Abstract
Stauntonia obovatifoliola Hayata subsp. urophylla is a novel edible and healthy fruit in China, commonly known as “Jiuyuehuang” (September yellow). The fully ripe fruit of S. obovatifoliola subsp. urophylla has a soft fruit pulp texture, golden flesh, and sweet flavor which is very popular with the locals. In this paper, we have investigated the fruit appearance quality, physiochemical quality, and nutritional quality of S. obovatifoliola subsp. urophylla that was harvested at six stages (S1: 60 DAFB, S2: 90 DAFB, S3: 130 DAFB, S4: 160 DAFB, S5: 190 DAFB, S6: 205 DAFB). An increase in fruit size (including single fruit weight, fruit length, and fruit diameter) was related to the ripeness stage of fruit development. The total soluble solids, firmness, dry matter, sugar and starch showed remarkable changes as the fruit approached ripening (S5–S6 stage). The main sugar components in the fruit were fructose, glucose, and maltose. The contents of fructose, glucose, and total sugars in S. obovatifoliola subsp. urophylla fruit progressively increased from the S1 to the S6 stage while increasing sharply from the S4 to the S5 stage. As for the content of maltose and starch, they both showed an increasing trend from the S1 to the S4 stage but decreased sharply at the S5 stage. The vitamin B, vitamin C, total phenolics, total flavonoids, and amino acid levels showed an overall downward trend during fruit development. To our knowledge, this is the first study to compare the phytochemical characteristics, nutrient composition, and antioxidant content during the different fruit development stages. The results of this study may provide a scientific basis for clarifying the growth and development characteristics of S. obovatifoliola subsp. urophylla fruit and the further utilization of these excellent medicinal and edible germplasm resources.
1. Introduction
Stauntonia obovatifoliola Hayata subsp. urophylla (Hand.-Mazz.) H. N. Qin is a perennial woody liana. It belongs to the Stauntonia genus, has large and edible fruits, and is endemic to China [1]. The species is widely distributed in the Yangtze River basin provinces of China (Guangdong, Guangxi, Hunan, Jiangxi, Fujian, Zhejiang, etc.) (Figure 1), normally occurring at an altitude of 500–1500 m in forest edges, roadsides, or along streams in valleys [2]. Its fruits are usually called September yellow (Jiuyuehuang in Chinese) by local villagers, which is due to the fruit appearing yellow in color when ripe in Chinese lunar September [1]. The fully ripe fruit of S. obovatifoliola subsp. urophylla has a soft fruit pulp texture, golden flesh, and a sweet flavor, tasting like a mixture of persimmon and litchi. S. obovatifoliola subsp. urophylla fruits are rich in sugars, crude proteins, vitamins, amino acids, mineral elements, fat, total dietary fiber, and reducing sugars [3,4]. In addition to its edible value, S. obovatifoliola subsp. urophylla is also a traditional Chinese medicinal material. Its stems and fruits are rich in phenolics, flavonoids, triterpenes, sterides, and polysaccharides, and is usually used as an important medicinal material to treat rheumatic arthralgia, neuralgia, headache, heat strangury, trigeminal neuralgia, sciatica, and trauma pain [5,6,7,8,9]. These excellent nutritional and chemical compositions indicate that S. obovatifoliola subsp. urophylla is an alternative functional fruit that is worthy of development and utilization.
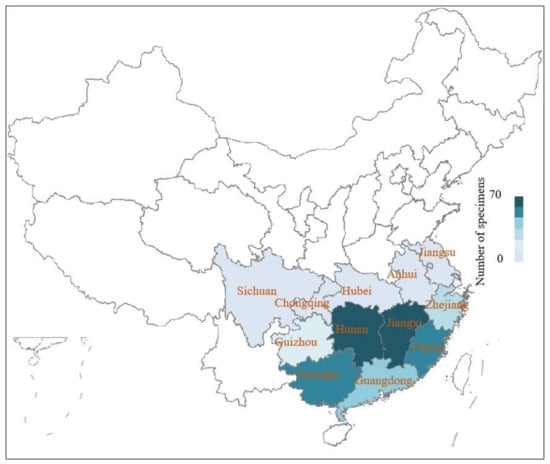
Figure 1.
The geographic distribution of Stauntonia obovatifoliola subsp. urophylla. The distribution heat map was made based on the specimen data of S. obovatifoliola subsp. urophylla in a Chinese virtual herbarium.
In recent years, S. obovatifoliola subsp. urophylla, as a characteristic health fruit, has been widely cultivated in China. Even so, S. obovatifoliola subsp. urophylla is still an underexploited wild fruit in the infancy stage of domestication. Only a few studies on S. obovatifoliola subsp. urophylla breeding, germplasm evaluation and cultivation have been attempted, with even most efforts on phytochemical analyses [1,3,5,10,11]. Moreover, most of the reports about S. obovatifoliola subsp. urophylla were found on the local flora with only a brief description [12,13,14,15,16]. The lack of information on the change patterns of the fruit’s physiological quality and physicochemical characteristics during fruit ripening impedes the development and utilization of S. obovatifoliola subsp. urophylla.
Fruit ripening is a very important physiological process in the later stage of fruit development which is usually accompanied by tremendous changes in the physical and chemical characteristics, such as sugar composition, fruit texture and color change, aromatic substances release, cell wall degradation, and so on, which ultimately affects the quality of fruit [17,18,19,20]. The analysis of these physical and chemical changes occurring during the development and ripening of fruit gives an insight into the underlying biochemical and physiological processes taking place [21] and is especially useful for determining fruit maturity. Although ripening is the process by which fruits attain their desirable color, flavor, nutritional quality, and textural properties, appropriate ripeness can effectively guarantee the commercial value of the fruit and prolong the storage time of the fruit. For instance, yellow peach will have a poor flavor if harvested too early, while the fruit can present a strong characteristic flavor when harvested late; however, if the olive fruit is harvested too late, the sugar content in the pulp will decrease and be prone to fibrosis [22,23]. In another example, in order to prolong the shelf life, winter jujube is usually harvested at the white maturity stage [24]. However, few studies are relevant to the change patterns in physicochemical indicators and nutrient composition of S. obovatifoliola subsp. urophylla at different fruit maturity stages. Therefore, it is necessary to explore the change patterns of S. obovatifoliola subsp. urophylla fruit’s physiological parameters and physicochemical characteristics during fruit ripening, which provide basic physiological information for a better understanding of the ripening process of S. obovatifoliola subsp. urophylla fruit.
