Mitigation of Chilling Stress by Ozone Pretreatment and Acclimation of Sweet Pepper Grown under Unheated Greenhouse Conditions
Abstract
1. Introduction
2. Materials and Methods
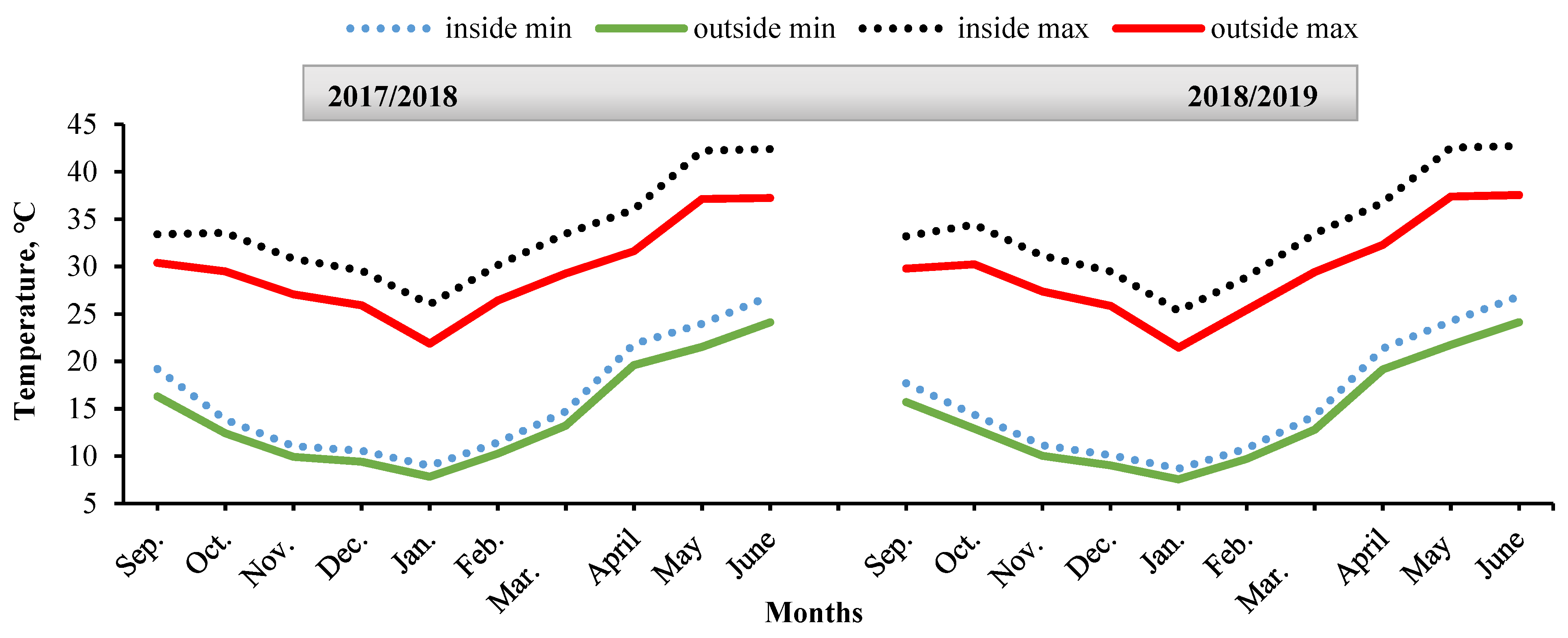
2.1. Climatic and Microclimatic Conditions
2.2. Treatments
- (A)
- Two sweet pepper hybrids (Zidenka and Lirica)
- (B)
- Four concentrations of ozonated water were compared to cold treatment and an untreated control.
- (C)
- Two application methods in two separate experiments (seed soaking and seedling spraying)
2.3. Studied Data
2.3.1. Plant Leaf Area
2.3.2. Plant Biochemical and Physiological Parameters
- (A)
- The relative chlorophyll content (SPAD) or green color content in the first fully expanded leaves was determined (without destroying them) using an SPAD501 apparatus (Minolta Corp, Ramsey, NJ, USA) for greenness measurements.
- (B)
- Membrane permeability (MP), a measure of semipermeability loss of the plasma membrane, was determined by assessing electrolyte leakage according to the method described by Valentovic et al. [36]. Five plants were randomly chosen per replicate and cut into uniformly sized discs; then, 0.5 g of the disc was taken from the middle portion of the youngest fully developed leaf, which was washed with distilled water to remove surface contamination. The discs were placed in closed tubes containing 20 mL of deionized water and incubated at 25 °C for 24 h. The electrical conductivity (EC) of the bathing solution (EC1) was determined. The samples were then boiled in a water bath at 120 °C for 20 min, and the electrical conductivity (EC2) was determined after cooling the solution to room temperature. Membrane permeability was calculated as a ratio as follows:
- (C)
- The relative water content (RWC) estimates the water content of fresh sampled leaf tissue relative the maximal water content it can hold at full turgidity (condition of being swollen). It was measured in fully developed leaves, which were cut and weighed immediately to determine the fresh weight (FW) and then immersed in double-distilled water for 16 h. The excess surface water was removed by drying with paper towels to determine the turgid weight (TW) of the leaves. The leaves were then oven-dried until a constant weight was recorded as the dry weight (DW). The RWC was determined according to Anjum [37]:
- (D)
- The proline (an amino acid) content was determined according to the method developed by Bates et al. [38], which involves filtering 0.5 g fresh leaf material homogenized in 10 mL of 3% aqueous sulfosalicylic acid through Whitman’s No. 1 filter paper. Then, 2 mL of filtered extract was taken for the analysis, and 2 mL acid ninhydrin and 2 mL glacial acetic acid were added. The reaction mixture was incubated in a boiling water bath for 1 h, and the reaction was finished in an ice bath. Then, 4 mL of toluene was added to the reaction mixture, and the organic phase was extracted and read at 520 nm by UV-visible spectrophotometer, using toluene as a blank.
- (E)
- Ascorbate peroxidase (APX) was measured in plants according to [39] using a spectrophotometer (model UV—160A, Shimadzu, Japan).
- (F)
- The chemical composition of samples of five fruits from each plot was determined 90 days after transplanting in both seasons by measuring the total soluble solids (TSS) and vitamin C (mg 100 g−1 FW) in fruit juice according to the methods described by the AOAC [40].
