Abstract
Thymus L. is of great interest in horticulture as ornamentals, spices, and medicinal plants, as well as in the extracts industry due to the richness in bioactive specialized metabolites. The natural hybrid T. × josephi-angeli Mansanet & Aguil. is produced in Spain, as its horticultural forms are very popular for domestic uses and gardening. However, its micropropagation and chemical composition have not been studied yet. Therefore, the main objective of this work was to develop a micropropagation procedure for T. × josephi-angeli, and to check whether the in vitro culture had an impact on the chemical profile of the plants. The results showed a high initiation rate (>91%) after two sterilization treatments were applied. Moreover, a micropropagation rate of around 21 new rooted explants per culture cycle was obtained in treatment M7 (Murashige and Skoog with 0.064 µM 6-(γ,γ-Dimethylallylamino)purine) when compared to the other 10 treatments performed. Acclimatization was successful in all three approaches tested (>75%), and all plants kept growing after 4 months of outdoor cultivation. Finally, 36 volatiles were identified, and the content of major compounds remained not statistically different in acclimatized plants when compared to the wild-type plants according to the analyses made by HS-SPME-GC/MS and SPME-GC/MS. This chemical stability points out the uniformity of the microplants and the suitability of the procedure applied in this study for T.×josephi-angeli horticultural production using in vitro techniques.
1. Introduction
The genus Thymus L. (Lamiaceae) is composed of approximately 400 described species in the world, with a remarkable diversity in infra-specific taxa and hybrids [1,2,3]. Iberian Thymus are of great ethnopharmacological interest, mainly as medicinal plants and spices [4,5,6], due to their high content of volatile compounds (thymol, carvacrol, etc.) in their essential oils, which have been related to the antimicrobial or antioxidant (among others) biological activities of their extracts [7]. Therefore, it is not surprising that a high number of genotypes, ecotypes, cultivars, and hybrid forms have been selected, domesticated, and produced in nurseries to be commercially exploited as ornamentals and medicinal plants/spices by the horticultural industry, as well as in the plant extracts and food technology industries [5,8]. Some examples of popularly used hybrid forms, due to their ornamental value and richness in specialized metabolites, are T. × citriodorus (Pers.) Schreb. [9,10,11], T. × oblongifolius Opiz [12], T. × mourae Paiva & Salgueiro [13], and cultivars such as T. vulgaris ‘Porlock’ [14], whose chemical constituents have been quite well characterized. T. × josephi-angeli Mansanet & Aguil. is a hybrid between T. vulgaris L. subsp. aestivus (Reut.) A. Bolòs & O. Bolòs and T. piperella L. that naturally grows in the Valencian Region of Spain [1,15]. It has been domesticated and popularized, and its horticultural forms are sold in many parts of the Iberian territory, especially in the Valencian Region, but its chemical composition has not been studied yet.
In this regard, in vitro culture techniques (such as micropropagation) are used in horticulture for massive plant production [16,17], and they are especially interesting for the production of aromatic species in the case of maintaining the chemical profile of the wild-type donor plants [18,19]. Micropropagation has been applied to a wide array of aromatic taxa, including the most interesting Thymus species in horticulture. Some examples are reviewed in Leal et al. [19]. In addition, endemic Iberian species with ethnobotanical and horticultural interests have been micropropagated, such as pebrella (T. piperella) [20] and cantueso (T. moroderi Pau ex Martínez) [18]. However, published works on the micropropagation of hybrids with horticultural interests are rather limited, and are completely lacking for T. × josephi-angeli, as well as for information on its chemical composition.
Plant hybrids often produce a greater diversity in bioactive specialized metabolites (when compared to parental plants) that result in the generation of elite genotypes with evolutionary advantages, higher resistance to herbivory, or better morphological or growth performances, as well as higher yields of target chemical compounds [21,22], which justifies the interest for their use in horticulture. For all these reasons, the aim of this work was to develop an in vitro multiplication protocol of a horticultural, commercial form of T. × josephi-angeli, as well as to characterize and compare the phytochemical profile of both in vitro cultured plantlets and wild-type plants, in order to provide new insights on the massive production of these materials for the horticultural industries.
2. Materials and Methods
2.1. Standards and Reagents
The reagents used for the in vitro culture experiments included commercial soap Ecco-Quimixel (Carlet, Spain), ethanol (VWR Chemicals, Fontenay-sos-Bois, France), a commercial solution of NaOCl (Yunae,-Químicas TJ, Alaquàs, Spain; active chlorine content 40 g/L), and Murashige and Skoog basal salts and vitamins (Duchefa Biochemie, Haarlem, The Netherlands). The plant agar and plant growth regulators (6-Benzylaminopurine, 6-(γ,γ-Dimethylallylamino)purine, and 3-Indoleacetic) were all purchased from Sigma-Aldrich (Barcelona, Spain). For the chemical analyses, methanol (HPLC grade) was supplied by Scharlau Chemie S.A. (Sentmenat, Spain). The following standards used for the chemical analyses were purchased also from Sigma-Aldrich (Madrid, Spain): α-pinene (CAS 80-56-8); camphene (CAS 79-92-5); α-terpinene (CAS 99-86-5); p-cymene (CAS 99-87-6); D-limonene (CAS 5989-27-5); γ-terpinene (CAS 99-85-4); terpinolene (CAS 586-62-9); linalool (CAS 78-70-6); camphor (CAS 76-22-2); endo-borneol (CAS 507-70-0); 4-terpinen-ol (CAS 562-74-3); α-terpineol (CAS 98-55-5); carvacrol (CAS 499-75-2); β-caryophyllene (CAS 87-44-5); humulene (CAS 6753-98-6); and 2,4-di-tert-butylphenol (CAS 96-76-4).
