Increasing γ-Aminobutyric Acid Content in Dwarf Cherry Tomato Using CRISPR/Cas9-Mediated Gene Editing
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Molecular Cloning
2.3. Tomato Transformation
2.4. Identification of Target Mutations
2.5. Off-Target Analysis
2.6. Determination of GABA and Other Free Amino Acid Content
2.7. Determination of Soluble Solid Content (SSC)
2.8. Statistical Analysis
3. Results
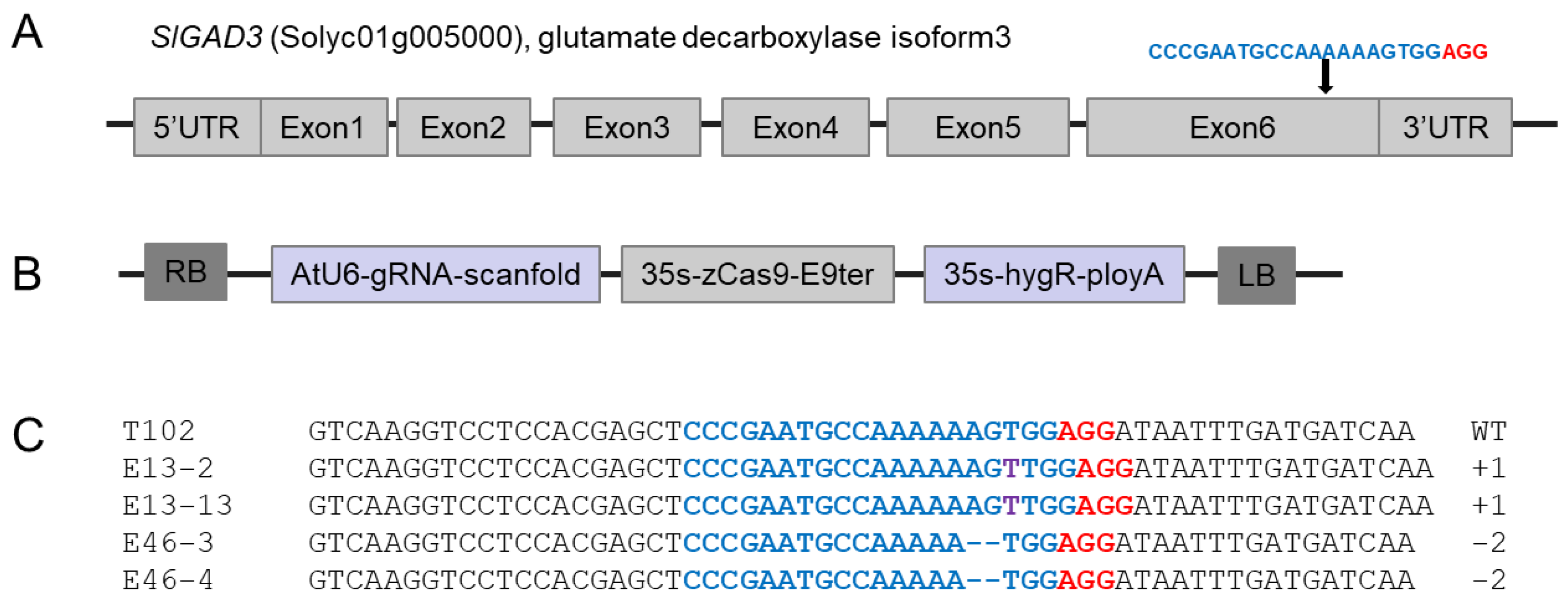
3.1. CRISPR/Cas9-Mediated Mutation of SlGAD3 in Tomatoes
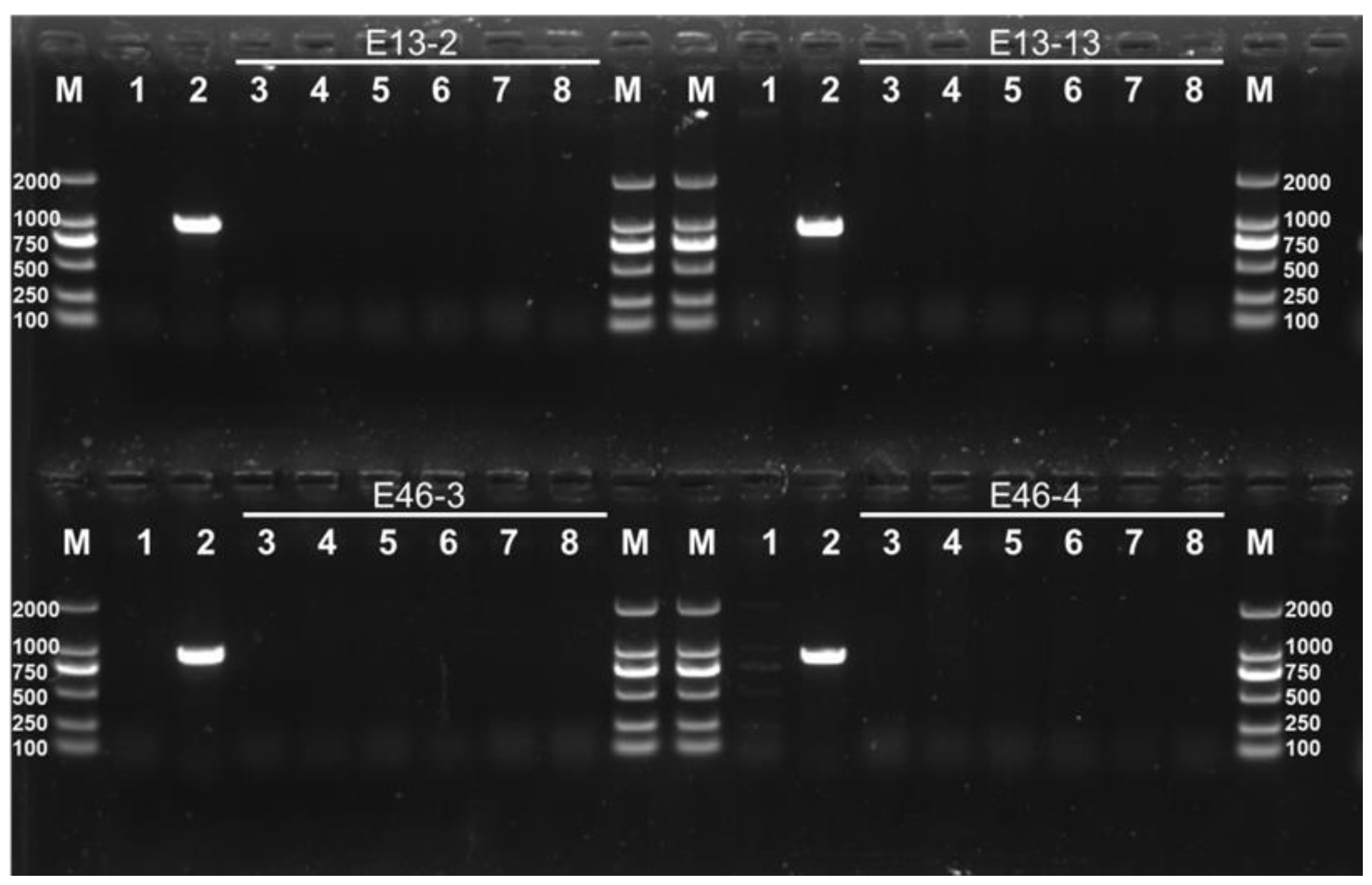
3.2. Stable Inheritance of Edited Gene
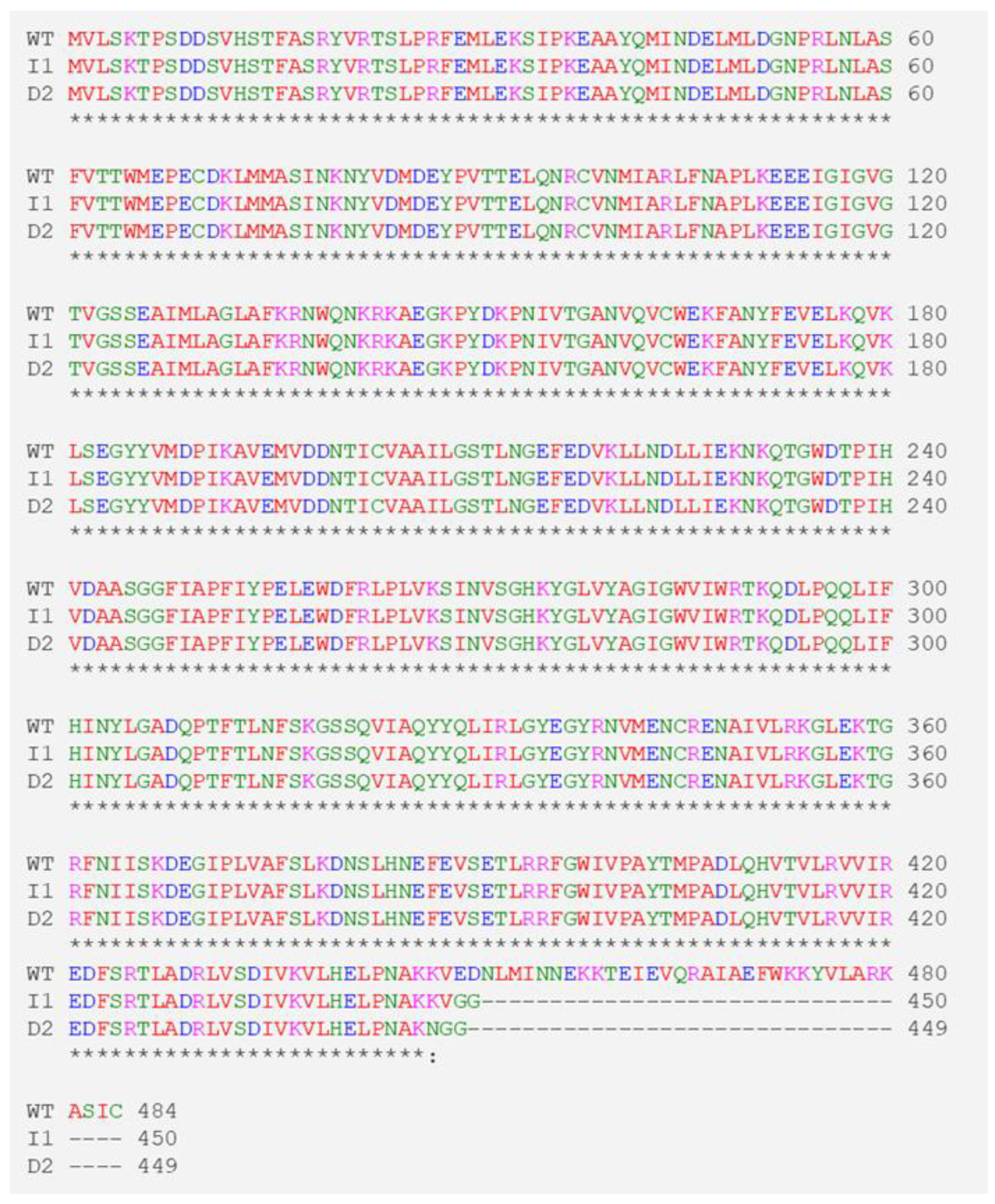
3.3. No Off-Target Site Edits Detected
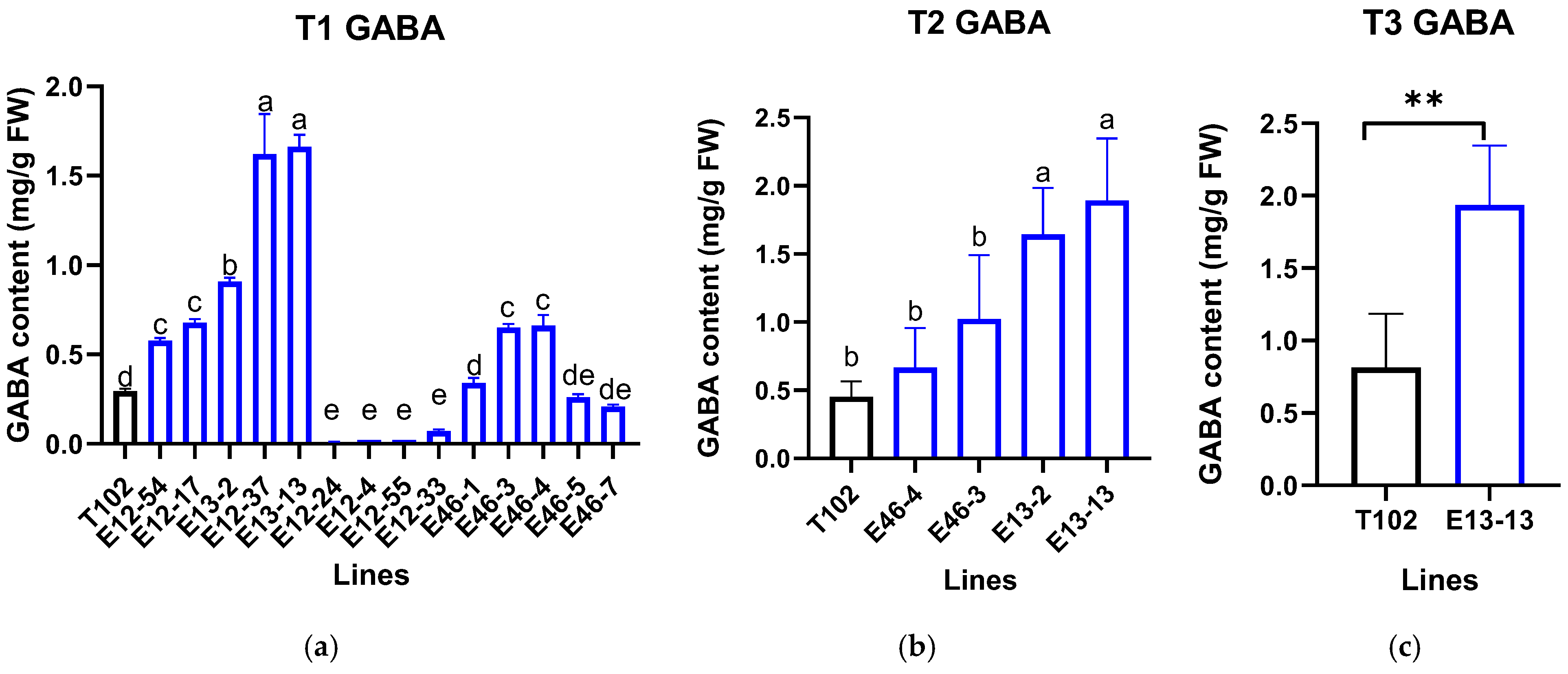
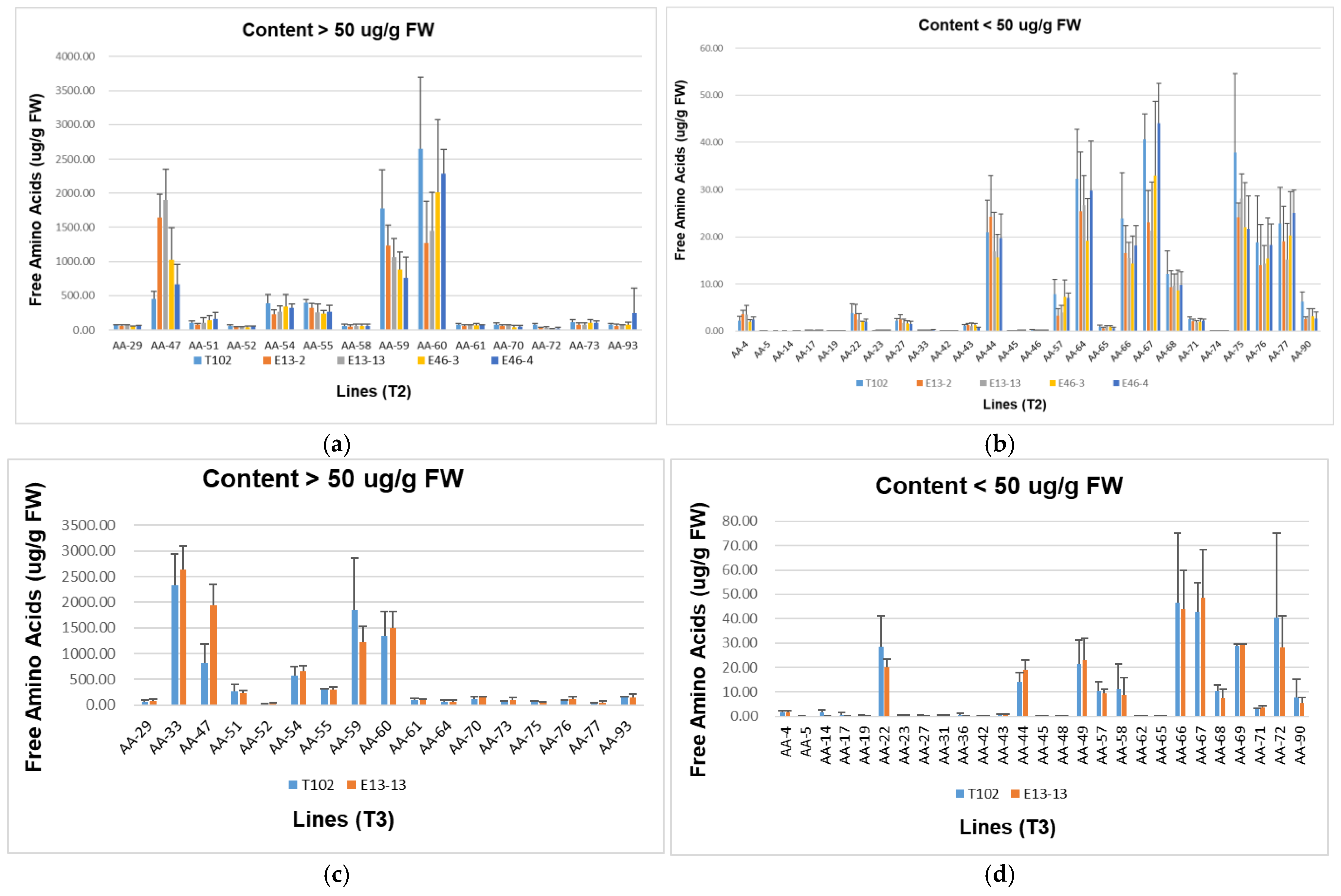
3.4. Content of GABA and Other Free Amino Acids
3.5. Analysis of the Plant Height, the Fruit Weight, and SSC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| M519 | MS basal medium with vitamin |
| IAA | Heteroauxin |
| TZT | Trans-zeaxanthin |
| IBA | Hormodin |
References
- Takahashi, H.; Tiba, M.; Iino, M.; Takayasu, T. The effect of gamma-aminobutyric acid on blood pressure. Jpn. J. Physiol. 1955, 5, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Siucinska, E. ɣ-Aminobutyric acid in adult brain: An update. Behav. Brain Res. 2019, 376, 112224. [Google Scholar] [CrossRef] [PubMed]
