Abstract
Blueberries are rich in a variety of functional substances and have high nutritional and health values, but they are not resistant to decline during fresh storage. Here, the effects of six Bacillus species on the storage quality of, and antioxidant levels in, the southern highbush blueberry ‘O’Neal’ fruit were investigated. Bacillus treatments reduced the fruit decay rate, slowed fruit quality decline, inhibited malondialdehyde accumulation, and increased superoxide dismutase and peroxidase activity levels. Bacillus altitudinis Y-14 had the best effect overall. Furthermore, the effects of 1-methylcyclopropene (1-MCP), B. altitudinis Y-14, and 1-MCP + B. altitudinis Y-14 treatments on the storage quality and antioxidant of rabbiteye blueberry ‘Brightwell’ fruit were investigated, and each treatment effectively reduced the decay rate and weight loss of fruit. When stored for 25 days, the decay rate of the 1-MCP + B. altitudinis Y-14 group was only 8.33%, significantly lower than that of the control. The three treatments delayed the decline in fruit quality, inhibited malondialdehyde accumulation, and increased superoxide dismutase and peroxidase activity levels. The 1-MCP + B. altitudinis Y-14 treatment was more conducive to prolonging the postharvest storage period of blueberries and had the best effect in delaying the decline in fruit quality. Thus, combined 1-MCP and B. altitudinis Y-14 treatment may be an effective way to improve the storage quality and extend the storage period of blueberries, which provides a new way for storing and transporting blueberries to reduce costs and improve economic benefits.
1. Introduction
Blueberry (Vaccinium spp.) fruit are rich in nutrients, including vitamins, anthocyanins, sugars, acids, and minerals. They also have anti-aging, cancer, brain nerve aging prevention, and eye fatigue alleviation effects. They are listed as one of the ‘Five Health Foods for Humans’ by the Food and Agriculture Organization of the United Nations [1]. China’s blueberry production has grown to a large-scale industry, and the number, variety, and structures of blueberry products are increasingly abundant. As a result, five major blueberry production areas have formed: Changbai Mountain, Liaodong Peninsula, Jiaodong Peninsula, Yangtze River Basin, and the southwest [2]. The blueberry fruit grown in the Yangtze River Basin generally mature during the high temperature and humidity of the summer. They have a high moisture content and can only be stored for 5–7 days at room temperature [3,4]. Mechanical damage and infection by pathogenic bacteria during harvesting can easily result in blueberries rotting and softening, which further shortens their storage life [5]. Therefore, how to preserve and store blueberries during production and shipping to market have become key factors restricting the development of the blueberry industry.
Increasing the storage capacity of fruit requires weakening their physiological and metabolic activities by some means in an appropriate storage space, such as reducing the consumption of nutrients by respiration and the activities of related enzymes, thereby maximizing fruit freshness. Low-temperature storage is still a main preservation method for blueberries due to its low cost, easy operation, and high stability [6]. Low temperature can effectively reduce fruit metabolic rates, delay fruit aging time, and prevent the growth and reproduction of pathogenic bacteria. Zhang et al. [7] reported that compared with 20 °C storage, 4 °C storage significantly reduced the blueberry decay rate and delayed the decline in fruit quality. 1-Methylcyclopropene (1-MCP) is an inhibitor of ethylene production that is widely used to maintain the quality of several climacteric fruit during storage [8,9]. 1-MCP competes with the receptor protein of ethylene and blocks the ethylene-binding receptor protein, which stops the ethylene information pathway. Thus, the ripening reaction related to ethylene is inhibited, the ripening and senescence processes of fruit are delayed, and their storage lives are prolonged [10,11]. 1-MCPs improve the postharvest fruit quality and maintain volatile compound levels, and thus they may represent a feasible method to extend the shelf lives of blueberries [12] and apples [13]. Ji et al. [14] reported that 1-MCPs inhibited the intensity of respiration and ethylene production, delayed the decline in anthocyanins, vitamin C (Vc), and other antioxidant substances in blueberries, and significantly reduced the decay rate. Zhang et al. [15] reported that 1-MCPs maintained the hardness of crisp plums at 4 °C and reduced the incidence of brown rot. In addition, biological control measures mainly use natural extracts, antagonistic microorganisms, or bioengineering technology to obtain green, safe, healthy, and non-toxic substances for postharvest fruit preservation and storage. Because of their wide sources, safety, non-toxicity, and green and environmental protection, biological control measures are the main trend in fruit preservation technology. The antibacterial mechanisms of antagonistic bacteria against pathogenic bacteria can be divided into nutrition and spatial competition, antibiotic secretion, and volatile metabolite secretion, hyperparasitic effects, and host resistance induction [16]. In total, 5% to 8% of the genes in the whole genomes of Bacillus species are dedicated to the synthesis of secondary metabolites, including peptides, lipopeptides, bacteriocins, and other bioactive substances, which means that Bacillus has a wide range of antagonistic effects. Qin [17] reported that a strain of Hanseniaspora uvarum that can inhibit six pathogens was isolated from rotten blueberries. Wei [18] screened a strain of Cryptococcus flavescens from the surface of rotten fruit and showed that it had good inhibitory effects on postharvest diseases and control effects on Botrytis cinerea and Alternaria alternata in blueberries. The strain’s mode of action was to compete with the pathogenic bacteria for nutrients and space, thereby inhibiting their growth. Kurniawan et al. [19] showed that antagonistic Bacillus and Pseudomonas bacteria inhibited B. cinerea and Alternaria sp. and that they effectively reduced the postharvest decay rate of blueberries.