2.2. Plant Sterilization and Culture Initiation
The donor plants (wild-type, WP) were purchased from a greenhouse in València and kept in outdoor conditions until April 2021. To perform the experiments, healthy stems (without symptoms of chlorosis, pathologies, or abnormalities) showing vigorous growth were cut and washed with tap water and commercial soap. Then, two surface sterilization procedures were applied based on the strength and duration of the NaOCl treatment. The first sterilization procedure (S1) consisted of immersion of the stems in a 70% (v/v) ethanol solution for 30 s. Then, the plant materials were immersed in a commercial solution of NaOCl at 7% (v/v) for 20 min. Finally, three rinses in sterile distilled water were performed: the first two washes lasted for 1 min each in order for a faster removal of NaOCl, whereas the last one lasted for 10 min. The second sterilization procedure (S2) also included a first immersion of the stems in 70% ethanol solution (v/v) for 30 s. Then, the stems were transferred to NaOCl at 15% (v/v) for 15 min. Finally, three rinses in sterile distilled water were performed as above described. To initiate the in vitro cultures, axillary buds of 0.1–0.2 cm in length were cut and taken as explants. The culture medium for initiation consisted of Murashige and Skoog basal salts and vitamins (MS) [23], plus 0.088 M sucrose (SU), and 0.021 M plant agar (PA) without plant growth regulators (PGRs). Each explant was sown into one 25 × 150-mm test tube (Auxilab S.L., San Ginés, Spain) and sealed with polypropylene caps containing 15 mL of medium. Conditions for culture initiation were as follows: first, cultivation in the dark for 2 days, followed by cultivation for 30 more days in a 16-h photoperiod of white-daylight illumination (8500 K) provided by T8 Gro-lux fluorescent tubes (Sylvania Lamps, Erlangen, Germany) with photosynthetically active radiation of 50 μmol m−2 s −1. The temperature during the whole experiment was maintained at 21 ± 1 °C in a culture chamber (EGCS 701 3S-Equitec, Madrid, Spain). Both sterilization and initiation of in vitro cultures were carried out in an aseptic environment provided by a horizontal laminar flow cabinet (AH-100, Burdinola, Bizkaia, Spain). The success of the sterilization was measured by counting the total contamination (either by bacteria, and/or fungi) versus axenic explants obtained (%), and the initiation success was evaluated by the total percentage of initiated explants showing growth restoration signs in terms of shoot elongation (in cm) and leaves produced (n°) after 30 days of culture. From those explants successfully initiated, a selected shoot showing vigorous growth was multiplied through several passages in the above described medium and conditions, in order to obtain a uniform stock of clonal and axenic plant material for further experiments.
2.3. Multiplication and Rooting
Multiplication experiments were based in 11 treatments applied on axillary buds taken from the initiated stock and transferred onto fresh MS medium containing 0.088 M SU and 0.021 M PA, supplemented or not supplemented with PGRs: the plantlets cultured in the absence of PGRs served as controls (medium M0). The PGR-treated explants were subjected to different concentrations of 6-Benzylaminopurine (BAP), 6-(γ,γ-Dimethylallylamino)purine (2iP), and 3-Indoleacetic acid (IAA) alone or in combination in the basal medium above described in order to design media M1-M10. The effects of PGR treatments on the plants’ in vitro morphogenesis and development, were assessed after 30 days of in vitro culture under the same culture conditions employed in the initiation experiments. The parameters measured included the number of newly developed shoots per explant (n°), shoot elongation (in cm), as well as nodes and leaves formed de novo per explant (n°). Additionally, the potential micropropagation rate (MR), defined as the number of new explants obtained after the cultivation period that can be used in the following in vitro passages to regenerate whole plantlets, was calculated for each treatment according to the following equation: MR = SN × NN, where SN is the number of newly produced shoots and NN the number of new nodes formed per shoot. Finally, rooting was studied by counting the roots produced (n°) and their elongation (in cm). All media pH for both initiation and multiplication and rooting were adjusted to 5.75 using solutions of 0.1 N NaOH or 0.1 N HCl before autoclaving for 20 min at 121 °C. The explants for rooting and multiplication were sown into autoclaved food jars containing 150 mL of medium, and sealed with aluminum caps.
2.4. Acclimatization
To assess the acclimatization response of the in vitro produced materials, the micropropagated and rooted plantlets were planted in polyethylene boxes containing a mixture of peat moss, as substrate, and vermiculite (5:3 ratio) in order to improve the substrate aeration and moisture retention. Then, the plantlets were subjected to three schemes of acclimatization ex vitro, based on the duration of the two stages designed: primary acclimatization (PA) and hardening (HA). PA was performed in a culture room at 22 °C, a 12-h photoperiod, and 90–100% relative humidity (HR). For HA, a gradual reduction of the RH was done by opening one or two windows on the lid of the boxes, and finally removing the lid. The three acclimatization schemes performed included: (1) 30 day PA followed by 30 day HA (AC1), (2) 30 day PA followed by 15 day HA (AC2), and (3) 15 day PA followed by 15 day HA (AC3). After each stage, the percentage of surviving plants was recorded. Finally, the plants were transferred to open field conditions, watered once a week, and the percentage of survival after 4 months was also recorded.
2.5. Plant Extraction and Chemical Analyses
The content of terpenes was studied for WT, acclimatized plants, as well as for those plantlets from in vitro treatments that showed the highest levels of plant development according to the multiplication and rooting experiments (M0 and M7). Plant samples were dried in the dark at room temperature conditions (≈22 °C). After drying, three replicates of a sample of 0.02 g from the plant material were subjected to two complementary chemical analyses as explained beneath. The plant extraction method and analytical methodologies were based on previous studies performed by the research group on similar chemical compounds [24,25,26].
Firstly, a solid phase micro-extraction (SPME) with subsequent analysis by gas chromatography coupled with mass spectrometry (GC/MS) using the headspace (HS) technique was performed. In this approach, the chemical characterization of the sample is direct, and extraction is not necessary because the analyses are based on the emission of plant volatile compounds. The samples were prepared as follows: 0.02 g of leaves was dried and pulverized. Then, the plant material was placed in a glass vial and subjected to heating and agitation with the SPME fiber. The volatile compounds adsorbed by the fiber were then analysed by GC/MS. This methodology allowed the qualitative determination of volatile terpenes present in plants, as the identification is not affected by the solvent, when compared to the analyses performed on plant extracts. Estimations of the content of phytochemicals were made through the calculation of percentage of peak area (% PA).
Secondly, 0.02 g of dried plant samples was extracted in 4 mL of 80% methanol (v/v) in test tubes. Tubes were then incubated for 30 min in an ultrasonic bath, and the extracts obtained were finally filtered (0.45 mm) and stored at 4 °C until analyzing by SPME-GC/MS. This approach allowed the quantification of the identified compounds to be carried out by means of an external standard calibration and, when combined with HS-SPME-GC/MS, it allows a better understanding of the chemical composition of plants.
