Abstract
To accurately evaluate the role of storage temperature in improving the quality of fresh-cut fruits and vegetables, the effects of two storage temperatures (5 °C and 15 °C) on the phenylpropanoid pathway and sucrose metabolism in fresh-cut melon (cv. Yugu) cubes were determined. A higher temperature (15 °C) expedited sucrose decomposition in the melon cubes at the early stage of storage, resulting in higher levels of glucose and fructose. This effect was corroborated by increased activities of acid invertase (AI), neutral invertase (NI), and sucrose synthase cleavage (SS-c), along with higher expressions of CmAI1/2, CmNI1/2, and CmSS1/2 in the melon cubes at 15 °C. Additionally, the higher activity and gene expression of hexokinase in melon cubes at 15 °C led to an increase in the utilization rate of sugars toward downstream metabolic pathways. Moreover, the melon cube storage at 15 °C elevated the activities and gene expressions of phenylalanine ammonia lyase (PAL), cinnamic acid 4-hydroxylase (C4H), and 4-coumaric acid: CoA ligase (4CL), thereby increasing the synthesis of phenolics. Sucrose showed a significant negative correlation with PAL, C4H, and 4CL, as well as with CmPAL5/7 and CmC4H1/3. However, hexokinase displayed a significant positive correlation with PAL, C4H, and 4CL, as well as with CmPAL1, CmPAL3-9, CmC4Hs, and Cm4CLs. These findings demonstrate that a higher-temperature storage of melon cubes can accelerate the phenylpropanoid pathway and sucrose metabolism by regulating the activity and gene expression of related enzymes, thereby inducing phenolic accumulation. These results also indicate that lower-temperature storage is not conducive to the conversion of sugars into phenolics in fresh-cut melon. Therefore, the temperature can be appropriately and briefly raised in the production and preservation process of fresh-cut melon to obtain higher levels of phenolics.
1. Introduction
Melon (Cucumis melo L.) has become one of the favorite fruits among consumers due to its health-promoting properties, unique taste, and pleasant flavor [1]. However, the shelf-life of melon and fresh-cut melon at 25 °C is relatively short, and they need to be stored at lower temperatures to extend their shelf-life and maintain their original quality. Temperature is an important factor in maintaining the postharvest quality of horticultural products; thus, low-temperature storage has become a classic method for storing fruits and vegetables [2]. In recent years, the market demand for fresh-cut melon is growing rapidly because of its freshness and convenience [3]. However, melon peeling accelerates its quality deterioration accompanied by water-soaking, microbial pollution, etc. [4]; this forces fresh-cut melon to be stored in lower-temperature environments during the processing, transportation, and sales.
The fruits can form protective tissue on the surface of cut wounding, gaining self-healing ability and effectively preventing microbial invasion. Studies have shown that phenolics and lignins were accumulated around the wound of apples to achieve self-healing [5]. The accumulation of phenolics helps to improve the antioxidant capacity of fruits and vegetables. In higher plants, the synthesis of phenolic compounds starts from the shikimic acid pathway and undergoes the phenylpropanoid pathway. The three steps catalyzed by phenylalanine ammonia lyase (PAL), cinnamic acid 4-hydroxylase (C4H), and 4-coumaric acid: CoA ligase (4CL) constitute the common metabolic component of phenylpropanoid metabolism. These are the core reactions in plant growth and development, stress defense, and important secondary metabolite biosynthesis [6,7]. These three enzymes can be activated when horticultural crops are stimulated by adverse external environments [8,9]. The activities and transcriptional levels of PAL, C4H, and 4CL in fresh-cut potato cubes were enhanced by the treatment of methyl jasmonate, resulting in higher contents of flavonoids and phenolics [10]. Storage temperature also affects the activity of enzymes related to phenylpropanoid metabolism in horticultural products and the synthesis speed of phenolic compounds. The comparison of fresh-cut cantaloupe melon stored at 0, 5, and 10 °C for 14 days showed that storage at 0 °C was optimal for avoiding the increase in microbial load and the loss of vitamin C, but storage at 10 °C could better maintain the balance of esters and phenolics [11]. Therefore, a lower storage temperature (0 °C–5 °C) is beneficial to maintain the appearance and commercialization of fresh-cut fruits, but it may actually be unfavorable for increasing the phenolics content.
In addition, sugars are converted into phosphoenolpyruvate and 4-phosphate erythritol through glycolysis and hexose monophosphate shunt, and further converted into phenylalanine and shikimic acid required for the synthesis of phenolic compounds [12]. Sucrose, fructose, and glucose are important components in postharvest fruits, and can provide essential energy and ingredients for the alterations in fruit taste and flavor [13]. The metabolism of these soluble sugars in plants is regulated by multiple enzymes. The synthesis and dissociation of sucrose in various cellular compartments are controlled by sucrose synthase synthesis (SS-s), sucrose synthase cleavage (SS-c), sucrose phosphate synthase (SPS), acid invertase (AI), and neutral invertase (NI) [14]. Glucose and fructose are then phosphorylated under the action of hexokinase (HXK) and fructokinase, respectively, to produce glucose-6-phosphate and fructose-6-phosphate, which enter other metabolic pathways, such as the phenylpropanoid pathway [15]. Previous studies have verified that sucrose is a substance in regulating the plant response to various stresses, and it can effectively improve the ability of fruit to resist chilling injury [16]. Zhang et al. [17] pointed out that the transcription levels of enzymes involved in sugar metabolism were markedly changed by storage temperatures, which resulted in the higher sweetness in Hami melons during storage at 21 °C compared with samples stored at 3 °C. However, compared to papaya fruit stored at 6 °C, storage at 1 °C could promote higher activities and gene expressions of SS, SPS, and NI, and decrease the activity and gene expression of AI, which led to the accumulation of sucrose and slower degradation of hexose, and contributed to the alleviation of chilling injury in papaya fruit [18].
