Physiological and Transcriptional Characteristics of Banana Seedlings in Response to Nitrogen Deficiency Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatment and Sampling
2.2. Determination of Chlorophyll SPAD Value
2.3. Determination of Dry Weight and Plant Height
2.4. Determination of Chlorophyll Fluorescence Parameters
2.5. Transcriptome Sequencing and Data Analysis
2.6. Data Analysis and Mapping
2.7. RNA Extraction and qRT-PCR Analysis
3. Results
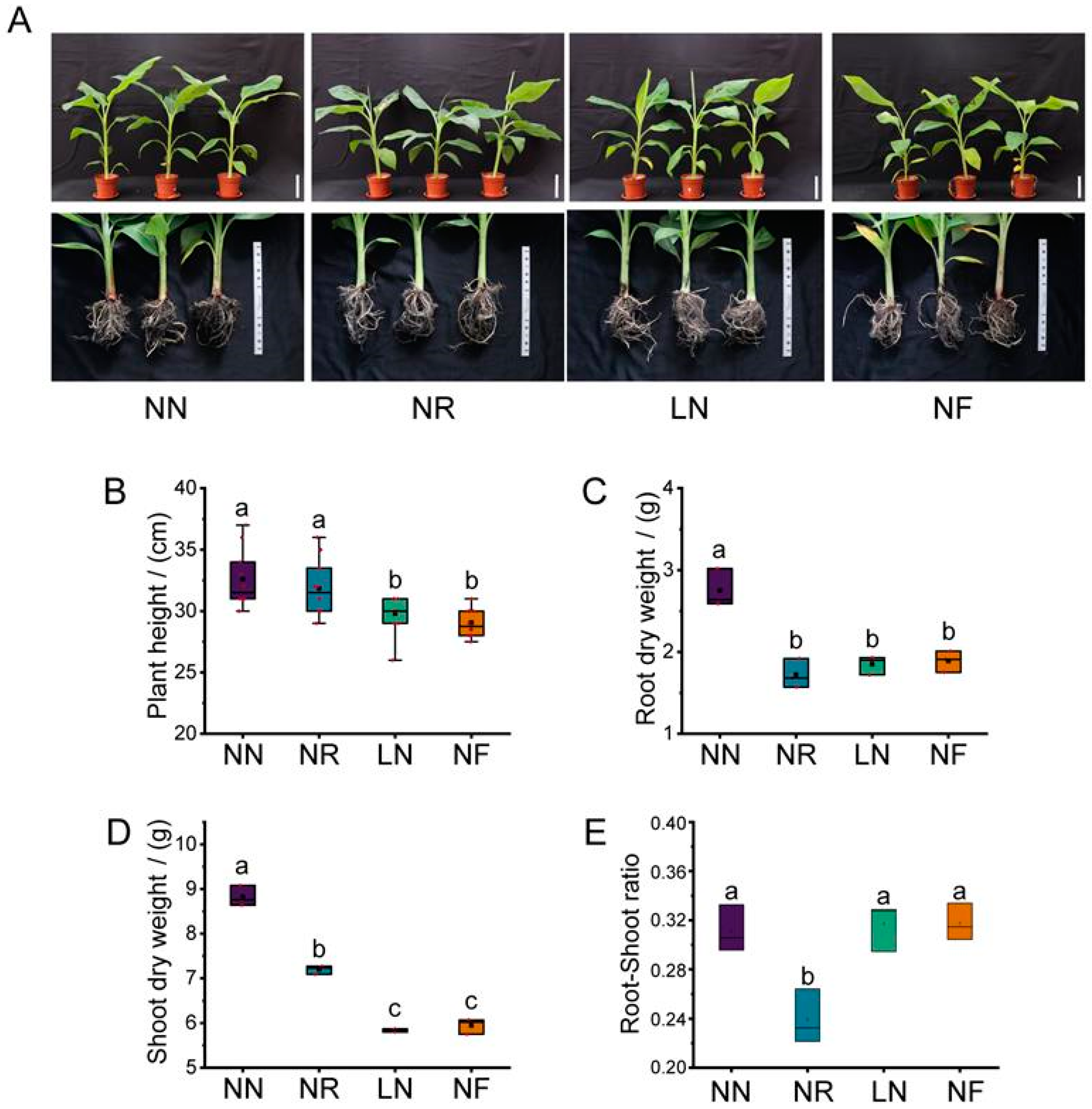
3.1. Effects of Different N Treatments on Plant Height and Dry Weight of Banana Seedlings
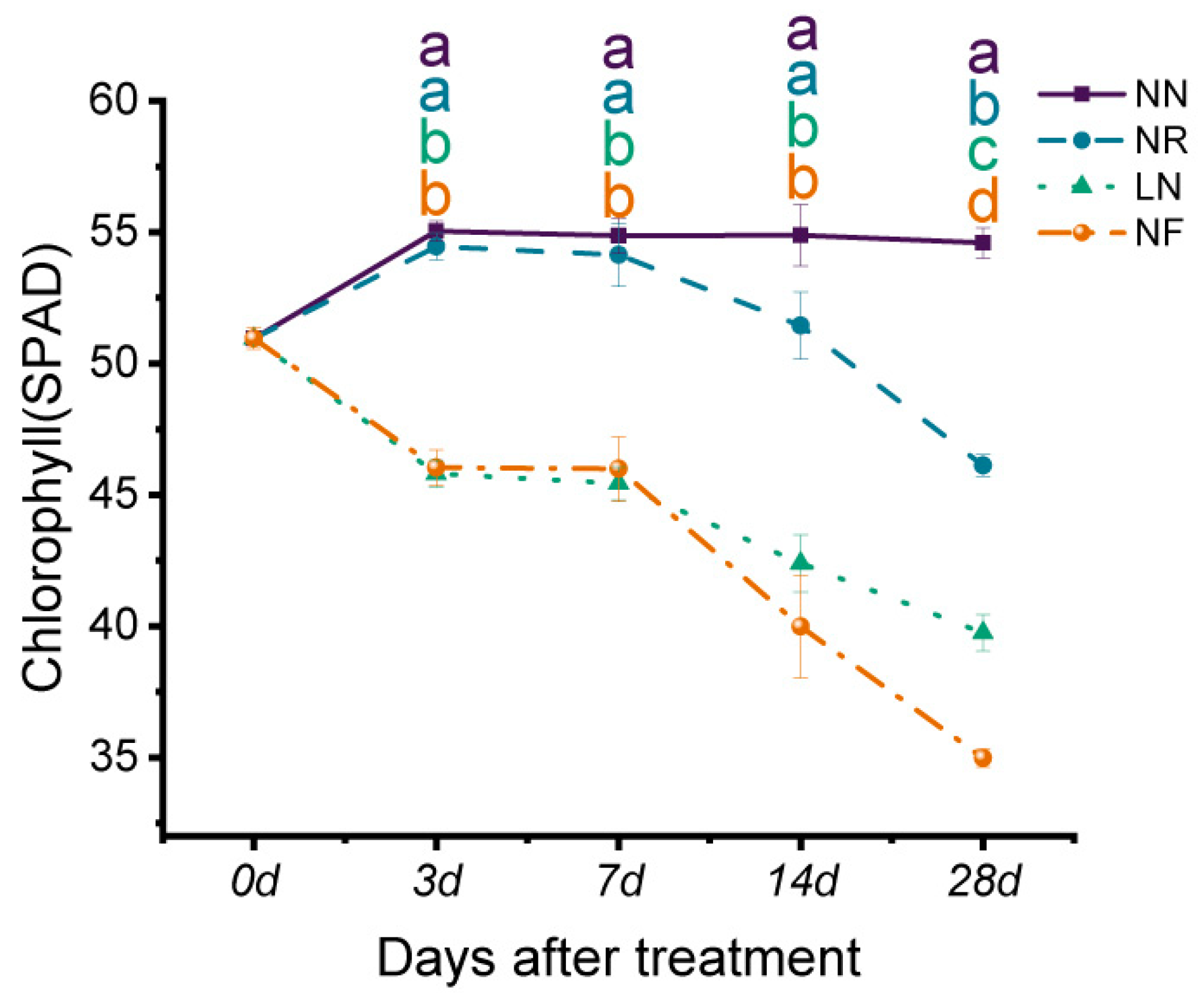
3.2. Effects of Different N Treatments on Chlorophyll Content of Banana Seedlings
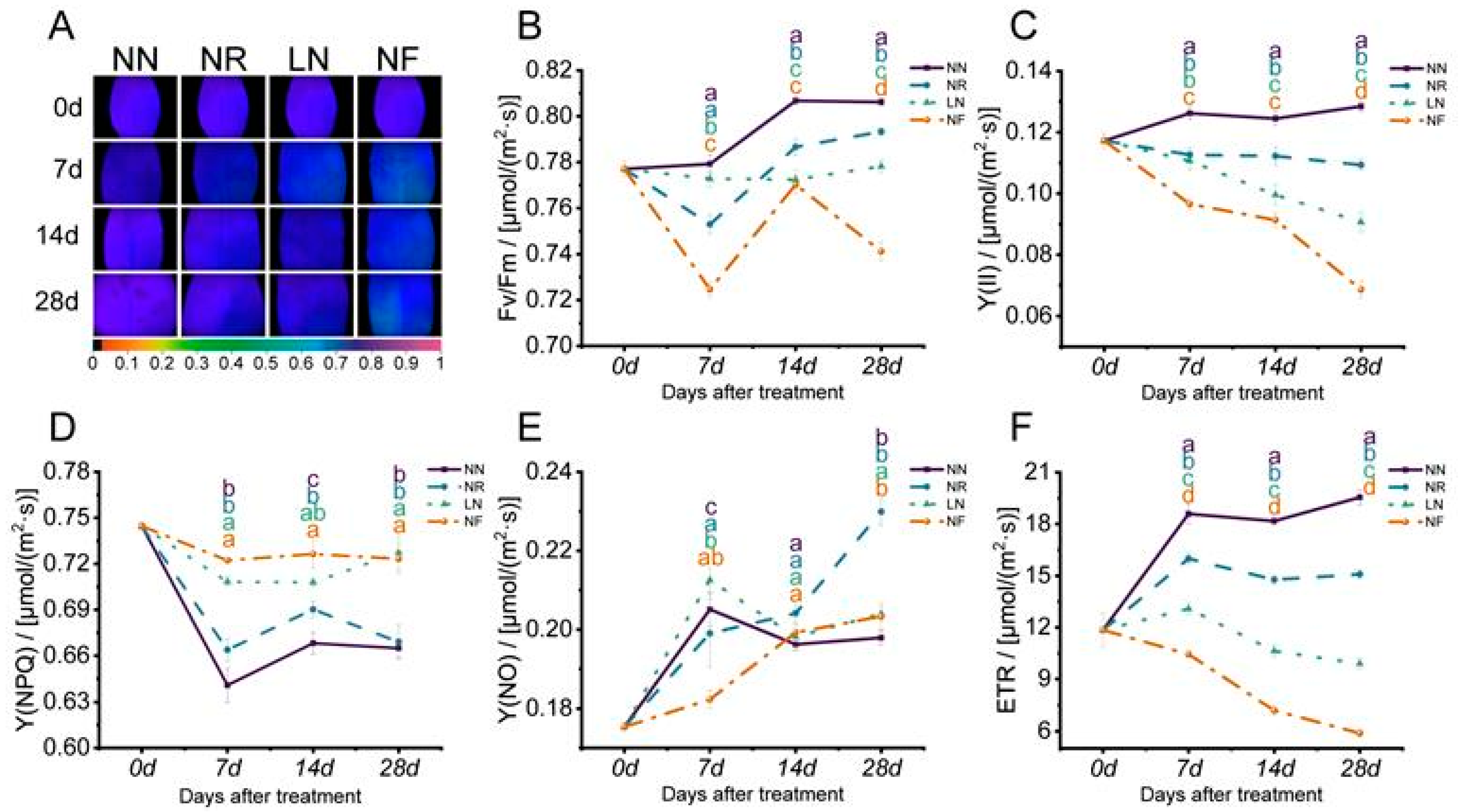
3.3. Different N Treatments’ Effects on Leaf Chlorophyll Fluorescence Parameters
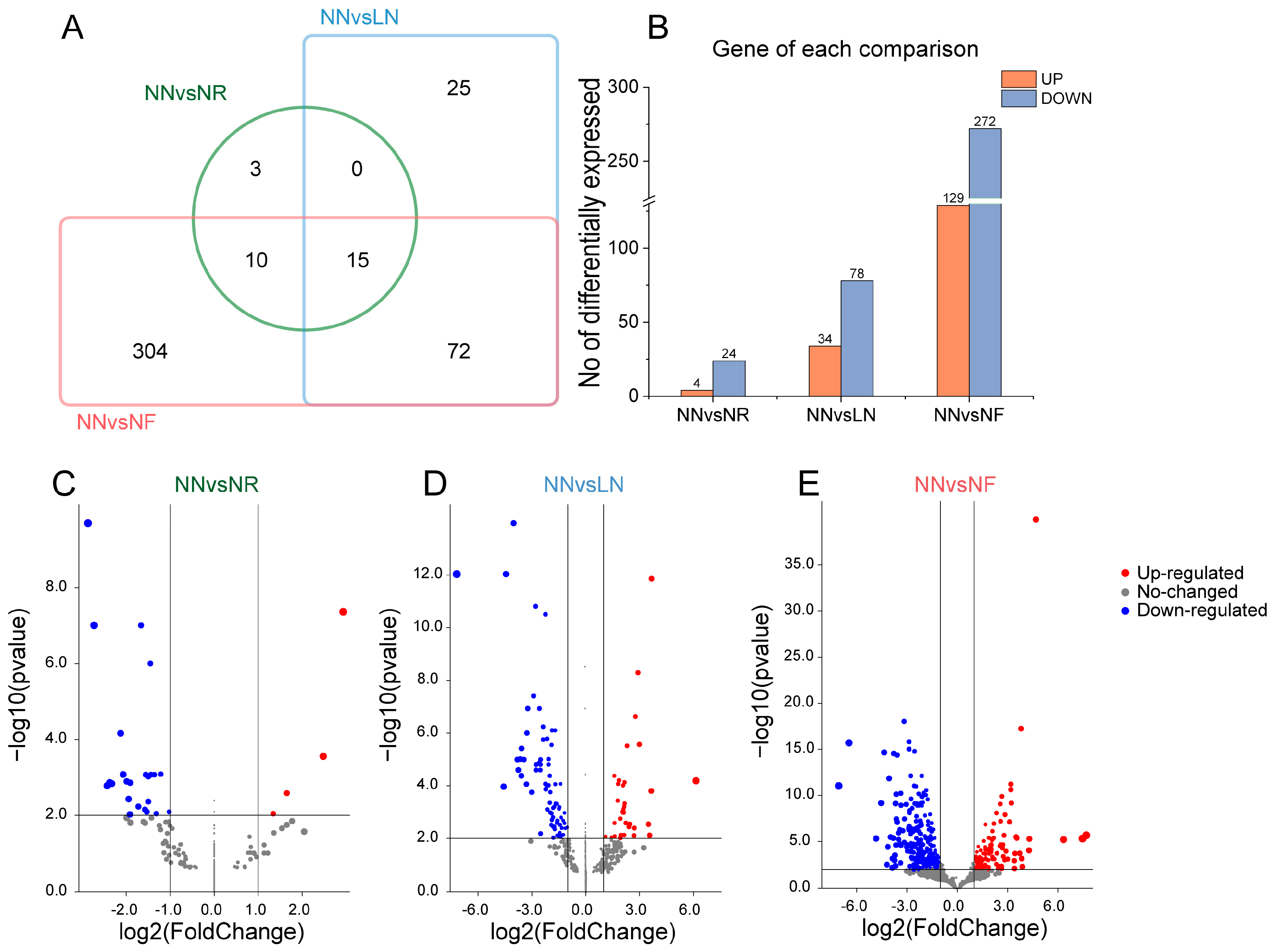
3.4. Screening and Annotation of Differentially Expressed Genes in Banana Roots after Exposure to Low N Stress
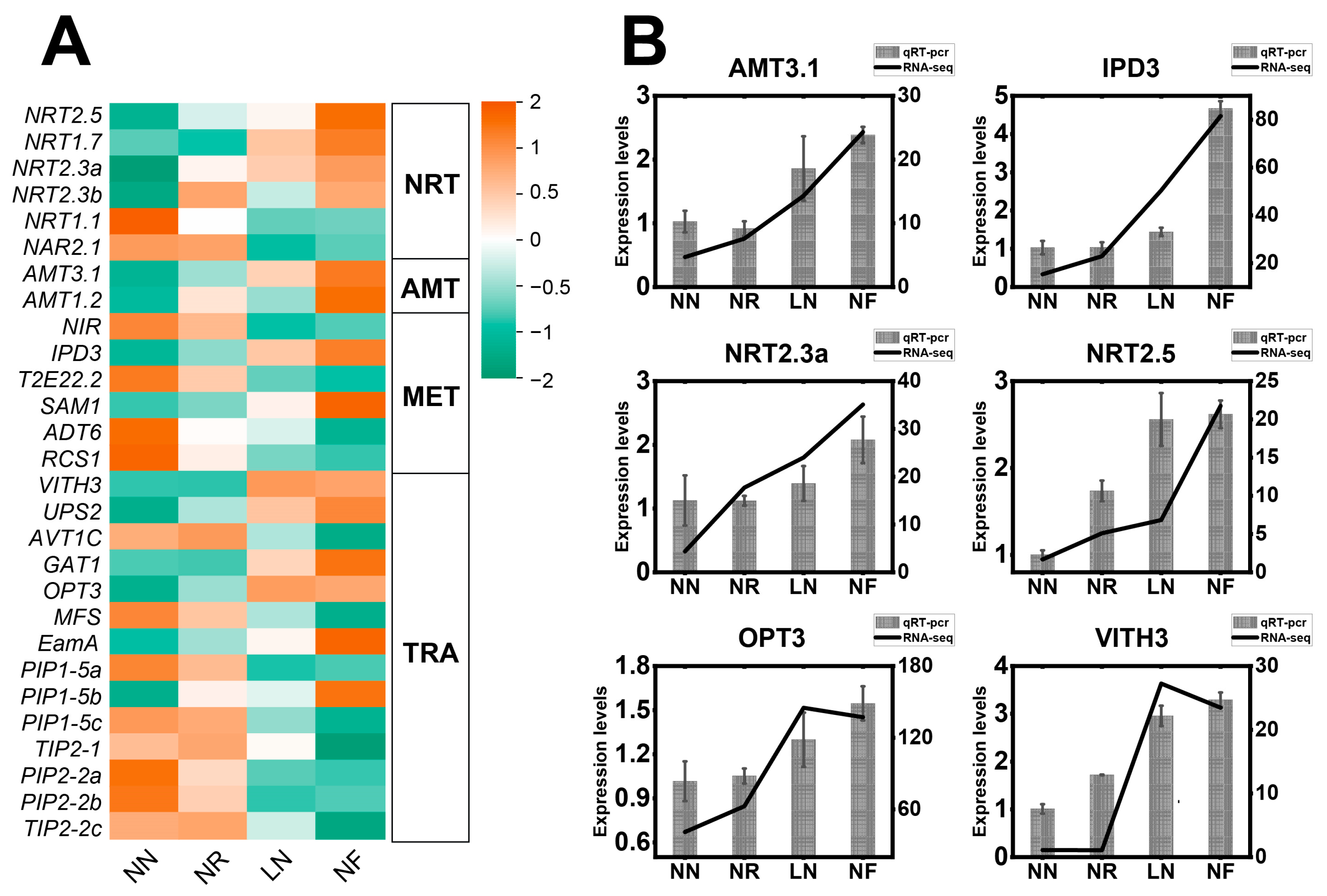
3.5. Expression Characteristics of DEGs Related to N Transport and N Metabolism
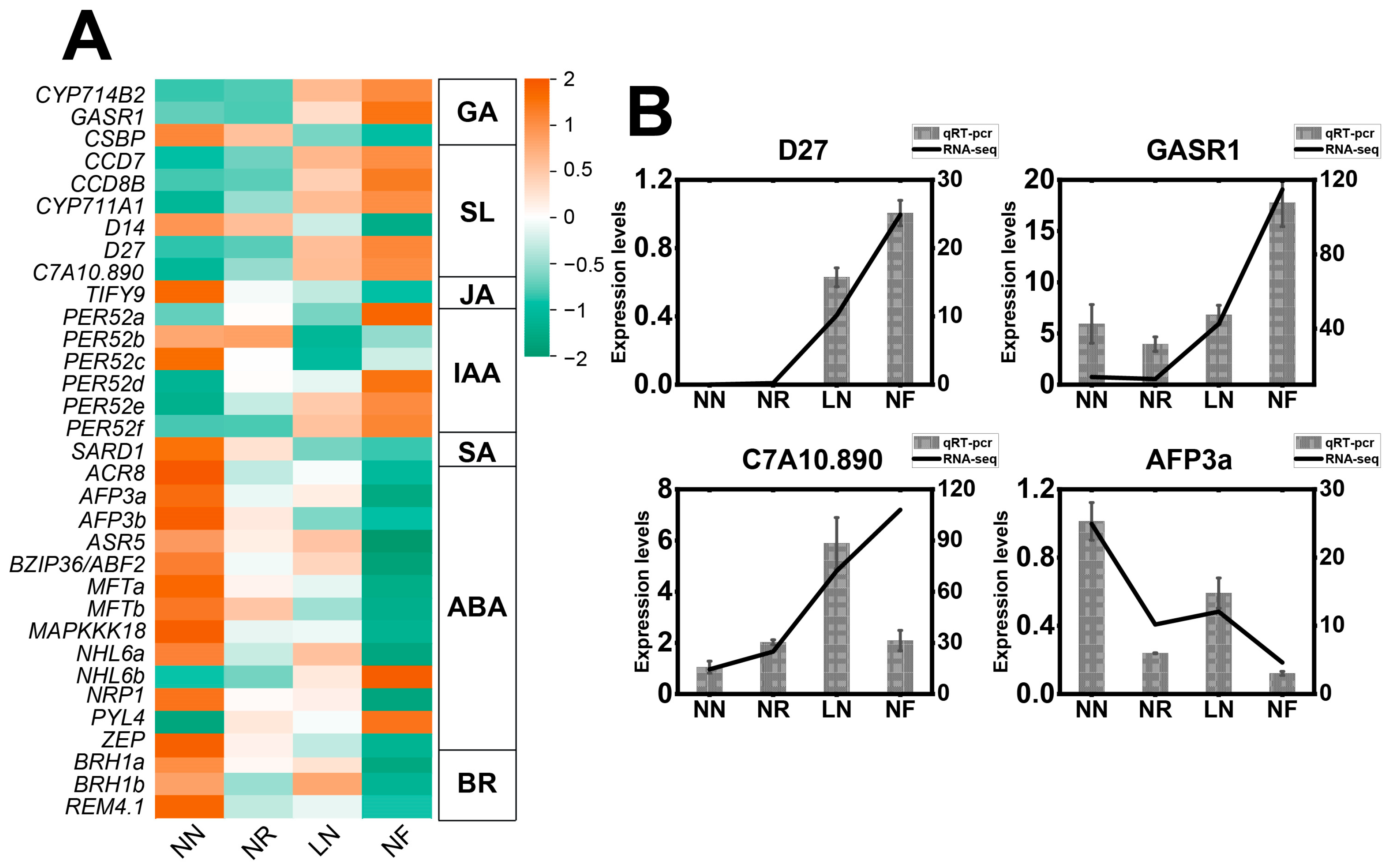
3.6. Expression Characteristics of DEGs Related to Phytohormones
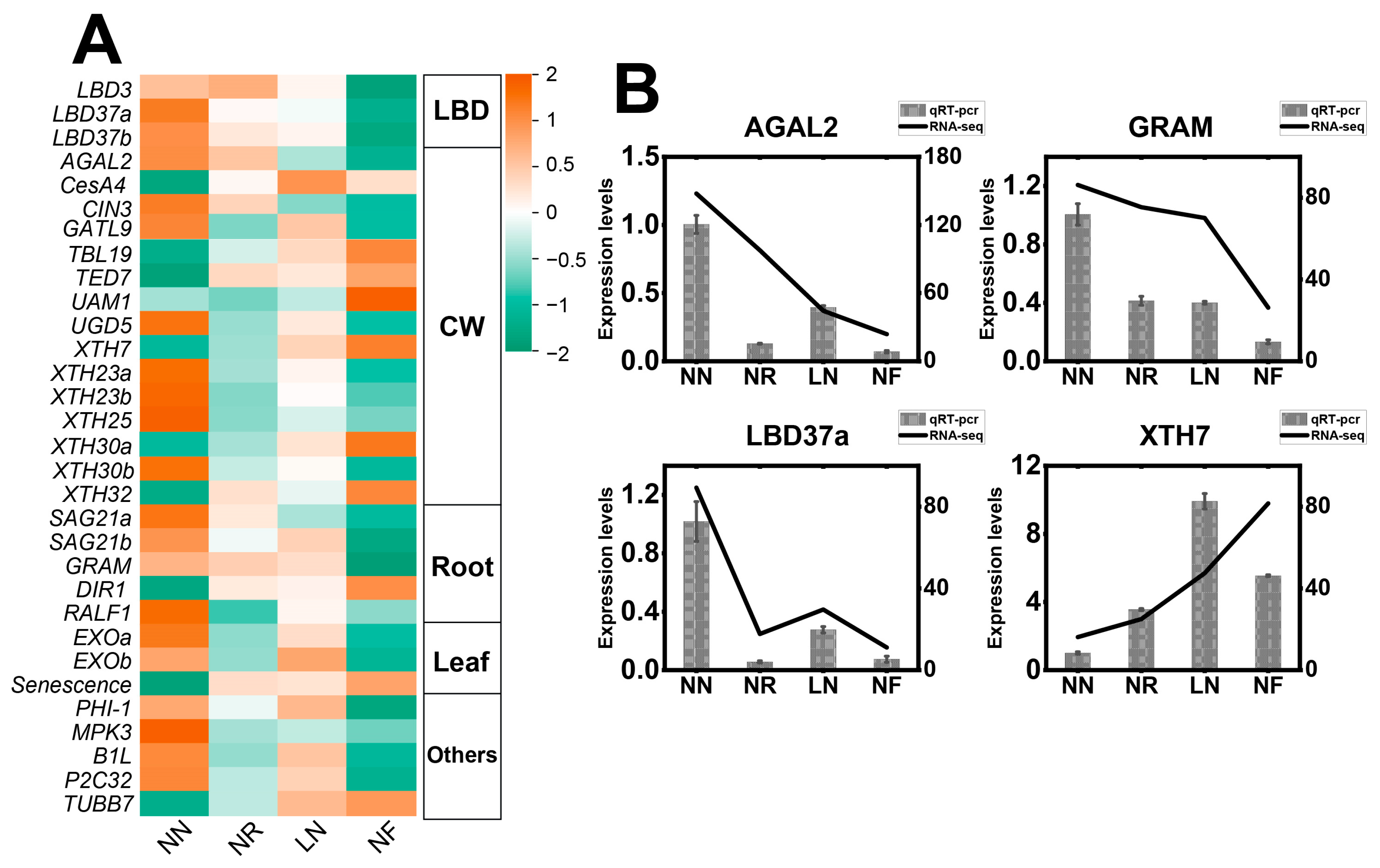
3.7. Analysis of Expression Characteristics of Plant Growth-Related Genes
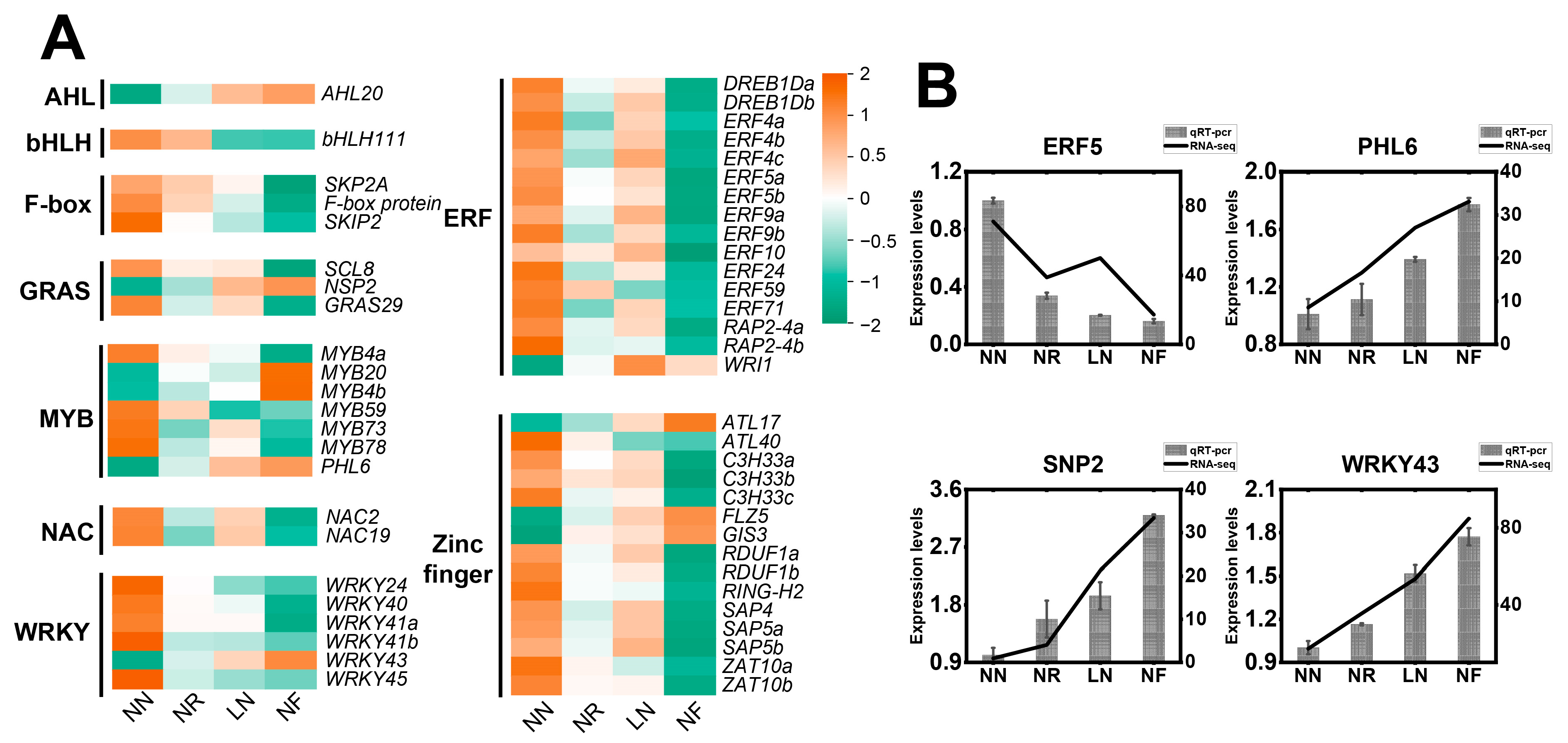
3.8. Expression Characteristics of Transcription Factors Involved in Low N Stress
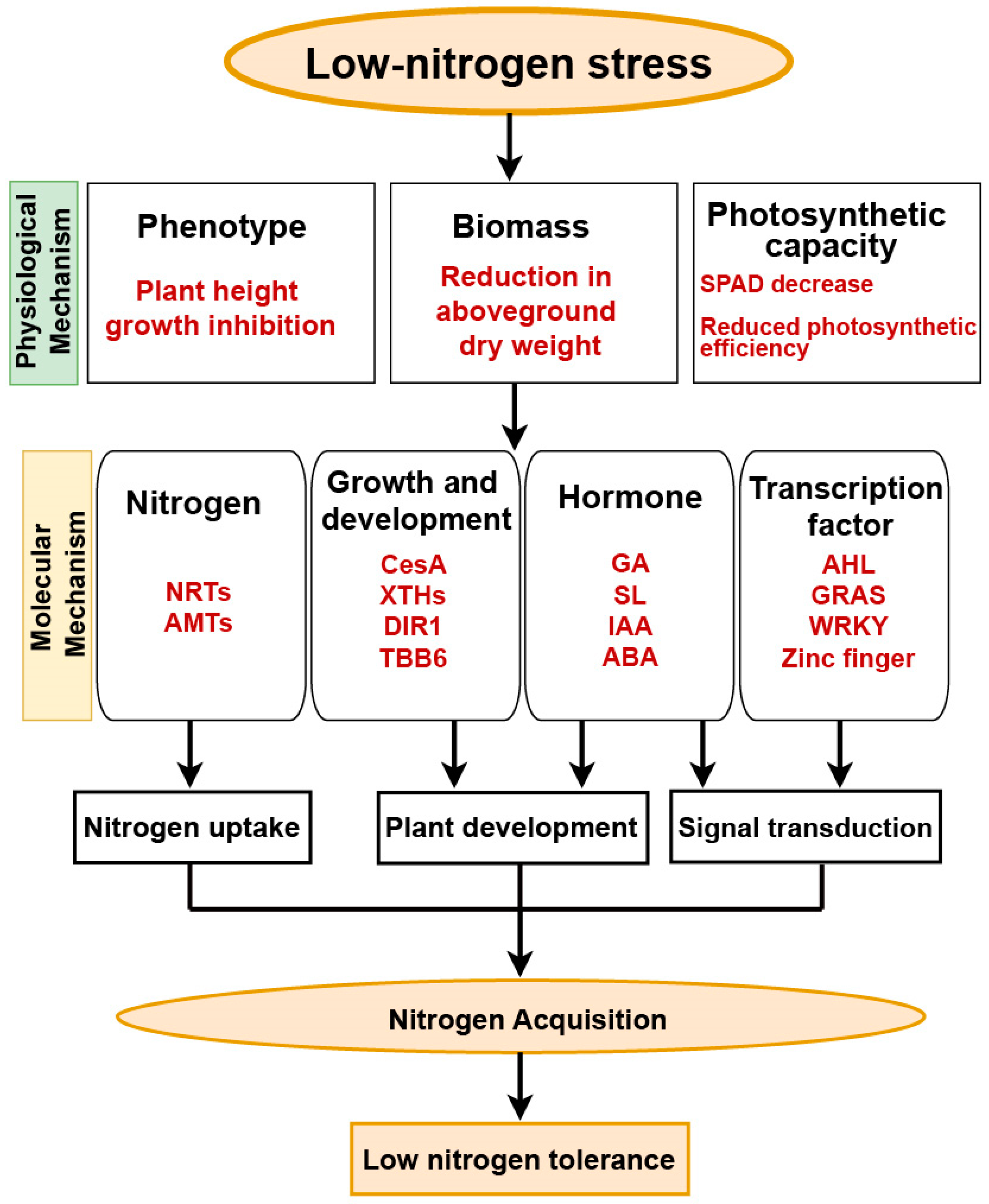
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Wang, M.; Li, W.; He, X.; Teng, W.; Ma, W.; Zhao, X.; Hu, M.; Li, H.; Zhang, Y.; et al. Reducing expression of a nitrate-responsive bZIP transcription factor increases grain yield and N use in wheat. Plant Biotechnol. J. 2019, 9, 1823–1833. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Yao, L.; Li, B.; Ma, X.; Si, E.; Yang, K.; Li, C.; Shang, X.; Meng, Y.; et al. Transcriptome and metabolome reveal the molecular mechanism of barley genotypes underlying the response to low nitrogen and resupply. Int. J. Mol. Sci. 2023, 5, 4706. [Google Scholar] [CrossRef]
- Remans, T.; Nacry, P.; Pervent, M.; Girin, T.; Tillard, P.; Lepetit, M.; Gojon, A. A central role for the nitrate transporter NRT2.1 in the integrated morphological and physiological responses of the root system to nitrogen limitation in Arabidopsis. Plant Physiol. 