Effect of Phenological Variation on the Phytochemical Composition and Antioxidant Activity of Different Organs of Capparis spinosa L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Sampling
2.2. Essential Oil Isolation
2.3. Gas Chromatography (GC)
2.4. GC-MS Chromatography
2.5. Preparation of Extracts
2.6. Determination of Total Phenolic Content
2.7. Determination of Total Flavonoid Content
2.8. Analysis of Phenolic Compounds
2.9. Antioxidant Activity (DPPH Radical Scavenging Assay)
2.10. Statistical Analysis
3. Results
3.1. Contents of Essential Oil and Extract
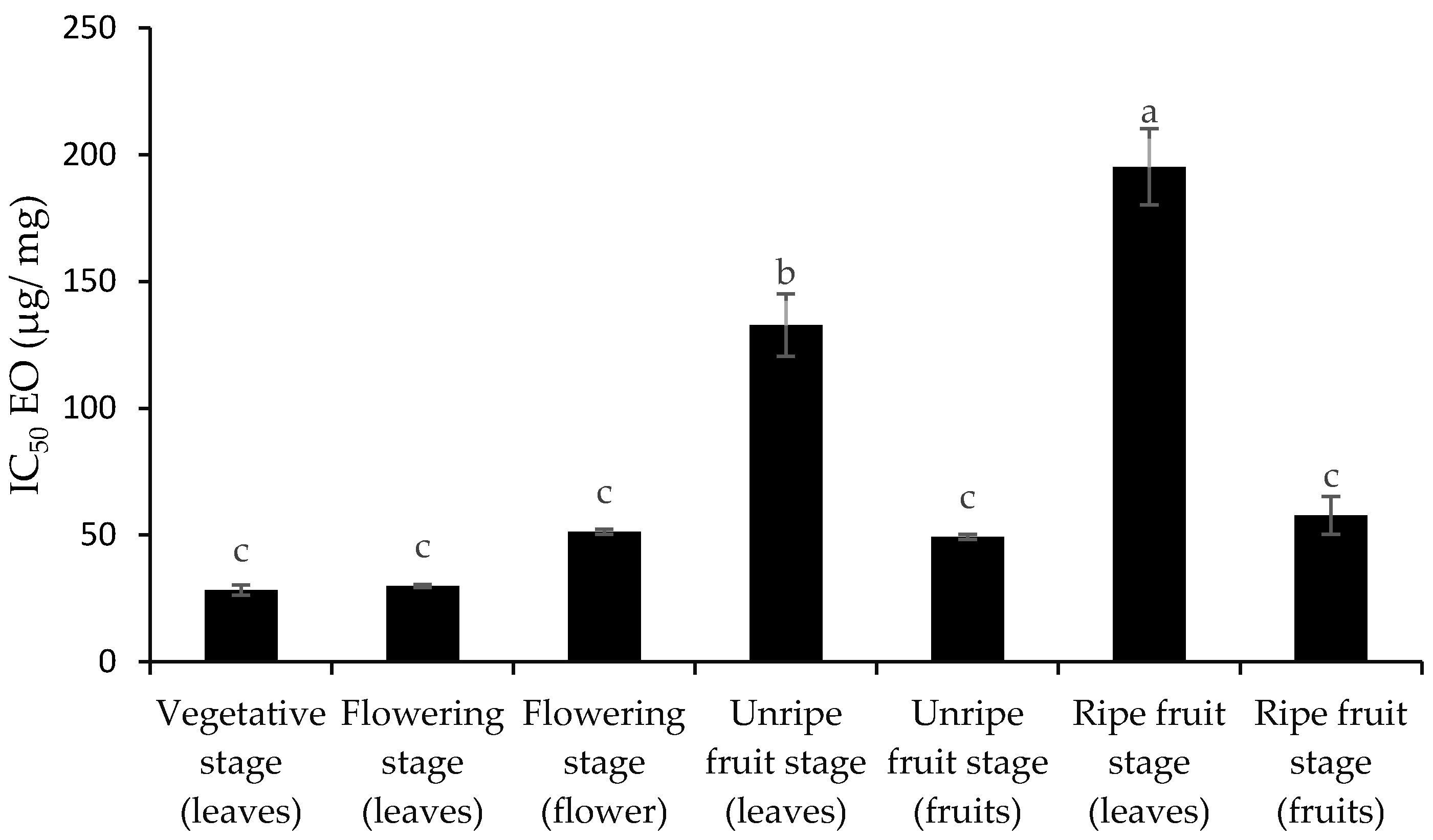
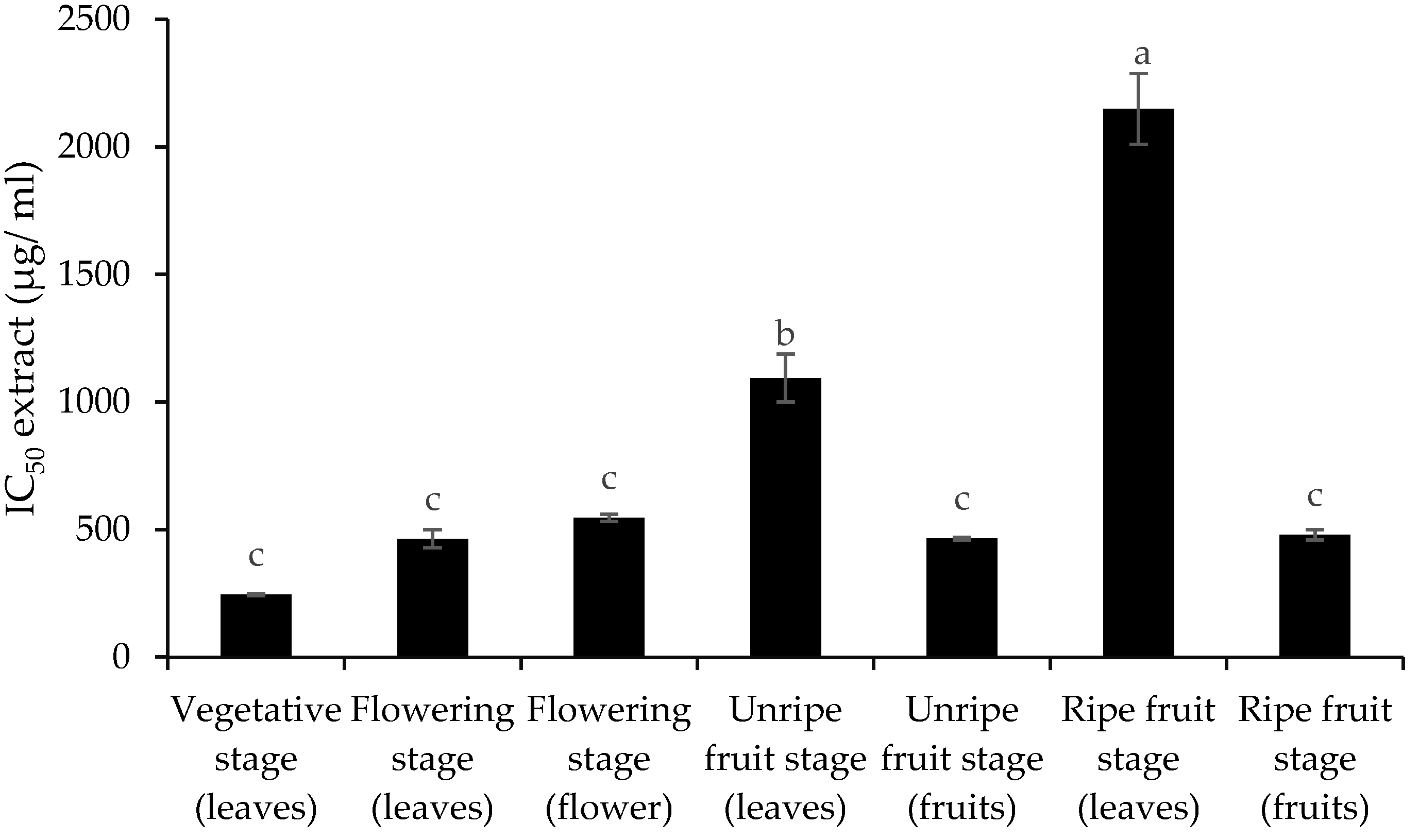
3.2. Total Phenol and Flavonoid Content and Antioxidant Activity
3.3. Essential Oils Composition
3.4. Phenolic Acids
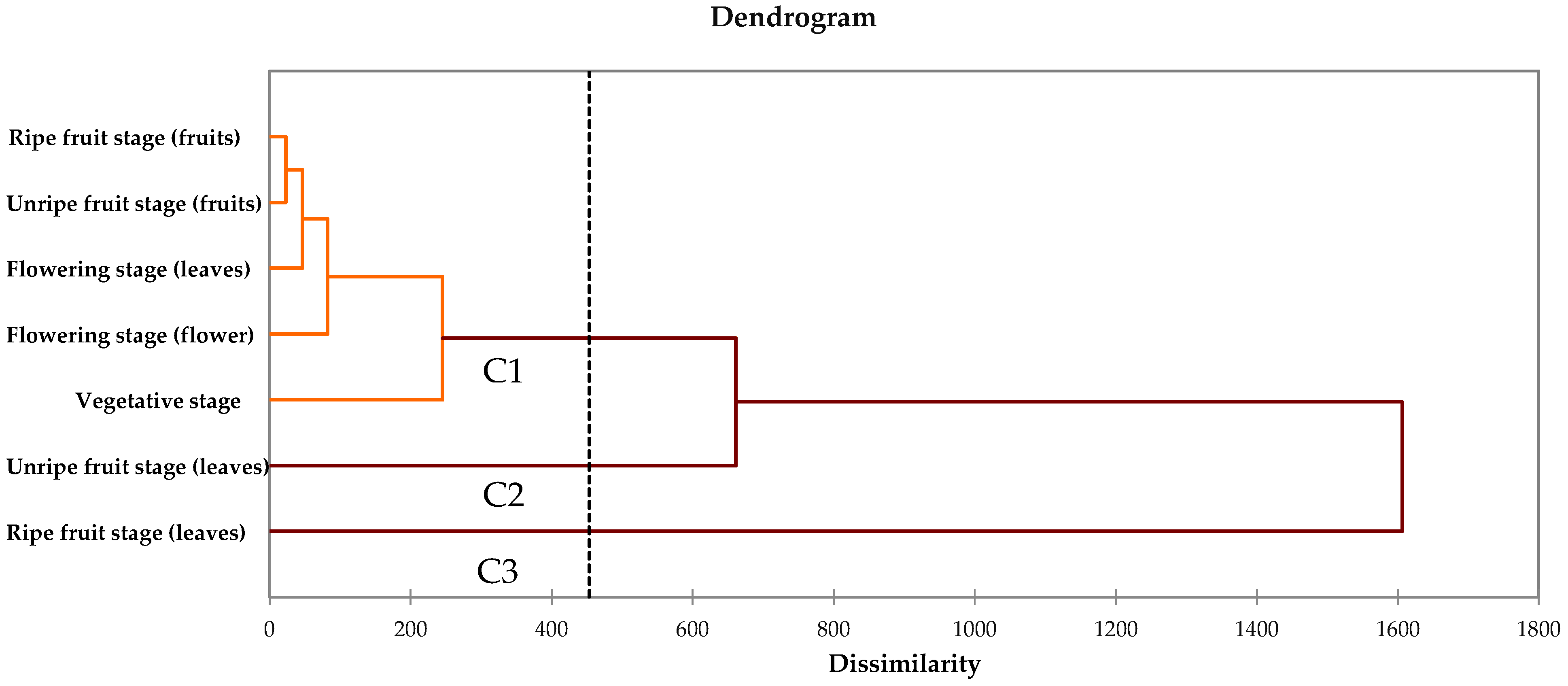
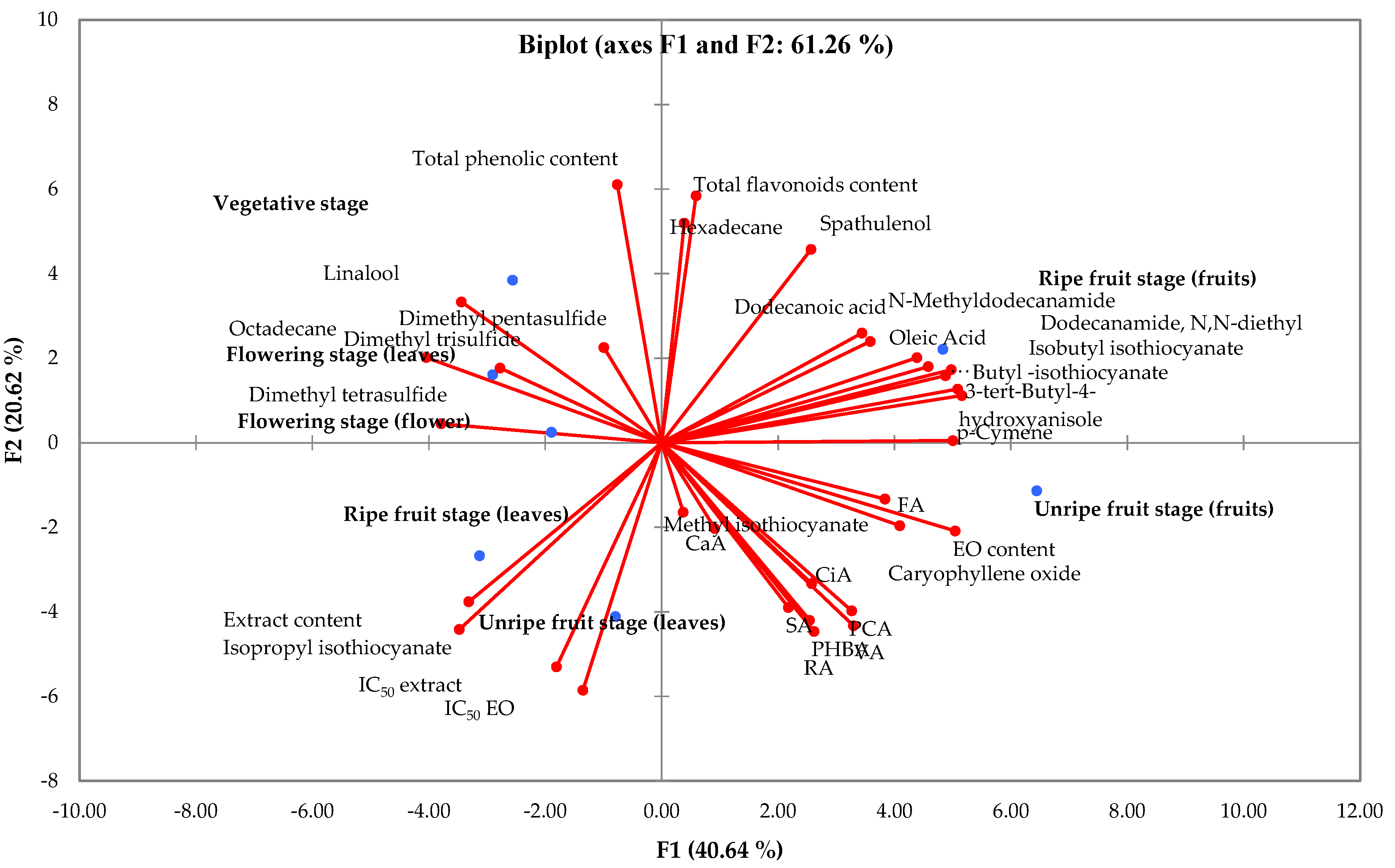
3.5. Cluster Analysis and Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Ma, Z.F. Phytochemical and pharmacological properties of Capparis spinose as a medicinal plants. Nutrients 2018, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, S.C.; Lal, M.A. Secondary metabolites. In Plant Physiology, Development and Metabolism; Springer Nature: Singapore, 2023; pp. 765–808. [Google Scholar]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A comprehensive review on the biological, agricultural and pharmaceutical properties of secondary metabolites based-plant origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef] [PubMed]
- Raissi, A.; Arbabi, M.; Ahmad, R.M. Capparis spinosa L., an important medicinal plant from Sistan and Baloochestan Province, Iran. In Proceedings of the Global Conference on New Approaches in Agriculture and Environment with the Focus on Sustainable Development and Safe Production, Shiraz, Iran, 28 January 2016. [Google Scholar]
- Sun, W.; Shahrajabian, M.H. Therapeutic potential of phenolic compounds in medicinal plants—Natural health products for human health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- Rivera, D.; Inocencio, C.; Obón, C.; Alcaraz, F. Review of food and medicinal uses of Capparis L. Subgenus Capparis (capparidaceae). Econ. Bot. 2003, 57, 515–534. [Google Scholar] [CrossRef]
- Liu, Y.T.; Sun, Y.T.; Cheng, X.M.; Wang, C.H.; Zhou, H.; Yang, T. Advances on the investigation of chemical constituents and pharmacological activities of Capparis spinosa L. Modernization of Traditional Chinese Medicine and Materia Materia. World Sci. Technol. 2019, 12, 2599–2608. [Google Scholar]
- Bina, F.; Bostani, A. Evaluation of the phenotypic variation in a caper (Capparis spinosa L.) population growing in south of Tehran using multivariate analysis. J. BioSci. Biotechnol. 2016, 5, 117–123. [Google Scholar]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Plant of the Millennium, Caper (Capparis spinosa L.), chemical composition and medicinal uses. Bull. Natl. Res. Cent. 2021, 45, 131. [Google Scholar] [CrossRef]
- Zarei, M.; Seyedi, N.; Maghsoudi, S.; Shahbi Nejad, M.; Sheibani, H. Green synthesis of Ag nanoparticles on the modified graphene oxide using Capparis spinosa fruit extract for catalytic reduction of organic dyes. Inorg. Chem. Commun. 2021, 123, 108327. [Google Scholar] [CrossRef]
- Kdimy, A.; El Yadini, M.; Guaadaoui, A.; Bourais, I.; El Hajjaji, S.; Le, H.V. Phytochemistry, Biological Activities, Therapeutic Potential, and Socio-Economic Value of the Caper Bush (Capparis spinosa L.). Chem. Biodivers. 2022, 19, 202200300. [Google Scholar] [CrossRef]