Since the optimum harvesting period is a considerable evaluation criterion for both food industries and consumers, the aim of the present study was to investigate the changes in the physicochemical characteristics, antioxidant content, and nutritional composition of S. obovatifoliola subsp. urophylla fruit at different maturity stages. The results of this work provide the basic dynamic change patterns of the fruit’s physicochemical characteristics and explore a proper maturity stage at the harvest of S. obovatifoliola subsp. urophylla fruit with better quality, longer shelf life, and better market acceptability simultaneously.
2. Materials and Methods
2.1. Plant Materials
The plant materials were grown from seeds which were collected from Jiangxi Province, China, with relatively good comprehensive traits. A total of 56 plant materials were planted in 3 × 1.5 m in a fruit garden in Jiujiang City (29°38′ N, 115°59′ E), Jiangxi Province, China. The fruits of S. obovatifoliola subsp. urophylla free from insects, pests, and diseases were randomly harvested at six developmental stages (S1: 60 days after full bloom (DAFB), S2: 90 DAFB, S3: 130 DAFB, S4: 160 DAFB, S5: 190 DAFB, S6: 205 DAFB) until the fruits became fully ripe (S6 stage) in November (Figure 2). The S. obovatifoliola subsp. urophylla trees were four years old. Open pollination and a sprinkling irrigation system were used in the experimental farm. Due to a single tree of S. obovatifoliola subsp. urophylla being unable to provide enough fruit samples for all six stages, different trees were used at each stage in this experiment. In total, twenty fruit trees were used in this study, and ten fruits were harvested from each tree; thirty fruits were harvested at each stage. The fruits harvested from the same tree were considered as a sample at each stage. Three fruits’ pulp was mixed into one biological replicate for sugars, vitamin B, vitamin C, total phenolics, total flavonoids, starch, and amino acid content analysis. We cut the fruit into slices with a knife and removed the peel and seeds to separate the pulp, and then the pulp was quickly treated with liquid nitrogen and stored at −80 °C until further analysis. In total, three biological replicates were conducted for each stage. The first sampling time was in June 2021, and the last sampling time was in November 2021. In this sampling period, the average daily temperature in Jiujiang was 20–28 °C, and the total precipitation was 471.80 mm.

Figure 2.
The fruits of Stauntonia obovatifoliola subsp. urophylla at different maturity stages.
2.2. Determination of Fruit Physical Parameters
The single fruit weight, fruit length, fruit diameter, and fruit shape index were evaluated immediately after the fruit samples were picked. The single fruit weight was recorded using an electronic balance. The fruit length and fruit diameter were measured using a digital caliper, and the ratio of fruit length to fruit diameter was the fruit shape index. The firmness of S. obovatifoliola subsp. urophylla fruit was determined by using a digital fruit firmness tester (GY-4, Zhejiang, China) equipped with a 3.5 mm cylinder probe, and the results were expressed as kg/cm2. The dry matter content and moisture content in S. obovatifoliola subsp. urophylla fruit were measured by the drying–weighing method. Thin slices of fresh fruit were weighed using an electronic balance and then dried at 70 °C in an oven until their weights became constant. The dry matter content is calculated as the percentage of dry weight to fresh weight. Conversely, the moisture content is the percentage of lost weight to fresh weight.
2.3. Determination of Biochemical Parameters
The total soluble solids content (TSS) of S. obovatifoliola subsp. urophylla fruit was measured by an ATAGO (PAL-1) handheld digital refractometer, and the results were expressed in °Brix. The content of titratable acid (TA) was measured by the acid–base neutralization titration method, and the values were calculated as % of citric acid. The protein content in S. obovatifoliola subsp. urophylla fruit pulp was measured by the Kjeldahl method, and the results of protein content were expressed as N × 6.25 [25].
2.4. Determination of Carbohydrates
The sugar components (glucose, fructose, maltose, and sucrose) were measured by high-performance liquid chromatography (HPLC-E2695, Waters, Milford, MA, USA) equipped with a differential refraction detector, and the sample preparation and chromatographic conditions were conducted as described in the previous study [26].
The starch content of S. obovatifoliola subsp. urophylla fruit pulp was also determined by the method described in our previous study [26]. Briefly, approximately 1 g of fruit pulp samples and 20 mL of 80% ethanol were added together and vortex mixed to promote particle dispersion. Subsequently, the sample was incubated in the water bath at 80 °C for 30 min and stirred continuously until cooling. Then, the sample was filtered with 80% ethanol, and the filter residue was washed in a centrifuge tube by using hot distilled water and placed in a boiling water bath for gelatinization. Then, we added 2 mL of cold 9.2 mol/L perchloric acid and incubated for 15 min in a boiling water bath, and then filtered the mixture. The filtrate was poured into a 100 mL volumetric flask, and the filtrate residue was washed with distilled water. The absorbance was read at 490 nm by a differential refraction detector.
2.5. Determination of Vitamin B, Vitamin C, Total Phenolics, and Total Flavonoids
The Vitamin B1, Vitamin B2, Vitamin B3, Vitamin B6, and Vitamin C contents of S. obovatifoliola subsp. urophylla fruit pulp were measured by HPLC (HPLC-E2695, Waters, Milford, MA, USA) equipped with a diode array detector. Briefly, approximately 0.5 g of fruit pulp samples were weighed into a 15 mL centrifuge tube, and 5 mL of 0.1% hydrochloric acid aqueous solution was added. After 2 min of shock, the sample was extracted by ultrasonic wave (20 kHz, 30 °C) for 20 min and then centrifuged at a low temperature (4 °C) at a rate of 8000 r/min for 10 min. After the supernatant was filtered by a 0.45 μm filter membrane, then the sample was detected by HPLC. The detection conditions were as follows: a C18 chromatographic column (4.6 mm × 250 mm, 5 μm) was used for analysis; the mobile phase consisted of monopotassium phosphate and acetonitrile (80% and 20%, respectively) at a flow rate of 1.00 mL/min. The column temperature was 30 °C, and the injection volume was 10 µL.