2006, 3, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.Y.; Tian, W.H.; Jin, C.W. A reevaluation of the contribution of NRT1.1 to nitrate uptake in Arabidopsis under low-nitrate supply. FEBS Lett. 2019, 15, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T.; Feria-Bourrellier, A.B.; Lafouge, F.; Lezhneva, L.; Boutet-Mercey, S.; Orsel, M.; Bréhaut, V.; Miller, A.; Daniel-Vedele, F.; Sakakibara, H.; et al. The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants. Plant Cell 2012, 1, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Fan, X.; Feng, H.; Miller, A.J.; Shen, Q.; Xu, G. Rice OsNAR2.1 interacts with OsNRT2.1, OsNRT2.2 and OsNRT2.3a nitrate transporters to provide uptake over high and low concentration ranges. Plant Cell Environ. 2011, 8, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Naz, M.; Fan, X.; Xuan, W.; Miller, A.J.; Xu, G. Plant nitrate transporters: From gene function to application. J. Exp. Bot. 2017, 10, 2463–2475. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Feng, H.; Tan, Y.; Xu, Y.; Miao, Q.; Xu, G. A putative 6-transmembrane nitrate transporter OsNRT1.1b plays a key role in rice under low nitrogen. J. Integr. Plant Biol. 2016, 6, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.C.; Lin, C.S.; Hsu, P.K.; Lin, S.H.; Tsay, Y.F. The Arabidopsis nitrate transporter NRT1.7, expressed in phloem, is responsible for source-to-sink remobilization of nitrate. Plant Cell 2009, 9, 2750–2761. [Google Scholar] [CrossRef]
- Ferreira, L.M.; De Souza, V.M.; Tavares, O.C.H.; Zonta, E.; Santa-Catarina, C.; de Souza, S.R.; Santos, L.A. OsAMT1.3 expression alters rice ammonium uptake kinetics and root morphology. Plant Biotechnol. Rep. 2015, 9, 221–229. [Google Scholar] [CrossRef]
- Hao, D.L.; Zhou, J.Y.; Yang, S.Y.; Qi, W.; Yang, K.J.; Su, Y.H. Function and regulation of ammonium transporters in plants. Int. J. Mol. Sci. 2020, 10, 3557. [Google Scholar] [CrossRef]
- Wen, B.; Zhao, X.; Gong, X.; Zhao, W.; Sun, M.; Chen, X.; Li, D.; Li, L.; Wei, X. The NAC transcription factor MdNAC4 positively regulates nitrogen deficiency-induced leaf senescence by enhancing ABA biosynthesis in apple. Mol. Hortic. 2023, 3, 5. [Google Scholar] [CrossRef]
- Cui, R.; Feng, Y.; Yao, J.; Shi, L.; Wang, S.; Xu, F. The transcription factor BnaA9.WRKY47 coordinates leaf senescence and nitrogen remobilization in Brassica napus. J. Exp. Bot. 2023, 74, 5606–5619. [Google Scholar] [CrossRef]
- Ge, L.; Dou, Y.; Li, M.; Qu, P.; He, Z.; Liu, Y.; Xu, Z.; Chen, J.; Chen, M.; Ma, Y. SiMYB3 in Foxtail Millet (Setaria italica) Confers Tolerance to Low-Nitrogen Stress by Regulating Root Growth in Transgenic Plants. Int. J. Mol. Sci. 2019, 22, 5741. [Google Scholar] [CrossRef]
- Pan, Y. Overexpression of ZmNF-YA13 Enhances Nitrogen Uptake in Tobacco and Maize. Master’s Thesis, Dalian University of Technology, Dalian, China, 2021. [Google Scholar]