- Hepsomali, P.; Groeger, J.A.; Nishihira, J.; Scholey, A. Effects of oral gamma-aminobutyric acid (GABA) administration on stress and sleep in humans: A systematic review. Front. Neurosci. 2020, 14, 923. [Google Scholar] [CrossRef]
- Everlien, I.; Yen, T.Y.; Liu, Y.C.; Di Marco, B.; Vázquez-Marín, J.; Centanin, L.; Alfonso, J.; Monyer, H. Diazepam binding inhibitor governs neurogenesis of excitatory and inhibitory neurons during embryonic development via GABA signaling. Neuron 2022, 110, 3139–3153. [Google Scholar] [CrossRef]
- Diez-Gutiérrez, L.; San Vicente, L.; Barrón, L.J.R.; Villarán, M.d.C.; Chávarri, M. Gamma-aminobutyric acid and probiotics: Multiple health benefits and their future in the global functional food and nutraceuticals market. J. Funct. Foods 2020, 64, 103669. [Google Scholar] [CrossRef]
- Sahab, N.R.M.; Subroto, E.; Balia, R.L.; Utama, G.L. γ-Aminobutyric acid found in fermented foods and beverages: Current trends. Heliyon 2020, 16, e05526. [Google Scholar] [CrossRef]
- Yee, C.S.; Ilham, Z.; Cheng, A.; Rahim, M.H.A.; Hajar-Azhari, S.; Yuswan, M.H.; Zaini, N.A.M.; Reale, A.; Renzo, T.D.; Wan-Mohtar, W.A.A.Q.I. Optimisation of fermentation conditions for the production of gamma-aminobutyric acid (GABA)-rich soy sauce. Heliyon 2024, 10, e33147. [Google Scholar] [CrossRef]
