yao, Y.; Catalano, J.; Centeno, S.A.; Dybowski, C.; Zumbulyadis, N.; Pruski, M. DNP-enhanced ultrawideline 207Pb solid-state NMR spectroscopy: An application to cultural heritage science. Dalton Trans. 2017, 46, 3535. [Google Scholar] [CrossRef]
- Proietti, N.; Capitani, D.; Di Tullio, V. Nuclear Magnetic Resonance, a Powerful Tool in Cultural Heritage. Magnetochemistry 2018, 4, 11. [Google Scholar] [CrossRef]
- Invernizzi, C.; Fiocco, G.; Iwanicka, M.; Kowalska, M.; Targowski, P.; Blümich, B.; Rehorn, C.; Gabrielli, V.; Bersani, D.; Licchelli, M.; et al. Non-invasive mobile technology to study the stratigraphy of ancient Cremonese violins: OCT, NMR-MOUSE, XRF and reflection FT-IR spectroscopy. Microchem. J. 2020, 155, 104754. [Google Scholar] [CrossRef]
- Capitani, D.; Di Tullio, V.; Proietti, N. Nuclear Magnetic Resonance to characterize and monitor Cultural Heritage. Prog. Nucl. Magn. Reson. Spetrosc. 2012, 64, 29–69. [Google Scholar] [CrossRef] [PubMed]
- Moretti, P.; Cartechini, L.; Miliani, C. Single-sided NMR: A non-invasive diagnostic tool for monitoring swelling effects in paint films subjected to solvent cleaning. Anal. Bioanal. Chem. 2020, 412, 1063–1075. [Google Scholar] [CrossRef]
- Oligschläger, D.; Waldow, S.; Haber, A.; Zia, W.; Blümich, B. Moisture dynamics in wall paintings monitoredby single-sided NMR. Magn. Reson. Chem. 2015, 53, 48–57. [Google Scholar] [CrossRef]
- Prati, S.; Sciutto, G.; Volpi, F.; Rehorn, C.; Vurro, R.; Blumich, B.; Mazzocchetti, L.; Giorgini, L.; Samori, C.; Galletti, P. Cleaning oil paintings: NMR relaxometry and SPME to evaluate the effects of green solvents and innovative green gels. New J. Chem. 2019, 43, 8229–8238. [Google Scholar] [CrossRef]
- Adams, A.; Blümich, B. Single-Sided NMR of Semicrystalline Polymers. Macromol. Symp. 2013, 327, 29–38. [Google Scholar] [CrossRef]