- Kulisic-Bilusic, T.; Schmöller, I.; Schnäbele, K.; Siracusa, L.; Ruberto, G. The anticarcinogenic potential of essential oil and aqueous infusion from caper (Capparis spinosa L.). Food. Chem. 2012, 132, 261–267. [Google Scholar] [CrossRef]
- Olas, B. The Current State of Knowledge about the Biological Activity of Different Parts of Capers. Nutrients 2023, 15, 623. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, R. Phytochemical and Pharmacological Screening on Roots of Capparis spinosa F: Capparidaceae. Ph.D. Thesis, Rajiv Gandhi University of Health Sciences, Bengaluru, India, 2010. [Google Scholar]
- Al-Snafi, A.E. Chemical constituents and pharmacological activities of Ammi majus and Ammi visnaga: A review. Int. J. Pharm. Ind. Res. 2013, 3, 257–265. [Google Scholar]
- Rezzan, A.; Gonca, T.; Nurettin, Y.; Emre, E. Characterisation of volatile compounds by spme and gc-fid/ms of capers (Capparis spinosa L.). Afr. J. Agric. Res. 2015, 10, 2213–2217. [Google Scholar]
- Koufan, M.; Belkoura, I.; Mazri, M.A. In vitro propagation of caper (Capparis spinosa L.): A review. Horticulturae 2022, 8, 737. [Google Scholar] [CrossRef]
- Afzali, S.F.; Sadeghi, H.; Taban, A. A comprehensive model for predicting the development of defense system of Capparis spinosa L.: A novel approach to assess the physiological indices. Sci. Rep. 2023, 13, 12413. [Google Scholar] [CrossRef]
- Moghaddasi, S.M.; Haddad Kashani, H.; Azarbad, Z. Capparis spinosa L. propagation and medicinal uses. Life Sci. J. 2012, 9, 684–686. [Google Scholar]
- Sun, Y.; Yang, T.; Wang, C. Capparis spinosa L. as a potential source of nutrition and its health benefits in foods: A comprehensive review of its phytochemistry, bioactivities, safety, and application. Food Chem. 2023, 409, 135258. [Google Scholar] [CrossRef]
- Annaz, H.; Sane, Y.; Bitchagno, G.T.M.; Ben Bakrim, W.; Drissi, B.; Mahdi, I.; El Bouhssini, M.; Sobeh, M. Caper (Capparis spinosa L.): An updated review on its phytochemistry, nutritional value, traditional uses, and therapeutic potential. Front. Pharmacol. 2022, 13, 878749. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Grimalt, M.; Legua, P.; Almansa, M.S.; Amoros, A.; Carbonell-Barachina, A.A.; Hernandez, F. Polyphenol compounds and biological activity of caper (Capparis spinosa L.) flowers buds. Plants 2019, 8, 539. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, Y.; Zhang, X. Preparation and characterization of electrospun nanofibre membranes incorporated with an ethanol extract of Capparis spinosa L. as a potential packaging material. Food Packag. Shelf Life 2022, 32, 100851. [Google Scholar] [CrossRef]
- Bacchetti, T.; Campagna, R.; Sartini, D.; Cecati, M.; Morresi, C.; Bellachioma, L.; Martinelli, E.; Rocchetti, G.; Lucini, L.; Ferretti, G.; et al. spinosa L. subsp. rupestris Phytochemical Profile and Effect on Oxidative Stress in Normal and Cancer Cells. Molecules 2022, 27, 6488. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Muhammad, G.; Hussain, M.A.; Zengin, G.; Alkharfy, K.M.; Ashraf, M.; Gilani, A.H. Capparis spinosa L.: A Plant with High Potential for Development of Functional Foods and Nutraceuticals/Pharmaceuticals. Int. J. Pharmacol. 2016, 12, 201–219. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Maggi, F.; Daglia, M.; Habtermariam, S.; Rstrelli, L.; Nabavi, S.M. Pharmacological effects of Capparis spinosa L. Phytother. Res. 2016, 30, 1733–1744. [Google Scholar] [CrossRef]