- Kurai, T.; Wakayama, M.; Abiko, T.; Yanagisawa, S.; Aoki, N.; Ohsugi, R. Introduction of the ZmDof1 gene into rice enhances carbon and nitrogen assimilation under low-nitrogen conditions. Plant Biotechnol. J. 2011, 8, 826–837. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, S.; Li, J.; Pei, R.; Tian, L.; Qi, J.; Azam, M.; Agyenim-Boateng, K.G.; Shaibu, A.S.; Liu, Y.; et al. Dual-function C2H2-type zinc-finger transcription factor GmZFP7 contributes to isoflavone accumulation in soybean. N. Phytol. 2023, 5, 1794–1809. [Google Scholar] [CrossRef]
- Liang, T.; Yuan, Z.; Fu, L.; Zhu, M.; Luo, X.; Xu, W.; Yuan, H.; Zhu, R.; Hu, Z.; Wu, X. Integrative Transcriptomic and proteomic analysis reveals an alternative molecular network of glutamine synthetase 2 corresponding to nitrogen deficiency in rice (Oryza sativa L.). Int. J. Mol. Sci. 2021, 14, 7674. [Google Scholar] [CrossRef] [PubMed]
- Takatsuka, H.; Umeda, M. Hormonal control of cell division and elongation along differentiation trajectories in roots. J. Exp. Bot. 2014, 10, 2633–2643. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, E.; Polverari, L.; Sabatini, S. Plant hormone cross-talk: The pivot of root growth. J. Exp. Bot. 2015, 4, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Steffens, B.; Rasmussen, A. The physiology of adventitious roots. Plant Physiol. 2016, 2, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Praveen, A.; Gupta, M. Nitric oxide confronts arsenic stimulated oxidative stress and root architecture through distinct gene expression of auxin transporters, nutrient related genes and modulates biochemical responses in Oryza sativa L. Environ. Pollut. 2018, 240, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, J.; Wang, X.; Fu, H.; Zhao, M.; Wang, H.; Shi, L. Photosynthetic characteristics and metabolic analyses of two soybean genotypes revealed adaptive strategies to low-nitrogen stress. J. Plant Physiol. 2018, 229, 132–141. [Google Scholar] [CrossRef]
- Collier, M.D.; Fotelli, M.N.; Nahm, M.; Kopriva, S.; Rennenberg, H.; Hanke, D.E.; Gessler, A. Regulation of nitrogen uptake by Fagus sylvatica on a whole plant level–interactions between cytokinins and soluble N compounds. Plant Cell Environ. 2003, 9, 1549–1560. [Google Scholar] [CrossRef]
- Gu, J.; Li, Z.; Mao, Y.; Struik, P.C.; Zhang, H.; Liu, L.; Wang, Z.; Yang, J. Roles of nitrogen and cytokinin signals in root and shoot communications in maximizing of plant productivity and their agronomic applications. Plant Sci. 2018, 274, 320–331. [Google Scholar] [CrossRef]
- Werner, T.; Nehnevajova, E.; Köllmer, I.; Novák, O.; Strnad, M.; Krämer, U.; Schmülling, T. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 2010, 12, 3905–3920. [Google Scholar] [CrossRef] [PubMed]