2.3.3. Flowering and Fruit Set Parameters
2.3.4. Fruit Yield and Quality
2.4. Soil Analysis
2.5. Agricultural Practices
2.6. Statistical Analyses
3. Results
3.1. Climatic and Microclimatic Conditions
3.2. Relative Water Content of Leaves (RWC)
3.3. Plant Leaf Area
3.4. Relative Chlorophyll Content of Leaves (SPAD)
3.5. Membrane Permeability
3.6. Proline Content of Leaves
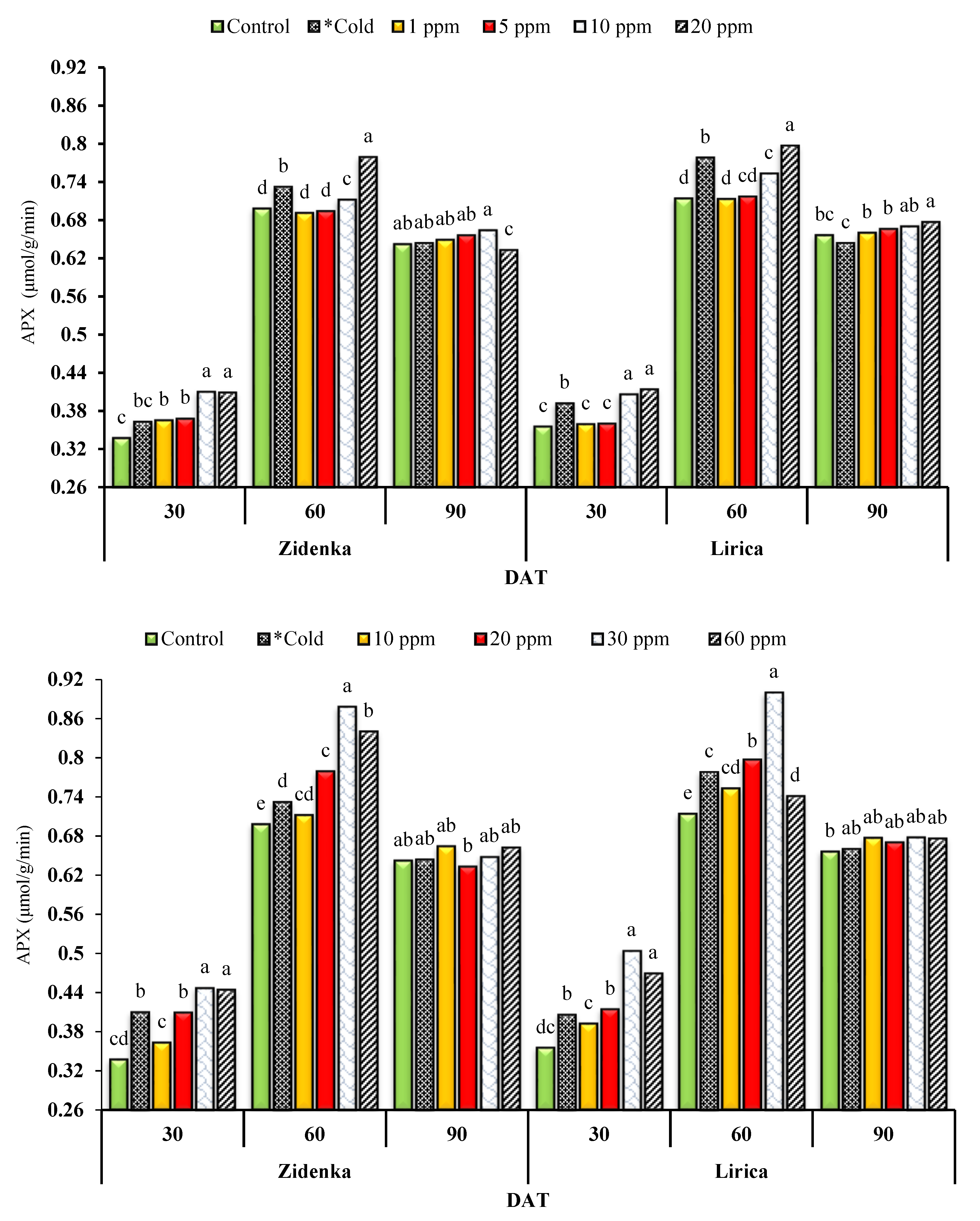
3.7. Ascorbate Peroxidase (APX)
3.8. Flowering and Fruit Set Parameters
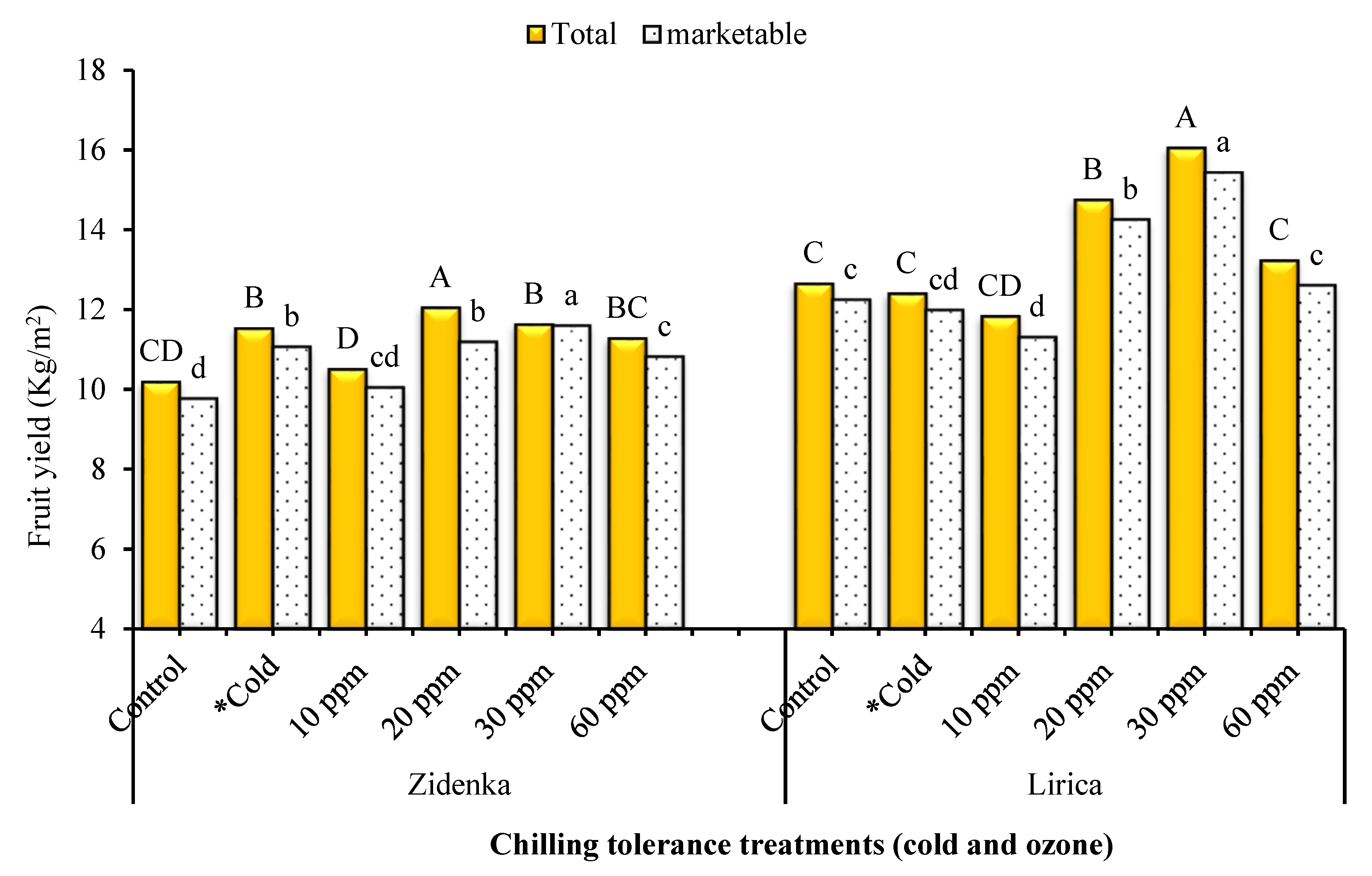
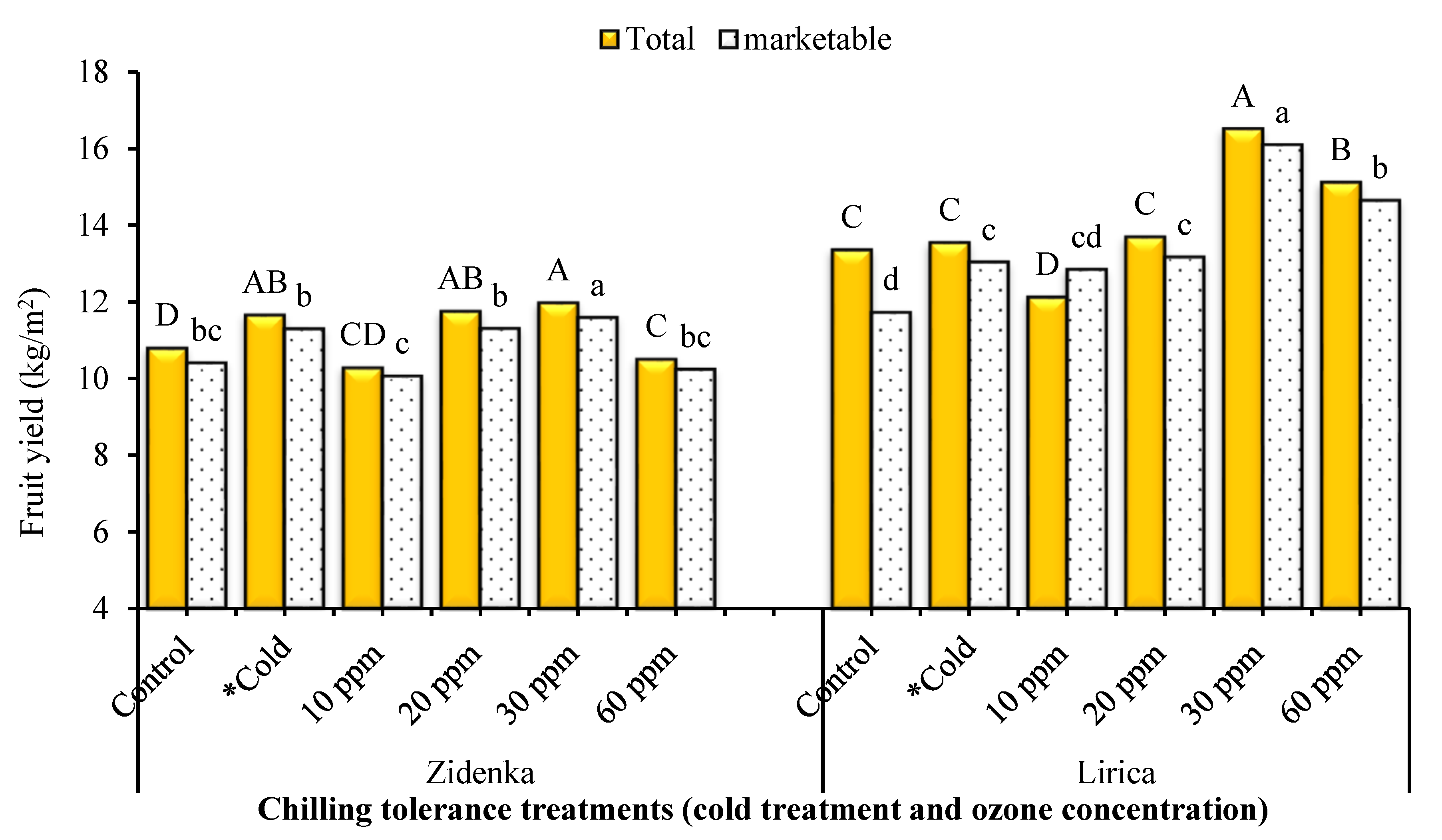
3.9. Fruit Yield and Its Components
3.10. Fruit Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, C.; Fang, M.; Zhai, B.; Ma, L.; Fu, A.; Gao, L.; Kou, X.; Meng, D.; Wang, Q.; Zheng, S.; et al. Regulations of M6A Methylation on Tomato Fruit Chilling Injury. Hortic. Plant J. 2021, 7, 434–442. [Google Scholar] [CrossRef]
- Rehman, R.N.U.; Malik, A.U.; Khan, A.S.; Hasan, M.U.; Anwar, R.; Ali, S.; Haider, M.W. Combined Application of Hot Water Treatment and Methyl Salicylate Mitigates Chilling Injury in Sweet Pepper (Capsicum annuum L.) Fruits. Sci. Hortic. 2021, 283, 110113. [Google Scholar] [CrossRef]
- El-Aidy, F.A.; Sharaf-Eldin, M.A. Modifying Microclimatic Conditions in Plastic Walk-in Tunnels through Solar Energy System for Improving Yield and Quality of Four Sweet Pepper Hybrids. Plasticulture 2015, 134, 6–22. [Google Scholar]
- Zhang, L.; Hao, X.; Li, Y.; Jiang, G. Response of Greenhouse Tomato to Varied Low Pre-Night Temperatures at the Same Daily Integrated Temperature. HortScience 2010, 45, 1654–1661. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, N.; Deswal, R. The Molecular Biology of the Low-Temperature Response in Plants. BioEssays 2005, 27, 1048–1059. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, P.; Huang, Y.; Shan, T.; Wang, L.; Li, Y.; Zheng, Y. Effect of Hot Water Combined with Glycine Betaine Alleviates Chilling Injury in Cold-Stored Loquat Fruit. Postharvest Biol. Technol. 2016, 118, 141–147. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.; Li, C.; Shao, T.; Jiang, X.; Zhao, H.; Ai, W. Postharvest Hot Water Dipping and Hot Water Forced Convection Treatments Alleviate Chilling Injury for Zucchini Fruit during Cold Storage. Sci. Hortic. 2019, 249, 219–227. [Google Scholar] [CrossRef]
- Sharaf-Eldin, M.A.; Alshallash, K.S.; Alharbi, K.R.; Alqahtani, M.M.; Etman, A.A.; Yassin, A.M.; Azab, E.S.; El-Okkiah, S.A.F. Influence of Seed Soaking and Foliar Application Using Ozonated Water on Two Sweet Pepper Hybrids under Cold Stress. Sustainability 2022, 14, 13453. [Google Scholar] [CrossRef]
- Giudice, G.; Moffa, L.; Varotto, S.; Cardone, M.F.; Bergamini, C.; De Lorenzis, G.; Velasco, R.; Nerva, L.; Chitarra, W. Novel and emerging biotechnological crop protection approaches. Plant Biotechnol. J. 2021, 19, 1495–1510. [Google Scholar] [CrossRef]
- FAO. Good Agricultural Practices for Greenhouse Vegetable Crops: Principles for Mediterranean Climate Areas. 2013. Available online: www.fao.org/3/a-i3284e (accessed on 1 October 2022).
- Consentino, B.B.; Sabatino, L.; Vultaggio, L.; Rotino, G.L.; La Placa, G.G.; D’Anna, F.; Leto, C.; Iacuzzi, N.; De Pasquale, C. Grafting of aubergines on underutilized solane species and the biostimulating action of Azospirillum brasilense modulate growth, yield, NUE and nutritional and functional traits. Horticulturae 2022, 8, 722. [Google Scholar] [CrossRef]
- Lefèvre, A.; Perrin, B.; Lesur-Dumoulin, C.; Salembier, C.; Navarrete, M. Challenges of Complying with Both Food Value Chain Specifications and Agroecology Principles in Vegetable Crop Protection. Agric. Syst. 2020, 185, 102953. [Google Scholar] [CrossRef]
- Tan, S.; Cao, J.; Xia, X.; Li, Z. Advances in 5-Aminolevulinic Acid Priming to Enhance Plant Tolerance to Abiotic Stress. Int. J. Mol. Sci. 2022, 23, 702. [Google Scholar] [CrossRef] [PubMed]
- Satyakam; Zinta, G.; Singh, R.K.; Kumar, R. Cold adaptation strategies in plants—An emerging role of epigenetics and antifreeze proteins to engineer cold resilient plants. Front. Genet. 2022, 13, 909007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, H.; Cao, J.; Jiang, W. Advances in Biochemical Mechanisms and Control Technologies to Treat Chilling Injury in Postharvest Fruits and Vegetables. Trends Food Sci. Technol. 2021, 113, 355–365. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, N.; Zhang, H.; Chen, C.; Li, L.; Dong, C.; Cheng, Y. Comparative Transcriptomic Analysis of Cantaloupe Melon under Cold Storage with Ozone Treatment. Food Res. Int. 2021, 140, 109993. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, I.J.; Rathore, D. Assessment of Ozone Toxicity on Cotton (Gossypium hirsutum L.) Cultivars: Its Defensive System and Intraspecific Sensitivity. Plant Physiol. Biochem. 2021, 166, 912–927. [Google Scholar] [CrossRef]
- González-Fernández, I.; Elvira, S.; Calatayud, V.; Calvo, E.; Aparicio, P.; Sánchez, M.; Alonso, R.; Bermejo Bermejo, V. Ozone Effects on the Physiology and Marketable Biomass of Leafy Vegetables under Mediterranean Conditions: Spinach (Spinacia oleracea L.) and Swiss Chard (Beta vulgaris L. Var. Cycla). Agric. Ecosyst. Environ. 2016, 235, 215–228. [Google Scholar] [CrossRef]