- Lo Bosco, F.; Guasrrasi, V.; Moschetti, M.; Germana, M.A.; Butera, D.; Corana, F.; Papetti, A. Nutraceutical value of pantelleria capers (Capparis spinosa L.). J. Food Sci. 2019, 84, 2337–2346. [Google Scholar] [CrossRef]
- Esmaeili, H.; Karami, A.; Maggi, F. Essential oil composition, total phenolic and flavonoids contents, and antioxidant activity of Oliveria decumbens Vent. (Apiaceae) at different phenological stages. J. Clean. Prod. 2018, 198, 91–95. [Google Scholar] [CrossRef]
- Grevsen, K.; Fretté, X.C.; Christensen, L.P. Content and composition of volatile terpenes, flavonoids and phenolic acids in Greek Oregano (Origanum vulgare L. ssp. hirtum) at different development stages during cultivation in cool temperate climate. Eur. J. Hortic. Sci. 2009, 74, 193–203. [Google Scholar] [CrossRef]
- Hazrati, S.; Mollaei, S.; Rabbi Angourani, H.; Hosseini, S.J.; Sedaghat, M.; Nicola, S. How do essential oil composition and phenolic acid profile of Heracleum persicum fluctuate at different phenological stages. Food Sci. Nutr. 2020, 8, 6192–6206. [Google Scholar] [CrossRef]
- Hazrati, S.; Rowshan, V.; Hosseini, S.J.; Sedaghat, M.; Mohammadi, H. Variation of essential oil composition and antioxidant activity in aerial parts of Stachys schtschegleevi Sosn at different growing stages. J. Essent. Oil Bear. Plants 2020, 23, 1054–1071. [Google Scholar] [CrossRef]
- Hazrati, S.; Hosseini, S.J.; Ebadi, M.T.; Nicola, S. Evolution of phytochemical variation in Myrtle (Myrtus communis L.) organs during different phenological stages. Horticulturae 2022, 8, 757. [Google Scholar] [CrossRef]
- Hazrati, S.; Mousavi, Z.; Nicola, S. Harvest time optimization for medicinal and aromatic plant secondary metabolites. Plant Physiol. Biochem. 2024, 212, 108735. [Google Scholar] [CrossRef]
- Mohaddab, M.; Genva, M.; Malika, F.; El-Goumi, Y.; Zeroual, A.; Fauconnier, M.L. Capparis spinosa: A rich source of phenolic compounds—A comprehensive review of its phytochemistry, health benefits, and biotechnological applications. Biocatal. Agric. Biotechnol. 2024, 61, 103409. [Google Scholar] [CrossRef]
- Grimalt, M.; Hernández, F.; Legua, P.; Almansa, M.S.; Amorós, A. Physicochemical composition and antioxidant activity of three Spanish caper (Capparis spinosa L.) fruit cultivars in three stages of development. Sci. Hortic. 2018, 240, 509–515. [Google Scholar] [CrossRef]
- Grimalt, M.; Sánchez-Rodríguez, L.; Hernández, F.; Legua, P.; Carbonell-Barrachina, Á.A.; Almansa, M.S.; Amorós, A. Volatile profile in different aerial parts of two caper cultivars (Capparis spinosa L.). J. Food Qual. 2021, 2021, 6620776. [Google Scholar] [CrossRef]
- Vahid, H.; Rakhshandeh, H.; Ghorbani, A. Antidiabetic properties of Capparis spinosa L. and its components. Biomed. Pharm. 2017, 92, 293–302. [Google Scholar] [CrossRef]
- Yahia, Y.; Benabderrahim, M.A.; Tlili, N.; Hannachi, H.; Ayadi, L.; Elfalleh, W. Comparison of three extraction protocols for the characterization of caper (Capparis spinosa L.) leaf extracts: Evaluation of phenolic acids and flavonoids by liquid chromatography–electrospray ionization–tandem mass spectrometry (LC–ESI–MS) and the antioxidant activity. Anal. Lett. 2020, 53, 1366–1377. [Google Scholar]
- Sonmezdag, A.S.; Kelebek, H.; Selli, S. Characterization of aroma-active compounds, phenolics, and antioxidant properties in fresh and fermented capers (Capparis spinosa) by GC-MS-olfactometry and LC-DAD-ESI-MS/MS. J. Food Sci. 2019, 84, 2449–2457. [Google Scholar] [CrossRef]
