Abstract
Two mononuclear ReIV complexes of general formula (PPh4)2[ReX6] [PPh4+ = tetraphenylphosphonium cation, X = Br (1) and I (2)] have been prepared and structurally and magnetically characterised. Both compounds crystallise in the triclinic system with space group Pī. Their structures are made up of hexahalorhenate(IV), [ReX6]2−, anions, and bulky PPh4+ cations. Each ReIV ion in 1 and 2 is six-coordinate and bonded to six halide ions in a quasi regular octahedral geometry. In their crystal packing, the [ReX6]2− anions are well separated from each other through the organic cations, generating alternated anionic and cationic layers, and no intermolecular Re−X···X−Re interactions are present. Variable-temperature dc magnetic susceptibility measurements performed on microcrystalline samples of 1 and 2 show a very similar magnetic behaviour, which is typical of noninteracting mononuclear ReIV complexes with S = 3/2. Ac magnetic susceptibility measurements reveal the slow relaxation of the magnetisation in the presence of external dc fields for 1 and 2, hence indicating the occurrence of the field-induced single-ion magnet (SIM) phenomenon in these hexabromo- and hexaiodorhenate(IV) complexes.
1. Introduction
The last decade has witnessed a rapid advance in the development of mononuclear Single-Molecule Magnets (SMMs), the so-called Single-Ion Magnets (SIMs), which are mainly discrete molecules that are based on one paramagnetic and highly anisotropic ion belonging mainly to the d-block or f-block metals and displaying slow relaxation of the magnetisation [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. These nanosized magnetic systems are often considered to be promising candidates for future technological applications, such as high-density information storage or quantum computing at the molecular level, among others [15,16].
In comparison, SIMs containing 4d/5d metal ions have been far less investigated than their 3d-based analogues [17,18,19]. Indeed, the first SIMs based on a 5d metal ion, namely, the (NBu4)2[ReX4(ox)] systems (X = Cl and Br; ox = oxalate anion), were published by some of us in 2013 [20]. Just one year later, Pedersen et al. reported the research work that deals with the study of the (PPh4)2[ReF6]·2H2O compound [21]. Afterwards, the photoluminescent (Th2imH)2[ReCl6] complex (Th2imH = protonated bisthienylethene) and the (NBu4)2[ReCl4(CN)2]·2DMA (DMA = N,N-dimethylacetamide) compound, that as the previously reported 5d-SIMs display field-induced slow magnetic relaxation, were also studied and published [22,23]. These last systems complete the short list of SIMs that are based on the highly anisotropic ReIV metal ion. Recently, additional 5d-SIMs containing the OsV and IrIV metal ions have also been reported [24,25,26].
We have studied further hexahalorhenate(IV) salts of the PPh4+ cation to develop our investigation in 5d-SIMs. Thus, by means of this bulky cation, the well-known intermolecular Re-X···X-Re (X = halide ion) interactions, with pathways that can mediate significant magnetic exchanges between neighbouring [ReX6]2− complexes in the crystal lattice, hence annulling the SIM behaviour of such systems, are avoided [18,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42].
Herein, we report the preparation, crystal structures, and magnetic properties of two mononuclear ReIV complexes of general formula (PPh4)2[ReX6] [PPh4+ = tetraphenylphosphonium cation, X = Br (1) and I (2)], moreover studying the effect of the halide ligand and the crystal packing on the magnetisation relaxation dynamics.
2. Results and Discussion
2.1. Description of the Crystal Structures
The two compounds (1 and 2) crystallise in the triclinic system with space group Pī (Table 1). Their structures are made up of hexahalorhenate(IV) [ReX6]2− [X = Br (1) and I (2)] anions and bulky PPh4+ cations, which are mainly held by electrostatic forces along with weak intermolecular X···H-C and X···π interactions. In their asymmetric unit, fragments of [ReX6]2− anions and entire PPh4+ cations are present (Figure 1).

Table 1.
Summary of the crystal data and structure refinement parameters for 1 and 2.
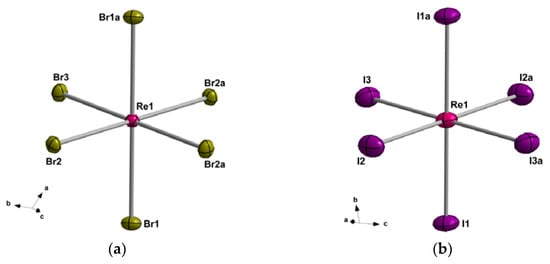
Figure 1.
Perspective view of the [ReBr6]2− and [ReI6]2− anions of the compounds 1 (a) and 2 (b). The PPh4+ cations have been omitted for clarity. The thermal ellipsoids are depicted at the 50% probability level.
