Integration of Green and Far-Red Light with Red-Blue Light Enhances Shoot Multiplication in Micropropagated Strawberry
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Light Treatments
2.2. Chlorophyll and Carotenoid Determination
2.3. Soluble Sugars and Proteins Determination
2.4. RNA Extraction and cDNA Library Construction
2.5. RNA-Seq and Differential Gene Enrichment Analysis
2.6. Validation of Gene Expression Profiles by qRT-PCR
2.7. Statistical Analysis
3. Results
3.1. Growth Parameters
3.2. Chlorophyll and Carotenoid Contents
3.3. Soluble Sugar and Protein Contents
3.4. Transcriptomic Analysis
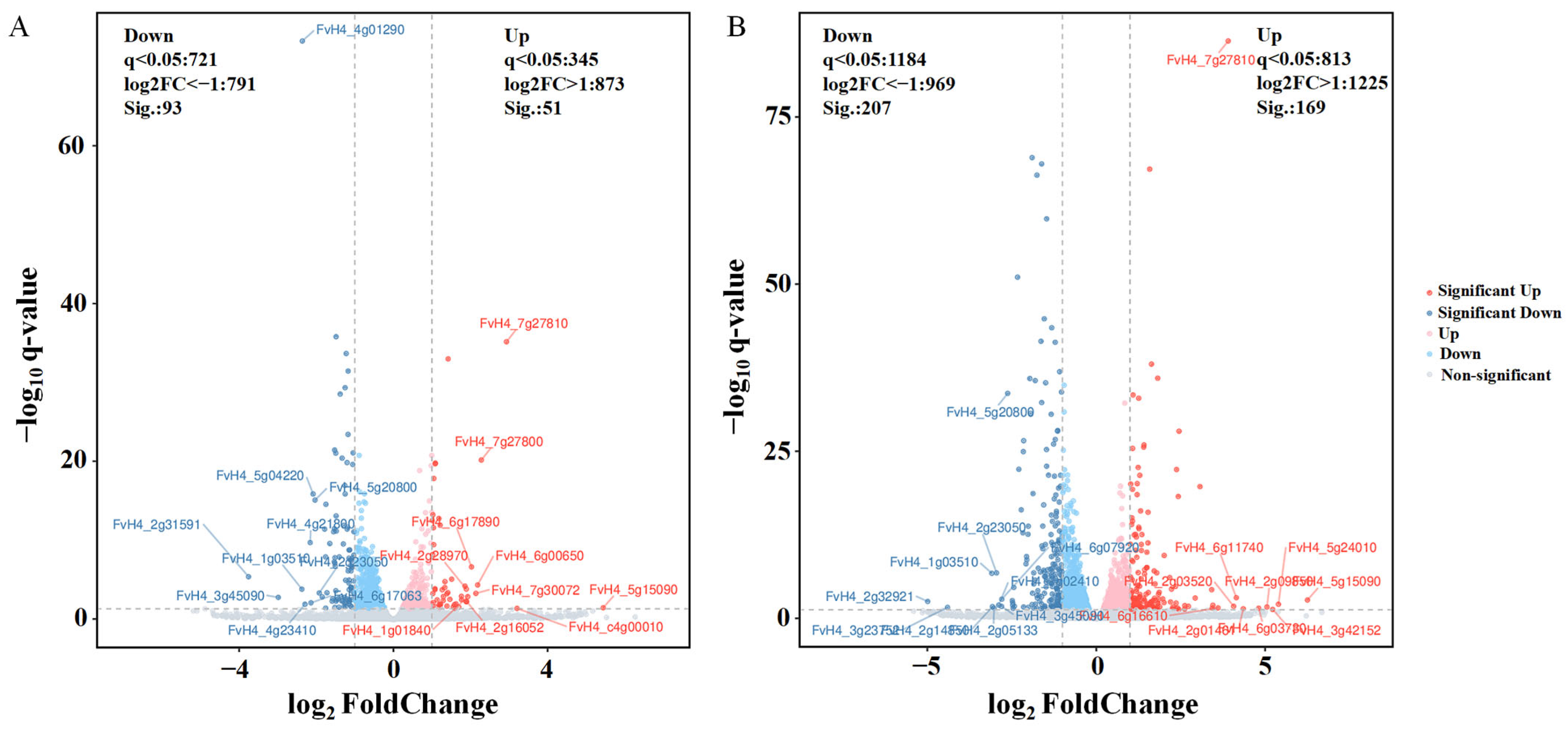
3.4.1. Volcano Plots of DEGs
3.4.2. GO Enrichment Analysis
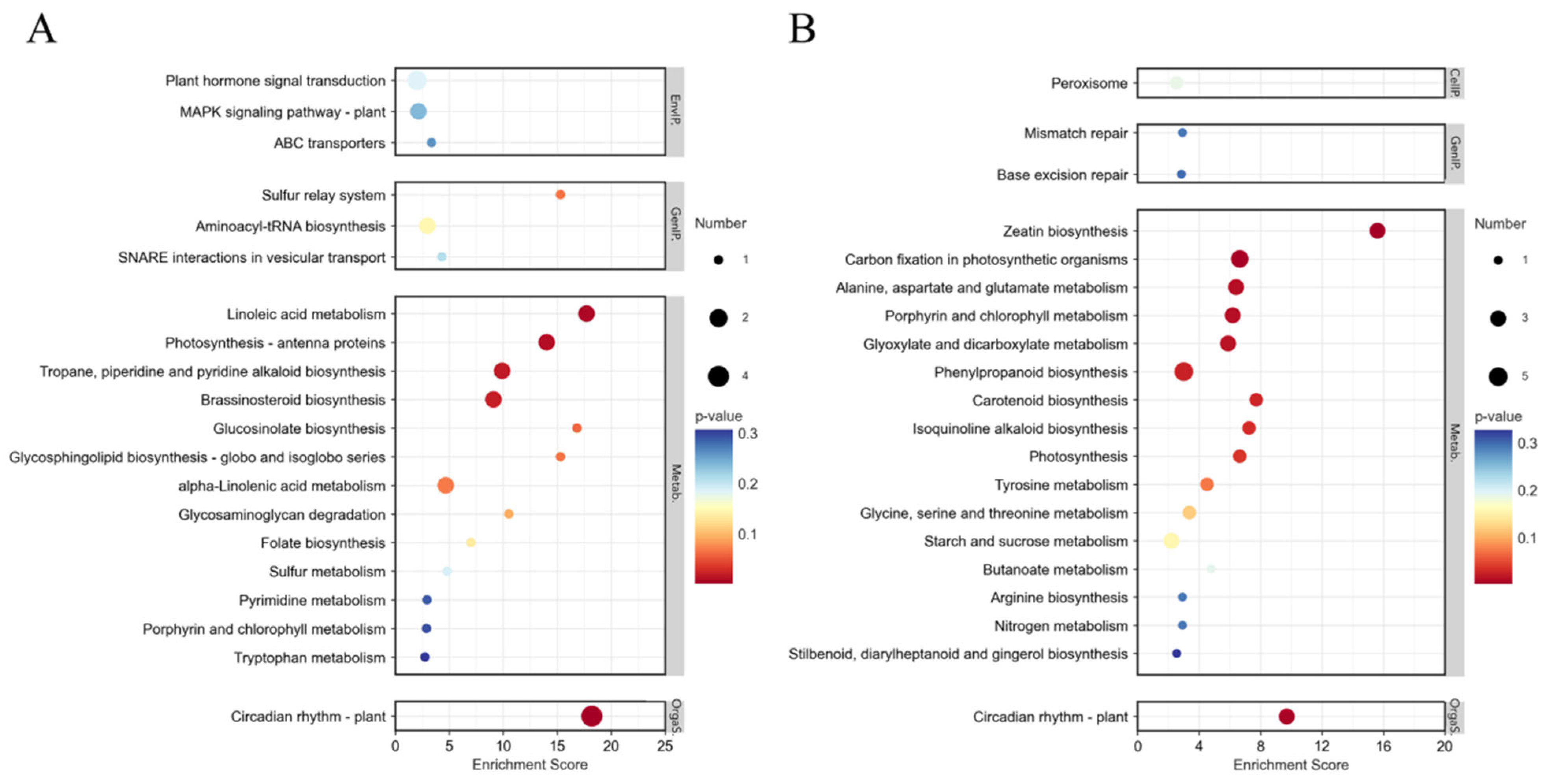
3.4.3. KEGG Enrichment Analysis
3.4.4. Top Genes Affected by Green and Far-Red Light
3.5. Expression Profile Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martin, R.R.; Tzanetakis, I.E. Characterization and recent advances in detection of strawberry viruses. Plant Dis. 2006, 90, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Tang, H. Effect of heat treatment combined with shoot tip culture on the virus-free of arena strawberry. J. Life Sci. 2012, 6, 168–174. [Google Scholar]
- Ashrafuzzaman, M.; Faisal, S.; Yadav, D.; Khanam, D.; Raihan, F. Micropropagation of strawberry (Fragaria ananassa) through runner culture. Bangladesh J. Agric. Res. 2013, 38, 467–472. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Heo Jeongwook, H.J.; Lee Chunwoo, L.C.; Debasis Chakrabarty, D.C.; Paek Keeyoeup, P.K. Growth responses of marigold and salvia bedding plants as affected by monochromic or mixture radiation provided by a light-emitting diode (LED). Plant Growth Regul. 2002, 38, 225–230. [Google Scholar]
- Ouzounis, T.; Fretté, X.; Ottosen, C.O.; Rosenqvist, E. Spectral effects of LEDs on chlorophyll fluorescence and pigmentation in Phalaenopsis ‘Vivien’ and ‘Purple Star’. Physiol. Plant. 2015, 154, 314–327. [Google Scholar] [CrossRef]
- Sabzalian, M.R.; Heydarizadeh, P.; Zahedi, M.; Boroomand, A.; Agharokh, M.; Sahba, M.R.; Schoefs, B. High performance of vegetables, flowers, and medicinal plants in a red-blue LED incubator for indoor plant production. Agron. Sustain. Dev. 2014, 34, 879–886. [Google Scholar] [CrossRef]