- Bakr, R.O.; El Bishbishy, M.H. Profile of bioactive compounds of Capparis spinosa var. aegyptiaca growing in Egypt. Rev. Bras. Farmacogn. 2016, 26, 514–520. [Google Scholar] [CrossRef]
- Alkhaibari, A.M.; Alanazi, A.D. Chemical composition and insecticidal, antiplasmodial, and anti-leishmanial activity of Capparis spinosa essential oil and its main constituents. Evid. Based Complement. Altern. Med. 2022, 2022, 6371274. [Google Scholar] [CrossRef]
- Merlino, M.; Condurso, C.; Cincotta, F.; Nalbone, L.; Ziino, G.; Verzera, A. Essential oil emulsion from caper (Capparis spinosa L.) leaves: Exploration of its antibacterial and anti-oxidant properties for possible application as a natural food preservative. Antioxidants 2024, 13, 718. [Google Scholar] [CrossRef]
- AlMousa, L.A.; AlFaris, N.A.; Alshammari, G.M.; ALTamimi, J.Z.; Alsyadi, M.M.; Alagal, R.I.; Yahya, M.A. Antioxidant and antimicrobial potential of two extracts from Capparis spinosa L. and Rumex nervosus and molecular docking investigation of selected major compounds. Saudi J. Biol. Sci. 2022, 29, 103346. [Google Scholar] [CrossRef]
- El Finou, H.; Salhi, N.; Zaid, A.; El Rhaffari, L. Phytotoxicity, antioxidant activity and chemical profile of aqueous extracts from Moroccan caper (Capparis spinosa L.). Sci. Afr. 2024, 24, 02176. [Google Scholar] [CrossRef]
- Rajhi, I.; Hernandez-Ramos, F.; Abderrabba, M.; Ben Dhia, M.T.; Ayadi, S.; Labidi, J. Antioxidant, Antifungal and Phytochemical Investigations of Capparis spinosa L. Agriculture 2021, 11, 1025. Agriculture 2021, 11, 1025. [Google Scholar]
- Ouhammou, M.; Adnany, E.M.E.; Mourjane, A.; Hammou, H.A.; Bouchdoug, M.; Jaouad, A.; Mahrouz, M. Physi-co-chemical analysis and antioxidant activity of Moroccan caper leaves (Capparis spinosa L.). Euro-Mediterranean. J. Environ. Integr. 2022, 7, 407–414. [Google Scholar] [CrossRef]
- Arrar, L.; Benzidane, N.; Krache, I.; Charef, N.; Khennouf, S.; Baghiani, A. Comparison between polyphenol contents and antioxidant activities of different parts of Capparis spinosa L. Pharmacogn. Commun. 2013, 3, 70. [Google Scholar]
- British Pharmacopoeia. Published on the Recommendation of the Medicines Commission Pursuant to the Medicines Act; Her Majesty’s Stationary Office: London, UK, 1998. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Chang, C.; Yang, M.; Wen, H.; Chern, J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Akroum, S.; Bendjeddou, D.; Satta, D.; Lalaoui, K. Antibacterial, antioxidant and acute toxicity tests on flavonoids extracted from some medicinal plants. Int. J. Green Pharm. 2010, 4, 165–169. [Google Scholar] [CrossRef]
- Chrystal, P.; Pereira, A.C.; Fernandes, C.C.; Souza, J.M.D.; Martins, C.H.G.; Potenza, J.; Crotti, A.E.M.; Miranda, M.L.D. Essential oil from Psidium cattleianum Sabine (Myrtaceae) fresh leaves: Chemical characterization and in vitro antibacterial activity against endodontic pathogens. Braz. Arch. Biol. Technol. 2020, 63, e20190196. [Google Scholar] [CrossRef]
- Gundewadi, G.; Reddy, V.R.; Bhimappa, B.B. Physiological and biochemical basis of fruit development and ripening—A review. J. Hill Agric. 2018, 9, 7–21. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, M.A. Fruit Development and Ripening. In Plant Physiology, Development and Metabolism; Springer: Singapore, 2023. [Google Scholar]