Identification of volatile metabolites was carried out by using the spectra library present in the software NIST Chemistry WebBook. A total of 19 compounds were quantified using the 14 terpene standards stated in Section 2.1. Mix solutions of standards were prepared using methanol 80% in a range of concentrations and injected into SPME-GS/MS using the same chromatographic conditions as the samples. Detected compounds were quantified using the corresponding standards. In absence of corresponding standards, a similar compound was used for quantification.
2.6. Experimental Design and Data Treatment
For the initiation experiments, a variable amount of 30–85 explants were used in the different trials performed per treatment and replicate. The multiplication and rooting experiments were set up using three replicates per treatment, containing ten explants each (n = 30). For the acclimatization experiments, three replicates containing twelve explants each were used (n = 36). All these experiments were performed in triplicate in order to assure the uniformity and reproducibility of the results. The statistical treatment of the results was done using the free software “Infostat” (National University of Córdoba, Argentina; https://www.infostat.com.ar/ (accessed on 25 April 2022)). Group analyses were carried out by ANOVA, and significant differences were revealed by using the Fisher’s Least Significant Difference Test (LSD) at 95% confidence (α = 0.05).
3. Results
3.1. Plant Sterilization and Culture Initiation
The sterilization procedures applied in this study resulted in an explant survival rate higher than 91% for both treatments (not statistically different among them). In addition, the parameters measured to assess the growth restoration (shoot elongation and leaves produced) did not show significant differences among them after 30 days of culture. Finally, contamination percentage (proliferation of bacteria and/or fungi during the initiation period) remained lower than 15% in both procedures (Table 1).

Table 1.
Sterilization procedures applied to axillary bud explants of Thymus × josephi-angeli Mansanet & Aguil. donor plants. The shoot elongation, number of produced leaves, and the percentage of explants that restored growth (mean ± SD) or became contaminated are shown for each treatment after 32 days of cultivation on a medium of Murashige and Skoog basal salts and vitamins [23] at a temperature of 21 ± 1 °C. Mean values within a column followed by the same letter are not significantly different by Fisher’s LSD test (α = 0.05).
3.2. Multiplication and Rooting
The results obtained after 30 days of in vitro multiplication using 11 combinations of PGRs showed that treatment M7 (0.064 µM 2iP) gave significantly higher performance (p < 0.01) for all morphogenic parameters measured (number of shoots formed per explant, shoot elongation, newly formed nodes, leaves and roots formed per explant, and root length) when compared to the other 10 treatments (Table 2; Figure 1). Moreover, M0 (without PGRs) offered suboptimal micropropagation results that were not statistically different to the M7 treatment for shoot elongation and number of newly formed leaves and roots (Table 2). Shoot elongation and root elongation decreased with the increase in cytokinin concentrations in an almost dose-dependent manner (especially for treatments based solely on 2iP), whereas the new shoots, nodes, and leaves formed per explant showed a more irregular trend. Attending to the micropropagation rate, treatment M7 gave the highest value, approximately 21 new explants for each passage in vitro, in accordance with the optimal values obtained from the morphogenic parameters measured. The treatment M1 showed the second highest micropropagation rates (9.31 explants), but rooting was nil in this treatment (Figure 1).

Table 2.
Effects of 11 concentrations of 6-benzylaminopurine (BAP), 6-(γ,γ-Dimethylallylamino)purine (2iP), and 3-Indoleacetic acid (IAA) on the in vitro morphogenesis of Thymus × josephi-angeli Mansanet & Aguil. after 30 days of cultivation (mean ± SD) on Murashige and Skoog basal salts and vitamins [23] at a temperature of 21 ± 1 °C. Mean values within a column followed by the same letter are not significantly different by Fisher’s LSD test (α = 0.05).

Figure 1.
In vitro propagation of Thymus × josephi-angeli Mansanet & Aguil. from axillary bud explants: elongated and rooted shoots after 30 days of culture onto solidified medium based in Murashige and Skoog basal salts and vitamins [23] with (a) 0.064 µM 6-(γ,γ-Dimethylallylamino)purine and 2iP (M7); (b) plant growth regulator-free medium (M0); (c) 6.57 µM 6-benzyladenine and BAP (M1); and (d) 0.69 µM 2iP (M9). Bars indicate 1 cm.
3.3. Acclimatization
Higher than 75% of microplants survived after the three acclimatization schemes were tested. According to the results obtained, the percentage of survival decreases with the reduction of the total acclimatization time. The highest survival was registered with the longest treatment, AC1 (consisting of 30 days of PA followed with 30 more days of HA, overall 60 days), whereas the lowest survival was obtained in the shortest treatment, AC3 (consisting of 15 days of PA and 15 more days of HA, overall 30 days). However, the statistical analyses revealed that the observed differences in the percentage of surviving microplants was not significant (p-value > 0.05) for the PA phase, nor for the HA phase (Table 3). All plants that survived after HA kept growing during the 4-months period of cultivation in outdoor conditions and were morphologically equal to the donor plants.

Table 3.
Percentage of micropropagated plants of Thymus × josephi-angeli Mansanet & Aguil. (mean ± SD) that survived after three acclimatization schemes (AC) based on the different durations of the primary acclimatization and hardening phases. Mean values within a column followed by the same letter are not significantly different by Fisher’s LSD test (α = 0.05).
3.4. Plant Extraction and Chemical Analyses
A total of 36 volatile compounds were identified by HS-SPME-GC/MS. The most abundant compounds according to percentage of peak area were p-cymene, γ-terpinene, thymol, and β-caryophyllene for all wild-type, in vitro cultured, and acclimatized plants (Table 4). Although significant differences in the peak area were detected in in vitro cultured plants for p-cymene and β-caryophyllene, no significant differences were found between the percentages of peak area in acclimatized plant samples when compared to the wild type plants for the four major compounds identified by HS-SPME-GC/MS (Figure 2 and Figure 3).

Table 4.
Percentage (peak area, PA) of the volatiles identified in Thymus × josephi-angeli Mansanet & Aguil. samples from the wild-type and in vitro cultured plants; M7, 0.064 µM of 6-(γ,γ-Dimethylallylamino)purine, and acclimatized plants. RT, retention time; ND, not detected compound. Samples were analyzed by HS-SPME-GC/MS. Data are presented as means ± SD. 1 Compounds identified by corresponding analytical standard.