In 1 and 2, each ReIV ion is six-coordinate and bonded to six halide ions in a quasi regular octahedral geometry. The Re-X bond lengths vary in the ranges of 2.498(1)–2.526(1) Å and 2.710(1)–2.746(1) Å for 1 and 2, respectively. The values of the X(1)-Re-X(2) and X(2)-Re-X(3) angles cover the ranges of 90.1(1)–91.6(1)° (1) and 88.3(1)–91.7(1)° (2). These structural data are in agreement with those that were previously reported for salts containing the anionic [ReX6]2− units [27,28,29,30,31,32,33,34,35,36,37,38,39]. The PPh4+ cations in 1 and 2 counterbalance the negative charges and show values of C-C and C-P bond lengths typical of the phenyl groups linked to the central phosphorous atom.
In the crystal packing of 1 and 2, the [ReX6]2− anions are well separated from each other through the bulky PPh4+ cations, which generate alternated anionic and cationic layers, respectively (Figure 2a,b). The shortest Re···Re separation is ca. 10.43 (1) and 10.85 Å (2). The shortest X···X distance is approximately 6.52 and 5.93 Å for 1 and 2, respectively. It is worth pointing out that the [ReBr6]2− anions are arranged in a very similar way in 1, that is, with all of the anionic units orientated in the same direction, whereas the [ReI6]2− anions display different orientations in the crystal of 2 (Figure 2).
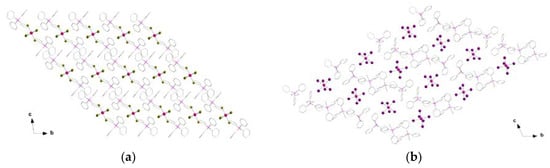
Figure 2.
View along the crystallographic a axis of a fragment of the crystal packing of 1 (a) and 2 (b) showing the arrangement of the [ReX6]2− anions (ball and stick model) and PPh4+ cations (wireframe model).
The PPh4+ cations in both of the compounds show several intermolecular phenyl–phenyl interactions that provide noticeable supramolecular conformations. Indeed, the sextuple phenyl embrace (SPE) supramolecular conformation is present in both 1 and 2 (Figure S1) [43,44,45]. The shortest P···P distance between PPh4+ cations displaying the SPE conformation is ca. 6.07 and 6.13 Å for 1 and 2, respectively. Additionally, weak intermolecular X···H-C (the shortest X···C distance being 3.66 (1) and 3.85 Å (2)) and X···π (halide-centroid distance of 3.66 (1) and 4.4 Å (2)) interactions between anions and cations contribute to stabilizing the crystal structure of the two compounds.
2.2. Magnetic Properties
2.2.1. Dc Magnetic Susceptibility
The magnetic properties of the reported compounds were studied through direct current (dc) magnetic susceptibility measurements, which were performed on the microcrystalline samples of 1 and 2 in the 2–300 K temperature range and under an external magnetic dc field of 0.5 T. Powder XRD previously confirmed the purity and homogeneity of these bulk samples (Figure S2). Both of the compounds exhibit a very similar behaviour that is represented in the form of χMT versus T plots (χM being the molar magnetic susceptibility per ReIV ion) for 1 and 2 in Figure 3. At room temperature, the χMT values are approximately 1.58 (1) and 1.54 cm3mol−1K (2), which fall into the range that was reported for systems containing a magnetically isolated ReIV (S = 3/2 with g = 1.80–1.90) metal ion [18,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. Upon cooling, the χMT values for these compounds decrease first slowly and, around 70–80 K, they decrease faster, reaching minimum values of approximately 0.95 (1) and 0.96 cm3mol−1K (2) at 2.0 K. These minimum values obtained at very low temperature are close to that expected for a magnetically isolated ReIV ion (ca. 1.0 cm3mol−1K), as previously reported [18]. The decrease of the χMT values observed for complexes 1 and 2 is mainly due to zero-field splitting (ZFS) effects, which are very significant in ReIV-based systems [18,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42].
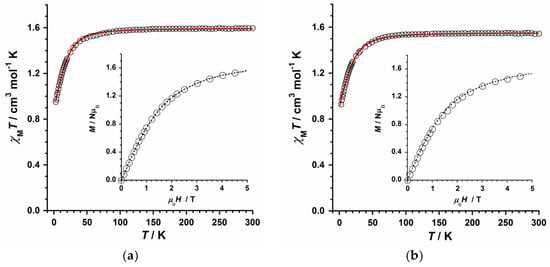
Figure 3.
Thermal variation of the χMT product for 1 (a) and 2 (b). The solid red line represents the theoretical fit of the experimental data and the inset shows the M versus H plot at 2.0 K obtained for 1 and 2, the dashed line being a guide to the eye.
A field dependence of the molar magnetisation (M) plot for 1 and 2 at 2.0 K is given in the respective insets of Figure 3a(1),b(2). In all the cases, the M values display a continuous increase with the applied magnetic field, the higher M values being 1.57 (1) and 1.53 µB (2) at 5.0 T, which are in agreement with those of similar mononuclear ReIV complexes that were reported in the literature [18,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. Given that no significant intermolecular interactions are observed in the crystal structures of 1 and 2, as indicated in the structure description, the shortest intermolecular Re-X···X-Re distances are covering the range of 5.93–6.52 Å, we have performed the treatment of the experimental data of the χMT versus T plots through the anisotropic Hamiltonian of Equation (1) (where Ŝz is the easy-axis spin operator, H is the applied field, β is the Bohr magneton, g is the Landé factor, and D is the ZFS for the ReIV ion).
