Abstract
Pepper anthracnose is a globally devastating fungal disease caused by Colletotrichum spp. In this study, we explored the molecular mechanisms underlying anthracnose resistance in Capsicum annuum by comparing a resistant variety 225 with a susceptible variety 307. Phenotypic analysis revealed that variety 225 displayed stronger resistance than variety 307. Through comparative transcriptome analysis and weighted gene co-expression network analysis (WGCNA), 17 gene modules were identified, among which the salmon module showed a strong association with resistance in variety 225. Within this module, 18 hub genes—including Ca59V2g00372.1 (CaTLP6), encoding a thaumatin-like protein (TLP)—were significantly upregulated upon infection. A genome-wide analysis identified 31 CaTLP genes in C. annuum, with members of group V (such as CaTLP6) exhibiting induced expression post-inoculation of Colletotrichum scovillei. Subcellular localization analysis indicated that group V CaTLP proteins were associated with the plasma membrane, suggesting a role in pathogen recognition. These findings highlight the significance of CaTLP genes, particularly those in group V, in pepper’s defense against anthracnose caused by C. scovillei and offer promising targets for breeding resistant cultivars.
1. Introduction
Pepper (Capsicum spp.) anthracnose is a globally prevalent disease that severely impacts pepper production, leading to an estimated annual yield loss of up to 50% worldwide [1]. Tropical and subtropical regions are particularly affected. In India, anthracnose results in a 10–54% reduction in pepper yield each year, while in Vietnam, it can cause yield losses of up to 80% [2]. The pathogens responsible for pepper anthracnose belong to the genus Colletotrichum in the subphylum Pezizomycotina, which contains many species and has a complex classification. The dominant anthracnose-causing species are C. truncatum, C. scovillei, and C. siamense [3]. In China, at least 15 species of Colletotrichum have been identified, with significant regional differences in the dominant species [4]. For example, in northern Guangdong, C. scovillei has been identified as the predominant species [5].
RNA sequencing (RNA-seq) and comparative transcriptomics are widely used to study plant responses and defense mechanisms to biotic stresses, including bacterial and fungal infections. For instance, Ralstonia solanacearum (Ras) is the pathogen responsible for bacterial wilt (BW) in Solanaceae crops. A study investigating the differentially expressed genes (DEGs) in the root tissues of the resistant tobacco cultivar D101 and the susceptible cultivar Changbohuang (CBH) after inoculation with Ras indicated that glutathione and flavonoids play crucial roles in the initial defense against Ras infection [6]. Similarly, a comparison of the root expression profiles in the susceptible cultivar Yunyan 87 and the resistant cultivar Fandi 3 at different stages after Ras inoculation produced similar findings. Notably, genes encoding WRKY6, WRKY11, ERF5, ERF15, and PR5 were significantly upregulated in the resistant variety after Ras infection [7].
Pathogenesis-related proteins (PRs) are constitutively or inducibly expressed in plants to defend against pathogen attacks or insect predation. To date, 17 PR families (PR1–PR17) have been identified based on their sequence, structure, and enzymatic properties [8]. Members of the PR5 family share sequence homology with thaumatin, a sweet-tasting protein first discovered in Thaumatococcus danielli Benth, and are hence termed thaumatin-like proteins (TLPs) [9]. Most TLPs contain the conserved motif G-X-[GF]-X-C-X-T-[GA]-D-C-X-(1,2)-G-X-(2,3)-C (PROSITE: PS00316), a highly conserved sequence in the thaumatin family [10]. They also have 10 or 16 conserved cysteine residues and a REDDD (arginine, glutamic acid, and three aspartic acid residues) motif, with five or eight disulfide bonds contributing to the stability of their structure [10,11].
Studies on TLPs in various plants have revealed their diverse roles in biotic and abiotic stress responses. TLPs have been linked to broad-spectrum resistance against various pathogens. For example, in garlic (Allium sativum L.), AsTLP7, AsTLP8, AsTLP9, and AsTLP21 were involved in the defense against Fusarium proliferatum attack [12]. Overexpression of the CsTLP gene of Camellia sinensis in potato (Solanum tuberosum L.) resulted in enhanced resistance to fungal pathogens such as Macrophomina phaseolina (necrotrophic) and Phytophthora infestans (hemibiotrophic) [13]. In transgenic potato tubers infected with M. phaseolina, expressions of phenylpropanoids, lipoxygenase, StPAL, StLOX, and StTLP were significantly upregulated, while the leaves of transgenic potatoes also exhibited resistance to P. infestans [13]. In rice (Oryza stiva L.), the expression patterns of OsTLP genes varied in response to various abiotic and biotic stresses. For example, under salt stress and hormone treatment, OsTLP genes exhibited differential expression, with only OsTLP1, OsTLP2, and OsTLP27 expressed after infection with brown planthopper (BPH), white back planthopper (SSB), and rice leaf folder (RFL) [14]. Additionally, several TLPs were involved in plant responses to different abiotic stresses. For instance, AnTLP13 from Ammopiptanthus nanus, an evergreen shrub from Central Asia, enhanced tolerance to cold stress when heterologously expressed in Escherichia coli, yeast cells, and tobacco leaves [15]. Similarly, BolTLP1 in broccoli (Brassica oleracea) exhibited induced expression under abiotic stress and positively regulated drought and salt tolerance [16].
Current studies indicate that anthracnose resistance in peppers are predominantly found in C. chinense (accession PRI95030 and PBC932) and C. baccatum (accession PBC81), with limited resistant resources available in the widely cultivated C. annuum [17,18,19,20]. Through systematic screening of 30 C. annuum varieties using needle inoculation assays, we identified ‘Goldfish yellow pepper’ (variety 225) as a resistant variety and ‘Big horn’ (variety 307) as susceptible to C. scovillei infection. Comparative analysis revealed significantly reduced lesion formation in variety 225, confirming its resistant phenotype. Transcriptomic profiling identified 17 co-expression modules associated with infection response, with the salmon module showing strong correlation with resistance in variety 225. Notably, CaTLP6 exhibited marked upregulation during 48–72 h post-inoculation in the resistant variety, suggesting its potential role in defense response. Based on the established roles of TLPs in plant immunity [12,13,14], we hypothesize that structural features and expression patterns of CaTLPs contribute to anthracnose resistance in pepper, similar to what has been observed in other pathosystems [6,7,13]. This study provides comprehensive insights into TLP-mediated resistance mechanisms in C. annuum and offers valuable genetic resources for breeding anthracnose-resistant varieties.
2. Materials and Methods
2.1. Pathogen Inoculation
Two C. annuum varieties, a resistant variety ‘Goldfish yellow pepper’ (variety 225) and a susceptible variety ‘Big horn’ (variety 307), were selected for this study. C. scovillei conidium suspension was prepared from freshly cultured fungal isolates and adjusted to a concentration of 1 × 106 conidia/mL using sterile distilled water.
Fruits and leaves from resistant variety 225 and susceptible variety 307 were divided into two experimental groups: (1) mechanical wounding via sterile needle puncture (control for physical damage) and (2) inoculation with C. scovillei conidium suspension. For pathogen inoculation, 10 μL of conidium suspension (1 × 106 conidia/mL) was applied to needle-punctured tissues. Control groups received identical wounding without fungal exposure. All treated samples were maintained under controlled environmental conditions (25 °C, 90% relative humidity, 12 h light/dark cycles) to facilitate infection. Three biological replicates of fruit tissues surrounding the inoculation site were collected at designated time points: 0 h (pre-inoculation control), 12 h, 24 h, 48 h, 72 h, and 96 h post-inoculation.