- Sharps, K.; Hayes, F.; Harmens, H.; Mills, G. Ozone-Induced Effects on Leaves in African Crop Species. Environ. Pollut. 2021, 268, 115789. [Google Scholar] [CrossRef]
- Peng, J.; Shang, B.; Xu, Y.; Feng, Z.; Calatayud, V. Effects of Ozone on Maize (Zea mays L.) Photosynthetic Physiology, Biomass and Yield Components Based on Exposure- and Flux-Response Relationships. Environ. Pollut. 2020, 256, 113466. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Wang, T.; Li, C. Metabolic Response of Soybean Leaves Induced by Short-Term Exposure of Ozone. Ecotoxicol. Environ. Saf. 2021, 213, 112033. [Google Scholar] [CrossRef]
- Yadav, D.S.; Agrawal, S.B.; Agrawal, M. Ozone Flux-Effect Relationship for Early and Late Sown Indian Wheat Cultivars: Growth, Biomass, and Yield. Field Crops Res. 2021, 263, 108076. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, H.; Dong, C.; Ji, H.; Zhang, X.; Li, L.; Ban, Z.; Zhang, N.; Xue, W. Effect of Ozone Treatment on the Phenylpropanoid Biosynthesis of Postharvest Strawberries. RSC Adv. 2019, 9, 25429–25438. [Google Scholar] [CrossRef]
- Shezi, S.; Samukelo Magwaza, L.; Mditshwa, A.; Zeray Tesfay, S. Changes in Biochemistry of Fresh Produce in Response to Ozone Postharvest Treatment. Sci. Hortic. 2020, 269, 109397. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, X.; Zhang, H.; Ban, Z.; Li, L.; Dong, C.; Ji, H.; Xue, W. Label-Free Quantitative Proteomics to Investigate the Response of Strawberry Fruit after Controlled Ozone Treatment. RSC Adv. 2019, 9, 676–689. [Google Scholar] [CrossRef]
- Rodrigues, A.A.Z.; de Queiroz, M.E.L.R.; Neves, A.A.; de Oliveira, A.F.; Prates, L.H.F.; de Freitas, J.F.; Heleno, F.F.; Faroni, L.R.D.A. Use of Ozone and Detergent for Removal of Pesticides and Improving Storage Quality of Tomato. Food Res. Int. 2019, 125, 108626. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Belz, R.G.; Calatayud, V.; De Marco, A.; Hoshika, Y.; Kitao, M.; Saitanis, C.J.; Sicard, P.; Paoletti, E.; Calabrese. Predicting the effect ofozone on vegetation via linear non-threshold (LNT), threshold and hormetic dose-response models. Sci. Total Environ. 2019, 649, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Calzone, A.; Podda, A.; Lorenzini, G.; Maserti, B.E.; Carrari, E.; Deleanu, E.; Hoshika, Y.; Haworth, M.; Nali, C.; Badea, O.; et al. Cross-Talk between Physiological and Biochemical Adjustments by Punica granatum cv. Dente di Cavallo Mitigates the Effects of Salinity and Ozone Stress. Sci. Total Environ. 2019, 656, 589–597. [Google Scholar] [CrossRef]
- Pellegrini, E.; Cotrozzi, L.; Neri, L.; Baraldi, R.; Carrari, E.; Nali, C.; Lorenzini, G.; Paoletti, E.; Hoshika, Y. Stress Markers and Physiochemical Responses of the Mediterranean Shrub Phillyrea Angustifolia under Current and Future Drought and Ozone Scenarios. Environ. Res. 2021, 201, 111615. [Google Scholar] [CrossRef]
- Pellegrini, E.; Campanella, A.; Cotrozzi, L.; Tonelli, M.; Nali, C.; Lorenzini, G. Ozone primes changes in phytochemical parameters in the medicinal herb Hypericum perforatum (St. John’ s wort). Ind. Crops Prod. 2018, 126, 119–128. [Google Scholar] [CrossRef]
- Endo, H.; Miyazaki, K.; Ose, K.; Imahori, Y. Hot Water Treatment to Alleviate Chilling Injury and Enhance Ascorbate-Glutathione Cycle in Sweet Pepper Fruit during Postharvest Cold Storage. Sci. Hortic. 2019, 257, 108715. [Google Scholar] [CrossRef]
- Airaki, M.; Leterrier, M.; Mateos, R.M.; Valderrama, R.; Chaki, M.; Barroso, J.B.; Del Rio, L.A.; Palma, J.M.; Corpas, F.J. Metabolism of Reactive Oxygen Species and Reactive Nitrogen Species in Pepper (Capsicum annuum L.) Plants under Low Temperature Stress. Plant Cell Environ. 2011, 35, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Hongna, C.; Leyuan, T.; Junmei, S.; Xiaori, H.; Xianguo, C. Exogenous Salicylic Acid Signal Reveals an Osmotic Regulatory Role in Priming the Seed Germination of Leymus chinensis under Salt-Alkali Stress. Environ. Exp. Bot. 2021, 188, 104498. [Google Scholar] [CrossRef]
- Kulak, M.; Jorrín-Novo, J.V.; Romero-Rodriguez, M.C.; Yildirim, E.D.; Gul, F.; Karaman, S. Seed Priming with Salicylic Acid on Plant Growth and Essential Oil Composition in Basil (Ocimum basilicum L.) Plants Grown under Water Stress Conditions. Ind. Crops Prod. 2021, 161, 113235. [Google Scholar] [CrossRef]
- Korkmaz, A.; Korkmaz, Y.; Demirkiran, A.R. Enhancing chilling stress tolerance of pepper seedlings by exogenous application of 5-aminolevulinic acid. Environ. Exp. Bot. 2010, 67, 495–501. [Google Scholar] [CrossRef]
- Valentovič, P.; Luxová, M.; Kolarovič, L.; Gašparíková, O. Effect of Osmotic Stress on Compatible Solutes Content, Membrane Stability and Water Relationsin Two Maize Cultivars. Plant Soil Environ. 2011, 52, 186–191. [Google Scholar] [CrossRef]