The total phenolics content in the S. obovatifoliola subsp. urophylla fruit pulp was determined with Folin–Ciocalteu reagent following the method of Razzaq et al. [27]. Additionally, the total flavonoids content was measured following the method of Zhao et al. [28]. The detailed detection methods could be found in our previous study [26].
2.6. Determination of Amino Acids
The amino acid content in the S. obovatifoliola subsp. urophylla fruit pulp was measured by HPLC. Briefly, approximately 0.5 g of ground sample was put into the hydrolysis tube, 6 mL of 6 mol/L hydrochloric acid was added into the hydrolysis tube, and 3–4 drops of phenol were added, and then filled with high-purity nitrogen and sealed. The hydrolysis tube was placed in a constant temperature drying oven and hydrolyzed at 115 °C for 23 h. Then, we took out the hydrolysis tube from the oven and cooled it, adjusted the PH = 7 with sodium hydroxide solution, and added water to 7 mL for further analysis. Subsequently, 10 μL of extract solution was absorbed into the derivative tube, and 70 μL AccQ-Fluor borate buffer was added for vortex mixing. Then, another 20 μL AccQ-Fluor derivator was added to the tube, and vortex mixing was maintained for 10 s. We placed the tube at room temperature for one minute and then heated it in the oven at 55 °C for 10 min. Finally, we took out the hydrolysis tube from the oven and cooled it to ambient temperature, then transferred it to the fully recovered sample bottle for machine determination. AccQ·Tag column (3.9 × 150 mm) for amino acid analysis was used. The detection conditions were as follows, Mobile phase A: sodium acetate buffer solution; Mobile phase B: acetonitrile; Mobile phase C: pure water. Gradient elution was performed according to the Table 1.

Table 1.
The elution gradient of mobile phase.
The column temperature was 37 °C, and the injection volume was 10 µL. The excitation wavelength of the fluorescence detector was 250 nm, and the emission wavelength was 395 nm.
2.7. Statistical Analysis
SPSS v20.0 software was used to analyze the difference significance of the data (one-way ANOVA, LSD test, p < 0.05), and the results were expressed as means ± standard errors.
3. Results
3.1. Dynamic Changes of Appearance Quality during Fruit Development
The measurement results of the appearance quality of S. obovatifoliola subsp. urophylla fruit at different development stages were shown in Table 2. The single fruit weight began to increase rapidly at 60 DAFB, and the growth trend slowed down after 160 DAFB, entering the slow growth stage, and the single fruit weight increased to 192.72 ± 10.51 g during the period of the S1 to the S4 stages. As the fruit was close to ripening, the single fruit weight increased more slowly and had the highest weight value at the S6 stage. The growth patterns of the fruit length and fruit diameter were basically the same, and the rapid growth period of them was from 60 DAFB to 130 DAFB, and then they entered the slow growth period. The fruit shape index did not change much throughout the development period; the fruit length was about twice as long as the fruit diameter, indicating that the fruit length and fruit diameter developed simultaneously during the fruit growth.

Table 2.
Dynamic changes in appearance quality during S. obovatifoliola subsp. urophylla fruit development.
3.2. Dynamic Changes of Total Soluble Solids, Titratable Acidity, Dry Matter, and Fruit Firmness during Fruit Development
The total soluble solids content fluctuated below 2° Brix in the early stages (S1–S4). Whereas there were no significant differences between those stages, thereafter, the total soluble solids content of the fruit pulp increased sharply during the S5 and S6 stages and reached the maximum value of 13.52° Brix in the ripening stage S6 (Figure 3A). Although the titratable acidity content maintained an increasing trend during the whole fruit development period, the titratable acidity content of the S. obovatifoliola subsp. urophylla fruit maintained at a low level, and the values fluctuated in the range of 0.62–0.84% during the period of the S1 to the S6 maturity stage (Figure 3B).
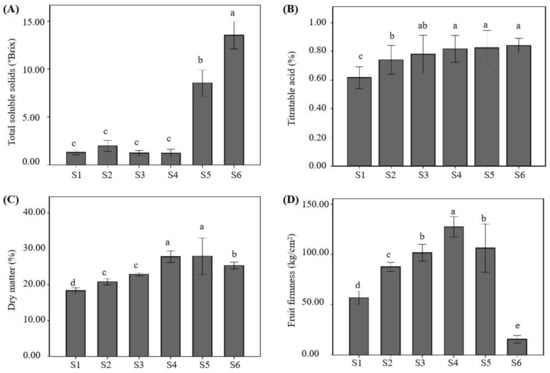
Figure 3.
Total soluble solids (A), titratable acidity (B), dry matter (C), and fruit firmness (D) in S. obovatifoliola subsp. urophylla fruit at different ripening stages. Means with different letters within the same fruit character indicate statistical differences at the p < 0.05 level using the LSD test. S1: 60 DAFB (days after full bloom), S2: 90 DAFB, S3: 130 DAFB, S4: 160 DAFB, S5: 190 DAFB, S6: 205 DAFB.
The content of dry matter content increased continuously in the early stage of fruit development (S1–S4) and reached the maximum value of 27.90% at the S4 stage but decreased slightly during the period from S5 to S6 (Figure 3C).
The fruit firmness of S. obovatifoliola subsp. urophylla was significantly (p < 0.05) affected by the fruit development stage. The fruit firmness also maintained an increasing trend during the early fruit development period and reached the maximum value of 127.53 kg/cm2 at the S4 stage but decreased sharply during the period from S5 to S6, and had a minimum value of 15.75 kg/cm2 at the S6 stage (Figure 3D).
