Abstract
Goji berry is a rich source of polyphenols, carotenoids and polysaccharides, contributing to its diverse biological activities. Due to their high water content and perishability, the berries are often processed into dried forms or juices. This study hypothesized that juice obtained from Serbian-grown goji berries would exhibit a distinctive phytochemical composition and significant in vitro antioxidant and hypoglycemic potential. Antioxidant activity was assessed using DPPH, ABTS, CUPRAC, FRAP and β-carotene bleaching assays, while hypoglycemic potential was evaluated via α-amylase and α-glucosidase inhibition. The analyzed goji juice exhibited mild acidity and moderate sweetness. In terms of bioactive composition, the juice contained high levels of polyphenols (194.50 ± 3.88 mg GAE/100 mL) exceeding the values reported for most commercial fruit juices, as well as notable levels of flavonoids (70.30 ± 5.11 mg CE/100 mL), carotenoids (289.53 ± 0.65 µg/100 mL) and polysaccharides (375.20 ± 12.46 mg Glu/100 mL), along with minerals, particularly potassium and copper. It also showed strong antioxidant capacity and concentration-dependent inhibition of α-amylase (IC50 = 5.28 ± 0.26 mg/mL) and α-glucosidase (IC50 = 10.12 ± 0.23 mg/mL). This study provides the first comprehensive characterization of Serbian-grown goji berry juice, confirming its potential as a functional ingredient.
1. Introduction
The genus Lycium (Solanaceae) comprises more than 100 species distributed worldwide []. Among them, Lycium barbarum L., L. chinense Mill., and L. ruthenicum Murr. are the most prominent species, with a long history of use in traditional medicine and human nutrition []. Today, Lycium barbarum is listed in several European and national pharmacopoeias as an official medicinal plant [,]. Lycium barbarum is a perennial woody shrub producing small (1–2 cm), bright red-orange, ellipsoid berries, commonly known as goji berry or wolfberry, containing more than ten seeds. The pulp is soft and juicy, becoming wrinkled when dried, with a flavor ranging from sweet to slightly bitter [,]. While the fruits can be consumed fresh, their high water content makes them highly perishable due to enzymatic and microbial degradation. Consequently, preservation strategies such as drying are widely applied, and the berries are further processed into various products, including teas, juices, yoghurts, and nutraceuticals. China remains the global leader in goji production, with the industry significantly contributing to local economies, particularly in rural areas [,,,]. Nevertheless, despite the growing global interest in goji berries and the expansion of their cultivation beyond Asia, most of available studies have focused on fruits grown in China or other Asian regions []. Consequently, data on the phytochemical composition and biological properties of goji berries cultivated under European conditions remain scarce. To address this gap, the present study provides the first comprehensive assessment of the phytochemical composition, antioxidant capacity, and hypoglycemic potential of goji berry juice produced from fruits cultivated in Serbia.
Goji berries are a notable source of nutrients, including protein, dietary fiber, essential amino acids, and fatty acids. They also contain stable derivatives of ascorbic acid (2-O-β-D-glucopyranosyl-L-ascorbic acid) and a wide range of minerals such as potassium, sodium, phosphorus, calcium, magnesium, and iron [,,]. To date, hundreds of compounds have been identified in Lycium species, with polysaccharides, carotenoids, and phenolic compounds representing the most biologically significant groups. These constituents are associated with diverse health-promoting effects, including antioxidant [], anti-inflammatory, immunomodulatory, cytotoxic [], hypolipidemic, hypoglycemic [] and prebiotic activities [,]. In particular, the inhibition of α-amylase and α-glucosidase represents a promising mechanism in the dietary management of early-stage diabetes, obesity, and metabolic syndrome, supporting their incorporation into functional foods such as teas and bakery products enriched with goji purée [,,]. Since oxidative stress and hyperglycemia are recognized as major contributors to chronic metabolic disorders, evaluating the antioxidant and hypoglycemic properties of goji berry juice provides valuable insight into its potential role in preventive nutrition and diabetes management. Properly balanced nutrition that includes goji berries and their derived products may help prevent the onset of these disorders and reduce the overall burden on the healthcare system [].
The chemical composition and bioactive potential of Lycium barbarum are known to vary considerably depending on plant genotype, pedoclimatic conditions, and cultivation practices. Environmental factors such as temperature fluctuations, soil mineral content, and sunlight exposure strongly influence the accumulation of secondary metabolites, thereby affecting both the nutritional value and biological activity of the fruit [].
Lycium barbarum polysaccharides, with molecular weights ranging from 24 to 241 kDa, are among the most extensively studied compounds. They are composed of monosaccharaides such as arabinose, glucose, galactose, mannose, xylose, and rhamnose, as well as 18 amino acids, and are responsible for various biological activities, including strong antioxidant, immunomodulatory, and metabolic regulatory effects [,,]. Carotenoids are the second most abundant bioactive group responsible for the characteristic red-orange pigmentation of the fruit. Zeaxanthin dipalmitate is the predominant carotenoid, exhibiting potent antioxidant activity as well as protective effects against hepatic and retinal damage [,]. Similarly, phenolic compounds, the largest class of plant secondary metabolites, contribute substantially to the antioxidant potential of goji berries. Flavonoids such as quercetin, kaempferol, and rutin, together with phenolic acids including chlorogenic and caffeic acids, are the major representatives [,].
In recent years, fruit juices have gained recognition as convenient carriers of bioactive compounds with high sensory appeal, representing an alternative to whole fruits. Their consumption is particularly beneficial for children and the elderly, positioning fruit juices as a promising natural source of bioactive compounds []. From this perspective, the present study aimed to investigate the phytochemical composition, and in vitro biological activities of juice obtained from goji berries cultivated in Serbia. The working hypothesis proposed that the specific agro ecological conditions of the Serbian growing region influence the accumulation of bioactive compounds and, consequently, the functional properties of the juice. To test this hypothesis, advanced analytical techniques, including HPLC-DAD, ICP-OES, and spectrophotometric assays, were employed within an integrative experimental design that combined physicochemical, phytochemical, antioxidant, and hypoglycemic assessments.