- Di Tullio, V.; Capitani, D.; Presciutti, F.; Gentile, G.; Gentile, G.; Brunetti, B.G.; Proietti, N. Non-invasive NMR stratigraphy of a multi-layered artefact: An ancient detached mural painting. Anal. Bioanal. Chem. 2013, 405, 8669–8675. [Google Scholar] [CrossRef]
- Di Tullio, V.; Proietti, N.; Gobbino, M.; Capitani, D.; Olmi, R.; Riminesi, C.; Giani, E. Non-destructive mapping of dampness and salts in degraded wall paintings in hypogeous buildings: The case of St. Clement at mass fresco in St. Clement Basilica, Rome. Anal. Bioanal. Chem. 2010, 396, 1885–1896. [Google Scholar] [CrossRef]
- Di Tullio, V.; Capitani, D.; Atrei, A.; Benetti, F.; Perra, G.; Presciutti, F.; Proietti, N.; Marchettini, N. Advanced NMR methodologies and micro-analytical techniques to investigate the stratigraphy and materials of 14th century Sienese wooden paintings. Microchem. J. 2016, 125, 208–218. [Google Scholar] [CrossRef]
- Spyros, A.; Anglos, D. Study of Aging in Oil Paintings by 1D and 2D NMR Spectroscopy. Anal. Chem. 2004, 76, 4929–4936. [Google Scholar] [CrossRef]
- Spinella, A.; Malagodi, M.; Saladino, M.L.; Weththimuni, M.L.; Caponetti, E.; Licchelli, M. A step forward in disclosing the secret of stradivari’s varnish by NMR spectroscopy. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3949–3954. [Google Scholar] [CrossRef]
- Kehlet, C.; Kuvvetli, F.; Catalano, A.; Dittmer, J. Solid-state NMR for the study of Asger Jorn’s paintings. Microchem. J. 2016, 125, 308–314. [Google Scholar] [CrossRef]
- Sfakianaki, S.; Kouloumpi, E.; Anglos, D.; Spyros, A. Egg yolk identification and aging in mixed paint binding media by NMR spectroscopy. Magn. Res. Chem. 2015, 53, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Catalano, J.; Yao, Y.; Murphy, A.; Zumbulyadis, N.; Centeno, S.A.; Dybowski, C. Nuclear Magnetic Resonance Spectra and 207Pb Chemical-Shift Tensors of Lead Carboxylates Relevant to Soap Formation in Oil Paintings. Appl. Spectrosc. 2014, 68, 280–286. [Google Scholar] [CrossRef]
