Abstract
The crocin in gardenia, as a medical plant, has drawnthe attention of researchers and scientists due to its color and high antioxidant activity. To optimize the extraction parameters of crocin from gardenia fruits, response surface methodology (RSM) was employed.The effects of four independent variables, namely extraction temperature (45–55 °C), time (40–60 min), percentage of gardenia fruits(15–25%), and ethanol concentration (50–60%),on a crocin compound were investigated. The extract from the gardenia fruit was dried at different temperatures (55–70 °C) by the foam-mat drying method. The optimal extraction parameters were an extraction temperature of 55 °C, time of 57 min, percent of fruits in solvent at 24%, and an ethanol concentration of 56%. The results showed that the dried gardenia powder had maintained the crocin content well(6.64 mg/g), and the product with low water activity and moisture content of 0.33 and 5.72%, respectively, is suitable for storage. The foam-mat dried product also maintains the natural color and characteristics inherent in the raw materials, which could also be used as supplemental ingredients for other food industries.
1. Introduction
Gardenia (Gardenia jasminoides Ellis) is a shrub in the family Rubiaceae, first grown in the southern regions of China and now widely distributed in parts of Asia. At present, in the Fuding area of Fujian province in China, there are 4500-hectare gardenia planting bases [1].The components in the fruit are mainly carotenoids, flavonoids, sterol iridoid and glycosides.The metabolites of G. jasminoides are used as a traditional natural medicine as a diuretic and for hemostasis, lowering blood pressure and enhancing blood circulation [2]. In the group of carotenoids, crocin is the component of interest in many studies. Crocins are water-soluble carotenoids responsible for the color of Gardenia, which are low in toxicity and have low allergic potential [3].
Crocin has been shown to have various pharmacological effects such as treatment against cardiovascular diseases [4], help with memory improvement, anticonvulsant, neuroprotective [5], and antidepressant effects [6], and inhibition of tumor cell proliferation [7], and it is increasingly focused on minimizing health risks [8]. In addition, crocin is also confirmed as non-toxic and chemically stable compared to other natural food colorants. The yellow pigments of this herb have been widely used to color soft drinks, cakes, candies, and noodles [2]. With the worldwide trend of replacing synthetic colorants with natural ones, crocin has created increasing demand and growing interest. In order to be able to use crocin as a food colorant, an extraction process needs to be performed. Common solvents used in extraction are water, ethanol, and acetone. The effectiveness of solvents in extracting bioactive compounds from plants will vary depending on the intended use. Ethanol of 70 and 80% was selected as the best extraction solvent for total crocin extraction from saffron [9]. However, crocin is an unstable compound under thermal process, which dramatically degrades at temperatures above 70 °C [10,11]
The goal of extracting crocin from gardenia is for use as an additive in food processing. Extraction in water often prolongs the time at high temperature to speed up mass transfer; however, the degradation of bioactive compounds at high extraction temperatures is also limited to thermally unstable compounds. In addition, the amount of organic solvents used in these extraction processes is quite substantial, and safety procedures should be followed during extraction, as well as for human health and the environment. Therefore, it is necessary to develop an environmentally friendly extraction technology using safe substances. Ethanol is considered safe for humans compared to methanol, so in this study, ethanol was used as the solvent for extraction. At mild temperature extraction and shorter time, the ability to recover bioactive compounds will be better. The development of an efficient and economical extraction method to obtain bioactive compounds from gardenia fruit also offers an opportunity to replace synthetic colorants. Several studies on techniques for extracting natural pigments from brightly colored fruits and vegetables have been carried out [12,13,14]. Extraction and purification of crocin from saffron was studied by Hadizadeh et al. [15].
The extract is often in a liquid state, making it difficult to use, store, and transport; therefore, the drying process is often applied to the production of powders, facilitating easy storage and diverse applications in food processing [16]. Normally, the extract can be converted to a powder by freeze-drying or spray-drying, which involve significant costs in terms of both money and energy [17,18]. Hot-air drying can be employed as a result, and its temperature is often lower than the initial material [19]. This method also has drawbacks, such as excessive energy use and poor sensory, nutritional, and functional properties. The effective moisture diffusivity is lowered as a result of the textural compactness and shrinkage processes that occur during traditional hot-air drying. It is important to shorten the time it takes for the food to lose water because the drying process exposes the food to heat for a long time period [20].