- Nakamura, U.; Nohmi, T.; Sagane, R.; Hai, J.; Ohbayashi, K.; Miyazaki, M.; Yamatsu, A.; Kim, M.; Iwasaki, Y. Dietary Gamma-Aminobutyric Acid (GABA) Induces Satiation by Enhancing the Postprandial Activation of Vagal Afferent Nerves. Nutrients 2022, 14, 2492. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, C.; Yang, J.; Wei, J.; Wu, J. Effect of Different Cooking Methods on the Antioxidant Capacity and Related Antioxidant of Common Vegetables. J. Food Sci. Biotech. 2008, 27, 50–56, (In Chinese with English Abstract). [Google Scholar]
- Gramazio, P.; Takayama, M.; Ezura, H. Challenges and Prospects of New Plant Breeding Techniques for GABA Improvement in Crops: Tomato as an Example. Front. Plant Sci. 2020, 11, 577980. [Google Scholar] [CrossRef]
- Takayama, M.; Ezura, H. How and why does tomato accumulate a large amount of GABA in the fruit. Front. Plant Sci. 2015, 6, 612. [Google Scholar] [CrossRef]
- Akihiro, T.; Koike, S.; Tani, R.; Tominaga, T.; Watanabe, S.; Iijima, Y.; Aoki, K.; Shibata, D.; Ashihara, H.; Matsukura, C.; et al. Biochemical mechanism on GABA accumulation during fruit development in tomato. Plant Cell Physiol. 2008, 9, 1378–1379. [Google Scholar] [CrossRef]