In summary, the molecular mechanisms of sugar and phenylpropanoid metabolism in fruits and vegetables are rather complicated. There is limited research on the relationship between sucrose metabolism and phenolic compound accumulation in fresh-cut fruits. It is currently unclear how the use of storage temperatures affects the sucrose metabolism and phenylpropanoid pathway to improve the quality of fresh-cut melon. Therefore, the purpose of this research was to reveal the molecular mechanism behind sucrose metabolism and the phenylpropanoid pathway in fresh-cut melon cubes during storage at different temperatures in order to evaluate how to precisely regulate temperature during the processing and storage to control or improve the quality of fresh-cut melon.
2. Materials and Methods
2.1. Melon Harvesting and Treatments
The melon fruit ‘Yugu’ (Cucumis melo L. var. cantalupensis Nand.) was harvested at the commercially mature stage (35 days after flowering) and transported to our laboratory within three hours. The intact melons were then cut into 2 cm × 2 cm × 2 cm cubes, which were packaged into transparent, environmentally friendly food-grade plastic boxes. Each box, containing only one fruit, weighed approximately 120 ± 10 g. Fresh-cut samples were stored at 5 °C (5 °C group) and 15 °C (15 °C group), respectively. Samples in the 15 °C group were sampled daily over a 4-day storage period, while those in the 5 °C group were sampled on days 1, 2, 4, and 8. At each time point, each group consisted of three biological replicates, with each replicate comprising 10 fruits. The samples were frozen by liquid nitrogen and stored at −80 °C for subsequent analyses. The experiments were repeated twice, and the melon fruit ‘Xizhoumi-17’ was used in the second iteration.
2.2. Determination of the Contents of the Total Phenolics and Soluble Sugars
Total phenolics were extracted and measured based on the method of Singleton and Rosi [19] with slight modifications. Three grams of melon powder was homogenized using 25 mL of chilled methanol for 12 h at 4 °C, followed by centrifugation at 12,000× g for 15 min at 4 °C. After centrifugation, 0.5 mL of supernatant was mixed with 1.0 mL of Folin–Ciocalteu reagent, 1.5 mL of deionized water, and 1 mL of 7.5% (w/v) Na2CO3, and then the reaction mixture was placed in darkness for 2 h at 25 °C. The absorbance at 765 nm was measured. The total phenolics content was expressed as gram of gallic acid equivalent per kilogram of fresh weight (g gallic acid kg−1).
Soluble sugars (fructose, glucose, and sucrose) were obtained and measured using our previously established method [20]. The contents of sugars were calculated using standard curves with the aid of an HPLC system (Model 2695, Waters, Milford, MA, USA) equipped with a refractive index detector (Model 2414, Waters, USA) and an InertSustain NH2 Column (5 μm, 4.6 × 250 mm, GL Sciences, Tokyo, Japan), and were expressed as g kg−1 on a fresh weight basis.
2.3. Assessment of Enzymes Activities in Sucrose Metabolism and Phenylpropanoid Pathway
Enzymes related to sucrose metabolism were extracted according to our experimental method [20] and all steps were conducted at 4 °C. Finally, the dialyzed supernatant was used as crude enzyme extracts for assessing enzyme activities involved in sugar metabolism. The activities of AI, NI, SS-c, SS-s, and SPS were determined using our previously established method [20]. One unit of enzyme activity was calculated as the amount of enzyme producing 1 μmol of glucose or sucrose per hour, and the results were reported as U mg−1 according to the protein content.
The HXK activity was determined using our previously established method [21]. Melon powder (2.0 g) was extracted using 4 mL of ice-cold HEPES-KOH buffer (pH 8.0, 50 mmol L−1, containing 2 mmol L−1 benzamidine, 2 mmol L−1 EDTA, 2.5 mmol L−1 dithiothreitol, 0.1 mmol L−1 leupeptin, 5 mmol L−1 MgCl2, 4% (w/v) polyvinyl pyrrolidone (PVP), 1% (v/v) Triton X-100, 2% (v/v) glycerin, and 0.1% (w/v) bovine serum albumin). The homogenate was centrifuged at 12,000× g for 10 min at 4 °C, and the supernatant was used to determine HXK activity. The reaction mixture contained 0.9 mL of 50 mmol L−1 HEPES-KOH (including 4 mmol L−1 MgCl2, 10 mmol L−1 glucose, 2.5 mmol L−1 ATP, 1 U glucose-6-phosphate dehydrogenase, and 0.4 mmol L−1 NADP) and 0.1 mL enzyme extract. HXK activity was calculated based on a 0.01 change in absorbance at 340 nm per min and reported as U g−1 according to the protein content.
The activities of PAL, C4H, and 4CL were obtained and analyzed using our previous described experimental method [21] and reported as U g−1 according to the protein content.
2.4. qRT-PCR Analysis
Eleven key genes of the phenylpropanoid pathway and twelve key genes of sucrose metabolism were sought from the CuGenDB (http://cucurbitgenomics.org/) (6 June 2021), and these genes were selected for gene expression analysis. Total RNA and first-strand cDNA were obtained using our previously established method [20]. Primer 6.0 software (PREMIER Biosoft International, San Francisco, CA, USA) was employed to design gene-specific primers (see Table S1); the specificity of these primers was confirmed through gel electrophoresis and melting curve analyses. β-actin was used as a housekeeping gene to normalize the expression of target genes using the “Comparative 2−ΔΔCT” method in the qRT-PCR procedure according to our previously established experimental methodology [20].
2.5. Statistical Analysis
The values were presented as the mean ± standard error (SE) of three replicates. The data from the 5 °C and 15 °C groups were analyzed using one-way analysis of variance (ANOVA) and Student’s t-test to determine statistical significance at the 0.05 level using SPSS 19.0 (SPSS Inc., Chicago, IL, USA). Pearson correlation analysis was conducted using Origin 2022 (OriginLab Corporation, Northampton, MA, USA).
3. Results
3.1. Soluble Sugars and Total Phenolics
As shown in Figure 1, the glucose and fructose contents in the 5 °C group showed a downward trend during storage. However, in the 15 °C samples, these levels initially rose, reaching their maximum values on days 1 and 2, respectively, followed by a dramatic decrease. In addition, the fructose and glucose contents in melon cubes at 15 °C were obviously higher than those at 5 °C on days 1 and 2 (Figure 1A,B, p < 0.05). The sucrose content in melon cubes decreased over time and was consistently lower in the 15 °C group (Figure 1C, p < 0.05). The total phenolics content in both the 5 °C and 15 °C group increased during storage, with notably higher levels maintained in the 15 °C group from day 2 to day 4 compared to the 5 °C group (Figure 1D, p < 0.05). These results mean that a higher-temperature storage (15 °C) of melon cubes accelerates the reduction in sucrose and increases the levels of monosaccharides (glucose and fructose) and total phenolics.
