Enhancing In Vitro Multiplication and Acclimatization of Blackberry (Rubus L.) Through Sterilization Optimizing and Growth Regulator Use
Abstract
1. Introduction
2. Materials and Methods
2.1. Optimization of Sterilization Method for Plant Explants
2.1.1. Plant Material and Explant Source
2.1.2. Surface Sterilization Protocols
2.2. Optimization of the Composition of the Nutrient Medium for Blackberry Culture Initiation
Culture Media and Growth Regulators
- -
- Medium 1: MS + 0.1 mg/L BAP;
- -
- Medium 2: MS + 0.5 mg/L BAP + 0.1 mg/L α-naphthaleneacetic acid (NAA) (Phyto Tech Labs, Lenexa, KA, USA);
- -
- Medium 3: MS + 1 mg/L BAP + 0.5 mg/L Kin.
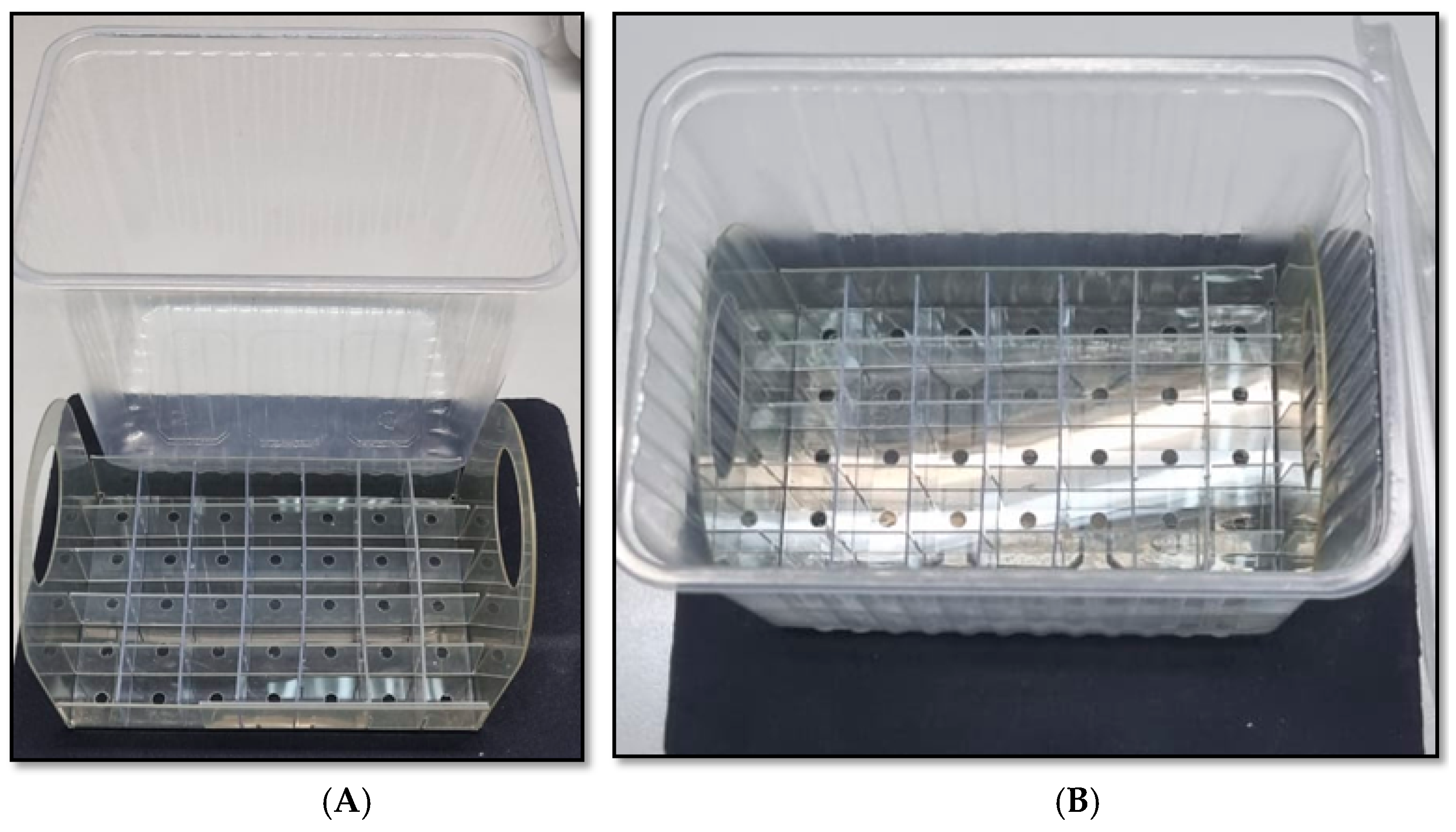
2.3. Rooting and Ex Vitro Acclimatization of Regenerated Plants in Experimental Agroboxes
2.4. Virus Detection by PCR
2.5. Experimental Design and Statistical Analysis
3. Results
3.1. Optimization of the Method of Sterilization of Plant Explants
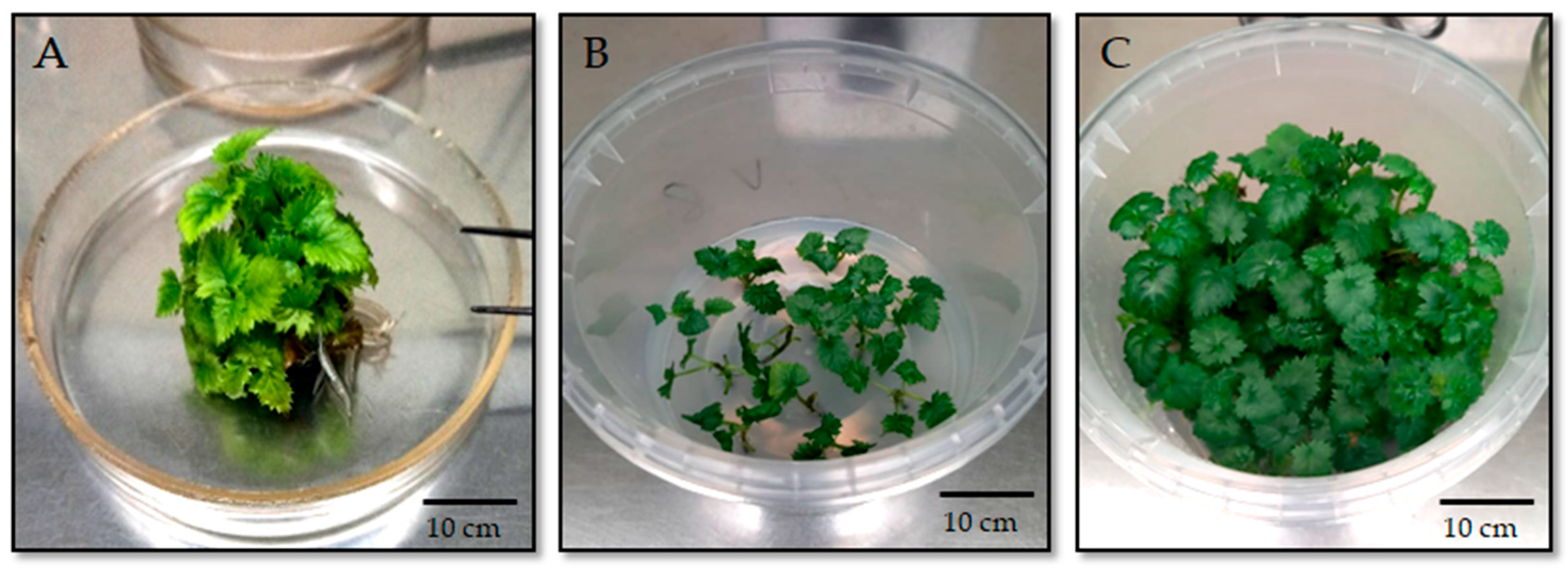
3.2. Optimization of Nutrient Medium Composition for Blackberry Introduction
3.3. Optimization of Nutrient Medium Composition for Shoot Multiplication
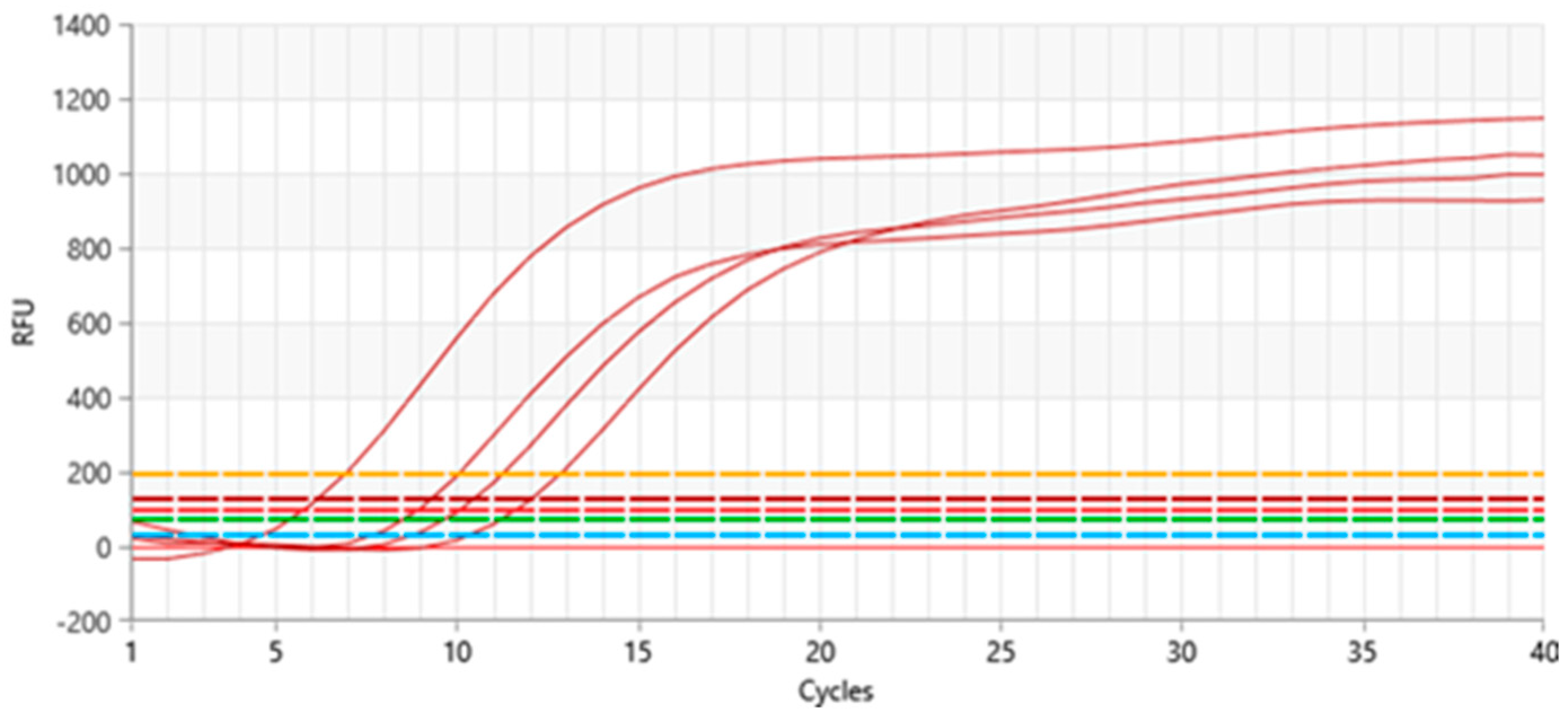
3.4. Virus Diagnostics of Donor and Micropropagated Plants
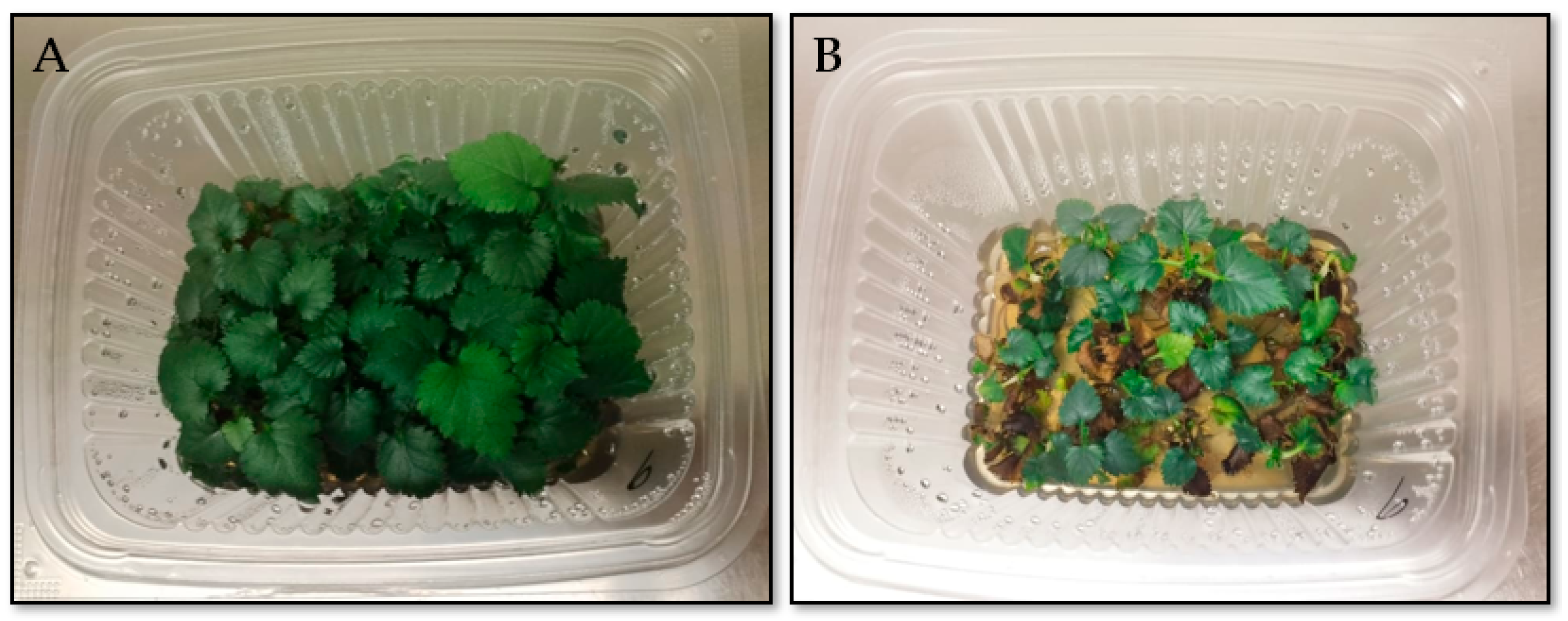
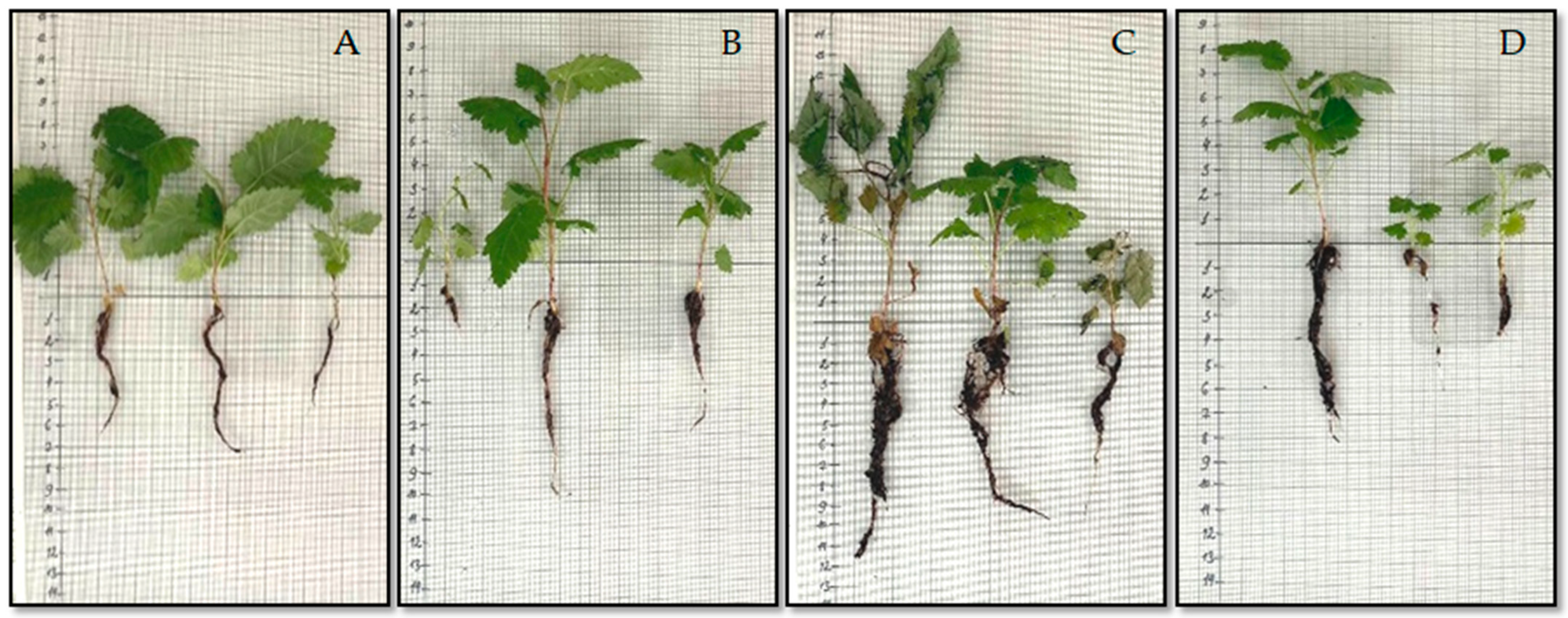
3.5. Adaptation of Regenerated Blackberry Plants Under In Vitro—Ex Vitro Conditions in Experimental Agroboxes
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dolgih, S.G.; Kabylbekova, B.J. Prospects for the production of certified planting material of fruit crops in Kazakhstan. Izdenister Natigeler 2023, 2, 133–143. [Google Scholar] [CrossRef]