- Köllmer, I.; Novák, O.; Strnad, M.; Schmülling, T.; Werner, T. Overexpression of the cytosolic cytokinin oxidase/dehydrogenase (CKX7) from Arabidopsis causes specific changes in root growth and xylem differentiation. Plant J. 2014, 3, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.B.; Filin, A.N. Cytokinins regulate root growth through its action on meristematic cell proliferation but not on the transition to differentiation. Funct. Plant Biol. 2018, 2, 215–221. [Google Scholar] [CrossRef]
- Smith, K.A.; Robertson, P.D. Effect of ethylene on root extension of cereals. Nature 1971, 5325, 148–149. [Google Scholar] [CrossRef]
- Ma, B.; Yin, C.C.; He, S.J.; Lu, X.; Zhang, W.K.; Lu, T.G.; Chen, S.Y.; Zhang, J.S. Ethylene-induced inhibition of root growth requires abscisic acid function in rice (Oryza sativa L.) seedlings. PLoS Genet. 2014, 10, e1004701. [Google Scholar] [CrossRef]
- García, M.J.; Romera, F.J.; Lucena, C.; Alcántara, E.; Pérez-Vicente, R. Ethylene and the regulation of physiological and morphological responses to nutrient deficiencies. Plant Physiol. 2015, 1, 51–60. [Google Scholar] [CrossRef]
- Drew, M.C.; He, C.J.; Morgan, P.W. Decreased ethylene biosynthesis, and induction of aerenchyma, by nitrogen- or phosphate-starvation in adventitious roots of Zea mays L. Plant Physiol. 1989, 1, 266–271. [Google Scholar] [CrossRef] [PubMed]
- He, C.J.; Morgan, P.W.; Drew, M.C. Enhanced sensitivity to ethylene in nitrogen-or phosphate-starved roots of Zea mays L. during aerenchyma formation. Plant Physiol. 1992, 1, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, F.; Yuan, L.; Mi, G. The physiological mechanism underlying root elongation in response to nitrogen deficiency in crop plants. Planta 2020, 4, 84. [Google Scholar] [CrossRef]
- Sun, J.; Li, W.; Li, C.; Chang, W.; Zhang, S.; Zeng, Y.; Zeng, C.; Peng, M. Effect of different rates of nitrogen fertilization on crop yield, soil properties and leaf physiological attributes in banana under subtropical regions of china. Front. Plant Sci. 2020, 11, 613760. [Google Scholar] [CrossRef]
- Huang, L.; Cheng, S.; Liu, H.; Zhao, Z.; Wei, S.; Sun, S. Effects of nitrogen reduction combined with organic fertilizer on growth and nitrogen fate in banana at seedling stage. Environ. Res. 2022, 1, 113826. [Google Scholar] [CrossRef]
- Chen, H. Study on Banana Nutrition Characteristics under N, P, K, Ca, Mg, S Deficiency and Banana Nutrition Diagnosis. Ph.D. Thesis, South China Agricultural University, Guangzhou, China, 2020. [Google Scholar]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 8, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.F.; Deng, J.S.; Shi, Y.Y. Investigation of SPAD meter-based indices for estimating rice nitrogen status. Comput. Electron. Agric. 2010, 71, S60–S65. [Google Scholar] [CrossRef]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta 1975, 1, 105–115. [Google Scholar] [CrossRef]