- Ayour, J.; Harrak, H.; Benichou, M. Cell wall enzymatic activity control: A reliable technique in the fruit ripening process. In New Discoveries in the Ripening Processes; IntechOpen: London, UK, 2023. [Google Scholar]
- Şanli, A.; Karadoğan, T.; Tosun, B.; Tonguç, M.; Erbaş, S. Growth Stage and drying methods affect essential oil content and composition of Pickling Herb (Echinophora tenuifolia subsp. sibthorpiana Tutin). J. Appl. Nat. Sci. 2016, 20, 143–149. [Google Scholar] [CrossRef]
- Chang, T.G.; Zhu, X.G. Source–sink interaction: A century old concept under the light of modern molecular systems biology. J. Exp. Bot. 2017, 68, 4417–4431. [Google Scholar] [CrossRef]
- Soltani Howyzeh, M.; Sadat Noori, S.A.; Shariati, J.V. Essential oil profiling of Ajowan (Trachyspermum ammi) industrial medicinal plant. Ind. Crop. Prod. 2018, 119, 255–259. [Google Scholar] [CrossRef]
- Daghbouche, S.; Ammar, I.; Rekik, D.M.; Djazouli, Z.E.; Zebib, B.; Merah, O. Effect of phenological stages on essential oil composition of Cytisus triflorus L’Her. J. King Saud Univ. Sci. 2020, 32, 2383–2387. [Google Scholar] [CrossRef]
- Chrétien, L.T.; Khalil, A.; Gershenzon, J.; Lucas-Barbosa, D.; Dicke, M.; Giron, D. Plant metabolism and defence strategies in the flowering stage: Time-dependent responses of leaves and flowers under attack. Plant Cell Environ. 2022, 45, 2841–2855. [Google Scholar] [CrossRef]
- Winde, I.; Wittstock, U. Insect herbivore counteradaptations to the plant glucosinolate–myrosinase system. Phytochemistry 2011, 72, 1566–1575. [Google Scholar] [CrossRef]
- Rahimi, F.; Rahmanpour, S. Overcoming glucosinolate-myrosinase-isothiocyanate defense system by plant pathogenic fungi. Int. J. Second. Metab. 2020, 7, 19–27. [Google Scholar] [CrossRef]
- Alsharif, B.; Babington, G.A.; Radulović, N.; Boylan, F. Volatiles of Capparis cartilaginea Decne. from Saudi Arabia. Plants 2022, 11, 2518. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Kurashige, N.S. A role for isothiocyanates in plant resistance against the specialist herbivore Pieris rapae. J. Chem. Ecol. 2003, 29, 1403–1415. [Google Scholar] [CrossRef]
- Olayanju, J.B.; Bozic, D.; Naidoo, U.; Sadik, O.A. A Comparative Review of Key Isothiocyanates and Their Health Benefits. Nutrients 2024, 16, 757. [Google Scholar] [CrossRef]
- Kulisic-Bilusic, T.; Blažević, I.; Dejanović, B.; Miloš, M.; Pifat, G. Evaluation of the antioxidant activity of essential oils from caper (Capparis spinosa) and sea fennel (Crithmum maritimum) by different methods. J. Food Biochem. 2010, 34, 286–302. [Google Scholar] [CrossRef]
- Afsharypuor, S.; Jeiran, K.; Jazy, A.A. First investigation of the flavour profiles of the leaf, ripe fruit and root of Capparis spinosa var. mucronifolia from Iran. Pharm. Acta Helv. 1998, 72, 307–309. [Google Scholar] [CrossRef]
- Mollica, A.; Stefanucci, A.; Macedonio, G.; Locatelli, M.; Luisi, G.; Novellino, E.; Zengin, G. Chemical composition and biological activity of Capparis spinosa L. from Lipari Island. S. Afr. J. Bot. 2019, 120, 135–140. [Google Scholar] [CrossRef]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The influence of environmental conditions on secondary metabolites in medicinal plants: A literature review. Chem. Biodivers. 2021, 18, 2100345. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Martel, A.B.; Strugnell, C.A. Environmental Factors Regulate Plant Secondary Metabolites. Plants 2023, 12, 447. [Google Scholar] [CrossRef]
