Abstract
We tested the effect of varying percentages (v/v) of peatmoss and compost (60/40, T1; 40/60, T2; and 20/80, T3) on growth and macronutrient concentration of lulo (Solanum quitoense Lam.) seedlings in a completely randomized experiment with ten replicates under greenhouse conditions. Lulo seedlings displayed higher plant height and stem diameter when grown in T1 and T2, as compared to T3. In root tissues, N concentration was higher in plants grown in T1, and the same trend was observed in leaves, though differences were not significant in the latter. All other nutrient concentrations analyzed in root tissues were higher in plants under T3. These results are directly related to a higher biomass production in roots as compared to shoots (52.5% higher) found in T3. In leaf tissues, however, significant increases in plants exposed to T3 were only evident for Ca and S concentrations (i.e., 10.6 and 2.6 g kg−1 DBW). Considering dry biomass weight (DBW), lulo plants exhibited a significant and positive correlation between shoot (ShDBW) and total dry biomass (TDBW), whereas low and negative correlations were observed between root DBW and ShDBW. Therefore, a peatmoss/compost ratio of 0.66 (40/60, T2) results in a better plant growth performance, ensuring a good plant nutrient status for lulo seedlings.
1. Introduction
Lulo (Solanum quitoense Lam.) is a horticultural species native to the Andes Mountains of South America, especially Colombia, Ecuador, and Peru, countries where its production and consumption are concentrated [1]. Its fruit is highly prized because of its flavor, aroma, and nutraceutical properties, including high contents of vitamins A and C and other antioxidant compounds [2,3]. These facts are leading to an increased domestic and international market demand [4,5]. Moreover, this fruit provides raw material for the food industry and active compounds for the pharmaceutical and novel food industries, among others [5]. When plants reach maturity, nearly 40% of the total dry biomass corresponds to stems, 33.8% to fruits, 15.54% to leaves, 10.56% to roots, and 0.4% to flowers [3].
Due to various technical constraints, crop production is insufficient to meet regional and international demands. Indeed, imports of this fruit in the United States have significantly risen in recent years. Moreover, research on its cultivation is still in its infancy [5]. In fact, the lulo-growing industry has developed as a result of initiatives taken by the growers themselves, without significant scientific support in terms of research, technology and innovation [6]. Therefore, there is a big need for carrying out research aimed at overcoming some technical limitations that currently limit its expansion and competitiveness, including studies on the effect of substrates on germination and production of healthy seedlings in containers under greenhouse or nursery conditions.
Horticultural crops produced in containers rely on adequate substrates for proper growth, while suitable mixture of materials is a sine qua non to obtain a good substrate that allows optimal conditions for plants [7,8]. The development of novel horticultural substrates is continually evolving to meet current constraints, demands, and trends in the production of most horticultural crops. In containers, plant roots are restricted to a small volume, so their demand for water, nutrients, and support is greater compared to those produced in the field where root growth occurs without space limitations [8]. In order to produce healthy and high-quality plants, while increasing the surface area available for absorption of water and nutrients, a vigorous root system is an absolutely indispensable condition [9,10].
Sexual propagation of lulo is the main method for obtaining plants, and the best protocol to obtain seeds is pulp fermentation [2]. Importantly, each lulo fruit may produce up to 1000 seeds [11]. After sowing, those seeds can germinate in 15–20 days. Once seeds are germinated, seedlings should remain in the nursery or greenhouse for approximately 30 days [12]. High-quality seedlings exhibit outstanding morphological characteristics such as thick stems, dark-green leaves, and large, soft roots [13], which in the end can improve fruit yield and quality at harvest.
Lulo plants require slightly acidic soil (pH 5.5 to 6.5) and respond to both organic and mineral fertilization [11]. For instance, lulo cv. La Selva produced higher yields when composted poultry manure was applied, whereas vermicompost had similar effects to mineral fertilization (N-P-K: 10-30-10) [14]. Importantly, a lack of nutrients in lulo plants negatively influences plant growth, causing a high biomass accumulation in the roots [15].
Regarding organic substrates, lulo seeds can germinate and produce healthy seedlings at a peatmoss/compost ratio of 1.50 to 0.66, which corresponds to 40% to 60% compost content in the substrate, without the application of chemical fertilizers [12]. Furthermore, seedling growth rates of lulo are improved when supplying 40% to 60% compost in the substrate [16]. Importantly, an optimal nutrient supply through an adequate organic substrate mixture may improve plant growth and biomass production [17,18,19], though just a few studies have addressed these responses in lulo at the seedling stage [5,12]. We hypothesize that different combinations of organic substrate mixtures may differentially affect the nutrient status and growth of lulo plants at early developmental stages. Hence, in this study we aimed to deeply analyze the macronutrient concentrations and growth parameters of lulo seedlings established in substrates with three different peatmoss/compost combinations (in percentage, v/v): 60/40 (T1), 40/60 (T2), and 20/80 (T3).
2. Materials and Methods
The experiment was carried out for two months in a rectangular greenhouse (30 × 12 m) with a gable roof covered with a shade net, which allowed 70% light transmittance. The experimental station was located in central Veracruz, Mexico at 18°50′ NL, 96°51′ WL and 650 m altitude. The local climate is tropical humid, with summer rainfall and an annual average temperature of 20 °C, maximum 35 °C and minimum 10 °C, and annual average rainfall over 1800 mm [20].