3.3. Dynamic Changes of Carbohydrate Contents during Fruit Development
Carbohydrate contents in S. obovatifoliola subsp. urophylla fruit showed significant changes during fruit development and ripening (Figure 4). Only three (fructose, glucose, and maltose) of the four tested soluble sugars were detected in the fruit pulp of S. obovatifoliola subsp. urophylla, of which sucrose was not detected by HPLC. The fructose contents were very low before the S5 stage and fluctuated from 0.10 g/100 g FW to 0.33 g/100 g FW. As Figure 4A showed, the fructose content accumulated rapidly during the S5 and S6 stages and reached the maximum value of 5.35 g/100 g FW at the mature stage (S6). Similarly, the glucose showed the same accumulation pattern as the fructose, mainly accumulated during the S5 and S6 stages and reaching the maximum value of 7.02 g/100 g FW at the S6 stage (Figure 4B). The maltose content of S. obovatifoliola subsp. urophylla fruit continuously accumulated from the S1 to the S3 stage (0.72–1.22 g/100 g FW) and reached the maximum value at the S3 stage, although it had a slight decrease at the S4 stage, whereafter, a sharp and significant decline during the S5 and S6 stages were detected (0.16 g/100 g FW and 0.08 g/100 g FW, respectively) (Figure 4C). The contents of the total sugars of the S. obovatifoliola subsp. urophylla fruit were relatively low and changed little in the early stages of fruit development (S1–S4) and began to increase significantly from the S5 stage and reached the maximum value at the S6 stage (12.45 g/100 g FW) (Figure 4D). The starch content of S. obovatifoliola subsp. urophylla fruit continuously accumulated from the S1 to the S4 stage (0.21–1.09 g/100 g FW) and reached the maximum value at the S4 stage, but had a sharp decrease at the S5 stage, and finally reached the minimum value at the mature stage (S6) (0.07 g/100 g FW) (Figure 4E).
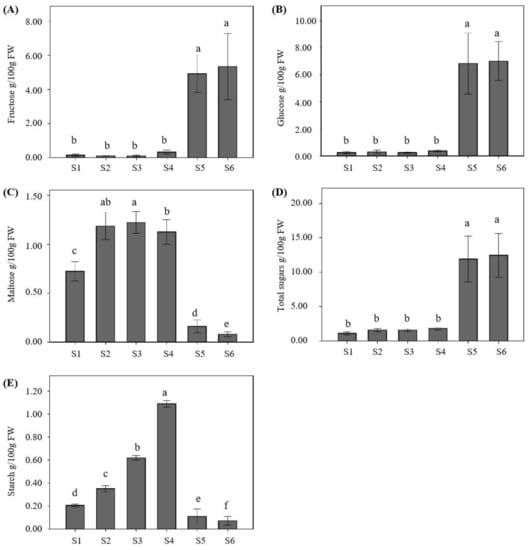
Figure 4.
Dynamic changes of concentrations of fructose (A), glucose (B), maltose (C), total sugars (the sum of glucose, fructose, and maltose) (D), and starch (E) during the fruit development of S. obovatifoliola subsp. urophylla. The different letters in each figure indicate significant differences at the p < 0.05 level using the LSD test. S1: 60 DAFB (days after full bloom), S2: 90 DAFB, S3: 130 DAFB, S4: 160 DAFB, S5: 190 DAFB, S6: 205 DAFB.
3.4. Dynamic Changes of Vitamin B, Vitamin C, Total Phenolics, Total Flavonoids, and Protein Contents during Fruit Development
The changes in antioxidant component and protein contents during S. obovatifoliola subsp. urophylla fruit development were shown in Table 3. The vitamin B1 content increased from the S1 to the S2 stage and reached the maximum value at the S2 stage (20.24 ± 1.68 mg/100 g FW); thereafter, the content of vitamin B1 continued to decline from the S2 to the S6 stage; particularly, between the S5 and S6 stages, a sharp and significant decline has been observed. The content of vitamin B2 has the maximum value at the S1 stage (191.21 ± 2.99 mg/100 g FW), then fluctuated between 31.08 ± 1.27 mg/100 g FW and 38.65 ± 1.57 mg/100 g FW; subsequently, the vitamin B2 content declined slightly and tended to be stable during the S5 and S6 stages. The vitamin B3 content also has the maximum value at the S1 stage (10.53 ± 0.71 mg/100 g FW), then fluctuated between 3.08 ± 0.16 mg/100 g FW and 4.68 ± 0.28 mg/100 g FW, and finally reached the minimum value at the S6 stage (2.03 ± 0.30 mg/100 g FW). As displayed in Table 3, the content of vitamin B6 at the six maturity stages declined continuously with the delaying of the fruit’s development, and finally reached the minimum value at the mature stage (S6) (0.13 ± 0.01 mg/100 g FW). The content of vitamin C indicated the same accumulation pattern as vitamin B1. That is, the content of vitamin C increased from the S1 to the S2 stage and reached the maximum value at the S2 stage (608.58 ± 7.28 mg/100 g FW). Thereafter, the content of vitamin C continued to decline from the S2 to the S6 stage; particularly, between the S4 and S5 stages, a sharp and significant decline was observed, and it finally tended to be stable during the S5 and S6 stages.

Table 3.
Dynamic changes of antioxidant component and protein content during S. obovatifoliola subsp. urophylla fruit development.
As shown in Table 3, the protein content continuously declined during the whole development period and reached the minimum value at the S6 stage (0.48 ± 0.00 g/100 g FW). The content of total phenolics declined from the S1 to the S2 stage, then increased at the S3 stage, and declined again at the S4 stage, whereas a significant rise was found from the S4 to the S5 stage; thereafter, there was no significant difference between the S5 and S6 stages. Similarly, the total flavonoids indicated the same accumulation pattern as the total phenolics in the early stage of fruit development (S1–S3) and then showed a continuing downward trend until reaching the minimum value at the mature stage (13.85 ± 0.80 mg/100 g FW).
3.5. Dynamic Changes of Amino Acid Composition during Fruit Development
We detected the content of 17 common free amino acids in the fruit pulp of S. obovatifoliola subsp. urophylla at different development stages, and the results were shown in Table 4. In general, the content of amino acids at the six development stages declined continuously with the fruit development and has the minimum value at the mature stage, including glutamic acid (Glu), glycine (Gly), alanine (Ala), tyrosine (Tyr), valine (Val), methionine (Met), lysine (Lys), isoleucine (Ile), leucine (Leu), proline (Pro), and phenylalanine (Phe). However, some amino acids showed a spike and then declined in an overall decline trend, such as aspartic acid (Asp) showing a spike at the S2 stage, threonine (Thr) showing a spike at the S4 stage, and cysteine (Cys) showing a spike at the S5 stage, whereas the content of serine (Ser), histidine (His), and arginine (Arg) have maximum values in the S1 stage and showed a spike at the S5 stage. The content of total amino acids (TAAs) declined continuously with the fruit development and has the minimum value at the mature stage (336.89 ± 11.98 mg/100 g). Similarly, essential amino acids (EAAs) indicated the same accumulation pattern as the TAAs. In particular, the proportion of EAAs, including Thr, Val, Met, Lys, Ile, Leu, and Phe, has a maximum value at the mature stage (38.94%).