- Anjum, S.A.; Farooq, M.; Xie, X.; Liu, X.; Ijaz, M.F. Antioxidant Defense System and Proline Accumulation Enables Hot Pepper to Perform Better under Drought. Sci. Hortic. 2012, 140, 66–73. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis. Association of Official Analytical Chemists, 15th ed.; AOAC: Washington, DC, USA, 1990. [Google Scholar]
- Bottomley, P.J.; Angle, J.S.; Weaver, R.W. Methods of Soil Analysis, Part 2: Microbiological and Biochemical Properties; John Wiley & Sons: Hoboken, NJ, USA, 2020; Volume 12, ISBN 089118810X. [Google Scholar]
- Yadav, S.K. Cold Stress Tolerance Mechanisms in Plants. A Review. Agron. Sustain. Dev. 2010, 30, 515–527. [Google Scholar] [CrossRef]
- İşeri, Ö.D.; Körpe, D.A.; Sahin, F.I.; Haberal, M. Hydrogen Peroxide Pretreatment of Roots Enhanced Oxidative Stress Response of Tomato under Cold Stress. Acta Physiol. Plant. 2013, 35, 1905–1913. [Google Scholar] [CrossRef]
- Shalaby, T.A.; Abd-Alkarim, E.; El-Aidy, F.; Hamed, E.-S.; Sharaf-Eldin, M.; Taha, N.; El-Ramady, H.; Bayoumi, Y.; dos Reis, A.R. Nano-Selenium, Silicon and H2O2 Boost Growth and Productivity of Cucumber under Combined Salinity and Heat Stress. Ecotoxicol. Environ. Saf. 2021, 212, 111962. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, F.; Hussein, L.; Mohammad, H.; Shiranirad, A.H.; Valadabadi, A.R.; Daneshian, J. Effects of Arbuscular Mycorrhizal Fungi, Different Levels of Phosphorus and Drought Stress on Water Use Efficiency, Relative Water Content and Proline Accumulation Rate of Coriander (Coriandrum sativum L.). J. Med. Plants Res. 2008, 2, 125–131. [Google Scholar]
- Filek, M.; Walas, S.; Mrowiec, H.; Rudolphy-Skórska, E.; Sieprawska, A.; Biesaga-Kościelniak, J. Membrane Permeability and Micro- and Macroelement Accumulation in Spring Wheat Cultivars during the Short-Term Effect of Salinity- and PEG-Induced Water Stress. Acta Physiol. Plant. 2011, 34, 985–995. [Google Scholar] [CrossRef]
- Chaudhary, I.J.; Rathore, D. Assessment of Dose–Response Relationship between Ozone Dose and Groundnut (Arachis hypogaea L.) Cultivars Using Open Top Chamber (OTC) and Ethylenediurea (EDU). Environ. Technol. Innov. 2021, 22, 101494. [Google Scholar] [CrossRef]
- Schmidt, R.; Kunkowska, A.B.; Schippers, J.H.M. Role of Reactive Oxygen Species during Cell Expansion in Leaves. Plant Physiol. 2016, 172, 2098–2106. [Google Scholar] [CrossRef]
- Wilkinson, S.; Mills, G.; Illidge, R.; Davies, W.J. How Is Ozone Pollution Reducing Our Food Supply? J. Exp. Bot. 2011, 63, 527–536. [Google Scholar] [CrossRef]
- Ainsworth, E.A. Understanding and improving global crop response to ozone pollution. Plant J. 2017, 90, 886–897. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Harayama, J.; Calabrese, E.J. Temperature-induced hormesis in plants. J. For. Res. 2019, 30, 13–20. [Google Scholar] [CrossRef]
- Jalal, A.; de Oliveira Junior, J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; dos Reis, A.R. Hormesis in plants: Physiological and biochemical responses. Ecotox. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef]
- Marchica, A.; Loré, S.; Cotrozzi, L.; Lorenzini, G.; Nali, C.; Pellegrini, E.; Remorini, D. Early Detection of Sage (Salvia officinalis L.) Responses to Ozone Using Reflectance Spectroscopy. Plants 2019, 8, 346. [Google Scholar] [CrossRef]
- Marchica, A.; Ascrizzi, R.; Flamini, G.; Cotrozzi, L.; Tonelli, M.; Lorenzini, G.; Nali, C.; Pellegrini, E. Ozone as eustress for enhancing secondary metabolites and bioactive properties in Salvia officinalis. Ind. Crops Prod. 2021, 170, 113730. [Google Scholar] [CrossRef]
- Modesti, M.; Baccelloni, S.; Brizzolara, S.; Aleandri, M.P.; Bellincontro, A.; Mencarelli, F.; Tonutti, P. Effects of treatments with ozonated water in the vineyard (cv Vermentino) on microbial population and fruit quality parameters. BIO Web Conf. 2019, 13, 04011. [Google Scholar] [CrossRef]
- Liheng, H.; Zhiqiang, G.; Runzhi, L. Pretreatment of Seed with H2O2 Enhances Drought Tolerance of Wheat (Triticum aestivum L.) Seedlings. Afr. J. Biotechnol. 2009, 8, 6151–6157. [Google Scholar] [CrossRef]
- Ali, B.; Hasan, S.A.; Hayat, S.; Hayat, Q.; Yadav, S.; Fariduddin, Q.; Ahmad, A. A Role for Brassinosteroids in the Amelioration of Aluminium Stress through Antioxidant System in Mung Bean (Vigna radiata L. Wilczek). Environ. Exp. Bot. 2008, 62, 153–159. [Google Scholar] [CrossRef]
- Nali, C.; Paoletti, E.; Marabottini, R.; Della Rocca, G.; Lorenzini, G.; Paolacci, A.R.; Ciaffi, M.; Badiani, M. Ecophysiological and Biochemical Strategies of Response to Ozone in Mediterranean Evergreen Broadleaf Species. Atmos. Environ. 2004, 38, 2247–2257. [Google Scholar] [CrossRef]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From Intracellular Signaling Networks to Cell Death: The Dual Role of Reactive Oxygen Species in Seed Physiology. Comptes Rendus Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef]
- Sanz, J.; Muntifering, R.B.; Bermejo, V.; Gimeno, B.S.; Elvira, S. Ozone and Increased Nitrogen Supply Effects on the Yield and Nutritive Quality of Trifolium Subterraneum. Atmos. Environ. 2005, 39, 5899–5907. [Google Scholar] [CrossRef]