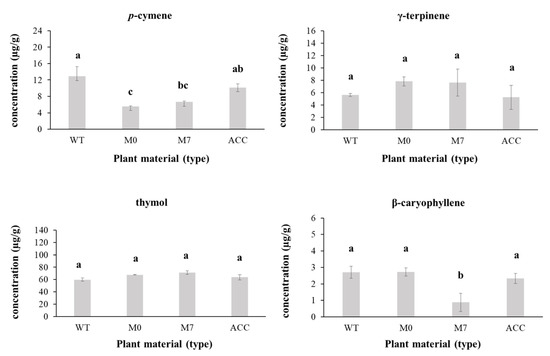
Figure 2.
Changes in the relative concentration of p-cymene, γ-terpinene, thymol, and β-caryophyllene (µg/g) in methanol extracts of Thymus × josephi-angeli Mansanet & Aguil. during the micropropagation process according to the analyses performed by SPME-GC/MS: WT (wild-type plants); M0 in vitro cultured plants in medium MS without plant growth regulators; M7 in vitro cultured plants in medium MS supplemented with 0.064 µM of 6-(γ,γ-Dimethylallylamino)purine; and ACC acclimatized plants. Mean values within a column followed by the same letter are not significantly different by Fisher’s LSD test (α = 0.05). Bars indicate SD.
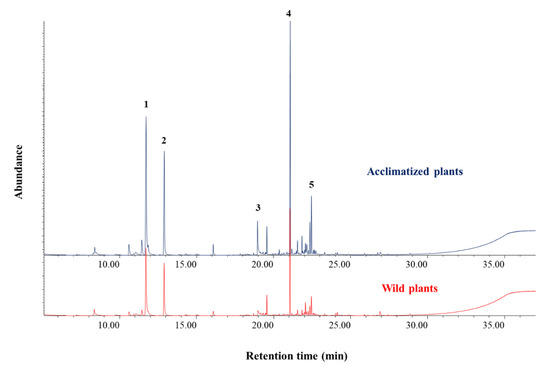
Figure 3.
Chromatographic profiles of the methanol extracts made from samples of wild-type plants (red) and acclimatized micropropagated plants (blue) of Thymus × josephi-angeli Mansanet & Aguil.: 1, p-cymene; 2, γ-terpinene; 3, thymol; 4, β-caryophyllene; 5, δ-cadinene.
Among the identified compounds, 19 were further quantified in methanol extracts by SPME-GC/MS. In these experiments, thymol and 2,4-di-tert-butylphenol were the most abundant (with concentrations higher than 100 µg/g), whereas p-cymene, α-terpinene, γ-terpinene, β-caryophyllene, and humulene were present in lower amounts in both wild-type plants and acclimatized microplants. The other 12 compounds analyzed were below either the limit of quantification or detection (Table 5).

Table 5.
Content (µg/g) in terpenes of the methanol extracts from wild-type and acclimatized plants of Thymus × josephi-angeli Mansanet & Aguil. analyzed by SPME-GC/MS. Data are presented as means ± SD. RT, retention time. LQ, limit of quantification. * semi-quantified as γ-terpinene; ** semi-quantified as isoborneol; *** semi-quantified as carvacrol.
4. Discussion
The two sterilization procedures applied in the present work did not show significant differences between them according to the parameters measured (shoot elongation and leaves produced), but provided more than 91% growth restoration and proliferation of microorganisms lower than 15%, thus showing their suitability for the initiation of the explants. Similar procedures, combining short immersion in ethanol followed by longer dipping in diluted bleach (10–20%), are frequently reported to be successful for in vitro initiation of nodal explants developed in young shoots from woody plants [27,28]. However, high contamination rates and low growth restoration (with the subsequent unsuccessful or difficult initiation of in vitro culture) are also reported for these type of starting materials [18], which makes it quite difficult to standardize a general approach for explants taken from shoots, and makes it necessary to test specifically the sterilization procedures in every in vitro assay.
Once the cultures were initiated and a stock of clonal and axenic plants was obtained, the in vitro multiplication and rooting of the T. × josephi-angeli form was studied under 11 PGR-based treatments. In these experiments, shoot and root elongation decreased with the increase in cytokinin concentrations in an almost dose-dependent manner (Table 2). These are the expected results due to the suppression of apical dominance as the cytokinin concentrations were increased in the culture media [29]. However, the number of new shoots produced per explant did not show the expected inverse trend (derived from lateral bud development as a consequence of the apical dominance suppression), as treatments having higher cytokinin concentrations did not produce a significantly higher number of shoots when compared to the other media and the untreated controls (for example, treatment M6), or the response was even significantly lower (treatment M10). In the present work, treatments consisting of the lowest cytokinin concentrations (M1 and M7) produced a higher number of new shoots per explant when compared to the untreated controls, and M7 (low cytokinin concentration) also when compared to those treatments having higher cytokinin concentrations. Attending to the type of cytokinin, treatments based solely on 2iP showed, generally, significantly higher morphogenic response for the new shoot formation, shoot elongation, as well as nodes and leaves production when compared to treatments based on BAP or combinations of BAP and 2iP (Table 2). From the physiological point of view, it is normally assumed that BAP and its conjugates are more stable, and active cytokinins more than 2iP [30,31], as the first ones are not degraded by cytokinin oxidases [30]. Therefore, BAP constitutes the mainly chosen PGR for in vitro multiplication [32]. The exogenous application of BAP provided optimal morphogenic results in Lamiaceae such as in the genera Clerodendrum L. [33], Sideritis L. [34,35], Mentha L. [36,37,38], and Thymus L. [19,20,39], although comparisons with 2iP were not made in these works. In some other works carried out in this botanical family, BAP was demonstrated to perform better than 2iP, and examples are found in Satureja L. [40], Mentha × piperita [41], and Teucrium capitatum L. [42]. In contrast to all this, other works showed that 2iP-treated plants performed a higher in vitro multiplication response when compared to other cytokinins such as BAP, as we obtained in the present work. Examples of this can be found for T. vulgaris [40] or Sideritis leucantha Cav. subsp. leucantha [27]. In this regard, Marco-Medina and Casas [18] also reported optimal morphogenic performance in medium without PGRs for T. moroderi, indicating the sensitivity of these explants to the exogenously applied PGRs, although they did not test 2iP. In addition, it is reported that BAP induced high percentages of vitrified shoots in T. lotocephalus G.López & R.Morales [43]. All these results suggest certain sensitivity of some species of Lamiaceae to relatively high concentrations of cytokinins, particularly BAP, during the regeneration process in vitro. In plants, cytokinins enhance the expression of cyclin-dependent kinases (CDKs), thus having an impact on the cell-cycle progression, which ultimately regulates the new tissue formation and morphogenesis [44,45]. 