Abstract
The genus Morus L., mulberry, is an interesting taxonomic group on account of its existing genetic variability, functional food potential and commercial importance. Mulberry trees are found in a wide range of areas in Serbia, accounting for a large phenotypic diversity in its genetic resources. Tree and fruit characteristics of more than 300 mulberry specimens were surveyed, and 15 genotypes of Morus alba, Morus nigra and Morus rubra species were selected for further analyses. These were located at various sites in the province of Vojvodina, Serbia. The present study was undertaken to investigate the diversity of the collected material aiming to pre-select genotypes suitable for landscaping/ornamental and/or fruit production purposes. Genotypes BP 3/9, DT1, ZP3 and MR1 have semi-vigorous growth, dropping growth habits, different leaf shapes (ovate, oval, cordate) and leaf color (from light to dark green), corresponding to ornamental mulberries. In addition, the semi-vigorous genotype ZD1 with a spreading tree and interesting palmate-lobed leaves was distinguished as a unique genotype for landscaping purposes. The most vigorous annual shoot growth was detected in the ZP3 genotype (118.5 cm), followed by DT1 (108.2 cm), MR1 (101.8 cm) and ZP1 (100.5 cm) genotypes. Contrary, genotype DJ1 exhibited the lowest annual growth with only a 32.9 cm average length of the shoots. Due to the greater fruit mass (4.2–6.1 g), sweetness and acidity balance as well as chemical composition, genotypes BP 1/4, DJ1, MG, MR1, DT1 and ZP3 may be recommended for fresh consumption, while genotypes DJ1, DT1, MR1, ZD1, ZP1 and BP 3/9 could be appropriate for home processing. According to fruit chemical analyses, the most promising genotypes were MR1 and DT1 combining high soluble solids content (21.2% and 18.5%, respectively), total sugar content (17.41% and 15.20%, respectively) and ascorbic acid content (42.24 and 49.28 mg/%, respectively). Additionally, DT1 genotype was also characterized by the highest total phenolic content (221.08 mg 27 GAE/100 g fresh weight). The most ornamental genotypes from this study (BP 3/9, DT1, ZD1, ZP3 and MR1) combined with their pomological and chemical characterization can be recommended for edible gardening purposes due to both aesthetic appearance and nutritive value of the fruits.
1. Introduction
A wide variety of underutilized fruit crops (including Morus sp.), which are neither commercially cultivated nor traded on a large scale, are mainly grown, commercialized and consumed locally [1]. Less known or underutilized fruit species are distributed in different parts of the world in temperate, subtropic and tropic conditions. They are attracting more and more popularity, and the number of studies on these species has increased, due to their edible as well as ornamental properties. These fruits often possess a high content of non-nutritive, nutritive, and bioactive compounds that are vital for human health. They are also accepted as healthy food because of the growth and harvest from natural populations or rural areas without the use of chemical fertilizers or pesticides. Exemplary studies include not only mulberries but carob tree (Ceratonia siliqua L.), honeysuckle (Lonicera caerulea L.) and wild sea buckthorn (Hippophae rhamnoides L.) that have distinct flavor and taste as well as excellent medical and aesthetic value [2,3,4,5].
Genus Morus is widespread in Asia, Europe, North and South America, and Africa. Mulberries can be found from between moderate and sub-tropical regions of the northern hemisphere, to the tropical regions of the southern hemisphere, because they can grow in a wide range of climatic, topographic and soil conditions. They are spread in all regions, from the tropics to the sub-Arctic, and from sea level to an altitude of 4000 m [1]. Mulberry is grown particularly in China, India, and Japan for sericulture, as the silkworms (Bombyx mori L.) feed exclusively on leaves of the Morus genus. In China, there are over a thousand mulberry cultivars available for sericulture originating from four main species, i.e., M. alba, M. multicaulis, M. bombycis and M. atropurpurea. Mulberry is also used as forage in animal production along with mainly fruit production for human consumption in Balkan countries [6,7]. In Turkey, mulberries have been cultivated for more than 400 years with predominant species of Morus alba (95%), 3% M. rubra (3%) and M. nigra (2%) [8], commonly eaten fresh, dried, or processed into molasses and jam for its delicious taste, pleasing color, low-calorie content, and high nutrient content [9].
The genetic diversity of mulberry in Northern Serbia, province of Vojvodina, is reflected in a large number of solitary trees that have never been investigated for their breeding potential. The expansion of mulberries in Vojvodina started in the XVII century. Later, former Yugoslavia was the fifth most important region for silk production with more than 2.5 million white mulberry trees during the 30 s of the XX century. In that time, in Serbia, mulberry trees were mainly grown for sericulture, but mulberry as an edible ornamental species was often planted in green belts, green corridors between villages, as well as in parks, alleys, or solitary trees in various green areas. The mulberry used for decorative purposes is often a grafted species with a hanging or pyramidal shape [10], interesting in landscape motives of urban and rural greenery. Coining the term ‘ornafruits’, Sahin [11] best described the potential of such greenery, referring to the fruit species that can be used for both fruit production and ornamental purposes. According to the same author, mulberries specifically can be used as a fast-growing tree cultivated for horticultural, pharmaceutical, industrial, and ornamental purposes, among which mulberries characterized by a weeping canopy are commonly used in landscape areas. In addition to the weeping form, M. alba var. tortuosa as a tortuous type can be applied as a small specimen tree or as the central element of garden compositions [12]. Owing to its drought hardiness and low maintenance cost, black mulberry is perfect for edible landscaping in changing environmental conditions [13]. While searching for appropriate tree species for urban habitats affected by climate change, Roloff et al. [14] suggested white mulberry as very suitable and black mulberry as suitable for novel plannings in cities. Investigating the profitability of food trees planted in urban public green areas, (providing the physiological, sociological, economic and aesthetic benefits), Lafontaine-Messier et al. [15] found black mulberry as a ‘value’ tree. In a Montreal case study, surveying public urban fruit trees including mulberries, Colinas et al. [16] showed that similarly to community gardens and food foraging, public fruit production could serve as a potentially cost-effective means of improving the resilience and pro-environmental behavior of communities. Suggesting species for edible landscaping in urban horticulture, Fetouh [17] listed white and black mulberry among species with multiple functions, such as for food, flavor, and ornamental appearance. Additionally, Poguberović et al. [18] suggested the usage of mulberry leaves as extracts for the preparation of environmentally friendly, non-toxic and low-cost adsorbents to remediate urban pollutants Ni(II) and Cu(II) from aqueous solutions, due to their efficiency as well as mulberry tree abundance and easiness to be found in Vojvodina. Although highly represented in both public and private green areas in Serbia, mulberries are predominantly preferred for fruits [19,20], mainly in private gardens.