Foam-mat drying can be considered a novel and very relevant drying technique, in which a liquid is converted into stable foam by foaming in the presence of a foaming agent and stabilizer, which are then dried to reduce the moisture content, reaching 2–2.5% [16]. The foam-drying technique presents several advantages such as shorter drying time due to the physical structure of the foam (honeycomb structure), resulting in easy and rapid removal of moisture from liquid foods and reduction of nutrient loss. The foam-drying advantage is only effective when the foam layer is mechanically and thermodynamically stable. The surface area of the dried product is increased due to foaming, and the time required to dry the foamed product is reduced compared to the non-foam-dried product [17]. Therefore, the extraction techniques related to the pigments from the gardenia fruit and the powder produced by the foam-mat drying technique are still limited. The objective of the study was to optimize the extraction conditions for crocin content from the gardenia fruit, determining the appropriate drying time for the extract from the foam-mat drying in order to maintain the powder quality and inherent natural color of the ingredients.
2. Materials and Methods
2.1. Sample Preparation
The main raw material used for the study was dried gardenia (moisture 9–10%)fruits (Figure 1). The drying process followed the recently published procedure of Bien et al. [18]. Briefly, the fruits were collected and directly transported to the laboratory within 3 h. Then, sample was dried at mild temperature (50 °C) until the moisture content reached 9–10%. The dried skin was removed, and the pulpwas ground with a blender. Collected samples were mixed to homogenize the sample used for the entire experiment and stored in a vacuum-sealed bag at room temperature (25 ± 2 °C) under subdued light for further extraction.

Figure 1.
Dried gardenia (left side) and peeled dried fruits (right side).
Albumin (Livewell, Bangkok, Thailand), carboxymethyl cellulose (99% purity, Fortune Biotech, Qingdao, China), and ethanol 96% (Cemaco, Can Tho, Vietnam) are food grade.
2.2. Box–Behnken Design
According to the preliminary study and literature review, a three-level-four-factor Box–Behnken design (BBD) was applied to determine the best combination of variables for the extraction of crocin in Gardenia Jasminoides Ellis.The total volume for each extraction was constant at 250 mL. Four selected independent variables were temperature (X1), time (X2), percentage of raw material in solvent (X3), and ethanol concentration (X4), which were usedin this experimental design. The crocin in the extract was determined as the response for the combination of the independent variables. The experimental factors and coding level are given in Table 1. The design consists of 30 runs with six center points (Table 2). Three replications were made.

Table 1.
Factor levels and coding of BBD combination experiment design.

Table 2.
Box–Behnken response surface design and responses value.
2.3. Extraction of Crocin from Gardenia Fruits
The prepared gardenia pulp was extracted in a water bath with temperature, time, percent of fruits in solvent, and ethanol concentration according to 30 treatments (as shown in Table 2). Each treatment was repeated 3 times. The extract was then filtered (Whatman Filter Paper No. 1) and analyzed for crocin content (mg/100 mL).
2.4. Foam-Mat Drying
The obtained extract was mixed with the albumin (as foam generation) with the content of 9.3%, and CMC (0.79%) was used as foam-stabilizing agent. The mixture was whipped and foamed with a mixer (Philips HR 3705-300W, Columbus, OH, USA) at the highest speed, fixing the batch volume at 200 mL.The whipping time was 19 min, and then the mixture was dried in oven dryer (MEMMERT, UN260, Bavaria, Germany) at different temperatures (55 to 70 °C) until final moisture content of dried foam-mat was reached (≈6%). Samples after collection were finely ground and sieved through a sieve with size of 0.5 mm. The crocin content (mg/g), aw, moisture content (%), and color of the foam-mat dried powder were analyzed and evaluated.
2.5. Crocin Content Determination
Total crocin was determined by UV–Vis spectrophotometry at 440 nm wavelength [19] using Equation (1):
where a is crocin content (mg/100 mL); A is the optical absorbance measured at 440 nm; F is the dilution (F = 100 times); M is the molar mass of crocin (M = 977 g/mol); l is the thickness of the pigment layer (1 cm); and ε is the molar absorption coefficient of the crocin pigment = 89.000 L/(cm·mol)
2.6. Data Analysis
Thirty different experimental pointswere obtained and analyzed by using the multiple regression analysis (quadratic models). A statistical analysis (Statgraphics) was used to fit the model to the observed data. The proposed model (Equation (2)) for crocin content (Y) was:
where bo is Y intercept (constant); bn is regression coefficient for linear effect of Xn on Y; bnn and bnm are regression coefficient for quadratic effect on Y; Xn, Xm are independent values.