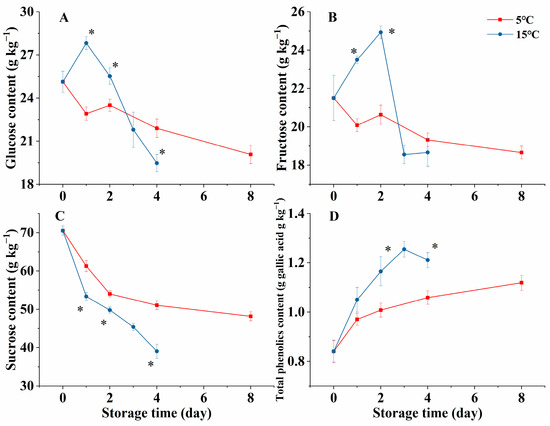
Figure 1.
Effect of storage temperatures (5 °C and 15 °C) on the levels of glucose (A), fructose (B), sucrose (C), and total phenolics (D) in fresh-cut melon cubes. Values are means ± SE (n = 3) and the bars represent the SE. * indicates statistically significant differences (p < 0.05) at the sampling point according to Student’s t-test.
3.2. Enzyme Activity Involved in Sucrose Metabolism and Phenylpropanoid Pathway
As shown in Figure 2A, AI activity in the 15 °C group rose dramatically during the initial 3 days of storage, and then declined rapidly on day 4. It remained lower in the 5 °C group compared to the 15 °C group throughout the storage period (p < 0.05). However, NI activity in the 15 °C group increased during storage and reached 67.69 U mg−1 at day 4; like AI, it was consistently lower in the 5 °C group (Figure 2B, p < 0.05). SS-c activity in both groups rose during the first two days and then declined; the activity in the 15 °C group was higher on days 2 and 4 compared to the 5 °C group (Figure 2C, p < 0.05). SS-s and SPS activities in melon cubes decreased over time. However, significantly higher SPS activity was observed in the melon cubes stored at 15 °C from day 2 to day 4 compared with those in the 5 °C group (Figure 2D,E, p ˂ 0.05). Although HXK activity in both groups rose markedly from day 0 to day 2 and then decreased during later storage, the HXK activity in the 15 °C group was consistently higher than in the 5 °C group (Figure 2F, p ˂ 0.05).
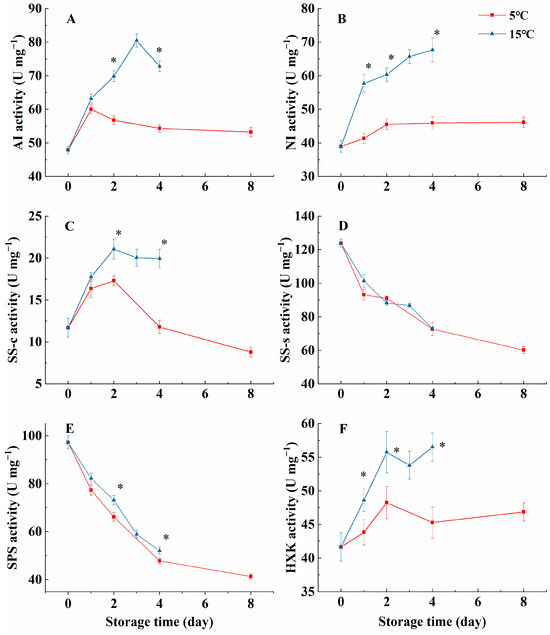
Figure 2.
Effect of storage temperatures (5 °C and 15 °C) on the activities of AI (A), NI (B), SS-c (C), SS-s (D), SPS (E), and HXK (F) in fresh-cut melon cubes. Values are means ± SE (n = 3) and the bars represent the SE. * indicates statistically significant differences (p < 0.05) at the sampling point according to Student’s t-test.
PAL, C4H, and 4CL activities are closely associated with the synthesis of phenolics. As demonstrated in Figure 3A, PAL activity in melon cubes increased over the storage period and was consistently higher in the 15 °C group than in the 5 °C group (p < 0.05). Similarly, C4H activity in melon cubes showed an increasing trend throughout the storage period, and it was higher in melon cubes stored at 15 °C compared with those stored at 5 °C (Figure 3B, p < 0.05). 4CL activity in melon cubes stored at 5 °C increased from day 0 to day 2 and then remained stable, reaching a maximum value of 1.59 U mg−1 on day 8. Similar to PAL and C4H, 4CL activity was notably stimulated by the higher-temperature storage (Figure 3C, p < 0.05).
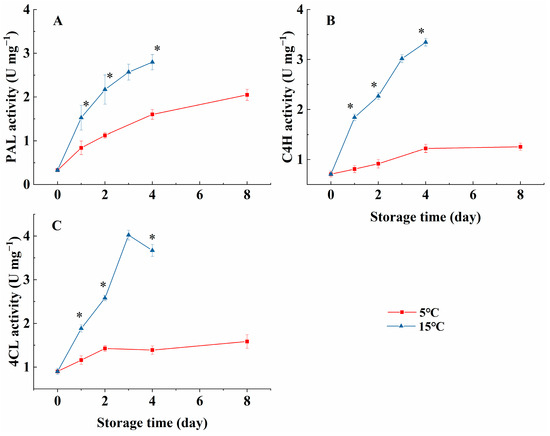
Figure 3.
Effect of storage temperatures (5 °C and 15 °C) on the PAL (A), C4H (B), and 4CL (C) activities in fresh-cut melon cubes. Values are means ± SE (n = 3) and the bars represent the SE. * indicates statistically significant differences (p < 0.05) at the sampling point according to Student’s t-test.