By assuming that g‖ = g⊥ = g for the two complexes, we have fitted the experimental magnetic susceptibility data of the compounds 1 and 2, affording the following parameters: |D| = 22.0(2) cm−1, g = 1.84(2) with R = 5.6 × 10−5 for 1 and |D| = 24.0(2) cm−1, g = 1.82(2) with R = 2.4 × 10−5 for 2 (R being the agreement factor defined as Σi[(χMT)iobs − (χMT)icalc]2/[(χMT)iobs]2). The solid red line, indicating the fit in Figure 3a(1),b(2), matches quite well the experimental curves in both cases. The g and D values that are computed for 1 and 2 are in agreement with those calculated for previously reported mononuclear ReIV complexes [18,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. The |D| value that is obtained for 2 is somewhat greater than that of the complex 1.
2.2.2. Ac Magnetic Susceptibility
Alternating current (ac) magnetic susceptibility measurements were performed on microcrystalline samples of 1 and 2 in the temperature range of 2–7 K and in a 3.5 G ac field oscillating at different frequencies. In both cases, no out-of-phase ac signals (χ″M) are observed at Hdc = 0 G. However, out-of-phase ac signals, with observable χ″M maxima, take place at low temperatures in 1 and 2 when an external dc magnetic field (Hdc = 1000 and 5000 G) is applied (Figure 4 and Figure 5). This magnetic behaviour would indicate that the two studied systems (1 and 2) exhibit slow relaxation of the magnetisation and, therefore, single-ion magnet (SIM) phenomenon [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21]. Nevertheless, the relaxation dynamics that the two compounds exhibit is not equally affected by the external dc magnetic fields. While the Hdc = 5000 G seems to be optimal for compound 1 (with the presence of more χ″M maxima that shift towards higher frequencies), this magnetic field results in being less useful for studying the magnetic relaxation in 2, where a decrease of the number of χ″M maxima in the χ″M versus ν plot occurs (Figure 4 and Figure 5). In both 1 and 2, the intensity of the χ″M peaks increases with increasing the external dc magnetic field.
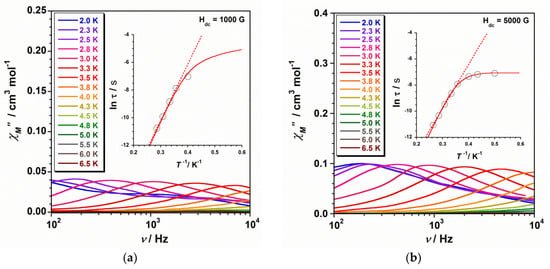
Figure 4.
Frequency dependence of the out-of-phase ac susceptibility signals under dc fields of 1000 (a) and 5000 G (b) for compound 1. The respective inset shows the ln(τ) versus 1/T plot for 1 with the fit to the Arrhenius law (dashed line) and the fit considering several mechanisms (solid line).
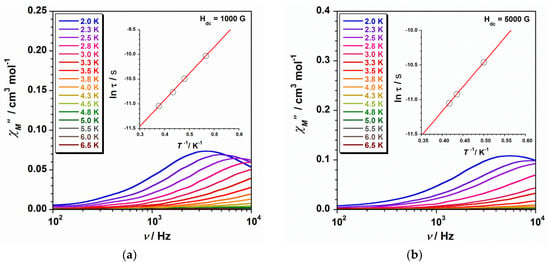
Figure 5.
Frequency dependence of the out-of-phase ac susceptibility signals under dc fields of 1000 (a) and 5000 G (b) for compound 2. The respective inset shows the ln(τ) versus 1/T plot for 2 with the fit performed through the Arrhenius law (solid line) corresponding to the Orbach mechanism.
The insets in Figure 4 and Figure 5 show the ln(τ) versus 1/T plot for complexes 1 and 2, respectively. In the high-temperature region, the experimental data that were obtained from the frequency-dependent χ″M peaks draw a straight line pretty much in both cases along the ranges of ca. 0.25–0.35 K−1 for 1 ca. 0.35–0.50 K−1 for 2. Consequently, these experimental data were fitted to the Arrhenius equation (τ = τoexp(Ueff/kBT), where τo is the preexponential factor, τ is the relaxation time, Ueff is the anisotropy (effective) energy barrier to the magnetisation reorientation, and kB is the Boltzmann constant) by considering that the magnetisation relaxation only involves an Orbach process [12]. In this way, we can evaluate the Ueff and τo parameters in this region for 1 and 2 and compare their values with those previously reported. These fits are indicated as dashed lines in the insets of Figure 4 and Figure 5, and Table 2 provides the Ueff and τo values thus obtained for 1 and 2.