- Bartolozzi, G.; Marchiafava, V.; Mirabello, V.; Peruzzini, M.; Picollo, M. Chemical curing in alkyd paints: An evaluation via FT-IR and NMR spectroscopies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 520–525. [Google Scholar] [CrossRef]
- Alam, T.M.; Jenkins, J.E. HR-MAS NMR Spectroscopy in Material Science. In Advanced Aspect of Spectroscopy; IntechOpen: Rijeka, Croatia, 2012; pp. 279–306. [Google Scholar]
- Viel, S.; Ziarelli, F.; Pagès, G.; Carrara, C.; Caldarelli, S. Pulsed field gradient magic angle spinning NMR self-diffusion measurements in liquids. J. Magn. Reson. 2008, 190, 113–123. [Google Scholar] [CrossRef]
- Cheng, L.L. Tissue and Cell Samples by HR-MAS NMR. In eMagRes; Wiley Online Library, John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 2. [Google Scholar]
- Bachmann, S.; Hellriegel, C.; Wegmann, J.; Händel, H.; Albert, K. Characterization of polyalkylvinyl ether phases by solid-state and suspended-state nuclear magnetic resonance investigations. Solid State Nucl. Mag. 2000, 17, 39–51. [Google Scholar] [CrossRef]
- Gaede, H.C.; Gawrisch, K. Lateral Diffusion Rates of Lipid, Water and a Hydrophobic Drug in a Multilamellar Liposome. Biophys. J. 2003, 85, 1734–1740. [Google Scholar] [CrossRef]
- Corsaro, C.; Mallamace, D.; Vasi, S.; Pietronero, L.; Mallamace, F.; Missori, M. The role of water in the degradation process of paper using 1H HR-MAS NMR spectroscopy. Phys. Chem. Chem. Phys. 2016, 18, 33335–33343. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, C.; Mallamace, D.; Łojewska, J.; Mallamace, F.; Pietronero, L.; Missori, M. Molecular degradation of ancient documents revealed by 1H HR-MAS NMR spectroscopy. Sci. Rep. 2013, 3, 2896. [Google Scholar] [CrossRef]