2.2. Sampling for RNA-Seq and Data Processing
The leaves of variety 225 and variety 307 were individually treated with wounding with the needle and inoculation using C. scovillei conidium suspension. Samples were collected from the needled/inoculated leaves at 0 h, 12 h, 24 h, 48 h, 72 h, and 96 h post-inoculation. For each time point and treatment combination, three biological replicates were collected to ensure experimental reproducibility. Total RNA was isolated using TRIzol Reagent (Invitrogen, Thermo Fisher Scientific, Carlsbad, CA, USA) following the manufacturer’s protocol. RNA quality and concentration were assessed using a NanoDrop 2000c Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The transcriptomes of the samples were deeply sequenced using second-generation sequencing technology by Biomarker Technologies Company (Beijing, China). The cleaned RNA-seq data have been deposited in the China National GeneBank DataBase (CNGBdb) (accession: CNP0007496).
Raw reads underwent stringent quality control using the NGS QC Toolkit (v2.3.3) [21], including adapter trimming and removal of low-quality reads (Q-score < 20). High-quality reads were aligned to the reference genome using HISAT2 (v2.2.1) [22], and transcriptomes were assembled de novo with StringTie (v2.2.0) [23]. Gene expression levels were normalized as Transcripts Per Million (TPM) to enable cross-sample comparisons of transcript abundance. This integrated workflow ensured robust detection of temporal gene expression changes in response to C. scovillei infection, distinguishing pathogen-specific responses from mechanical stress artifacts.
2.3. Co-Expression Network Construction and Hub Gene Identification
Weighted gene co-expression network analysis (WGCNA) was performed using the WGCNA package (v1.73) in R (v4.3.0) [24]. TPMs derived from RNA-seq data were used as input. Genes with a median absolute deviation (MAD) below the 50th percentile across samples were excluded to reduce noise. Potential outlier genes were further removed via the “goodSamplesGenes” function in WGCNA.
A signed network was constructed using a soft thresholding power of 9 to approximate scale-free topology. Co-expressed gene modules were identified through hierarchical clustering with the dynamic tree-cutting algorithm. Parameters included a minimum module size of 30 genes, a deepSplit setting of 3 for branch sensitivity, and a merging threshold of 0.25 (module eigengene dissimilarity). Modules significantly correlated with phenotypic traits (Pearson correlation coefficient ≥ 0.8, p ≤ 0.05) were prioritized for downstream analysis.
Within trait-associated modules, gene significance (GS) and module membership (MM) were calculated to quantify gene–trait relationships and intramodular connectivity, respectively. Hub genes were defined as those meeting thresholds of GS > 0.4, MM > 0.8, and p < 0.05. Weighted interactions between hub genes were exported to Cytoscape (v3.10.0) [25] for network visualization. Key regulatory nodes within the network were identified using the CytoNCA plugin (v2.1.6) [26] based on betweenness centrality (BC), a measure of node influence in mediating information flow.
2.4. Identification and Chromosomal Distribution of 31 CaTLPs
TLP genes in C. annuum were identified using the Hidden Markov Model (HMM) profile of the TLP domain (PF00314) from the Pfam database. The search was performed against the genome of C. annuum inbred line CA59 in the CNGB (accession: CNP0001129) and National Center for Biotechnology Information (accession: PRJNA788020) [27]. Genes were named CaTLP1 to CaTLP31 according to their chromosomal positions. Gene duplication events (segmental and tandem) were analyzed using WGDI (v0.5.6) with default parameters [28]. Segmental duplications were defined as paralogs located on non-syntenic chromosomal blocks, while tandem duplications were identified as adjacent genes within 100 kb on the same chromosome.
2.5. Physicochemical Characterization of CaTLP Proteins
Protein sequences of the 31 CaTLPs were analyzed for amino acid length, molecular weight (MW), theoretical isoelectric point (pI), Grand Average of Hydropathicity (GRAVY), and instability index using the ProtParam tool in Biopython (v1.78). Subcellular localization was predicted using DeepLoc-2.1, and signal peptides were identified with SignalP 6.0. Proteins with instability indices > 40 were classified as unstable.
2.6. Phylogenetic and Structural Analysis
A neighbor-joining (NJ) phylogenetic tree was constructed using MEGA11 with 1000 bootstrap replicates [29]. The analysis included 31 CaTLPs, 43 rice OsTLPs, and 25 Arabidopsis AtTLPs, and the OsTLPs and AtTLPs were previously identified by Zhao [30]. Conserved motifs were identified using MEME Suite (v5.5.2) with a maximum of 10 motifs and default parameters [31]. Gene structures (intron/exon organization) were visualized using TBtools (v2.309) based on genome annotation files [32].
2.7. Expression Analysis and qRT–PCR of CaTLPs
The relative expression levels of CaTLPs in pepper leaves following fungal inoculation were obtained from the transcriptome analysis of fungal-inoculated samples described above. The expression patterns of 31 CaTLPs were clustered and visualized using the “pheatmap” package (v1.0.12) in R, while the expression patterns of selected CaTLPs were illustrated using the “ggplot2” package in R.
Two micrograms of total RNA for each sample were reverse transcribed using HiScript® III Reverse Transcriptase (Vazyme Biotech Co., Ltd., Nanjing, China). Real-time PCR was conducted on a CFX Connect™ real-time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd., Nanjing, China). CaUBQ5 (Ca59V2g02917.1) was used as the reference gene. RNA samples used for qRT–PCR were the same as those used for RNA-seq, with three independent biological replicates collected for each treatment and time point. Each biological replicate was further assessed in triplicate as technical replicates. All primers used are listed in Table S1.
2.8. Subcellular Localization of CaTLPs
The coding sequences of CaTLPs were amplified and inserted into vector p1300S–GFP (modified from pCAMBIA1300), driven by the CaMV35S promoter. The fusion constructs were transferred into Agrobacterium tumefaciens strain GV3101 and then injected into 4-week-old tobacco leaves. AtPIP2A protein (AT3G53420) fused with mCherry was used as a plasma membrane marker and was co-transformed with CaTLPs in tobacco [33]. After 2 days, fluorescence was detected and imaged using laser confocal fluorescence microscopy (Olympus FV 1200, Tokyo, Japan). The excitation wavelengths for GFP and mCherry were 488 and 543 nm, respectively, while the emission wavelengths were 498–548 nm and 560–631 nm, respectively.
3. Results
3.1. Disease Symptoms Caused by C. scovillei in Variety 225 and 307
The fruits and leaves of resistant variety 225 and susceptible variety 307 were individually treated with needling and inoculation using C. scovillei conidium suspension. Seven d after fruit inoculation, large lesions with orange sporulation appeared at the inoculation sites of variety 307, while no significant lesions were observed in variety 225 (Figure 1a,b). Twenty d post-inoculation on the leaves, prominent lesions developed at the inoculation sites of variety 307, whereas no obvious lesions were found in variety 225 (Figure 1c,d). Needle puncture treatment on both varieties, whether on fruits or leaves, did not result in any visible lesion formation. These findings confirm that variety 225 exhibits stronger resistance to C. scovillei compared to variety 307, suggesting the potential involvement of specific resistance genes in variety 225.