2. Materials and Methods
2.1. Sample
The goji berry juice was produced from batches of fully ripe Lycium barbarum fruits harvested in August 2022 from a private plantation in Belgrade, Serbia (44°49′14″ N and 20°27′44″ E). Goji plants were grown on well-drained loamy soil enriched with organic compost to improve nutrient content, while irrigation was maintained through a drip system to ensure optimal water availability. The plantation is situated in a continental climate zone characterized by cold winters and hot summers, providing adequate sunlight, precipitation, and temperature during the vegetative period for the development of high-quality fruits. Fully ripe berries were hand-picked and stored in airtight containers in a cool, dark place until processing into juice by direct pressing. According to the Regulation on fruit juices and related products intended for human consumption, the product belongs to the category of cassava fruit juices []. All analyses were conducted on concentrated or diluted juice samples. For evaluation of anti-enzyme activities goji berry juice was air-dried at room temperature (22–25 °C) for 48 h under dark, ventilated conditions.
2.2. Physicochemical Analysis
The pH and total soluble solids (TSS) were measured directly using a pH meter (Wissenschaftlich-Technische Werkstätten, Weilheim, Germany) and a refractometer (HI 96811 Digital Brix Refractometer, Hanna Instruments®, Woonsocket, RI, USA), respectively. Titratable acidity (TA) was determined by titration with 0.1 M NaOH (Merck KGaA, Darmstadt, Germany) and expressed as % citric acid per 100 mL of juice [].
2.3. Determination of Bioactive Compounds
2.3.1. Total Phenolic Content (TPC)
Total phenolic content (TPC) was measured using the Folin–Ciocalteu (Sigma-Aldrich, St. Louis, MO, USA) method following Singleton and Rossi []. Results are expressed as mg gallic acid equivalents (GAE)/100 mL.
2.3.2. Total Flavonoid Content (TFC)
Total flavonoid content (TFC) was determined spectrophotometrically with AlCl3 reagent (Merck KGaA, Darmstadt, Germany) and catechin standard (Sigma-Aldrich, St. Louis, MO, USA) []. Results are expressed as mg catechin equivalents (CE)/100 mL.
2.3.3. Total Carotenoid Content (TCC)
Sample was extracted with acetone: water (4:1, v/v; Merck KGaA, Darmstadt, Germany), and total carotenoid content was quantified spectrophotometrically following Braniša et al. []. Results are expressed as µg/100 mL.
2.3.4. 2-O-β-D-Glucopyranosyl-L-Ascorbic Acid (AA-2βG)
2-O-β-D-glucopyranosyl-L-ascorbic acid (AA-2βG) was quantified by HPLC-DAD (Agilent 1260 Infinity, Agilent Technologies, Santa Clara, CA, USA) according to Trang []. Results are expressed as mg AA-2βG/100 mL.
2.3.5. Total Polysaccharides (TPoly)
Total polysaccharides content (TPoly) was determined by the phenol–sulphuric acid method [], using D-glucose (Sigma-Aldrich, St. Louis, MO, USA) as the standard. Results are expressed as mg Glu/100 mL.
2.4. Mineral Composition Analysis
The mineral content of goji berry juice was determined using inductively coupled plasma optical emission spectrometry (ICP-OES, Thermo Fisher Scientific, Cambridge, UK) after microwave-assisted acid digestion with ultrapure nitric acid (65%) (Merck KGaA, Darmstadt, Germany) and hydrogen peroxide (35%) (Sigma-Aldrich, St. Louis, MO, USA), following a modified protocol []. After digestion, samples were diluted to 50 mL with ultrapure water. Calibration was performed using standard solutions of known concentrations (Merck KGaA, Darmstadt, Germany). Results were reported as the mean of three consecutive measurements, expressed in mg or µg per 100 mL of juice. The nutritional relevance of minerals was evaluated in relation to recommended dietary allowances (RDA) established by EFSA, enabling assessment of the potential contribution of goji juice to daily nutritional requirements.
2.5. Antioxidant Analysis
The antioxidant potential of goji juice was evaluated using complementary assays that reflect different mechanisms of action. These assays provide insight into hydrogen atom transfer (HAT) and single electron transfer (SET) mechanisms, offering a comprehensive view of the juice’s antioxidant capacity.
2.5.1. DPPH Radical Scavenging Assay
The free radical scavenging potential of goji berry juice was evaluated using the DPPH assay according to Norma et al. []. Absorbance was measured at 490 nm, and a standard curve was generated using Trolox (200–700 µM, Sigma-Aldrich, St. Louis, MO, USA). Results are expressed as µM Trolox equivalents (TE) per 100 mL of juice.
2.5.2. FRAP Assay
Total antioxidant capacity was determined using the ferric reducing antioxidant power (FRAP) method []. Trolox standards (100–800 µM, Sigma-Aldrich, St. Louis, MO, USA) were used for calibration, and absorbance was recorded at 630 nm. Antioxidant capacity is reported as µM TE/100 mL of juice.
2.5.3. CUPRAC Assay
Cupric ion reducing antioxidant capacity (CUPRAC) was assessed following the protocol of Apak et al. []. Absorbance was measured at 450 nm, with Trolox standards (Sigma-Aldrich, St. Louis, MO, USA) ranging from 200 to 700 µM for calibration. Results are expressed as mM TE/100 mL of juice.