Table 4.
Dynamic changes of amino acid composition during S. obovatifoliola subsp. urophylla fruit development.
4. Discussion
Stauntonia obovatifoliola subsp. urophylla is a novel edible and healthy fruit and has tremendous potential for exploitation and utilization. In this study, the dynamic changes in the fruit quality, sugar composition and content, antioxidant component and content, and amino acids content of S. obovatifoliola subsp. urophylla during fruit development were detected and analyzed.
The detection results of the fruit’s appearance quality showed that the fruit increased rapidly in the early stages of development. The single fruit weight of S. obovatifoliola subsp. urophylla at the S2, S3, S4, S5, and S6 stages increased by 495.60%, 59.49%, 44.09%, 19.64%, and 4.26%, respectively. For the fruit length, the growth rates at each stage were 76.27%, 14.43%, 15.79%, 2.32%, and 3.99%, respectively. The highest growth rate of fruit diameter also occurred at the S2 stage (63.93%), and the lowest growth rate was found at the S4 stage (2.66%), whereafter, the growth rate showed a spike at the S5 stage (S5–S6, 10.44%, and 5.52%, respectively). The results of the fruit shape index showed that the fruit shape index of S. obovatifoliola subsp. urophylla was basically stable between 1.8–2.2 during the fruit development period, and the difference between each stage was not obvious. In particular, the values of fruit weight, fruit length, and fruit diameter at the mature stage were much higher than those that grew in the wild [3]. Moreover, most of the wild S. obovatifoliola subsp. urophylla fruits are small and have a low edible ratio, low stress resistance, and poor appearance quality. Fortunately, the wide range of geographical distribution of S. obovatifoliola subsp. urophylla provides substantial genetic diversity and rich wild germplasm resources for breeders to select superior genotypes with excellent comprehensive characteristics through resource exploration and evaluation.
Total soluble solids and titratable acid are the main components of fruit flavor quality and nutritional composition which were also considered as crucial parameters of fruit ripening [29]. Additionally, the importance of detecting the change of TSS, TA, and TSS/TA has also been demonstrated by many studies in different fruits, such as strawberry, sweet cherry, orange, mulberry, etc. [30,31,32]. In this experiment, the TSS content of the S. obovatifoliola subsp. urophylla fruit showed an increasing trend during the whole fruit development period. Exactly, the TSS content kept at low levels during the early fruit development period (S1–S4), while the TSS content accumulated sharply during the S5–S6 stages, which was mainly due to the breakdown of starch and the accumulation of sugars. Meanwhile, the TA content of the S. obovatifoliola subsp. urophylla fruit remained at low levels during the fruit ripening process, which is consistent with previous reports on S. obovatifoliola subsp. urophylla fruit [3]. Thus, the high ratio of TSS to TA resulted in the sweet flavor of S. obovatifoliola subsp. urophylla fruit. Moreover, the TSS content changed sharply when the fruit neared ripening, which could be considered as an alternative maturity indicator of S. obovatifoliola subsp. urophylla fruit.
The dry matter content not only is a significant parameter to evaluate the carbon incorporation at different development stages of fruits but is also an important indicator of fruit flavor quality and texture [33,34]. The dry matter content of the S. obovatifoliola subsp. urophylla fruit continuously increased in the early stages and then declined significantly at the mature stage, which was mainly due to the direct relation between an increase in fruit size and the degradation of starch. Fruit firmness usually has a significant impact on fruits’ market value, consumer acceptance, and shelf life [26,35]. The firmness declined sharply when the fruit of S. obovatifoliola subsp. urophylla neared maturity and softening. Unlike Akebia fruits (a relative genus of Stauntonia), the S. obovatifoliola subsp. urophylla fruit did not crack when fully ripe; thus, it could be picked after full maturity (S6 stage), and it tasted better as a fresh fruit. However, considering the long-distance transportation, we suggest that the best time point to harvest the fruit may be at the S5 stage as the fruit is maintained at a suitable hardness that could bear long-distance transportation.
Sugars are not only important components of fruit flavor and nutritional composition but also play important signaling factor roles during plant growth and are also involved in regulating gene expression during plant development [36,37]. The major sugars identified in S. obovatifoliola subsp. urophylla fruit pulp are fructose, glucose, and maltose. The contents of fructose, glucose, and total sugars showed the same accumulation trend, which firstly increased slightly in the early fruit development periods (S1–S4), thereafter increased sharply at the S5 stage, and there were no significant dynamic changes at the S5 and S6 stages. The changing trends of maltose and starch during the fruit’s development and ripening were basically similar; both continuously accumulated during the early fruit development periods but declined sharply as the fruit approached ripening. This opposite change trend of monosaccharides and polysaccharides was mainly due to the hydrolysis of carbohydrates as the fruit ripened, similar to strawberry, sweet cherry, bananas, etc. [30,38,39].
Vitamins, apart from being an important nutrient in daily diet, are also potent antioxidant components [40,41,42]. The results of this study showed that the fruit of S. obovatifoliola subsp. urophylla was rich in vitamin B (B1, B2, B3, B6), vitamin C, phenolics, and flavonoids. In particular, the content of vitamin B2 was the most abundant vitamin B at the mature stage, while the vitamin C content at the mature stage was in accordance with a previous report [6]. The vitamin content of S. obovatifoliola subsp. urophylla fruit showed an overall downward trend during fruit development, but there was a large fluctuation in this process, especially the vitamin C content, which decreased sharply when the fruit was near ripening (S5–S6). Phenolics and flavonoids compounds are important secondary metabolites of plants, which can help plants’ resistance to bacteria and protect cells from oxidative damage [43,44]. In this study, the content of phenolics declined from the S1 to the S2 stage, followed by a subsequent increase in fluctuation. The flavonoids content declined firstly from the S1 to the S2 stage and increased at the S3 stage, followed by a subsequent sustained decrease in further fruit development in S. obovatifoliola subsp. urophylla fruit. The dynamic change of antioxidant content was closely related to fruit development and ripening; the content of antioxidants could also be influenced by structural genes, temperature, light intensity, etc. [45,46]. The general decrease in the content of vitamins, phenolics, and flavonoids is mainly due to the oxidation of oxidase during fruit maturity or the effect of dilution as fruit increases in size [47,48].