- Hummer, K.E.; Janick, J. Rosaceae: Taxonomy, economic importance, genomics. In Genetics and Genomics of Rosaceae; Folta, K.M., Gardiner, S.E., Eds.; Springer: New York, NY, USA, 2009; pp. 1–17. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plantderived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef] [PubMed]
- Zia-Ul-Haq, M.; Riaz, M.; De Feo, V.; Jaafar, H.Z.E.; Moga, M. Rubus fruticosus L.: Constituents, Biological Activities and Health Related Uses. Molecules 2014, 19, 10998–11029. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Hancock, J.F.; Luby, J.J.; Beaudry, R. Fruits of temperate climates/Fruits of the Ericaceae. In Encyclopedia of Food Science, Food Technology and Nutrition, 2nd ed.; Trugo, L., Finglas, P.M., Caballero, B., Eds.; Academic Press: London, UK, 2003; pp. 2762–2768. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef]
- Seeram, N.P. Emerging research supporting the positive effects of berries on human health and disease prevention. J. Agric. Food Chem. 2012, 60, 5685–5686. [Google Scholar] [CrossRef]
- Haytowitz, D.B.; Pehrsson, P.R. USDA’s National Food and Nutrient Analysis Program (NFNAP) produces high-quality data for USDA food composition databases: Two decades of collaboration. Food Chem. 2018, 238, 134–138. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Heinonen, I.M.; Meyer, A.S.; Frankel, E.N. Antioxidant activity of berry phenolics on human low-density lipoprotein and liposome oxidation. J. Agric. Food Chem. 2008, 46, 4107–4112. [Google Scholar] [CrossRef]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Hellstrom, J.K.; Torronen, A.R.; Mattila, P.H. Proanthocyanidins in common food products of plant origin. J. Agric. Food Chem. 2009, 57, 7899–7906. [Google Scholar] [CrossRef]