The reference equation was selected to fit to the data, based on the R2 value, the lack of the fit, and model p-value (p < 0.05) obtained from the multiple regression. In addition, the normal distribution of the residuals and the plot of actual values versus predicted values were employed. Analysis of variance (ANOVA) was applied as a method of statistical analysis of responses, where probability critical level (p-value) of 0.05 was considered to reflect the statistical significance of the parameters of the proposed model. Analysis of all experimental points was carried out in triplicate, and the results were expressed as mean value and standard deviations.
3. Results and Discussion
3.1. Crocin Content of Gardenia
The crocin content in the fruit is about 12.76 mg/g dry weight. This value has shown the great potential of this material in the extraction of natural color compounds. The results of the analysis are similar to the published results of He, Cheng, Chen and Zhou [4] on the same material, where the analyzed crocin content was 10.08 (mg/g dry weight). Huang et al. [20] analyzed the crocin content in gardenia fruit as 36.97 (mg/g dry weight),which is 2.82 times higher than the analytical result. This might be because of the differenceswith planting materials in each country and the climate and soil conditions, which lead to the crocin content on the same material showing different values.
3.2. Extraction Optimization of Crocin—Modeling the Effects
Using the RSM and experiments of design of four variables and six central points, a total of 30 runs (experiments) were employed. The relationship between crocin content and extraction temperature, time, percent of gardenia, and ethanol concentration was presented in the multiple regression equation. The statistical significance of the equation was checked through analysis of variance (ANOVA) at the 95% level (Table 3). In addition, it can be observed that only double interaction X1X3, X1X4 did not have significant (p > 0.05) crocin content. The independent variables and the quadratic and interaction effects all significantly affected the crocin content in the extract (p < 0.05). The unimportant terms are already omitted. In addition, the points on the plot of the residuals (difference between experimental and predicted values) for each parameter form a nearly linear pattern, which indicates that the normal distribution is a good model for the datasets. The final regression equation showed the relationship between crocin content and thefour dependent variables (according to the coded factors) for the quadratic model of response surface according to the Box–Behnken design for the extraction, which is described in Equation (3).The fit of the model is also evaluated through the p-value of lack of fit. A good correlation model needs a fit between the actual data and the model’s prediction, so a model established with a lack of fit test that is insignificant is desirable [21,22].
Y = 86.968 − 16.782X1 + 3.859X2 + 7.259X3+ 9.592X4 + 0.158X12 + 0.031X1X2 − 0.067X22 + 0.06X2X3 + 0.011X2X4 − 0.449X32 + 0.192X3X4 − 0.132X42
R2 = 95.89%; R2(adjusted for d.f.) = 95.26%; SEE: 0.721564

Table 3.
ANOVA for the response surface quadratic model for crocin content.
Here, Y is crocin content (mg/100 mL), X1 is extraction temperature (°C), X2 is extraction time (min), X3 is percent of gardenia in solvent (%), and X4 is ethanol concentration (%).
With the refitted equation, the high values of R2(93.47%) and adjusted R2(92.94%) for the response variable were observed. The lackoffit is insignificant (p = 0.24 > 0.05), suggesting that the selected model is accurate enough to explain the behavior and predict the crocin content (mg/100 mL).
The Pareto plot in Figure 2 showed the percentage of gardenia fruit used and its quadratic interaction strongly affecting the crocin content with the large bar. The other variables appeared to have effect on crocin in this experiment, except X1X3 and X1X4. These results again confirmed all independent variables have more significant effect on crocin content in extraction.
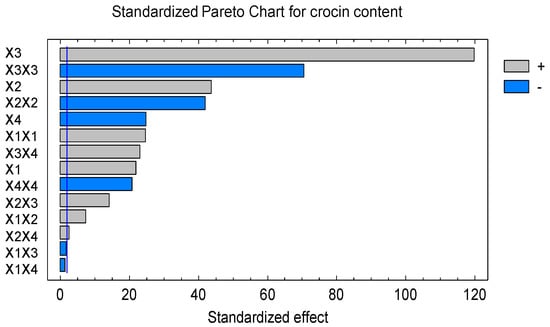
Figure 2.
Standardized Pareto chart for crocin content (the gardenia extract was dried at different temperatures, 55 to 70 °C, by the foam-mat drying).
The fit of the model was also checked through the coefficient of determination R2. By substituting the empirical values of the variables into Equation (3), the crocin content can be predicted. The high correlation achieved between the experimental data and the predictive data for the extraction is shown in Figure 3.