- Albergaria, E.T.; Oliveira, A.F.M.; Albuquerque, U.P. The effect of water deficit stress on the composition of phenolic compounds in medicinal plants. S. Afr. J. Bot. 2020, 131, 12–17. [Google Scholar] [CrossRef]
- Tlili, N.; Mejri, H.; Anouer, F.; Saadaoui, E.; Khaldi, A.; Nasri, N. Phenolic Profile and Antioxidant Activity of Capparis spinosa Seeds Harvested from Different Wild Habitats. Ind. Crop. Prod. 2015, 76, 930–935. [Google Scholar] [CrossRef]
- Ghafoor, K.; Juhaimi, F.A.; Özcan, M.M.; Uslu, N.; Babiker, E.E.; Ahmed, I.A.M. Bioactive Properties and Phenolic Compounds in Bud, Sprout, and Fruit of Capparis Spp. Plants. J. Food Process. Preserv. 2020, 44, e14357. [Google Scholar] [CrossRef]
- Naghiloo, S.; Movafeghi, A.; Delazar, A.; Nazemiyeh, H.; Asnaashari, S.; Dadpour, M.R. Ontogenetic variation of total phenolics and antioxidant activity in roots, leaves and flowers of Astragalus compactus Lam. (Fabaceae). BioImpacts BI 2012, 2, 105. [Google Scholar]
- Jiang, X.; Liu, Y.; Li, W.; Zhao, L.; Meng, F.; Wang, Y.; Tan, H.; Yang, H.; Wei, C.; Wan, X.; et al. Tissue-specific, development-dependent phenolic compounds accumulation profile and gene expression pattern in tea plant [Camellia sinensis]. PLoS ONE 2013, 8, 62315. [Google Scholar] [CrossRef]
- Saffaryazdi, A.; Ganjeali, A.; Farhoosh, R.; Cheniany, M. Variation in phenolic compounds, α-linolenic acid and linoleic acid contents and antioxidant activity of purslane (Portulaca oleracea L.) during phenological growth stages. Physiol. Mol. Biol. Plants 2020, 26, 1519–1529. [Google Scholar] [CrossRef]
- Feduraev, P.; Riabova, A.; Skrypnik, L.; Pungin, A.; Tokupova, E.; Maslennikov, P.; Chupakhina, G. Assessment of the role of PAL in lignin accumulation in wheat (Triticum aestivum L.) at the early stage of ontogenesis. Int. J. Mol. Sci. 2021, 22, 9848. [Google Scholar] [CrossRef]
- Ferreyra, M.L.F.; Serra, P.; Casati, P. Recent advances on the roles of flavonoids as plant protective molecules after UV and high light exposure. Physiol. Plant 2021, 173, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical scavenging mechanisms of phenolic compounds: A quantitative structure-property relationship (QSPR) study. Front. Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef] [PubMed]
- Mathesius, U. Flavonoid functions in plants and their interactions with other organisms. Plants 2018, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Dehghanian, Z.; Habibi, K.; Dehghanian, M.; Aliyar, S.; Lajayer, B.A.; Astatkie, T.; Minkina, T.; Keswani, C. Reinforcing the bulwark: Unravelling the efficient applications of plant phenolics and tannins against environmental stresses. Heliyon 2022, 8, e09094. [Google Scholar] [CrossRef]
- Biswas, A.; Dey, S.; Xiao, A.; Huang, S.; Birhanie, Z.M.; Deng, Y.; Liu, L.; Li, D. Phytochemical content and antioxidant activity of different anatomical parts of Corchorus olitorius and C. capsularis during different phenological stages. Heliyon 2023, 9, e16494. [Google Scholar] [CrossRef]
- Knoblauch, M.; Peters, W.S. What actually is the Münch hypothesis? A short history of assimilate transport by mass flow. J. Integr. Plant Biol. 2017, 59, 292–310. [Google Scholar] [CrossRef]
- Savina, T.; Lisun, V.; Feduraev, P.; Skrypnik, L. Variation in phenolic compounds, antioxidant and antibacte-rial activities of extracts from different plant organs of meadowsweet (Filipendula ulmaria (L.) Maxim.). Molecules 2023, 28, 3512. [Google Scholar] [CrossRef]