2.1. Experimental Design and Treatments
The experimental design was completely randomized with ten replicates. Each experimental unit was represented by four seedlings, with 10 repetitions per treatment, and concomitantly, a total of 40 seedlings for each treatment. Each repetition per treatment was completely randomized in four germination trays of 128 cavities each, as depicted in Figure 1.
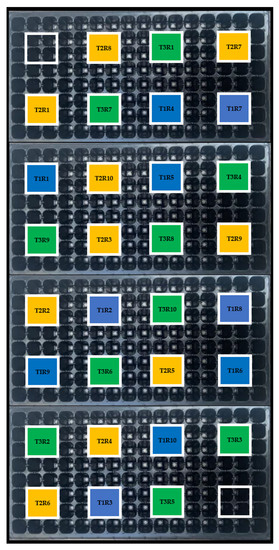
Figure 1.
Randomized distribution of treatments and repetitions in the experiment designed to test the effect of different peatmoss/compost combinations on growth and macronutrient concentrations of lulo (Solanum quitoense Lam.) seedlings under greenhouse conditions. Treatments are as follows (percentage of peatmoss/compost, v/v): T1: 60/40; T2: 40/60; T3: 20/80. Colors of the boxes within the tray represent the three different treatments tested: Blue for T1; Yellow for T2; and Green for T3.
The treatments consisted of different combinations of peatmoss and composted sugarcane filter cake (in percentage, v/v): treatment 1 (T1): 60/40 (peatmoss/compost); treatment 2 (T2): 40/60 (peatmoss/compost); and treatment 3 (T3): 20/80 (peatmoss/compost). The nutrient concentrations of these substrates are shown in Table 1, while the physical and chemical properties are displayed in Table 2.

Table 1.
Mineral composition of the substrate mixtures composed of peatmoss and compost at different percentages (v/v) used to grow lulo (Solanum quitoense Lam.) seedlings.

Table 2.
Physical and chemical properties of the substrate mixtures composed of peatmoss and compost at different percentages (v/v) used to grow lulo (Solanum quitoense Lam.) seedlings.
2.2. Seed Extraction and Fermentation
Lulo seeds extracted from vine-ripened fruits were first fermented for 48 h in 500 mL glass flasks using tap water. Fermented seeds were washed with distilled water and then dried at room temperature for 48 h.
2.3. Experiment Establishment
Dried seeds were sown in the substrate mixtures according to treatments, using four 128-cavity germination trays for each treatment tested. One seed was deposited in each cavity (Figure 1). Trays were kept under moderate shade (70% of transmittance) in the greenhouse and irrigated daily with tap water until seedlings reached 5 cm in height on average (60 days after sowing).
2.4. Growth Parameters
Sixty days after seed sowing, we measured plant height (PH) with a ruler, and stem diameter (SD) using a digital caliper. Subsequently, seedlings were harvested and dissected into leaves and stems (shoots) and roots, and dried in an air-forced oven (Riossa, HCF-125D, Monterrey, Mexico) for 48 h at 72 °C. After drying, samples were weighed in an OHAUS Adventurer Pro AV213C analytical balance (Parsippany, NJ, USA) to determine dry biomass weight of shoots (ShDBW) and roots (RDBW). By summing ShDBW plus RDBW we calculated the total dry biomass weight (TDBW).
2.5. Macronutrient Analyses in Plant Tissues
Macronutrient concentrations were analyzed in dried and mill-ground tissues of leaves and roots, as described by Alcántar-González and Sandoval-Villa [21]. Accordingly, concentrations of P, K, Ca, Mg, and S were quantified by inductively coupled plasma optical emission spectroscopy (ICP-OES; Agilent 725-ES; Santa Clara, CA, USA), in the extracts resulting from the double digestion of samples with nitric (HNO3) and perchloric (HClO4) acids. Nitrogen concentration was estimated using the micro-Kjeldahl method.
2.6. Statistical Analyses
The assumption of normality of data was determined by the Shapiro–Wilk and Kolmogorov–Smirnov tests at 5% significance, while the homogeneity of variance was estimated by the Bartlett test.
Plant height, stem diameter, shoot and total dry biomass weight, and nutrient concentration in roots were subjected to analysis of variance (PROC ANOVA) using SAS 9.1 statistical software [22]. Means comparison was carried out using Tukey’s test and statistical significance was obtained at a 95% confidence level (α = 0.05).
Data from root dry biomass weight and foliar nutrient concentrations did not meet the normality and homogeneity assumptions, so Wilcoxon’s rank summation tests were performed using SAS 9.1 statistical software [22].
For the means corresponding to total dry biomass weight (TDBW), that of shoots (ShDBW) and roots (RDBW), as well as plant height (PH), and stem diameter (SD), we estimated the Pearson correlations among them using the SAS package [22].
3. Results and Discussion
Physical and chemical properties of organic substrates have tremendous impacts on plant growth and development [9,10]. Under our experimental conditions, all primary macronutrients in the substrates increased by raising the content of compost in the substrate mixtures (Table 1). Nitrogen concentrations rose from 1.38% to 1.51% in T1 and T3, respectively. In turn, extractable P concentration rose from 4.52 to 6.58 mg kg−1 in T1 and T3, respectively, while soluble K concentration increased from 233.43 mg L−1 in T1 to 256.89 mg L−1 in T2 and 274.09 mg L−1 in T3. Interestingly, we observed an increase in the nitrate (NO3−) concentration from 1022 mg kg−1 in T1 to 1316 mg kg−1 in T3.