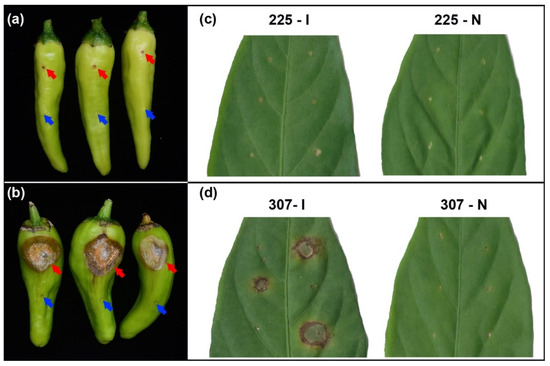
Figure 1.
Phenotype after inoculation with Colletotrichum scovillei and needling treatment in resistant variety 225 and susceptible variety 307. (a) Phenotype of fruits of variety 225 after inoculation with C. scovillei (top, red arrow) and needling (bottom, blue arrow) treatment. (b) Phenotype of fruits of variety 307 after inoculation with C. scovillei (top, red arrow) and needling (bottom, blue arrow) treatment. (c) Phenotype of leaves from variety 225 after inoculation with C. scovillei (225–I) and needling (225–N) treatment. (d) Phenotype of leaves from variety 307 after inoculation with C. scovillei (307–I) and needling (307–N) treatment.
3.2. Identification of Gene Modules Responsive to Colletotrichum Infection in Pepper
To investigate the response mechanisms of resistant variety 225 and susceptible variety 307 to C. scovillei infection, we performed leaf inoculation and needling treatments on six leaf-stage plants of both varieties. The needling treatment was designed as a control to eliminate the effects of physical damage. Leaf samples were collected at 0 h, 12 h, 24 h, 48 h, 72 h, and 96 h post-treatment for RNA-seq analysis, resulting in a total of 412 Gbp of sequencing data (Table S2).
WGCNA was conducted on the expression levels of these transcripts, revealing a total of 17 gene modules. Among these, the expression pattern of the salmon module was highly correlated with the inoculation treatment in variety 225 (correlation = 0.43, p = 2.74 × 10−4) (Figure 2a), suggesting that genes in this module may play a key role in the specific response mechanism to C. scovillei infection in variety 225. This module contained 81 genes (Table S3), with 18 hub genes having a connectivity (weight) greater than 0.80, selected as key genes based on WGCNA results (Table S4).
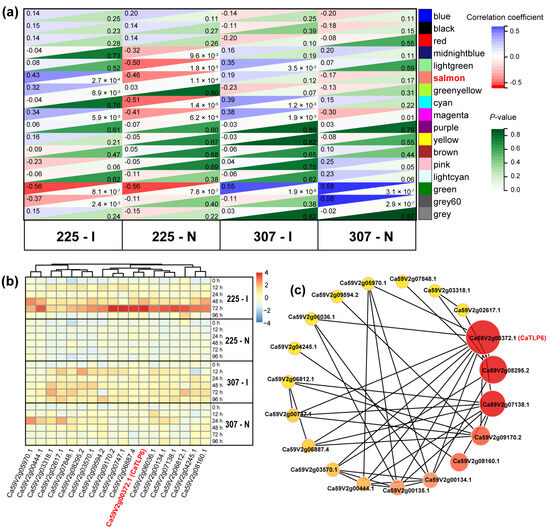
Figure 2.
Identification of hub genes related to C. scovillei infection in pepper. (a) Correlation analysis between modules and traits using Pearson’s correlation coefficient. The co-expression modules are represented in different colors. Numbers to the left of each box indicate the correlation values, and numbers to the right represent the p-values. (b) Heatmap of TPM values of 18 hub genes from the salmon module at different time points after various treatments. (c) Co-expression network of the 18 hub genes from the salmon module. The redder color indicates higher connectivity. 225–I: variety 225 after inoculation with C. scovillei; 225–N: variety 225 after needling; 307–I: variety 307 after inoculation with C. scovillei; 307–N: variety 307 after needling.
Analysis of the expression of these 18 hub genes revealed a significant upregulation of gene expression at 48 h and/or 72 h post-inoculation in variety 225. In contrast, the expression levels of these genes did not significantly change in variety 225 after needle puncture treatment or in variety 307 after both needle puncture and inoculation treatments (Figure 2b). The co-expression network analysis indicated that Ca59V2g00372.1 (CaTLP6), with the highest connectivity, could be a central gene in the response of variety 225 to C. scovillei infection (Figure 2c).
3.3. Distribution and Duplication of CaTLP Genes in the C. annuum Genome
Ca59V2g00372 (CaTLP6) is a member of the TLP (thaumatin-like protein) gene family, which has been proposed as a molecular marker for resistance to fungal diseases [34]. Using the Hidden Markov Model (HMM) of the TLP motif (Pfam: PF00314), we identified and predicted TLP proteins in the public China National GeneBank Database (accession number CNP0001129) [27]. A total of 31 candidate genes encoding TLP proteins were identified (Table S5). These genes were named CaTLP1 to CaTLP31 based on their chromosomal locations (Figure 3a and Table 1). Chromosome mapping showed that CaTLP genes are unevenly distributed across the 12 chromosomes, with at least one CaTLP gene on each chromosome. Chromosome 1 (Chr01) contained the highest number of CaTLP genes, with nine genes (CaTLP1–CaTLP9).
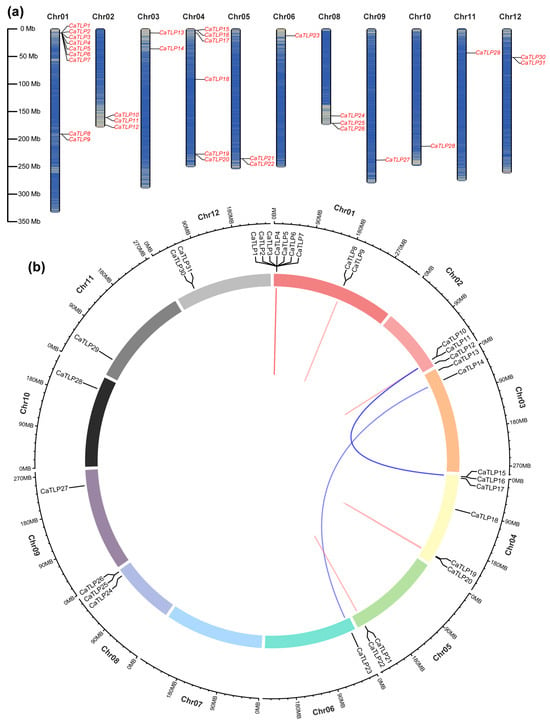
Figure 3.
Distribution of TLP genes on Capsicum annuum chromosomes. (a) Distribution of 31 CaTLP genes on 12 chromosomes. The scale represents megabases (Mb). The chromosome numbers (Chr01–Chr12) are indicated above each vertical bar. (b) Distribution of segmental and tandem duplicated TLP genes in the C. annuum genome. Segmental duplications are connected by blue lines, while tandem duplications are indicated by red lines.