Berry fruits have received great attention over the past decade since many studies have highlighted their ability to impact human health and reduce the risk of certain types of diseases [21] and the effect of aging. Berries provide significant health benefits because of their high levels of polyphenols, vitamins, minerals, and fiber [22,23]. Many studies revealed that mulberry is a good source of bioactive compounds such as carotenoids, alkaloids [1,24,25], phenolics, vitamins, fatty acids and minerals [7,26,27,28,29]. Antioxidant, antimicrobial and anticarcinogenic activities are mainly linked to the presence of phenolics [30].
The present research targets morphological traits of vegetative growth parameters, pomological and chemical evaluation of selected mulberry genotypes to determine the genotypes most suitable for ornamental gardening purposes, with one word coined as ‘ornafruits’. Thus, the objective of the present study was to evaluate Morus species from the Northern region of Serbia and to identify the genotypes with both superior landscaping (ornamental) and pomological (functional food) characteristics.
2. Materials and Methods
2.1. Plant Material
More than 300 mulberry genotypes growing in comparable environmental conditions were visited, and preliminary tree and fruit characteristics were qualitatively scored in situ, by the researcher panel consisting of landscape architects, horticulturalists, pomologists and breeders. The dendrological analysis included qualitative parameters—tree vigor (low, semi or vigorous), tree habit (upright, spreading or dropping), rotten trunk and branches presence (present or absent), phytopathological and entomological damages (present or absent), vitality and decorative value. Scores for vitality and decorativeness were provided by panelists according to the five-level scale (1—very low, 2—low, 3—medium, 4—high and 5—very high). Mulberry genotypes of specific overall appearance and observed morphological properties (highly scored for interesting growth habit, absence of disease and pest symptoms, decorativeness and vitality) were pre-selected from their in situ locations. The core collection in this survey resulted in 15 genotypes (Table 1) selected from various locations in the province of Vojvodina, Serbia, situated mainly in small private rural front and backyards as well as both rural and urban ornamental front yard gardens (Table 1). One exception was the genotype JP2 selected from the public school front yard.

Table 1.
Sampling locations in Northern Serbia and list of Morus species studied.
Edible mulberry germplasm genotypes from Northern Serbia were identified and classified into three species M. alba, M. nigra and M. rubra, of which the majority belonged to M. alba. Vegetative parts for analysis were sampled in June, after intensive vegetative growth completion. Research related to morphological and pomological traits was conducted at the Faculty of Agriculture in Novi Sad, while the chemical investigation of fruits and determination of the content of specific compounds were performed at the Institute for Medicinal Plants Research ‘Dr Josif Pančić’ in Belgrade. For this research, berries were picked at the biologically ripe stage, determined by the harvesting easiness (resistance of the fruit stem to the fruit pulling) as well as fruit softness. Harvest time was determined between the 10th and 15th of June, depending on the genotype. Since two genotypes—BPJ and SK1—proved to be male specimens, further fruit analysis was not performed, resulting in 13 genotypes pomologically described.
2.2. Morphometric Analyses of Vegetative Parts and Fruit
Vegetative samples were collected after intensive spring growth was finished and the final size was achieved. Fruits were collected in their full ripening stage and transported to the laboratory in a hand refrigerator. Vegetative and pomological characterization of genotypes was carried out according to the international FAO descriptor [31] as in Kadri et al. [32]. Measurement of appropriate parameters was conducted on 15 samples of each genotype. The following morphometric and pomological parameters were studied: annual branches length (cm), branch thickness (mm), internodes length (cm), leaf length (cm), leaf width (cm), leaf shape index, petiole length (cm) and width (mm), a mass of ten leaves (g), fruit mass (g), fruit width (mm) and height (mm), peduncle length (mm) and width (mm) and fruit shape index. In addition, dry matter content in fruits was determined (%). Petiole length (cm) and width (mm), fruit height and width (mm) as well as peduncle length (mm) and width (mm) were measured by a Mitutoyo digital caliper (accuracy of ±0.01 mm), while dry matter content (%) was determined using a handheld E-Line refractometer ‘ATC Range’.
2.3. Chemical Analysis of Mulberry Fruits
The chemical composition of fruits from selected genotypes was also analyzed. Fruit samples were used to determine total soluble solids content (SSC), total acidity (TA), total sugar content, reducing sugar, sucrose, ascorbic acid, total anthocyanin content (TAC) and total phenolic contents (TPC). Soluble solids (SS) content was determined by a refractometer (Atago, pocket PAL-1. Kyoto, Japan). Titratable acidity (TA) was determined by titrating 10 g of berries with 0.1 N NaOH up to pH 7.0. Acidity was expressed as the percent of malic acid. An iodometric titration method was performed for the determination of ascorbic acid (AA), and the results were expressed as milligram ascorbic acid/100 g fresh mass. Total sugars (TS), inverted sugar (IS) and sucrose (S) were determined by the Luff–Schoorl method in %. The total phenolic content was estimated by Folin–Ciocalteu method with slight modifications [33]. Juice samples (10 g equivalent of fresh berry) were extracted with MeOH for 30 min on the ultrasonic bath and then filtered. Two hundred microliters of extract was added to 1 mL of 1:10 diluted Folin–Ciocalteu reagent. After 4 min, 800 μL of sodium carbonate (75 g/L) was added. After 2 h of incubation at room temperature, the absorbance at 765 nm was measured. Gallic acid (0–100 mg/L) was used for the calibration of a standard curve. The results were expressed as milligrams of gallic acid equivalents per 100 grams of fresh mass (mg GAE/100 g FW). Total anthocyanin content was investigated according to the procedure described in European Pharmacopoeia 9.0 (E.R 9.0) with slight modifications. Then, 95 mL of methanol was added to 10 g of berry juice and mechanically stirred for 30 min, and then filtered into a 100 mL volumetric flask. The filter was rinsed and diluted to 100 mL with methanol. A 50-fold dilution of this solution in a 0.1% v/v solution of hydrochloric acid in methanol was prepared. The absorbance of the solution was measured at 528 nm, using a 0.1% v/v solution of hydrochloric acid in methanol as the compensation liquid.
