Integrative Metabolome and Transcriptome Analyses Reveal the Pericarp Coloration Mechanisms in Bitter Melon (Momordica charantia L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Procedures
2.2. LC–MS-Based Widely Targeted Metabolomics Analysis
2.3. RNA Extraction, Library Construction, and Sequencing
2.4. Differentially Expressed Genes (DEGs) and Functional Enrichment Analysis
2.5. Quantitative RT-PCR Analysis
2.6. Data Analysis
3. Results
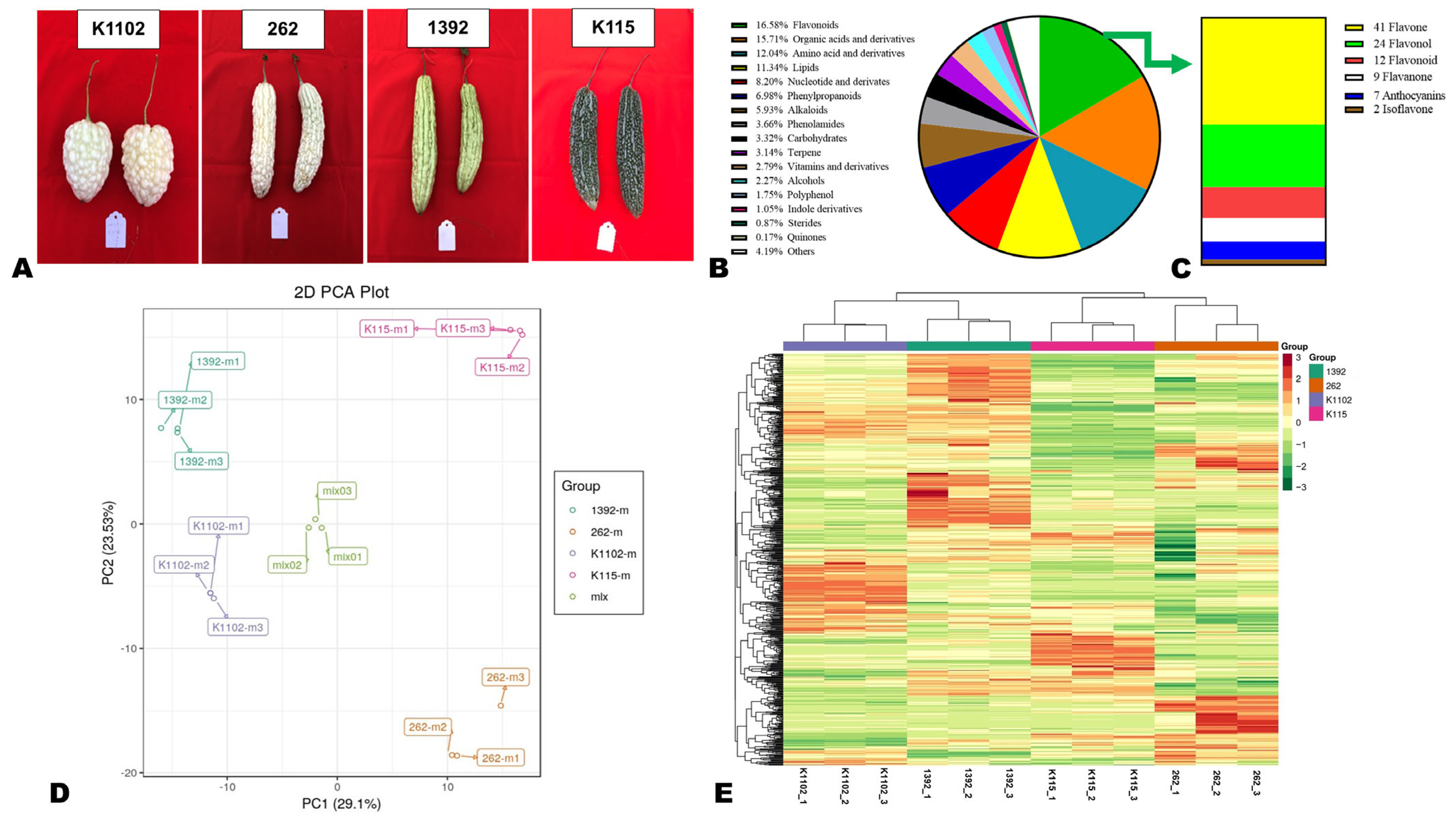
3.1. Metabolite Profiles of White, Grey White, Light Green, and Dark Green Bitter Melon Pericarps
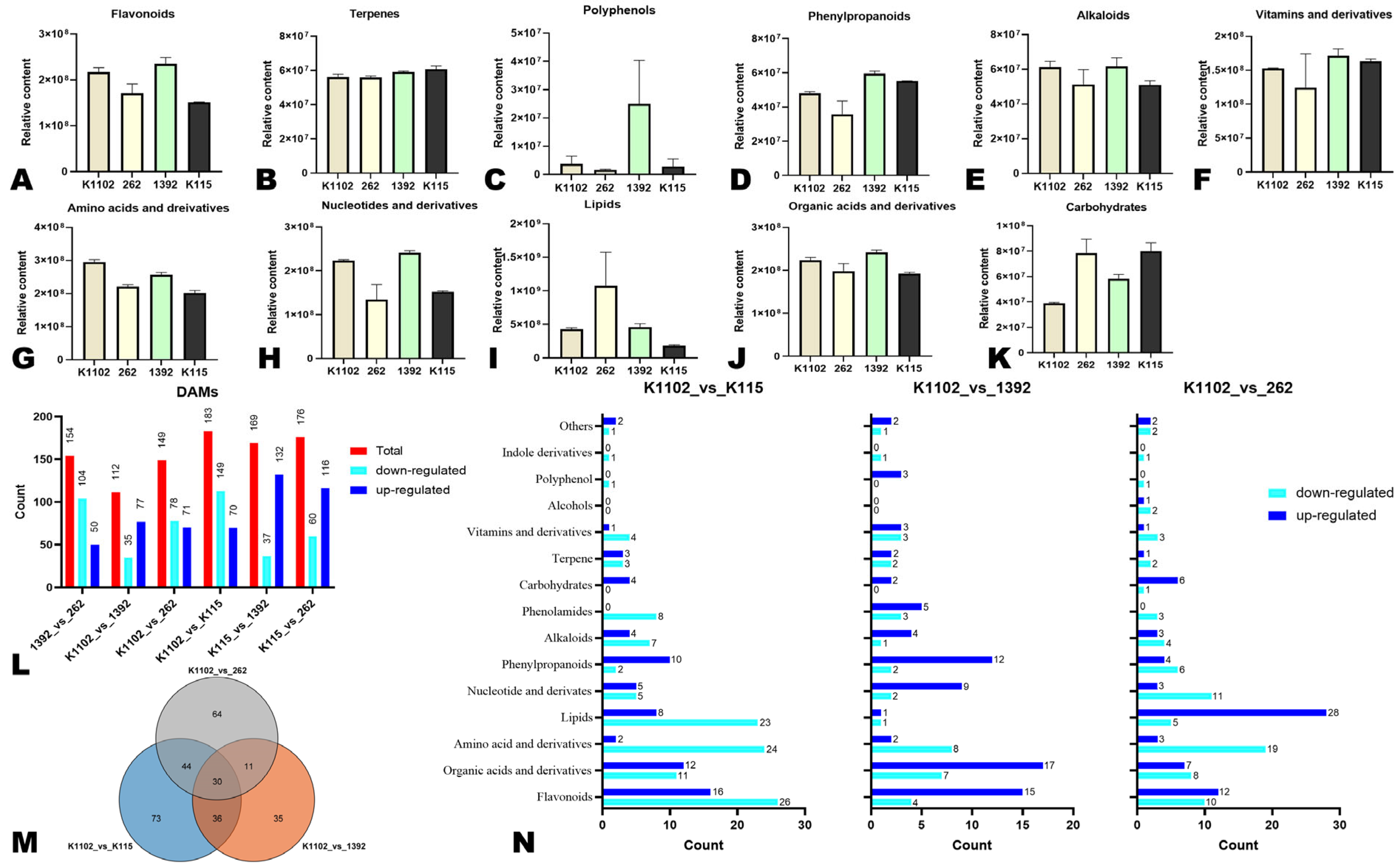
3.2. Variation in Important Metabolic Classes and Differentially Accumulated Metabolites (DAMs)
3.3. Major Flavonoid Pigments in the Different Colored Bitter Melon Pericarps
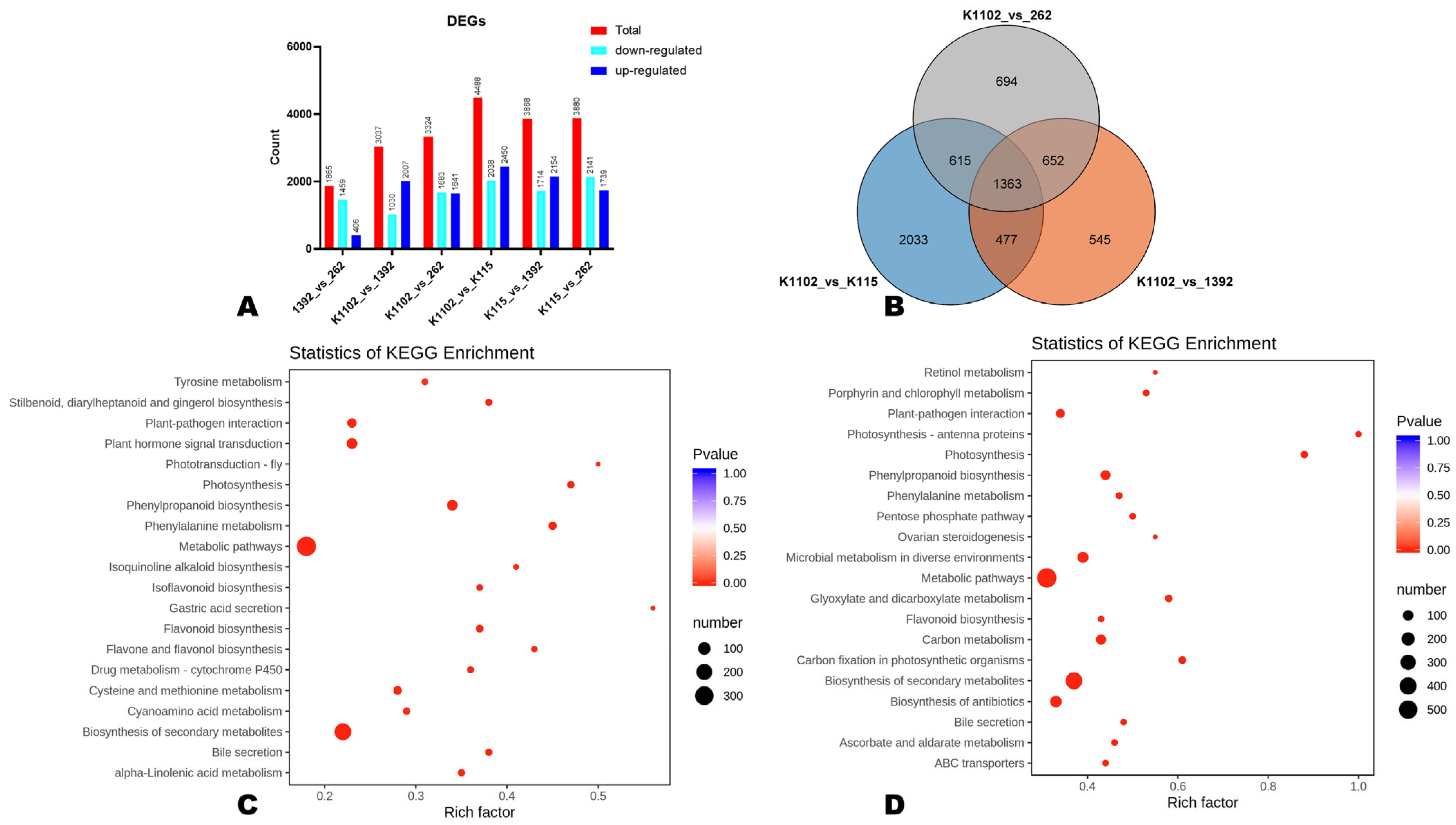