Other chemical and physical properties of these substrate mixtures have been reported elsewhere [12,16], and are summarized in Table 2. In general, bulk density, pH, and electrical conductivity (EC), as well as the Na+, Cl−, and HCO3− concentrations increased by raising the compost volume in the substrate mixture. These results are consistent with other studies. For instance, manure composting involved an increase in pH, EC, cation exchange capacity (CEC), and some anions [23]. The release of mineral salts due to the decomposition of organic matter (OM) and the concentration effect due to a net loss of dry mass lead to an increase in EC values [24]. Additionally, inorganic forms of nutrients (i.e., nitrates and sulfates) and the increase in organic acids (e.g., acetic acid) during composting may contribute to increase the EC values [25]. In this study, the EC values found in the substrate mixtures (T1: 1.74; T2: 2.11; and T3: 2.43 dS m−1) were lower than the upper limit (4 dS m−1) considered tolerable by plants of medium salt sensitivity [26]. Such EC values may be related to the accumulation of partially oxidized OM, such as low molecular weight organic acids [25]. The presence of a high percentage of C-rich raw materials favors OM oxidation, which eventually becomes highly stabilized [24], which may improve plant health and vigor when properly managed.
3.1. Plant Growth Analyses and Correlation among Variables
The treatments tested differentially affected plant height and stem diameter, with the highest means found in T1 and T2 (Figure 2). Treatment 3 displayed the lowest mean for both variables. Importantly, both growth variables in T3 were statistically different to T1 and T2.
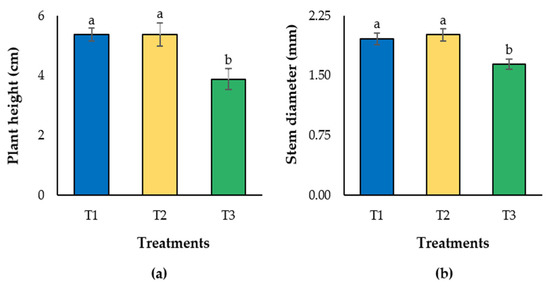
Figure 2.
Plant height (a) and stem diameter (b) of lulo (Solanum quitoense Lam.) seedlings grown in different combinations of peatmoss and compost. Columns are means of 10 seedlings per treatment randomly selected, one per repetition. Bars on the columns indicate standard deviation. Different letters on the columns indicate significant differences among treatments. Treatments are as follows (percentage of peatmoss/compost, v/v): T1: 60/40; T2: 40/60; T3: 20/80.
Lulo seedlings respond both to the application of vermicompost and composted poultry manure as well as to diammonium phosphate (DAP) [14]. In fact, the application of 1 kg organic matter (OM) per plant (preferably composted) at the bottom of the hole into which the plants are transplanted in the field is strongly recommended by the Colombian Fundación Codesarrollo [27].
However, a high compost content in the substrate mixtures for lulo plants may result in drastic reductions in growth, including significant decreases in leaf area ratio (LAR), specific leaf area (SLA), and leaf ratio (LR) [16]. Furthermore, both plant height and stem diameter are lower in plants under treatment containing 80% compost (T3), in comparison to treatments containing less compost in the substrate mixtures (i.e., either 40% or 60%) [12].
These behaviors can be explained in light of the physical and chemical properties of the substrates used. For instance, as shown in Table 2, bulk density was higher (0.30 g cm−3) in the substrate contained in treatment 3 (peatmoss/compost, 20/80) in comparison to the other treatments (i.e., 0.21 g cm−3 for T1 and 0.25 g cm−3 for T2). It is well known that bulk density has a tremendous effect on plant growth, and the closer to 0.15 g cm−3, the better it is [28]. Accordingly, lulo plants grown in T3 (with the highest value for bulk density) displayed smaller size than those grown in T1 and T2 (Figure 2). Likewise, tomato plants grown in composted filter cake having a bulk density of 0.36 g cm−3 displayed growth penalties, while those grown in peatmoss with a bulk density of 0.15 g cm−3 developed better [29]. Moreover, total porosity and air space were lower in the substrate mixture containing 80% compost (T3). Total porosity and air space are probably the most important physical properties affecting substrate quality. While it is generally recommended that the air space value be above 10%, the substrate mixture in T3 containing 80% compost had an air space of only 8%, which may explain in part the negative effects on growth parameters observed in our study.
Concerning chemical properties of the substrate mixtures, pH (6.54) and electrical conductivity (EC) (2.43 dS m−1) were higher in the substrate mixture containing 80% compost (T3) in comparison to those values observed in T1 and T2 (Table 2). As lulo prefers soils with low pH (between 5 and 6), better growth can be expected in substrates with lower pH [5]. Accordingly, plants displayed better growth when established in substrate mixtures with lower pH (i.e., T1 with 6.36 and T2 with 6.51) [16]. In addition, EC reached 2.43 dS m−1 in T3, whereas T1 displayed 1.74 dS m−1, and in T2 it was 2.11 dS m−1. Electrical conductivity is directly correlated with salts contained in the growth media [28], and lulo plants are drastically affected by salinity [30]. Therefore, this fact also helps explain the reduction in growth parameters previously reported [16]. Likewise, cation exchange capacity (CEC) and organic matter (OM) in the substrates are strongly correlated, as organic components in the substrate improve CEC through an increase in available negative charges. As such, OM build-up in soil usually positively impacts soil fertility. Again, a general reduction in the CEC of substrates was observed as the compost percentage increased, and the OM content decreased (Table 2).