Amino acids are important nutrient elements for most of life and also play some key roles in various biological reactions, such as signaling pathways, ATP generation, redox balance, nucleotide synthesis, cellular immunity, etc. [49,50,51,52,53]. In this study, 17 common free amino acids and 7 essential amino acids were detected by HPLC. The content of amino acids showed an overall downward trend during fruit development, which might be related to the conversion and expenditure of amino acids. Another reason may be the dilution effect of increasing fruit size. The abundance of amino acids in the fruit of S. obovatifoliola subsp. urophylla documented its excellent medicinal and edible value.
Compared to some common fruits, S. obovatifoliola subsp. urophylla has more advantages in certain nutrients (Table 5). For example, the total soluble solids, total sugars, fructose, and glucose are higher than apple, peach, kiwifruit, and strawberry, indicating that the S. obovatifoliola subsp. urophylla fruit has a sweeter flavor than some common fruits. S. obovatifoliola subsp. urophylla fruit has more protein than apple and cherry but less than banana, grape, peach, kiwifruit, and strawberry. As for the content of total amino acids, S. obovatifoliola subsp. urophylla is on par with grape, kiwifruit, strawberry, and cherry whereas less than apple, banana, and peach. Surprisingly, the contents of vitamin B1, vitamin B2, and vitamin B3 of S. obovatifoliola subsp. urophylla are much higher than those common fruits, whereas it has less vitamin C than those common fruits. It should be pointed out that the determination of the nutritional composition of S. obovatifoliola subsp. urophylla in this paper is not comprehensive, but it can still prove that S. obovatifoliola subsp. urophylla is worthy of being exploited and utilized as a medicinal and edible fruit crop.

Table 5.
The nutrient composition of S. obovatifoliola subsp. urophylla fruit at the ripening stage [54,55].
5. Conclusions
In conclusion, the dynamic changes in the fruit’s appearance, physiochemical quality, and nutritional quality during the development of S. obovatifoliola subsp. urophylla were detected and analyzed. This study suggested that the maturity stage had a significant effect on nutritional properties and physiochemical parameters during S. obovatifoliola subsp. urophylla fruit ripening and softening. Particularly, the values of TSS, firmness, dry matter, fructose, glucose, maltose, starch, vitamin B, vitamin C, total phenolics, and total flavonoids of S. obovatifoliola subsp. urophylla fruit showed significant changes during the transition to physiological maturity. In view of the nutrient content and pulp texture, the fruit of S. obovatifoliola subsp. urophylla picked at the S5 maturity stage was more suitable for long-distance transportation. The results of this study laid a foundation for clarifying the growth and development characteristics of S. obovatifoliola subsp. urophylla fruit and the further utilization of these excellent medicinal and edible germplasm resources.
Author Contributions
Writing—original draft preparation, T.J.; investigation, C.F.; conceptualization, writing—review and editing, S.Z.; funding acquisition, P.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Plant Germplasm Innovation Program of Chinese Academy of Sciences (KFJ-BRP-007-001) and Youth Fund of Lushan Botanical Garden, the Chinese Academy of Sciences (2021 ZWZX07).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zou, S.; Yao, X.; Zhong, C.; Gao, P.; Wang, Z.; Huang, H. Phenotypic characterization of Stauntonia obovatifoliola Hayata subsp. urophylla germplasm: A potential new fruit crop. Genet. Resour. Crop Evol. 2020, 67, 1037–1050. [Google Scholar]
- Editorial Commission of Chinese Flora of Chinese Academy of Sciences. Flora of China; Science Press: Beijing, China, 2001. [Google Scholar]
- Wang, Y.; Xing, W.; He, X.; Duan, W.; Huang, W.; Gong, C. Differential comparison among different geographical provenances of Stauntonia urophylla. J. Cent. South Univ. For. Technol. 2015, 35, 55–60. [Google Scholar]
- Li, R.; Ru, Y.; Feng, L.; Wang, Z.; He, X.; Zhang, X. A comparative study of nutrient composition, bioactive properties and phytochemical characteristics of Stauntonia obovatifoliola flesh and pericarp. Front. Nutr. 2022, 9, 1013971. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.M.; Yang, T.M. Chemical base of Yao’s medication principle. J. South-Cent. Univ. Natl. 2010, 29, 37–40. [Google Scholar]
- Peng, X.B.; Gao, W.L.; Hu, D.Q.; Ma, F.F.; Fu, L.G.; Deng, Q.; Wei, Y. Chemical constituents from the aerial part of Stauntonia obovatifoliola Hayata subsp. urophylla. J. Chin. Med. Mater. 2013, 36, 1795–1798. [Google Scholar]
- Lu, X.R.; Qiu, F.; Pan, X.Q.; Li, J.; Wang, M.Y.; Gong, M.X. Simultaneous quantitative analysis of nine triterpenoid saponins for the quality control of Stauntonia obovatifoliola Hayata subsp. intermedia stems. J. Sep. Sci. 2014, 37, 3632–3640. [Google Scholar] [CrossRef]