- Koike, S.; Matsukura, C.; Takayama, M.; Asamizu, E.; Ezura, H. Suppression of ɣ-Aminobutyric Acid (GABA) Transaminases Induces Prominent GABA Accumulation, Dwarfism and Infertility in the Tomato (Solanum lycopersicum L.). Plant Cell Physiol. 2013, 54, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Takayama, M.; Koike, S.; Kusano, M.; Matsukura, C.; Saito, K.; Ariizumi, T.; Ezura, H. Tomato glutamate decarboxylase genes SlGAD2 and SlGAD3 play key roles in regulating ɣ -aminobutyric acid levels in tomato (Solanum lycopersicum). Plant Cell Physiol. 2015, 56, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Takayama, M.; Matsukura, C.; Ariizumi, T.; Ezura, H. Activating glutamate decarboxylase activity by removing the autoinhibitory domain leads to hyper ɣ -aminobutyric acid (GABA) accumulation in tomato fruit. Plant Cell Rep. 2017, 36, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Cao, B.L.; Wei, J.W.; Gong, B. Redesigning a-nitrosylated pyruvate-dependent GABA transaminase 1 to generate high-malate and saline-alkali-tolerant tomato. New Phytol. 2024, 242, 2148–2162. [Google Scholar] [CrossRef]
- Nonaka, S.; Aral, C.; Takayama, M.; Matsukura, C.; Ezura, H. Efficient increase of ɣ-aminobutyric acid (GABA) content in tomato fruits by targeted mutagenesis. Sci. Rep. 2017, 7, 7057. [Google Scholar] [CrossRef]