2iP is a naturally occurring cytokinin in plants that has almost 10 times less affinity than BAP for the CRE1/AHK4 cytokinin receptor, as described in Arabidopsis [46]. This would explain why BAP is generally seen as a more active cytokinin than 2iP [30,31]. However, particularities in the different cytokinin perception by the plant cells and signal transduction at the molecular level by a differential genetic expression might be associated with a wide array of factors. For instance, Herrera-Isidron et al. [47] recently proposed differential cytokinin perception depending on certain developmental events during the organogenesis in potatoes. In addition, it is still not clear whether cytokinin signaling takes place from one or more than one site at the cellular level (endoplasmic reticulum and/or plasma membrane), if the action is equal in all tissues or if it depends on the tissue, its developmental stage, and/or the action of signalling molecules from different origins [48], or if synergies exist between endogenous levels of hormones and exogenously applied PGRs [49]. Therefore, how exogenously applied BAP and 2iP (among other cytokinins) might act at the cellular or tissue level, and how this interaction is specifically translated into morphogenesis in vitro for different genotypes or plant species, cannot be stated. In any case, it seems reasonable to think that there is a certain sensitivity to BAP in some members of Lamiaceae, including the hybrid subject of this work, and, for those cases, alternative exogenously applied cytokinins (such as 2iP) at low concentrations offer higher morphogenic responses. This fact strongly contrasts to what is widely reported for other plant families such as Amaryllidaceae, where optimal developmental parameters are achieved with BAP at higher concentrations (>6 µM BAP) than in many Lamiaceae [50,51,52,53]. The optimal root development and elongation were achieved during the multiplication phase in treatments M7 and M0. As both shooting and rooting are developed in parallel during the same culture passage, it is not necessary to perform a specific rooting stage for the micropropagation of T. × josephi-angeli, unlike what is reported for Thymus species [20], among other Lamiaceae [27], which constitutes a clear advantage for its commercial production.
From the horticultural point of view, it is certainly more interesting to obtain a greater number of new explants after one culture cycle (as they can be easily rooted and acclimated ex vitro) rather than solely new shoots per explant. Therefore, the micropropagation rate (MR) was defined in the present work as the number of new explants obtained after the cultivation period that can be used in the following in vitro passages to regenerate whole plantlets (number of newly produced shoots x the number of new nodes formed per shoot). In this sense, treatment M7 (2iP = 0.064 µM) also showed optimal results, as the micropropagation rate was almost 21 new explants to be used in the following subcultures (or directly rooted and acclimatized), more than double when compared to the second best treatment (M1, BAP = 6.57 µM, micropropagation rate = 9.31). The results obtained during the in vitro cultivation, the morphogenic performance, and the acclimatization success are comparable to the results reported for other Thymus species, including the parental species of T. × josephi-angeli, T. vulgaris and T. piperella [19,20], thus showing the suitability of the procedure applied in this study for the horticultural industries.
The chemical analyses performed by HS-SPME-GC/MS allowed the identification of 36 volatile compounds in the samples of T. × josephi-angeli. According to this technique, the major compounds, in descending order, were thymol, p-cymene, γ-terpinene, carvacrol, linalool, thymoquinone, and β-caryophyllene, where solely thymol constituted around 60% (peak area) of the volatiles in all samples screened (wild-type, in vitro cultured, and acclimatized), whereas the other six compounds ranged from 1.5% to 10% of peak area. The SPME-GC/MS analyses of the extracts and terpene quantification confirmed most of the qualitative results on volatiles obtained in the direct measurements made by HS-SPME-GC/MS. To combine both approaches allowed a better understanding of the volatile composition of the plant extracts. Both methods revealed thymol as the major component, by far, being six times more abundant than the second one. In addition, results on p-cymene, γ-terpinene, and β-caryophyllene (major components in these plant samples) are in agreement in both approaches. However, 2,4-di-tert-butylphenol was revealed as the second most abundant compound in methanol extracts analyzed by SPME-GC/MS (with concentrations around 100 µg/g), but this compound could not be identified by HS-SPME-GC/MS. This fact is most likely due to the inability of the fiber to adsorb (and detect) this compound at the given heating temperature in the HS-SPME-GC/MS technique. In contrast, the use of methanol at 80% (v/v) allowed its extraction and further identification and quantification. In line with this, carvacrol, linalool, and thymoquinone were identified by HS-SPME-GC/MS, but these compounds were not confirmed in the methanol extracts according to the analyses made by SPME-GC/MS. In any case, both approaches agreed for the major compounds, with thymol as the most abundant in T. × josephi-angeli samples. Thymol is also the most abundant terpene in T. vulgaris [54], and it constitutes the second most abundant component (after p-cymene) in the T. piperella chemotypes from the central counties of the Valencian Region [55], where the commercial hybrid is produced. Therefore, the results obtained in the chemical analyses performed in this work also point out the hybrid origin of T. × josephi-angeli, between T. vulgaris and T. piperella, as is described for morphological characters in the original description of the hybrid [15]. The culture conditions during the in vitro regeneration processes may have an impact on the composition of volatile compounds of aromatic plants [17,56]. Among the major compounds screened in the present work, thymol and γ-terpinene showed an increase in their content during the in vitro culture, whereas p-cymene and β-caryophyllene contents decreased. This is in line with the preliminary results that we performed on this commercial form of T. × josephi-angeli [57]. In the present work, these changes were only significant in the cases of p-cymene (treatments M0 and M7) and β-caryophyllene (M7). However, the content of these volatiles was restored after the ex vitro transference of the plantlets, and remained not statistically different in acclimatized plants (cultivated in outdoor conditions for 4 months) when compared to the wild-type plants. Moreover, according to the determinations made by SPME-GC/MS analyses of the extracts, the chemical profile and relative amounts of the identified compounds do not vary among wild-type and acclimatized plants (Table 4). This chemical stability points out the uniformity of the microplants produced using the approach presented in this study. Given the commercial importance of Thymus in the horticultural industry [19] and the high-added value of thymol (the most abundant terpene in T. × josephi-angeli) in several fields of applications [54], the results obtained in the present work highlight the suitability of the in vitro culture techniques not only for the industrial production of T. × josephi-angeli as a commercial plant with interests as ornamentals or domestic spices and medicinal plants, but also to provide high-added value materials for the plant extracts industry.