3.3. Gene Expression in Sucrose Metabolism
The CmAI1/2 expressions in the 15 °C group were up-regulated from day 0 to day 3 and then down-regulated as displayed in Figure 4. Compared with the 5 °C group, higher-temperature storage increased the expressions of CmAI1/2 by 0.71 and 1.79 times on the 2nd day, respectively (p < 0.05). Similar to CmAI1/2, the expressions of CmNI1/2 in the 15 °C group were increased firstly and then decreased, reaching their maximum values (2.99 and 9.36) on the second and third days, respectively. Their expressions remained higher than those in the 5 °C group throughout the storage period (Figure 4, p < 0.05).
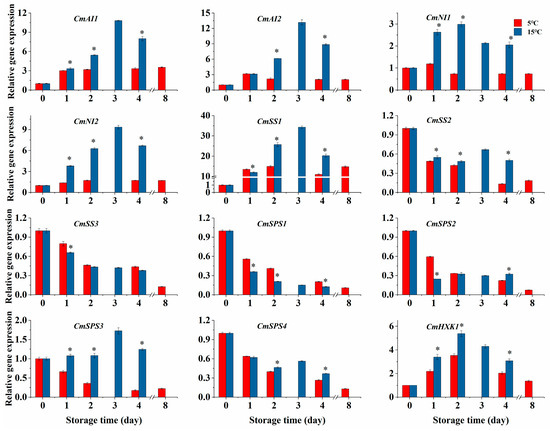
Figure 4.
Effect of storage temperatures (5 °C and 15 °C) on the expressions of CmAI1/2, CmNI1/2, CmSS1/2/3, CmSPS1/2/3/4, and CmHXK1 in fresh-cut melon cubes. Values are means ± SE (n = 3) and the bars represent the SE. * indicates statistically significant differences (p < 0.05) at the sampling point according to Student’s t-test.
The CmSS1/2/3 expressions in melon cubes were influenced to varying degrees by higher-temperature storage. CmSS1 expression in the 5 °C group increased from day 0 to day 2 and decreased transiently on day 4, followed by an increase on day 8, while its expression in the 15 °C group was significantly enhanced from day 2 to day 4 in comparison with the 5 °C group (Figure 4, p < 0.05). As displayed in Figure 4, the higher-temperature storage of melon cubes prevented the decrease in CmSS2 expression compared with the 5 °C group. However, CmSS3 expression decreased over storage time at both temperatures and, in the 15 °C group, it was only down-regulated on day 1 compared with the 5 °C group. The expressions of CmSPS1/2/4 in the 5 °C group declined over time, while CmSPS3 expression decreased in the initial 4 days and increased slightly on day 8. Compared with the 5 °C group, the higher-temperature storage of melon cubes accelerated the reduction in CmSPS1 expression and increased the expression of CmSPS3, while the CmSPS4 expression in the 15 °C group was enhanced on days 2 and 4. In addition, compared with the 5 °C group, CmSPS2 expression in the 15 °C group decreased on day 1 and increased on day 4. The CmHXK1 expression in both groups rose dramatically in the initial 2 days and declined until the end of storage, with significantly higher levels in the 15 °C group (Figure 4, p < 0.05).
3.4. Gene Expression in Phenylpropanoid Pathway
As demonstrated in Figure 5, the CmPAL1/2/4/5 expressions in the 15 °C group enhanced noteworthily from day 0 to day 2, followed by a decrease, while the expressions of CmPAL3//6/7/8/9 in the 15 °C group were significantly increased during the first three days and then declined at the end of storage. Moreover, in comparison with the 5 °C group, higher-temperature storage increased the expressions of CmPAL1-9 in melon cubes, and the expressions of CmPAL1-9 in the 15 °C group were increased by 7.75, 11.34, 3.10, 10.97, 17.34, 1.64, 3.00, 9.00, and 3.78 times, respectively, on the second day (Figure 5, p < 0.05).
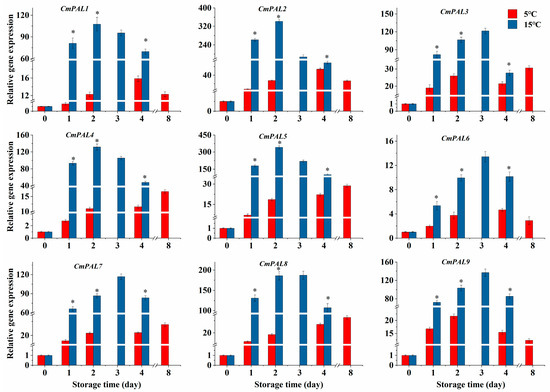
Figure 5.
Effect of storage temperatures (5 °C and 15 °C) on the expressions of CmPAL1-9 in fresh-cut melon cubes. Values are means ± SE (n = 3) and the bars represent the SE. * indicates statistically significant differences (p < 0.05) at the sampling point according to Student’s t-test.
The CmC4Hs expressions in the 5 °C group increased over the storage time (Figure 6). However, the CmC4H1/4 expressions in the 15 °C group enhanced dramatically during the initial three days of storage and declined rapidly on the fourth day, while CmC4H2 expression was only up-regulated from day 0 to day 2 and CmC4H3 expression increased throughout the storage period. Overall, the expressions of CmC4Hs were elevated to varying degrees by higher-temperature storage in comparison with the 5 °C group (Figure 6, p < 0.05). Similarly, the expressions of Cm4CLs in fresh-cut cubes at 15 °C were also significantly risen in comparison with those at 5 °C, and the expressions of Cm4CL1/2/3 were enhanced by 11.42, 14.27, and 3.31 times, respectively, on the second day of higher-temperature storage (Figure 6, p < 0.05).
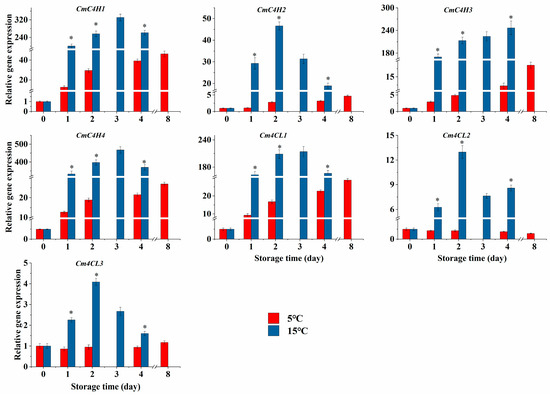
Figure 6.