- Di Tullio, V.; Zumbulyadis, N.; Centeno, S.A.; Catalano, J.; Wagner, M.; Dybowski, C. Water Diffusion and Transport in Oil Paints as Studied by Unilateral NMR and 1H High-Resolution MAS-NMR Spectroscopy. Chem. Phys. Chem. 2020, 21, 113. [Google Scholar] [CrossRef]
- Capitani, D.; Proietti, N.; Gobbino, M.; Soroldoni, L.; Casellato, U.; Valentini, M.; Rosina, E. An integrated study for mapping the moisture distribution in an ancient damaged wall painting. Anal. Bioanal. Chem. 2009, 395, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- Gauri, K.L.; Bandyopadhyay, J.K. Carbonate Stone: Chemical Behaviour, Durability and Conservation; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Canevali, C.; Fasoli, M.; Bertasa, M.; Botteon, A.; Colombo, A.; Di Tullio, V.; Capitani, D.; Proietti, N.; Scalarone, D.; Sansonetti, A. A multi-analytical approach for the study of copper stain removal by agar gels. Microchem. J. 2016, 129, 249–258. [Google Scholar] [CrossRef]
- Baglioni, M.; Giorgi, R.; Berti, D.; Baglioni, P. Smart cleaning of cultural heritage: A new challenge for soft nanoscience. Nanoscale 2012, 4, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.R.; Carmalt, C.J.; Parkin, I.P. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef]
- Presciutti, F.; Perlo, J.; Casanova, F.; Glöggler, S.; Miliani, C.; Blümich, B.; Brunetti, B.G.; Sgamellotti, A. Noninvasive nuclear magnetic resonance profiling of painting layers. Appl. Phys. Lett. 2008, 93, 033505. [Google Scholar] [CrossRef]
- Schneider, J.; Lopes, L.V.S.; Tambelli, C.E.; Donoso, J.P.; Lozano, H.; Gonzalez, G. NMR Study of Slow Motions of HDA Hydrocarbons Chains Inside Lamellar Structures. Mol. Cryst. Liq. Cryst. 2006, 483, 130–140. [Google Scholar] [CrossRef]