Although many antagonistic microorganisms have biocontrol effects on the postharvest diseases of fruit, there are still deficiencies, and their inhibitory effects are not as good as those of chemical reagents. The antagonistic effects of microorganisms may also be affected by the environment, the bacterial concentration, and even the fruit variety [20]. Therefore, combining biocontrol bacteria and chemical means is one way to improve the antagonistic effects of biocontrol bacteria. This can not only strengthen the control effect, but also reduce the use of chemical reagents. Therefore, this study was designed to treat blueberries with six Bacillus strains and to study the preservation effects on blueberries during cool storage. The selected Bacillus and 1-MCP, both jointly or separately, were used to treat blueberries, and the preservation effects during cool storage were studied. The combined Bacillus and 1-MCP has the advantages of contributing to safety, efficiency, and green and environmental protection, which is the main trend in the future development of fruit storage and preservation technology. The results provide a basis for further investigations and have applications in blueberry postharvest storage and preservation.
2. Materials and Methods
2.1. Materials
The six Bacillus strains, B. subtulis 21, B. cereus SS2-4A, B. cereus YJ-DA, B. altitudinis Y-14, B. altitudinis LY-3, and B. tropicus YJ-4D, used in the experiment were screened, isolated, and purified from rotten blueberry, pear, and rice surfaces. They were identified using a combination of physiological and biochemical characteristics as well as nucleotide sequence alignments. The strains were stored at −80 °C in A813 laboratory, College of Life Science, Anhui Normal University.
In total, 500 μL of activated bacterial solution (109 cells/mL) was added to 500 mL of sterilized Luria–Bertani liquid medium (including 10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl) and cultured for 10 h at 37 °C and 135 r/min.
The ‘O’Neal’ (Vaccinium corymbosum L.) blueberries were harvested on 24 May 2022 and the ‘Brightwell’ (Vaccinium virgatum Ait.) blueberries were harvested on 21 June 2022 at the blueberry base of Gecun, Jishan Town, Nanling County, Anhui Province (30°47′ N, 118°08′ E). After harvest, the mature blueberries were placed in a precooling chamber for 2 h at 21 °C and then transported back to the laboratory in a styrofoam box containing ice bags. Blueberries of uniform size with no mechanical damage or disease and of a similar maturity were selected for experiments.
2.2. Methods
The selected ‘O’Neal’ blueberries were randomly distributed into the following seven groups: (1) control with ddH2O treatment before refrigeration (CK); (2) B. subtulis 21 treatment (B1); (3) B. cereus SS2-4A treatment (B2); (4) B. cereus YJ-DA treatment (B3); (5) B. altitudinis Y-14 treatment (B4); (6) B. altitudinis LY-3 treatment (B5); and (7) B. tropicus YJ-4D treatment (B6). Each group included three biological replicates. The B1–6 groups were soaked in the appropriate Bacillus solution (500 μL 109 cells/mL of activated bacterial solution was added to 500 mL of sterilized Luria–Bertani liquid medium and obtained after 10 h of incubation, mentioned above) for 3 min, and the control group was soaked in ddH2O for 3 min. After drying, blueberries were put into a 180 × 140 × 40 mm (length × width × height) low-density polyethylene plastic food packaging box (approximately 120 berries/box) and stored at 4 °C (relative humidity~60%, light intensity~25 Lux). Samples were taken every 5 days during storage for the determinations and analyses of the related indicators.
The selected ‘Brightwell’ blueberries were randomly distributed into the following four groups: (1) control with no treatment before refrigeration (CK); (2) 1-MCP (MCP): two 10 cm × 10 cm pieces of 1-MCP-treated paper (Xianyang Xiqin Biotechnology Co., Ltd., Xianyang, China) were placed at the bottom and top of the plastic food packaging box containing the blueberries; (3) B. altitudinis Y-14 treatment (B4): blueberries were soaked in the B. altitudinis Y-14 solution (mentioned above) for 3 min and then removed and drained; and (4) combined treatment of 1-MCP and B. altitudinis Y-14 (MCP + B4): blueberries were soaked in the B. altitudinis Y-14 solution for 3 min, drained, and put into the packaging box (180 × 140 × 40 mm, mentioned above). And two 10 cm × 10 cm pieces of 1-MCP-treated paper were placed at the bottom and top of the packaging box. Each group included three biological replicates. The blueberries were stored at 4 °C (relative humidity~60%, light intensity~25 Lux) and were sampled every 5 days during storage for the determinations and analyses of the related indicators.
The decomposition rate of blueberries was calculated according to Equation (1).
The decay rate (%) = (number of rotten blueberries/total number of blueberries) × 100%
And the fruit weight loss rate was calculated according to Equation (2).
Weight loss rate (%) = [(initial weight − final weight)/initial weight] × 100%
Total soluble solids (TSS) content was determined using a hand refractometer and titratable acid (TA) content was determined by continuous titration with an acidimeter [21]. The anthocyanin content was determined using the pH differential method [22,23], the vitamin C (ascorbic acid, Vc) content was determined using a UV spectrophotometer (Unico UV2150, Shanghai, China) [24], and the malondialdehyde (MDA) level was determined using thiobarbituric acid colorimetry. The activities of peroxidase (POD) and superoxide dismutase (SOD) were determined using the guaiacol and nitro-blue tetrazolium methods, respectively [25].
The measured data are reported as the average values and standard deviations of three repetitions. SPSS 20.0 (IBM, Armonk, NY, USA) was used for multiple data comparisons, and the one-way ANOVA and Tukey methods were used to calculate the significant differences (p < 0.05).