Though roots of lulo seedlings grown under the highest compost content (80%) developed the greatest length (12.11 cm) in comparison to plants grown in substrates containing either 40 (7.14 cm) or 60% (8.03 cm) [12], other growth parameters including leaf area ratio (LAR), specific leaf area (SLA), and leaf ratio (LR) were lower in plants under T3, which has been associated with higher electrical conductivity (Table 1) and Na+, Cl-, and HCO3. concentrations found in the substrate mixture of T3 [16].
Like plant height, shoot dry biomass weight of seedlings grown in T3 was 24.4 and 22.4% lower than those observed in T1 and T2, respectively (Figure 3a). Likewise, total dry biomass weight of seedlings exposed to T3 was significantly lower than that observed in T1, with a reduction of 22.8% (Figure 3b).
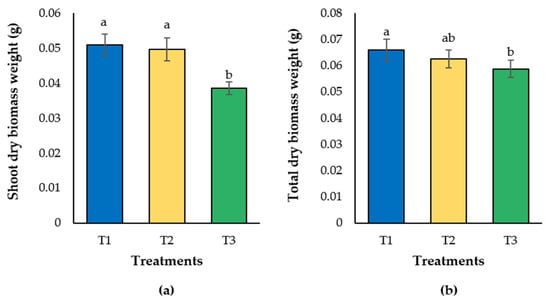
Figure 3.
Shoot (a) and total dry biomass weight (b) of lulo (Solanum quitoense Lam.) seedlings grown in different combinations of peatmoss and compost. Columns are means of 10 seedlings per treatment, randomly selected, one per repetition. Bars on the columns indicate standard deviation. Different letters on the columns in each subfigure indicate significant differences among treatments. Treatments are as follows (percentage of peatmoss/compost, v/v): T1: 60/40; T2: 40/60; T3: 20/80.
Contrary to the responses observed in ShDBW and TDBW, T3 increased RBDW by 34.4 and 55.7% compared to T1 and T2, respectively (Figure 4). The mean ratios of ShDBW and RDBW were 3.4, 3.87, and 1.9 in T1, T2, and T3, respectively. Therefore, in T1, T2, and T3, RDBW represented 29.5, 26.2, and 52.5%, respectively, that of ShDBW. The root is an organ of pivotal importance for plants, since it has several functions, principally to anchor and support the plant to the soil or the substrate. Moreover, it has a key role in nutrient and water absorption, hormone synthesis, food storage, and tolerance to osmotic stress. The shoot/root ratio is a morphological attribute that reflects the integrity and complexity of the plant, and each genotype, even within the same species, may display specific values for this ratio [31]. Plants exposed to different abiotic stress factors may exhibit significant changes regarding biomass partitioning. For instance, in Eucalyptus camaldulensis plants exposed to moderate (8 dS m−1) or high (16 dS m−1) saline stress, root biomass represented 30.6 and 34.6% of the total biomass, respectively, compared to the control (2 dS m−1), whose root produced 25% of the total biomass produced [32]. Under drought conditions (water potential of soil at −60 MPa), two wheat (Triticum aestivum) cultivars (YM13 and YN19) displayed a root/shoot ratio of 0.395, while the control (water potential of soil at −20 MPa) exhibited a ratio of 0.35 [33]. Similarly, nutrient deficiencies may inhibit shoot growth, with a concomitantly increased root growth to explore more area in the soil or growth medium [34]. In our experiment, T3 did not stimulate shoot growth because of the less favorable physical (less porosity and air space) and chemical (lower CEC and OM, but higher EC) properties, as described in Table 2. The higher biomass production in roots (52.5% higher) as compared to shoots observed in T3 represents an adaptive mechanism of plants to improve nutrient uptake under low nutrient availability conditions imposed by a reduction in total air space and ion exchange capacity, and an increased electrical conductivity resulting from the higher percentage of compost in T3, as compared to T1 and T2.
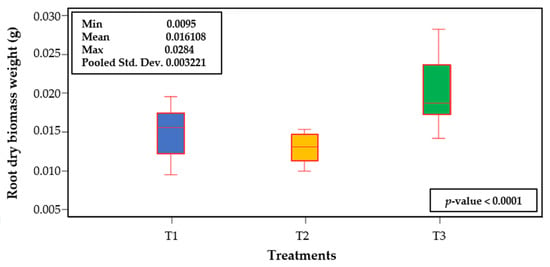
Figure 4.
Box diagram of root dry biomass weight of lulo (Solanum quitoense Lam.) seedlings grown in different combinations of peatmoss and compost. Data of 10 seedlings per treatment, randomly selected, one per repetition. Wilcoxon’s rank summation test (p ≤ 0.05). Treatments are as follows (percentage of peatmoss/compost, v/v): T1: 60/40; T2: 40/60; T3: 20/80.
Organic substrates are more susceptible to compaction than inorganic ones, and soil compaction results in poor root development. Importantly, optimized mixtures of growing substrates guarantee optimal conditions for plant growth by providing improved physical and chemical properties [35]. The tested substrates significantly influenced the growth responses observed in lulo plants in our study (Figure 2). Thus, plants grown in T1 (with the lowest compost content tested) and T2 (with the mean compost content proven), reached higher mean values for height and stem diameter compared to T3 (which contained the highest percentage of compost tested) (Figure 2). These responses are in full agreement with those observed previously [12], demonstrating that substrate characteristics are critical for plant growth and development [28].