- Li, R.; Li, R.; Li, X.; Fu, D.; Zhu, B.; Tian, H.; Luo, Y.; Zhu, H. Multiplexed CRISPR/Cas9-mediated metabolic engineering of ɣ -aminobutyric acid levels in Solanum lycopersicum. Plant Biotechnol. J. 2018, 16, 415–427. [Google Scholar] [CrossRef]
- Lee, J.; Nonaka, S.; Takayama, M.; Ezura, H. Utilization of a Genome-Edited Tomato (Solanum lycopersicum) with High Gamma Aminobutyric Acid Content in Hybrid Breeding. J. Agric. Food Chem. 2018, 66, 963–971. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, S.; Niu, Q.; Li, Y.; Niu, X.; Wang, P.; Zhu, J.-K.; Lang, Z. Targeted mutagenesis of SlGAD3 generates very high levels of GABA in commercial tomato cultivars. aBIOTECH 2025. [Google Scholar] [CrossRef]
- Xing, H.-L.; Dong, L.; Wang, Z.-P.; Zhang, H.-Y.; Han, C.-Y.; Liu, B.; Wang, X.-C.; Chen, Q.-J. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. Plant Biol. 2014, 14, 327. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Liu, X.; Pan, F.; Hu, J.; Han, Y.; Bi, R.; Zhang, C.; Liu, Y.; Wang, Y.; Liang, Z.; et al. Dissection of major QTLs and candidate genes for seedling stage salt/drought tolerance in tomato. BMC Genom. 2024, 25, 1170. [Google Scholar] [CrossRef]
- Cui, L.; Zheng, F.; Zhang, D.; Li, C.; Li, M.; Ye, J.; Zhang, Y.; Wang, T.; Ouyang, B.; Hong, Z.; et al. Tomato methionine sulfoxide reductase B2 functions in drought tolerance by promoting ROS scavenging and chlorophyll accumulation through interaction with Catalase 2 and RBCS3B. Plant Sci. 2022, 318, 111206. [Google Scholar] [CrossRef]