2.4. Statistical Analysis
The obtained data were statistically processed by the analysis of variance, using STATISTICA 14 software (Tibco, Palo Alto, CA, USA). The significance of differences between the mean values of investigated parameters was determined by Duncan’s multiple range tests with the confidence of p ≤ 0.05.
3. Results and Discussion
3.1. Vegetative Characterization of Investigated Mulberry Genotypes
Given the horticultural and phytonutrient traits of mulberries, it is important to examine morpho-chemical characteristics of selected genotypes, before their selection, registration and propagation. In our country, there are no varieties selected based on combined nutritional and horticultural values, nor are there varieties recommended for commercial orchard cultivation. Hence, the complete morphological and pomological characterization of selected genotypes may serve the purpose of selecting interesting varieties such as fruits or ‘ornafruits’ [11]. Meena et al. [34] suggested mulberry among others (karonda, custard apple, khejri, mulberry, etc.) as suitable for backyard or kitchen gardening. Following the mentioned, qualitative vegetative growth characteristics were observed to select genotypes with an overall attractive appearance (Table 2). Genotypes BP 3/9, DT1, ZP3 and MR1 have semi-vigorous growth, dropping growth habits, different leaf shapes (ovate, oval, cordate) and leaf color (from light to dark green), corresponding to ornamental mulberries. Semi-vigorous genotype ZD1 with a spreading tree and interesting palmate-lobed leaves were distinguished as a unique genotype for edible gardening or landscaping purposes.

Table 2.
Tree and leaf qualitative characteristics of mulberry genotypes.
To provide a complete picture of the morphological properties of the collected material, vegetative parts were analyzed. According to the data, variations were noticed among and within genotypes for some of the traits (Table 3). The most vigorous annual shoot growth was detected in the ZP3 genotype (118.5 cm), followed by DT1 (108.2 cm), MR1 (101.8 cm) and ZP1 (100.5 cm) genotypes. Contrary, genotype DJ1 exhibited the lowest annual growth with only a 32.9 cm average length of the shoots. The coefficient of variation CV over 40% for annual branches clearly shows that there were variations among genotypes, and high standard deviation (SD) indicates variations within genotypes. When observed between species, M. alba genotypes had smaller mean values (65.2 cm) followed by M. nigra (74.4 cm), compared to one M. rubra (101.8 cm). The widest average branch thickness was determined for ZP1 (8.1 cm), followed by ZP2, MG1 and ZP1 with 5.5, 5.2 and 5.1 cm, respectively. The average internode length ranged from 2.8 cm (DJ1) to 8.3 cm (DT1), with an absolute maximum of 10.4 cm in ZP3 and an absolute minimum of 2.0 cm in DJ1. The highest average value for leaf length was determined for ZP1 (18.1 cm), followed by MG1 (17.9 cm), ZP2 (17.2 cm) and PB1 (16.4 cm). The widest average value for leaf width was determined for the same genotypes, ZP1 (11.1 cm), followed by MG1 (14.1 cm), ZP2 (12.4 cm), SK1 (11.8 cm) and PB1 (11.2 cm). Mean averages for leaf length and leaf width amounted to 14.1 and 10.9 cm (respectively), with an absolute maximum of 22.0 and 21.0 cm for leaf length and leaf width determined in genotype ZP1.

Table 3.
Morphometric characteristics of branches and leaves sampled from studied genotypes.
Ten leaf masses followed the same trend as determined for leaf length and width. The greatest average value for ten leaves mass was determined in ZP1 (40.1 g), followed by MG1 (34.9 cm), ZP2 (28.8 cm) and PB1 (23.8 cm). An absolute maximum of 51.4 g was determined for ZP1 and an absolute minimum of 6.2 g for ZD1. It was previously reported by Pentón et al. [35] that the genus Morus is known for variations of morphometric traits, which our study confirmed.
The diversity of studied genotypes is confirmed through most studied traits, and it is reflected in large differences in the mean values, a wide range between absolute minimum and absolute maximum values and a high coefficient of variation. Genetic variability among genotypes can be attributed to the heterozygote nature of genotypes [36]. Investigating the morphological variation of 110 M. alba genotypes, Farahani et al. [37] determined that most of the traits exhibited significant differences among the studied genotypes. On the contrary, the most stable parameter was the leaf shape index (quantitative ratio between leaf height and width) for which the standard deviation (SD) was 0.2, and the coefficient of variation (CV) was 14.6%. In general, leaf shape (Figure 1) as a qualitative characteristic is considered a stable taxonomic characteristic most frequently used for plant determination; thus, its stability is presumed to be high within the genotypes of the same species, previously confirmed for cherries [38] and sweet potato [39]. In mulberries, Lo Bianco and Mirabella [40] successfully used leaf morphometric descriptors, images and multivariate analysis for discriminating mulberry cultivars. Although mulberry leaves are heteromorphic, accompanied by qualitative characterization (margins incision, leaf blade smoothness, leaf blade shininess and similar), quantitate measurements can help in the determination of true-to-type genotypes. In a very recent study [41], high heritability (h2) was recorded for mulberry leaf properties, ranging from 0.95 to 0.99.