We analyzed the correlations among total dry biomass weight (TDBW), that of shoots (ShDBW) and roots (RDBW), as well as plant height (PH) and stem diameter (SD). Lulo plants exhibited a significant and positive correlation between ShDBW and TDBW. Nevertheless, RDBW showed a low and negative correlation with ShDBW (Table 3). Because of the positive correlation between ShDBW and TDBW, we can observe a more efficient dry biomass production in the aboveground parts of the plant, at the expense of the root. Accordingly, higher dry biomass accumulation was recorded in the foliage of two lulo varieties during the first 100 days of growth, resulting in an 80/20 shoot/root ratio [36], which is in full agreement with our results. Indeed, lulo plants grown in substrates containing either 40/60 or 60/40 (v/v) peatmoss/compost ratios accumulated higher dry biomass weight in the aboveground parts in comparison to the roots [16]. Furthermore, in tomato (which also belongs to the Solanaceae family) plants exposed to different shade treatments, a higher accumulation of photoassimilates in leaves than roots was reported [37]; such biological compounds formed by assimilation using light-dependent reactions stimulated aboveground growth.

Table 3.
Correlation among growth variables of lulo plants (Solanum quitoense Lam.) grown in different peatmoss/compost percentages (v/v) under greenhouse conditions for two months.
Lulo displays high genetic variability [38], which is observed at the morphological and physiological levels [39]. Such variability is a great advantage for future breeding programs aimed to find elite materials to expand the cultivation of this species to other regions. Furthermore, such diversity also supposes different responses to agronomic and nursery practices among genotypes. Exploring such diversity is a daunting task to be addressed in future approaches to find optimal growing-substrate mixtures for germination and growth of healthy and vigorous seedlings.
3.2. Macronutrient Concentrations in Plant Tissues
Treatments significantly affected nutrient concentrations in roots (Table 4). Nitrogen concentration in these tissues decreased as the percentage of compost in the substrate mixture increased. Indeed, the highest N concentration was obtained in roots of plants under T1 containing the lowest compost content (40%), which may be attributed to a possible better balance between NO3− and NH4+ in the substrate mixture for these tissues (Table 1). This species positively responds to increasing N applications, applied to both the soil and the leaves [6]. Conversely, all other macronutrients in roots were higher in plants under T3, which contained 80% compost.

Table 4.
Macronutrient concentrations in root tissues of lulo (Solanum quitoense Lam.) seedlings grown in substrates containing different percentages (v/v) of peatmoss and compost under greenhouse conditions.
Regarding macronutrients in leaves, the results of the Wilcoxon’s rank summation test demonstrate that there were no significant differences among treatments regarding the concentrations of N, P, K, and Mg (Table 5). Nitrogen is the most crucial macronutrient for plant growth at the vegetative stage and its deficiency results in general chlorosis as well as a reduction in leaf area and number of leaves [40]. As shown in Table 5, N concentration was statistically similar in leaves of all treatments. Interestingly, nitrate (NO3−) concentrations in the substrate mixture were the highest in T3 (peatmoss/compost: 20/80). It is well known that some species of the Solanaceae family prefer a medium nitrate/ammonium ratio for optimum growth [41,42,43]. This may be the case for lulo plants, as plant height and root length values were the lowest in T3 [16], which contained the highest NO3−/NH4+ ratio (31.33) compared to T1 (i.e., 24.33) and T2 (7.41) (Table 1). Indeed, the standard Hoagland nutrient solution containing a nitrate/ammonium ratio of 6.0/0.5 results in significant reductions in growth, whereas a lower NO3−/NH4+ ratio (i.e., 3.5/3.0) causes growth stability in tomato plants [44].

Table 5.
Concentrations of N, P, K, and Mg in leaf tissues of lulo (Solanum quitoense Lam.) seedlings grown in substrates containing different percentages (v/v) of peatmoss and compost under greenhouse conditions.
Similarly, P concentrations in leaves (Table 5) were directly correlated with the P levels found in the substrates (Table 1). Nevertheless, the average P levels in our study (~15 g kg−1 DBW) are much lower than those reported by Flórez et al. [45] (~32 g kg−1 DBW). This difference may be due to the experimental conditions. In our study, plants were maintained in the same treatments (different mixtures of peatmoss and compost) for two months after sowing. By contrast, Flórez et al. [45] applied treatments (peatmoss, sand, and agricultural soil either alone or mixed) when plants had already reached two months of age and then maintained those plants under experimentation for 12 weeks (three months), meaning the plants finished the experiment at five months of age.
Although K concentrations in the substrate mixtures were different (Table 1), no statistical differences were observed in K concentrations in leaves. The same trend was observed for Mg concentrations (Table 5).
In leaves, the Wilcoxon’s rank summation test demonstrates that the treatments evaluated were significantly different with respect to Ca and S concentrations (Figure 5). The highest Ca concentration was observed in plant leaves under T3 (80% compost), reaching 10.64 g kg−1 DBW. The lowest concentration for this element was observed in T1, with a mean of 8.05 g kg−1 DBW (Figure 5a). These concentrations are lower than that observed by Flórez et al. [45], which reached 26 g kg−1 DBW in some treatments. Similarly, the Wilcoxon’s rank summation test proved that the S concentrations in leaves was different among treatments (Figure 5b), with the highest mean found in T3 (2.57 g kg−1 DBW), followed by T2 (2.31 g kg−1 DBW) and T1 (1.79 g kg−1 DBW). In lulo, the application of 28 ppm P increased plant height and diminished anthocyanin content in leaves, while 33 ppm S avoided chlorosis and growth penalties [40].