Effect of storage temperatures (5 °C and 15 °C) on expressions of CmC4H1-4 and Cm4CL1/2/3 in fresh-cut melon cubes. Values are means ± SE (n = 3) and the bars represent the SE. * indicates statistically significant differences (p < 0.05) at the sampling point according to Student’s t-test.
3.5. Correlation Analysis
The Pearson correlation analysis of metabolites, enzymes, and genes related to sucrose metabolism and the phenylpropanoid pathway is shown in Figure 7. Sucrose content was negatively correlated with the levels of enzymes and genes involved in the phenylpropanoid pathway, with significant correlation coefficients of −0.96, −0.80, and −0.78 between sucrose and PAL, C4H, and 4CL, respectively (p < 0.05). In addition, significant correlations were found between sucrose and the expressions of CmPAL5, CmPAL7, CmC4H1, and CmC4H3, with correlation coefficients of −0.75, −0.72, −0.69, and −0.67, respectively (p < 0.05). Conversely, the activity and expression of HXK were positively correlated with levels of enzymes and genes involved in the phenylpropanoid pathway. Meanwhile, there was a significant correlation between HXK and PAL, C4H, and 4CL, with correlation coefficients of 0.88, 0.91, and 0.89, respectively (p < 0.05). Moreover, HXK had a significant correlation with CmPAL1, CmPAL3-9, CmC4Hs, and Cm4CLs, and the correlation coefficients were all greater than 0.65. These results suggest that sucrose decomposition is beneficial for phenolic synthesis in fresh-cut melon.
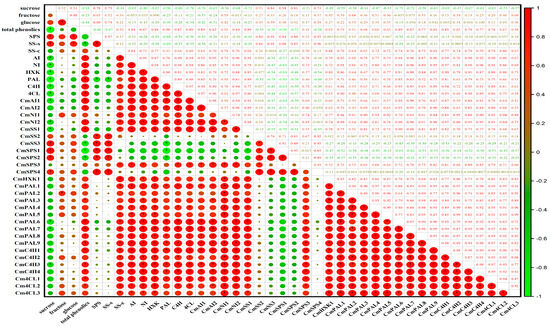
Figure 7.
Pearson correlation analysis of data in fresh-cut melon cubes storage at 5 °C and 15 °C. * indicates statistically significant differences (p < 0.05). The numbers are the correlation coefficients between metabolites, enzymes, and genes during the storage of fresh-cut melon. The size of the circle represents the value of the correlation coefficient.
4. Discussion
It has been reported that cut wounding could promote the accumulation of phenolics in horticultural products. In addition, some studies have confirmed that higher temperatures enhance the phenolic accumulation induced by cut wounding in pitaya [8,9], onion [22], and carrot [23]. However, research on the molecular mechanisms underlying phenolic accumulation induced by cut wounding in Cucurbitaceae species has been lacking. In our study, we explored the enzyme and genetic functional changes in the phenylpropanoid pathway and sucrose metabolism under different storage temperature conditions, and how these changes may affect the total phenolics content of the fresh-cut melon fruit ‘Yu gu’. Although cutting accelerated the quality deterioration of melon, it can still maintain high quality in the first two days of storage at 15 °C, as demonstrated in our previous study [20]. Our results indicate that a higher-temperature storage of melon accelerates sucrose decomposition, increases the production of fructose and glucose, and intensifies the phosphorylation of hexose. These processes elevate the level of sugar conversion, accompanied by the increase in the phenylpropanoid pathway and phenolics content.
The physiological metabolism of fruits during preservation and storage is quite complicated. Storage at lower temperatures, a classic storage method, significantly affects fruit sugars content, firmness, aromatic substances, etc., and these cause changes in fruit quality [24]. Many reports have shown that melon is often stored at a low temperature to maintain its freshness and extend its postharvest life [25]. Sugars are not only quality indicators of melon but also play critical roles in regulating fruit flavor and stress responses [26]. Li et al. [26] indicated that tomato fruit treated with methyl jasmonate improved its post-ripening quality via regulating the enzyme activity and transcript levels in sugar metabolism. In the present study, fresh-cut melon storage at 15 °C maintained lower sucrose levels and higher hexose levels early in storage compared with melon stored at 5 °C. These imply that higher-temperature storage can effectively accelerate the sucrose decomposition, which may be related to the increased rate of sugar conversion by higher temperature as indicated by the enhanced AI, NI, and SS-c activities. Sucrose, MYB, and miRNA synergistically regulated the phenylpropanoid pathway in potatoes, affecting the biosynthesis of flavonoids, where overexpression of StAN1 enhanced the invertase activity, allowing sucrose to produce hexose required for flavonoid biosynthesis through the roles of regulation and metabolism [27]. Meanwhile, a significant correlation between the invertase activity and the total phenolics content was found, as shown in Figure 7. Combining the above results, we speculated that a higher temperature can promote sucrose decomposition, which is beneficial for phenolic synthesis. This was in accordance with the finding that nitrous oxide induced higher phenolics levels during the storage of red raspberries via regulating sucrose metabolism enzymes [28]. When plants are subjected to external stress, the altered enzyme activity is usually accompanied by changes in related genes. In order to gain a deeper understanding of how higher temperature regulates sucrose metabolism, the transcription levels of key genes in sucrose metabolism were evaluated in the current research. The results manifested that higher temperature up-regulated the expressions of CmAI1/2, CmNI1/2, CmSS1/2, and CmSPS3/4; these increased gene expressions correspond directly to the AI, NI, SS-c, and SPS activities, and exhibited positive correlations with the corresponding enzymes.