2.5.4. ABTS Radical Scavenging Assay
The ABTS decolorization assay [] was used to determine the juice’s ability to quench ABTS radicals. Calibration was performed using Trolox (0.2–1.5 mM, Sigma-Aldrich, St. Louis, MO, USA), and absorbance was measured at 630 nm. Results are reported as mM TE/100 mL of juice.
2.5.5. β-Carotene Bleaching Assay
The ability of goji berry juice to inhibit lipid peroxidation was evaluated using the β-carotene bleaching assay []. Absorbance was recorded immediately (t = 0 min) and after 120 min of incubation at 490 nm. Antioxidant activity is expressed as the percentage inhibition of β-carotene degradation.
2.6. Evaluation of Anti-Enzymatic Activity
The inhibitory effects of goji juice extracts were assessed against key carbohydrate hydrolysing enzymes. These assays evaluate the potential of goji bioactive compounds to modulate postprandial glucose metabolism, providing insight into their hypoglycemic potential.
2.6.1. α-Amylase Inhibition
α-Amylase inhibitory activity of dried goji berry juice was assessed using a modified method []. Samples and α-amylase solution (type VI-B, ≥10 units/mg; Sigma-Aldrich, St. Louis, MA, USA) were prepared in phosphate buffer (pH 6.9). After incubation with starch solution (Merck KGaA, Darmstadt, Germany) and treatment with 3,5-dinitrosalicylic acid reagent (Sigma-Aldrich, St. Louis, MA, USA), absorbance was measured at 540 nm. Percentage inhibition was calculated as:
where Ac is the control absorbance and As is the sample absorbance. IC50 values were obtained from inhibition–concentration curves using linear regression. The reference inhibitor, acarbose (Sigma-Aldrich, St. Louis, MO, USA), was analyzed in parallel with the goji berry juice sample under the same assay conditions, and its IC50 values were determined for direct comparison.
% of inhibition = [(Ac − As)/Ac] × 100
2.6.2. α-Glucosidase Inhibition
α-Glucosidase inhibitory activity was determined following a modified protocol []. Diluted samples were incubated with p-nitrophenyl-α-D-glucopyranoside (Sigma-Aldrich, St. Louis, MO, USA) and α-glucosidase enzyme (≥10 units/mg; Sigma-Aldrich, St. Louis, MO, USA) at 37 °C. The reaction was stopped with Na2CO3 (Merck KGaA, Darmstadt, Germany), and absorbance was measured at 405 nm. Percentage inhibition and IC50 values were calculated as described above, with acarbose (Sigma-Aldrich, St. Louis, MO, USA) as the reference standard. The reference inhibitor, acarbose, was analyzed in parallel with the goji berry juice sample under the same assay conditions, and its IC50 values were determined for direct comparison.
2.7. Statistical Analysis
Results are presented as mean values ± standard deviation, based on three independent technical replicates, with a 95% confidence interval. Differences in mineral composition and antioxidant activities of goji juice were evaluated using one-way ANOVA. When significant differences were detected (p < 0.05), Tukey’s Honestly HSD test was applied for post hoc comparisons. Statistical analyses were conducted using SPSS software, version 26.0 (SPSS Inc., Chicago, IL, USA, available at: https://www.ibm.com/spss).
3. Results and Discussion
3.1. Physicochemical Characteristics and Bioactive Compounds Content
Table 1 summarizes the physicochemical characteristics and content of key bioactive compounds in goji berry juice. These parameters, including pH, total soluble solids, total acidity, and major classes of bioactive compounds, provide essential information on the nutritional quality and functional potential of the product.

Table 1.
Physicochemical characteristics and bioactive compounds content of goji berry juice (mean ± SD; n = 3).
The measured pH value of 4.95 places goji berry juice among low-acid juices, which is technologically relevant as it affects microbiological stability and pasteurization requirements. Compared to other fruit juices, the pH of goji juice is higher than that of raspberry, red grape, or apple juice (pH 3.0–3.5) []. A higher pH contributes to a milder taste, enhancing consumer acceptance, especially among those who prefer less acidic beverages. The TSS content of goji berry juice was placing it within the range of medium-sweet fruit juices. This level is lower compared to chokeberry juice [], but comparable to or slightly higher than values reported for raspberry and currant juices []. The TSS content is strongly influenced by the ripening stage of the fruit and cultivation conditions, and represents one of the key parameters determining taste intensity and overall sensory acceptability of the final product. TA of the juice was 0.11% citric acid per 100 mL, confirming its mild acidity. Organic acids are critical for color stability, flavor balance, and the refreshing character of fruit juices. Combining TSS and TA values, the calculated TSS/TA ratio was 84.62, which is generally considered the most reliable indicator of the balance between sweetness and acidity. A high TSS/TA ratio indicates that goji berry juice is distinctly sweet, with only mild acidity, providing a pleasant and palatable flavor. Literature suggests that fruit juices with higher TSS/TA ratios are often associated with greater consumer acceptability and higher commercial potential [].