3.4. Comparative Transcriptome Sequencing and Differentially Expressed Genes (DEGs)
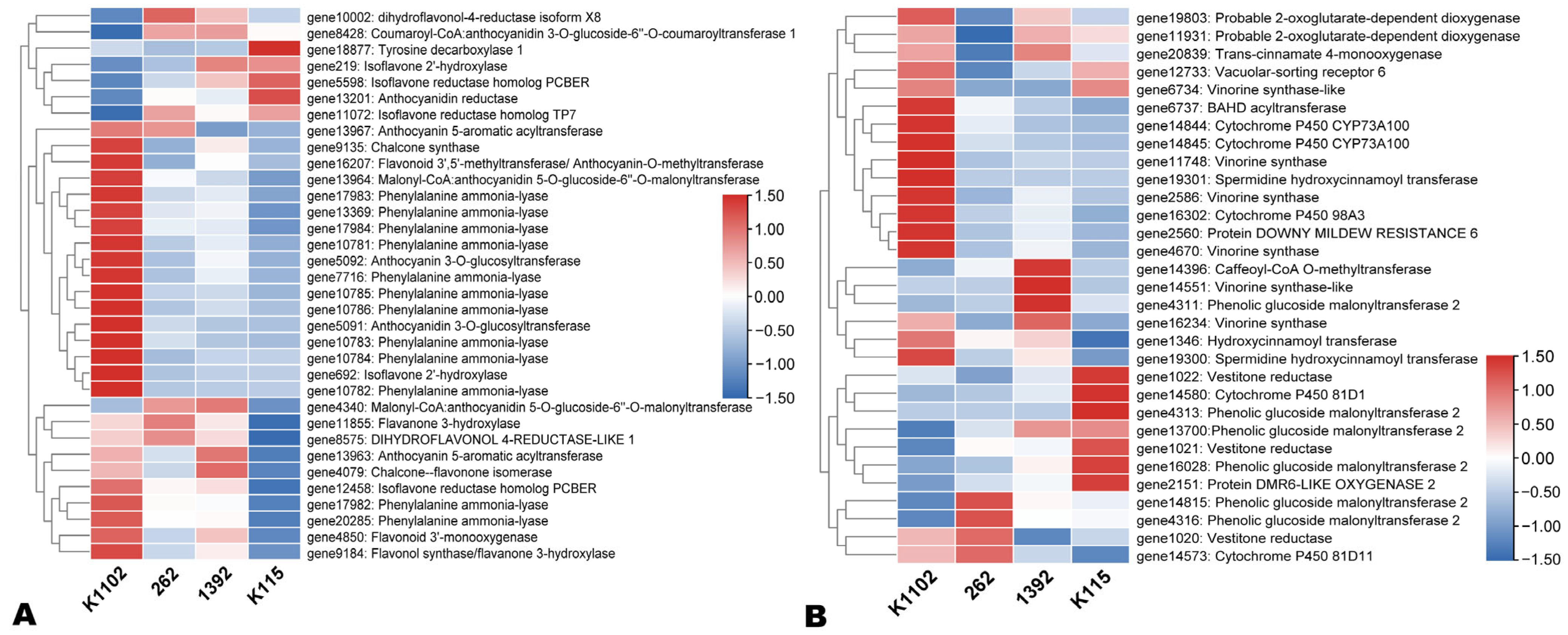
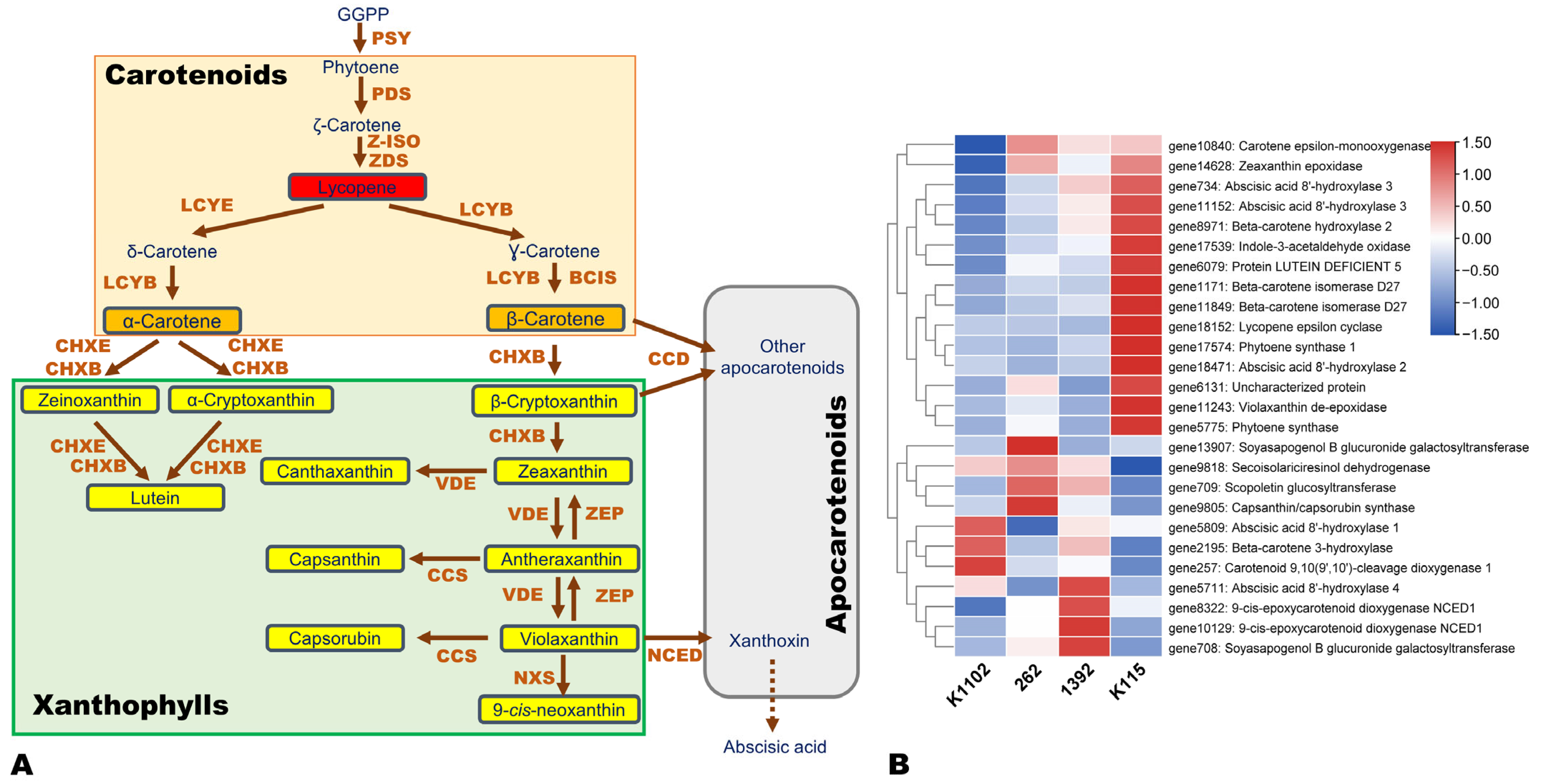
3.5. Expression Patterns of Flavonoid, Carotenoid, and Other Pigment Biosynthesis-Related DEGs
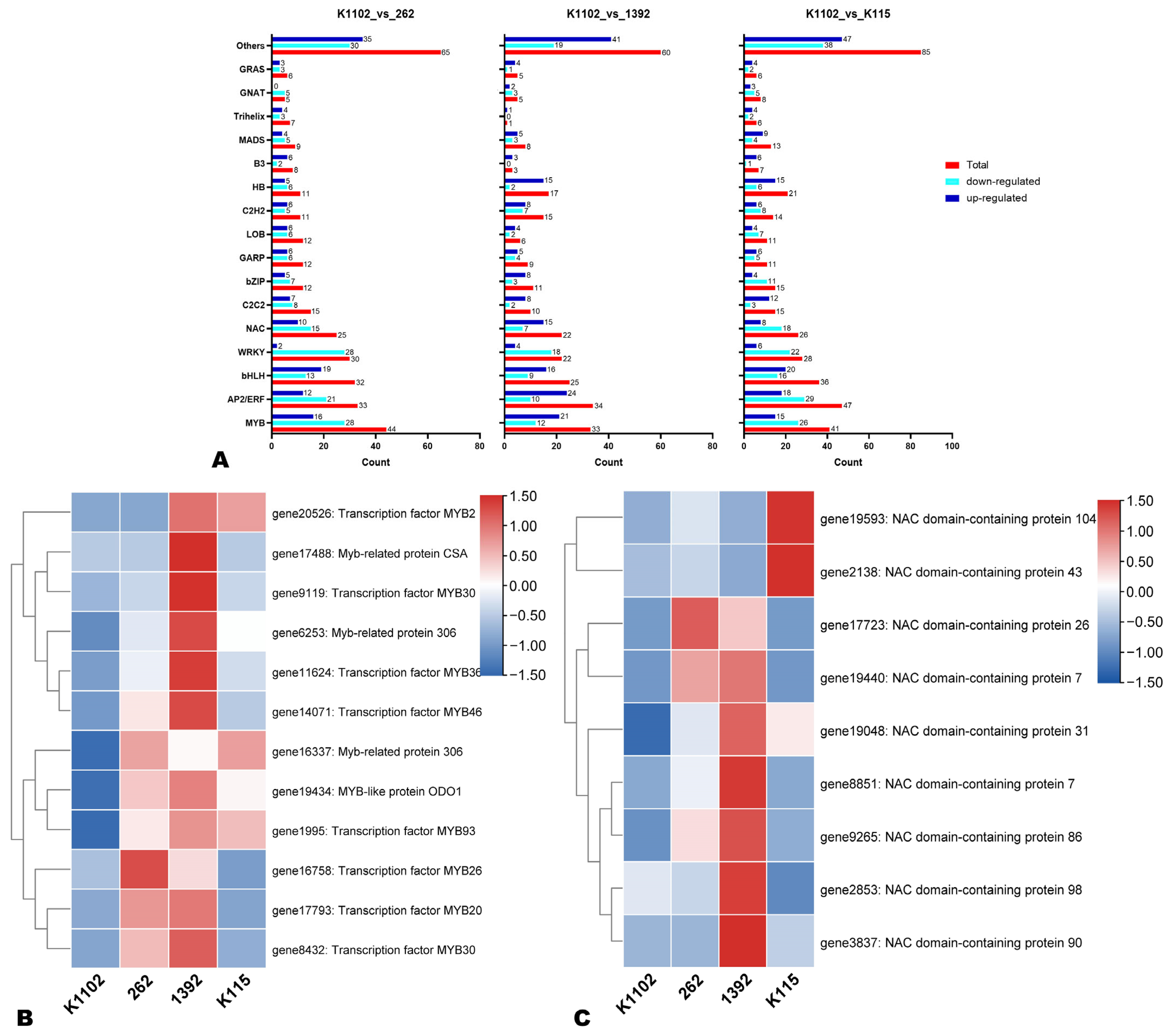
3.6. Potential Candidate Transcription Factors (TFs) Regulating Bitter Melon Pericarp Pigmentation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, J.; Yang, Y.; Luo, S.; Wang, L.; Huang, R.; Wen, Q.; Han, X.; Miao, N.; Cheng, J.; Liu, Z.; et al. Whole-Genome Sequencing Provides Insights into the Genetic Diversity and Domestication of Bitter Gourd (Momordica Spp.). Hortic. Res. 2020, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Navarrete, M.; Méndez-Del Villar, M.; Ramos-González, E.J.; Pérez-Rubio, K.G. Momordica charantia: A Review of Its Effects on Metabolic Diseases and Mechanisms of Action. J. Med. Food 2021, 24, 1017–1027. [Google Scholar] [CrossRef]