Many studies have shown that the accumulation of soluble sugars in postharvest fruits increases their quality and flavor [29]. Fresh-cut melon cubes had superior quality in the initial two days of storage at 15 °C [20,30], characterized by higher hexose levels. Moreover, our results indicate that melon cube storage at 15 °C accelerated hexose decomposition after two days of storage; this may be due to the higher storage temperature inducing a higher respiration rate in melon cubes, in which glucose is oxidized to form simpler molecules, such as CO2, water, and the release of energy required for plant biochemical processes [31,32]. HXK, an important rate-limiting enzyme in glycolysis, acts a critical role in transmitting sugar signals and regulating the ability to resist stress in plants [33,34]. The increased activity and gene expression of HXK in melon cubes at 15 °C could enhance the glycolysis by stimulating the phosphorylation of hexose, producing more necessary substrates and energy for secondary metabolic pathways [21,35,36]. Furthermore, exogenous glucose treatment of apple tissues revealed that MdHXK1 can phosphorylate and stabilize MdbHLH3, thereby regulating anthocyanin accumulation [37]. In the current study, the total phenolics content was positively correlated with the HXK activity and CmHXK1 expression. Based on the above results, the increased CmHXK1 expression in melon cubes may promote the accumulation of phenolics through transmitting sugar signals.
Phenolics, synthesized by PAL, C4H, and 4CL enzymes via the phenylpropanoid pathway, act as critical secondary metabolites affecting the flavor, color, and antioxidant capacity in fresh-cut products [38,39]. Many studies have indicated that horticultural products can produce more phenolic substances via enhancing the phenylpropanoid pathway after mechanical injury, which leads to an increase in their nutritional value and antioxidant capacity, such as in melon [21] and potato [10]. A study on fresh-cut pitaya fruit suggested that the gene expressions and activities of PAL, C4H, and 4CL were increased to enhance the phenylpropanoid pathway by methyl salicylate fumigating, which increased the contents of total flavonoids and phenolics [40]. In the current study, it was also found that the PAL, C4H, and 4CL activities and total phenolics content in fresh-cut melon cubes stored at 15 °C were significantly higher than those stored at 5 °C, and these enzymes were positively correlated with total phenolics content. Changes in plant enzyme activity are often closely related to gene levels. Research has confirmed that the gene expressions and activities of PAL, C4H, and 4CL in pitaya fruit were increased simultaneously under the induction of cut wounding; similar findings were also found in fresh-cut melon according to our previous reports [9,21]. In this study, the transcription levels of CmPALs, CmC4Hs, and Cm4CLs in melon cubes were up-regulated by a higher storage temperature, which is consistent with the activity of these three enzymes. These findings are similar to an earlier study where higher-temperature storage increased the expressions of StPAL1 and St4CL to heighten the formation of lignins and phenolics during the healing of potato [41]. From this, it can be seen that lower storage temperatures may actually be unfavorable for activating the phenylpropanoid pathway and accumulating phenolics. Our research indicates that fresh-cut melon cubes obtained preferred quality and flavor when they produced more soluble sugars and phenolic substances during the early two days of storage at 15 °C [20]. To gain a clearer understanding of the impact of higher-temperature storage (15 °C) on the phenylpropanoid pathway and sucrose metabolism in fresh-cut melon, a hypothetical model was established as illustrated in Figure 8. Therefore, we can temporarily store fresh-cut melon cubes at a temperature of 15 °C for 1–2 days, and then transfer them to 4 °C, which may maintain better sweetness and higher commercial values. However, further research is needed on how to precisely control temperature and time for storing fresh-cut melon to ensure their high nutritional values, as well as how these genes participate in the synthesis of phenolic compounds in fruits methodically. Additionally, the ripening process in fruits is very complex, involving physiological and biochemical reactions that contribute to the formation of fruit flavor. These reactions trigger the reprogramming of multiple ripening-associated genes, leading to changes in fruit flavor and quality indicators [42]. For example, ethylene plays a very important role in all these events, particularly in climacteric fruits like melon [43,44]. The findings in this study are not independent of the various biochemical and molecular processes that occur during ripening. Therefore, further research is also needed on the interaction between temperature and signaling molecules to reveal the mechanism of quality changes during the storage of fresh-cut products. This study provides new ideas and a basis for precise quality control in the production and preservation process of fresh-cut melon, and further reveals the molecular mechanisms of the phenylpropanoid pathway and sucrose metabolism during the storage of melon cubes at different temperatures.
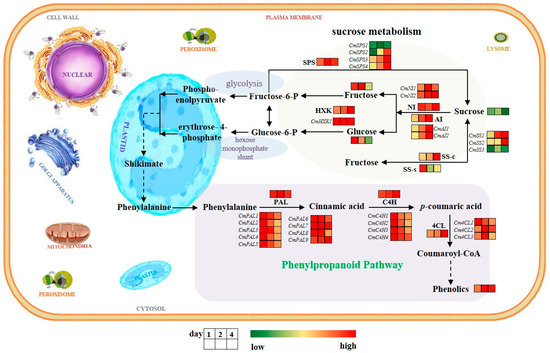
Figure 8.
A hypothetical model is used to further understand the effect of temperatures on phenylpropanoid pathway and sucrose metabolism in fresh-cut melon. Red and green indicate higher-temperature storage significantly increased or decreased, respectively, the levels of metabolites, enzymes, or genes in melon cubes (p < 0.05) compared with the 5 °C group. Yellow means no significant difference (p < 0.05).
5. Conclusions
The current study revealed molecular changes in the phenylpropanoid pathway and sucrose metabolism during the storage of melon cubes at 5 °C and 15 °C. Our results suggest that the higher-temperature storage (15 °C) of fresh-cut melon cubes can accelerate the sucrose decomposition to supply the necessary substrates required for the phenylpropanoid pathway, thereby inducing phenolic accumulation. These results will contribute to the improvement and maintenance of nutrient levels during fresh-cut melon processing and storage by precisely regulating the temperature. The high correlation analysis data between these enzymes and genes will help to further elucidate the regulatory mechanism of phenolic synthesis in fresh-cut melon.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10050488/s1, Table S1. Primers used in this study.