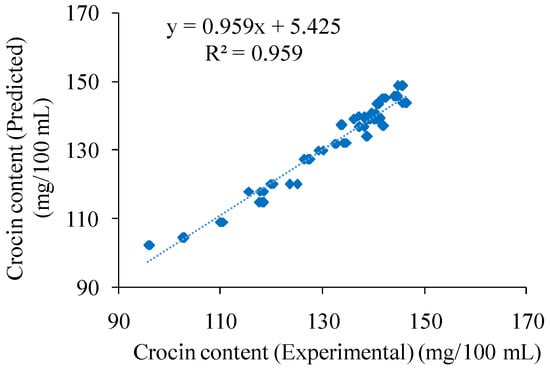
Figure 3.
Correlation between the experimental and the predicted crocin content using the model described in Equation (3).
Response surface modeling (Figure 4) shows that the variables of temperature, time, the percentage of material in solvent, and ethanol concentration all have an influence on the crocin content in the extract.Specifically, the increasing of the temperature, time, material/solvent ratio, and ethanol concentration led to an increase in the crocin content in the extract. The optimal crocin content found from the model was 153.25 mg/100 mL, corresponding to temperature, time, material in solvent, and ethanol concentration of 55°C, 57 min, 24%, and 56%, respectively.
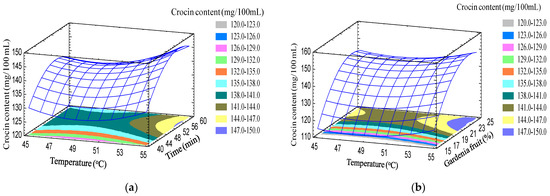
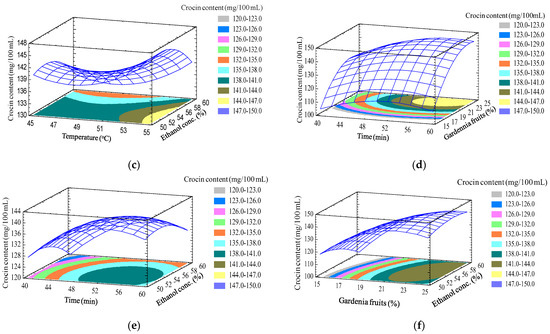
Figure 4.
Response surface of foam density as a function oftemperature, time, gardenia percent in solvent, and ethanol concentration. (a) The gardenia percent and ethanol concentration fixed at 20% and 50%, respectively; (b) the time and ethanol concentration fixed at 50 min and 50%, respectively; (c) the time and gardenia fruit percent fixed at 50 min and 20%, respectively; (d) temperature and ethanol concentration fixed at 50 °C and 50%, respectively; (e) temperature and gardenia fruits percent fixed at 50 °C and 20%, respectively; (f) time and temperature fixed at 50 min and 50 °C, respectively.
The results obtained from this study are relatively consistent with previous studies. Heating increases the obtained crocin content. The reason can be explained by the higher the temperature, the more effective the extraction is because the release of compounds will increase, and the solvent has a higher ability to dissolve the substances at high temperature. At the same time, the increased extraction efficiency was due to the increased diffusion of the solvent into the internal structure of the sample when the temperature was high. Therefore, by increasing the solubility and diffusion of compounds and reducing solvent viscosity, mass transfer and solvent penetration were increased into the cells [22]. On the other hand, Mohamad et al. [23] also suggested that high temperature can reduce the free cell barriers to weaken the wall on the cell membrane, resulting in easier contact of the solvent with the active ingredients, increasing the extraction capacity.
Extraction time has a great influence on extraction efficiency, since crocin content increases with increasing extraction time. However, when the time is too long, the extraction efficiency will not increase but decrease. This can be explained by Fick’s second law of diffusion: as extraction time increases, the content of substances in the material diffuses from the cell to the outside [24]. However, the extraction efficiency will not increase after a certain period of time. This time period depends on other extraction conditions such as solvent, extraction temperature, and the ratio of solvent material, as well as the nature of the material and compound to be extracted [25]. At the same time, when the extraction time is prolonged, the biological compounds inside and outside the material are close to reaching equilibrium, so the obtained extract has a slow increase in crocin content later on. In addition, these compounds can be oxidized by adverse environmental factors (temperature, light, and oxygen) [26].