Table 1.
Characterization of the predicted TLPs in Capsicum annuum.
Gene duplication analysis revealed three pairs of segmental duplications (CaTLP14/CaTLP23, CaTLP10/CaTLP15, and CaTLP11/CaTLP16) and four pairs of tandem duplications (CaTLP8/CaTLP9, CaTLP10/CaTLP11, CaTLP19/CaTLP20, and CaTLP21/CaTLP22) (Figure 3b). Notably, seven CaTLP genes (CaTLP1–CaTLP7) underwent multiple tandem duplication events.
3.4. Physicochemical Characteristics of 31 CaTLPs
To further elucidate the main physicochemical properties of 31 CaTLP proteins, we analyzed their amino acid length, molecular weight (MW), theoretical isoelectric point (pI), Grand Average of Hydropathicity (GRAVY), instability index, subcellular localization, and signal peptides. The average length of CaTLP proteins is 266 amino acids, with an average molecular weight of 28.53 kDa. The smallest protein is CaTLP22, with a molecular weight of 13.65 kDa and a length of 123 amino acids, while the largest one is CaTLP26, with a molecular weight of 44.36 kDa and a length of 436 amino acids. The pI values range from 4.29 (CaTLP11) to 9.37 (CaTLP22), with 12 CaTLPs being acidic proteins and the rest basic. Of the 31 CaTLPs, 66.67% (20/31) are hydrophilic (negative GRAVY values), while the others are hydrophobic. Instability index analysis classified 16 proteins as stable and 15 as unstable. Subcellular localization predictions indicated that twenty-two CaTLPs are extracellular, while nine are localized at the plasma membrane. Additionally, 22 CaTLPs possess signal peptide sequences (Table 1).
3.5. Phylogenetic Analysis of TLPs from Different Plant Species
To examine the evolutionary relationships within the CaTLP protein family, we constructed a phylogenetic tree using 31 CaTLPs, 43 OsTLPs from rice, and 25 AtTLPs from Arabidopsis (Table S6, Figure 4). Based on the structural characteristics, 31 CaTLPs were classified into 10 distinct subgroups (Group I to X), following the phylogenetic classification for TLP proteins in Arabidopsis and rice [30]. Each subgroup contained at least one CaTLP protein. Group V contained the highest number of CaTLPs (nine members), while Groups II and VI each contained four, Groups VII and I contained three, Groups VIII and X each had two, and Groups III, IV, and IX had one each. Notably, the first seven CaTLPs (CaTLP1–CaTLP7) on Chr01 were closely related in the phylogenetic tree, similar to the TLP proteins OsTLP37–OsTLP43 in rice. Some of these proteins have been reported to be involved in resistance to fungal diseases. For example, protein encoded by LOC_Os03g46070 (identified as OsTLP10 of Group V in this study) was involved in resistance to rice blast disease [35]. Another protein encoded by Os12g0628600 (also known as LOC_Os12g43380 and identified as OsTLP37 of Group V in this study) was associated with resistance to Colletotrichum gloeosporioides f. sp. Manihotis [36].
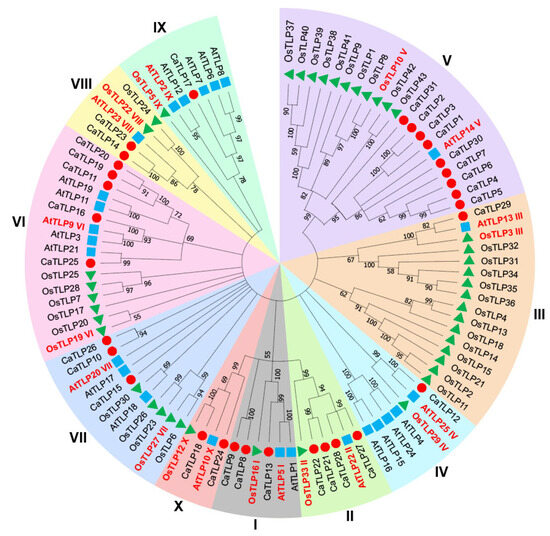
Figure 4.
Phylogenetic analysis of TLPs. CaTLPs (red circles) represent Capsicum annuum TLPs; AtTLPs (blue squares) represent Arabidopsis thaliana TLPs; OsTLPs (green triangles) represent Oryza sativa TLPs. The tree was constructed using amino acid sequences by the neighbor-joining (NJ) method, with TLP proteins classified into 10 groups (I to X). Protein names and Roman numerals in red indicate phylogenetic clades as described by Zhao [30].
3.6. Analysis of the Protein and Gene Structure of CaTLPs
To further elucidate the structural features of CaTLP proteins, we conducted a conserved motif analysis on 31 CaTLP proteins. A phylogenetic tree was constructed for only CaTLP proteins using Geneious (v2025.0.3). Combined with the TLP protein grouping information, it was evident that closely related CaTLPs possessed similar types and combinations of motifs (Figure 5a). In this study, we identified a total of 10 distinct motifs. Notably, Motif 4, which contains the thaumatin family signature domain (PROSITE: PS00316), was present in all CaTLP proteins except for CaTLP22 (Figure 5b). Only the CaTLPs from Groups IV, IX, X, and some members of Groups II, VI, and VIII contained all 10 motifs. In contrast, Groups V and VII lacked Motif 7. Gene structure analysis of the 31 CaTLP-encoding genes revealed that most CaTLP genes (70.97%) contained introns. However, nearly all genes in Group V CaTLPs were intron-less, except for CaTLP31 (Figure 5c). These findings suggest that CaTLPs in Group V may have distinct protein and gene structures compared to other CaTLPs.
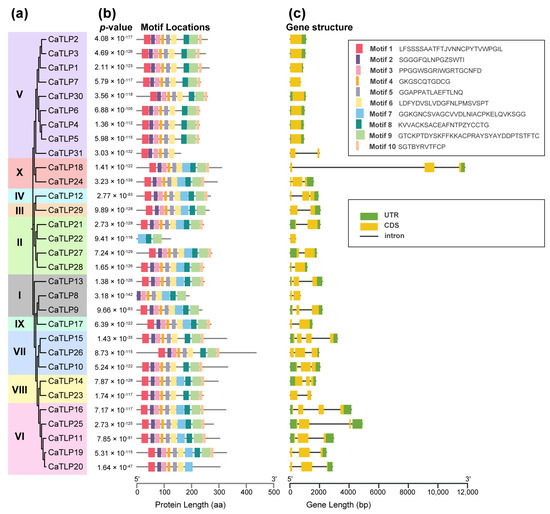
Figure 5.
Phylogenetic analysis, conserved motifs, and gene structure of the 31 CaTLPs. (a) Phylogenetic tree of 31 CaTLPs using Geneious. (b) Motif composition and distribution of CaTLPs based on the phylogenetic relationship. Ten different motifs indicated by different colors were identified by MEME. The length of box and line is proportional to protein length. (c) Structure of the 31 CaTLP genes from the TBtools. UTRs and CDSs are indicted by green and yellow boxes, respectively, and introns are shown as black lines. The length of box and line is proportional to gene length.