- Lin, J.C.; Nosal, M.; Muntifering, R.B.; Krupa, S.V. Alfalfa Nutritive Quality for Ruminant Livestock as Influenced by Ambient Air Quality in West-Central Alberta. Environ. Pollut. 2007, 149, 99–103. [Google Scholar] [CrossRef]
- Yadegari, L.Z.; Heidari, R.; Carapetian, J. The Influence of Cold Acclimation on Proline, Malondialdehyde (MDA), Total Protein and Pigments Contents in Soybean (Glycine max) Seedlings. J. Biol. Sci. 2007, 7, 1436–1441. [Google Scholar] [CrossRef][Green Version]
- Esra, K.O.Ç.; İŞLEK, C.; Üstün, A.S. Effect of Cold on Protein, Proline, Phenolic Compounds and Chlorophyll Content of Two Pepper (Capsicum annuum L.) Varieties. Gazi Univ. J. Sci. 2010, 23, 1–6. [Google Scholar]
- Zhang, Z.J.; Li, H.Z.; Zhou, W.J.; Takeuchi, Y.; Yoneyama, K. Effect of 5-Aminolevulinic Acid on Development and Salt Tolerance of Potato (Solanum tuberosum L.) Microtubers in Vitro. Plant Growth Regul. 2006, 49, 27–34. [Google Scholar]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. ISBN 9789048126651. [Google Scholar]
- Farooq, M.; Wahid, A.; Lee, D.-J.; Ito, O.; Siddique, K.H.M. Advances in Drought Resistance of Rice. Crit. Rev. Plant Sci. 2009, 28, 199–217. [Google Scholar] [CrossRef]
- Farooq, M.; Aziz, T.; Wahid, A.; Lee, D.-J.; Siddique, K.H.M. Chilling Tolerance in Maize: Agronomic and Physiological Approaches. Crop Pasture Sci. 2009, 60, 501. [Google Scholar] [CrossRef]
- Parida, A.K.; Dagaonkar, V.S.; Phalak, M.S.; Aurangabadkar, L.P. Differential Responses of the Enzymes Involved in Proline Biosynthesis and Degradation in Drought Tolerant and Sensitive Cotton Genotypes during Drought Stress and Recovery. Acta Physiol. Plant. 2008, 30, 619–627. [Google Scholar] [CrossRef]
- Yan, K.; Chen, W.; He, X.; Zhang, G.; Xu, S.; Wang, L. Responses of Photosynthesis, Lipid Peroxidation and Antioxidant System in Leaves of Quercus mongolica to Elevated OZONE. Environ. Exp. Bot. 2010, 69, 198–204. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, C.; Chen, J.; Qiu, J.; Huang, Z.; Wang, Q.; Ye, Y. Cold acclimation improves photosynthesis by regulating the ascorbate–glutathione cycle in chloroplasts of Kandelia obovate. J. For. Res. 2019, 30, 755–765. [Google Scholar] [CrossRef]
- Flowers, M.D.; Fiscus, E.L.; Burkey, K.O.; Booker, F.L.; Dubois, J.-J.B. Photosynthesis, Chlorophyll Fluorescence, and Yield of Snap Bean (Phaseolus vulgaris L.) Genotypes Differing in Sensitivity to Ozone. Environ. Exp. Bot. 2007, 61, 190–198. [Google Scholar] [CrossRef]
- Han, Y.J.; Gharibeshghi, A.; Mewis, I.; Förster, N.; Beck, W.; Ulrichs, C. Plant Responses to Ozone: Effects of Different Ozone Exposure Durations on Plant Growth and Biochemical Quality of Brassica Campestris L. Ssp. Chinensis. Sci. Hortic. 2020, 262, 108921. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Iqbal, N.; Masood, A.; Per, T.S.; Khan, N.A. Salicylic Acid Alleviates Adverse Effects of Heat Stress on Photosynthesis through Changes in Proline Production and Ethylene Formation. Plant Signal. Behav. 2013, 8, e26374. [Google Scholar] [CrossRef]
- Rajametov, S.N.; Lee, K.; Jeong, H.-B.; Cho, M.-C.; Nam, C.-W.; Yang, E.-Y. The Effect of Night Low Temperature on Agronomical Traits of Thirty-Nine Pepper Accessions (Capsicum annuum L.). Agronomy 2021, 11, 1986. [Google Scholar] [CrossRef]





| Treatment | Relative Water Content (%) | ||||||
|---|---|---|---|---|---|---|---|
| 30 Days after Transplanting | 60 Days after Transplanting | 90 Days after Transplanting | |||||
| Seed Soaking | Seedling Spray | Seed Soaking | Seedling Spray | Seed Soaking | Seedling Spray | ||
| 2017/2018 | |||||||
| Zidenka cv. | Control | 72.43 d | 71.50 f | 81.10 c | 79.46 e | 81.00 b | 81.13 b |
| * Cold | 75.46 b | 76.13 b | 82.06 b | 81.90 bc | 81.20 b | 81.30 b | |
| 1 ppm O3 | 72.60 d | 71.40 f | 81.16 c | 79.40 e | 81.30 b | 81.20 b | |
| 5 ppm O3 | 72.10 d | 71.50 f | 81.23 c | 79.56 e | 81.50 b | 81.40 b | |
| 10 ppm O3 | 74.26 c | 73.06 e | 81.56 c | 81.66 c | 81.40 b | 81.50 b | |
| 20 ppm O3 | 75.20 b | 75.70 bc | 82.30 b | 82.03 b | 82.06 a | 82.16 a | |
| Lirica cv. | Control | 74.30 c | 74.40 d | 82.00 bc | 81.00 c | 81.06 b | 80.96 ab |
| * Cold | 74.26 c | 76.13 b | 82.56 b | 81.33 c | 81.06 b | 81.26 b | |
| 1 ppm O3 | 74.06 c | 74.36 d | 82.00 bc | 81.10 c | 81.26 b | 81.06 bc | |
| 5 ppm O3 | 74.30 c | 74.50 d | 82.03 b | 81.43 c | 81.80 ab | 81.73 b | |
| 10 ppm O3 | 75.46 b | 75.06 c | 84.43 a | 84.20 a | 81.73 ab | 81.80 b | |
| 20 ppm O3 | 78.63 a | 78.33 a | 85.23 a | 85.13 a | 82.06 a | 82.33 a | |
| Significance | * | * | * | * | * | * | |
| 2018/2019 | |||||||
| Zidenka cv. | Control | 71.20 i | 71.50 g | 79.60 e | 79.63 f | 81.00 cd | 81.13 c |
| * Cold | 76.60 e | 76.13 d | 81.90 d | 81.20 c | 81.20 c | 81.30 c | |
| 10 ppm O3 | 73.33 g | 73.06 f | 82.00 cd | 79.86 f | 81.40 c | 81.50 c | |
| 20 ppm O3 | 76.23 e | 75.70 e | 83.26 c | 80.03 e | 82.06 b | 82.16 b | |
| 30 ppm O3 | 81.36 b | 81.00 b | 89.73 a | 81.93 bc | 83.00 a | 83.13 ab | |
| 60 ppm O3 | 77.20 d | 81.13 b | 84.63 b | 82.56 b | 82.93 b | 83.23 a | |
| Lirica cv. | Control | 74.30 fg | 74.40 ef | 80.86 e | 79.83 f | 81.06 cd | 80.96 d |