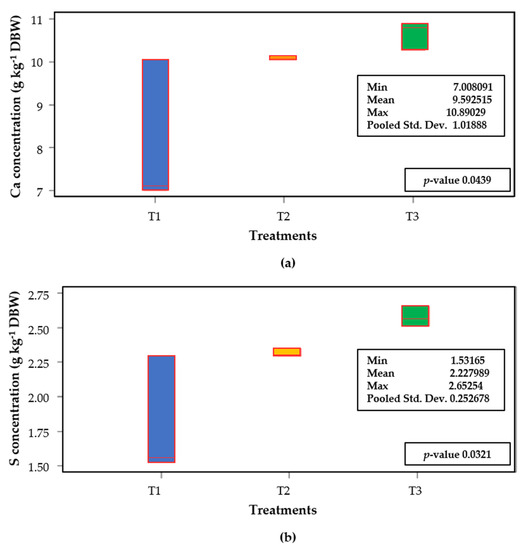
Figure 5.
Box diagram of Ca (a) and S (b) concentrations in leaves of lulo (Solanum quitoense Lam.) seedlings grown in different combinations of peatmoss and compost. Data of five samples (each composed sample was derived from two repetitions per treatment). Wilcoxon’s rank summation test (p ≤ 0.05). Treatments are as follows (percentage of peatmoss/compost, v/v): T1: 60/40; T2: 40/60; T3: 20/80.
4. Conclusions
Herewith we have demonstrated that different combinations of organic substrate mixtures (i.e., peatmoss and composted sugarcane filter cake) differentially affected growth and macronutrient concentrations of lulo seedlings. Importantly, seedlings grew better in substrates containing up to 60% compost in the substrate mixture. In leaf tissues, all macronutrients analyzed except S displayed higher concentrations than in root tissues. Nitrogen concentration was higher in roots of plants grown with the lowest compost content (40%), and the same trend was observed in leaves, though differences were not significant in the latter. In root tissues, all other nutrient concentrations (P, K, Ca, Mg, and S) were higher in plants under T3 (containing the highest compost content). Nevertheless, in leaf tissues only Ca and S displayed higher concentrations in plants under T3. Though the higher content of compost in T3 resulted in higher concentrations of most macronutrients analyzed in roots and of some of them in leaves of plants under this treatment, this increased nutrient concentration did not improve growth parameters. The correlation analyses of growth variables demonstrated a significant and positive relationship between shoot dry biomass weight (ShDBW) and total dry biomass weight (TDBW), though root dry biomass weight (RDBW) showed a low and negative correlation with ShDBW. Therefore, the balance between substrate mixtures must be kept at a peatmoss/compost ratio of 0.66 in order to achieve better plant growth performance, ensuring a good plant nutrient status. To the best of our knowledge, this is the first study reporting the effect of organic growing substrate mixtures on growth and nutrient status of lulo seedlings under greenhouse conditions.
Author Contributions
Conceptualization: L.I.T.-T. and F.C.G.-M.; methodology: J.C.G.-A. and M.G.P.-S.; validation: F.C.G.-M. and L.I.T.-T.; formal analysis: L.I.T.-T. and F.C.G.-M.; investigation: J.C.G.-A. and M.G.P.-S.; resources: L.I.T.-T.; writing—original draft preparation: L.I.T.-T.; writing—review and editing F.C.G.-M.; visualization: F.C.G.-M.; supervision: F.C.G.-M. and L.I.T.-T.; project administration: L.I.T.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Medina-Cano, C.I.; Lobo-Arias, M.; Martínez-Bustamante, E. State of knowledge review on the productive function of lulo (Solanum quitoense Lam.) in Colombia. Corpoica Cienc. Tecnol. Agropec. 2009, 10, 167–179. [Google Scholar] [CrossRef]
- Ramírez, F.; Davenport, T.L. Underutilized, Fruits of the Andes. In Advances in Environmental Research; Daniels, J.A., Ed.; Nova Science Publishers: New York, NY, USA, 2014; pp. 69–88. [Google Scholar]
- Criollo-Escobar, H.; Moncayo-Palacios, M.F.; Lagos-Burbano, T.C. Phenology and growth of lulo (Solanum quitoense Lam.) plants grafted onto Solanum hirtum Vahl. Rev. Colomb. Cienc. Hortíc. 2020, 14, 291–300. [Google Scholar] [CrossRef]
- IICA. Naranjilla. Guía Práctica para la Exportación a Estados Unidos. Instituto Interamericano de Cooperación para la Agricultura. 2007. Available online: http://repositorio.iica.int/bitstream/handle/11324/7813/BVE19040120e.pdf?sequence=1&isAllowed=y (accessed on 7 May 2022).