3. Result and Analysis
3.1. Experiment 1
3.1.1. Effects of Different Bacillus on Decay and Weight Loss Rates, as Well as TSS and Anthocyanin Contents, in Blueberries
Compared with the control, the B1–6 treatment groups, except B5, significantly inhibited the decay rate and weight loss rate of ‘O’Neal’ blueberries, especially the B4 group. On the 25th day, the decay rate of the CK reached 49.7%, whereas that of the B4 group was only 13.03% (Figure 1A). The weight loss rate of the control reached 37.63%, whereas that of the B4 group reached only 22.89% (Figure 1B).
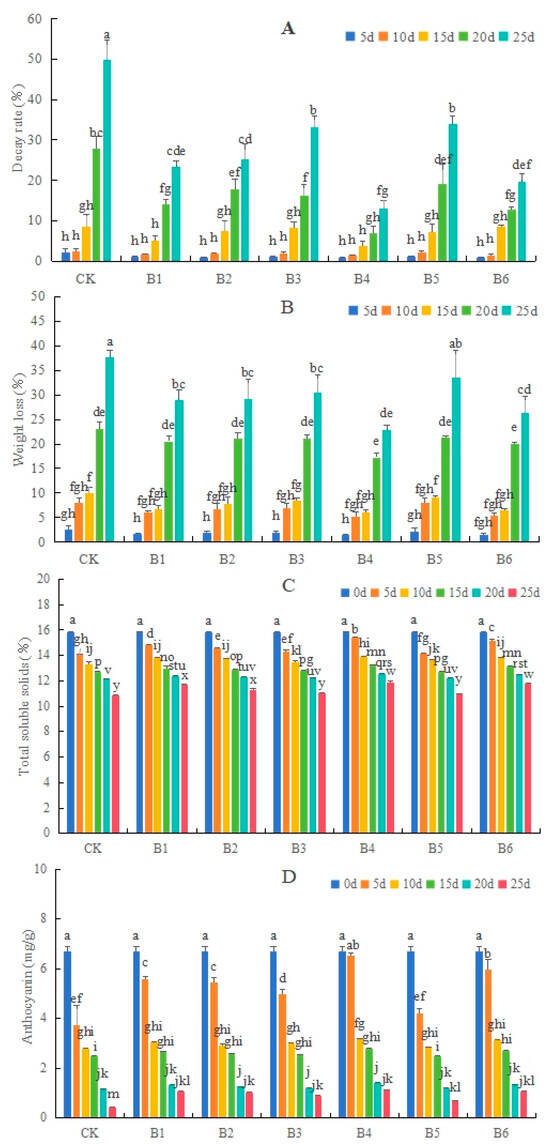
Figure 1.
Effects of different Bacillus species on the decay (A) and weight loss (B) rates as well as total soluble solids (C) and anthocyanin (D) contents of ‘O’Neal’ blueberries under 4 °C storage conditions. CK, control group; B1, B. subtulis 21 treatment; B2, B. cereus SS2-4A treatment; B3, B. cereus YJ-DA treatment; B4, B. altitudinis Y-14 treatment; B5, B. altitudinis LY-3 treatment; B6, B. tropicus YJ-4D treatment. Different lowercase letters above the bars indicate significant differences at the p < 0.05 level.
The TSS and anthocyanin contents of ‘O’Neal’ blueberries decreased with the extension of the 4 °C storage period. The six biocontrol bacterial treatments significantly delayed the reductions in TSS and anthocyanin contents in blueberries, especially the B4 group. On the 25th day, the anthocyanin contents in the B1–6 groups were significantly greater than in the CK (Figure 1C,D).
3.1.2. Effects of Different Bacillus on Vc and MDA Contents, and POD and SOD Activity Levels, in Blueberries
The Vc content of blueberries in each group decreased with the extension of storage time. Biocontrol bacterial treatments delayed the decline in the Vc content, with the B4 group having the longest delay (Figure 2A).
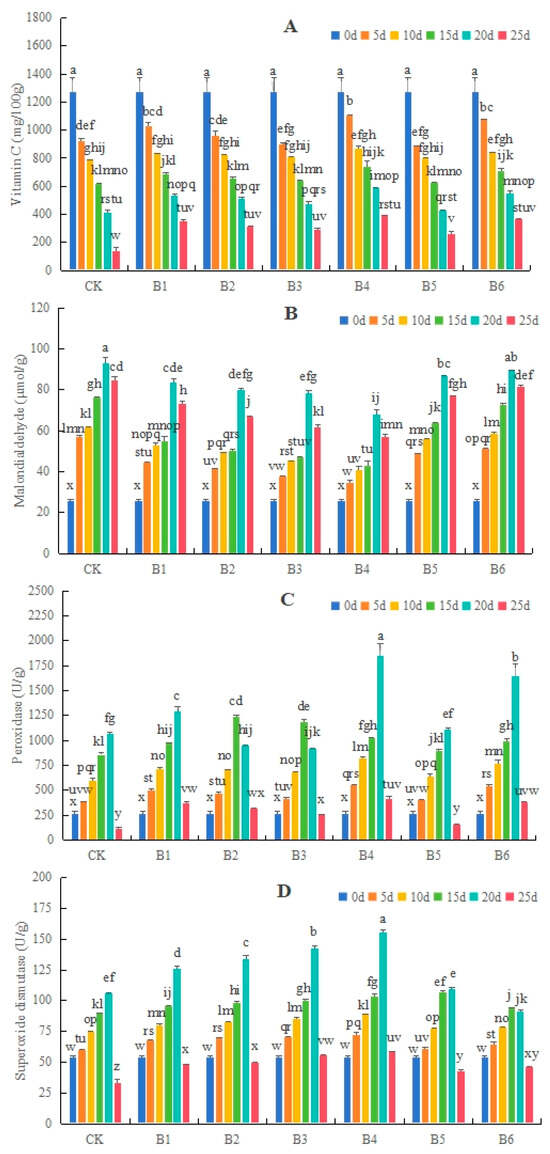
Figure 2.