- Vlaisavljević, S.; Kaurinović, B.; Popović, M.; Vasiljević, S. Profile of phenolic compounds in Trifolium pratense L. extracts at different growth stages and their biological activities. Int. J. Food Prop. 2017, 20, 3090–3101. [Google Scholar] [CrossRef]
- Farhadi, N.; Babaei, K.; Farsaraei, S.; Moghaddam, M.; Pirbalouti, A.G. Changes in essential oil compositions, total phenol, flavonoids and antioxidant capacity of Achillea millefolium at different growth stages. Ind. Crop. Prod. 2020, 152, 112570. [Google Scholar] [CrossRef]
- Grimalt, M.; Hernández, F.; Legua, P.; Amorós, A.; Almansa, M.S. Antioxidant activity and the physicochemical composition of young caper shoots (Capparis spinosa L.) of different Spanish cultivars. Sci. Hortic. 2022, 293, 110646. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in plants: Structure, biosynthesis, abiotic stress regulation, and practical applications. Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, S.B.; Sierra, L.J.; Fernández-Alonso, J.L.; Romero, A.K.; Martínez, J.R.; Stashenko, E.E. Antioxidant Properties and Secondary Metabolites Profile of Hyptis colombiana at Various Phenological Stages. Molecules 2023, 28, 6767. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic acid derivatives and their biological efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef]
- Feng, L.S.; Cheng, J.B.; Su, W.Q.; Li, H.Z.; Xiao, T.; Chen, D.A.; Zhang, Z.L. Cinnamic acid hybrids as anticancer agents: A mini-review. Arch. Pharm. 2022, 355, 2200052. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Aslam Gondal, T.; Imran, A.; Shahbaz, M.; Muhammad Amir, R.; Wasim Sajid, M.; Batool Qaisrani, T.; Atif, M.; Hussain, G.; et al. Therapeutic potential of rosmarinic acid: A comprehensive review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef]
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savova, M.S.; Koycheva, I.K.; Balcheva-Sivenova, Z.P.; Vasileva, S.M.; Georgiev, M.I. Rosmarinic acid-From bench to valuable applications in food industry. Trends Food Sci. Technol. 2021, 117, 182–193. [Google Scholar] [CrossRef]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic implications of caffeic acid in cancer and neurological diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef]
- De-la-Cruz Chacón, I.; Riley-Saldaña, C.A.; González-Esquinca, A.R. Secondary metabolites during early development in plants. Phytochem. Rev. 2013, 12, 47–64. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Maróstica, M.R., Jr. Chapter 2—Phenolic Compounds: Structure, Classification, and Antioxidant Power. Bioact. Compd. 2019, 33–50. [Google Scholar] [CrossRef]
- Hazrati, S.; Ebadi, M.T.; Mollaei, S.; Khurizadeh, S. Evaluation of volatile and phenolic compounds, and antioxidant activity of different parts of Ferulago angulata (schlecht.) Boiss. Ind. Crop. Prod. 2019, 140, 111589. [Google Scholar] [CrossRef]
- Ringnér, M. What is principal component analysis? Nat. Biotechnol. 2008, 26, 303–304. [Google Scholar] [CrossRef]

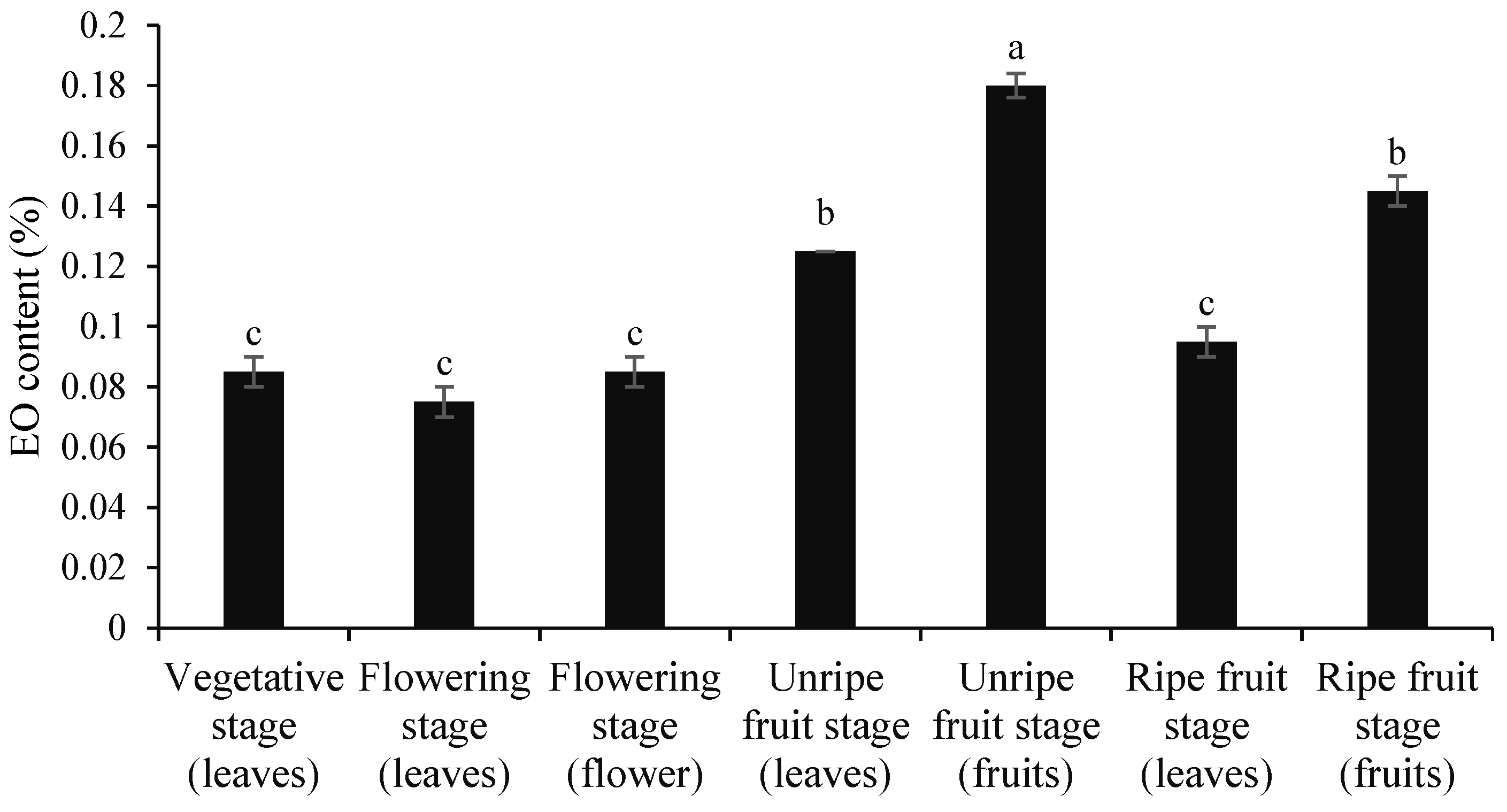
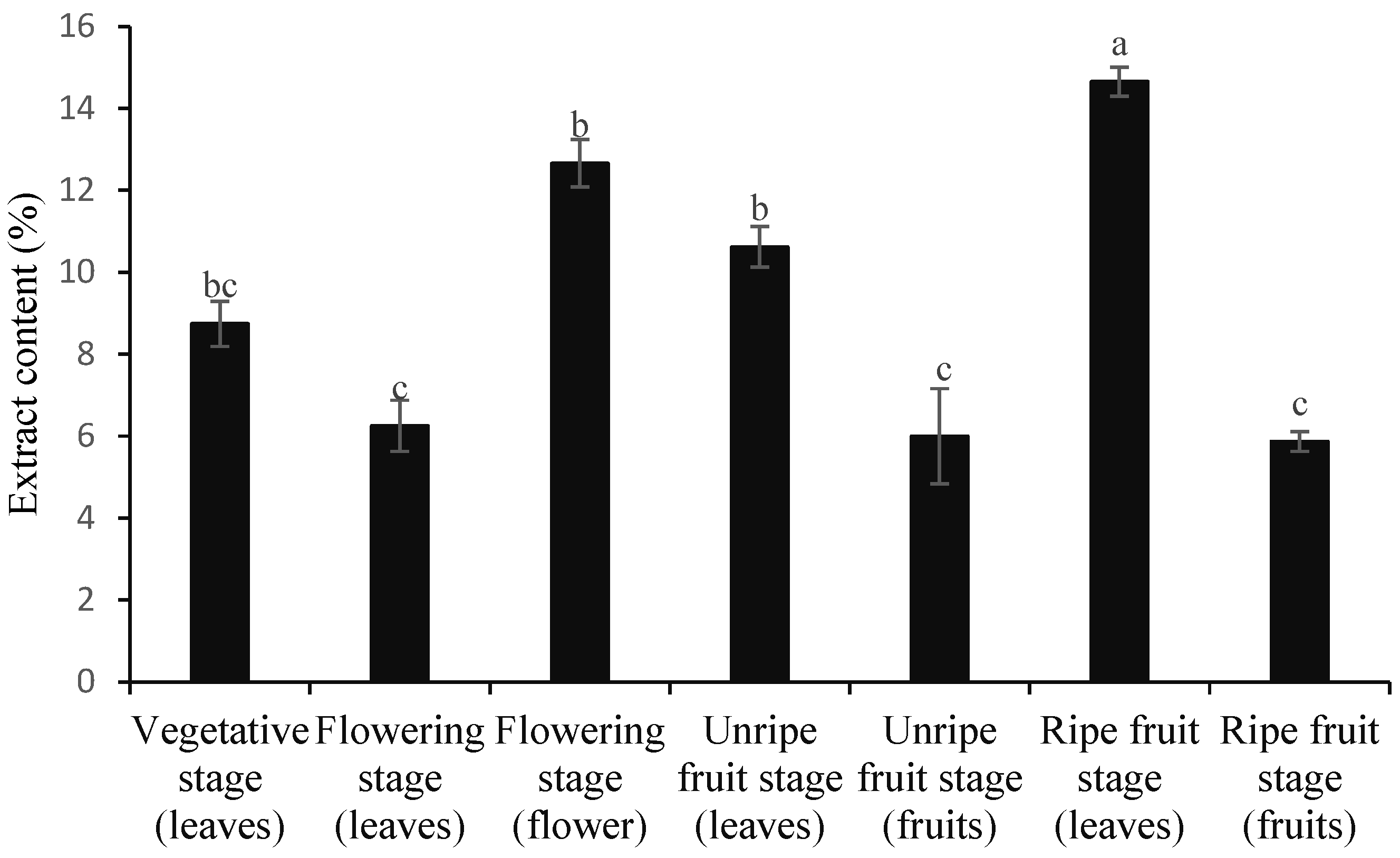
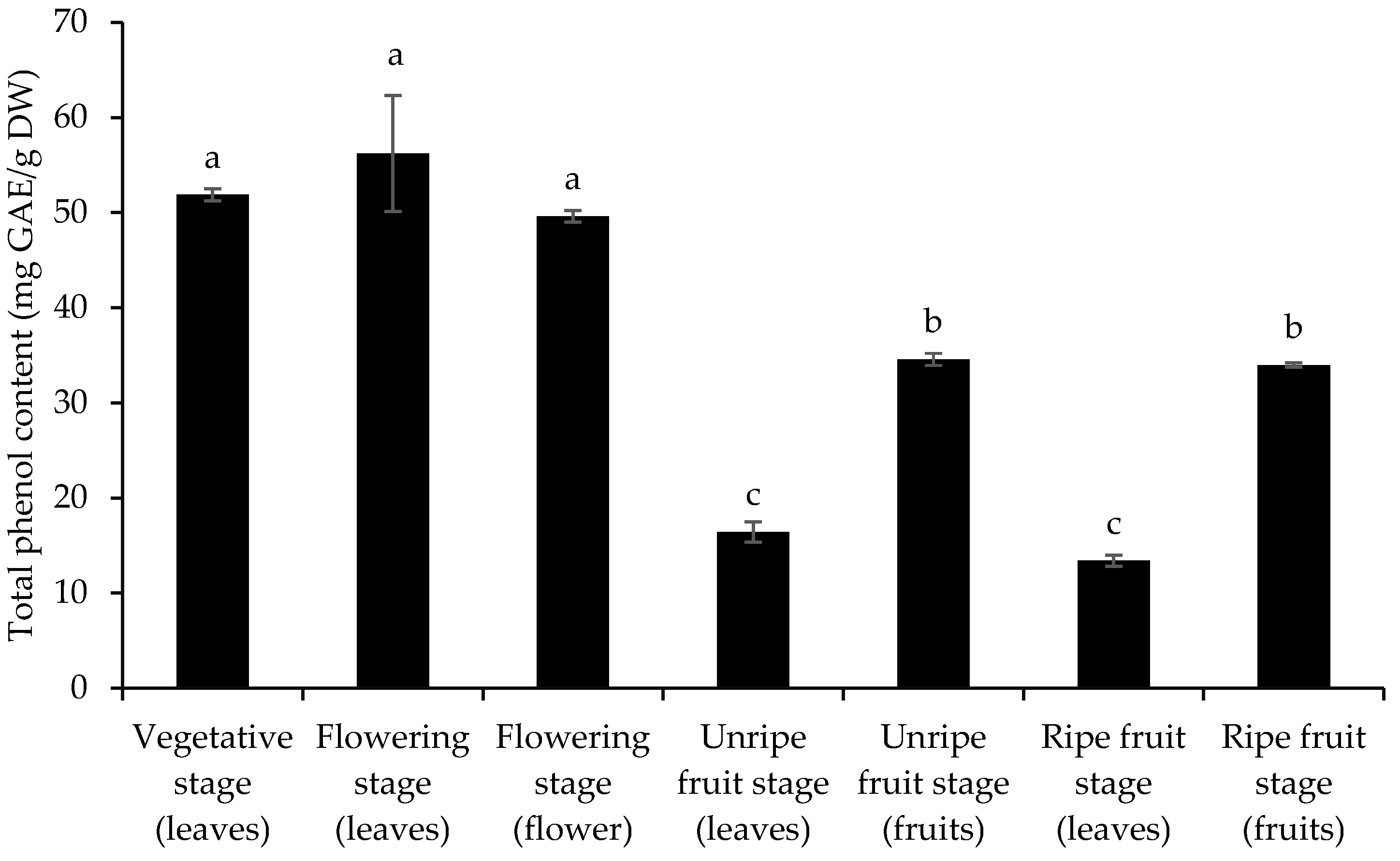
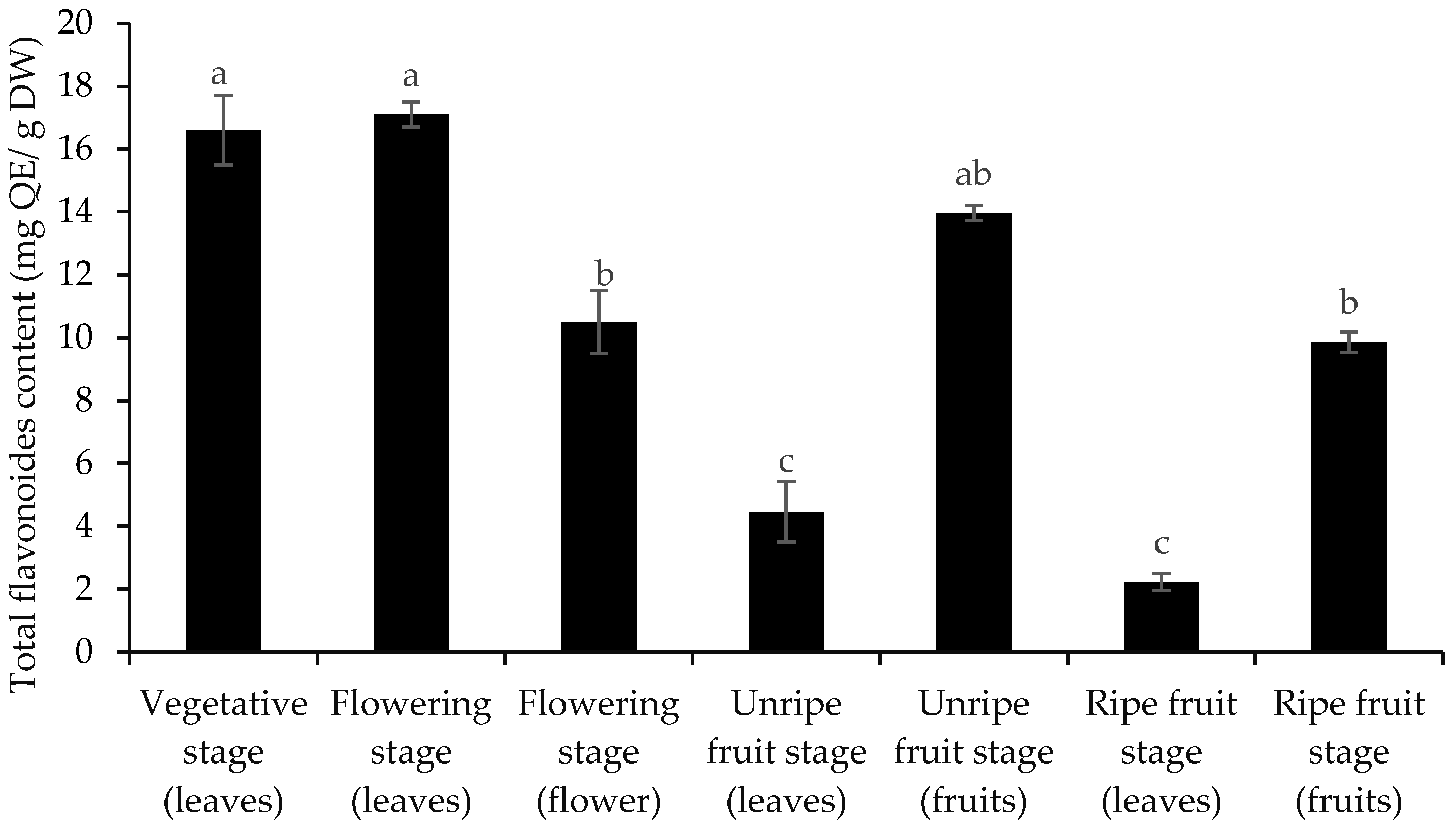
| No | Compounds | KI | Vegetative Stage (Leaves) | Flowering Stage (Leaves) | Flowering Stage (Flower) | Unripe Fruit Stage (Leaves) | Unripe Fruit Stage (Fruits) | Ripe Fruit Stage (Leaves) | Ripe Fruit Stage (Fruits) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Methyl isothiocyanate | 705 | 27.5 | 32.7 | 41.6 | 32.5 | 32.8 | 35.7 | 36.7 |