- Erhardt, D.; Tumosa, C.S.; Mecklenburg, M.F. Long-Term Chemical and Physical Processes in Oil Paint Films. Stud. Conserv. 2005, 50, 143–150. [Google Scholar] [CrossRef]
- Von den Berg, J.D.J.; van den Berg, K.J.; Boon, J.J. Identification of non-cross-linked compounds in methanolic extracts of cured and aged linseed oil-based paint films using gas chromatography-mass spectrometry. J. Chromatogr. A 2002, 950, 195–211. [Google Scholar] [CrossRef]
- Catalano, J.; Murphy, A.; Yao, Y.; Yap, G.P.A.; Zumbulyadis, N.; Centeno, S.A.; Dybowski, C. Coordination geometry of lead carboxylates—Spectroscopic and crystallographic evidence. Dalton Trans. 2015, 44, 2340. [Google Scholar] [CrossRef]
- Casadio, F.; Keune, K.; Noble, P.; Van Loon, A.; Hendriks, E.; Centeno, S.A.; Osmond, G. Metal Soaps in Art; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Mills, J.S.; White, R. The Organic Chemistry of Museum Objects, 2nd ed.; Oxford Butterworths-Heinemann: Oxford, UK, 1999; p. 206. ISBN 0-7506-1693-8. [Google Scholar]
- Dietemann, P.; Higgitt, C.; Kalin, M.; Edelmann, M.J.; Knochenmuss, R.; Zenobi, R. Aging and yellowing of triterpenoid resin varnishes e Influence of aging conditions and resin composition. J. Cult. Herit. 2009, 10, 30–40. [Google Scholar] [CrossRef]
- Romero-Noguera, J.; Martín-Sánchez, I.; Doménech-Carbó, M.T.; Osete-Cortina, L.; López-Miras, M.M.; Bolívar-Galiano, F. Analytical characterisation of the biodeterioration of diterpenoid labdanic varnishes used in pictorial techniques: Sandarac and Manila copal. Int. Biodeterior. Biodegrad. 2014, 90, 99–105. [Google Scholar] [CrossRef]
- Scalarone, D.; Duursma, M.C.; Boon, J.J.; Chiantore, O. MALDI-TOF mass spectrometry on cellulosic surfaces of fresh and photo-aged di- and triterpenoid varnish resins. J. Mass Spectrom. 2005, 40, 1527–1535. [Google Scholar] [CrossRef]