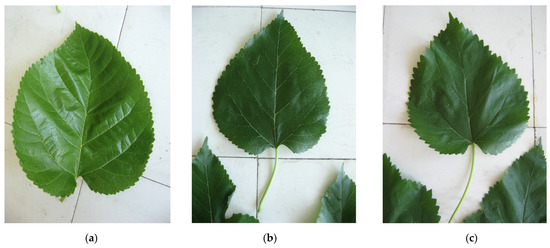
Figure 1.
Leaf shape of white mulberry—DJ1 ‘ovate’ (a), black mulberry—ZP3 ‘cordate’ (b) and red mulberry—MR1 ‘cordate’ (c).
3.2. Pomological Characterization of Investigated Mulberry Genotypes
Results obtained for the characterization of fruit mass, height and width, fruit shape index, peduncle length and width as well as dry matter content are presented in Table 4 and Figure 2. Fruit mass, as one of the most important agronomic traits and one of the main objectives of selection, significantly varied among genotypes, with a high coefficient of variation (43.7%). Previously, Özgen et al. [42] reported a lower coefficient of variation (CV) for fruit mass up to 26% of M. alba, M. rubra and M. nigra genotypes from Turkey, indicating a possible greater genetic distance of pertinent genotypes from Serbia. Fruit mass ranged from 1.4 g (DT1) to 6.1 g (DJ1), with an average of 3.5 g. An average value over 4.00 g was achieved in genotypes MG1, MR1, PB1 and BP1/4. The absolute maximum for fruit mass appeared among samples from genotype DJ and amounted to 8.3 g, while an absolute minimum of 1.1 g appeared among samples from genotype DT1. Our pomological results were comparable with previously provided data from Turkish [7,42] and Spanish [43] researchers with a remark that there were samples with fruit mass values (absolute minimum and absolute maximum) lower and higher than those previously reported ranges. Some of our genotypes even surpassed the results obtained in the most recent studies, including mulberry selections with 0.75–5.02 g [37,44] as well as the commercial cultivar ‘Chinese Long’ with 6.26 g [45]. Our results showed that fruits of M. rubra can reach a maximal 6.4 g, and an average of 4.9 g, corroborating the previous results of Ercisli and Orhan [7] and Özgen et al. [42], with the fruit mass of M. alba, M. rubra and M. nigra genotypes in the ranges of 2.14–4.37 g and 3.3–8.2 g, per a study, respectively. Sanchez et al. [43] reported that M. nigra genotypes had bigger fruits, while in the pertinent study, black mulberry achieved above-average results. Differences in results are not surprising considering genotypic and different environmental effects.

Table 4.
Pomological traits of Morus sp. genotypes.
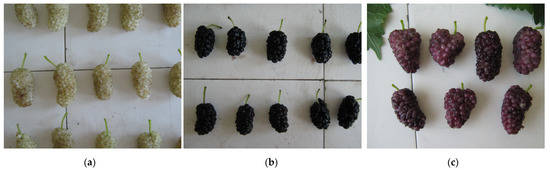
Figure 2.
Fruit (berry) appearance of white mulberry—DJ1 (a), black mulberry—ZP3 (b) and red mulberry—MR1 (c).
Considering the mean values of fruit height, genotypes DJ1 and MG1 were distinguished by 33.2 and 34.6 mm (respectively), again surpassing the results of previously mentioned fruit investigations [7,42,43]. The average for all genotypes’ fruit width was 14.0 mm and ranged from 10.2 (DT1) to 17.6 mm (DJ1). An absolute maximum for a fruit width of 21.3 mm was noted within samples of genotype DT1. Özgen et al. [42] reported fruit height and width in the ranges of 23.6–35.0 and 16.1–21.1 mm, respectively, while the range for fruit height was 20.5–30.3 mm in M. alba and M. nigra [43]. Our M. alba genotypes had higher values of fruit height compared to the Spanish type. Significant variations among genotypes considering peduncle length and width were noted according to high coefficients of variation. Dry matter content ranged from 11.7% (MN1) to 24.9% (ZD1) with an average of 16.5%, while the absolute maximum was 28.2% (ZD1) and the minimum was 11.2% (MN1). These results are similar to Farahani et al. [37] who found a variation from 9.3–32%.
3.3. The Chemical Composition of Fruits Belonging to Selected Genotypes
Although fruit samples were collected and immediately stored in a hand refrigerator until reaching the laboratory, some perishable genotypes showed a fast decline and very weak transportability and thus were excluded from further analysis. The antioxidant activities of mulberry fruits and their link to the presence of phenolics have been investigated and confirmed by various researchers in previous studies [20,46,47]. As high phenolic content and high antioxidant activity increase the nutritive and phytomedicinal potentials of mulberry, it was important to examine the chemical value of the fruits from the selected genotypes. High values of fruit mass do not necessarily mean high content of bioactive compounds, and in that sense, it is not the only important trait to rely on when it comes to selection. Table 5 provides the results of the fruit chemical analysis for genotypes MN1, ZP3, DT1, MR1, ZP2, ZP1 and BP 3/9. Soluble solids content (SSC) in white mulberry genotypes ranged from 11.5 (MN1) to 15.8% (ZP3). The highest SSC (21.2%) was associated with purple fruits belonging to red mulberry genotype MR1. These results are in agreement with previously reported data [7,42], although some studies had shown that purple mulberry fruits had lower soluble solid content than white and black mulberry [7,26]. The variation of SSC in mulberry fruits could be a result of the heterozygote nature of genotypes and the effect on different environmental conditions where the genotypes were grown [36]. The highest values for the SSC besides MR1 were determined for DT1 and BP 3/9. Since genotypes MR1, DT1 and BP 3/9 also had a high amount of dry matter content 19.8%, 19.1%, and 16.9%, respectively (determined by hand refractometer), those could be recommended for processing.

Table 5.
Chemical composition of investigated mulberry fruits.