| * Cold | 76.70 e | 76.50 d | 82.66 c | 81.16 c | 81.06 cd | 81.26 c | |
| 10 ppm O3 | 75.46 f | 75.06 e | 84.53 b | 80.70 e | 81.73 c | 81.80 c | |
| 20 ppm O3 | 78.63 cd | 78.23 c | 85.20 b | 81.36 c | 82.06 b | 82.33 b | |
| 30 ppm O3 | 83.83 a | 82.90 a | 91.76 a | 83.70 ab | 84.06 a | 84.40 a | |
| 60 ppm O3 | 79.30 c | 82.20 a | 84.66 b | 84. 93 a | 83.60 a | 83.23 a | |
| Significance | * | * | * | * | * | * | |
| Treatment | Leaf Area (m2 Plant−1) | ||||
|---|---|---|---|---|---|
| 30 Days after Transplanting | 60 Days after Transplanting | ||||
| Seed Soaking | Seedling Spray | Seed Soaking | Seedling Spray | ||
| 2017/2018 | |||||
| Zidenka cv. | Control | 15.68 a | 15.47 a | 30.01 e | 31.09 d |
| * Cold | 15.00 a | 15.00 a | 33.62 b | 31.40 d | |
| 1 ppm O3 | 15.88 a | 15.31 a | 30.56 e | 31.58 d | |
| 5 ppm O3 | 15.88 a | 16.03 a | 30.64 e | 31.25 d | |
| 10 ppm O3 | 16.86 a | 17.01 a | 32.43 c | 34.15 bc | |
| 20 ppm O3 | 17.51 a | 17.29 a | 36.58 a | 35.73 b | |
| Lirica cv. | Control | 15.90 a | 15.27 a | 31.09 d | 33.57 c |
| * Cold | 15.34 a | 15.42 a | 31.40 d | 35.72 b | |
| 1 ppm O3 | 16.19 a | 16.00 a | 31.58 d | 33.31 c | |
| 5 ppm O3 | 16.26 a | 17.32 a | 31.25 d | 33.62 c | |
| 10 ppm O3 | 17.03 a | 17.37 a | 34.15 b | 40.39 a | |
| 20 ppm O3 | 17.95 a | 15.84 a | 35.75 a | 36.37 b | |
| Significance | ns | ns | * | * | |
| 2018/2019 | |||||
| Zidenka cv. | Control | 18.50 cd | 17.55 d | 26.12 h | 28.77 f |
| * Cold | 15.92 e | 18.07 cd | 31.55 g | 42.42 c | |
| 10 ppm O3 | 19.13 c | 19.49 b | 34.00 f | 38.26 d | |
| 20 ppm O3 | 19.77 bc | 19.64 b | 41.32 d | 44.20 b | |
| 30 ppm O3 | 21.59 a | 19.28 b | 43.96 c | 47.03 a | |
| 60 ppm O3 | 19.92 bc | 18.77 c | 45.04 b | 34.09 e | |
| Lirica cv. | Control | 16.85 d | 16.71 e | 25.73 h | 26.84 g |
| * Cold | 15.98 de | 15.89 e | 30.38 g | 27.80 f | |
| 10 ppm O3 | 20.18 b | 19.13 bc | 37.55 e | 33.34 e | |
| 20 ppm O3 | 20.96 ab | 21.94 a | 43.91 c | 37.75 d | |
| 30 ppm O3 | 21.65 a | 20.83 ab | 49.36 a | 48.33 a | |
| 60 ppm O3 | 21.05 a | 18.20 c | 44.93 b | 38.84 d | |
| Significance | * | * | * | * | |
| Treatment | Relative Chlorophyll Content (SPAD) | ||||||
|---|---|---|---|---|---|---|---|
| 30 Days after Transplanting | 60 Days after Transplanting | 90 Days after Transplanting | |||||
| Seed Soaking | Seedling Spray | Seed Soaking | Seedling Spray | Seed Soaking | Seedling Spray | ||
| 2017/2018 | |||||||
| Zidenka cv. | Control | 43.06 e | 41.43 e | 49.46 e | 49.30 e | 58.30 c | 58.46 b |
| * Cold | 49.10 c | 46.60 c | 52.20 c | 54.60 bc | 58.33 c | 58.13 bc | |
| 1 ppm O3 | 42.56 ef | 41.50 e | 49.63 e | 49.36 e | 58.13 c | 58.33 b | |
| 5 ppm O3 | 43.86 e | 41.30 e | 49.76 e | 49.40 e | 58.13 c | 58.13 bc | |
| 10 ppm O3 | 48.13 d | 43.46 d | 54.10 b | 51.46 d | 58.13 c | 58.13 bc | |
| 20 ppm O3 | 53.20 ab | 47.13 bc | 54.83 b | 55.13 b | 59.10 b | 59.10 ab | |
| Lirica cv. | Control | 48.50 cd | 48.13 b | 52.06 c | 53.90 c | 58.80 bc | 58.50 b |
| * Cold | 48.03 d | 47.60 bc | 52.60 c | 55.60 b | 59.70 ab | 59.70 b | |
| 1 ppm O3 | 48.50 cd | 48.30 b | 52.33 c | 55.13 b | 59.70 ab | 59.70 a | |
| 5 ppm O3 | 48.40 cd | 48.60 b | 52.36 c | 56.40 a | 60.13 a | 60.13 a | |
| 10 ppm O3 | 52.73 b | 49.50 a | 54.70 b | 56.13 a | 60.13 a | 60.13 a | |
| 20 ppm O3 | 54.76 a | 50.13 a | 57.40 a | 56.20 a | 60.13 a | 61.06 a | |
| Significance | * | * | * | * | * | * | |
| 2018/2019 | |||||||
| Zidenka cv. | Control | 41.20 e | 42.30 e | 51.20 g | 51.30 e | 58.30 c | 58.46 c |
| * Cold | 41.80 e | 46.60 c | 51.80 g | 56.60 c | 58.33 c | 58.13 c | |
| 10 ppm O3 | 48.13 d | 43.66 d | 58.13 e | 53.46 d | 58.13 c | 58.13 c | |
| 20 ppm O3 | 49.10 c | 47.13 b | 59.10 d | 57.13 b | 59.10 b | 59.10 bc | |
| 30 ppm O3 | 53.13 ab | 48.76 a | 63.13 b | 58.76 a | 60.13 a | 60.13 ab | |
| 60 ppm O3 | 48.20 d | 45.66 cd | 58.20 e | 55.66 c | 59.76 ab | 59.23 bc | |
| Lirica cv. | Control | 48.50 cd | 48.13 ab | 58.50 de | 58.13 ab | 58.80 c | 58.80 c |
| * Cold | 44.60 e | 47.60 ab | 54.60 f | 57.60 b | 59.70 ab | 59.70 b | |
| 10 ppm O3 | 52.73 b | 45.90 cd | 62.73 bc | 55.90 c | 60.13 a | 60.13 ab | |
| 20 ppm O3 | 53.20 ab | 47.13 b | 63.20 b | 57.13 b | 60.13 a | 61.06 a | |
| 30 ppm O3 | 54.76 a | 49.10 a | 64.76 a | 59.10 a | 60.36 a | 60.36 a | |
| 60 ppm O3 | 52.13 b | 48.56 a | 62.13 c | 58.56 a | 60.00 a | 59.83 b | |
| Significance | * | * | * | * | * | * | |
| Treatment | Membrane Permeability (%) | ||||||
|---|---|---|---|---|---|---|---|
| 30 Days after Transplanting | 60 Days after Transplanting | 90 Days after Transplanting | |||||
| Seed Soaking | Seedling Spray | Seed Soaking | Seedling Spray | Seed Soaking | Seedling Spray | ||
| 2017/2018 | |||||||
| Zidenka cv. | Control | 18.23 a | 18.23 a | 22.13 a | 22.56 a | 21.06 a | 21.20 a |
| * Cold | 16.36 c | 16.36 c | 19.63 c | 19.70 c | 19.96 b | 20.66 ab | |
| 1 ppm O3 | 18.30 a | 18.23 a | 21.50 b | 22.53 a | 17.13 e | 16.66 cd | |
| 5 ppm O3 | 18.03 a | 18.36 a | 21.76 ab | 22.60 a | 17.96 e | 16.43 d | |
| 10 ppm O3 | 17.23 b | 17.23 b | 21.76 ab | 21.53 b | 19.66 b | 19.50 b | |