Author Contributions
Conceptualization, methodology, investigation, and visualization, Z.W. and Y.L.; writing—original draft preparation, Z.W. and Z.Y. (Zengyan Yang); data curation and validation, Y.G. and Z.Z.; resources and funding acquisition, C.X. and Z.Y. (Zhifang Yu); project administration and writing—reviewing and editing, L.W., Y.G., and Z.Y. (Zhifang Yu). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Hebei Province (C2022408017), Science and Technology Project of Hebei Education Department (QN2022174), and Doctoral Scientific Research Startup Fund of Langfang Normal University (XBQ202141).
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interests.
References
- Martínez, C.; Valenzuela, J.L.; Jamilena, M. Genetic and Pre- and Postharvest Factors Influencing the Content of Antioxidants in Cucurbit Crops. Antioxidants 2021, 10, 894. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Flores, F.B. Employing phytosulfokine α (PSKα) for delaying broccoli florets yellowing during cold storage. Food Chem. 2021, 355, 129626. [Google Scholar] [CrossRef] [PubMed]
- Spadafora, N.D.; Cocetta, G.; Cavaiuolo, M.; Bulgari, R.; Dhorajiwala, R.; Ferrante, A.; Spinardi, A.; Rogers, H.J.; Müller, C.T. A complex interaction between pre-harvest and post-harvest factors determines fresh-cut melon quality and aroma. Sci. Rep. 2019, 9, 2745. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.H.; Bashir, O.; Khan, S.; Wahid, A.; Makroo, H.A. 4—Fresh-cut products: Processing operations and equipments. In Fresh-Cut Fruits and Vegetables; Siddiqui, M.W., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 77–97. [Google Scholar]
- Zhang, X.; Zong, Y.; Li, Z.; Yang, R.; Li, Z.; Bi, Y.; Prusky, D. Postharvest Pichia guilliermondii treatment promotes wound healing of apple fruits. Postharvest Biol. Technol. 2020, 167, 111228. [Google Scholar] [CrossRef]
- Dong, N.-Q.; Lin, H.-X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.M.; Chapple, C. The phenylpropanoid pathway in Arabidopsis. Arab. Book 2011, 9, e0152. [Google Scholar] [CrossRef] [PubMed]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Díaz-López, V.; Artés, F.; Artés-Hernández, F. Effects of UV-B and UV-C combination on phenolic compounds biosynthesis in fresh-cut carrots. Postharvest Biol. Technol. 2017, 127, 99–104. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Wang, L.; Wang, J.; Jin, P.; Zheng, Y. Methyl jasmonate primes defense responses against wounding stress and enhances phenolic accumulation in fresh-cut pitaya fruit. Postharvest Biol. Technol. 2018, 145, 101–107. [Google Scholar] [CrossRef]
- Zhou, F.; Jiang, A.; Feng, K.; Gu, S.; Xu, D.; Hu, W. Effect of methyl jasmonate on wound healing and resistance in fresh-cut potato cubes. Postharvest Biol. Technol. 2019, 157, 110958. [Google Scholar] [CrossRef]
- Amaro, A.L.; Spadafora, N.D.; Pereira, M.J.; Dhorajiwala, R.; Herbert, R.J.; Müller, C.T.; Rogers, H.J.; Pintado, M. Multitrait analysis of fresh-cut cantaloupe melon enables discrimination between storage times and temperatures and identifies potential markers for quality assessments. Food Chem. 2018, 241, 222–231. [Google Scholar] [CrossRef]
- Hu, W.; Sarengaowa; Guan, Y.; Feng, K. Biosynthesis of Phenolic Compounds and Antioxidant Activity in Fresh-Cut Fruits and Vegetables. Front. Microbiol. 2022, 13, 906069. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, Y.; Zhou, D.; Zhang, Q.; Pan, L.; Tu, K. Transcriptomics analysis provides insights into metabolisms of sugars and carotenoids of nectarine fruit subjected to different temperature storage. Sci. Hortic. 2022, 304, 111262. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Wei, M.; Ge, Y.; Tang, Q.; Xue, W.; Zhang, S.; Wang, W.; Lv, J. Effects of trisodium phosphate treatment after harvest on storage quality and sucrose metabolism in jujube fruit. J. Sci. Food Agric. 2019, 99, 5526–5532. [Google Scholar] [CrossRef] [PubMed]
- Granot, D.; David-Schwartz, R.; Kelly, G. Hexose Kinases and Their Role in Sugar-Sensing and Plant Development. Front. Plant Sci. 2013, 4, 44. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Jiang, S.; Xu, F.; Wang, H.; Wei, Y.; Shao, X. PpINH1, an invertase inhibitor, interacts with vacuolar invertase PpVIN2 in regulating the chilling tolerance of peach fruit. Hortic. Res. 2020, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shan, C.; Song, W.; Cai, W.; Zhou, F.; Ning, M.; Tang, F. Transcriptome analysis of starch and sucrose metabolism change in Gold Queen Hami melons under different storage temperatures. Postharvest Biol. Technol. 2021, 174, 111445. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, Q.; Pan, Y.; Zhang, Z.; Yuan, R.; Nie, Y. Abnormal behavior of chilling injury in postharvest papaya fruit is associated with sugar metabolism. J. Food Sci. 2022, 87, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar] [CrossRef]
- Wu, Z.; Tu, M.; Yang, X.; Xu, J.; Yu, Z. Effect of cutting and storage temperature on sucrose and organic acids metabolism in postharvest melon fruit. Postharvest Biol. Technol. 2020, 161, 111081. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, Z.; Li, R.; Wang, L.; Xie, C.; Wu, Z. Cut-Wounding Promotes Phenolic Accumulation in Cucumis melo L. Fruit (cv. Yugu) by Regulating Sucrose Metabolism. Horticulturae 2023, 9, 258. [Google Scholar] [CrossRef]