The total acidity (TA) ranged from 0.13 to 0.43%. TA of black mulberry genotypes was between 0.40 and 0.43%, whereas these values were between 0.13 and 0.38% in white mulberry genotypes. The TA of purple mulberry MR1 was 0.19%. Purple mulberry had lower acidity compared to the majority of the investigated black and white mulberry genotypes. Previous reports showed higher values of total acidity in other regions [1,7,26,30,42]. Considering SSC and acidity together, genotypes DT1 and ZP3 may be recommended for fresh fruit production since they have attractive dark-colored fruits in combination with high fruit SSC and TA, which gives them a pleasant taste.
The highest amount of total sugar content (TSC) was associated with the purple mulberry fruits of MR1 (17.41%). Furthermore, M. alba genotypes appear to have a higher amount of sugar (12.08%-15.20%) than M. nigra genotypes (8.97%-12.08%). A similar trend was also observed for reducing sugar. Elmacı and Altuğ [48] reported for M. nigra that sugar content ranged from 11.3% to 16.2%, which was comparable to our results, while Imran et al. [1] found a lower amount of sugar in mulberry fruits. The presence of important amounts of sugars in mulberries should encourage their use as natural sugar sources in different food recipes. Sucrose ranged from 1.05 (MR1) to 2.09% (BP 3/9). Sucrose is present in low amounts due to the state of maturity of the berries, as the concentration of sucrose decreases regularly in mulberry fruits as maturity progresses [43].
A proper equilibrium between organic acids and sugars is essential for the pleasing flavor of fruits. Organic acids have an important influence on taste because their presence reduces the sweetness of fruits, exposing their sourness. The content of ascorbic acid ranged from 17.60 (ZP1) to 49.28 mg/% (DT1) and was variable in genotypes within and among genotypes. Considerably different contents of ascorbic acid have been reported by various authors [7,36,42,49]. The differences between genotypes in terms of the content of ascorbic acid might be caused by genetic factors as well as ecological factors—temperature, light, humidity, etc. [49]. Genotypes DT1, MR1 and ZP3 are characterized by a great amount of ascorbic acid, which indicates their selective value. The mulberry fruit is consumed in both fresh and processed forms. Traditionally, mulberry fruits are used for making juices, syrup, compotes, jams and fruit brandy (‘rakija’). Fruits that are used for making fruit brandy and jams are supposed to satisfy some sensory properties in terms of taste and aroma intensity, and genotypes should be high yielding. Genotypes with very sweet fruits and strong aromas are preferred. Genotypes that fulfill most of these terms are DJ, DT, MR, ZD1 and ZP2.
Anthocyanin pigments in mulberry fruits have a dual role. They affect the coloration of fruits during the last ripening stage and are considered secondary metabolites with potential nutritional value. Anthocyanins are considered very good antioxidant agents with high activity, which is attributed to their peculiar structure [27]. Results showed that three black-colored fruits contained anthocyanins (TAC) in the range of 0.05–0.12%. These genotypes also showed higher amounts of total phenolic content (147.84–221.08 mg GAE/100 g fresh weight), several times higher than the content of phenolics found in the pink- and white-colored mulberries. Pink- and white-colored mulberry samples were characterized by the absence of anthocyanins, and total phenolic content (TPC) values ranged from 16.51 to 63.23 mg GAE/100 g fresh weight.
The variation of TPC in the fruits depends on many factors, such as the degree of maturity at harvest, genetic differences, and environmental conditions during fruit development. According to our results, the highest content of total phenolics was found in the black-colored white mulberry genotype DT1 (TPC = 221.08 mg GAE/100 g fresh mass), while the lowest TPC value was obtained for the white-colored white mulberry genotype BP 3/9 (TPC = 16.51 mg GAE/100 g fresh mass). A previous study on M. alba grown in Vojvodina, North Serbia, reported higher TPC (43.84 to 326.29 mg GAE/100 g frozen fruit) [20], while a study of M. nigra from Southeast Serbia found lower TPC (90.26–118.84 mg GAE/ 100 g fresh mass) [25] compared to the present study. The TPC of M. nigra selected from a natural population in the vicinity of Belgrade, Serbia, was 177.51 mg GAE/100 g frozen fruit [29]. When compared to other publications, the investigated genotypes contained a significantly lower amount of total phenolics in fruits [1,7,27,36,42]. Although, phenolic compounds could be considered the main bioactive compounds contributing to the antioxidant activity of mulberry fruits, our chemical analyses showed that the most promising genotypes were MR1 and DT1 combining high SSC, total sugar content and ascorbic acid content. Genotype DT1 also had the highest TPC, although it is characterized by a small fruit mass due to the vigorousness of its tree and high yielding.
Fruit properties (both appearance and nutritive value) are important when deciding which species or cultivars to apply for edible landscaping (or urban foraging) purposes. According to Al-Mayahi et al. [50], the top gardening motives in Oman were esthetic, shading, the joy of hobby, functional-food source, physical exercise and environmental protection.
Homan [51] stated that well-known examples of urban foraged plants are mulberries as well as blackberries, apples, acorns and sweet chestnuts. Quantifying the nutritive value of four common urban species (serviceberry, mulberry, apple and black walnut), Bunge et al. [52] noted that urban foraging is an under-explored aspect of the alternative functional food movement.
Getting the general public accustomed to edible ornamentals as well as their nutritional values might increase their usage. Haight [53] concluded that only half of the participants in their study knew that quince and mulberry are edible. Amani-Beni et al. [54] noted that the integration of fruit-bearing trees, including mulberries, in the Akbarieh garden in Iran was a functional-food urban gardening solution.
Besides rural gardens where traditionally vegetables, flowers, spices, aromatic and medicinal herbs, decorative dendrological species, fruit species and vines can be grown separately or in combination on a considerable share of land, urban gardening recently comes in a form of a small kitchen or even Nutri-garden. Nutri-gardens are advanced forms of kitchen gardens in which vegetables are grown along with fruit, herbs, spices and other useful plants such as medicinal plants as a supplementary source of food or small income [55]. With the proven content of reducing sugars, total anthocyanins- and phenolic-investigated mulberry genotypes can have added value to their decorativeness and can be a part of both rural and urban edible gardening.