- Du, X.; Finn, C.; Qian, M.C. Distribution of volatile composition in ‘Marion’(Rubus species hyb) blackberry pedigree. J. Agric. Food Chem. 2010, 58, 1860–1869. [Google Scholar] [CrossRef]
- Winter, J.M.; Tang, Y. Synthetic biological approaches to natural product biosynthesis. Curr. Opin. Biotechnol. 2012, 23, 736–743. [Google Scholar] [CrossRef]
- Reyes-Carmona, J.; Yousef, G.G.; Martínez- Peniche, R.A.; Lila, M.A. Antioxidant capacity of fruit extracts of blackberry (Rubus sp.) produced in different climatic regions. J. Food Sci. 2005, 70, s497–s503. [Google Scholar] [CrossRef]
- Strik, B.C.; Clark, J.R.; Finn, C.E.; Bañados, M.P. Worldwide Blackberry Production. HortTechnology 2007, 17, 205–213. [Google Scholar] [CrossRef]
- Kabdrakhmanova, S.K.; Kabdrakhmanova, A.K.; Shaimardan, E.; Akatan, K.; Beisebekov, M.M.; Selenova, B.S.; Aubakirova, R.A.; Maussumbayeva, A.; Thomas, S.; Seilkhanov, T.M. Growth Stimulating and Fungicidal Properties of Succinic Acid Complexes with Silver, Copper and Boron Ions During Pre-Sowing Treatment of Soybean Seeds. Eng. Sci. 2023, 26, 973. [Google Scholar] [CrossRef]
- Nyzhnyk, T.; Kiedrzyński, M.; Kiedrzyńska, E.; Kots, S. Salicylic and succinic acids as inducers of phytoimmunity in winter wheat for the management of powdery mildew (Blumeria graminis (DC) Speer f. sp. tritici). BMC Plant Biol. 2025, 25, 376. [Google Scholar] [CrossRef]
- Kaume, L.; Howard, L.R.; Devareddy, L. The blackberry fruit: A review on its composition and chemistry, metabolism and bioavailability, and health benefits. J. Agric. Food Chem. 2012, 60, 5716–5727. [Google Scholar] [CrossRef]
- Takeda, F.; Peterson, D.L. Considerations for machine-harvesting eastern thorn less blackberries for fresh market: Trellis designs, cane training system, and mechanical harvester development. HortTechnology 1999, 9, 16–22. [Google Scholar] [CrossRef]