- Sharma, K.; Ko, E.Y.; Assefa, A.D.; Ha, S.; Nile, S.H.; Lee, E.T.; Park, S.W. Temperature-dependent studies on the total phenolics, flavonoids, antioxidant activities, and sugar content in six onion varieties. J. Food Drug Anal. 2015, 23, 243–252. [Google Scholar] [CrossRef]
- Han, C.; Li, J.; Jin, P.; Li, X.; Wang, L.; Zheng, Y. The effect of temperature on phenolic content in wounded carrots. Food Chem. 2017, 215, 116–123. [Google Scholar] [CrossRef]
- Hong, K.; Xu, H.; Wang, J.; Zhang, L.; Hu, H.; Jia, Z.; Gu, H.; He, Q.; Gong, D. Quality changes and internal browning developments of summer pineapple fruit during storage at different temperatures. Sci. Hortic. 2013, 151, 68–74. [Google Scholar] [CrossRef]
- Wang, J.; Mao, L.-C.; Li, X.-W.; Lv, Z.; Liu, C.-H.; Huang, Y.-Y.; Li, D.-D. Oxalic acid pretreatment reduces chilling injury in Hami melons (Cucumis melo var. reticulatus Naud.) by regulating enzymes involved in antioxidative pathways. Sci. Hortic. 2018, 241, 201–208. [Google Scholar] [CrossRef]
- Li, J.; Min, D.; Li, Z.; Fu, X.; Zhao, X.; Wang, J.; Zhang, X.; Li, F.; Li, X.; Guo, Y. Regulation of Sugar Metabolism by Methyl Jasmonate to Improve the Postharvest Quality of Tomato Fruit. J. Plant Growth Regul. 2022, 41, 1615–1626. [Google Scholar] [CrossRef]
- Lin, S.; Singh, R.K.; Moehninsi; Navarre, D.A. R2R3-MYB transcription factors, StmiR858 and sucrose mediate potato flavonol biosynthesis. Hortic. Res. 2021, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Liu, Z.; Wang, J.; Zhu, S.; Huang, D. Nitric oxide modulates sugar metabolism and maintains the quality of red raspberry during storage. Sci. Hortic. 2019, 256, 108611. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, C.; Brummell, D.A.; Qi, S.; Lin, Q.; Bi, J.; Duan, Y. Salicylic acid treatment mitigates chilling injury in peach fruit by regulation of sucrose metabolism and soluble sugar content. Food Chem. 2021, 358, 129867. [Google Scholar] [CrossRef]
- Wu, Z.; Tu, M.; Yang, X.; Xu, J.; Yu, Z. Effect of cutting on the reactive oxygen species accumulation and energy change in postharvest melon fruit during storage. Sci. Hortic. 2019, 257, 108752. [Google Scholar] [CrossRef]
- Fonseca, S.C.; Oliveira, F.A.R.; Brecht, J.K. Modelling respiration rate of fresh fruits and vegetables for modified atmosphere packages: A review. J. Food Eng. 2002, 52, 99–119. [Google Scholar] [CrossRef]
- Aguayo, E.; Escalona, V.H.; Rtés, F.A. Metabolic Behavior and Quality Changes of Whole and Fresh Processed Melon. J. Food Sci. 2004, 69, SNQ148–SNQ155. [Google Scholar] [CrossRef]
- Durán-Soria, S.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Sugar Signaling During Fruit Ripening. Front. Plant Sci. 2020, 11, 564917. [Google Scholar] [CrossRef]
- Xu, W.; Wei, Y.; Wang, X.; Han, P.; Chen, Y.; Xu, F.; Shao, X. Molecular cloning and expression analysis of hexokinase genes in peach fruit under postharvest disease stress. Postharvest Biol. Technol. 2021, 172, 111377. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Wang, J.; Wang, L.; Han, C.; Jin, P.; Zheng, Y. Methyl jasmonate enhances wound-induced phenolic accumulation in pitaya fruit by regulating sugar content and energy status. Postharvest Biol. Technol. 2018, 137, 106–112. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Min, D.; Ji, N.; Zhang, X.; Li, F.; Zheng, Y. Transcriptomic analysis reveals key genes associated with the biosynthesis regulation of phenolics in fresh-cut pitaya fruit (Hylocereus undatus). Postharvest Biol. Technol. 2021, 181, 111684. [Google Scholar] [CrossRef]
- Hu, D.-G.; Sun, C.-H.; Zhang, Q.-Y.; An, J.-P.; You, C.-X.; Hao, Y.-J. Glucose Sensor MdHXK1 Phosphorylates and Stabilizes MdbHLH3 to Promote Anthocyanin Biosynthesis in Apple. PLoS Genet. 2016, 12, e1006273. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Q.; Chen, W.; Guo, Q.; Xia, Y.; Wu, D.; Jing, D.; Liang, G. Melatonin treatment maintains quality and delays lignification in loquat fruit during cold storage. Sci. Hortic. 2021, 284, 110126. [Google Scholar] [CrossRef]
- Xin, Q.; Liu, B.; Sun, J.; Fan, X.; Li, X.; Jiang, L.; Hao, G.; Pei, H.; Zhou, X. Heat Shock Treatment Promoted Callus Formation on Postharvest Sweet Potato by Adjusting Active Oxygen and Phenylpropanoid Metabolism. Agriculture 2022, 12, 1351. [Google Scholar] [CrossRef]
- Li, B.; Li, M.; Liu, J.; Sun, W.; Min, D.; Li, F.; Li, X. Methyl salicylate pretreatment maintains quality and antioxidant capacity of fresh-cut pitaya fruit by modulating phenylpropanoid metabolism and antioxidant system. Sci. Hortic. 2023, 309, 111705. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, J.; Mao, L.; Li, Q.; Wang, L.; Lin, Q. Low temperature reduces potato wound formation by inhibiting phenylpropanoid metabolism and fatty acid biosynthesis. Front. Plant Sci. 2023, 13, 1109953. [Google Scholar] [CrossRef]
- Alós, E.; Rodrigo, M.J.; Zacarias, L. Chapter 7—Ripening and Senescence. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 131–155. [Google Scholar]
- Pech, J.C.; Bouzayen, M.; Latché, A. Climacteric fruit ripening: Ethylene-dependent and independent regulation of ripening pathways in melon fruit. Plant Sci. 2008, 175, 114–120. [Google Scholar] [CrossRef]
- Wang, R.; Lammers, M.; Tikunov, Y.; Bovy, A.G.; Angenent, G.C.; de Maagd, R.A. The rin, nor and Cnr spontaneous mutations inhibit tomato fruit ripening in additive and epistatic manners. Plant Sci. 2020, 294, 110436. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).