Table 2.
Ueff and τo values obtained through the dc applied magnetic fields of 1000 and 5000 G and the Arrhenius law for 1 and 2.
The Ueff values for 1 are similar between them at both 1000 and 5000 G, and much higher (approximately five/six times) than that of compound 2 (Table 2). Besides, the energy barrier value of 2 remains practically unaffected with increasing the dc applied magnetic field. The τo parameter for 1 and 2 shows values that are in agreement with those that were reported for similar ReIV complexes displaying SIM behaviour [20,21,22,23].
In the low-temperature region of the ln(τ) versus 1/T plots, curved lines are only observed for 1, which are better defined when the Hdc = 5000 G is applied (Figure 4 and Figure 5). These features would account for the occurrence in such conditions of several relaxation processes, especially in compound 1. In consequence, the whole experimental curve from these ln(τ) versus 1/T plots was fitted through Equation (2), where four mechanisms for spin-lattice relaxation of magnetisation can be considered, namely, Orbach (τo−1exp(-Ueff/kBT)), direct (AT), Raman (CTn), and Quantum Tunnelling (QTM) [12].
All the four mechanisms were considered during the fitting process of the ln(τ) versus 1/T curve for 1 that was obtained at the optimal magnetic field of 5000 G, whereas in the case of the treatment of the experimental data acquired with Hdc = 1000 G, the fourth term (QTM) was kept equal to zero (Table 3). The least-squares fit of the experimental data of 1 through Equation (2) leads to the set of parameters listed in Table 3. From these results, it is worthy to point out that the Ueff values thus obtained for compound 1 are somewhat higher than those calculated through the Arrhenius law and listed in Table 2. Besides, the Ueff parameter for 1 remains higher than that of 2. Indeed, the effective energy barrier value obtained at the optimal dc field (Hdc = 5000 G) for 1 [43.3 K (30.1 cm−1)] is even larger than those previously reported for the hexahalo (PPh4)2[ReF6]·2H2O and (Th2imH)2[ReCl6] compounds [21,22]. The computed values of τo and τQTM for 1 agree with those that were reported for other 5d-SIMs [20,21,22,23]. On the other hand, the n values for 1 lie between 4.3 and 5.5 and fall into the range typical of metal ions with relaxation through optical and acoustic Raman-like process (n being equal to 9 for the Raman relaxation of Kramer ions) [12]. These n values are very close to those that were obtained for similar 5d-SIMs (Table 3) [24,25,26].

Table 3.
Parameters of the magnetic relaxation obtained through the dc applied magnetic fields of 1000 and 5000 G and the Equation (2) for 1.
The frequency-dependent ac magnetic susceptibility data of compounds 1 and 2 were modelled to give the Cole-Cole plots that are shown in Figure 6. The obtained values for the α parameter are in the ranges of 0.09–0.17 (1) and 0.06–0.11 (2), with these values suggesting a narrow distribution of the relaxation times for these mononuclear ReIV complexes [20,21,22,23,24,25].
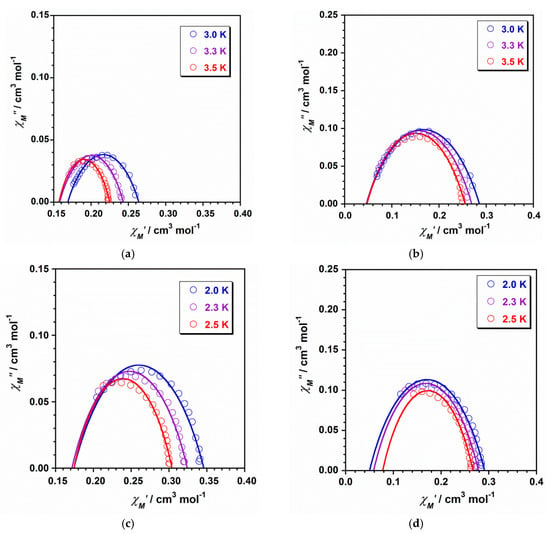
Figure 6.
Cole–Cole plots for 1 (a,b) and 2 (c,d) obtained from the experimental data of the out-of-phase ac susceptibility signals under dc fields of 1000 (a–c) and 5000 G (b–d).
3. Materials and Methods
3.1. Reagents and Instruments
All of the manipulations were performed under aerobic conditions, using materials as received (reagent grade). The ReIV precursors, namely, the K2ReBr6 and K2ReI6 salts, were prepared following the synthetic methods described in the literature [29,30].
Elemental analyses (C, H, N) were performed by the Central Service for the Support to Experimental Research (SCSIE) at the University of Valencia. Infrared spectra of 1 and 2 were recorded with a PerkinElmer Spectrum 65 FT-IR spectrometer in the 4000–400 cm−1 region. The powder X-ray diffraction (PXRD) patterns of 1 and 2 confirmed the homogeneity of their bulk samples (Figure S2). Variable-temperature, solid-state direct current (dc) magnetic susceptibility data down to 2.0 K were collected on a Quantum Design MPMS-XL SQUID magnetometer that was equipped with a 5 T dc magnet. Experimental magnetic data were corrected for the diamagnetic contributions of the involved atoms by using Pascal’s constants [46].