The driving force of the extraction process is the difference in the concentration of the constituents in the raw material and in the solvent [22]. Therefore, the selection of the appropriate ratio of raw materials and solvents is of great significance in the extraction process. Research results show that when increasing the solvent volume, the amount of crocin content in the extract increases and reaches the highest value at the rate of 24%. However, when continuing to increase the solvent volume, the crocin content in the extract tended to decrease slightly. This is probably because the amount of colorant dissolved in the solvent has reached the maximum, and when continuing to increase the solvent volume, the increase in colorant content is not significant. Too large a solvent volume is likely to saturate and reduce the amount of colorant that diffuses into the solvent. Because increasing the ratio between the raw materials and the solvent leads to the difference in the concentration gradient of the substances being extracted in the raw materials with the extraction solvent, the extraction efficiency decreases [27]. Therefore, the percentage of material 24% can be considered as the optimal ratio to ensure both extraction efficiency and solvent savings.
The selection of the appropriate solvent is an important step in the extraction because it directly affects the extraction efficiency [21]. Many previous studies have shown that ethanol has the ability to promote rapid diffusion thanks to its polar properties in the molecular structure, which helps to thoroughly extract components and has anti-inflammatory activity [27]. At the same time, a number of studies have also shown that ethanol is likely to be an optimal solvent for extraction due to its safety in human health and environmental friendliness [28]. With the results obtained from this experiment, the optimal ethanol concentration for the extraction of crocin from gardenia fruit was 56%. Because the structure of crocin consists of two parts, the hydrophilic glycosyl radical and the hydrophobic polyene of crocetin acid, it is soluble in solvents including water and ethanol. If the amount of water is large, the hydrophobic part is difficult to dissolve; if the amount of ethanol is high, the hydrophilic part is difficult to dissolve [29]. In addition, extraction of metabolites by using an ethanol–water solvent was simple and cost effective, which could be applied in various scales of industry [30]. Sharmila et al. [31] also reported that organic solvents combined with water can create optimal conditions for the extraction of natural color compounds. The studies of Yilmaz et al. [32] also showed that the appropriate concentration of ethanol for extraction of natural color compounds is usually in the range of 50% to 80%. Therefore, ethanol with 56% concentration was selected for the extraction of crocin from gardenia fruit.
In addition, the antioxidant properties of extract are also one of the most important to determine their quality. The result showed that under optimal extraction conditions, the gardenia extract has 87.73% inhibition of DPPH (1,1-diphenyl-2-picryl-hydrazyl, 0.2 mM), which shows the high antioxidant activity. It could be seen that under these operations the antioxidant properties of Gardenia jasminoides Ellis could be maintained.
3.3. Effect of Drying Temperature (Foam-Mat Drying Method Was Employed) on the Quality of Gardenia Powder
The content of crocin and water, together with measured water activity, is shown in Table 4. The effects of foam-mat drying temperature and time on the color of gardenia powder are presented in Figure 5. Drying air temperature greatly affects the drying process of the sponge; too low or too high temperature is detrimental to the crocin content in gardenia powder.

Table 4.
Crocin content, moisture content, and water activity of foam-mat dried gardenia powder at different drying temperatures and times.

Figure 5.
The color of the foam-mat dried gardenia fruit powder at different drying temperatures.
The crocin content in the dried powder product tends to decrease as the drying temperature increases. Specifically, when the drying temperature increased to 55, 60, 65, and 70 °C, the crocin content in the dried powder decreased to 6.82, 6.64, 5.60, and 4.17 mg/g, respectively. The crocin content obtained showed a significant difference between the drying temperatures, but between 55 and 60 °C, the difference was not significant. With drying temperatures at 55 and 60 °C, the crocin content in the product was highest; however, when drying at 55 °C, it will take longer time (5 h) than the rest of the samples. In contrast, at 65 and 70 °C, the drying time is fast, and the crocin content is significantly reduced. It maybe due to the bioactive compound being easily changed, oxidized, and decomposed at high temperature for a long time [33]. Therefore, the powder sample obtained from foam-mat drying at 60 °C was selected. The results obtained are quite similar to the study of Kandasamy et al. [34], where the optimum drying temperature for the papaya powder foam-drying process was 60 °C for 180 min, and the product met the desired quality requirements.