- Nhut, D.T.; Takamura, T.; Watanabe, H.; Okamoto, K.; Tanaka, M. Responses of strawberry plantlets cultured in vitro under superbright red and blue light-emitting diodes (LEDs). Plant Cell Tissue Organ Cult. 2003, 73, 43–52. [Google Scholar] [CrossRef]
- Kim, H.-H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-light supplementation for enhanced lettuce growth under red-and blue-light-emitting diodes. HortScience 2004, 39, 1617–1622. [Google Scholar] [CrossRef]
- Wang, Y.; Folta, K.M. Contributions of green light to plant growth and development. Am. J. Bot. 2013, 100, 70–78. [Google Scholar] [CrossRef]
- Park, Y.G.; Jeong, B.R. Both the quality and positioning of the night interruption light are important for flowering and plant extension growth. J. Plant Growth Regul. 2020, 39, 583–593. [Google Scholar] [CrossRef]
- Tan, T.; Li, S.; Fan, Y.; Wang, Z.; Ali Raza, M.; Shafiq, I.; Wang, B.; Wu, X.; Yong, T.; Wang, X. Far—Red light: A regulator of plant morphology and photosynthetic capacity. Crop J. 2022, 10, 300–309. [Google Scholar] [CrossRef]
- Zhen, S.; van Iersel, M.W. Far-red light is needed for efficient photochemistry and photosynthesis. J. Plant Physiol. 2017, 209, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Li, Y.; Jeong, B.R. Foliar silicon spray to strawberry plants during summer cutting propagation enhances resistance of transplants to high temperature stresses. Front. Sustain. Food Syst. 2022, 6, 938128. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, H.; Gao, M.; He, R.; Liu, X.; Su, W.; Liu, H. Far-red-light-induced morphology changes, phytohormone, and transcriptome reprogramming of Chinese kale (Brassica alboglabra Bailey). Int. J. Mol. Sci. 2023, 24, 5563. [Google Scholar] [CrossRef]
- Paik, I.; Huq, E. Plant photoreceptors: Multi-functional sensory proteins and their signaling networks. Proc. Semin. Cell Dev. Biol. 2019, 92, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-regulated plant growth and development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar]
- Leivar, P.; Monte, E. PIFs: Systems integrators in plant development. Plant Cell 2014, 26, 56–78. [Google Scholar] [CrossRef]
- Lin, K.-H.; Huang, M.-Y.; Huang, W.-D.; Hsu, M.-H.; Yang, Z.-W.; Yang, C.-M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Inzé, D.; De Veylder, L. Cell cycle regulation in plant development. Annu. Rev. Genet. 2006, 40, 77–105. [Google Scholar] [CrossRef] [PubMed]