| 2 | Isopropyl isothiocyanate | 835 | 13.2 | 32.9 | 13.2 | 36.2 | 9.4 | 33.8 | 4.9 |
| 3 | Isobutyl isothiocyanate | 919 | 0.5 | nd | 1 | nd | 6.7 | nd | 9.2 |
| 4 | Dimethyl trisulfide | 943 | 6.4 | 3.9 | 5.9 | 3.2 | 4.2 | 4.2 | 1.5 |
| 5 | Butyl-isothiocyanate | 975 | nd | nd | 0.7 | nd | 2.8 | nd | 3.2 |
| 6 | p-Cymene | 1020 | 0.8 | 0.7 | 1.1 | 0.6 | 6.1 | 0.8 | 3.3 |
| 7 | Linalool | 1095 | 4.4 | 5.4 | 0.5 | 1.7 | nd | 1.7 | 0.6 |
| 8 | Dimethyl tetrasulfide | 1204 | 12.4 | 10.4 | 18.3 | 9.5 | 5.2 | 8.5 | 1.8 |
| 9 | Dimethyl pentasulfide | 1426 | 12.3 | 2.9 | 3.7 | 6.5 | 5.3 | 2.9 | 2.4 |
| 10 | N-Methyldodecanamide | 1486 | nd | nd | nd | nd | 1.2 | nd | 7 |
| 11 | 3-tert-Butyl-4-hydroxyanisole | 1496 | nd | nd | nd | nd | 5.1 | nd | 5.6 |
| 12 | Dodecanamide, N,N-diethyl | 1504 | nd | nd | nd | nd | 2.6 | nd | 6.3 |
| 13 | Dodecanoic acid | 1565 | 0.2 | nd | nd | nd | 0.9 | nd | 6.3 |
| 14 | Spathulenol | 1577 | 2.3 | nd | 0.6 | nd | 1.8 | nd | 1.3 |
| 15 | Caryophyllene oxide | 1582 | 0.5 | 1.2 | 1.1 | 1.1 | 4.6 | 1.3 | 1.4 |
| 16 | Hexadecane | 1600 | 8.4 | 1.3 | 5.9 | nd | 4.4 | nd | 2.8 |
| 17 | α-Cadinol | 1655 | 1.1 | nd | nd | nd | 2.7 | nd | 1.4 |
| 18 | Octadecane | 1800 | 3.5 | 3.1 | 2.1 | 2.1 | 1.7 | 2.3 | 0.8 |
| 19 | Oleic acid | 2132 | nd | nd | nd | nd | 1.8 | nd | 2.8 |
| Total | - | 93.5 | 94.5 | 95.7 | 93.4 | 99.3 | 91.2 | 99.3 |
| Different Organs in Different Phenological Stages | PCA | PHBA | VA | CaA | FA | CiA | RA | SA | Total |
|---|---|---|---|---|---|---|---|---|---|
| Vegetative stage | nd | 0.2 | nd | nd | nd | 0.5 | 0.2 | 0.1 | 1 |
| Flowering stage (leaves) | nd | 0.18 | 0.05 | nd | nd | 0.4 | 1.2 | nd | 1.83 |
| Flowering stage (flower) | nd | nd | nd | 3.4 | nd | 3.2 | 1.3 | 0.2 | 8.1 |
| Unripe fruit stage (leaves) | 1.3 | 0.8 | 4.23 | 1.2 | nd | 2.8 | 2.7 | 0.4 | 13.43 |
| Unripe fruit stage (fruits) | 2.1 | 0.81 | 5.81 | 1.9 | 1.1 | 4.5 | 2.8 | 0.3 | 19.32 |
| Ripe fruit stage (leaves) | nd | 0.2 | 0.3 | nd | nd | 0.3 | 0.2 | nd | 1 |
| Ripe fruit stage (fruits) | nd | 0.18 | 0.5 | 0.1 | nd | 0.5 | 0.7 | 0.1 | 2.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazrati, S.; Mousavi, Z.; Mollaei, S.; Angourani, H.R.; Nicola, S. Effect of Phenological Variation on the Phytochemical Composition and Antioxidant Activity of Different Organs of Capparis spinosa L. Horticulturae 2025, 11, 702. https://doi.org/10.3390/horticulturae11060702
Hazrati S, Mousavi Z, Mollaei S, Angourani HR, Nicola S. Effect of Phenological Variation on the Phytochemical Composition and Antioxidant Activity of Different Organs of Capparis spinosa L. Horticulturae. 2025; 11(6):702. https://doi.org/10.3390/horticulturae11060702
Chicago/Turabian StyleHazrati, Saeid, Zahra Mousavi, Saeed Mollaei, Hossein Rabbi Angourani, and Silvana Nicola. 2025. "Effect of Phenological Variation on the Phytochemical Composition and Antioxidant Activity of Different Organs of Capparis spinosa L." Horticulturae 11, no. 6: 702. https://doi.org/10.3390/horticulturae11060702
APA StyleHazrati, S., Mousavi, Z., Mollaei, S., Angourani, H. R., & Nicola, S. (2025). Effect of Phenological Variation on the Phytochemical Composition and Antioxidant Activity of Different Organs of Capparis spinosa L. Horticulturae, 11(6), 702. https://doi.org/10.3390/horticulturae11060702