- Dzhangaliev, A.D.; Mukanova, G.S.; Salova, N.T. Wild fruit plants of Kazakhstan and food security of the country. Rep. Natl. Acad. Sci. Repub. Kazakhstan 2008, 3, 5–10. [Google Scholar]
- Kukusheva, A.N. Practical Training in Fruit Growing in Northern Kazakhstan: A Textbook for Agronomic Specialties of Higher Educational Institutions; Kereku: Pavlodar, Kazakhstan, 2016; p. 124. [Google Scholar]
- Martins, M.S.; Gonçalves, A.C.; Alves, G.; Silva, L.R. Blackberries and Mulberries: Berries with Significant Health-Promoting Properties. Int. J. Mol. Sci. 2023, 24, 12024. [Google Scholar] [CrossRef]
- Čechovičienė, I.; Šlepetienė, A.; Gumbytė, M.; Paulauskienė, A.; Tarasevičienė, Ž. Composition and Physicochemical Properties of Pomace of Various Cultivars of Blackberry (Rubus fruticosus L.). Horticulturae 2024, 10, 38. [Google Scholar] [CrossRef]
- Yilmaz, K.U.; Zengin, Y.; Ercisli, S.; Serce, S.; Gunduz, K.; Sengul, M.; Asma, B.M. Some selected physico-chemical characteristics of wild and cultivated blackberry fruits (Rubus fruticosus L.) from Turkey. Rom. Biotechnol. Lett. 2009, 14, 4152–4163. [Google Scholar]
- Milošević, T.; Milošević, N.; Glišić, I.; Mladenović, J. Fruit quality attributes of blackberry grown under limited environmental conditions. Plant Soil Environ. 2012, 58, 322–327. [Google Scholar] [CrossRef]
- Strik, B.C.; Clark, J.R.; Finn, C.E.; Bañados, M.P. Worldwide Production of Blackberries. Acta Hortic. 2008, 777, 209–218. [Google Scholar] [CrossRef]
- Ark, J.R.; Strik, B.; Thompson, A.E.; Finn, C.E. Progress and Challenges in Primocane-Fruiting Blackberry Breeding and Cultural Management. Acta Hortic. 2012, 926, 387–392. [Google Scholar] [CrossRef]