- Folta, K.M.; Carvalho, S.D. Photoreceptors and control of horticultural plant traits. HortScience 2015, 50, 1274–1280. [Google Scholar] [CrossRef]
- Zhang, T.; Maruhnich, S.A.; Folta, K.M. Green light induces shade avoidance symptoms. Plant Physiol. 2011, 157, 1528–1536. [Google Scholar] [CrossRef]
- Jang, I.T.; Lee, J.H.; Shin, E.J.; Nam, S.Y. Evaluation of growth, flowering, and chlorophyll fluorescence responses of Viola cornuta cv. Penny Red Wing according to spectral power distributions. J. People Plants Environ. 2023, 26, 335–349. [Google Scholar] [CrossRef]
- Park, B.G.; Lee, J.H.; Shin, E.J.; Kim, E.A.; Nam, S.Y. Light quality influence on growth performance and physiological activity of Coleus cultivars. Int. J. Plant Biol. 2024, 15, 807–826. [Google Scholar] [CrossRef]
- Geisler, M.; Aryal, B.; Di Donato, M.; Hao, P. A critical view on ABC transporters and their interacting partners in auxin transport. Plant Cell Physiol. 2017, 58, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Tanaka, A. Tetrapyrrole biosynthesis in higher plants. Annu. Rev. Plant Biol. 2007, 58, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hahida, S.-N.; Yoshihara, T. Effect of green light wavelength and intensity on photomorphogenesis and photosynthesis in Lactuca sativa. Environ. Exp. Bot. 2012, 75, 128–133. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.-O. Spectral effects of artificial light on plant physiology and secondary metabolism: A review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef]
- Schenkels, L.; Saeys, W.; Lauwers, A.; De Proft, M.P. Green light induces shade avoidance to alter plant morphology and increases biomass production in Ocimum basilicum L. Sci. Hortic. 2020, 261, 109002. [Google Scholar] [CrossRef]
- Xu, D. COP1 and BBXs-HY5-mediated light signal transduction in plants. New Phytol. 2020, 228, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- McClung, C.R. Plant circadian rhythms. Plant Cell 2006, 18, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [PubMed]
- Clouse, S.D. Brassinosteroid signal transduction: From receptor kinase activation to transcriptional networks regulating plant development. Plant Cell 2011, 23, 1219–1230. [Google Scholar] [CrossRef]
- Goldschmidt-Clermont, M.; Bassi, R. Sharing light between two photosystems: Mechanism of state transitions. Curr. Opin. Plant Biol. 2015, 25, 71–78. [Google Scholar] [CrossRef]