- Gómez-Merino, F.C.; Trejo-Téllez, L.I.; García-Albarado, J.C.; Cadena-Íñiguez, J. Lulo (Solanum quitoense [Lamarck]) as a new landscape crop in the Mexican agro-ecosystem. Rev. Mex. Cienc. Agríc. 2014, 9, 1741–1753. [Google Scholar]
- Jaime-Guerrero, M.; Álvarez-Herrera, J.; Fischer, G. Physiology and crop aspects of lulo (Solanum quitoense Lam.) in Colombia: A review. Rev. Investig. Agra. Amb. 2022, 13, 131–148. [Google Scholar] [CrossRef]
- Pérez-López, H.; Gómez-Merino, F.C.; Trejo-Téllez, L.I.; García-Morales, S.; Rivera-Olivares, L.Y. Agricultural lignocellulosic waste and volcanic rock combinations differentially affect seed germination and growth of pepper (Capsicum annuum L.). BioResources 2014, 9, 3977–3992. [Google Scholar] [CrossRef][Green Version]
- Trejo-Téllez, L.I.; García-Albarado, J.C.; Méndez-Urbano, D.; Pérez-Sato, J.A.; Gómez-Merino, F.C. Plant growth and nitrogen concentration of Tillandsia species produced in organic, volcanic, and lignocellulosic substrates. J. Plant Nutr. 2018, 41, 2547–2559. [Google Scholar] [CrossRef]
- Jackson, B.E.; Wright, R.D.; Seiler, J.R. Changes in chemical and physical properties of pine tree substrate and pine bark during long-term nursery crop production. HortScience 2009, 44, 791–799. [Google Scholar] [CrossRef]
- Jackson, B.E.; Wright, R.D.; Barnes, M.C. Methods of constructing a pine tree substrate from various wood particle sizes, organic amendments, and sand for desired physical properties and plant growth. HortScience 2010, 45, 103–112. [Google Scholar] [CrossRef]
- Quinchia, C.F.; Cabrera, C.A. Manual Técnico del Cultivo del Lulo (Solanum quitoense Lam.) en el Departamento de Huila; Gobernación del Huila: Neiva, Colombia, 2002; 34p. [Google Scholar]
- Gómez-Merino, F.C.; Trejo-Téllez, L.I.; García-Albarado, J.C.; Morales-Ramos, V. Lulo (Solanum quitoense [Lamarck]) as a new element of the landscape in Mexico: Germination and growth on organic soils. Rev. Mex. Cienc. Agríc. 2013, 5, 877–887. [Google Scholar]
- Oda, M. Raising of vigorous and valuable seedlings. Regul. Plant Growth Dev. 2007, 42, 176–182. [Google Scholar]
- Ramírez, V.H.; Duque, N.N. Response of the lulo fruit cv. La Selva (Solanum quitoense × Solanum hirtum) at the aerobic organic and inorganic fertilizer applications. Acta Agron. 2010, 59, 155–161. [Google Scholar]
- Parra-Coronado, A.; Ardila-Roa, G.H.; Restrepo-Díaz, H. The physiological response of lulo plants (Solanum quitoense var. septentrionale) to soil and foliar applications of nutrients. Int. J. Fruit Sci. 2014, 15, 148–160. [Google Scholar] [CrossRef]
- Gómez-Merino, F.C.; Trejo-Téllez, L.I.; Ladewig, P. Seedlings growth rates of lulo (Solanum quitoense [Lamarck]) in organic substrates. Rev. Mex. Cienc. Agríc. 2014, 9, 1787–1793. [Google Scholar]
- Carlile, W.R.; Cattivello, C.; Zaccheo, P. Organic growing media: Constituents and properties. Vadose Zone J. 2015, 14, 1–13. [Google Scholar] [CrossRef]
- Fortis-Hernández, M.; Antonio-Ordóñez, E.; Preciado-Rangel, P.; Gallegos-Robles, M.A.; Vázquez-Vázquez, C.; Reyes-Gonzáles, A.; Esparza-Rivera, J.R. Effect of substrates formulated with organic materials on yielding, commercial and phytochemical quality, and benefit-cost ratio of tomato (Solanum lycopersicum L.) produced under greenhouse conditions. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 11999. [Google Scholar] [CrossRef]
- Bartley, P.C.; Fonteno, W.C.; Jackson, B.E. A Review and analysis of horticultural substrate characterization by sieve analysis. HortScience 2022, 57, 715–725. [Google Scholar] [CrossRef]
- Soto-Esparza, M. Localidades y Climas del Estado de Veracruz; Instituto Nacional de Investigaciones sobre Recursos Bióticos: Xalapa, Mexico, 1986; 137p. [Google Scholar]
- Alcántar-González, G.; Sandoval-Villa, M. Handbook of Chemical Analyses of Plant Tissues; Mexican Society of Soil Science: Chapingo, Mexico, 1999; 156p. [Google Scholar]
- SAS Institute. SAS/STAT 13.2 Users Guide. What’s New in SAS/STAT 13.2; SAS Institute Inc.: Cary, NC, USA, 2014. [Google Scholar]
- Gil, M.V.; Carballo, M.T.; Calvo, L.F. Fertilization of maize with compost from cattle manure supplemented with additional mineral nutrients. Waste Manag. 2008, 28, 1432–1440. [Google Scholar] [CrossRef]
- Silva, M.E.F.; de Lemos, L.T.; Nunes, O.C.; Cunha-Queda, A.C. Influence of the composition of the initial mixtures on the chemical composition, physicochemical properties and humic-like substances content of composts. Waste Manag. 2013, 34, 21–27. [Google Scholar] [CrossRef]