3.7. Expression Patterns of CaTLP Genes
To further investigate the expression patterns of TLP genes in pepper, we examined the expression changes of 31 CaTLP genes in variety 225 and 307 following inoculation and needling treatments by mining the RNA-seq data. The results showed that four CaTLP genes (CaTLP8, CaTLP21, CaTLP22, and CaTLP31) were not expressed. Among the expressed CaTLPs, Group V CaTLPs in both variety 225 and 307 were induced to varying degrees after inoculation but did not respond significantly to needling treatment, while the other CaTLPs did not exhibit a specific response to inoculation (Figure 6a).
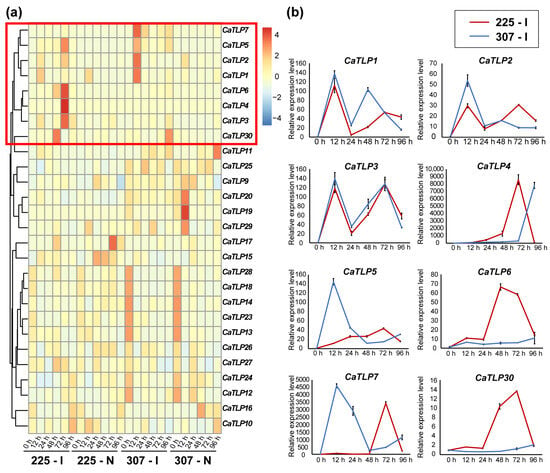
Figure 6.
Expression levels of CaTLP genes after inoculation and needling treatments. (a) Heatmap of expression profiles of the 31 CaTLPs in variety 225 and 307 following inoculation and needling treatments, and the TPM value for each gene was normalized. (b) qRT–PCR analysis of the expression profiles of group V CaTLPs in variety 225 and 307 following inoculation treatments.
We then tested the expression levels of Group V CaTLPs using qRT–PCR to validate the RNA-seq results. The expression trends obtained from qRT–PCR were consistent with those from RNA-seq, supporting the reliability of our transcriptomic data (Figure 6b). The expression patterns of CaTLP1, CaTLP2, and CaTLP3 were similar in both cultivars, being rapidly induced 12 h after inoculation and fluctuating thereafter. In variety 225, all eight CaTLPs were up-regulated to varying extents. Specifically, CaTLP1 and CaTLP6 had the highest expression levels at 12 h and 48 h post-inoculation, respectively, while CaTLP2, CaTLP3, CaTLP4, CaTLP5, CaTLP7, and CaTLP30 peaked at 72 h post-inoculation. In variety 307, CaTLP6 and CaTLP30 were not significantly induced, while CaTLP1, CaTLP2, CaTLP3, CaTLP5, and CaTLP7 peaked at 12 h post-inoculation, and CaTLP4 was activated at 96 h post-inoculation. The peak of CaTLPs expression in variety 307 mainly appeared in the early stage (12 h after inoculation), while that in variety 225 mainly appeared in the late stage (72 h after inoculation), which may have different effects on the two varieties against fungal diseases.
3.8. Localization Characteristics of Group V CaTLPs
To investigate the functional localization of Group V CaTLP proteins in plant cells, we conducted a subcellular localization analysis using GFP-tagged CaTLP proteins expressed in tobacco leaves (Figure 7). A GFP protein driven by the 35S promoter served as a control, while a plasma membrane protein AtPIP2A fused with red fluorescent protein (mCherry) was used as a plasma membrane marker. The GFP protein alone was observed in the plasma membrane, cytoplasm, and nuclei, while the fluorescent signals of CaTLPs–GFP predominantly overlapped with the red fluorescence. Specifically, the fluorescence signal of CaTLP5–GFP completely overlapped with the red fluorescence, whereas the other CaTLPs showed partial overlap with the red fluorescence and tended to diffuse into the surrounding area. These observations suggest that most CaTLPs are localized to the plasma membrane, the cytoplasm, and the extracellular matrix, which is consistent with the predicted localization results (Table 1). Previous studies have shown that RlemTLP and CsTLP1, predicted to be extracellular proteins, are located at the plasma membrane’s periphery and in the cytoplasm, and exhibit antifungal activity [37,38]. Taken together, these findings suggest that Group V CaTLP proteins are primarily localized to the plasma membrane and surrounding regions, supporting their potential role in plant defense mechanisms.
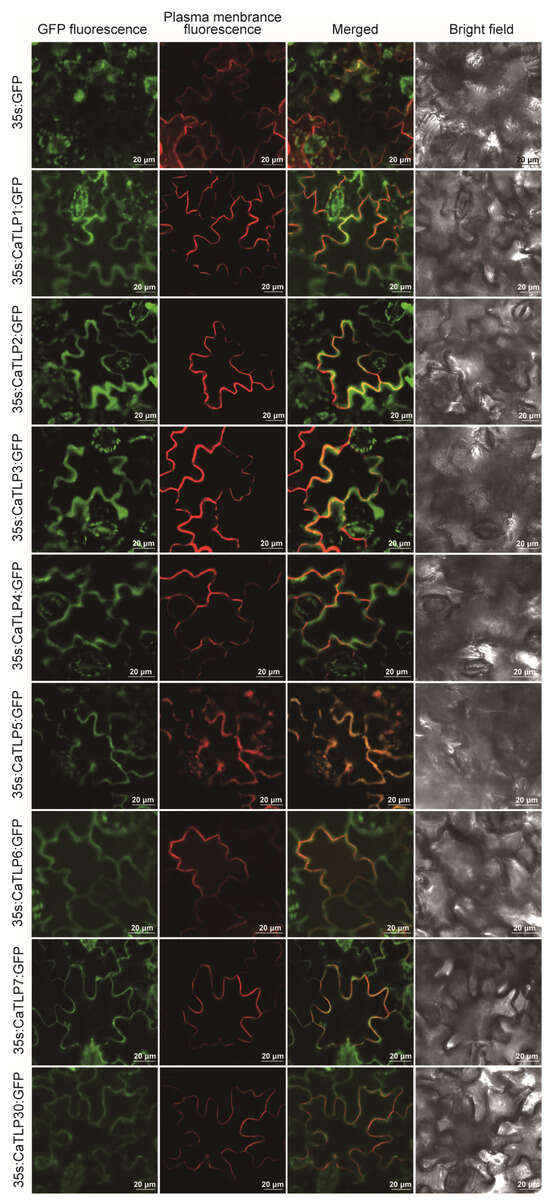
Figure 7.
The subcellular localization analysis of the CaTLP1, CaTLP2, CaTLP3, CaTLP4, CaTLP5, CaTLP6, CaTLP7, and CaTLP30 in Nicotiana benthamiana. The transient expression of the 35S::CaTLP–GFP fusing construct and the 35S::GFP construct in N. benthamiana. Green fluorescent protein (GFP) fluorescence was observed using a confocal microscope. Scale bar = 20 μm.
4. Discussion
Pepper (C. annuum) is an important economic crop in the Solanaceae family. Understanding its genetic basis and exploring genetic resources for resistance to anthracnose are of great significance. In this study, we investigated the molecular mechanisms underlying the resistance of C. annuum variety 225 to C. scovillei, the fungal pathogen responsible for anthracnose. By comparing the resistant variety 225 with the susceptible variety 307, we gained insights into the genes and proteins involved in defense responses. Our findings reaffirm the importance of genetic resistance in pepper and highlight potential candidates for future breeding programs aimed at improving resistance.