3.2. Preparation of the Compounds
3.2.1. Synthesis of (PPh4)2[ReBr6] (1)
PPh4Br (41.9 mg, 0.1 mmol) was dissolved in a 1.0 M HBr solution (5.0 mL) and slowly added to a solution of K2ReBr6 (37.2 mg, 0.05 mmol) dissolved in a 1.0 M HBr solution (25.0 mL). The thus generated yellow solid was filtered off and then washed with cold isopropanol and diethyl ether. Orange crystals of 1 were grown in a MeCN:isopropanol (1:2, 20.0 mL, v/v) mixture. Yield: ca. 92.0%. Anal. Calcd. for C48H40P2Br6Re (1): C, 42.88 and H, 3.00. Found: C, 43.02 and H, 2.96. IR peaks (KBr pellets, ν/cm−1): 3055(m), 1584(m), 1482(m), 1439(s), 1107(s), 996(m), 722(vs), 690(s), and 529(vs).
3.2.2. Synthesis of (PPh4)2[ReI6] (2)
The synthesis of 2 was very similar to that of 1. PPh4I (46.6 mg, 0.1 mmol) was dissolved in a 1.0 M HI solution (5.0 mL) and slowly added to a solution of K2ReI6 (0.30 mg, 0.05 mmol) that was dissolved in a 1.0 M HI solution (25.0 mL). A brown solid was formed, which was filtered off and washed with cold isopropanol and diethyl ether. Dark-purple crystals of 2 were grown in a CH3CN:CH2Cl2 (1:1, 30.0 mL, v/v) mixture. Yield: ca. 80.0%. Anal. Calcd. for C48H40P2I6Re (2): C, 35.45 and H, 2.48. Found: C, 35.53 and H, 2.42. IR peaks (KBr pellets, ν/cm−1): 3051(m), 1584(m), 1482(m), 1435(s), 1107(s), 996(m), 722(vs), 689(s), and 526(vs).
3.3. X-ray Data Collection and Structure Refinement
X-ray diffraction data from single crystals of dimensions 0.28 × 0.26 × 0.20 (1) and 0.18 × 0.13 × 0.08 mm3 (2) were collected on a Bruker D8 Venture diffractometer with graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å). Crystal parameters and refinement results for 1 and 2 are summarized in Table 1. The data were processed through SAINT [47] reduction and SADABS [48] multi-scan absorption software. The structure was solved with the SHELXS structure solution program through the Patterson method. The model was refined with version 2013/4 of SHELXL against F2 on all data by full-matrix least squares [49,50,51]. In the two samples, all non-hydrogen atoms were anisotropically refined. All of the hydrogen atoms of the PPh4+ cations were set in calculated positions and refined isotropically by using the riding model. The graphical manipulations were performed with the DIAMOND program [52]. The CCDC codes are 1956543 and 1956544 for 1 and 2, respectively.
4. Conclusions
In summary, the X-ray structures and magnetic properties of two mononuclear ReIV complexes, of general formula (PPh4)2[ReX6] [PPh4+ = tetraphenylphosphonium cation, X = Br (1) and I (2)], have been reported. In their crystal lattices, the paramagnetic [ReX6]2− anions are well separated from each other by means of the bulky PPh4+ cations and no significant intermolecular Re−X···X−Re interactions are present. The study of the relaxation dynamics reveals that 1 and 2 are not equally affected by the external dc magnetic fields, the bromo-derivative complex 1 exhibiting the higher value of the energy barrier (Ueff) for the reverse of the magnetisation in this family of hexahalo [ReX6]2− compounds. Indeed, the Ueff value for 1 is higher than those previously reported for ReIV-based SIMs. Hence, the information generated by these results could be very useful in designing future magnetic materials that are based on ReIV SIMs.
Supplementary Materials
The following are available online at https://www.mdpi.com/2312-7481/6/2/20/s1, Figure S1: Sextuple phenyl embrace (SPE) supramolecular conformation of the PPh4+ cations, Figure S2: Plots of the theoretical and experimental XRD patterns profile.
Author Contributions
Conceptualization, R.G. and J.M.-L.; funding acquisition, R.G., F.L. and J.M.-L.; methodology, C.R.-D.; A.S.-P.; M.O.-A.; N.M. and J.M.-L.; investigation, C.R.-D.; A.S.-P.; M.O.-A.; N.M.; F.L. and J.M.-L.; formal analysis, C.R.-D.; A.S.-P.; M.O.-A.; N.M. and J.M.-L.; writing-original draft preparation, R.G. and J.M.-L.; writing-review and editing, R.G. and J.M.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministerio de Ciencia e Innovación (Spain, grant numbers MDM-2015-0538 and CTQ2016-75068P) and the Agencia Nacional de Investigación e Innovación (Uruguay, grant number FCE-1-2017-1-136539).