- Lu, X.R.; Wang, X.M.; Wang, Z.M.; Chen, X.Q.; Wang, M.Y.; Gong, M.X. Triterpenoid Saponins from Stauntonia obovatifoliola Hayata ssp. urophylla. Helv. Chim. Acta 2015, 98, 245–252. [Google Scholar] [CrossRef]
- Thomas, S.S.; Cha, Y.S.; Kim, K.A. Perilla oil alleviates high-fat diet-induced inflammation in the colon of mice by suppressing nuclear factor-kappa b activation. J. Med. Food 2020, 23, 818–826. [Google Scholar] [CrossRef]
- Chen, X.D.; Tian, W.; Deng, R.H.; Liu, Y.; Deng, H.Y. Chemical constituents from Stauntonia obovatifoliola subsp. Urophylla. Chin. Tradit. Herb. Drugs 2013, 44, 671–673. [Google Scholar]
- Zhou, W.J.; Chen, Q.P.; Zeng, Y.B.; Wang, G.X. Content Determination of Oleanolic Acid in Stauntonia obovatifoliola Hayata subsp. urophylla by RP-HPLC. Chin. J. Inf. TCM 2015, 22, 92–94. [Google Scholar]
- Shan, Z.; Mu, Z.; Qin, H. Study on the species identification and distribution of Lardizabalaceae medicinal plants in Jiangxi province. China Med. Pharm. 2018, 8, 53–57. [Google Scholar]
- Wang, Y.; He, X.; Li, J.; Gong, C.; Zhou, W. Evaluation of adaptability on germplasm resources of Lardizabalaceae. South China For. Sci. 2018, 46, 24–27. [Google Scholar]
- Wu, L.; Xu, W.; He, S. Species and distributuin of medicinal plants of Lardizabalaceae in China. J. Anhui Agric. Sci. 2010, 38, 14325–14326. [Google Scholar]
- You, C.; Huang, H.; Mei, Y. Study of Lardizabalaceae resources and cultivation in southern Jiangxi Province. South China For. Sci. 2018, 46, 22–24. [Google Scholar]
- Zhong, W.; Cao, L.; Zhong, W.; Mu, Z.; Du, X.; Zhong, G. Survey resources of medicinal plants of Lardizabalaceae in Jiangxi Province. J. Jiangxi Univ. TCM 2016, 28, 63–64. [Google Scholar]
- Lelièvre, J.M.; Latchè, A.; Jones, B.; Bouzayen, M.; Pech, J.C. Ethylene and fruit ripening. Physiol. Plant. 1997, 101, 727–739. [Google Scholar] [CrossRef]
- Palma, J.M.; Corpas, F.J.; del Rio, L.A. Proteomics as an approach to the understanding of the molecular physiology of fruit development and ripening. J. Proteomics 2011, 74, 1230–1243. [Google Scholar] [CrossRef]
- Liu, R.; How-Kit, A.; Stammitti, L.; Teyssier, E.; Rolin, D.; Mortain-Bertrand, A.; Halle, S.; Liu, M.; Kong, J.; Wu, C.; et al. A DEMETER-like DNA demethylase governs tomato fruit ripening. Proc. Natl. Acad. Sci. USA 2015, 112, 10804–10809. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Nicolas, P.; Fernandez-Pozo, N.; Ma, Q.; Evanich, D.J.; Shi, Y.; Xu, Y.; Zheng, Y.; Snyder, S.I.; Martin, L.B.B.; et al. High-resolution spatiotemporal transcriptome mapping of tomato fruit development and ripening. Nat. Commun. 2018, 9, 364. [Google Scholar] [CrossRef]
- Dabesor, P.A.; Sanni, D.M.; Kolawole, A.O.; Enujiugha, V.N.; Lawal, O.T.; Edeh, A.T. Changes in physicochemical properties and enzymes associated with ripening of snake tomato (Trichosanthes Cucumerina L.) fruit. Biocatal. Agric. Biotechnol. 2022, 40, 102313. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, X.; Zhang, L.P.; Gao, Z.S.; Jia, H.J. Effect of both harvest maturities and storage temperatures on fruit quality of‘Jinxiu’ yellow peach. Sci. Technol. Food Ind. 2015, 36, 334–338. [Google Scholar]
- Kong, X.J.; Lin, H.T.; Zhou, H.; Lin, Y.F.; Chen, Y.H.; Wang, H. Study on the optimum harvesting date of fresh-eating Chinese olive fruit and its quality assessment parameters. Storage Process 2016, 16, 6–14. [Google Scholar]
- Zozio, S.; Servent, A.; Cazal, G.; Mbeguie-A-Mbeguie, D.; Ravion, S.; Pallet, D.; Abel, H. Changes in antioxidant activity during the ripening of jujube (Ziziphus mauritiana Lamk). Food Chem. 2014, 150, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Khan, H.; Shah, M.; Khan, R.; Khan, F. Chemical composition and antioxidant activity of certain Morus species. J. Zhejiang Univ. Sci. B 2010, 11, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Gao, P.; Jia, T.; Huang, H. Physicochemical Characteristics and Nutritional Composition during Fruit Ripening of Akebia trifoliata (Lardizabalaceae). Horticulturae 2022, 8, 326. [Google Scholar] [CrossRef]
- Razzaq, K.; Khan, A.S.; Malik, A.U.; Shahid, M. Ripening period influences fruit softening and antioxidative system of ‘Samar Bahisht Chaunsa’ mango. Sci. Hortic. 2013, 160, 108–114. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Zhu, X.; Hou, Y.Y.; Pan, Y.F.; Shi, L.; Li, X.H. Effects of harvest maturity stage on postharvest quality of winter jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit during cold storage. Sci. Hortic. 2021, 277, 109778. [Google Scholar] [CrossRef]
- Saquet, A.A. Storage of pears. Sci. Hortic. 2019, 246, 1009–1016. [Google Scholar] [CrossRef]
- Mahmood, T.; Anwar, F.; Abbas, M.; Boyce, M.C.; Saari, N. Compositional Variation in Sugars and Organic Acids at Different Maturity Stages in Selected Small Fruits from Pakistan. Int. J. Mol. Sci. 2012, 13, 1380–1392. [Google Scholar] [CrossRef]
- Riaz, M.; Zamir, T.; Rashid, N.; Jamil, N.; Rizwan, S.; Masood, Z.; Mushtaq, A.; Tareen, H.; Khan, M.; Ali, M. Comparative study of nutritional quality of orange (Citrus sinensis) at different maturity stages in relation to significance for human health. Am. Eur. J. Toxicol. Sci. 2015, 7, 209–213. [Google Scholar]