| 20 ppm O3 | 15.20 d | 15.20 d | 26.60 e | 21.40 b | 19.03 b | 18.80 bc | |
| Lirica cv. | Control | 17.93 ab | 17.93 ab | 20.32 bc | 19.73 c | 18.60 c | 19.73 b |
| * Cold | 16.56 c | 16.56 c | 18.90 cd | 18.66 d | 18.50 c | 18.60 bc | |
| 1 ppm O3 | 18.13 a | 17.56 ab | 21.06 b | 19.70 c | 18.26 c | 18.13 c | |
| 5 ppm O3 | 18.26 a | 17.86 ab | 20.53 bc | 19.60 c | 17.40 e | 17.23 c | |
| 10 ppm O3 | 17.40 b | 17.40 b | 17.73 d | 17.93 de | 18.03 c | 18.03 c | |
| 20 ppm O3 | 15.66 d | 15.66 d | 17.00 d | 17.13 e | 17.60 e | 17.60 cd | |
| Significance | * | * | * | * | * | * | |
| 2018/2019 | |||||||
| Zidenka cv. | Control | 18.23 a | 18.23 a | 22.13 a | 22.56 a | 21.06 a | 21.20 a |
| * Cold | 16.36 c | 16.36 c | 19.63 d | 19.70 c | 19.96 ab | 20.06 b | |
| 10 ppm O3 | 17.23 b | 17.23 b | 21.50 b | 22.53 a | 19.66 b | 19.50 b | |
| 20 ppm O3 | 15.20 d | 15.20 d | 21.76 ab | 22.60 a | 19.03 b | 18.80 bc | |
| 30 ppm O3 | 14.46 e | 14.46 e | 21.70 ab | 21.53 b | 18.26 c | 18.13 c | |
| 60 ppm O3 | 16.30 c | 14.96 d | 20.60 c | 21.40 b | 17.40 d | 17.23 cd | |
| Lirica cv. | Control | 17.93 a | 17.93 a | 20.23 c | 22.53 a | 18.60 bc | 19.73 b |
| * Cold | 16.56 c | 16.56 c | 18.90 de | 18.66 e | 18.50 bc | 18.60 bc | |
| 10 ppm O3 | 17.40 b | 17.40 ab | 21.06 b | 19.73 c | 18.03 c | 18.03 c | |
| 20 ppm O3 | 15.66 d | 15.66 d | 20.53 c | 19.60 c | 17.60 d | 17.60 c | |
| 30 ppm O3 | 14.06 e | 14.06 e | 17.73 e | 17.93 f | 17.13 d | 16.66 d | |
| 60 ppm O3 | 17.23 b | 14.80 e | 17.00 e | 17.13 e | 17.96 cd | 16.43 d | |
| Significance | * | * | * | * | * | * | |
| Treatment | No. Flowers/Plant | No. Fruits/Plant | No. Seeds/Fruit | Fruits Set % | |||||
|---|---|---|---|---|---|---|---|---|---|
| Seed Soaking | Seedling Spray | Seed Soaking | Seedling Spray | Seed Soaking | Seedling Spray | Seed Soaking | Seedling Spray | ||
| 2017/2018 | |||||||||
| Zidenka cv. | Control | 5.00 c | 5.00 bc | 1.66 e | 1.66 d | 261 e | 261 d | 38.77 e | 34.86 f |
| * Cold | 4.66 cd | 4.66 c | 3.00 c | 3.00 bcd | 263 e | 263 d | 65.00 c | 65.00 d | |
| 1 ppm O3 | 4.33 d | 5.33 b | 2.66 cd | 3.66 bc | 307 cd | 254 d | 65.41 c | 69.44 c | |
| 5 ppm O3 | 5.33 bc | 5.00 bc | 2.33 d | 3.00 bcd | 290 d | 277 cd | 55.16 d | 69.11 c | |
| 10 ppm O3 | 4.66 cd | 5.00 bc | 3.00 c | 3.66 bc | 315 c | 303 bc | 56.00 d | 93.65 a | |
| 20 ppm O3 | 6.00 ab | 5.66 b | 4.33 ab | 5.33 a | 322 b | 339 c | 73.40 b | 88.29 b | |
| Lirica cv. | Control | 4.66 cd | 4.66 c | 3.00 c | 3.33 bc | 280 d | 280 cd | 63.44 bc | 79.65 b |
| * Cold | 3.66 e | 3.66 d | 2.33 d | 2.33 cd | 313 c | 313 c | 66.60 b | 68.33 c | |
| 1 ppm O3 | 5.66 b | 5.00 bc | 4.33 ab | 2.33 cd | 311 c | 360 b | 78.66 b | 50.62 e | |
| 5 ppm O3 | 5.33 bc | 5.00 bc | 5.66 a | 4.66 b | 314 c | 356 b | 57.93 d | 83.45 b | |
| 10 ppm O3 | 6.33 ab | 5.66 b | 3.66 b | 5.33 a | 341 ab | 412 a | 77.98 b | 91.62 ab | |
| 20 ppm O3 | 7.00 a | 7.33 a | 5.66 a | 6.00 a | 364 a | 403 a | 99.16 a | 97.77 a | |
| Significance | * | * | * | * | * | * | * | * | |
| 2018/2019 | |||||||||
| Zidenka cv. | Control | 5.20 bc | 4.76 bc | 2.06 d | 2.16 de | 283 d | 283 d | 40.47 e | 45.50 e |
| * Cold | 4.86 c | 4.93 b | 2.96 cd | 3.13 cd | 276 d | 276 de | 60.83 cd | 63.82 c | |
| 10 ppm O3 | 4.53 cd | 5.53 ab | 2.66 d | 3.23 cd | 322 c | 268 e | 59.37 cd | 59.55 d | |
| 20 ppm O3 | 5.26 bc | 5.43 ab | 2.66 d | 3.13 cd | 304 c | 324 c | 52.45 d | 59.56 d | |
| 30 ppm O3 | 5.93 ab | 6.50 a | 4.60 d | 4.86 b | 348 b | 355 bc | 78.28 b | 75.30 b | |
| 60 ppm O3 | 5.93 ab | 5.80 a | 4.53 b | 5.53 ab | 329 b | 318 c | 74.08 bc | 92.13 a | |
| Lirica cv. | Control | 5.03 c | 5.20 ab | 3.33 c | 3.46 bc | 294 d | 294 d | 65.99 c | 73.13 bc |
| * Cold | 4.20 d | 4.20 c | 2.66 d | 2.73 d | 328 bc | 328 c | 64.42 c | 63.97 c | |
| 10 ppm O3 | 5.53 b | 4.86 b | 4.53 b | 2.66 d | 327 bc | 374 b | 79.69 b | 54.80 d | |
| 20 ppm O3 | 5.20 bc | 5.53 ab | 5.83 a | 4.86 b | 329 b | 376 b | 81.92 b | 88.21 ab | |
| 30 ppm O3 | 7.26 a | 6.66 a | 5.80 a | 6.16 a | 382 a | 423 a | 99.24 a | 93.19 a | |
| 60 ppm O3 | 6.66 a | 6.33 a | 4.06 bc | 5.76 ab | 358 a | 421 a | 61.26 c | 91.50 a | |
| Significance | * | * | * | * | * | * | * | * | |
| Treatment | + Early Yield (kg m−2) | Fruits per m−2 for Early Yield | Average Fruit Weight (g) for Total Yield | ||||
|---|---|---|---|---|---|---|---|
| Seed Soaking | Seedling Spray | Seed Soaking | Seedling Spray | Seed Soaking | Seedling Spray | ||
| 2017/2018 | |||||||
| Zidenka cv. | Control | 4.76 d | 5.75 bc | 24.44 ab | 26.41 ab | 164 cd | 172 cd |
| * Cold | 4.80 d | 5.49 c | 24.53 ab | 21.60 c | 175 c | 173 cd | |
| 1 ppm O3 | 4.63 d | 4.52 c | 19.75 d | 25.82 b | 177 c | 172 cd | |
| 5 ppm O3 | 5.04 c | 4.88 d | 24.83 b | 21.98 cd | 169 c | 186 c | |
| 10 ppm O3 | 5.05 c | 5.23 c | 23.60 bc | 23.84 c | 179 c | 169 d | |
| 20 ppm O3 | 5.89 bc | 5.14 cd | 26.44 a | 23.55 c | 175 c | 171 cd | |
| Lirica cv. | Control | 4.36 e | 4.80 d | 16.81 e | 19.75 d | 220 a | 205 b |
| * Cold | 4.57 de | 5.50 c | 17.60 e | 22.03 cd | 212 a | 206 b | |
| 1 ppm O3 | 5.24 c | 5.90 bc | 21.27 d | 20.99 d | 200 b | 206 b | |