Acknowledgments
A.S.-P., M.O.-A. and J.M.-L. thank the Spanish “FPU fellowships”, “FPI fellowships” and “Ramón y Cajal” Programmes, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Craig, G.A.; Murrie, M. 3d single-ion magnets. Chem. Soc. Rev. 2015, 44, 2135–2147. [Google Scholar]
- Frost, J.M.; Harrimana, K.L.M.; Murugesu, M. The rise of 3-d single-ion magnets in molecular magnetism: Towards materials from molecules? Chem. Sci. 2016, 7, 2470–2491. [Google Scholar]
- Colacio, E.; Ruiz, J.; Ruiz, E.; Cremades, E.; Krzystek, J.; Carretta, S.; Cano, J.; Guidi, T.; Wernsdorfer, W.; Brechin, E.K. Slow Magnetic Relaxation in a CoII–YIII Single-Ion Magnet with Positive Axial Zero-Field Splitting. Angew. Chem. Int. Ed. 2013, 52, 9130–9134. [Google Scholar]
- Vallejo, J.; Pascual-Álvarez, A.; Cano, J.; Castro, I.; Julve, M.; Lloret, F.; Krzystek, J.; De Munno, G.; Armentano, D.; Wernsdorfer, W.; et al. Field-induced hysteresis and quantum tunneling of the magnetization in a mononuclear manganese(III) complex. Angew. Chem. Int. Ed. 2013, 52, 14075–14079. [Google Scholar]
- Zadrozny, J.M.; Xiao, D.J.; Atanasov, M.; Long, G.J.; Grandjean, F.; Neese, F.; Long, J.R. Magnetic blocking in a linear iron(I) complex. Nat. Chem. 2013, 5, 577–581. [Google Scholar]
- Rechkemmer, Y.; Breitgoff, F.D.; van der Meer, M.; Atanasov, M.; Hakl, M.; Orlita, M.; Neugebauer, P.; Neese, F.; Sarkar, B.; van Slageren, J. A four-coordinate cobalt(II) single-ion magnet with coercivity and a very high energy barrier. Nat. Commun. 2016, 7, 10467. [Google Scholar]
- Atzori, M.; Tesi, L.; Benci, S.; Lunghi, A.; Righini, R.; Taschin, A.; Torre, R.; Sorace, L.; Sessoli, R. Quantum Coherence Times Enhancement in Vanadium(IV)-based Potential Molecular Qubits: The Key Role of the Vanadyl Moiety. J. Am. Chem. Soc. 2017, 139, 4338–4341. [Google Scholar]
- Liu, J.; Chen, Y.-C.; Liu, J.-L.; Vieru, V.; Ungur, L.; Jia, J.-H.; Chibotaru, L.F.; Lan, Y.; Wernsdorfer, W.; Gao, S.; et al. A Stable Pentagonal Bipyramidal Dy(III) Single-Ion Magnet with a Record Magnetization Reversal Barrier over 1000 K. J. Am. Chem. Soc. 2016, 138, 5441–5450. [Google Scholar]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. A Dysprosium Metallocene Single-Molecule Magnet Functioning at the Axial Limit. Angew. Chem. Int. Ed. 2017, 56, 11445–11449. [Google Scholar]
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular magnetic hysteresis at 60 kelvin in dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäk, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar]
- McAdams, S.G.; Ariciu, A.-M.; Kostopoulos, A.K.; Walsh, J.P.S.; Tuna, F. Molecular single-ion magnets based on lanthanides and actinides: Design considerations and new advances in the context of quantum technologies. Coord. Chem. Rev. 2017, 346, 216–239. [Google Scholar]
- Canaj, A.B.; Dey, S.; Regincós Martí, E.; Wilson, C.; Rajaraman, G.; Murrie, M. Insight into D6h Symmetry: Targeting Strong Axiality in Stable Dysprosium(III) Hexagonal Bipyramidal Single-Ion Magnets. Angew. Chem. Int. Ed. 2019, 58, 1–7. [Google Scholar]
- Escalera-Moreno, L.; Baldoví, J.J.; Gaita-Ariño, A.; Coronado, E. Exploring the High-Temperature Frontier in Molecular Nanomagnets: From Lanthanides to Actinides. Inorg. Chem. 2019, 58, 11883–11892. [Google Scholar]
- Miller, J.S.; Gatteschi, D. Molecule-based magnets. Chem. Soc. Rev. 2011, 40, 3065–3066. [Google Scholar]
- Troiani, F.; Affronte, M. Molecular spins for quantum information technologies. Chem. Soc. Rev. 2011, 40, 3119–3129. [Google Scholar]
- Wang, X.-Y.; Avendaño, C.; Dunbar, K.R. Molecular magnetic materials based on 4d and 5d transition metals. Chem. Soc. Rev. 2011, 40, 3213–3238. [Google Scholar]