- Fu, X.; Li, X.; Ali, M.; Zhao, X.; Min, D.; Liu, J.; Li, F.; Zhang, X. Methionine sulfoxide reductase B5 plays vital roles in tomato defense response against Botrytis cinerea induced by methyl jasmonate. Postharvest Biol. Tec. 2023, 196, 112165. [Google Scholar] [CrossRef]
- Tarrago, L.; Laugier, E.; Rey, P. Protein-repairing Mehionine Sulfoxide Reductases in Photosynthetic Organisms: Gene Organization, Reduction Mechanisms, and Physiological Roles. Mol. Plant 2009, 2, 202–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Wang, J.; Ma, F.; Wang, L.; Zhan, X.; Li, G.; Hu, S.; Khan, A.; Dang, H.; et al. Promoting gamma-aminibutyric acid accumulation to enhances saline-alkali tolerance in tomato. Plant Physiol. 2024, 196, 2089–2104. [Google Scholar] [CrossRef] [PubMed]

| T0 Lines | Target Mutation |
|---|---|
| E5 | 95% + 1 bp, 5%WT |
| E10 | 39.8%WT, 25.5% + 1 bp, 15.9% − 16 bp, 18.8% − 77 bp |
| E11 | 24.8%WT, 42.4% − 11 bp, 32.8% − 70 bp |
| E12 | 52.5%WT, 28.2% + 1 bp, 19.2% − 77 bp |
| E13 | 100% + 1 bp |
| E17 | 30.9% + 1 bp, 28.8% − 1 bp, 27.8% − 4 bp, 12.6%WT |
| E19 | 8.7%WT, 17.7% + 15 bp, 20.6% − 11 bp, 16% − 12 bp, 37.1% − 70 bp |
| E34 | 55.5% + 1 bp, 20% − 3 bp, 24.4%WT |
| E36 | 86.5% + 1 bp, 13.5%WT |