5. Conclusions
In conclusion, a satisfactory procedure of in vitro propagation for T. × josephi-angeli plants is presented here. The initiation of explants in axenic conditions was successful (>91% growth restoration) after sterilization, consisting of dipping explants in 70% (v/v) ethanol for 30 s, followed by immersion in NaOCl either at 7% (v/v) for 20 min or at 15% (v/v) for 15 min. The highest morphogenic capacity was obtained in medium MS with 0.064 µM 2iP, showing significantly higher values of shoot and root elongation, as well as number of new shoots, nodes, leaves, and roots produced per explant, with an optimal micropropagation rate of 21 new explants produced per culture cycle. Rooted explants were obtained during the multiplication phase, and acclimatization was successful (>75%) by transferring the microplants to a sterile soil mixture of peat moss and vermiculite (5:3 ratio) in all three approaches tested. The chemical analyses performed allowed the identification of 36 volatile compounds, and thymol, p-cymene, γ-terpinene, and β-caryophyllene constituted the major compounds according to the determinations made by HS-SPME-GC/MS and SPME-GC/MS, where thymol was the most abundant (≈60% peak area and concentrations around 500 µg/g). The content of these compounds is not significantly different in acclimatized plants when compared to wild-type plants, which points out the uniformity of the plants produced in vitro. Finally considered, the results presented in this study constitute the first ones on tissue culture and phytochemistry of T. × josephi-angeli. These results are of great interest not only due to the scientific novelty and the multiple interests of the hybrid taxon here studied, but also due to the potential application of this approach to produce uniform and thymol-rich plants for the horticultural and plant extracts industries.
Author Contributions
Conceptualization, E.A. and J.J.-V.; methodology, E.A. and J.J.-V.; formal analysis, R.d.M.J.-M. and J.J.-V.; investigation, E.A., R.d.M.J.-M. and J.J.-V.; data curation, E.A., R.d.M.J.-M. and J.J.-V.; writing—original draft preparation, J.J.-V.; writing—review and editing, E.A. and J.J.-V.; supervision, J.J.-V.; project administration, J.J.-V.; funding acquisition, J.J.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research study was possible as a part of the funding from the Vice Rectorate for Research of UCV (Universidad Católica de Valencia) to Instituto de Investigación en Medio Ambiente y Ciencia Marina IMEDMAR-UCV with code number 2020-999-004. Also, this grant provided the funding to support the APC for the publication of this work, along with funding from IMEDMAR-UCV with code number 2016-006-002.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the funding provided by the Gobierno de Aragón and Fondo Social Europeo to the GUIA group [financiación grupo GUIA T53-17R] that partly supported the chemical analyses performed in this work. In addition, the authors would like to thank Pedro Pablo Ferrer Gallego for his help with the taxonomical treatment of the plant materials, and Borja Oller Estela and Raquel Muñoz del Rincón for their help in the laboratory. Finally, we appreciate the time invested by the anonymous reviewers in revising the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morales, R. Thymus L. In Flora iberica; Real Jardín Botánico CSIC: Madrid, Spain, 2010; Volume 12, pp. 349–409. [Google Scholar]
- Khajuria, A.K.; Bisht, N.; Bhagat, N. In vitro organogenesis and plant regeneration of Thymus serpyllum L.: An important aromatic medicinal plant. In Vitro Cell. Dev. Biol.—Plant 2020, 56, 652–661. [Google Scholar] [CrossRef]
- Kosakowska, O.; Bączek, K.; Przybył, J.L.; Pawełczak, A.; Rolewska, K.; Węglarz, Z. Morphological and chemical traits as quality determinants of common thyme (Thymus vulgaris L.), on the example of ‘Standard Winter’ cultivar. Agronomy 2020, 10, 909. [Google Scholar] [CrossRef]
- Font Quer, P. Plantas Medicinales; Editorial Labor: Barcelona, Spain, 1962. [Google Scholar]
- Morales, R.; Tardío, J.; Aceituno, L.; Molina, M.; Pardo-De-Santayana, M. Biodiversidad y Etnobotánica en España; Real Jardín Botánico CSIC: Madrid, Spain, 2011; pp. 157–207. Available online: http://147.96.59.157/rsehn/cont/publis/boletines/130.pdf (accessed on 9 October 2022).
- Pardo de Santayana, M.; Morales, R.; Tardío, J.; Molina, M. Inventario Español de los Conocimientos Tradicionales Relativos a la Biodiversidad. Fase II; Ministerio de Agricultura y Pesca, Alimentación y Medio Ambiente: Madrid, Spain, 2018.
- Ballester, C. Composición Química y Propiedades Antibacterianas y Antioxidantes de Aceites Esenciales de Especies de Thymus Procedentes de Cultivo Ecológico y su Aplicación a Películas de Quitosano. Ph.D. Thesis, Universidad Miguel Hernández, Elche, Spain, 2016. Available online: https://dialnet.unirioja.es/servlet/tesis?codigo=64432 (accessed on 9 October 2022).