4. Conclusions
Since mulberry fruits are a source of functional food with therapeutic and nutritional applications, breeding strategies and selection from the existing gene pool aim to distinguish genotypes with high ornamental and pomological quality. Genotypes BP 3/9, DT1, ZP3 and MR1 have semi-vigorous growth, dropping growth habits, different leaf shapes (ovate, oval, cordate) and leaf color (from light to dark green), corresponding to the ornamental aspects of mulberries. Combined with the pomological and chemical characterization, those genotypes can be recommended for edible gardening purposes due to both the aesthetic appearance and nutritive value of their fruits. Some selected mulberry genotypes showed promising and interesting physicochemical properties for both fresh consumption and processing. For instance, if the appropriateness for fresh consumption is based on ‘appearance’ (higher fruit mass and attractive color), the best genotypes would be BP 1/4, DJ1, MG and MR1. Considering SSC and acidity together, genotypes DT1 and ZP3 may also be recommended for fresh fruit production. However, if classification is based on high intensity of sweetness, the best cultivars would be DJ1, DT1, MR1, ZD1, and ZP1, while genotypes MR1, DT1 and BP 3/9, due to the high SSC and high amount of dry matter content, could be recommended for processing into juices, syrup, compotes, jams or fruit brandy. According to the fruit’s chemical composition and functional food value, the most promising genotypes were MR1 and DT1 where DT1 distinguishes itself for the highest content of total phenolics and total anthocyanins. Besides chemically analyzed genotypes, ones with more perishable fruits should not be discarded, but further studies are recommended for home gardening and edible landscaping purposes where fruits would be used for fresh consumption in the immediate residence vicinity.
Author Contributions
Conceptualization, M.L.; methodology, K.Š. and M.L.; formal analysis, G.Z., T.N., M.P. and S.M.; investigation, M.L. and S.B.; resources, M.K., M.L. and K.Š.; writing—original draft preparation, M.K., M.L. and K.Š.; writing—review and editing, M.L., S.B. and K.Š.; visualization, M.K., T.N. and M.P.; supervision, M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia, contract numbers 451-03-9/2021-14/200117 and 451-03-68/2022-14/200003. In addition, this manuscript covered one of the research topics conducted by the researchers gathered in the Center of excellence Agro-Ur-For at the Faculty of Agriculture in Novi Sad, supported by the Ministry of Science, Technological Development and Innovations, contract number 451-03-1627/2022-16/17.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Imran, M.; Khan, I.; Shah, M.; Khan, R.; Khan, F. Chemical composition and antioxidant activity of certain Morus species. J. Zhejiang Univ. Sci. B 2010, 11, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Eyduran, S.P.; Ercisli, S.; Akin, M.; Beyhan, Ö.; Geçer, M.K. Organic acids, sugars, vitamin c, antioxidant capacity, and phenolic compounds in fruits of white (Morus alba L.) and black (Morus nigra L.) mulberry genotypes. J. Appl. Bot. Food Qual. 2014, 88, 134–138. [Google Scholar]
- Bolaric, S.; Müller, I.D.; Vokurka, A.; Cepo, D.V.; Ruscic, M.; Srecec, S.; Kremer, D. Morphological and molecular characteri-zation of Croatian carob tree (Ceratonia siliqua L.) germplasm. Turk. J. Agric. For. 2021, 45, 807–818. [Google Scholar] [CrossRef]
- Grygorieva, O.; Klymenko, S.; Kuklina, A.; Vinogradova, Y.; Vergun, O.; Sedlackova, V.H.; Brindza, J. Evaluation of Lonicera caerulea L. genotypes based on morphological characteristics of fruits germplasm collection. Turk. J. Agric. For. 2021, 45, 850–860. [Google Scholar] [CrossRef]
- Ilhan, G.; Gundogdu, M.; Karlović, K.; Židovec, V.; Vokurka, A.; Ercişli, S. Main agro-morphological and biochemical berry characteristics of wild-grown Sea Buckthorn (Hippophae rhamnoides L. ssp. caucasica Rousi) genotypes in Turkey. Sustainability 2021, 13, 1198. [Google Scholar] [CrossRef]
- Singhal, B.K.; Khan, M.A.; Dhar, A.; Baqual, F.M.; Bindroo, B.B. Approaches to industrial exploitation of mulberry (Mulberry sp.) fruits. J. Fruit Ornam. Plant Res. 2010, 18, 83–99. [Google Scholar]
- Ericsli, S.; Orhan, E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 2007, 103, 1380–1384. [Google Scholar] [CrossRef]
- Ercisli, S. A short review of the fruit germplasm resources of Turkey. Genet. Res. Crop. Evol. 2004, 51, 419–435. [Google Scholar] [CrossRef]
- Can, A.; Kazankaya, A.; Orman, E.; Gundogdu, M.; Ercisli, S.; Choudhary, R.; Karunakaran, R. Sustainable mulberry (Morus nigra L., Morus alba L. and Morus rubra L.) production in Eastern Turkey. Sustainability 2021, 13, 13507. [Google Scholar] [CrossRef]
- Ljubojević, M.; Ognjanov, V.; Sentić, I.; Dulić, J. Fruit Species in Landscape Design (In Serbian: Voćne vrste u Pejzažnom Projektovanju); University of Novi Sad, Faculty of Agriculture: Novi Sad, Serbia, 2018. [Google Scholar]