Effects of different Bacillus species on the vitamin C (A) and malondialdehyde (B) contents as well as peroxidase (C) and superoxide dismutase (D) activities of ‘O’Neal’ blueberries under 4 °C storage conditions. CK, control group; B1, B. subtulis 21 treatment; B2, B. cereus SS2-4A treatment; B3, B. cereus YJ-DA treatment; B4, B. altitudinis Y-14 treatment; B5, B. altitudinis LY-3 treatment; B6, B. tropicus YJ-4D treatment. Different lowercase letters above the bars indicate significant differences at p < 0.05 level.
Under the 4 °C storage conditions, the MDA contents of blueberries increased at first and then decreased, with the peak occurring on the 20th day. The changes in MDA contents in blueberries treated with six biocontrol bacteria were slower than in the CK, and the peak value of MDA in the B4 group was significantly lower than those in other treatment groups (Figure 2B). The POD and SOD activities in each group of blueberries increased first and then decreased, with the peak value appearing on the 15th or 20th day. The peak values of POD and SOD activities in blueberries treated with biocontrol bacteria were higher than those of the CK, except for POD in the B5 group and SOD in the B5 and B6 groups (Figure 2C,D).
3.2. Experiment 2
3.2.1. Effects of MCP and B4 on the Decay and Weight Loss Rates, as Well as the TSS, Anthocyanin, and TA Contents, in Blueberries
Compared with the CK, the MCP, B4, and MCP + B4 groups showed significant inhibitions in the decay and weight loss rates of ‘Brightwell’ blueberries, especially in the MCP+B4 group. On the 25th day, the decay rate of the CK group was 27.78%, whereas that of the MCP + B4 group was only 8.33%. In addition, the weight loss rate of the CK group was 61.27%, whereas that of the MCP + B4 group was 37.78% (Figure 3A,B).
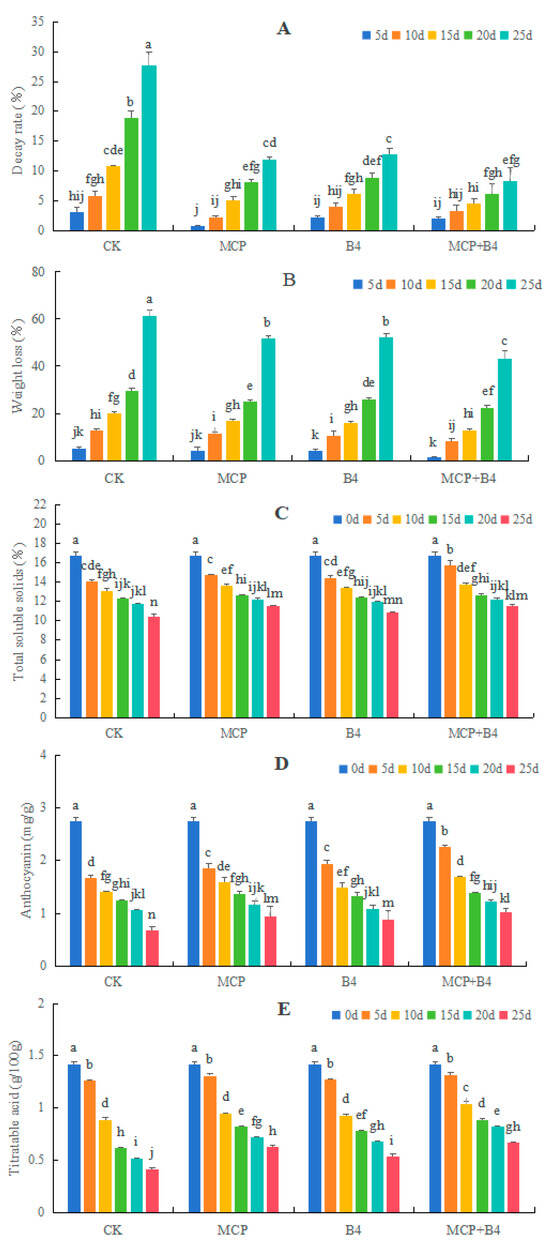
Figure 3.
Effects of MCP and B4 on the decay (A) and weight loss (B) rates, as well as total soluble solids (C), anthocyanin (D), and titratable acid (E) contents of ‘Brightwell’ blueberries under 4 °C storage condition. CK, control group; MCP, 1-MCP; B4, B. altitudinis Y-14 treatment; MCP + B4, combined treatment of 1-MCP and B. altitudinis Y-14. Different lowercase letters above the bars indicate significant differences at p < 0.05 level.
Under the 4 °C storage conditions, the TSS, anthocyanin, and TA contents of ‘Brightwell’ blueberries decreased with the extension of the storage period. The MCP, B4, and MCP + B4 treatment groups showed delayed declines in TSS, anthocyanin, and TA levels, especially in the MCP + B4 group (Figure 3C–E).
3.2.2. Effects of MCP and B4 on Vc and MDA Contents, as Well as POD and SOD Activity Levels, in Blueberries
Under the 4 °C storage conditions, the Vc content in each group decreased with the extension of storage time. The MCP, B4, and MCP + B4 groups showed delayed declines in the Vc content, and the MCP + B4 group had the longest delay (Figure 4A). The MDA content and the POD and SOD activity levels in blueberries increased first and then decreased during storage, with the peak occurring on the 20th day. At the later stage of storage, the POD and SOD activities in blueberries of the MCP + B4 group were significantly higher than those in the other treatment groups (Figure 4B–D).