| Gene id | Primer | Primer Sequence (5′ → 3′) | Annealing Temperature (°C) | Amplicon Size (bp) |
|---|---|---|---|---|
| FvH4_7g27810 | CDKSF4-F | ACAGTGTGGTGGTGTTCCTC | 59 | 210 |
| CDKSF4-R | GACGCTTTCCTTGTTGCTCC | |||
| FvH4_5g15090 | PKS3-F | GGATTGATCAGGACGGCGAA | 60 | 292 |
| PKS3-R | GAACCACTGCAGACAAAGCC | |||
| FvH4_4g21800 | HY5-F | ACAAGCCCGTGAGAGGAAAA | 59 | 181 |
| HY5-R | ACAGCTTGCACACTGATGATT | |||
| FvH4_6g17063 | UGT73C1-F | CCAAAGGGCTACAAGAGCCA | 60 | 256 |
| UGT73C1-R | ACCTCAATGCGATCTGGCAA | |||
| FvH4_2g23840 | IPT3-F | CATGGTTGGTACATTAATTTGGTCA | 59 | 257 |
| IPT3-R | CCGTCATTTCTCAGGTTCCCA |
| Light Quality | Number of Shoots/Plant | Plant Height (cm) | Shoot Fresh Weight (g/Plant) | Leaf Weight (g/Plant) | Callus Weight (g/Plant) |
|---|---|---|---|---|---|
| RB | 1.378 ± 0.067 b * | 5.107 ± 0.090 b | 0.460 ± 0.024 a | 0.162 ± 0.005 a | 0.052 ± 0.004 b |
| RGB | 1.712 ± 0.119 a | 5.240 ± 0.092 b | 0.434 ± 0.023 a | 0.150 ± 0.013 a | 0.049 ± 0.001 b |
| RBFR | 1.914 ± 0.067 a | 6.027 ± 0.038 a | 0.460 ± 0.019 a | 0.151 ± 0.011 a | 0.059 ± 0.002 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Huang, P.; Qiu, X.; Zhu, F.; Chen, H.; Wang, S.; He, J.; Pang, Y.; Ma, H.; Wang, F. Integration of Green and Far-Red Light with Red-Blue Light Enhances Shoot Multiplication in Micropropagated Strawberry. Horticulturae 2025, 11, 701. https://doi.org/10.3390/horticulturae11060701
Li Y, Huang P, Qiu X, Zhu F, Chen H, Wang S, He J, Pang Y, Ma H, Wang F. Integration of Green and Far-Red Light with Red-Blue Light Enhances Shoot Multiplication in Micropropagated Strawberry. Horticulturae. 2025; 11(6):701. https://doi.org/10.3390/horticulturae11060701
Chicago/Turabian StyleLi, Yali, Ping Huang, Xia Qiu, Feiyu Zhu, Hongwen Chen, Si Wang, Jiaxian He, Yadan Pang, Hui Ma, and Fang Wang. 2025. "Integration of Green and Far-Red Light with Red-Blue Light Enhances Shoot Multiplication in Micropropagated Strawberry" Horticulturae 11, no. 6: 701. https://doi.org/10.3390/horticulturae11060701
APA StyleLi, Y., Huang, P., Qiu, X., Zhu, F., Chen, H., Wang, S., He, J., Pang, Y., Ma, H., & Wang, F. (2025). Integration of Green and Far-Red Light with Red-Blue Light Enhances Shoot Multiplication in Micropropagated Strawberry. Horticulturae, 11(6), 701. https://doi.org/10.3390/horticulturae11060701