| E39 | 46.3% + 1 bp, 53.7% − 5 bp |
| E41 | 49.7% + 1 bp, 24.1% − 1 bp, 26.2% − 2 bp |
| E46 | 100% − 2 bp |
| E48 | 62.5%WT, 17.3% + 1 bp, 20.2% − 1 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, D.; Meng, Z.; Kong, F.; Gao, N.; Liu, K.; Deng, Y.; Zong, L.; Zhang, X.; Han, Z. Increasing γ-Aminobutyric Acid Content in Dwarf Cherry Tomato Using CRISPR/Cas9-Mediated Gene Editing. Horticulturae 2025, 11, 1423. https://doi.org/10.3390/horticulturae11121423
Yu D, Meng Z, Kong F, Gao N, Liu K, Deng Y, Zong L, Zhang X, Han Z. Increasing γ-Aminobutyric Acid Content in Dwarf Cherry Tomato Using CRISPR/Cas9-Mediated Gene Editing. Horticulturae. 2025; 11(12):1423. https://doi.org/10.3390/horticulturae11121423
Chicago/Turabian StyleYu, Danna, Zhiqiang Meng, Fanhui Kong, Ning Gao, Kanglong Liu, Yun Deng, Li Zong, Xingping Zhang, and Zhiguo Han. 2025. "Increasing γ-Aminobutyric Acid Content in Dwarf Cherry Tomato Using CRISPR/Cas9-Mediated Gene Editing" Horticulturae 11, no. 12: 1423. https://doi.org/10.3390/horticulturae11121423
APA StyleYu, D., Meng, Z., Kong, F., Gao, N., Liu, K., Deng, Y., Zong, L., Zhang, X., & Han, Z. (2025). Increasing γ-Aminobutyric Acid Content in Dwarf Cherry Tomato Using CRISPR/Cas9-Mediated Gene Editing. Horticulturae, 11(12), 1423. https://doi.org/10.3390/horticulturae11121423