The analyzed goji juice contained substantial concentrations of total phenolic (194.5 mg GAE/100 mL) and flavonoids (70.3 mg CE/100 mL). The total carotenoid content reached 289.5 ± 0.65 µg/100 mL, while polysaccharides were present in notably high amounts (375.22 mg Glu/100 mL). Comparison with literature data reveals consistency with previously reported values. Boleslawska et al. [] examined commercial goji-based products in Europe and observed total phenolic contents in juices ranging from 100 to 272 mg GAE/100 mL and flavonoid levels between 9 and 18.3 mg quercetin equivalents (QE)/100 mL. The values obtained in the present study fall within this range but indicate a comparatively higher flavonoid content. Similarly, Jumlah et al. reported that total phenolic contents of fresh and commercial fruit juices from apple, grape, guava, mango, pineapple, pomegranate, and orange varied between 13.38 and 130.39 mg GAE/100 mL, substantially lower than those measured in goji berry juice, highlighting its superior polyphenol profile []. The compositional characteristics of Serbian goji berry juice can be partly attributed to the specific agro ecological and climatic conditions in which the plants were cultivated. Polyphenol synthesis is strongly influenced by environmental factors such as light intensity, temperature variation, water availability, and soil composition. Fruits highly exposed to sunlight and UV radiation tend to accumulate greater amounts of polyphenols than those grown in shaded areas, due to enhanced activation of phenylpropanoid pathways []. High temperature amplitudes during fruit development induce physiological stress that stimulates polyphenol biosynthesis, whereas prolonged exposure to elevated temperatures can reduce total phenolic content, while lower temperatures generally promote it. Water availability is another key determinant, as water deficit conditions stimulate secondary metabolite production, including phenolic compounds, as a part of the plant’s stress response. In addition, soil fertility and fertilization regime substantially influence metabolite accumulation. Fruits obtained from soils with lower nitrogen content or under organic cultivation practices, such as the use of compost without pesticides, typically exhibit higher polyphenol levels due to mild nutrient stress that enhances secondary metabolism [].
The carotenoid content observed aligns with the average values for red goji berries []. Moreover, carotenoid accumulation in red Lycium barbarum fruits increased markedly during ripening, emphasizing the role of fruit developmental stage in determining carotenoid levels []. From this perspective, it is important to emphasize that variety and harvest time are significant factors that could influence the carotenoid content in goji berries []. Vitamin C (L-ascorbic acid) is a potent natural antioxidant, but its application is limited by low stability and bioavailability. A naturally occurring derivative, 2-O-β-D-glucopyranosyl-L-ascorbic acid (AA-2βG), present in Lycium fruits, has attracted attention as a more stable alternative with demonstrated antioxidant, anti-aging, and antitumor activities []. The concentration of AA-2βG in goji berry juice is higher than in blueberry (Vaccinium corymbosum L.) juice. The vitamin C content in juice obtained by pressing the pulp ranges from 4.54 to 5.99 mg/100 g []. In terms of polysaccharides, the content in Serbian goji juice was more than twice that reported for tea prepared from black goji berries (150 mg/100 mL) [], confirming its richness in this bioactive fraction.
3.2. Mineral Composition
Mineral composition is an important parameter in assessing the nutritional value of fruit-based products. Goji berry juice contains several essential macro- and microelements, which are particularly relevant to human health. The detailed mineral profile is presented in Table 2.

Table 2.
Mineral composition of goji berry juice (mean ± SD; n = 3).
Among the quantified elements, potassium (K) was the most abundant mineral, followed by phosphorus (P), sulfur (S), sodium (Na), calcium (Ca), and magnesium (Mg). Trace elements such as zinc (Zn), copper (Cu), and iron (Fe) were also detected in notable amounts. These findings are consistent with the results of Llorent-Martínez et al. [], who reported potassium as the predominant mineral in goji juice (187 mg/100 mL), followed by phosphorus, sodium, calcium, and magnesium. The absolute concentrations observed in our sample were higher than those found in other commercial fruit juices such as acai and pomegranate, indicating the superior mineral richness of goji juice. In addition, six commercial fruit juices were found to contain substantially lower concentrations of K, Mg, Zn, and Cu compared to our results—potassium being four to forty times lower, magnesium about two times lower, and zinc and copper up to ten times lower [].
According to EFSA dietary reference values for selected minerals are as follows: potassium—3500 mg/day, phosphorus—550 mg/day, magnesium—375 mg/day, calcium—1000 mg/day, iron—14 mg/day, zinc—10 mg/day, and copper—1 mg/day. In relation to dietary recommendations, an intake of 200 mL of goji berry juice provides more than 15% of the Recommended Daily Allowance (RDA) for potassium and copper, highlighting its value as a valuable dietary source of these essential minerals. To facilitate a clearer nutritional interpretation, the contributions of the analyzed minerals to RDA values were presented graphically in Figure 1.
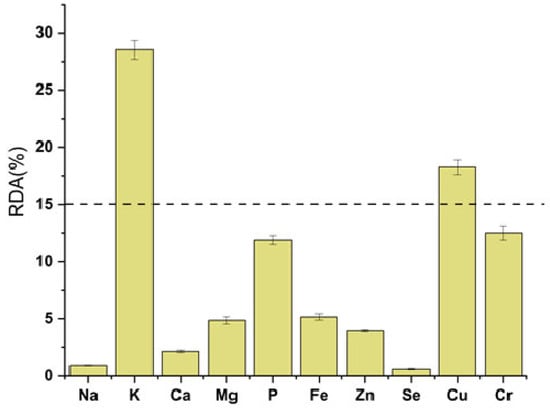
Figure 1.
Contribution of mineral content in 200 mL of goji berry juice to the Recommended Daily Allowance (RDA).
3.3. Antioxidant Properties
Oxidative stress, defined as an imbalance between excessive production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) and the capacity of endogenous antioxidant defenses, is a key factor implicated in the development of chronic conditions such as neurodegenerative disorders, diabetes, and cardiovascular diseases [,]. The protective effects of fruits are strongly linked to their content of bioactive compounds, particularly phenolic compounds, carotenoids, and vitamin C, which exert antioxidant activity through multiple mechanisms. Phenolic compounds primarily contribute through their hydroxyl groups, acting as hydrogen or electron donors, while carotenoids provide protection via singlet oxygen quenching due to their conjugated double bonds. In addition, these constituents help prevent lipid peroxidation and stimulate endogenous antioxidant enzymes, collectively enhancing the overall antioxidant capacity of the fruits [,]. The antioxidant potential of goji berry juice was evaluated using five complementary in vitro assays (Table 3). The juice exhibited a pronounced ability to neutralize free radicals (DPPH, ABTS), strong reducing capacity (FRAP, CUPRAC), and a significant inhibitory effect on lipid peroxidation in the β-carotene bleaching assay.