- Sanchez, E.M.; Calin-Sanchez, A.; Carbonell-Barrachina, A.A.; Melgarejo, P.; Hernandez, F.; Martinez-Nicolas, J.J. Physicochemical characterisation of eight Spanish mulberry clones: Processing and fresh market aptitudes. Int. J. Food Sci. Technol. 2014, 49, 477–483. [Google Scholar] [CrossRef]
- Chen, M.Y.; Zhang, P.; Zhao, T.T.; Han, F.; Liu, X.L.; Zhong, C.H. Relationship between harvest indices and fruit quality traits in Actinidia chinensis ‘Jintao’. Plant Sci. J. 2019, 37, 621–627. [Google Scholar]
- Nardozza, S.; Gamble, J.; Axten, L.G.; Wohlers, M.W.; Clearwater, M.J.; Feng, J.Q.; Harker, F.R. Dry matter content and fruit size affect flavour and texture of novel Actinidia deliciosa. J. Sci. Food Agric. 2011, 91, 742–748. [Google Scholar] [CrossRef]
- Khodabakhshian, R.; Emadi, B.; Khojastehpour, M.; Golzarian, M.R. Determining quality and maturity of pomegranates using multispectral imaging. J. Saudi Soc. For. Agric. Sci. 2015, 16, 322–331. [Google Scholar] [CrossRef]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef]
- Araya, T.; Noguchi, K.; Terashima, I. Effects of carbohydrate accumulation on photosynthesis differ between sink and source leaves of Phaseolus vulgaris L. Plant Cell Physiol. 2006, 47, 644–652. [Google Scholar] [CrossRef]
- Sturm, K.; Koron, D.; Stampar, F. The composition of fruit of different strawberry varieties depending on maturity stage. Food Chem. 2003, 83, 417–422. [Google Scholar] [CrossRef]
- Phillips, K.M.; McGinty, R.C.; Couture, G.; Pehrsson, P.R.; McKillop, K.; Fukagawa, N.K. Dietary fiber, starch, and sugars in bananas at different stages of ripeness in the retail market. PLoS ONE 2021, 16, e0253366. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- Zhang, L.H.; Li, S.F.; Dong, Y.; Zhi, H.H.; Zong, W. Tea polyphenols incorporated into alginate-based edible coating for quality maintenance of Chinese winter jujube under ambient temperature. LWT Food Sci. Technol. 2016, 70, 155–161. [Google Scholar] [CrossRef]
- Mditshwa, A.; Fawole, O.A.; Vries, F.; Kobus, V.D.M.; Crouch, E.; Opara, U.L. Impact of dynamic controlled atmospheres on reactive oxygen species, antioxidant capacity and phytochemical properties of apple peel (cv. Granny Smith). Sci. Hortic. 2017, 216, 169–176. [Google Scholar] [CrossRef]
- Jin, P.; Wu, X.; Xu, F.; Wang, X.L.; Wang, J.; Zheng, Y.H. Enhancing Antioxidant Capacity and Reducing Decay of Chinese Bayberries by Essential Oils. J. Agric. Food Chem. 2012, 60, 3769–3775. [Google Scholar] [CrossRef] [PubMed]
- Kirigia, D.; Winkelmann, T.; Kasili, R.; Mibus, H. Nutritional composition in African nightshade (Solanum scabrum) influenced by harvesting methods, age and storage conditions. Postharvest Biol. Technol. 2019, 153, 142–151. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.L.; Liu, Z.G.; Zhao, Z.H.; Zhao, J.; Wang, Z.T.; Zhou, G.F.; Liu, P.; Liu, M.J. Transcriptome and metabolome profiling unveil the mechanisms of Ziziphus jujuba Mill. peel coloration. Food Chem. 2020, 312, 125903. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, L.; Karppinen, K.; Escobar, A.L.; Haggman, H.; Jaakola, L. Light-controlled flavonoid biosynthesis in fruits. Front. Plant Sci. 2014, 5, 534. [Google Scholar] [CrossRef]
- Shwartz, E.; Glazer, I.; Bar-Ya’akov, I.; Matityahu, I.; Bar-Ilan, I.; Holland, D.; Amir, R. Changes in chemical constituents during the maturation and ripening of two commercially important pomegranate accessions. Food Chem. 2009, 115, 965–973. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Changes in physical properties, chemical and elemental composition and antioxidant capacity of pomegranate (cv. Ruby) fruit at five maturity stages. Sci. Hortic. 2013, 150, 37–46. [Google Scholar] [CrossRef]
- Koopman, W.J.; Nijtmans, L.G.; Dieteren, C.E.; Roestenberg, P.; Valsecchi, F.; Smeitink, J.A.; Willems, P.H. Mammalian mitochondrial complex I: Biogenesis, regulation, and reactive oxygen species generation. Antioxid. Redox Signal. 2010, 12, 1431–1470. [Google Scholar] [CrossRef]
- Laxman, S.; Sutter, B.M.; Wu, X.; Kumar, S.; Guo, X.; Trudgian, D.C.; Mirzaei, H.; Tu, B.P. Sulfur amino acids regulate translational capacity and metabolic homeostasis through modulation of tRNA thiolation. Cell 2013, 154, 416–429. [Google Scholar] [CrossRef]
- Buck, M.D.; Sowell, R.T.; Kaech, S.M.; Pearce, E.L. Metabolic instruction of immunity. Cell 2017, 169, 570–586. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Yuan, Z.; Radford, S.J.; Liu, C.; Libutti, S.K.; Zheng, X.F.S. Amino acids-Rab1A-mTORC1 signaling controls whole-body glucose homeostasis. Cell Rep. 2021, 34, 108830. [Google Scholar] [CrossRef]
- Kelly, B.; Pearce, E.L. Amino assets: How amino acids support immunity. Cell Metab. 2020, 32, 154–175. [Google Scholar] [CrossRef]
- Zhu, S.R.; Yang, G.; Yang, D.; Li, L.b.; Mo, R.J.; Li, X.L.; Zhang, Y.F. Comparative analysis of organic nutrients between Holboellia latifolia and nine common fruits. J. Kunming Univ. 2022, 44, 70–74. [Google Scholar]
- Yang, Y.X. China Food Composition Tables, 6th ed.; Peking University Medical Press: Beijing, China, 2018. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).