- Carvalho, T.; Thomsen, M.R.; Clark, J.R. Commercial Fresh Blackberry Shipping Market Growth And Price Trends in The United States. Fruit News 2010, 10, 8–10. [Google Scholar]
- WITS. Kazakhstan Raspberries, Blackberries...etc, Frozen Imports by Country in 2023. Available online: https://wits.worldbank.org/trade/comtrade/en/country/KAZ/year/2023/tradeflow/Imports/partner/ALL/product/081120 (accessed on 7 August 2025).
- Dziedzic, E.; Jagła, J. Micropropagation of Rubus and Ribes spp. Methods Mol. Biol. 2013, 11013, 149–160. [Google Scholar] [CrossRef]
- Hunkova, J.; Libiakova, G.; Gajdosova, A. Shoot proliferation ability of selected cultivars of Rubus spp. as influenced by genotype and cytokinin concentration. J. Cent. Eur. Agric. 2016, 17, 379–390. [Google Scholar] [CrossRef]
- Reed, B.M.; Poothong, S.; Hall, H. Propagation of Blackberries and Related Rubus Species. In Blackberries and Their Hybrids; CABI: Wallingford, UK, 2017; pp. 101–112. Available online: https://www.cabidigitallibrary.org/doi/abs/10.1079/9781780646688.0101 (accessed on 10 November 2025).
- Gomes, H.T.; Bartos, P.M.C.; Andrade, M.T.D.; Almeida, R.F.; Lacerda, L.F.D.; Scherwinski-Pereira, J.E. In Vitro Conservation of Blackberry Genotypes under Minimal Growth Conditions and Subsequent Large-Scale Micropropagation. Pesqui. Agropecuária Bras. 2017, 52, 1286–1290. [Google Scholar] [CrossRef]
- Bobrowski, V.L.; Mello-Farias, P.; Petters, J. Micropropagation of Blackberries (Rubus Sp.) cultivars. Curr. Agric. Sci. Technol. 1996, 2, 17–20. Available online: https://periodicos.ufpel.edu.br/index.php/CAST/article/view/146 (accessed on 10 November 2025).