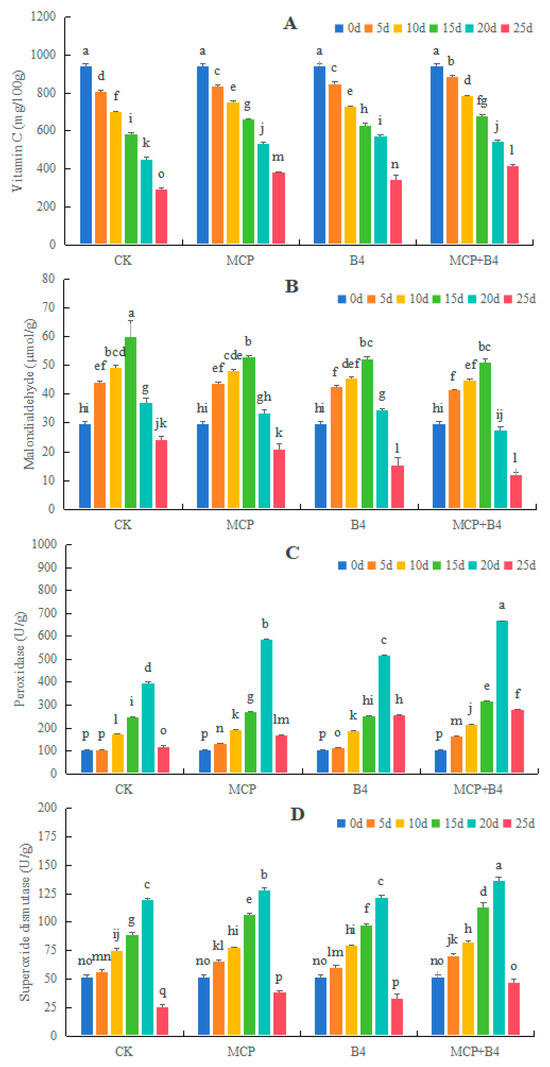
Figure 4.
Effects of MCP and B4 on vitamin C (A) and malondialdehyde (B) contents, as well as peroxidase (C) and superoxide dismutase (D) activities of ‘Brightwell’ blueberries under 4 °C storage conditions. CK, control group; MCP, 1-MCP; B4, B. altitudinis Y-14 treatment; MCP + B4, combined treatment of 1-MCP and B. altitudinis Y-14. Different lowercase letters above the bars indicate significant differences at p < 0.05 level.
4. Discussion
Postharvest pathogen infection can lead to the decay and deterioration of fruit and vegetables. At present, the use of antagonistic microorganisms to inhibit the invasion of postharvest pathogens is being investigated. The main biocontrol bacteria that can be used to control postharvest damage caused by pathogens are yeast and Bacillus. Because Bacillus sp. produce spores, they have strong stress resistance levels and stable antagonistic effects [26]. Bacillus can also produce antibiotics, bacteriocins, cell-degrading enzymes, antibacterial proteins, and other antagonistic substances to inhibit the growth and reproduction of pathogenic bacteria [27]. Consequently, Bacillus has been widely used in the field of biological preservation and control. In this study, six kinds of Bacillus were isolated and identified from rotten blueberries, pear, and rice. They were used to treat fruit and their storage performances were determined to identify the most effective strain. The different Bacillus treatments delayed the decay and water loss of blueberries to varying degrees. When stored for 25 days, the decay rate of the control group was nearly 50%, whereas that of the B. altitudinis Y-14 treatment was only 13%. The change in weight loss was basically consistent with the decay rate. During storage, the quality of berries decreased with the extension in storage time, the TSS, Vc, and anthocyanin contents presented downward trends, and all of the Bacillus treatments had significantly delay effects compared with the control. Zhang et al. [28] found that Bacillus beretii treatments effectively reduced the decay rate of grape berries, maintained the skin color and a high TSS content, delayed the degradation of TA and Vc, and effectively maintained the storage quality of berries, which was similar to the results of Bacillus treatments in this experiment.
If the change in the decay rate can be used determine whether the blueberries were infected by pathogens during storage, then lipid peroxidation is also an important indicator to judge whether the blueberries are normal during storage. MDA is the main product of lipid peroxidation. Consequently, if the MDA content increases, the fruit cells have been damaged to some degree. In this study, the MDA content first decreased and then increased, and the Bacillus treatments significantly inhibited MDA accumulation. Antagonistic bacteria can inhibit the decay of blueberries, inhibit the adhesion of pathogenic bacteria, and improve the antioxidant enzyme activity levels. During normal blueberry metabolism, the reactive oxygen species (ROS) scavenging system maintains a dynamic balance, but when the pathogen invades or the physiological metabolism becomes unbalanced during aging, the ROS will accumulate rapidly, and the enzyme systems, such as SOD and POD, in the fruit remove too much ROS [29]. In this experiment, the POD and SOD activity levels shared a consistent trend, first increasing and then decreasing. In the early and middle stages of fruit storage, with the continuous production of ROS, such as free radicals, in the fruit, the activity of the ROS-scavenging enzyme system increased, which was conducive to the timely removal of O2.− in the fruit, protecting the fruit cells from toxic bacteria, reducing the degree of fruit cell damage and membrane lipid peroxidation, maintaining MDA at a low level, and reducing the degree of fruit senescence. At the later stage of fruit storage, as fruit age, the activities of antioxidant enzymes decreased, and the MDA content increased. The six Bacillus treatments used in this study improved the antioxidant enzyme activity levels and inhibited MDA accumulation to varying degrees, with B. altitudinis Y-14 having the most significant treatment affect. Similarly, related experiments showed that Bacillus effectively inhibit the growth and reproduction of pathogenic bacteria during fruit storage. Li [30] tested the antibacterial activities of three kinds of Bacillus and chitosan against isolated spoilage bacteria. The three Bacillus treatments had antibacterial effects on 14 strains of spoilage bacteria, with the mixed bacterial treatment having the best antibacterial effect. Arrebola et al. [31] isolated a strain of Bacillus amyloliquefaciens from the surface of fruit, and the lipopeptide extract of this strain had a strong inhibitory activity against seven kinds of postharvest pathogens of citrus. Alvindia et al. [32] isolated B. amyloliquefaciens DGA14 from the surface of banana fruit, and this strain had an obvious inhibitory effect on banana crown rot.