- Oquist, G.; Chow, W.S. On the relationship between the quantum yield of Photosystem II electron transport, as determined by chlorophyll fluorescence and the quantum yield of CO2-dependent O2 evolution. Photosynth. Res. 1992, 1, 51–62. [Google Scholar] [CrossRef]
- Van Kooten, O.; Snel, J.F. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 1990, 3, 147–150. [Google Scholar] [CrossRef]
- Grzybowski, M.W.; Zwiener, M.; Jin, H.; Wijewardane, N.K.; Atefi, A.; Naldrett, M.J.; Alvarez, S.; Ge, Y.; Schnable, J.C. Variation in morpho-physiological and metabolic responses to low nitrogen stress across the sorghum association panel. BMC Plant Biol. 2022, 1, 433. [Google Scholar] [CrossRef]
- Li, X.; Zou, J.; Jin, C.; Jin, H. Transcriptomics analysis reveals Xanthoceras sorbifolia Bunge leaves’ adaptation strategy to low nitrogen. Trees 2023, 37, 1153–1166. [Google Scholar] [CrossRef]
- Zinta, R.; Tiwari, J.K.; Buckseth, T.; Thakur, K.; Goutam, U.; Kumar, D.; Challam, C.; Bhatia, N.; Poonia, A.K.; Naik, S.; et al. Root system architecture for abiotic stress tolerance in potato: Lessons from plants. Front. Plant Sci. 2022, 13, 926214. [Google Scholar] [CrossRef]
- Kiba, T.; Krapp, A. Plant nitrogen acquisition under low availability: Regulation of uptake and root architecture. Plant Cell Physiol. 2016, 4, 707–714. [Google Scholar] [CrossRef]
- Devi, L.L.; Pandey, A.; Gupta, S.; Singh, A.P. The interplay of auxin and brassinosteroid signaling tunes root growth under low and different nitrogen forms. Plant Physiol. 2022, 3, 1757–1773. [Google Scholar] [CrossRef]
- Agüera, E.; Cabello, P.; de la Haba, P. Induction of leaf senescence by low nitrogen nutrition in sunflower (Helianthus annuus) plants. Physiol. Plant 2010, 3, 256–267. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. A RNA-Seq analysis of the response of photosynthetic system to low nitrogen supply in maize leaf. Int. J. Mol. Sci. 2017, 12, 2624. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Zhao, Y.; Li, Y.; Zhang, G.; Peng, Z.; Zhang, J. Enhancing auxin accumulation in maize root tips improves root growth and dwarfs plant height. Plant Biotechnol. J. 2018, 1, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 4, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 2, 201–247. [Google Scholar]
- Luo, L.; Zhao, C.; Zheng, D.; Wang, E.; Liang, J.; Yin, C. Nitrogen uptake preference and allocation in Populus cathayana in response to drought stress. Environ. Exp. Bot. 2023, 213, 105415. [Google Scholar] [CrossRef]