- Taji, A.; Prakash, N.; Lakshmanan, P. In Vitro Plant Breeding; CRC Press: Boca Raton, FL, USA, 2002; p. 182. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Al-Ali, A.M.; Rihan, H.Z.; Alshahrani, T.; Alwahibi, M.S.; Almutairi, K.F.; Naidoo, Y.; Fuller, M.P. Effects of Artificial Light Spectra and Sucrose on the Leaf Pigments, Growth, and Rooting of Blackberry (Rubus fruticosus) Microshoots. Agronomy 2023, 13, 89. [Google Scholar] [CrossRef]
- Viswanath, M.; Ravindra Kumar, K.; Chetanchidambar, N.M.; Mahesh, S.S.N.M. Regeneration mechanisms in plant tissue culture: A review. J. Pharm. Innov. 2023, 12, 2948–2952. [Google Scholar]
- Fathy, H.M.; Abou El-Leel, O.F.; Amin, M.A. Micropropagation and biomass production of Rubus fruticosus L. (blackberry) plant. Middle East J. Appl. Sci. 2018, 8, 1215–1228. [Google Scholar]
- Singh, J.; Kumar, A. Plant tissue culture and its application in agriculture as biotechnological tool. Int. J. Curr. Microbiol. Appl. Sci. 2020, 11, 274–284. [Google Scholar]
- Kefayeti, S.; Kafkas, E.; Ercisli, S. Micropropagation of ‘Chester thornless’ Blackberry Cultivar using Axillary Bud Explants. Not. Bot. Horti Agrobot. 2019, 47, 162–168. [Google Scholar] [CrossRef]
- Clapa, D.; Hârța, M.; Szabo, K.; Teleky, B.E.; Pamfil, D. The Use of Wheat Starch as Gelling Agent for In Vitro Proliferation of Blackberry (Rubus fruticosus L.) Cultivars and the Evaluation of Genetic Fidelity after Repeated Subcultures. Horticulturae 2023, 9, 902. [Google Scholar] [CrossRef]
- García-Gonzáles, R.; Quiroz, K.; Caligari, P.D.S.; Carrasco, B. Plant tissue culture: Current status, opportunities and challenges. Cienc. E Investig. Agrar. 2010, 37, 5–30. [Google Scholar] [CrossRef]
- Ahmed, M.E.S.A.E.N.; Elaziem, A.; Abd Elaziem, T.M. In vitro regeneration and improving kaempferol accumulation in blackberry (Rubus fruticosus L.) callus and suspension cultures. Egypt. J. Chem. 2022, 65, 369–383. [Google Scholar] [CrossRef]
- Samaan, M.S.F.; Nasser, M.A.E. Micropropagation of Blackberry (Rubus fruticosus.) cv. Karaka Black. Egypt. J. Hortic. 2022, 49, 187–198. [Google Scholar] [CrossRef]
- Aly, A.A.; El-Desouky, W.; El-Leel, O.F.A. Micropropagation, phytochemical content and antioxidant activity of gamma-irradiated blackberry (Rubus fruticosus L.) plantlets. Vitr. Cell. Dev. Biol.-Plant 2022, 58, 457–469. [Google Scholar] [CrossRef]
- Da Silva, I.A.O.; Biasi, L. A Double-phase culture medium and plant growth regulators in the micropropagation of blackberries. Comun. Sci. 2022, 13, e3613. [Google Scholar] [CrossRef]
- Kumar, A.; Bhuj, B.D.; Dhar, S.; Dixit, K.M.J.; Singh, S.P. Micropropagation of Fruit Crops: A Review. Adv. Crop. Sci. Tech. 2023, 11, 595. [Google Scholar]
- Hunková, J.; Gajdošová, A.; Szabóová, M. Effect of Mesos Components (MgSO4, CaCl2, KH2PO4) on In Vitro Shoot Growth of Blackberry, Blueberry, and Saskatoon. Plants 2020, 9, 935. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.A.; Canellas, L.P.; Olivares, F.L. Humic Acids Modify Root Architecture in Arabidopsis thaliana through H+-ATPase and TOR Pathways. Chem. Biol. Technol. Agric. 2025, 12, 18. [Google Scholar] [CrossRef]
- Verma, K.K.; Zeng, Y.; Song, X.-P.; Singh, M.; Wu, K.-C.; Rajput, V.D.; Li, Y.-R. Nanosilicon: An Approach for Abiotic Stress Mitigation and Sustainable Agriculture. Front. Plant Sci. 2022, 13, 1025974. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.K.; Song, X.-P.; Singh, M.; Huang, H.-R.; Bhatt, R.; Xu, L.; Kumar, V.; Li, Y.-R. Influence of Nanosilicon on Drought Tolerance in Plants. Front. Plant Sci. 2022, 13, 1014816. [Google Scholar] [CrossRef]
- Kolchenko, M.; Kapytina, A.; Kerimbek, N.; Pozharskiy, A.; Nizamdinova, G.; Khusnitdinova, M.; Taskuzhina, A.; Gritsenko, D. Genetic Characterization of Raspberry Bushy Dwarf Virus Isolated from Red Raspberry in Kazakhstan. Viruses 2023, 15, 975. [Google Scholar] [CrossRef]