4.1. Highly Resistance in Variety 225 Contrasts with Severe Symptom Development in Susceptible Variety 307 upon C. scovillei Infection
In our experiments, inoculated pepper leaves and fruits with C. scovillei in vitro revealed that both the leaves and fruits of variety 225 exhibited strong resistance to anthracnose, while variety 307 was more susceptible (Figure 1), which indicates that different varieties have distinct sensitivity to the disease. In resistant variety 225, inoculation did not cause significant lesions on fruits and leaves, whereas the susceptible variety 307 showed extensive lesions with orange sporulation (Figure 1). These observations are consistent with previous studies suggesting that genetic resistance mechanisms play a central role in defending against fungal pathogens like Colletotrichum [39,40].
The formation of lesions in variety 307 but not in variety 225 suggests that variety 225 may activate more robust defense responses, including pathogen recognition and immune pathway activation. Notably, no lesions appeared on the fruits of variety 307 after needling treatment, which emphasizes that physical damage alone does not explain the observed differences in pathogen response. This indicates that the resistance of variety 225 is likely genetically controlled and involves complex immune signaling pathways that are not triggered by mechanical damage.
4.2. Hub Gene Ca59V2g00372.1 Specifically Regulates the Activation of the Defense Network in the Resistant Variety 225
To explore the natural defense mechanisms of pepper against anthracnose, we performed RNA-seq analysis to study the transcriptional responses of the two varieties to C. scovillei infection. Given that variety 225 showed greater resistance to anthracnose, we focused on its genetic response between 48 and 72 h post-inoculation. WGCNA of the expression profiles of 94,966 transcripts identified 17 modules, with one module, the salmon module, showing a strong correlation with the inoculation response in variety 225 (Figure 2a). This suggests that the salmon module is crucial in mediating the resistance mechanisms in variety 225.
Within this module, 18 hub genes were identified that exhibited upregulation after 48 h and 72 h of inoculation in variety 225, but not in variety 307 or after needling treatment. This indicates that the response in variety 225 to C. scovillei infection is time dependent and likely involves a cascade of gene activations leading to immune responses. Notably, the hub gene Ca59V2g00372.1 (CaTLP6), with high connectivity within the gene network, emerged as a key player in the resistance of variety 225 (Figure 2c). Given its central position in the network, Ca59V2g00372.1 may be involved in pathogen recognition and defense activation. Similar hub genes have been identified in other crops as central regulators of defense pathways [41], making this gene a candidate for further functional characterization and potential manipulation in crop breeding.
4.3. Group V CaTLPs Emerge as Key Resistance Determinants Through Pathogen-Induced Expression and Membrane Localization
A significant finding of this study was the characterization of the CaTLP gene family in C. annuum. TLPs are widely distributed in plants and typically have functionally differentiated subfamilies [10]. The number of TLP genes varies across species; for example, genome-wide analyses have identified 31 TLP genes in Ammopiptanthus nanus, 32 in garlic (Allium sativum L.), 33 in grape (Vitis vinifera L.), 36 in Qingke (Hordeum vulgare L.), 41 in strawberry (Fragaria × ananassa), 81 in bamboo (Phyllostachys edulis (Carrière) J.Houz.), and 126 in wheat (Triticum aestivum) [12,15,42,43,44,45,46]. In this study, we identified 31 CaTLP genes in the C. annuum genome. Phylogenetic analysis revealed that CaTLPs can be divided into 10 subclasses (Group I to Group X).
The 31 CaTLP genes identified in this study were unevenly distributed across the 12 chromosomes of the C. annuum genome, with notable tandem and segmental duplications (Figure 3). Comparative genomic analysis of the TLP family across six plant species showed that tandem and segmental duplications play a key role in the expansion of this gene family [47]. Moreover, positive selection of certain TLP nucleotides may have accelerated functional divergence, resulting in the formation of gene subgroups. Taken together, gene duplications likely contributed to the expansion of the CaTLP genes family, potentially enhancing pathogen defense.
The expression pattern analysis of the CaTLP genes (particularly in Group V) suggests that these genes are specifically induced by C. scovillei inoculation but not by physical damage (needling). This selective activation indicates that Group V CaTLPs may be involved in pathogen recognition and defense. Moreover, differential expression between variety 225 and variety 307 (Figure 6) further supports the notion that these genes contribute to resistance in variety 225, with expression levels peaking later in the resistant variety compared to the susceptible one. This pattern is consistent with other studies showing TLP gene activation during pathogen infection [42].
Additionally, the subcellular localization of Group V CaTLP proteins in the plasma membrane, the cytoplasm, and the extracellular matrix (Figure 6b), as reported in previous studies [37,38,48], suggests that these proteins may function in the recognition of the pathogen at the cell surface, which is the critical first step in plant immune responses. The plasma membrane is a key site for pathogen detection and subsequent activation of downstream defense pathways, such as the production of reactive oxygen species (ROS) and the reinforcement of the cell wall [49].
4.4. Gene Duplication Drives Expansion of Group V CaTLPs with Distinct Regulatory Potential
Phylogenetic analysis grouped CaTLPs into 10 distinct subgroups, with the largest subgroup (Group V) containing nine CaTLPs. Many of these genes are arranged in tandem on chromosome 1, supporting the hypothesis that gene duplication has contributed to the expansion and functional diversity of the TLP family (Figure 4). Gene structure analysis revealed that most CaTLP genes contain introns, although Group V members were less likely to contain introns (Figure 5). This structural difference may indicate functional divergence among the CaTLP genes and suggest that Group V members could have a distinct regulatory mechanism.
4.5. Group V CaTLPs May Serve as Prime Targets for Molecular Breeding of Anthracnose-Resistant Pepper Cultivars
The identification of hub genes within the salmon module, particularly those involved in the CaTLP gene family, opens the way for further functional studies. Many studies have reported that TLP genes play an important role in plant resistance to fungal infection. For instance, in peanut (Arachis hypogaea), AdTLP showed enhanced anti-fungal activity towards the late leaf spot pathogen, Phaeoisariopsis personata [39]. Overexpression of TLP29 from grape ‘Shang–24’ in Arabidopsis enhanced its resistance to powdery mildew and the bacterium Pseudomonas syringae pv. tomato DC3000 [43]. The rice gene OsTLP10, which is upregulated in response to rice blast disease, was known to positively regulate disease resistance [36]. Similarly, in pepper, the expression of PepTLP (identified as CaTLP2 in this study) was stimulated by jasmonic acid treatment, wounding, and fungal infection [35].
Previous studies have proposed TLPs as molecular markers for resistance to fungal pathogens [35], and our results support their role in resisting C. scovillei infection. Exploring the roles of Group V CaTLPs in resistance to C. scovillei through gene knockout and overexpression studies could provide deeper insights into the molecular mechanisms underlying pepper resistance. Moreover, these genes could be utilized as markers for marker-assisted selection (MAS), facilitating the development of new varieties with enhanced resistance to C. scovillei. Future studies should also focus on the expansion and functional diversification of the TLP gene family in C. annuum to better understand how these genes contribute to resistance under different environmental conditions and against various pathogens. Additionally, investigating the regulatory networks controlling the expression of these genes and their interactions with other immune pathways will be crucial for understanding how plants balance resistance and growth.