- Carlen, C.; Schaller, M.; Carron, C.A.; Vouillamoz, J.F.; Baroffio, C.A. The new Thymus vulgaris L. hybrid cultivar ‘Varico 3’ compared to five established cultivars from Germany, France and Switzerland. Acta Hortic. 2010, 860, 161–166. [Google Scholar] [CrossRef]
- Omidbaigi, R.; Sefidkon, F.; Hejazi, M. Essential oil composition of Thymus x citriodorus L. cultivated in Iran. Flavour Fragr. J. 2005, 20, 237–238. [Google Scholar] [CrossRef]
- Jurevičiūtė, R.; Ložienė, K.; Bruno, M.; Maggio, A.; Rosselli, S. Composition of essential oil of lemon thyme (Thymus × citriodorus) at different hydrodistillation times. Nat. Prod. Res. 2018, 33, 80–88. [Google Scholar] [CrossRef]
- Vaičiulyte, V.; Ložiene, K.; Sivicka, I. Effect of organic matter fertilizers on the composition of volatiles, morphometrical and anatomical parameters of essential oil-bearing Thymus × citriodorus cultivated in an open field conditions. Horticulturae 2022, 8, 917. [Google Scholar] [CrossRef]
- Loziene, K.; Vaiciuniene, J.; Venskutonis, P.R. Chemical composition of the essential oil of an interspecific hybrid of thyme (Thymus x oblongifolius Opiz) growing wild in Lithuania. J. Essent. Oil Res. 2002, 14, 308–311. [Google Scholar] [CrossRef]
- Salgueiro, L.R.; Vila, R.; Tomàs, X.; Cañigueral, S.; Paiva, J.; Proença da Cunha, A.; Adzet, T. Essential oil composition and variability of Thymus lotocephalus and Thymus x mourae. Biochem. Syst. Ecol. 2000, 28, 457–470. [Google Scholar] [CrossRef][Green Version]
- Mirza, M.; Baher, Z.F. Chemical composition of essential oil from Thymus vulgaris Hybrid. J. Essent. Oil Res. 2003, 15, 404–405. [Google Scholar] [CrossRef]
- Mansanet, J.; Aguilella-Palasís, A.; Mateu-Andrés, I. Dos especies híbridas nuevas: Thymus x Josephi Angeli J. Mansanet & A. Aguilella y Helianthemum x Carmen Joanae J. Mansanet & I. Mateu. Mediterr. Ser. Estud. Biol. 1985, 8, 83–88. [Google Scholar] [CrossRef][Green Version]
- Grigoriadou, K.; Krigas, N.; Sarropoulou, V.; Papanastasi, K.; Tsoktouridis, G.; Maloupa, E. In vitro propagation of medicinal and aromatic plants: The case of selected Greek species with conservation priority. In Vitro Cell. Dev. Biol.—Plant 2019, 55, 635–646. [Google Scholar] [CrossRef]
- Hussain, M.S.; Fareed, S.; Ansari, S.; Rahman, M.A.; Ahmad, I.Z.; Saeed, M. Current approaches toward production of secondary plant metabolites. J. Pharm. Pharm. Sci. 2012, 4, 10–20. [Google Scholar] [CrossRef]
- Marco-Medina, A.; Casas, J.L. In vitro multiplication and essential oil composition of Thymus moroderi Pau ex Martinez, an endemic Spanish plant. Plant Cell Tiss. Organ Cult. 2015, 120, 99–108. [Google Scholar] [CrossRef]
- Leal, F.; Taghouti, M.; Nunes, F.; Silva, A.; Coelho, A.C.; Matos, M. Thymus plants: A review—Micropropagation, molecular and antifungal activity. In Active Ingredients from Aromatic and Medicinal Plants; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Sáez, F.; Sánchez, P.; Piqueras, A. Micropropagation of Thymus piperella. Plant Cell Tissue Organ Cult. 1994, 39, 269–272. [Google Scholar] [CrossRef]
- Cheng, D.; Vrieling, K.; Klinkhamer, P.G. The effect of hybridization on secondary metabolites and herbivore resistance: Implications for the evolution of chemical diversity in plants. Phytochem. Rev. 2011, 10, 107–117. [Google Scholar] [CrossRef][Green Version]
- Gary, F.P. Breeding and engineering trees to accumulate high levels of terpene metabolites for plant defense and renewable chemicals. Front. Plant Sci. 2018, 9, 1672. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Wrona, M.; Blasco, S.; Becerril, R.; Nerín, C.; Sales, E.; Asensio, E. Antioxidant and antimicrobial markers by UPLC®–ESI-Q-TOF-MSE of a new multilayer active packaging based on Arctostaphylos uva-ursi. Talanta 2019, 196, 498–509. [Google Scholar] [CrossRef]
- Asensio, E.; Vitales, D.; Pérez, I.; Peralba, L.; Viruel, J.; Montaner, C.; Vallès, J.; Garnatje, T.; Sales, E. Phenolic compounds content and genetic diversity at population level across the natural distribution range of bearberry (Arctostaphylos uva-ursi, Ericaceae) in the Iberian Peninsula. Plants 2020, 9, 1250. [Google Scholar] [CrossRef]
- Song, X.-C.; Canellas, E.; Asensio, E.; Nerín, C. Predicting the antioxidant capacity and total phenolic content of bearberry leaves by data fusion of UV-Vis spectroscopy and UHPLC/Q-TOF-MS. Talanta 2020, 213, 120831. [Google Scholar] [CrossRef]
- Juan-Vicedo, J.; Ramírez-Luna, J.E.; Piqueras, A.; Casas, J.L. Micropropagation and cryopreservation by vitrification of the Spanish endemic medicinal plant Sideritis leucantha Cav. subsp. leucantha (Lamiaceae). In Vitro Cell. Dev. Biol.—Plant 2021, 57, 1057–1065. [Google Scholar] [CrossRef]
- Juan-Vicedo, J.; Serrano-Martínez, F.; Cano-Castillo, M.; Casas, J.L. In vitro propagation, genetic assessment, and medium-term conservation of the coastal endangered epecies Tetraclinis articulata (Vahl) Masters (Cupressaceae) from adult trees. Plants 2022, 11, 187. [Google Scholar] [CrossRef]
- Bhojwani, S.S.; Dantu, P.K. Plant Tissue Culture: An Introductory Text; Springer: New Delhi, India, 2013; ISBN 9788132210269. [Google Scholar]
- Kieber, J.J.; Schaller, G.E. Cytokinins. Arab. Book 2014, 12, e0168. [Google Scholar] [CrossRef]
- Hart, D.S.; Keightley, A.; Sappington, D.; Nguyen, T.M.P.; Chritton, C.; Seckinger, G.R.; Torres, K.C. Stability of adenine-based cytokinins in aqueous solution. In Vitro Cell. Dev. Biol.—Plant 2016, 52, 1–9. [Google Scholar] [CrossRef]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. In Vitro Cell. Dev. Biol.—Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Nataraj, M.; Mafatlal, M.K.; Teixeira da Silva, J.A. Micropropagation of Clerodendrum L. species: A review. Rend. Fis. Acc. Lincei 2016, 27, 169–179. [Google Scholar] [CrossRef]
- Papafotiou, M.; Kalantzis, A. Seed germination and in vitro propagation of Sideritis athoa. Acta Hortic. 2009, 813, 471–476. [Google Scholar] [CrossRef]
- Shtereva, L.A.; Vassilevska-Ivanova, R.D.; Kraptchev, B.V. In vitro cultures for micropropagation, mass multiplication and preservation of an endangered medicinal plant Sideritis scardica Griseb. Bot. Serb. 2015, 39, 111–120. Available online: https://botanicaserbica.bio.bg.ac.rs/arhiva/pdf/2015_39_2_633_full.pdf (accessed on 9 October 2022).