- McGavin, W.J.; Mitchell, C.; Cock, P.J.A.; Wright, K.M.; MacFarlane, S.A. Raspberry leaf blotch virus, a putative new member of the genus Emaravirus, encodes a novel genomic RNA. J. Gen. Virol. 2012, 93 Pt 2, 430–437. [Google Scholar] [CrossRef]
- Fan, N.; Zhao, C.; Yue, L.; Ji, H.; Wang, X.; Xiao, Z.; Rasmannc, S.; Wang, Z. Nanosilicon alters oxidative stress and defence reactions in plants: A meta-analysis, mechanism and perspective. Environ. Sci. 2022, 9, 3742–3755. [Google Scholar] [CrossRef]
- Miao, G.; Han, J.; Han, T. Silicon Nanoparticles and Apoplastic Protein Interaction: A Hypothesized Mechanism for Modulating Plant Growth and Immunity. Plants 2025, 14, 1630. [Google Scholar] [CrossRef] [PubMed]
- Seyed Hajizadeh, H.; Azizi, S.; Aghaee, A.; Karakus, S.; Kaya, O. Nano-silicone and Ascophyllum nodosum-based biostimulant down-regulates the negative effect of in vitro induced-salinity in Rosa damascena. BMC Plant Biol. 2023, 23, 560. [Google Scholar] [CrossRef] [PubMed]
- Hasanaklou, N.T.; Mohagheghi, V.; Hasanaklou, H.T.; Ma’mani, L.; Malekmohammadi, M.; Moradi, F.; Dalvand, Y. Seed nano-priming using silica nanoparticles: Effects in seed germination and physiological properties of Stevia Rebaudiana Bertoni. Chem. Biol. Technol. Agric. 2023, 10, 96. [Google Scholar] [CrossRef]
- Eghlima, G.; Mohammadi, M.; Ranjabr, M.E.; Nezamdoost, D.; Mammadov, A. Foliar application of nano-silicon enhances drought tolerance rate of pot marigold (Calendula officinalis L.) by regulation of abscisic acid signaling. BMC Plant Biol. 2024, 24, 1220. [Google Scholar] [CrossRef]
- Martin, R.R.; MacFarlane, S.; Sabanadzovic, S.; Quito, D.; Poudel, B.; Tzanetakis, I.E. Viruses and Virus Diseases of Rubus. Plant Dis. 2013, 97, 168–182. [Google Scholar] [CrossRef]
- Tang, J.; Ng, F.; Kanchiraopally, D.; Ward, L. Development of TaqMan real-time RT-PCR for sensitive detection of diverse Raspberry ringspot virus isolates. J. Virol. Methods 2020, 278, 113821. [Google Scholar] [CrossRef] [PubMed]
- Gong, E.S.; Li, B.; Li, B.; Podio, N.S.; Chen, H.; Li, T.; Sun, X.; Gao, N.; Wu, W.; Yang, T.; et al. Identification of key phenolic compounds responsible for antioxidant activities of free and bound fractions of blackberry varieties’ extracts by boosted regression trees. J. Sci. Food Agric. 2022, 102, 984–994. [Google Scholar] [CrossRef]
- Arozarena, Í.; Ortiz, J.; Hermosín-Gutiérrez, I.; Urretavizcaya, I.; Salvatierra, S.; Córdova, I.; Marín-Arroyo, M.R.; Noriega, M.J.; Navarro, M. Color, ellagitannins, anthocyanins, and antioxidant activity of Andean blackberry (Rubus glaucus Benth.) wines. J. Agric. Food Chem. 2012, 60, 7463–7473. [Google Scholar] [CrossRef]
- Foster, T.M.; Bassil, N.V.; Dossett, M.; Worthington, M.L.; Graham, J. Genetic and genomic resources for Rubus breeding: A roadmap for the future. Hortic. Res. 2019, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Dönmez, B.A.; Polat, Ş.; Hamakhan, A.; Kafkas, E. Methods of Blackberry Propagation In Vitro Condition. BIO Web Conf. 2024, 85, 01009. [Google Scholar] [CrossRef]
- Vujović, T.; Ružić, Đ.; Cerović, R.; Leposavić, A.; Karaklajić-Stajić, Ž.; Mitrović, O.; Žurawicz, E. An Assessment of the Genetic Integrity of Micropropagated Raspberry and Blackberry Plants. Sci. Hortic. 2017, 225, 454–461. [Google Scholar] [CrossRef]
- Rani, V.; Raina, S.N. Genetic Fidelity of Organized Meristem-Derived Micropropagated Plants: A Critical Reappraisal. Vitr. Cell. Dev. Biol.-Plant 2000, 36, 319–330. [Google Scholar] [CrossRef]
- Debnath, S.C.; Vyas, P.; Goyali, J.C.; Igamberdiev, A.U. Morphological and Molecular Analyses in Micropropagated Berry Plants Acclimatized under Ex vitro Condition. Can. J. Plant Sci. 2012, 92, 1065–1073. [Google Scholar] [CrossRef]

| Cultivar | Medium 1, Pcs Total/Survived (Percentage) | Medium 2, Pcs Total/Survived (Percentage) | Medium 3, Pcs Total/Survived (Percentage) |
|---|---|---|---|
| ‘Natchez’ | 50/45 (90% ± 2.5%) a | 50/31 (62% ± 4.0%) b | 50/11 (22% ± 3.3%) c |