- Lanquar, V.; Loqué, D.; Hörmann, F.; Yuan, L.; Bohner, A.; Engelsberger, W.R.; Lalonde, S.; Schulze, W.X.; von Wirén, N.; Frommer, W.B. Feedback inhibition of ammonium uptake by a phospho-dependent allosteric mechanism in Arabidopsis. Plant Cell 2009, 11, 3610–3622. [Google Scholar] [CrossRef]
- Léran, S.; Varala, K.; Boyer, J.C.; Chiurazzi, M.; Crawford, N.; Daniel-Vedele, F.; David, L.; Dickstein, R.; Fernandez, E.; Forde, B.; et al. A unified nomenclature of NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family members in plants. Trends Plant Sci. 2014, 1, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.E.; Groten, K. The costs and benefits of plant–arbuscular mycorrhizal fungal interactions. Annu. Rev. Plant Biol. 2022, 73, 649–672. [Google Scholar] [CrossRef]
- Xie, K.; Ren, Y.; Chen, A.; Yang, C.; Zheng, Q.; Chen, J.; Wang, D.; Li, Y.; Hu, S.; Xu, G. Plant nitrogen nutrition: The roles of arbuscular mycorrhizal fungi. J. Plant Physiol. 2022, 269, 153591. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, L.; Staehelin, C.; Li, Y.; Ruan, J.; Liang, Z.; Xie, Z.; Wang, W.; Xie, J.; Huang, S. The LYSIN MOTIF-CONTAINING RECEPTOR-LIKE KINASE 1 protein of banana is required for perception of pathogenic and symbiotic signals. N. Phytol. 2019, 223, 15888. [Google Scholar] [CrossRef]
- Lanfranco, L.; Bonfante, P.; Genre, A. The mutualistic interaction between plants and arbuscular mycorrhizal fungi. Microbiol. Spectr. 2016, 6, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Lanfranco, L.; Fiorilli, V.; Venice, F.; Bonfante, P. Strigolactones cross the kingdoms: Plants, fungi, and bacteria in the arbuscular mycorrhizal symbiosis. J. Exp. Bot. 2018, 9, 2175–2188. [Google Scholar] [CrossRef]
- Bogatek, R.; Gniazdowska, A. ROS and phytohormones in plant-plant allelopathic interaction. Plant Signal. Behav. 2007, 4, 317–318. [Google Scholar] [CrossRef]
- Qi, Y.; Wei, H.; Gu, W.; Shi, W.; Jiang, L.; Deng, L.; Liu, X. Transcriptome profiling provides insights into the fruit color development of wild Lycium ruthenicum Murr. from Qinghai-Tibet Plateau. Protoplasma 2021, 1, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 2011, 4, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Foo, E.; Reid, J.B. Strigolactones: New physiological roles for an ancient signal. J. Plant Growth Regul. 2013, 32, 429–442. [Google Scholar] [CrossRef]