Table 3.
In vitro antioxidant activity of goji berry juice assessed by various assays (mean ± SD; n = 3).
Specifically, the DPPH radical scavenging activity reached 403.2 µmol Trolox equivalents (TE) per 100 mL, while the ferric reducing antioxidant power (FRAP) was 630.3 µmol TE/100 mL. The obtained values are consistent with those reported by Boleslawska et al., where DPPH radical scavenging activity in commercial goji juices ranged from 135 to 405 µmol TE/100 mL []. However, certain fruit juices, such as guava, have been shown to possess even higher antioxidant capacity. For instance, reported DPPH scavenging activity of 770 µmol TE/100 mL and FRAP values of 843 µmol TE/100 mL for fresh guava juice, exceeding the antioxidant potential of goji berry juice []. Values for the antioxidant activity of goji berries assessed by CUPRAC and ABTS assays may fall within different intervals, depending on the variety and geographical origin of the plant [,].
3.4. Hypoglycemic Potential
Effective regulation of blood glucose levels is fundamental to the prevention and management of type 2 diabetes mellitus, which accounts for nearly 95% of all diabetes cases. One well-established therapeutic strategy involves the inhibition of digestive enzymes such as pancreatic α-amylase and intestinal α-glucosidase. These enzymes catalyze the hydrolysis of oligosaccharides and disaccharides into glucose, and their inhibition delays carbohydrate digestion and absorption, thereby mitigating postprandial hyperglycemia [,,]. A concentration-dependent inhibitory effect of goji berry juice against α-amylase and α-glucosidase was assessed, and the results are presented in Figure 2.
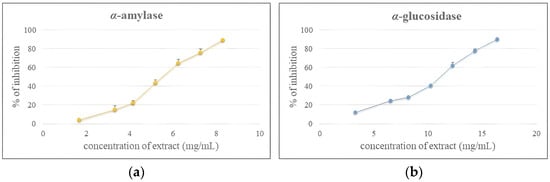
Figure 2.
Concentration-dependent inhibitory activity of goji berry juice extract against α-amylase (a) and α-glucosidase (b).
Although less potent than the pharmaceutical reference acarbose (IC50 = 5.17 μg/mL for α-amylase and 0.11 mg/mL for α-glucosidase), goji berry juice demonstrated inhibitory activity with IC50 values of 5.28 mg/mL and 10.12 mg/mL, respectively, highlighting its potential as a valuable natural source of enzyme inhibitors. Supporting evidence shows that hydroethanolic extracts of L. chinense fruits exhibit inhibitory activity with IC50 values of 33.4 mg/mL for α-amylase and 9.9 mg/mL for α-glucosidase, consistent with the present findings []. Similarly, wide range of fruits possess α-amylase and α-glucosidase inhibitory capacity, further supporting the hypoglycemic potential of goji-based products []. The bioactivity of goji berries in this regard is largely attributed to polysaccharides, which are consistently identified as the predominant metabolites in fruit extracts. These polysaccharides are believed to contribute to enzyme inhibition by steric interference and through direct interactions with the active sites of α-amylase and α-glucosidase []. For instance, various L. barbarum preparations including fruit decoctions, crude polysaccharide extracts, and purified polysaccharide fractions significantly reduced blood glucose levels in alloxan-induced diabetic rabbits, with the purified polysaccharides showing the most pronounced effect []. Compared with other fruit juices, Aronia melanocarpa juice obtained via enzymatic hydrolysis exhibited even stronger inhibition of α-amylase (IC50 = 2.326 mg/mL), suggesting that although goji berry juice demonstrates considerable inhibitory potential, other berry-derived products may display greater hypoglycemic efficacy []. Additionally it is possible to highlight inhibitory activity against α-amylase and α-glucosidase of other products obtained from L. barbarum such as bud and leaf tea, as well as ciabatta bread enriched with goji purée [,].
4. Conclusions
This study sets foundations for future studies which will be related to the investigation of biological activity of specific compounds from goji juice. This study provides the first evidence on the nutritional profile and bioactivity of goji berry (Lycium barbarum L.) juice produced in Serbia, highlighting its richness in polyphenols, carotenoids, polysaccharides, and essential minerals, particularly potassium and copper. The juice exhibited strong antioxidant capacity and notable inhibitory effects on α-amylase and α-glucosidase, emphasizing its potential as a valuable functional food for supporting antioxidant defense and glycemic control. Compared with other fruit juices, Serbian goji juice generally contained higher levels of bioactive compounds, establishing it as a valuable functional food with beneficial health effects. The results are based on in vitro assays conducted on juice obtained from single batch and should be interpreted as preliminary. Future in vivo studies are necessary to confirm the biological mechanisms and validate the potential of Serbian goji juice as a functional dietary component.