- Kononenko, I. Molecular Characterization and Ageing of the Sandarac Resin and Its Principal Component Communic Acid. Analytical Chemistry. Université Pierre et Marie Curie—Paris VI: Paris, France, Unpublished Thesis. 2017. [Google Scholar]
- Echard, J.P.; Bertrand, L.; von Bohlen, A.; Le Hô, A.S.; Paris, C.; Bellot-Gurlet, L.; Soulier, B.; Lattuati-Derieux, A.; Thao, S.; Robinet, L.; et al. Cover Picture: The Nature of the Extraordinary Finish of Stradivari’s Instruments. Angew. Chem. Int. Ed. 2010, 49, 1. [Google Scholar] [CrossRef]
- Ioannidis, K.; Melliou, E.; Prokopios, M. High-Throughput 1H-Nuclear Magnetic Resonance-Based Screening for the Identification and Quantification of Heartwood Diterpenic Acids in Four Black Pine (Pinus nigra Arn.) Marginal Provenances in Greece. Molecules 2019, 24, 3603. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bollard, M.E.; Keun, H.; Antti, H.; Beckonert, O.; Ebbels, T.M.; Lindon, J.C.; Holmes, E.; Tang, H.; Nicholson, G.K. Spectral editing and pattern recognition methods applied to high-resolution magic-angle spinning 1H nuclear magnetic resonance spectroscopy of liver tissues. Anal. Biochem. 2003, 323, 26–32. [Google Scholar] [CrossRef]
- Barbero, L.M.; Morelli, F.R.; Rivosecchi, V.; Bertolino, G.; Pola, F.; Mastinu, G.; Salvagnini, S.; Bolpagni, P.; D’Angelo, L. Capogrossi: A Retrospective; Marsilio: Venice, Italy, 2013; p. 394. [Google Scholar]
- Barnaba, C.; Bertorello, C. I Dipinti ad olio su muro del Passignano nella Cappella Rivaldi in Santa Maria della Pace a Roma. Rivisitazione di un Restauro della metà degli anni ’80 del ‘900. In Proceedings of the IV National Congress of the Italian Group of International Institute for Conservation (IGIIC), Lo Stato dell’Arte, L’Aquila, Italy, 20–22 October 2016. [Google Scholar]
- Blümich, B.; Perlo, J.; Casanova, F. Mobile single-sided NMR. Progr. Nucl. Magn. Res. Sp. 2008, 52, 197–269. [Google Scholar] [CrossRef]