- Yoneyama, K.; Mori, N.; Sato, T.; Yoda, A.; Xie, X.; Okamoto, M.; Iwanaga, M.; Ohnishi, T.; Nishiwaki, H.; Asami, T.; et al. Conversion of carlactone to carlactonoic acid is a conserved function of MAX1 homologs in strigolactone biosynthesis. N. Phytol. 2018, 4, 1522–1533. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Xie, X.; Kim, H.I.; Kisugi, T.; Nomura, T.; Sekimoto, H.; Yokota, T.; Yoneyama, K. How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta 2012, 235, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Wang, S.; Song, W.; Zhang, J.; Wang, Y.; Liu, Q.; Yu, J.; Ye, Y.; Li, S.; Chen, J.; et al. Enhanced sustainable green revolution yield via nitrogen-responsive chromatin modulation in rice. Science 2020, 6478, eaaz2046. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, Y.; Zhu, L.; Jiang, B.; Wang, H.; Gao, R.; Friml, J.; Xiao, G. Strigolactones act downstream of gibberellins to regulate fiber cell elongation and cell wall thickness in cotton (Gossypium hirsutum). Plant Cell 2022, 12, 4816–4839. [Google Scholar] [CrossRef]
- Guo, J.; Sun, B.; He, H.; Zhang, Y.; Tian, H.; Wang, B. Current Understanding of bHLH Transcription Factors in Plant Abiotic Stress Tolerance. Int. J. Mol. Sci. 2021, 9, 4921. [Google Scholar] [CrossRef]
- Kidokoro, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional regulatory network of plant cold-stress responses. Trends Plant Sci. 2022, 9, 922–935. [Google Scholar] [CrossRef]
- Hirsch, S.; Oldroyd, G.E. GRAS-domain transcription factors that regulate plant development. Plant Signal. Behav. 2009, 8, 698–700. [Google Scholar] [CrossRef]
- Bejarano, I.; Marino, D.; Coleto, I. Arabidopsis MYB28 and MYB29 transcription factors are involved in ammonium-mediated alterations of root-system architecture. Plant Signal. Behav. 2021, 4, 1879532. [Google Scholar] [CrossRef]
- Gulzar, F.; Fu, J.; Zhu, C.; Yan, J.; Li, X.; Meraj, T.A.; Shen, Q.; Hassan, B.; Wang, Q. Maize WRKY transcription factor ZmWRKY79 positively regulates drought tolerance through elevating ABA biosynthesis. Int. J. Mol. Sci. 2021, 18, 10080. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, D.; Majee, A.; Singh, S.; Roohi; Asif, M.H.; Sane, A.P.; Sane, V.A. Identification of tomato root growth regulatory genes and transcription factors through comparative transcriptomic profiling of different tissues. Physiol. Mol. Biol. Plant 2021, 6, 1173–1189. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Z.; Dong, W.; Liu, S.; Tian, L.; Li, J.; Du, H. MYB transcription factors becoming mainstream in plant roots. Int. J. Mol. Sci. 2022, 16, 62. [Google Scholar] [CrossRef]