Author Contributions
Conceptualization, T.I. and N.K.; methodology, T.I., N.K., M.M. and I.K.; software, U.Č.; data curation, T.I. and N.K.; writing—original draft preparation, T.I., N.K. and I.K.; writing—review and editing, T.I., N.K., U.Č., I.K. and B.V.; visualization, T.I. and U.Č. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science, Technological Development and Innovation, Republic of Serbia through two Grant Agreements with University of Belgrade-Faculty of Pharmacy No 451-03-136/2025-03/200161 and No 451-03-137/2025-03/200161 and Ministry of Education, Science, and Technological Development of the Republic of Serbia Grant Agreement 451-03-136/2025-03/200003.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TSS | total soluble solids |
| TA | titratable acidity |
| TPC | total phenolic content |
| TFC | total flavonoid content |
| TCC | total carotenoid content |
| AA | 2βG-2-O-β-D-glucopyranosyl-L-ascorbic acid |
| TPoly | total polysaccharide |
| GAE | gallic acid equivalent |
| CE | catechin equivalent |
| Glu | glucose equivalent |
| RDA | Recommended Daily Allowance |
| TE | trolox equivalents |
References
- Qian, D.; Zhao, Y.; Yang, G.; Huang, L. Systematic review of chemical constituents in the genus Lycium (Solanaceae). Molecules 2017, 22, 911. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Tao, W.; Zhang, X.; Gao, X.; Yong, J.; Zhao, J.; Zhang, L.; Li, Y.; Duan, J. Lycium ruthenicum studies: Molecular biology, phytochemistry and pharmacology. Food Chem. 2018, 240, 759–766. [Google Scholar] [CrossRef]
- Tudor, A.D.; Bolohan, C.; Tudor, V.; Teodorescu, I.R. Main active components of goji berry and their nutritional importance—A review. J. Appl. Life Sci. Environ. 2023, 55, 111–132. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, R.; Shi, Z.; Yang, L.; Fang, S. Phenotypic and phytochemical variations in wolfberry varieties and their harvest times. Horticulturae 2025, 11, 1138. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, Y.; Wang, Y.; Gao, F.; Chen, Z. Lycium barbarum: A traditional chinese herb and a promising anti-aging agent. Aging Dis. 2017, 8, 778. [Google Scholar] [CrossRef]
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010, 76, 7–19. [Google Scholar] [CrossRef]
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A. Goji berry (Lycium barbarum): Composition and health effects—A review. Pol. J. Food Nutr. Sci. 2016, 66, 67–75. [Google Scholar] [CrossRef]
- Yao, R.; Heinrich, M.; Weckerle, C.S. The genus Lycium as food and medicine: A botanical, ethnobotanical and historical review. J. Ethnopharmacol. 2018, 212, 50–66. [Google Scholar] [CrossRef]
- Vidović, B.B.; Milinčić, D.D.; Marčetić, M.D.; Djuriš, J.D.; Ilić, T.D.; Kostić, A.Ž.; Pešić, M.B. Health benefits and applications of goji berries in functional food products development: A review. Antioxidants 2022, 11, 248. [Google Scholar] [CrossRef]
- Yu, Z.; Xia, M.; Lan, J.; Yang, L.; Wang, Z.; Wang, R.; Tao, H.; Shi, Y. A comprehensive review on the ethnobotany, phytochemistry, pharmacology and quality control of the genus Lycium in China. Food Funct. 2023, 14, 2998–3025. [Google Scholar] [CrossRef]
- Ilić, T.; Dodevska, M.; Marčetić, M.; Božić, D.; Kodranov, I.; Vidović, B. Chemical characterization, antioxidant and antimicrobial properties of goji berries cultivated in Serbia. Foods 2020, 9, 1614. [Google Scholar] [CrossRef]
- Niro, S.; Fratianni, A.; Panfili, G.; Falasca, L.; Cinquanta, L.; Alam, M.R. Nutritional evaluation of fresh and dried goji berries cultivated in Italy. Ital. J. Food Sci. 2017, 29, 398–408. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, B.; Li, P.; Wei, W.; Dang, J.; Liu, Z.; Tao, Y.; Han, H.; Shao, Y.; Yue, H. Fatty acid and phytosterol composition, and biological activities of Lycium ruthenicum Murr. seed oil. J. Food Sci. 2018, 83, 2448–2456. [Google Scholar] [CrossRef]
- Ma, Z.F.; Zhang, H.; Teh, S.S.; Wang, C.W.; Zhang, Y.; Hayford, F.; Wang, L.; Ma, T.; Dong, Z.; Zhang, Y.; et al. Goji berries as a potential natural antioxidant medicine: An insight into their molecular mechanisms of action. Oxidative Med. Cell. Longev. 2019, 2019, 2437397. [Google Scholar] [CrossRef]
- Tang, W.M.; Chan, E.; Kwok, C.Y.; Lee, Y.K.; Wu, J.H.; Wan, C.W.; Chan, R.Y.-K.; Yu, P.H.-F.; Chan, S.-W. A review of the anticancer and immunomodulatory effects of Lycium barbarum fruit. Inflammopharmacology 2012, 20, 307–314. [Google Scholar] [CrossRef]
- Ilić, T.; Đuričić, I.; Kodranov, I.; Ušjak, L.; Kolašinac, S.; Milenković, M.; Marčetić, M.; Božić, D.D.; Vidović, B.B. Nutritional value, phytochemical composition and biological activities of Lycium barbarum L. fruits from Serbia. Plant Foods Hum. Nutr. 2024, 79, 662–668. [Google Scholar] [CrossRef]
- Ilić, T.; Krgović, N.; Božić, D.D.; Samardžić, S.; Marčetić, M.; Zdunić, G.; Vidović, B.B. Polyphenols profile and in vitro biological activities of black goji berries (Lycium ruthenicum Murr.). J. Berry Res. 2024, 14, 15–28. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, Y.; Wan, P.; Chen, C.; Chen, D.; Zeng, X.; Cao, Y. Prebiotic effects in vitro of anthocyanins from the fruits of Lycium ruthenicum Murray on gut microbiota compositions of feces from healthy human and patients with inflammatory bowel disease. LWT 2021, 149, 111829. [Google Scholar] [CrossRef]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef]