- Hernández, T.; Masciandaro, G.; Moreno, J.I.; García, C. Changes in organic matter composition during composting of two digested sewage sludges. Waste Manag. 2006, 26, 1370–1376. [Google Scholar] [CrossRef]
- Lasaridi, K.; Protopapa, I.; Kotsou, M.; Pilidis, G.; Manios, T.; Kyriacou, A. Quality assessment of composts in the Greek market: The need for standards and quality assurance. J. Environ. Manag. 2006, 80, 58–65. [Google Scholar] [CrossRef]
- Fundación Codesarrollo. Alianza Productiva de lulo en los Municipios de Santa Rosa y Dosquebradas en el Departamento de Risaralba; Ministerio de Cultura y Desarrollo, Fundación Codesarrollo: Pereira, Colombia, 2006; 172p. [Google Scholar]
- Cabrera, R.I. Properties, use and management of growing media for container plant production. Rev. Chapingo Ser. Hortic. 1999, 5, 5–11. [Google Scholar] [CrossRef]
- Berrospe-Ochoa, E.A.; Ordaz-Chaparro, V.M.; Rodríguez-Mendoza, M.N.; Quintero-Lizaola, R. Filter mud as growth medium on tomato seedling. Rev. Chapingo Ser. Hortic. 2012, 18, 141–156. [Google Scholar]
- Flórez, S.L.; Miranda-Larrispa, D.; Chaves, B. Growth of lulo (Solanum quitoense Lam.) plants affected by salinity and substrates. Rev. Bras. Frutic. Jaboticabal 2008, 30, 402–408. [Google Scholar] [CrossRef][Green Version]
- Bláha, L. Importance of root-shoot ratio for crops production. J. Agron. Agric. Sci. 2019, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, F.; Bakhsh, R.; Qadir, I. Trade-off between shoot and root dry weight along with a steady CO2 assimilation rate ensures the survival of Eucalyptus camaldulensis under salt stress. J. For. Sci. 2020, 66, 452–460. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Y.; Ding, Y.; Pan, R.; Shen, W.; Yu, X.; Xiong, F. The relationship between characteristics of root morphology and grain filling in wheat under drought stress. PeerJ 2021, 9, e12015. [Google Scholar] [CrossRef]
- Lynch, J.; Marschner, P.; Rengel, Z. Effect of internal and external factors on root growth and development. In Marschner´s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Academic Press: London, UK, 2012; pp. 331–346. [Google Scholar]
- Cruz-Crepo, E.; Can-Chulim, A.; Sandoval-Villa, M.; Bugarín-Montoya, R.; Robles-Bermúdez, A.; Juárez-López, P. Sustratos en la horticultura. Rev. BioCiencias 2013, 2, 17–26. [Google Scholar]
- Medina-Cano, C.I.; Martínez-Bustamante, E.; Lobo-Árias, M.; Vargas-Arcila, M.O. Distribución de la materia seca durante la ontogenia del lulo (Solanum quitoense Lam.) a plena exposición solar en el bosque húmedo montano bajo del oriente antioqueño, Colombia. Rev. Fac. Nac. Agron.-Medellín 2008, 61, 4256–4268. [Google Scholar]
- Páez, A.; Paz, V.; López, J.C. Growth and physiological responses of tomato plants cv. Río Grande during May to July season. Effect of shading. Rev. Fac. Agron. Univ. Zulia 2000, 17, 173–184. [Google Scholar]
- Morillo-Coronado, A.C.; Tovar-León, Y.P.; Morillo-Coronado, Y. Characterization of lulo (Solanum quitoense Lam.) genetic diversity in the department of Boyaca, Colombia. Acta Agron. 2017, 66, 430–435. [Google Scholar] [CrossRef]
- Morillo, A.; Rodríguez, A.; Morillo, Y. Morphological characterization of lulo (Solanum quitoense Lam.) in the municipality of Pachavita, Boyacá. Acta Biol. Colomb. 2019, 24, 291–298. [Google Scholar] [CrossRef]
- Vargas-Bolívar, M.I.; Calderón-Medellín, L.A.; Pérez-Trujillo, M.M. Efecto de las deficiencias de algunos nutrimentos en plantas de lulo (Solanum quitoense var. quitoense) en etapa de vivero. Rev. Fac. Cienc. Básicas 2009, 5, 64–81. [Google Scholar]
- Nasraoui-Hajaji, A.; Chaffei-Haouari, C.; Ghorbel, M.H.; Gouia, H. Growth and nitrate assimilation in tomato (Solanum lycopersicon) grown with different nitrogen source and treated with cadmium. Acta Bot. Gallica Bot. Lett. 2011, 158, 3–11. [Google Scholar] [CrossRef]
- Sárdi, K. Nutrient Management; Szechenyi Terv: Pannonia, Hungary, 2011; 107p. [Google Scholar]
- Rivera-Espejel, E.A.; Sandoval-Villa, M.; Rodríguez-Mendoza, M.N.; Trejo-López, C.; Gasga-Peña, R. Tomato fertilization using ammonium and nitrate in split roots in hydroponics. Rev. Chapingo Ser. Hortic. 2014, 20, 57–70. [Google Scholar] [CrossRef]
- Martínez-Andújar, C.; Ghanem, M.E.; Albacete, A.; Pérez-Alfocea, F. Response to nitrate/ammonium nutrition of tomato (Solanum lycopersicum L.) plants overexpressing a prokaryotic NH4+-dependent asparagine synthetase. J. Plant Physiol. 2013, 170, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Flórez, S.L.; Miranda, D.; Chaves, B. Nutrient dynamics in the vegetative growth phase of lulo (Solanum quitoense Lam.) in response to NaCl salinity. Agron. Colomb. 2008, 26, 205–216. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).