- Sahin, M. Ornafruit: Fruit species for ornamental purposes. In Ornamental Plants: With Their Features and Usage Principles; Cig, A., Ed.; Iksad Publications: Ankara, Turkey, 2020; pp. 397–424. [Google Scholar]
- Ljubojević, M. Horticulturalization of the 21st century cities. Sci. Hortic. 2021, 288, 110350. [Google Scholar] [CrossRef]
- Ahlawat, T.; Patel, N.L.; Agnihotri, R.; Patel, C.R.; Tandel, Y. Black Mulberry (Morus nigra). In Underutilized Fruit Crops: Importance and Cultivation; Narendra Publishing House: Delhi, India, 2016; pp. 195–212. ISBN 978-93-86110-09-1. [Google Scholar]
- Roloff, A.; Korn, S.; Gillner, S. The Climate-Species-Matrix to select tree species for urban habitats considering climate change. Urban For. Urban Green. 2009, 8, 295–308. [Google Scholar] [CrossRef]
- Lafontaine-Messier, M.; Gélinas, N.; Olivier, A. Profitability of food trees planted in urban public green areas. Urban For. Urban Green. 2016, 16, 197–207. [Google Scholar] [CrossRef]
- Colinas, J.; Bush, P.; Manaugh, K. The socio-environmental impacts of public urban fruit trees: A Montreal case-study. Urban For. Urban Green. 2019, 45, 126132. [Google Scholar] [CrossRef]
- Fetouh, M.I. Edible landscaping in urban horticulture. In Urban Horticulture; Nandwani, D., Ed.; Springer: Cham, Switzerland, 2018; pp. 141–173. [Google Scholar]
- Poguberović, S.S.; Krčmar, D.M.; Dalmacija, B.D.; Maletić, S.P.; Tomašević-Pilipović, D.D.; Kerkez, D.V.; Rončević, S.D. Removal of Ni (II) and Cu (II) from aqueous solutions using ‘green’ zero-valent iron nanoparticles produced by oak and mulberry leaf extracts. Water Sci. Technol. 2016, 74, 2115–2123. [Google Scholar] [CrossRef]
- Bošnjaković, D.; Ognjanov, V.; Ljubojević, M.; Barać, G.; Predojević, M.; Mladenović, E.; Čukanović, J. Biodiversity of wild fruit species of Serbia. Genetika 2012, 44, 81–90. [Google Scholar] [CrossRef]
- Natić, M.M.; Dabić, Č.D.; Papetti, A.; Fotrić, M.M.; Ognjanov, V.; Ljubojević, M.; Tešić, L.Ž. Analysis and characterisation of phytochemicals in mulberry (Morus alba) fruits grown in Vojvodina, North Serbia. Food Chem. 2015, 171, 128–136. [Google Scholar] [CrossRef]
- Marchetti, L.; Truzzi, E.; Frosi, I.; Papetti, A.; Cappellozza, S.; Saviane, A.; Pellati, F.; Bertelli, D. In vitro bioactivity evaluation of mulberry leaf extracts as nutraceuticals for the management of diabetes mellitus. Food Funct. 2022, 13, 4344–4359. [Google Scholar] [CrossRef]
- Zhao, Y. Berry Fruit: Value-Added Products for Health Promotion; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Asano, N.; Yamashita, T.; Yasuda, K.; Ikeda, K.; Kizu, H.; Kameda, Y.; Kato, A.; Nash, R.J.; Lee, H.S.; Ryu, K.S. Polyhydroxylated alkaloids isolated from mulberry trees (Morus alba L.) and silkworms (Bombyx mori L.). J. Agric. Food Chem. 2001, 49, 4208–4213. [Google Scholar] [CrossRef]
- Kostić, D.A.; Dimitrijević, D.S.; Mitić, S.S.; Mitić, M.N.; Stojanović, G.S.; Živanović, A.V. A survey on macro-and micro-elements, phenolic compounds, biological activity and use of Morus spp. (Moraceae). Fruits 2013, 68, 333–347. [Google Scholar] [CrossRef]
- Ercisli, S.; Tosun, M.; Duralija, B.; Voća, S.; Sengul, M.; Turan, M. Phytochemical content of some black (Morus nigra L.) and purple (Morus rubra L.) mulberry genotypes. Food Technol. Biotech. 2010, 48, 102–106. [Google Scholar]
- Bae, S.H.; Suh, H.J. Antioxidant activities of five different mulberry cultivars in Korea. LWT-Food Sci. Technol. 2007, 40, 955–962. [Google Scholar] [CrossRef]
- Song, W.; Wang, H.J.; Bucheli, P.; Zhang, P.F.; Wei, D.Z.; Lu, Y.H. Phytochemical profiles of different mulberry (Morus sp.) species from China. J. Agr. Food Chem. 2009, 57, 9133–9140. [Google Scholar] [CrossRef]
- Pavlović, A.V.; Dabić, D.C.; Momirovic, N.M.; Dojčinović, B.P.; Milojković-Opsenica, D.M.; Tešić, Z.L.; Natić, M.M. Chemical composition of two different extracts of berries harvested in Serbia. J. Agr. Food Chem. 2013, 61, 4188–4194. [Google Scholar] [CrossRef] [PubMed]
- Calín-Sánchez, Á.; Martínez-Nicolás, J.J.; Munera-Picazo, S.; Carbonell-Barrachina, Á.A.; Legua, P.; Hernández, F. Bioactive compounds and sensory quality of black and white mulberries grown in Spain. Plant. Food Hum. Nutr 2013, 68, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Sohn, K.W. Conservation status of sericulture germplasm resources in the world. In Conservation Status of Mulberry (Morus spp.) Genetic Resources in the World; Agriculture and Consumer Protection FAO: Rome, Italy, 2003; Volume 43, p. 11. [Google Scholar]
- Kadri, A.; Saleh, S.; Elbitar, A.; Chehade, A. Genetic diversity assessment of ancient mulberry (Morus spp.) in Lebanon using morphological, chemical and molecular markers (SSR and ISSR). Adv. Hort. Sci. 2021, 35, 243–253. [Google Scholar] [CrossRef]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites; Blackwell Scientific: Hoboken, NJ, USA, 1994. [Google Scholar]