Thus, the six Bacillus strains used in this study all affected the physiological indices of blueberries, delayed the declines in TSS, Vc, and anthocyanin contents in blueberries, inhibited the accumulation of MDA, and improved the activities of SOD and POD. The effects of Bacillus on prolonging the storage period of postharvest blueberries were also confirmed. In the comparison of their treatment effects, B. altitudinis Y-14 had the best effect on prolonging the shelf life of ‘O’Neal’ blueberries.
At present, combinations of antagonistic microorganisms and a variety of preservation methods have attracted attention. For example, Shi et al. [33] reported that the effect of natamycin combined with a 1-MCP improves the storability of winter jujube fruit. Mohamed et al. [34] treated papaya with 1-MCP combined with B. amyloliquefaciens. The combination reduced the incidences of papaya stem soft rot and anthracnose, and effectively maintained fruit freshness, quality, and taste. In this experiment, B. altitudinis Y-14, the best strain screened in the early stage, was combined with 1-MCP to treat ‘Brightwell’ blueberries. The results confirmed that the combination of a biocontrol bacteria and 1-MCP was more conducive to prolonging the postharvest storage period of blueberries and delaying the decline in fruit quality during storage. During storage, the combined 1-MCP and B. altitudinis Y-14 treatment delayed the increase in the decay rate of blueberries, reduced the quality loss during storage, slowed down the declines in TSS, Vc, and anthocyanin contents, inhibited the accumulation of MDA, and improved the activities of POD and SOD antioxidant enzymes. These results were similar to those of Liu et al. [35] in kiwifruit treated with B. subtilis strains. Of course, ripening was delayed by the 1-MCP and controlled atmosphere storage, as evidenced by the lower ethylene production, respiration rate, fermentative products, and sensory evaluation scores, as well as the slower softening, compared with fruit stored in regular air [36].
The Vc content in fruit directly affects its antioxidant capacity because Vc assists the antioxidant enzyme system in removing the oxygen free radicals produced during the physiological metabolism to avoid cellular damage [37]. When fruit are damaged by pathogen invasion, the defense enzymes are activated [38]. For example, SOD and POD play important roles in resistance to pathogen infection. SOD scavenges ROS to reduce fruit damage, and POD catalyzes the redox reaction, with H2O2 as the oxidant, to remove the H2O2 produced by bacteria in fruit. It also participates in the synthesis of antibacterial substances, such as lignin [39]. Here, the activity levels of SOD and POD in blueberries exposed to pathogens increased during storage. Compared with the CK group, the SOD and POD activities in 1-MCP, B. altitudinis Y-14, and B. altitudinis Y-14 + 1-MCP groups were higher, with the peak value in the B. altitudinis Y-14 + 1-MCP group being the highest. A relatively high enzyme activity was maintained in the late storage, indicating that 1-MCP and B. altitudinis Y-14 had a synergistic effect on the induction of defense enzymes, and the synergistic effect delayed the senescence of blueberries during storage. Similarly, the combination treatment of 1-MCP and ultraviolet C performs better than either ultraviolet C or 1-MCP alone in apricot fruit quality preservation [40]. The individual and combined melatonin and 1-MCPs had positive effects on mango [41] and apricot [42] fruit by enhancing the activities of enzymatic and nonenzymatic antioxidants. The application of 1-MCP was shown to be effective in reducing the loss of firmness in the minikiwi during storage, with fruit treated with 1-MCP having higher antioxidant activity levels and a higher content of biologically active compounds [43].
In conclusion, the six kinds of Bacillus effectively reduced the decay rate of ‘O’Neal’ blueberries under 4 °C storage, delayed the loss of fruit quality and the declines in fruit quality indicators such as Vc and anthocyanin, inhibited the accumulation of MDA, and improved the activities of POD and SOD. Among them, B. altitudinis Y-14 had the greatest effect on prolonging the shelf life of ‘O’Neal’ blueberries under cool storage conditions. Under 4 °C conditions, 1-MCP, B. altitudinis Y-14, and B. altitudinis Y-14 + 1-MCP groups reduced the decay and weight loss rates of ‘Brightwell’ blueberries, delayed the decline in fruit quality represented by TSS, Vc, and anthocyanin contents, and improved the activity levels of POD and SOD, with the B. altitudinis Y-14 + 1-MCP group having the greatest effect.