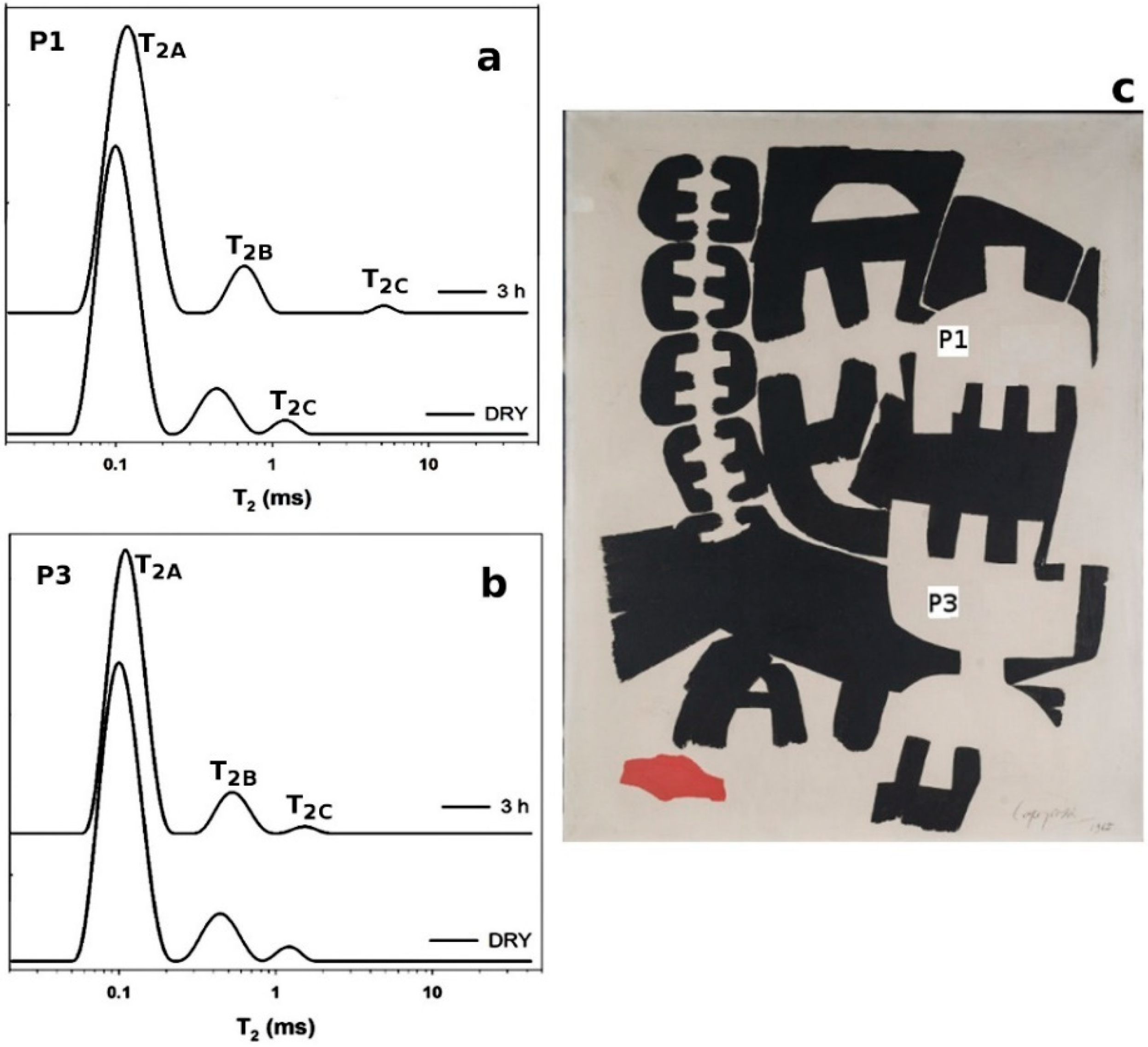
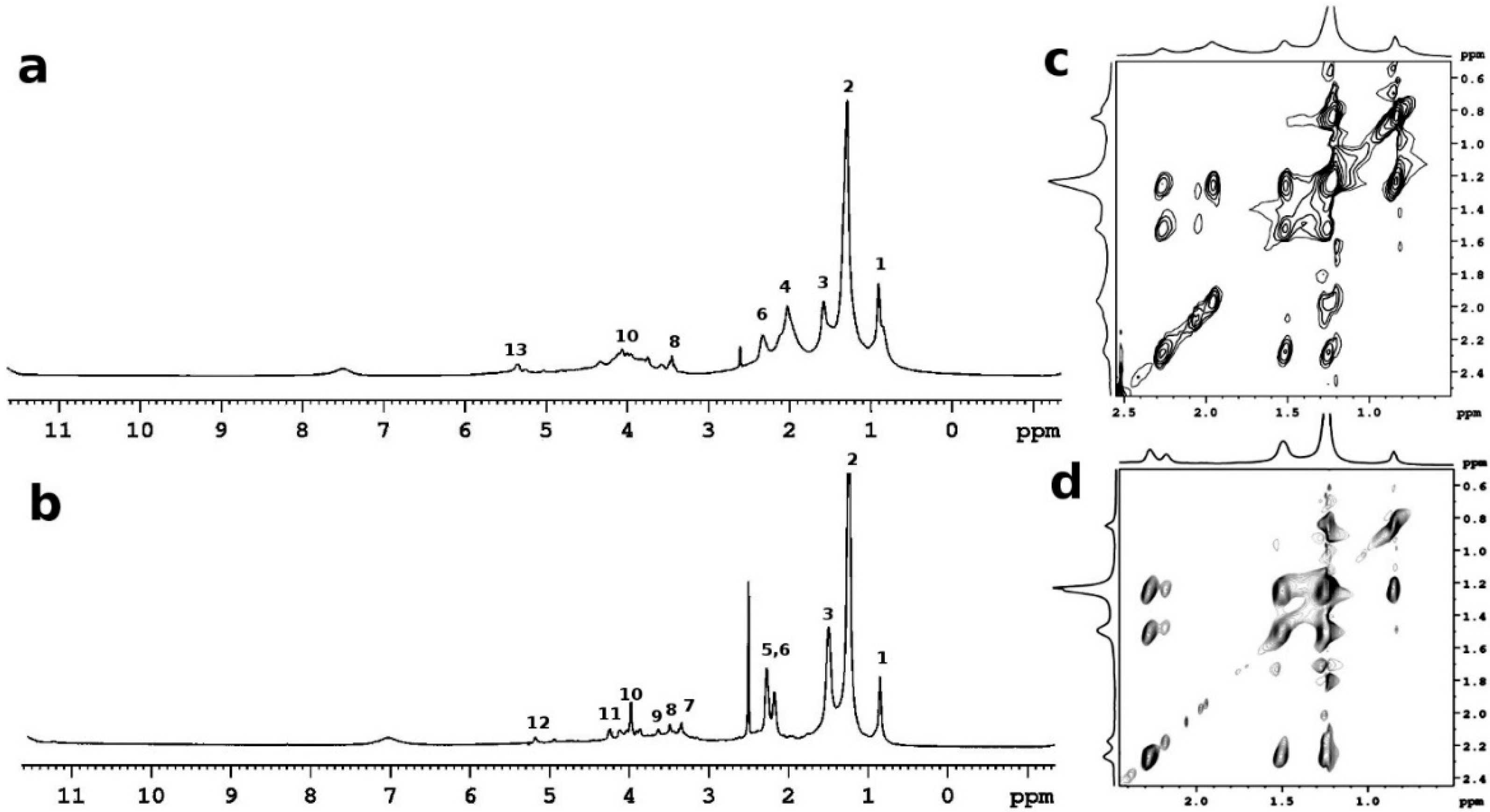
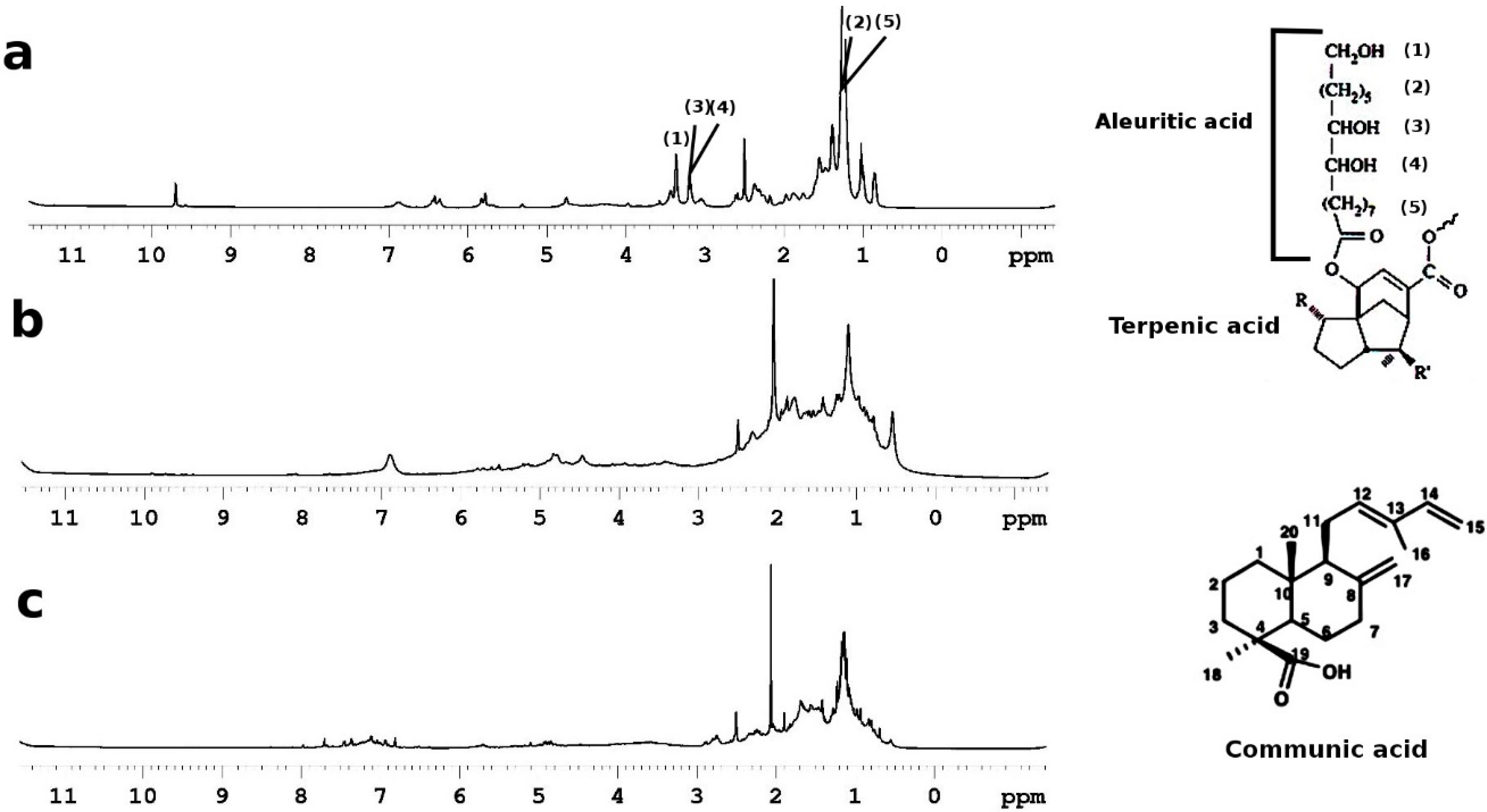

| Area * | WA | T2A | WB | T2B | WC | T2C |
|---|---|---|---|---|---|---|
| P1 (b) | 85% | 0.10 | 12% | 0.50 | 3% | 1.2 |
| P1 (a) | 89% | 0.12 | 10% | 0.70 | 1% | 5.0 |
| P3 (b) | 85% | 0.10 | 12% | 0.47 | 3% | 1.0 |
| P3 (a) | 87% | 0.11 | 12% | 0.54 | 1% | 1.6 |
| δ (ppm) | Group | |
|---|---|---|
| 1 | 0.85 | -CH3 |
| 2 | 1.20–1.40 | (CH2)x, CH2CH3 CH2CH2CH3 |
| 3 | 1.50 | CH2CH2COO- |
| 4 | 2.02 | CH2CH= |
| 5 | 2.20 | CH2COOH |
| 6 | 2.30 | CH2COOR |
| 7 | 3.5 | CH2OH |
| 8 | 3.66 | CH2OH |
| 9 | 3.8 | CHOH |
| 10 | 3.98 | CHOH, CHO- |
| 11 | 4.3/4.1 | CH2OCOR |
| 12 | 5.20 | CHOCOR |
| 13 | 5.30 | CH=CH |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Tullio, V.; Proietti, N. New Insights to Characterize Paint Varnishes and to Study Water in Paintings by Nuclear Magnetic Resonance Spectroscopy (NMR). Magnetochemistry 2020, 6, 21. https://doi.org/10.3390/magnetochemistry6020021
Di Tullio V, Proietti N. New Insights to Characterize Paint Varnishes and to Study Water in Paintings by Nuclear Magnetic Resonance Spectroscopy (NMR). Magnetochemistry. 2020; 6(2):21. https://doi.org/10.3390/magnetochemistry6020021
Chicago/Turabian StyleDi Tullio, Valeria, and Noemi Proietti. 2020. "New Insights to Characterize Paint Varnishes and to Study Water in Paintings by Nuclear Magnetic Resonance Spectroscopy (NMR)" Magnetochemistry 6, no. 2: 21. https://doi.org/10.3390/magnetochemistry6020021
APA StyleDi Tullio, V., & Proietti, N. (2020). New Insights to Characterize Paint Varnishes and to Study Water in Paintings by Nuclear Magnetic Resonance Spectroscopy (NMR). Magnetochemistry, 6(2), 21. https://doi.org/10.3390/magnetochemistry6020021