- Sicari, V.; Romeo, R.; Mincione, A.; Santacaterina, S.; Tundis, R.; Loizzo, M.R. Ciabatta bread incorporating goji (Lycium barbarum L.): A new potential functional product with impact on human health. Foods 2023, 12, 566. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, L.; Mi, J.; Wei, J.; Luo, Q.; Lu, L.; Yan, Y. Chemical composition and inhibitory effect of Lycium barbarum L. bud tea and leaf tea on pancreatic lipase and α-amylase activity. Foods 2025, 14, 3167. [Google Scholar] [CrossRef]
- Ciupei, D.; Colişar, A.; Leopold, L.; Stănilă, A.; Diaconeasa, Z.M. Polyphenols: From classification to therapeutic potential and bioavailability. Foods 2024, 13, 4131. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhou, S.; Liu, J.; McLean, R.J.C.; Chu, W. Prebiotic, immuno-stimulating and gut microbiota-modulating effects of Lycium barbarum polysaccharide. Biomed. Pharmacother. 2020, 121, 109591. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Ma, Y.; Liu, J.; Fan, Y.; Zheng, A.; Gao, P.; Wang, L.; Liu, D. Assessment of the bioaccessibility of carotenoids in goji berry (Lycium barbarum L.) in three forms: In vitro digestion model and metabolomics approach. Foods 2022, 11, 3731. [Google Scholar] [CrossRef]
- Covaci, E.; Senila, M.; Leopold, L.F.; Olah, N.K.; Cobzac, C.; Ivanova-Petropulos, V.; Balabanova, B.; Cadar, O.; Becze, A.; Ponta, M.; et al. Characterization of Lycium barbarum L. berry cultivated in North Macedonia: A chemometric approach. J. Berry Res. 2020, 10, 223–241. [Google Scholar] [CrossRef]
- Mocan, A.; Cairone, F.; Locatelli, M.; Cacciagrano, F.; Carradori, S.; Vodnar, D.C.; Crișan, G.; Simonetti, G.; Cesa, S. Polyphenols from Lycium barbarum (Goji) fruit european cultivars at different maturation steps: Extraction, HPLC-DAD analyses, and biological evaluation. Antioxidants 2019, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- Jumlah, P.; Fenolik, K.; Antioksidan, A.; Buah-Buahan Segar, J. Comparison of total phenolic contents (TPC) and antioxidant activities of fresh fruit juices, commercial 100% fruit juices and fruit drinks. Sains Malays. 2016, 45, 1319–1327. [Google Scholar]
- Republic of Serbia. Regulation on Fruit Juices and Certain Related Products Intended for Human Consumption. Official Gazette of the Republic of Serbia [Internet]. 2018. Available online: http://demo.paragraf.rs/demo/combined/Old/t/t2019_12/t12_0478.htm (accessed on 28 September 2025).
- SRPS EN 12147:2005. Fruit and Vegetable Juices—Determination of Titrated Acidity [Internet]. Available online: https://iss.rs/sr_Cyrl/project/show/iss:proj:17248 (accessed on 28 September 2025).
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Braniša, J.; Jenisová, Z.; Porubská, M.; Jomová, K.; Valko, M. Spectrophotometric determination of chlorophylls and carotenoids. an effect of soni-cation and sample processing. J. Microbiol. Biotech. Food Sci. 2014, 3, 61–64. [Google Scholar]
- Trang, H.K. Development of HPLC Methods for the Determination of Water-Soluble Vitamins in Pharmaceuticals and Fortified Food Products. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2013. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Norma, P.M.; Virginia, N.M.; Ral, R.H.; Jos, C.E.; Cristbal, N.A. A microassay for quantification of 2,2-diphenyl-1-picrylhydracyl (DPPH) free radical scavenging. Afr. J. Biochem. Res. 2014, 8, 14–18. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Esin Karademir, S.; Erçağ, E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C.F.R. Antioxidant properties and phenolic profile of the most widely appreciated cultivated mushrooms: A comparative study between in vivo and in vitro samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Ibrahim, A.; Dahiru, N.J.; Mohammed, H.S. Alpha amylase inhibitory potential and mode of inhibition of oils from Allium sativum (Garlic) and Allium cepa (Onion). Clin. Med. Insights Endocrinol. Diabetes 2020, 13, 117955142096310. [Google Scholar] [CrossRef] [PubMed]
- Indrianingsih, A.W.; Tachibana, S.; Itoh, K. In vitro evaluation of antioxidant and α-glucosidase inhibitory assay of several tropical and subtropical plants. Procedia Environ. Sci. 2015, 28, 639–648. [Google Scholar] [CrossRef]
- McKee, L.H.; Garcia-Whitehead, C.; Remmenga, M. Quality characteristics of red raspberry fruit spread made with four sweeteners. Plant Foods Hum. Nutr. 2002, 57, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Tolić, M.T.; Jurčević, I.L.; Krbavčić, I.P.; Marković, K.; Vahčić, N. Phenolic content, antioxidant capacity and quality of chokeberry (Aronia melanocarpa) product. Food Technol. Biotechnol. 2015, 53, 171–179. [Google Scholar] [CrossRef]
- Meng, X.; Ye, D.; Pan, Y.; Zhang, T.; Liang, L.; Liu, Y.; Ma, Y. Optimisation of not-from-concentrate goji juice processing using fuzzy mathematics and response surface methodology and its quality assessment. Appl. Sci. 2024, 14, 8393. [Google Scholar] [CrossRef]
- Boleslawska, I.; Kosewski, G.; Jagielski, P.; Dobrzynska, M.; Przyslawski, J. Analysis of antioxidant capacity and polyphenol content of Goji fruit products available on the European market. Acta Pol. Pharm.-Drug Res. 2021, 78, 345–351. [Google Scholar] [CrossRef]