Author Contributions
Study design, drafting the manuscript: S.W., Y.Z., F.H. and J.X. Data acquisition and analysis, manuscript preparation and editing: Y.Z., S.W., M.W., Y.L. and L.L. Manuscript revision and final version approval: F.H. and J.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Key Research and Development Program of Anhui Province (202204c06020029), and the University Synergy Innovation Program of Anhui Province (GXXT-2023-055).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gao, H.Y.; Xu, L.; Chen, H.J.; Fang, X.J. Research progress on postharvest quality control and antioxidant activity of blueberry. Chin. J. Food Sci. 2013, 13, 1–8. [Google Scholar]
- Zhang, J. Research on Inner Quality Evaluation of Northern Highbush Blueberry. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2020. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, F.S.; Xu, H.; Tao, L. Preliminary report on preservation technology of original base in fresh blueberries. Agric. Prod. Process. 2009, 4, 12–13. [Google Scholar] [CrossRef]
- Zhu, L.; Ling, J.G. Research progress of preservation technology of blueberry at home and abroad. Food Ferment. Ind. 2011, 37, 173–176. [Google Scholar] [CrossRef]
- Fang, X.J.; Wu, W.J.; Mu, H.L.; Chen, H.J.; Zheng, X.L.; Gao, H.Y. Effects of exogenous abscisic acid treatment on physiological response of blueberry under low temperature stress. Chin. J. Food Sci. 2023, 23, 232–242. [Google Scholar] [CrossRef]
- Jiang, L.Q.; Hu, T.J.; Zheng, J.X. Experiment, application and research of cold-storage and fruit technology for fruit and vegetable. Grain Oil Process. Food Mach. 2003, 8, 65–66. [Google Scholar]
- Zhang, H. Effects of Chitosan and Ultraviolet Treatment on Storage Tolerance of Blueberry Fruit and Its Physiological Mechanism. Master’s Thesis, Anhui Normal University, Wuhu, China, 2017. [Google Scholar]
- Zhang, J.; Ma, Y.; Dong, C.; Terry, L.A.; Watkins, C.B.; Yu, Z.; Cheng, Z.M. Meta-analysis of the effects of 1-methylcyclopropene (1-MCP) treatment on climacteric fruit ripening. Hortic. Res. 2020, 7, 208. [Google Scholar] [CrossRef]
- Satekge, T.K.; Magwaza, L.S. Postharvest application of 1-methylcyclopropene (1-MCP) on climacteric fruits: Factors affecting efficacy. Int. J. Fruit Sci. 2022, 22, 595–607. [Google Scholar] [CrossRef]
- Jiang, Q.J.; Xie, W.; Chen, Y.Z.; Wang, C.T.; Zhang, X.X. Research progress of 1-MCP on berry quality and postharvest physiology. Farm Prod. Process. 2017, 441, 64–65. [Google Scholar] [CrossRef]
- Blankenship, S.M.; Dole, J.M. 1-Methylcyclopropene: A review. Postharvest Biol. Technol. 2003, 28, 1–25. [Google Scholar] [CrossRef]
- Xu, F.; Liu, Y.; Dong, S.; Wang, S. Effect of 1-methylcyclopropene (1-MCP) on ripening and volatile compounds of blueberry fruit. J. Food Process. Preserv. 2020, 44, e14840. [Google Scholar] [CrossRef]
- Kwon, J.-G.; Yoo, J.; Win, N.M.; Maung, T.-T.; Naing, A.H.; Kang, I.-K. Fruit quality attributes of ‘Arisoo’ and ‘Picnic’ apples as influenced by 1-methylcyclopropene concentration and its application frequency during cold storage. Horticulturae 2021, 7, 477. [Google Scholar] [CrossRef]
- Ji, S.J.; Zhou, Q.; Ma, C.; Cheng, S.C. Effect of 1-MCP treatment on shelf quality change of blueberry at room temperature. Food Sci. 2014, 35, 322–327. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; He, X.X.; Xiong, J.L.; Peng, M.Y.; Huang, M.; Liu, R.P.; Guan, W.Q. Identification of pathogenic bacteria of brown rot of brittle plum in Wushan and its control effect by 1-MCP. Southwest Chin. J. Agric. Sci. 2023, 36, 84–90. [Google Scholar] [CrossRef]
- Francesco, A.D.; Martini, C.; Mari, M. Biological control of postharvest diseases by microbial antagonists: How many mechanisms of action? Eur. J. Plant Pathol. 2016, 145, 711–717. [Google Scholar] [CrossRef]
- Qin, S.W. Isolation, Identification and Biological Control of Pathogenic Fungi Latent Infection of Blueberry Fruit. Master’s Thesis, Dalian University of Technology, Dalian, China, 2017. [Google Scholar]
- Wei, Y.Y. Study on Pathogens of Postharvest Diseases of Blueberry and the Yeast HMQAUSZ01 for Biocontrol of Postharvest Diseases of Fruits and Vegetables. Master’s Thesis, Qingdao Agricultural University, Qingdao, China, 2017. [Google Scholar]
- Kurniawan, O.; Wilson, K.; Mohamed, R.; Avis, T.J. Bacillus and Pseudomonas spp. provide antifungal activity against gray mold and Alternaria rot on blueberry fruit. Biol. Control Theory Appl. Pest Manag. 2018, 126, 136–141. [Google Scholar] [CrossRef]
- Chan, Z.L.; Qin, G.Z.; Xu, X.B.; Li, B.Q.; Tian, S.P. Proteome approach to characterize proteins induced by antagonist yeast and salicylic acid in peach fruit. J. Proteome Res. 2007, 6, 1677–1688. [Google Scholar] [CrossRef]
- Yan, Q.C.; Jiang, X.M. Determination of total acid and amino nitrogen in fruit juice by continuous titration with acidometer. Shandong Anim. Husb. Vet. 2012, 33, 8–9. [Google Scholar] [CrossRef]