- Meena, V.S.; Gora, J.S.; Singh, A.; Ram, C.; Meena, N.K.; Pratibha; Rouphael, Y.; Basile, B.; Kumar, P. Underutilized fruit crops of Indian arid and semi-arid regions: Importance, conservation and utilization strategies. Horticulturae 2022, 8, 171. [Google Scholar] [CrossRef]
- Pentón, G.; Martín, G.; Pérez, A.; Noda, Y. Morphoagronomic performance of mulberry (Morus alba L.) varietes during the establishment. Pastos Forrajes 2007, 30, 315–325. [Google Scholar]
- Ercisli, S.; Orhan, E. Some physico-chemical characteristics of black mulberry (Morus nigra L.) genotypes from Northeast Anatolia region of Turkey. Sci. Hortic. 2008, 116, 41–46. [Google Scholar] [CrossRef]
- Farahani, M.; Salehi-Arjmand, H.; Khadivi, A.; Akramian, M. Chemical characterization and antioxidant activities of Morus alba var. nigra fruits. Sci. Hortic. 2019, 253, 120–127. [Google Scholar] [CrossRef]
- Ognjanov, V.; Ljubojević, M.; Ninić-Todorović, J.; Bošnjaković, D.; Barać, G.; Čukanović, J.; Mladenović, E. Morphometric diversity in dwarf sour cherry germplasm in Serbia. J. Hortic. Sci. Biotechnol. 2012, 87, 117–122. [Google Scholar] [CrossRef]
- Gupta, S.; Rosenthal, D.M.; Stinchcombe, J.R.; Baucom, R.S. The remarkable morphological diversity of leaf shape in sweet potato (Ipomoea batatas): The influence of genetics, environment, and G×E. New Phytol. 2020, 225, 2183–2195. [Google Scholar] [CrossRef] [PubMed]
- Lo Bianco, R.; Mirabella, F. Use of leaf and fruit morphometric analysis to identify and classify white mulberry (Morus alba L.) genotypes. Agriculture 2018, 8, 157. [Google Scholar] [CrossRef]
- Sharma, V.; Kumari, A.; Thakur, I.K.; Chandel, M.; Bhatia, A.K.; Kumar, A. Variations in morphometric characteristics of white mulberry (Morus alba L.). J. Pharmacogn. Phytochem. 2021, 10, 312–315. [Google Scholar]
- Özgen, M.; Güneş, M.; Akça, Y.; Türemiş, N.; Ilgin, M.; Kizilci, G.; Erdoğan, Ű.; Serçe, S. Morphological characterization of several Morus species from Turkey. Hort. Environ. Biotechnol. 2009, 50, 9–13. [Google Scholar]
- Sanchez, E.M.; Calin-Sanchez, A.; Carbonell-Barrachina, A.A.; Melgarejo, P.; Hernandez, F.; Martinez-Nicolas, J.J. Physicochemical characterization of eight Spanish mulberry clones: Processing and fresh market aptitudes. Int. J. Food Sci. Technol. 2014, 49, 477–483. [Google Scholar] [CrossRef]
- Hashemi, S.; Khadivi, A. Morphological and pomological characteristics of white mulberry (Morus alba L.) accessions. Sci. Hortic. 2020, 259, 108827. [Google Scholar] [CrossRef]
- Nayab, S.; Razzaq, K.; Ullah, S.; Rajwana, I.A.; Amin, M.; Faried, H.N.; Akhtar, G.; Khan, A.S.; Asghar, Z.; Hassan, H.; et al. Genotypes and harvest maturity influence the nutritional fruit quality of mulberry. Sci. Hortic. 2020, 266, 109311. [Google Scholar] [CrossRef]
- Chen, H.; Chen, J.; Yang, H.; Chen, W.; Gao, H.; Lu, W. Variation in total anthocyanin, phenolic contents, antioxidant enzyme and antioxidant capacity among different mulberry (Morus sp.) cultivars in China. Sci. Hortic. 2016, 213, 186–192. [Google Scholar] [CrossRef]
- Lee, Y.; Hwang, K.T. Changes in physicochemical properties of mulberry fruits (Morus alba L.) during ripening. Sci. Hortic. 2017, 217, 189–196. [Google Scholar] [CrossRef]
- Elmaci, Y.; Altuğ, T. Flavor evaluation of three black mulberry (Morus nigra) cultivars using GC/MS, chemical and sensory data. J. Sci. Food Agric. 2002, 82, 632–635. [Google Scholar] [CrossRef]
- Gundogdu, M.; Muradoglu, F.; Sensoy, R.I.G.; Yilmaz, H. Determination of fruit chemical properties of Morus nigra L., Morus alba L. and Morus rubra L. by HPLC. Sci. Hortic. 2011, 132, 37–41. [Google Scholar] [CrossRef]
- Al-Mayahi, A.; Al-Ismaily, S.; Gibreel, T.; Kacimov, A.; Al-Maktoumi, A. Home gardening in Muscat, Oman: Gardeners’ practices, perceptions and motivations. Urban For. Urban Green. 2019, 38, 286–294. [Google Scholar] [CrossRef]
- Homan, J. Edible Landscapes: Relocalising Food and Bringing Nature into North London. In Urban Biodiversity and Ecological Design for Sustainable Cities; Ito, K., Ed.; Springer: Tokyo, Japan, 2021. [Google Scholar]
- Bunge, A.; Diemont, S.A.W.; Bunge, J.A.; Harris, S. Urban foraging for food security and sovereignty: Quantifying edible forest yield in Syracuse, New York using four common fruit- and nut-producing street tree species. J. Urban Ecol. 2019, 5, juy028. [Google Scholar] [CrossRef]
- Haight, B.J. Lovely homegrown menus: Substituting beautiful edibles for ornamentals in residential landscapes. Ph.D. Thesis, Washington State University, Washington, DC, USA, 2006. [Google Scholar]
- Amani-Beni, M.; Xie, G.; Yang, Q.; Russo, A.; Khalilnezhad, M.R. Socio-cultural appropriateness of the use of historic Persian gardens for modern urban edible gardens. Land 2022, 11, 38. [Google Scholar] [CrossRef]
- Shubha, K.; Mukherjee, A.; Anand, S.; Koley, T.K.; Kumar, U. Nutri-garden for achieving Sustainable Development Goals (SDGs). Food Sci. Rep. 2020, 1, 25–27. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).