- Martínez-Lillo, J.; Faus, J.; Lloret, F.; Julve, J. Towards multifunctional magnetic systems through molecular-programmed self assembly of Re(IV) metalloligands. Coord. Chem. Rev. 2015, 289, 215–237. [Google Scholar]
- Ferrando-Soria, J.; Vallejo, J.; Castellano, M.; Martínez-Lillo, J.; Pardo, E.; Cano, J.; Castro, I.; Lloret, F.; Ruiz-García, R.; Julve, M. Molecular magnetism, quo vadis? A historical perspective from a coordination chemist viewpoint. Coord. Chem. Rev. 2017, 339, 17–103. [Google Scholar]
- Martínez-Lillo, J.; Mastropietro, T.F.; Lhotel, E.; Paulsen, C.; Cano, J.; De Munno, G.; Faus, J.; Lloret, F.; Julve, M.; Nellutla, S.; et al. Highly Anisotropic Rhenium(IV) Complexes: New Examples of Mononuclear Single-Molecule Magnets. J. Am. Chem. Soc. 2013, 135, 13737–13748. [Google Scholar]
- Pedersen, K.S.; Sigrist, M.; Sørensen, M.A.; Barra, A.-L.; Weyhermüller, T.; Piligkos, S.; Thuesen, C.A.; Vinum, M.G.; Mutka, H.; Weihe, H.; et al. [ReF6]2−: A Robust Module for the Design of Molecule-Based Magnetic Materials. Angew. Chem. Int. Ed. 2014, 53, 1351–1354. [Google Scholar] [CrossRef]
- Gong, D.-P.; Chen, J.-F.; Zhao, Y.; Cao, D.-K. Dalton Trans. 2016, 45, 3443–3449.
- Feng, X.; Liu, J.-L.; Pedersen, K.S.; Nehrkorn, J.; Schnegg, A.; Holldack, K.; Bendix, J.; Sigrist, M.; Mutka, H.; Samohvalov, D.; et al. Multifaceted magnetization dynamics in the mononuclear complex [Re IV Cl 4 (CN) 2] 2−. Chem. Commun. 2016, 52, 12905–12908. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Bendix, J.; Tressaud, A.; Durand, E.; Weihe, H.; Salman, Z.; Morsing, T.J.; Woodruff, D.N.; Lan, Y.; Wernsdorfer, W.; et al. Iridates from the molecular side. Nat. Commun. 2016, 7, 12195. [Google Scholar]
- Sanchis-Perucho, A.; Martínez-Lillo, J. Ferromagnetic exchange interaction in a new Ir(IV)-Cu(II) chain based on the hexachloroiridate(IV) anion. Dalton Trans. 2019, 48, 13925–13930. [Google Scholar] [CrossRef]
- Su, Q.-Q.; Fan, K.; Huang, X.-D.; Xiang, J.; Cheng, S.-C.; Ko, C.-C.; Zheng, L.M.; Kurmood, M. Field-induced slow magnetic relaxation in low-spin S = 1/2 mononuclear osmium(V) complexes. Dalton Trans. 2020, 49, 4084–4092. [Google Scholar] [CrossRef]
- Woodall, C.H.; Craig, G.A.; Prescimone, A.; Misek, M.; Cano, J.; Faus, J.; Probert, M.R.; Parsons, S.; Moggach, S.; Martínez-Lillo, J.; et al. Pressure induced enhancement of the magnetic ordering temperature in rhenium(IV) monomers. Nat. Commun. 2016, 7, 13870. [Google Scholar] [CrossRef]
- Chiozzone, R.; González, R.; Kremer, C.; De Munno, G.; Cano, J.; Lloret, F.; Julve, M.; Faus, J. Synthesis, Crystal Structure, and Magnetic Properties of Tetraphenylarsonium Tetrachloro(oxalato)rhenate(IV) and Bis(2,2‘-bipyridine)tetrachloro(μ-oxalato)copper(II)rhenium(IV). Inorg. Chem. 1999, 38, 4745–4752. [Google Scholar] [CrossRef]
- González, R.; Chiozzone, R.; Kremer, C.; De Munno, G.; Nicolò, F.; Lloret, F.; Julve, M.; Faus, J. Magnetic Studies on Hexaiodorhenate(IV) Salts of Univalent Cations. Spin Canting and Magnetic Ordering in K2[ReI6] with Tc = 24 K. Inorg. Chem. 2003, 42, 2512–2518. [Google Scholar] [CrossRef]
- González, R.; Chiozzone, R.; Kremer, C.; Guerra, F.; De Munno, G.; Lloret, F.; Julve, M.; Faus, J. Magnetic Studies on Hexahalorhenate(IV) Salts of Ferrocenium Cations [Fe(C5R5)2]2[ReX6] (R = H, CH3; X = Cl, Br, I). Inorg. Chem. 2004, 43, 3013–3019. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Armentano, D.; De Munno, G.; Lloret, F.; Julve, M.; Faus, J. A Two-Dimensional ReIVAgI Compound: X-ray Structure and Magnetic Properties. Cryst. Growth Des. 2006, 6, 2204–2206. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Armentano, D.; De Munno, G.; Marino, N.; Lloret, F.; Julve, M.; Faus, J. A self-assembled tetrameric water cluster stabilized by the hexachlororhenate(IV) anion and diprotonated 2,2′-biimidazole: X-ray structure and magnetic properties. CrystEngComm 2008, 10, 1284–1287. [Google Scholar] [CrossRef]