- An, L.; Zhou, Z.; Sun, L.; Yan, A.; Xi, W.; Yu, N.; Cai, W.; Chen, X.; Yu, H.; Schiefelbein, J.; et al. A zinc finger protein gene ZFP5 integrates phytohormone signaling to control root hair development in Arabidopsis. Plant J. 2012, 3, 474–490. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, W.; De Smet, I.; Lewis, D.R.; Löfke, C.; Jansen, L.; Goeminne, G.; Vanden Bossche, R.; Karimi, M.; De Rybel, B.; Vanholme, B.; et al. Transcription factor WRKY23 assists auxin distribution patterns during Arabidopsis root development through local control on flavonol biosynthesis. Proc. Natl. Acad. Sci. USA 2012, 5, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.J.; Yan, J.Y.; Li, C.X.; Li, G.X.; Wu, Y.R.; Zheng, S.J. Transcription factor WRKY46 modulates the development of Arabidopsis lateral roots in osmotic/salt stress conditions via regulation of ABA signaling and auxin homeostasis. Plant J. 2015, 1, 56–69. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Nicolas, P.; Fernandez-Pozo, N.; Ma, Q.; Evanich, D.J.; Shi, Y.; Xu, Y.; Zheng, Y.; Snyder, S.I.; Martin, L.B.B.; et al. High-resolution spatiotemporal transcriptome mapping of tomato fruit development and ripening. Nat. Commun. 2018, 1, 364. [Google Scholar] [CrossRef]
- Silverstone, A.L.; Ciampaglio, C.N.; Sun, T. The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. Plant Cell 1998, 2, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, A.B.; Lombardo, F.; Miwa, H.; Perry, J.A.; Bunnewell, S.; Parniske, M.; Wang, T.L.; Downie, J.A. Lotus japonicus nodulation requires two GRAS domain regulators, one of which is functionally conserved in a non-legume. Plant Physiol. 2006, 4, 1739–1750. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Cai, B.; Zhang, X.; Zhang, B.; Feng, J.; Zhou, D.; Chen, Y.; Zhang, M.; Qi, D.; Wang, W.; et al. Physiological and Transcriptional Characteristics of Banana Seedlings in Response to Nitrogen Deficiency Stress. Horticulturae 2024, 10, 290. https://doi.org/10.3390/horticulturae10030290
Zhao L, Cai B, Zhang X, Zhang B, Feng J, Zhou D, Chen Y, Zhang M, Qi D, Wang W, et al. Physiological and Transcriptional Characteristics of Banana Seedlings in Response to Nitrogen Deficiency Stress. Horticulturae. 2024; 10(3):290. https://doi.org/10.3390/horticulturae10030290
Chicago/Turabian StyleZhao, Lei, Bingyu Cai, Xiaohan Zhang, Bencheng Zhang, Junting Feng, Dengbo Zhou, Yufeng Chen, Miaoyi Zhang, Dengfeng Qi, Wei Wang, and et al. 2024. "Physiological and Transcriptional Characteristics of Banana Seedlings in Response to Nitrogen Deficiency Stress" Horticulturae 10, no. 3: 290. https://doi.org/10.3390/horticulturae10030290
APA StyleZhao, L., Cai, B., Zhang, X., Zhang, B., Feng, J., Zhou, D., Chen, Y., Zhang, M., Qi, D., Wang, W., Xie, J., & Wei, Y. (2024). Physiological and Transcriptional Characteristics of Banana Seedlings in Response to Nitrogen Deficiency Stress. Horticulturae, 10(3), 290. https://doi.org/10.3390/horticulturae10030290