5. Conclusions
This study provides a comprehensive analysis of the molecular mechanisms underlying the resistance of C. annuum variety 225 to C. scovillei infection. Through phenotypic observations, gene expression profiling, and functional genomics, we identified key genes and gene families, including TLPs, that play critical roles in defense responses. Our findings not only deepen our understanding of pepper immunity but also lay the foundation for developing more resistant pepper varieties through targeted breeding and genetic engineering. These results highlight the importance of integrating genomics and functional analyses to address challenges in plant disease management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11060703/s1, Table S1: Information of the primers used in this study; Table S2: Information of next-generation sequencing; Table S3: Information of 81 salmon module genes identified in this study; Table S4: Description of 18 Hub genes; Table S5: Description of 31 CaTLP proteins; Table S6: List of TLP proteins of Arabidopsis thaliana, Oryza sativa, and Capsicum annuum used in this study.
Author Contributions
Data curation, H.W., J.Z. (Jian Zeng), C.M., W.H., C.L., Y.Z. and J.Z. (Jie Zheng); Funding acquisition, H.W., Y.Z., Z.Z. and J.Z. (Jie Zheng); Investigation, L.Y., X.Z., and J.L. (Jiaxian Lin); Methodology, H.W., J.Z. (Jian Zeng), C.M., W.H., C.L., Y.Z. and J.Z. (Jie Zheng); Resources, J.L. (Jianjun Lei), Z.Z., and J.Z. (Jie Zheng); Software, H.W., J.Z. (Jian Zeng), and J.Z. (Jie Zheng); Validation, H.W., J.Z. (Jian Zeng) and J.Z. (Jie Zheng); Writing—original draft, H.W. and J.Z. (Jie Zheng); Writing—review and editing, H.W., Y.Z. and J.Z. (Jie Zheng). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Open Fund of the Guangdong Provincial Key Laboratory of Utilization and Conservation of Food and Medicinal Resources in Northern Region, grant number FMR2022004Z and FMR2023002M; Open Fund of Key laboratory of Biology and Germplasm Enhancement of Horticultural Crops in South China, Ministry of Agriculture and Rural Areas, grant number NYBK2023003; Science and Technology Planning Project of Shaoguan, grant number230329168030903.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Than, P.P.; Jeewon, R.; Hyde, K.D.; Pongsupasamit, S.; Mongkolporn, O.; Taylor, P.W.J. Characterization and pathogenicity of Colletotrichum species associated with anthracnose on chilli (Capsicum spp.) in Thailand. Plant Pathol. 2008, 57, 562–572. [Google Scholar] [CrossRef]
- Saxena, A.; Raghuwanshi, R.; Gupta, V.K.; Singh, H.B. Chilli anthracnose: The epidemiology and management. Front. Microbiol. 2016, 7, 1527. [Google Scholar] [CrossRef]
- Mongkolporn, O. Breeding Strategies for Anthracnose Resistance. In Capsicum, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Diao, Y.Z.; Zhang, C.; Liu, F.; Wang, W.Z.; Liu, L.; Cai, L.; Liu, X.L. Colletotrichum species causing anthracnose disease of chili in China. Persoonia 2017, 38, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhuang, Y.; Guo, Z.; Xu, Y.; Zhong, J.; Huang, W.; Hu, F.; Li, H.; Lei, J.; Wu, H. Isolation, identification and fungicide screening of capsicum anthracnose pathogen in Nanxiong county. China Veg. 2022, 10, 58–65. (In Chinese) [Google Scholar]
- Gao, W.; Chen, R.; Pan, M.; Tang, W.; Lan, T.; Huang, L.; Chi, W.; Wu, W. Early transcriptional response of seedling roots to Ralstonia solanacearum in tobacco (Nicotiana tabacum L.). Eur. J. Plant Pathol. 2019, 155, 527–536. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Sun, G.; Li, X.; Chen, Z.; Feng, J.; Yang, Y. Digital gene expression analysis of the response to Ralstonia solanacearum between resistant and susceptible tobacco varieties. Sci. Rep. 2021, 11, 3887. [Google Scholar] [CrossRef]
- van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, B.J.; Hooft van Huijsduijnen, R.A.; Bol, J.F. A tobacco mosaic virus-induced tobacco protein is homologous to the sweet-tasting protein thaumatin. Nature 1986, 321, 531–532. [Google Scholar] [CrossRef]
- Liu, J.J.; Sturrock, R.; Ekramoddoullah, A.K. The superfamily of thaumatin-like proteins: Its origin, evolution, and expression towards biological function. Plant Cell Rep. 2010, 29, 419–436. [Google Scholar] [CrossRef]
- Hu, X.; Reddy, A.S. Cloning and expression of a PR5-like protein from Arabidopsis: Inhibition of fungal growth by bacterially expressed protein. Plant Mol. Biol. 1997, 34, 949–959. [Google Scholar] [CrossRef]
- Anisimova, O.K.; Kochieva, E.Z.; Shchennikova, A.V.; Filyushin, M.A. Thaumatin-like protein (TLP) genes in garlic (Allium sativum L.): Genome-wide identification, characterization, and expression in response to Fusarium proliferatum infection. Plants 2022, 11, 748. [Google Scholar] [CrossRef] [PubMed]
- Acharya, K.; Pal, A.K.; Gulati, A.; Kumar, S.; Singh, A.K.; Ahuja, P.S. Overexpression of Camellia sinensis thaumatin-like protein, CsTLP in potato confers enhanced resistance to Macrophomina phaseolina and Phytophthora infestans infection. Mol. Biotechnol. 2013, 54, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Zhu, H.; Chen, Y.; Xi, C.; Shah, A.Z.; Ge, L. Comprehensive bioinformatics and expression analysis of the TLP gene family revealed its role in regulating the response of Oryza sativa to Nilaparvata lugens, Laodelphax striatellus, and Jinggangmycin. Agronomy 2022, 12, 1297. [Google Scholar] [CrossRef]
- Liu, Q.; Sui, X.; Wang, Y.; Zhu, M.; Zhou, Y.; Gao, F. Genome-wide analyses of thaumatin-like protein family genes reveal the involvement in the response to low-temperature stress in Ammopiptanthus nanus. Int. J. Mol. Sci. 2023, 24, 2209. [Google Scholar] [CrossRef]
- He, L.; Li, L.; Zhu, Y.; Pan, Y.; Zhang, X.; Han, X.; Li, M.; Chen, C.; Li, H.; Wang, C. BolTLP1, a thaumatin-like protein gene, confers tolerance to salt and drought stresses in broccoli (Brassica oleracea L. var. Italica). Int. J. Mol. Sci. 2021, 22, 11132. [Google Scholar] [CrossRef]
- Voorrips, R.E.; Finkers, R.; Sanjaya, L.; Groenwold, R. QTL mapping of anthracnose (Colletotrichum spp.) resistance in a cross between Capsicum annuum and C. chinense. Theor. Appl. Genet. 2004, 109, 1275–1282. [Google Scholar] [CrossRef]