The foam-drying technique presents several advantages such as shorter drying time due to the physical structure of the foam (honeycomb structure), resulting in easy and rapid removal of moisture from liquid foods, reducingthe loss of nutrients and moisture in the end product by about 2–2.5% [16]. The analysis results showed that when the drying temperature increases, the moisture content of the powder tends to decrease. Specifically, the moisture content decreased from 6.64% to 5.75% when the temperature increased from 55 to 70 °C. At a drying temperature of 55 °C, the moisture content of the powder decreased very slowly and needed a longer time (reached 6.64% after 5.5 drying hours) than the rest of the samples. Drying at 60 and 65 °C for 4 h, the moisture content of the powder was 5.82% and 5.78%, respectively, which had no significant difference between them. When drying at 70 °C for 3.5 h, the foam system has a moisture content suitable for grinding into powder; however, when drying at high temperature for a long time, the powder is easily changed, oxidized, and decomposed [33,35,36]. According to Wilkowska et al. [37], blueberry powder with a moisture content of 3.9% was satisfactory in terms of quality and suitable for storage. Franceschinis et al. [38] also found that blackberry powder with 6% moisture content was satisfactory.
High drying temperature causes more free water to escape because the heat delivery rate of hot air to the foam mat is increased, which in turn leads to an increase in the free water migration rate [39]. When the temperature increases from 55 to 70 °C, aw decreases by 0.34, 0.33, 0.328, and 0.311, respectively. The water activity of the dried fruit pulp is low, which is convenient for preservation. Bacteria cannot grow at aw < 0.85, and yeast and mold cannot grow at aw < 0.70 and <0.65, respectively [40]. In this case, the water activity and powder moisture content of foam-mat dried powder at drying temperatures from 55 to 70 °C are satisfactory.The obtained results are similar to the published results of Rao et al. [41]; powder with aw from 0.2 to 0.3 reduces microbial growth and oxidative and enzymatic activities. In related studies on soluble powder products, it was also shown that fruit powder had aw ranging from 0.2 to 0.3 [42]; yogurt products dried at 50, 60, and 70 °C had aw values ranging from 0.32 to 0.35.
4. Conclusions
In this study, the extraction process by ethanol and drying by foam-drying method were quite suitable to recover powder from gardenia fruit extract. RSM proved an effective technique for analyzing interactions among factors and optimizing the processes or products where multiple variables may influence the outputs. This work determined the optimum extraction conditions (ethanol concentration (56%), extraction temperature (55 °C), time (57 min), and percent of fruits in solvent (24%)) for a natural gardenia yellow pigment by the RSM method to improve extraction yields of crocin. The mathematical models which can describe and predict the experimental data of the extraction would be extremely helpful in the extraction process of the natural products. There is a strong interest in the food industry in studying the extraction optimization of natural plants to produce higher quality products. The possible uses of crocin extract powders in food systems, chemical composition, and storage potential should be studied in future projects.
Author Contributions
Conceptualization, N.M.T., N.V.T. and V.Q.M.; methodology, N.M.T. and N.V.T.; software, N.V.T. and P.H.N.; validation, N.M.T. and N.V.T.; formal analysis, P.H.N.; investigation, P.H.N.; writing—original draft preparation, N.V.T., N.M.T., P.H.N. and V.Q.M.; writing—review and editing, N.M.T., N.V.T. and V.Q.M.; supervision, N.M.T. and V.Q.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, N.; Bian, Y.; Yao, L. Essential Oils of Gardenia jasminoides J. Ellis and Gardenia jasminoides f. longicarpa Z.W. Xie & M. Okada Flowers: Chemical Characterization and Assessment of Anti-Inflammatory Effects in Alveolar Macrophage. Pharmaceutics 2022, 14, 966. [Google Scholar] [CrossRef]