- Pierantozzi, P.; Torres, M.; Contreras, C.; Stanzione, V.; Tivani, M.; Gentili, L.; Mastio, V.; Searles, P.; Brizuela, M.; Fernández, F.; et al. Phenolic content and profile of olive fruits: Impact of contrasting thermal regimes in non-Mediterranean growing environments. Eur. J. Agron. 2025, 164, 127506. [Google Scholar] [CrossRef]
- Islam, T.; Yu, X.; Badwal, T.S.; Xu, B. Comparative studies on phenolic profiles, antioxidant capacities and carotenoid contents of red goji berry (Lycium barbarum) and black goji berry (Lycium ruthenicum). Chem. Cent. J. 2017, 11, 59. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, S.; Sun, W.; Wu, M.; Hu, W.; Shen, X.; Wang, Y. Comparative analysis of carotenoid accumulation in two goji (Lycium barbarum L. and L. ruthenicum Murr.) fruits. BMC Plant Biol. 2014, 14, 269. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Zhang, X.; Liu, J.; Hao, Y.; Yang, X.; Wang, Y. Comparative evaluation of the antioxidant effects of the natural vitamin C analog 2-O-β-D-glucopyranosyl-L-ascorbic acid isolated from Goji berry fruit. Arch. Pharm. Res. 2011, 34, 801–810. [Google Scholar] [PubMed]
- Nadulski, R.; Masłowski, A.; Mazurek, A.; Sobczak, P.; Szmigielski, M.; Żukiewicz-Sobczak, W.; Niedziółka, I.; Mazur, J. Vitamin C and lutein content of northern highbush blueberry (Vaccinium corymbosum L.) juice processed using freezing and thawing. J. Food Meas. Charact. 2019, 13, 2521–2528. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, B.; Wen, H.; Tao, Y.; Shao, Y. Phytochemical profiles, nutritional constituents and antioxidant activity of black wolfberry (Lycium ruthenicum Murr.). Ind. Crops Prod. 2020, 154, 112692. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Fernández-de Córdova, M.L.; Ortega-Barrales, P.; Ruiz-Medina, A. Characterization and comparison of the chemical composition of exotic superfoods. Microchem. J. 2013, 110, 444–451. [Google Scholar] [CrossRef]
- Dehelean, A.; Magdas, D.A. Analysis of mineral and heavy metal content of some commercial fruit juices by inductively coupled plasma mass spectrometry. Sci. World J. 2013, 2013, 215423. [Google Scholar] [CrossRef]
- De Mello Andrade, J.M.; Fasolo, D. Polyphenol antioxidants from natural sources and contribution to health promotion. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 253–265. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780123984562000207 (accessed on 28 September 2025).
- Krgović, N.; Miljak, V.; Kukić, M.; Simić, M.; Miljak, M.; Kotur, S. Influence of immortelle (Helichrysum italicum (Roth) G. Don) and lavender (Lavandula angustifolia Mill.) hydrolates on oxidative stress and antioxidative parameters in human serum. Arh. Za Farm. 2025, 75, 202–220. [Google Scholar] [CrossRef]
- Kapoor, S.; Aggarwal, P. Effect of processing and storage on bioactive compounds and antioxidant activity of carrot juice. J. Appl. Hortic. 2014, 16, 80–84. [Google Scholar] [CrossRef]
- Ruffo, M.; Parisi, O.; Amone, F.; Malivindi, R.; Gorgoglione, D.; De Biasio, F.; Scrivano, L.; Pezzi, V.; Puoci, F. Calabrian goji vs. Chinese goji: A comparative study on biological properties. Foods 2017, 6, 30. [Google Scholar] [CrossRef]
- Solomando González, J.C.; Rodríguez Gómez, M.J.R.; Ramos García, M.; Nicolás Barroso, N.; Calvo Magro, P. Characterization and selection of Lycium barbarum cultivars based on physicochemical, bioactive, and aromatic properties. Horticulturae 2025, 11, 924. [Google Scholar] [CrossRef]
- Oboh, G.; Akinyemi, A.; Ademiluyi, A. Inhibition of α-amylase and α-glucosidase activities by ethanolic extract of Telfairia occidentalis (fluted pumpkin) leaf. Asian Pac. J. Trop. Biomed. 2012, 2, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef]
- Zdunić, G.; Aradski, A.A.; Gođevac, D.; Živković, J.; Laušević, S.D.; Milošević, D.K.; Šavikin, K. In vitro hypoglycemic, antioxidant and antineurodegenerative activity of chokeberry (Aronia melanocarpa) leaves. Ind. Crops Prod. 2020, 148, 112328. [Google Scholar] [CrossRef]
- Kruczek, A.; Ochmian, I.; Krupa-Małkiewicz, M.; Lachowicz, S. Comparison of morphological, antidiabetic and antioxidant properties of goji fruits. Acta Univ. Cibiniensis. Ser. E Food Technol. 2020, 24, 1–14. [Google Scholar] [CrossRef]
- Podsędek, A.; Majewska, I.; Redzynia, M.; Sosnowska, D.; Koziołkiewicz, M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. J. Agric. Food Chem. 2014, 62, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Kou, R.; Zuo, G.; Liu, J.; Di, D.; Guo, M. Structural properties and hypoglycaemic activity of polysaccharides extracted from the fruits of Lycium barbarum L. using various extraction media. Ind. Crops Prod. 2022, 188, 115725. [Google Scholar] [CrossRef]
- Luo, Q.; Cai, Y.; Yan, J.; Sun, M.; Corke, H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004, 76, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, X.g.; Wang, J.z.; Wang, F.j. Optimization of enzymatic hydrolysis for production of Aronia melanocarpa juice and its functional properties. Sci. Technol. Food Ind. 2020, 41, 125–137. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).