- Lin, S.W.; Gniffke, P.A.; Wang, T.C. Inheritance of resistance to pepper anthracnose caused by Colletotrichum acutatum. Acta Hortic. 2007, 760, 329–334. [Google Scholar] [CrossRef]
- Lee, J.; Hong, J.H.; Do, J.W.; Yoon, J.B. Identification of QTLs for resistance to anthracnose to two Colletotrichum species in pepper. J. Crop Sci. Biotechnol. 2010, 13, 227–233. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, Z.; Cao, Y.; Yu, H.; Feng, X.; Zhang, B.; Wang, L. Research progress on genetic breeding of pepper resistant to anthracnose. China Veg. 2022, 2, 17–24. (In Chinese) [Google Scholar]
- Patel, R.K.; Jain, M. NGS QC toolkit: A toolkit for quality control of next generation sequencing data. PLoS ONE 2012, 7, e30619. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Kovaka, S.; Zimin, A.V.; Pertea, G.M.; Razaghi, R.; Salzberg, S.L.; Pertea, M. Transcriptome assembly from long-read RNA-seq alignments with StringTie2. Genome Biol. 2019, 20, 278. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Tang, Y.; Li, M.; Wang, J.; Pan, Y.; Wu, F.X. CytoNCA: A cytoscape plugin for centrality analysis and evaluation of protein interaction networks. Biosystems 2015, 127, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wang, J.; Zhu, Z.; Liu, Y.; Chen, J.; Zhou, Y.; Liu, F.; Lei, J.; Gaut, B.S.; Cao, B.; et al. The 3D architecture of the pepper genome and its relationship to function and evolution. Nat. Commun. 2022, 13, 3479. [Google Scholar] [CrossRef]
- Sun, P.; Jiao, B.; Yang, Y.; Shan, L.; Li, T.; Li, X.; Xi, Z.; Wang, X.; Liu, J. WGDI: A user-friendly toolkit for evolutionary analyses of whole-genome duplications and ancestral karyotypes. Mol. Plant 2022, 15, 1841–1851. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Zhao, J.P.; Su, X.H. Patterns of molecular evolution and predicted function in thaumatin-like proteins of Populus trichocarpa. Planta 2010, 232, 949–962. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Xu, C.Y. BiFC assay for detecting protein-protein interaction in tobacco leaves. Bio-101 2018, e1010133. (In Chinese) [Google Scholar] [CrossRef]
- Kim, Y.S.; Park, J.Y.; Kim, K.S.; Ko, M.K.; Cheong, S.J.; Oh, B.J. A thaumatin-like gene in nonclimacteric pepper fruits used as molecular marker in probing disease resistance, ripening, and sugar accumulation. Plant Mol. Biol. 2002, 49, 125–135. [Google Scholar] [CrossRef]
- Tezuka, D.; Kawamata, A.; Kato, H.; Saburi, W.; Mori, H.; Imai, R. The rice ethylene response factor OsERF83 positively regulates disease resistance to Magnaporthe oryzae. Plant Physiol. Biochem. 2019, 135, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Odeny Ojola, P.; Nyaboga, E.N.; Njiru, P.N.; Orinda, G. Overexpression of rice thaumatin-like protein (Ostlp) gene in transgenic cassava results in enhanced tolerance to Colletotrichum gloeosporioides f. sp. manihotis. J. Genet. Eng. Biotechnol. 2018, 16, 125–131. [Google Scholar] [CrossRef]
- Garcia-Casado, G.; Collada, C.; Allona, I.; Soto, A.; Casado, R.; Rodriguez-Cerezo, E.; Gomez, L.; Aragoncillo, C. Characterization of an apoplastic basic thaumatin-like protein from recalcitrant chestnut seeds. Physiol. Plant. 2001, 110, 172–180. [Google Scholar] [CrossRef]
- Singh, N.K.; Kumar, K.R.; Kumar, D.; Shukla, P.; Kirti, P.B. Characterization of a pathogen induced thaumatin-like protein gene AdTLP from Arachis diogoi, a wild peanut. PLoS ONE 2013, 8, e83963. [Google Scholar] [CrossRef]
- Ma, W.; Gao, X.; Han, T.; Mohammed, M.T.; Yang, J.; Ding, J.; Zhao, W.; Peng, Y.L.; Bhadauria, V. Molecular genetics of anthracnose resistance in maize. J. Fungi 2022, 8, 540. [Google Scholar] [CrossRef]
- de Brito, M.V.; Torres, K.K.B.; Sousa, J.V.M.; França, G.B.; Costa, M.F.; da Costa, G.A.L.; Ferreira, G.N.C.; da Silva, V.B.; de Sá, G.H.; Lopes, Â.C.d.A.; et al. Inheritance of genetic resistance to anthracnose in lima beans: Analysis and implications for breeding. J. Phytopathol. 2025, 173, e70036. [Google Scholar] [CrossRef]
- Jayaprakash, A.; Roy, A.; Thanmalagan, R.R.; Arunachalam, A.; Ptv, L. Immune response gene co-expression network analysis of Arachis hypogaea infected with Aspergillus flavus. Genomics 2021, 113, 2977–2988. [Google Scholar] [CrossRef]
- Yan, X.; Qiao, H.; Zhang, X.; Guo, C.; Wang, M.; Wang, Y.; Wang, X. Analysis of the grape (Vitis vinifera L.) thaumatin-like protein (TLP) gene family and demonstration that TLP29 contributes to disease resistance. Sci. Rep. 2017, 7, 4269. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Zhou, X.; Zhang, X.; Li, X.; Zhang, P.; He, Y. Genome-wide identification and characterization of thaumatin-like protein family genes in wheat and analysis of their responses to Fusarium head blight infection. Food Prod. Process. Nutr. 2022, 4, 24. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Z.; Yin, W.; Xu, K.; Wang, S.; Shang, Q.; Sa, W.; Liang, J.; Wang, L. Genome-wide analysis of the Thaumatin-like gene family in Qingke (Hordeum vulgare L. var. nudum) uncovers candidates involved in plant defense against biotic and abiotic stresses. Front. Plant Sci. 2022, 13, 912296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Miao, L.; Yang, X.; Jiang, G. Genome-wide characterization and expression of the TLP gene family associated with Colletotrichum gloeosporioides inoculation in Fragaria x ananassa. PeerJ 2022, 10, e12979. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, H.; He, S.; Zhang, P.; Ma, X. Genome-wide identification and characterization of the TLP gene family in Phyllostachys edulis and association with witches’ broom disease resistance in bamboo. Int. J. Mol. Sci. 2023, 24, 10257. [Google Scholar] [CrossRef]
- Cao, J.; Lv, Y.; Hou, Z.; Li, X.; Ding, L. Expansion and evolution of thaumatin-like protein (TLP) gene family in six plants. Plant Growth Regul. 2015, 79, 299–307. [Google Scholar] [CrossRef]
- Li, P.; Xu, Y.; Wang, K.; Guo, W.; Gu, Y.; Lyu, S.; Huang, J.; Lin, H.; Huang, C.; Xu, Z.; et al. Genome-wide identification of TLP gene family and their roles in Carya cathayensis sarg in response to Botryosphaeria dothidea. Front. Plant Sci. 2022, 13, 849043. [Google Scholar] [CrossRef]
- Camejo, D.; Guzman-Cedeno, A.; Moreno, A. Reactive oxygen species, essential molecules, during plant-pathogen interactions. Plant Physiol. Biochem. 2016, 103, 10–23. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).