- Desai, S.; Tatke, P. Charantin: An Important Lead Compound from Momordica charantia for the Treatment of Diabetes. J. Pharmacogn. Phytochem. 2015, 3, 163–166. [Google Scholar]
- Jia, S.; Shen, M.; Zhang, F.; Xie, J. Recent Advances in Momordica charantia: Functional Components and Biological Activities. Int. J. Mol. Sci. 2017, 18, 2555. [Google Scholar] [CrossRef] [PubMed]
- Sur, S.; Ray, R.B. Bitter Melon (Momordica charantia), a Nutraceutical Approach for Cancer Prevention and Therapy. Cancers 2020, 12, 2064. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Z.; Yang, G.; Ho, C.T.; Li, S. Momordica charantia: A Popular Health-Promoting Vegetable with Multifunctionality. Food Funct. 2017, 8, 1749–1762. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.; Geetha, T.; Burnett, D.; Broderick, T.L.; Babu, J.R. The Effects of Momordica charantia on Type 2 Diabetes Mellitus and Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 4643. [Google Scholar] [CrossRef]
- Saeed, F.; Afzaal, M.; Niaz, B.; Arshad, M.U.; Tufail, T.; Hussain, M.B.; Javed, A. Bitter Melon (Momordica charantia): A Natural Healthy Vegetable. Int. J. Food Prop. 2018, 21, 1270–1290. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, S.; Singh, R. Targeting Novel Coronavirus SARS-CoV-2 Spike Protein with Phytoconstituents of Momordica charantia. J. Ovarian Res. 2021, 14, 126. [Google Scholar] [CrossRef]
- Yang, J.; Weng, Y.; Li, H.; Kong, Q.; Wang, W.; Yan, C.; Wang, L. Epidermal Patterning Factor 2-Like (McEPFL2): A Putative Candidate for the Continuous Ridge (Cr) Fruit Skin Locus in Bitter Gourd (Momordica charantia L.). Genes 2022, 13, 1148. [Google Scholar] [CrossRef]