- Feng, G.T. Extraction, Purification, Component Identification and Antioxidant of Blueberry Anthocyanins. Master’s Thesis, Guizhou University, Guiyang, China, 2016. [Google Scholar]
- Song, D.Q.; Meng, X.J.; Wang, C.Y.; Wang, G.Q.; Lv, C.M. Determination of blueberry anthocyanins by pH differential method. J. Shenyang Agric. Univ. 2013, 44, 231–233. [Google Scholar] [CrossRef]
- Wang, H.J. Study on the Stability of Vitamin C and Determination of Its Content in Fruits, Vegetables and Fruit Juices by Ultraviolet Spectrophotometry. Master’s Thesis, Shanxi Medical University, Taiyuan, China, 2015. [Google Scholar] [CrossRef]
- Li, H.S. Experimental Principles and Techniques of Plant Physiology and Biochemistry; Higher Education Press: Beijing, China, 2003; pp. 167–169. [Google Scholar]
- Wang, X.R. Burkholderia contaminans Biological Control and Mechanism of Postharcrop Gray Mold of Strawberry. Master’s Thesis, Shanxi Agricultural University, Taiyuan, China, 2019. [Google Scholar] [CrossRef]
- Zheng, X.X. Study on the Control and Bacteriostatic Effect of Bacillus against Postharvest Streptospora of Apricot and Peach. Master’s Thesis, Tianjin University of Commerce, Tianjin, China, 2019. [Google Scholar]
- Zhang, Y.M.; Niu, X.X.; Zou, Q.; Yang, H.M.; Chu, M.; Shi, Y.W. Effects of Bacillus Velez on storage quality of postharvest grape fruit. Storage Process 2022, 22, 1–8. [Google Scholar]
- Wang, C.H. Effects of Biocontrol Bacterium (Bacillus amyloliquefaciens) LY1 on Preservation and Postharvest Disease Control of Longan Fruit and Its Mechanism. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2012. [Google Scholar]
- Li, W.X. Study on the Bacteriostatic Effects of Spoilage Bacteria and Bacillus on Kiwi Fruit During Storage. Master’s Thesis, Jiamusi University, Jiamusi, China, 2022. [Google Scholar] [CrossRef]
- Arrebola, E.; Jacobs, R.; Korsten, L. Iturin a is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against postharvest fungal pathogens. J. Appl. Microbiol. 2010, 108, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Alvindia, D.G.; Natsuaki, K.T. Biocontrol activities of Bacillus amyloliquefaciens DGA14 isolated from banana fruit surface against banana crown rot-causing pathogens. Crop Prot. 2009, 28, 236–242. [Google Scholar] [CrossRef]
- Shi, H.; Ding, R.H.; Luo, Q.L.; Fu, J.; He, X.E. Study on storage tolerance of winter jujube fruit treated with natamycin combined with 1-MCP. Storage Process 2021, 21, 26–33. [Google Scholar]
- Mohamed, S.O.; Dharini, S.; Lise, K. Effect of bioinhibitor Bacillus amylolytica combined with 1-methylcyclopropylene treatment on postharvest disease control and quality maintenance of fruits. Storage Process 2011, 2, 56. [Google Scholar]
- Liu, K.; Zhao, H.L.; Zong, N.; Li, H.H.; Liu, Y.S.; Miao, M. Evaluation of inhibition and preservation effect of Bacillus subtilis BS-1 strain on postharvest soft rot of kiwi fruit. Storage Process 2021, 21, 40–49. [Google Scholar] [CrossRef]
- Horzum, Ö.; Güneş, N.T. Influence of storage technology and 1-methylcyclopropene on postharvest behavior of ‘Ankara’ pear. Tur. J. Agric. For. 2024, 48, 81–94. [Google Scholar] [CrossRef]
- Zuo, C.G.; Wang, J.Y.; Niu, X.X.; Guan, L.H.; Liu, P.; Yang, H.M.; Chu, M.; Wang, N.; Lin, Q.; Shi, Y.W. Effects of Bacillus Belez-BG-2 on storage quality and defense enzyme activity of muskmelon with thick skin. Microbiol. Bull. 2022, 49, 4171–4185. [Google Scholar] [CrossRef]
- Zheng, J.R.; Li, Y.Z. Research progress of induced resistance in plants. J. Mt. Agric. Biol. 2022, 41, 51–58. [Google Scholar] [CrossRef]
- Chen, J.Q.; Zhang, Y.M.; Zhang, L.M.; Han, H.G.; Shi, J.Y. Effects of different packaging methods on storage quality and antioxidant activity of fresh garlic. Food Res. Dev. 2021, 42, 28–34. [Google Scholar]
- Lv, Y.; Fu, A.; Song, X.; Wang, Y.; Chen, G.; Jiang, Y. 1-Methylcyclopropene and UV-C treatment effect on storage quality and antioxidant activity of ‘Xiaobai’ apricot fruit. Foods 2023, 12, 1296. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Wang, C.; Yi, P.; Li, L.; Wu, G.; Huang, F.; Huang, M.; Gan, T. The effects of combined 1-methylcyclopropene and melatonin treatment on the quality characteristics and active oxygen metabolism of mango fruit during storage. Foods 2023, 12, 1979. [Google Scholar] [CrossRef]
- Guo, S.; Li, T.; Wu, C.; Fan, G.; Wang, H.; Shen, D. Melatonin and 1-methylcyclopropene treatments on delay senescence of apricots during postharvest cold storage by enhancing antioxidant system activity. J. Food Process. Preserv. 2021, 45, e15863. [Google Scholar] [CrossRef]
- Krupa, T.; Kistechok, A.; Tomala, K. Estimating the physicochemical and antioxidant properties of hardy kiwi (Actinidia arguta) treated with 1-methylcyclopropene during storage. Agriculture 2023, 13, 1665. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).