- Chen, L.; Li, M.; Yang, Z.; Tao, W.; Wang, P.; Tian, X.; Li, X.; Wang, W. Gardenia jasminoides Ellis: Ethnopharmacology, phytochemistry, and pharmacological and industrial applications of an important traditional Chinese medicine. J. Ethnopharmacol. 2020, 257, 112829. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, H.; Tian, X.; Zhao, C.; Cai, L.; Liu, Y.; Jia, L.; Yin, H.-X.; Chen, C. Antioxidant potential of crocins and ethanol extracts of Gardenia jasminoides ELLIS and Crocus sativus L.: A relationship investigation between antioxidant activity and crocin contents. Food Chem. 2008, 109, 484–492. [Google Scholar] [CrossRef]
- He, M.-L.; Cheng, X.-W.; Chen, J.-K.; Zhou, T.-S. Simultaneous Determination of Five Major Biologically Active Ingredients in Different Parts of Gardenia jasminoides Fruits by HPLC with Diode-Array Detection. Chromatographia 2006, 64, 713–717. [Google Scholar] [CrossRef]
- Ochiai, T.; Shimeno, H.; Mishima, K.-i.; Iwasaki, K.; Fujiwara, M.; Tanaka, H.; Shoyama, Y.; Toda, A.; Eyanagi, R.; Soeda, S. Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2007, 1770, 578–584. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Jahanian, Z. Effect of Crocus sativus L. (saffron) stigma and its constituents, crocin and safranal, on morphine withdrawal syndrome in mice. Phytother. Res. 2010, 24, 726–730. [Google Scholar] [CrossRef]
- Magesh, V.; Singh, J.P.V.; Selvendiran, K.; Ekambaram, G.; Sakthisekaran, D. Antitumour activity of crocetin in accordance to tumor incidence, antioxidant status, drug metabolizing enzymes and histopathological studies. Mol. Cell. Biochem. 2006, 287, 127–135. [Google Scholar] [CrossRef]
- Sarfarazi, M.; Jafari, S.M.; Rajabzadeh, G.; Feizi, J. Development of an environmentally-friendly solvent-free extraction of saffron bioactives using subcritical water. LWT 2019, 114, 108428. [Google Scholar] [CrossRef]
- Tong, Y.; Jiang, Y.; Guo, D.; Yan, Y.; Jiang, S.; Lu, Y.; Bathaie, S.Z.; Wang, P. Homogenate Extraction of Crocins from Saffron Optimized by Response Surface Methodology. J. Chem. 2018, 2018, 9649062. [Google Scholar] [CrossRef]
- Budisa, N.; Schulze-Makuch, D. Supercritical Carbon Dioxide and Its Potential as a Life-Sustaining Solvent in a Planetary Environment. Life 2014, 4, 331–340. [Google Scholar] [CrossRef]
- Karasu, S.; Bayram, Y.; Ozkan, K.; Sagdic, O. Extraction optimization crocin pigments of saffron (Crocus sativus) using response surface methodology and determination stability of crocin microcapsules. J. Food Meas. Charact. 2019, 13, 1515–1523. [Google Scholar] [CrossRef]
- Maran, J.P.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Extraction of natural anthocyanin and colors from pulp of jamun fruit. J. Food Sci. Technol. 2015, 52, 3617–3626. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thuy, N.M.; Ben, T.C.; Minh, V.Q.; Van Tai, N. Effect of extraction techniques on anthocyanin from butterfly pea flowers (Clitoria ternatea L.) cultivated in Vietnam. J. Appl. Biol. Biotechnol. 2021, 9, 173–180. [Google Scholar] [CrossRef]
- Thuy, N.M.; Han, D.H.N.; Minh, V.Q.; Van Tai, N. Effect of extraction methods and temperature preservation on total anthocyanins compounds of Peristrophe bivalvis L. Merr leaf. J. Appl. Biol. Biotechnol. 2022, 10, 146–153. [Google Scholar] [CrossRef]
- Hadizadeh, F.; Mohajeri, S.A.; Seifi, M. Extraction and purification of crocin from saffron stigmas employing a simple and efficient crystallization method. Pak. J. Biol. Sci. 2010, 13, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Paridhi, G.; JD, B.; DM, K. Optimization of process conditions for the development of tomato foam by box-behnken design. Food Nutr. Sci. 2012, 3, 925–930. [Google Scholar] [CrossRef]
- Wilson, R.A.; Kadam, D.M.; Chadha, S.; Sharma, M. Foam mat drying characteristics of mango pulp. Int. J. Food Sci. Nutr. Eng. 2012, 2, 63–69. [Google Scholar] [CrossRef]
- Bien, T.; Luyen, B.; Han, N. Preparative separation and purification of geniposide from Gardenia jasminoides ellis fruit using macroporous adsorption resin D101. Pharm. Sci. Asia 2018, 42, 29–36. [Google Scholar] [CrossRef][Green Version]
- Thuy, N.T.T.; Hien, N.T. Extraction and study of stability of crocin colorant from gardenia fruit. Vietnam J. Agric. Sci. 2016, 14, 1978–1985. [Google Scholar]
- Huang, H.; Zhu, Y.; Fu, X.; Zou, Y.; Li, Q.; Luo, Z. Integrated natural deep eutectic solvent and pulse-ultrasonication for efficient extraction of crocins from gardenia fruits (Gardenia jasminoides Ellis) and its bioactivities. Food Chem. 2022, 380, 132216. [Google Scholar] [CrossRef]