- Armentano, D.; Martínez-Lillo, J. Hexachlororhenate(IV) salts of ruthenium(III) cations: X-ray structure and magnetic properties. Inorg. Chim. Acta 2012, 380, 118–124. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Kong, J.; Julve, M.; Brechin, E.K. Self-Assembly of the Hexabromorhenate(IV) Anion with Protonated Benzotriazoles: X-ray Structure and Magnetic Properties. Cryst. Growth Des. 2014, 14, 5985–5990. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Pedersen, A.H.; Faus, J.; Julve, M.; Brechin, E.K. Effect of Protonated Organic Cations and Anion−π Interactions on the Magnetic Behavior of Hexabromorhenate(IV) Salts. Cryst. Growth Des. 2015, 15, 2598–2601. [Google Scholar] [CrossRef]
- Armentano, D.; Martínez-Lillo, J. Aquapentachlororhenate(IV): A singular and promising building block for metal assembly. RSC Adv. 2015, 5, 54936–54940. [Google Scholar] [CrossRef]
- Armentano, D.; Martínez-Lillo, J. Anion-Assisted Crystallization of a Novel Type of Rhenium(IV)-Based Salt. Cryst. Growth Des. 2016, 16, 1812–1816. [Google Scholar] [CrossRef]
- Pedersen, A.H.; Julve, M.; Brechin, E.K.; Martínez-Lillo, J. Self-assembly of the tetrachlorido (oxalato) rhenate (IV) anion with protonated organic cations: X-ray structures and magnetic properties. CrystEngComm 2017, 19, 503–510. [Google Scholar] [CrossRef]
- Pedersen, A.H.; Geoghegan, B.L.; Nichol, G.S.; Lupton, D.W.; Murray, K.S.; Martínez-Lillo, J.; Gass, I.A.; Brechin, E.K. Hexahalorhenate(IV) salts of metal oxazolidine nitroxides. Dalton Trans. 2017, 46, 5250–5259. [Google Scholar] [CrossRef]
- Armentano, D.; Barquero, M.A.; Rojas-Dotti, C.; Moliner, N.; De Munno, G.; Brechin, E.K.; Martínez-Lillo, J. Enhancement of Intermolecular Magnetic Exchange through Halogen Halogen Interactions in Bisadeninium Rhenium(IV) Salts. Cryst. Growth Des. 2017, 17, 5342–5348. [Google Scholar] [CrossRef]
- Rojas-Dotti, C.; Moliner, N.; González, R.; Martínez-Lillo, J. Hexakis(dimethylformamide)iron(II) complex cation in hexahalorhenate(IV)-based salts: Synthesis, X-ray structure and magnetic properties. J. Coord. Chem. 2018, 71, 737–747. [Google Scholar] [CrossRef]
- Armentano, D.; Sanchis-Perucho, A.; Rojas-Dotti, C.; Martínez-Lillo, J. Halogen⋯halogen interactions in the self-assembly of one-dimensional 2,2′-bipyrimidine-based CuIIReIV systems. CrystEngComm 2018, 20, 4575–4581. [Google Scholar] [CrossRef]
- Dance, I.; Scudder, M. Supramolecular Motifs: Concerted Multiple Phenyl Embraces between Ph4P+ Cations Are Attractive and Ubiquitous. Chem. Eur. J. 1996, 2, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Dance, I.; Scudder, M. Supramolecular motifs: Sextuple aryl embraces in crystalline [M(2,2′-bipy)3] and related complexes. J. Chem. Soc. Dalton Trans. 1998, 1341–1350. [Google Scholar] [CrossRef]
- Orts-Arroyo, M.; Castro, I.; Lloret, F.; Martínez-Lillo, J. Molecular Self-Assembly in a Family of Oxo-Bridged Dinuclear Ruthenium(IV) Systems. Cryst. Growth Des. 2020, 20, 2044–2056. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- SAINT. Bruker Analytical X-ray Systems; Version 6.45; SAINT: Madison, WI, USA, 2003. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl.Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- SHELXTL-2013/4. Structure Determination Software Programs; Bruker Analytical X-ray Instruments Inc.: Madison, WI, USA, 2013. [Google Scholar]
- DIAMOND 4.5.0. Crystal Impact GbR, Crystal Impact; Brandenburg GbR, Kreuzherrenstr: Bonn, Germany, 2018. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).