- Phatak, S.V.; Heble, M.R. Organogenesis and terpenoid synthesis in Mentha arvensis. Fitoterapia 2002, 73, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, A.; Parshuram, S.; Manoranjan, K. Micro-propagation and biochemical analysis of Spear Mint (Mentha spicata). Indian J. Innov. Dev. 2015, 1, 489–493. [Google Scholar]
- Mehta, J.; Naruka, R.; Sain, M.; Dwivedi, A.; Sharma, D.; Mirza, J. An efficient protocol for clonal micropropagation of Mentha piperita L. (Pipperment). Asian J. Plant Sci. Res. 2012, 2, 518–523. [Google Scholar]
- Ozudogru, E.A.; Kaya, E.; Kirdok, E.; Issever-Ozturk, S. In vitro propagation from young and mature explants of thyme (Thymus vulgaris and T. longicaulis) resulting in genetically stable shoots. In Vitro Cell. Dev. Biol.—Plant 2011, 47, 309–320. [Google Scholar] [CrossRef]
- Arrebola, M.L.; Socorro, O.; Barcelò-Muńoz, A.; Simón-Pérez, E.; Pliego-Alfaro, F. Micropropagation of Satureja obovata Lag. Hortic Sci. 1997, 32, 1278–1280. [Google Scholar] [CrossRef]
- Vaidya, B.N.; Asanakunov, B.; Shahin, L.; Jernigan, H.L.; Joshee, N.; Dhekney, S.A. Improving micropropagation of Mentha × piperita L. using a liquid culture system. In Vitro Cell. Dev. Biol.-Plant 2019, 55, 71–80. [Google Scholar] [CrossRef]
- Papafotiou, M.; Martini, A.N. In vitro seed and clonal propagation of the Mediterranean aromatic and medicinal plant Teucrium capitatum. HortScience 2016, 51, 403–411. [Google Scholar] [CrossRef]
- Coelho, N.; Gonçalves, S.; González-Benito, M.E.; Romano, A. Establishment of an in vitro propagation protocol for Thymus lotocephalus, a rare aromatic species of the Algarve (Portugal). Plant Growth Regul. 2012, 66, 69–74. [Google Scholar] [CrossRef]
- Stals, H.; Inzé, D. When plant cells decide to divide. Trends Plant Sci. 2001, 6, 359–364. [Google Scholar] [CrossRef]
- Schaller, G.E.; Street, I.H.; Kieber, J.J. Cytokinin and the cell cycle. Curr. Opin. Plant Biol. 2014, 21, 7–15. [Google Scholar] [CrossRef]
- Romanov, G.A.; Lomin, S.N.; Schmülling, T. Biochemical characteristics and ligand-binding properties of Arabidopsis cytokinin receptor AHK3 compared to CRE1/AHK4 as revealed by a direct binding assay. J. Exp. Bot. 2006, 57, 4051–4058. [Google Scholar] [CrossRef]
- Herrera-Isidron, L.; Valencia-Lozano, E.; Rosiles-Loeza, P.Y.; Robles-Hernández, M.G.; Napsuciale-Heredia, A.; Cabrera-Ponce, J.L. Gene expression analysis of microtubers of potato Solanum tuberosum L. induced in cytokinin containing medium and osmotic stress. Plants 2021, 10, 876. [Google Scholar] [CrossRef]
- Romanov, G.A.; Lomin, S.N.; Schmülling, T. Cytokinin signaling: From the ER or from the PM? That is the question! New Phytol. 2018, 218, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Mantilla, G.; Lorenzo, G.A.; Mascarini, L. Hormonal endogenous changes in response to the exogenous 6-benzylaminopurine application in pre- and post-harvesting lilium flower stalks. Ornam. Hortic. 2021, 27, 357–364. [Google Scholar] [CrossRef]
- Hussey, G. In vitro propagation of Narcissus. Ann. Bot. 1982, 49, 707–719. [Google Scholar] [CrossRef]
- Langen-Gerrits, M.M.; de Klerk, G.J. Micropropagation of flower bulbs: Lily and Narcissus. Methods Mol. Biol. 1999, 111, 141–147. [Google Scholar] [CrossRef]
- Juan-Vicedo, J.; Pavlov, A.; Ríos, S.; Casas, J.L. In vitro culture and micropropagation of the Baetic-Moroccan endemic plant Lapiedra martinezii Lag. (Amaryllidaceae). In Vitro Cell. Dev. Biol.-Plant 2019, 55, 725–732. [Google Scholar] [CrossRef]
- Juan-Vicedo, J.; Pavlov, A.; Ríos, S.; Casas, J.L. Micropropagation of five endemic, rare and/or endangered Narcissus species from the Iberian Peninsula (Spain and Portugal). Acta Biol. Crac. Ser. Bot. 2021, 63, 55–61. [Google Scholar] [CrossRef]
- Escobar, E.; Pérez, M.; Romanelli, G.; Blustein, G. Thymol bioactivity: A review focusing on practical applications. Arab. J. Chem. 2020, 13, 9243–9269. [Google Scholar] [CrossRef]
- Blanquer, A.; Boira, H.; Soler, V.; Perez, I. Variability of the essential oil of Thymus piperella. Phytochemistry 1998, 47, 1271–1276. [Google Scholar] [CrossRef]
- Bekircan, T.; Yaşar, A.; Yıldırım, S.; Sökmen, M.; Sökmen, A. Effect of cytokinins on in vitro multiplication, volatiles composition and rosmarinic acid content of Thymus leucotrichus Hal. shoots. 3 Biotech 2018, 8, 180. [Google Scholar] [CrossRef]
- Juan-Méndez, R.; Asensio-Casas, E.; Juan-Vicedo, J. Thymol elicitation during in vitro regeneration of axillary bud explants from a Thymus piperella L. commercial hybrid. In MOL2NET’22, Conference on Molecular, Biomedical & Computational Sciences and Engineering, Proceedings of the Congress NIECXSM-08: North-Ibero-America-Europe Congress on Exp. & Simul. Methods, Valencia, Spain; Miami, FL, USA, 2022, 8th ed.; MDPI: Basel, Switzerland, 2022. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).