| ‘Black Magic’ | 50/45 (90% ± 2.5%) a | 50/23 (46% ± 4.1%) c | 50/12 (24% ± 3.4%) c |
| ‘Osage’ | 50/43 (86% ± 2.9%) a | 50/27 (54% ± 4.1%) b,c | 50/6 (12% ± 2.5%) d |
| ‘Heaven Can Wait’ | 50/42 (84% ± 3.0%) a | 50/20 (40% ± 4.0%) c | 50/8 (16% ± 2.9%) c,d |
| Content and Concentration of Hormones, mg/L | Cultivar | Shoot Height, cm | Number of Shoots, (Per Explant). |
|---|---|---|---|
| BAP 0.5 | ‘Natchez’ | 5.43 ± 0.26 a | 5.8 ± 0.6 a |
| ‘Black Magic’ | 5.30 ± 0.06 a | 5.3 ± 0.2 a | |
| GA3 0.1 | ‘Osage’ | 5.27 ± 0.30 a | 5.7 ± 0.5 a |
| Heaven Can Wait’ | 5.32 ± 0.08 a | 5.5 ± 0.2 a | |
| BAP 0.5 | ‘Natchez’ | 5.36 ± 0.58 a | 5.2 ± 0.4 a |
| ‘Black Magic’ | 4.07 ± 0.12 b | 4.8 ± 0.2 ab | |
| GA3 0.3 | ‘Osage’ | 3.87 ± 0.03 b | 4.9 ± 0.2 ab |
| ‘Heaven Can Wait’ | 3.36 ± 0.09 c | 4.3 ± 0.4 c | |
| BAP 0.5 | ‘Natchez’ | 3.80 ± 0.53 b | 3.9 ± 0.5 bc |
| ‘Black Magic’ | 3.47 ± 0.15 c | 3.4 ± 0.2 c | |
| GA3 0.5 | ‘Osage’ | 3.87 ± 0.03 b | 3.1 ± 0.4 c |
| ‘Heaven Can Wait’ | 3.63 ± 0.14 bc | 3.7 ± 0.5 c | |
| BAP 1.0 | ‘Natchez’ | 3.92 ± 0.04 b | 2.9 ± 0.5 d |
| ‘Black Magic’ | 3.45 ± 0.15 c | 2.5 ± 0.2 d | |
| GA3 0.5 | ‘Osage’ | 3.62 ± 0.03 bc | 2.1 ± 0.4 d |
| ‘Heaven Can Wait’ | 3.76 ± 0.12 b | 2.6 ± 0.2 d |
| Substrate | Cultivar | Shoot Length, cm | Number of Internodes, Pcs | Number of Roots, Pcs | Average Root Length, cm |
|---|---|---|---|---|---|
| Control, H2O | Natchez | 2.37 ± 0.09 f | 6.60 ± 0.29 b | 6.57 ± 0.64 e | 4.60 ± 0.58 b |
| Osage | 2.30 ± 0.06 f | 6.23 ± 0.07 b | 6.27 ± 0.38 e | 5.50 ± 0.45 b | |
| Heaven Can Wait | 2.03 ± 0.12 f | 6.27 ± 0.22 b | 5.53 ± 0.29 f | 5.27 ± 0.30 b | |
| Black Magic | 2.21 ± 0.06 f | 6.45 ± 0.65 b | 6.35 ± 0.41 e | 5.36 ± 0.29 b | |
| Humic acid (0.01%) | Natchez | 5.43 ± 0.26 c | 6.73 ± 0.34 b | 8.30 ± 0.84 d | 6.23 ± 0.43 a |
| Osage | 3.87 ± 0.03 e | 5.43 ± 0.03 c | 12.03 ± 0.13 c | 6.50 ± 0.31 a | |
| Heaven Can Wait | 5.30 ± 0.06 c | 6.40 ± 0.10 b | 11.77 ± 1.4 c | 6.43 ± 0.19 a | |
| Black Magic | 4.65 ± 0.15 d | 6.25 ± 0.18 b | 9.64 ± 0.34 d | 6.35 ± 0.23 a | |
| Nanosilicon (0.01%) | Natchez | 8.47 ± 0.15 a | 7.70 ± 0.26 a | 15.67 ± 0.58 b | 3.83 ± 0.12 c |
| Osage | 8.10 ± 0.15 a | 7.57 ± 0.22 a | 13.70 ± 0.44 c | 3.80 ± 0.53 c | |
| Heaven Can Wait | 7.10 ± 0.15 b | 7.30 ± 0.29 a | 17.67 ± 1.17 f | 4.07 ± 0.12 c | |
| Black Magic | 7.15 ± 0.15 b | 7.21 ± 0.23 a | 14.71 ± 0.21 c | 3.93 ± 0.14 c | |
| Succinic acid (0.1%) | Natchez | 4.60 ± 0.21 d | 3.66 ± 0.45 e | 7.33 ± 0.05 e | 3.40 ± 0.08 d |
| Osage | 4.02 ± 0.16 d | 3.41 ± 0.61 e | 7.20 ± 0.12 e | 2.23 ± 0.41 e | |
| Heaven Can Wait | 4.20 ± 0.18 d | 3.45 ± 0.36 e | 7.35 ± 0.24 e | 3.31 ± 0.23 d | |
| Black Magic | 3.61 ± 0.09 e | 3.30 ± 0.34 e | 7.52 ± 0.11 e | 3.45 ± 0.24 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malakhova, N.; Tezekbayeva, B.; Kiyan, V.; Yefremova, Y. Enhancing In Vitro Multiplication and Acclimatization of Blackberry (Rubus L.) Through Sterilization Optimizing and Growth Regulator Use. Horticulturae 2025, 11, 1422. https://doi.org/10.3390/horticulturae11121422
Malakhova N, Tezekbayeva B, Kiyan V, Yefremova Y. Enhancing In Vitro Multiplication and Acclimatization of Blackberry (Rubus L.) Through Sterilization Optimizing and Growth Regulator Use. Horticulturae. 2025; 11(12):1422. https://doi.org/10.3390/horticulturae11121422
Chicago/Turabian StyleMalakhova, Natalya, Botakoz Tezekbayeva, Vladimir Kiyan, and Yuliya Yefremova. 2025. "Enhancing In Vitro Multiplication and Acclimatization of Blackberry (Rubus L.) Through Sterilization Optimizing and Growth Regulator Use" Horticulturae 11, no. 12: 1422. https://doi.org/10.3390/horticulturae11121422
APA StyleMalakhova, N., Tezekbayeva, B., Kiyan, V., & Yefremova, Y. (2025). Enhancing In Vitro Multiplication and Acclimatization of Blackberry (Rubus L.) Through Sterilization Optimizing and Growth Regulator Use. Horticulturae, 11(12), 1422. https://doi.org/10.3390/horticulturae11121422