- Van Tai, N.; Linh, M.N.; Thuy, N.M. Optimization of extraction conditions of phytochemical compounds in “Xiem” banana peel powder using response surface methodology. J. Appl. Biol. Biotechnol. 2021, 9, 56–62. [Google Scholar] [CrossRef]
- Al-Farsi, M.A.; Lee, C.Y. Optimization of phenolics and dietary fibre extraction from date seeds. Food Chem. 2008, 108, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, M.; Ali, M.; Ahmad, A. Modelling for extraction of major phytochemical components from Eurycoma longifolia. J. Appl. Sci. 2010, 10, 2572–2577. [Google Scholar] [CrossRef][Green Version]
- Cracolice, M.S.; Peters, E.I. Introductory Chemistry: An Active Learning Approach; Cengage Learning: Singapore, 2020. [Google Scholar]
- Silva, E.M.; Rogez, H.; Larondelle, Y. Optimization of extraction of phenolics from Inga edulis leaves using response surface methodology. Sep. Purif. Technol. 2007, 55, 381–387. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Shi, J.; Mazza, G.; Le Maguer, M. Functional Foods: Biochemical and Processing Aspects, Volume 2; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.d.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Bathaie, S.Z.; Farajzade, A.; Hoshyar, R. A review of the chemistry and uses of crocins and crocetin, the carotenoid natural dyes in saffron, with particular emphasis on applications as colorants including their use as biological stains. Biotech. Histochem. 2014, 89, 401–411. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Sharmila, G.; Muthukumaran, C.; Suriya, E.; Muppidathi Keerthana, R.; Kamatchi, M.; Kumar, N.M.; Anbarasan, T.; Jeyanthi, J. Ultrasound aided extraction of yellow pigment from Tecoma castanifolia floral petals: Optimization by response surface method and evaluation of the antioxidant activity. Ind. Crops Prod. 2019, 130, 467–477. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Oxygen radical absorbance capacities of grape/wine industry byproducts and effect of solvent type on extraction of grape seed polyphenols. J. Food Compos. Anal. 2006, 19, 41–48. [Google Scholar] [CrossRef]
- Asami, D.K.; Hong, Y.-J.; Barrett, D.M.; Mitchell, A.E. Comparison of the Total Phenolic and Ascorbic Acid Content of Freeze-Dried and Air-Dried Marionberry, Strawberry, and Corn Grown Using Conventional, Organic, and Sustainable Agricultural Practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, P.; Varadharaju, N.; Shaik, K.; Moitra, R. Preparation of Papaya Powder under Foam-Mat Drying Technique using Egg Albumin as Foaming Agent. Int. J. Bio.-Resour. Stress Manag. 2012, 3, 324–331. [Google Scholar]
- Thuy, N.M.; Tien, V.Q.; Tuyen, N.N.; Giau, T.N.; Minh, V.Q.; Tai, N.V. Optimization of Mulberry Extract Foam-Mat Drying Process Parameters. Molecules 2022, 27, 8570. [Google Scholar] [CrossRef] [PubMed]
- Thuy, N.M.; Tien, V.Q.; Van Tai, N.; Minh, V.Q. Effect of Foaming Conditions on Foam Properties and Drying Behavior of Powder from Magenta (Peristropheroxburghiana) Leaves Extracts. Horticulturae 2022, 8, 546. [Google Scholar] [CrossRef]
- Wilkowska, A.; Ambroziak, W.; Czyżowska, A.; Adamiec, J. Effect of Microencapsulation by Spray Drying and Freeze Drying Technique on the Antioxidant Properties of Blueberry (Vaccinium myrtillus) Juice Polyphenolic Compounds. Pol. J. Food Nutr. Sci. 2016, 66, 11–16. [Google Scholar] [CrossRef]
- Franceschinis, L.; Salvatori, D.M.; Sosa, N.; Schebor, C. Physical and Functional Properties of Blackberry Freeze- and Spray-Dried Powders. Dry. Technol. 2014, 32, 197–207. [Google Scholar] [CrossRef]
- Demir, V.; Gunhan, T.; Yagcioglu, A.K.; Degirmencioglu, A. Mathematical Modelling and the Determination of Some Quality Parameters of Air-dried Bay Leaves. Biosyst. Eng. 2004, 88, 325–335. [Google Scholar] [CrossRef]
- Perera, C.O. Selected Quality Attributes of Dried Foods. Dry. Technol. 2005, 23, 717–730. [Google Scholar] [CrossRef]
- Rao, M.A.; Rizvi, S.S.; Datta, A.K.; Ahmed, J. Engineering Properties of Foods; CRC press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Breda, C.A.; Sanjinez-Argandoña, E.J.; Correia, C.d.A.C. Shelf life of powdered Campomanesia adamantium pulp in controlled environments. Food Chem. 2012, 135, 2960–2964. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).