| 5 ppm O3 | 6.04 b | 5.36 c | 23.26 bc | 19.56 de | 209 ab | 207 b | |
| 10 ppm O3 | 6.11 b | 6.69 b | 22.63 c | 23.35 c | 206 ab | 218 a | |
| 20 ppm O3 | 7.71 a | 8.98 a | 25.37 a | 28.24 a | 222 a | 229 a | |
| Significance | * | * | * | * | * | * | |
| 2018/2019 | |||||||
| Zidenka cv. | Control | 5.11 c | 5.64 d | 25.55 b | 25.39 b | 173 c | 175 d |
| * Cold | 5.14 c | 5.61 d | 25.36 b | 26.70 ab | 184 c | 180 cd | |
| 10 ppm O3 | 5.04 c | 4.92 e | 20.43 e | 22.34 c | 183 c | 176 d | |
| 20 ppm O3 | 5.49 cd | 5.32 d | 25.67 b | 22.73 c | 178 c | 196 c | |
| 30 ppm O3 | 6.61 b | 6.08 c | 27.91 a | 26.30 ab | 184 c | 179 d | |
| 60 ppm O3 | 5.01 cd | 5.38 d | 22.23 d | 23.20 c | 187 c | 181 d | |
| Lirica cv. | Control | 4.56 d | 4.59 e | 17.72 e | 18.99 de | 230 a | 212 b |
| * Cold | 4.97 cd | 5.93 cd | 17.38 f | 22.78 c | 223 ab | 215 b | |
| 10 ppm O3 | 5.71 bc | 6.42 c | 21.99 de | 21.70 d | 211 b | 213 b | |
| 20 ppm O3 | 6.58 b | 4.98 e | 24.05 c | 17.33 e | 220 ab | 218 b | |
| 30 ppm O3 | 8.49 a | 9.87 a | 26.23 ab | 29.19 a | 234 a | 241 a | |
| 60 ppm O3 | 6.65 b | 7.28 b | 23.39 cd | 24.14 b | 203 b | 230 a | |
| Significance | * | * | * | * | * | * | |
| Treatment | Length (cm) | Diameter (cm) | TSS (%) | Vitamin C (mg 100 g−1 FW) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Seed Soaking | Seedling Spray | Seed Soaking | Seedling Spray | Seed Soaking | Seedling Spray | Seed Soaking | Seedling Spray | ||
| 2017/2018 | |||||||||
| Zidenka cv. | Control | 9.10 c | 8.00 cd | 8.33 ab | 8.00 b | 10.00 a | 10.00 ab | 650 ab | 650 ab |
| * Cold | 9.60 bc | 8.63 c | 8.00 b | 8.63 ab | 10.06 a | 10.06 ab | 654 a | 654 ab | |
| 1 ppm O3 | 10.16 ab | 8.16 c | 9.00 a | 8.16 b | 9.26 b | 9.60 bc | 607 b | 627 b | |
| 5 ppm O3 | 9.00 c | 9.03 b | 9.00 a | 8.06 b | 9.10 b | 10.06 ab | 593 b | 654 ab | |
| 10 ppm O3 | 9.70 bc | 10.13 ab | 8.00 a | 8.00 b | 9.00 bc | 10.33 a | 585 b | 671 a | |
| 20 ppm O3 | 13.00 a | 11.86 a | 9.00 a | 9.00 a | 10.16 a | 10.06 ab | 661 a | 654 ab | |
| Lirica cv. | Control | 8.83 cd | 8.46 c | 6.50 c | 7.00 c | 9.10 b | 9.10 c | 591 b | 602 c |
| * Cold | 8.33 d | 9.00 bc | 8.00 b | 7.00 c | 9.50 b | 9.50 bc | 619 b | 621 bc | |
| 1 ppm O3 | 10.00 b | 10.00 b | 8.00 b | 9.00 a | 10.00 a | 10.20 a | 650 ab | 663 a | |
| 5 ppm O3 | 10.00 b | 12.00 a | 8.10 ab | 8.16 b | 9.96 ab | 9.46 bc | 647 ab | 615 bc | |
| 10 ppm O3 | 10.00 b | 11.00 ab | 8.83 ab | 8.66 ab | 10.00 a | 10.40 a | 650 ab | 670 a | |
| 20 ppm O3 | 12.33 a | 12.00 a | 9.00 a | 9.33 a | 10.26 a | 10.40 a | 661 a | 678 a | |
| Significance | * | * | * | * | * | * | * | * | |
| 2018/2019 | |||||||||
| Zidenka cv. | Control | 10.20 b | 10.20 b | 7.60 a | 7.60 b | 8.06 a | 8.06 a | 645 a | 645 a |
| * Cold | 10.66 ab | 9.40 c | 8.66 a | 7.80 b | 7.90 a | 8.26 a | 632 a | 657 a | |
| 10 ppm O3 | 9.33 c | 10.33 b | 8.20 a | 8.06 ab | 8.06 a | 8.83 a | 648 a | 657 a | |
| 20 ppm O3 | 9.66 bc | 10.53 b | 8.26 a | 8.60 a | 8.30 a | 8.53 a | 660 a | 658 a | |
| 30 ppm O3 | 9.33 c | 11.53 a | 8.36 a | 8.66 a | 8.53 a | 8.43 a | 682 a | 679 a | |
| 60 ppm O3 | 11.26 a | 11.00 ab | 8.10 a | 7.86 b | 8.03 a | 9.09 a | 680 a | 675 a | |
| Lirica cv. | Control | 9.60 bc | 9.60 c | 8.06 a | 8.06 ab | 8.80 a | 8.80 a | 656 a | 656 a |
| * Cold | 9.90 b | 10.10 bc | 8.96 a | 8.30 a | 8.50 a | 8.70 a | 665 a | 665 a | |
| 10 ppm O3 | 10.80 ab | 9.76 c | 8.73 a | 8.66 a | 8.50 a | 8.43 a | 661 a | 661 a | |
| 20 ppm O3 | 11.13 a | 10.20 b | 8.76 a | 9.00 a | 8.70 a | 8.80 a | 661 a | 676 a | |
| 30 ppm O3 | 11.73 a | 11.76 a | 9.13 a | 9.26 a | 9.23 a | 9.30 a | 696 a | 702 a | |
| 60 ppm O3 | 11.66 a | 11.30 a | 8.20 a | 8.46 a | 8.83 a | 9.53 a | 686 a | 699 a | |
| Significance | * | * | ns | * | ns | ns | ns | ns | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharaf-Eldin, M.A.; Etman, A.A.; Yassin, A.M.; Elsayed, S.; Scholz, M.; Yaseen, Z.M. Mitigation of Chilling Stress by Ozone Pretreatment and Acclimation of Sweet Pepper Grown under Unheated Greenhouse Conditions. Horticulturae 2022, 8, 1189. https://doi.org/10.3390/horticulturae8121189
Sharaf-Eldin MA, Etman AA, Yassin AM, Elsayed S, Scholz M, Yaseen ZM. Mitigation of Chilling Stress by Ozone Pretreatment and Acclimation of Sweet Pepper Grown under Unheated Greenhouse Conditions. Horticulturae. 2022; 8(12):1189. https://doi.org/10.3390/horticulturae8121189
Chicago/Turabian StyleSharaf-Eldin, Mohamed A., Abdelwahab A. Etman, Ali Mahmoud Yassin, Salah Elsayed, Miklas Scholz, and Zaher Mundher Yaseen. 2022. "Mitigation of Chilling Stress by Ozone Pretreatment and Acclimation of Sweet Pepper Grown under Unheated Greenhouse Conditions" Horticulturae 8, no. 12: 1189. https://doi.org/10.3390/horticulturae8121189
APA StyleSharaf-Eldin, M. A., Etman, A. A., Yassin, A. M., Elsayed, S., Scholz, M., & Yaseen, Z. M. (2022). Mitigation of Chilling Stress by Ozone Pretreatment and Acclimation of Sweet Pepper Grown under Unheated Greenhouse Conditions. Horticulturae, 8(12), 1189. https://doi.org/10.3390/horticulturae8121189