- Rao, P.G.; Behera, T.K.; Gaikwad, A.B.; Munshi, A.D.; Srivastava, A.; Boopalakrishnan, G. Vinod Genetic Analysis and QTL Mapping of Yield and Fruit Traits in Bitter Gourd (Momordica charantia L.). Sci. Rep. 2021, 11, 4109. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yu, L.; Liu, W.; Zhang, J.; Wang, N.; Chen, X. Research Progress of Fruit Color Development in Apple (Malus domestica Borkh.). Plant Physiol. Biochem. 2021, 162, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Bo, K.; Wei, S.; Wang, W.; Miao, H.; Dong, S.; Zhang, S.; Gu, X. QTL Mapping and Genome-Wide Association Study Reveal Two Novel Loci Associated with Green Flesh Color in Cucumber. BMC Plant Biol. 2019, 19, 243. [Google Scholar] [CrossRef] [PubMed]
- Gebretsadik, K.; Qiu, X.; Dong, S.; Miao, H.; Bo, K. Molecular Research Progress and Improvement Approach of Fruit Quality Traits in Cucumber. Theor. Appl. Genet. 2021, 134, 3535–3552. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, K.G.; Shivashankara, K.S.; Roy, T.K.; Dinesh, M.R.; Geetha, G.A.; Pavithra, K.C.G.; Ravishankar, K.V. Profiling of Anthocyanins and Carotenoids in Fruit Peel of Different Colored Mango Cultivars. J. Food Sci. Technol. 2018, 55, 4566–4577. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, J.; Chen, Z.; Jiang, J.; Jackson, A. Characterization of Carotenoids and Phenolics during Fruit Ripening of Chinese Raspberry (Rubus chingii Hu). RSC Adv. 2021, 11, 10804–10813. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.H.; Yang, F.Y.; Lu, B.G.; Zhao, W.W.; Jiang, T.; Feng, L.; Chen, X.J.; Ming, R. Exploring the Differential Mechanisms of Carotenoid Biosynthesis in the Yellow Peel and Red Flesh of Papaya. BMC Genom. 2019, 20, 49. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhao, M.; Hu, Y.; Meng, F.; Song, X.; Tigabu, M.; Chiang, V.L.; Sederoff, R.; Ma, W.; et al. Molecular and Metabolic Insights into Anthocyanin Biosynthesis for Leaf Color Change in Chokecherry (Padus virginiana). Int. J. Mol. Sci. 2021, 22, 10697. [Google Scholar] [CrossRef]
- Fu, X.; Cheng, S.; Liao, Y.; Huang, B.; Du, B.; Zeng, W.; Jiang, Y.; Duan, X.; Yang, Z. Comparative Analysis of Pigments in Red and Yellow Banana Fruit. Food Chem. 2018, 239, 1009–1018. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Chen, L.; Hu, B.; Qin, Y.; Hu, G.; Zhao, J. Advance of the Negative Regulation of Anthocyanin Biosynthesis by MYB Transcription Factors. Plant Physiol. Biochem. 2019, 136, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Zhang, J.; Liu, Z.; Wang, H.; Tu, H.; Zhou, J.; Luo, X.; Chen, Q.; He, W.; et al. Integrated Transcriptome and Metabolome Analyses Provide Insights into the Coloring Mechanism of Dark-Red and Yellow Fruits in Chinese Cherry [Cerasus pseudocerasus (Lindl.) G. Don]. Int. J. Mol. Sci. 2023, 24, 3471. [Google Scholar] [CrossRef]
- Tang, P.; Giusti, M.M. Black Goji as a Potential Source of Natural Color in a Wide PH Range. Food Chem. 2018, 269, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.L.; Maia, B.H.L.N.S.; Ferriani, A.P.; Teixeira, S.D. Flavonoids: Classification, Biosynthesis and Chemical Ecology. In Flavonoids—From Biosynthesis to Human Health; InTech: London, UK, 2017; pp. 3–16. [Google Scholar]
- Khoo, H.E.; Prasad, K.N.; Kong, K.W.; Jiang, Y.; Ismail, A. Carotenoids and Their Isomers: Color Pigments in Fruits and Vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef] [PubMed]
- Stra, A.; Almarwaey, L.O.; Alagoz, Y.; Moreno, J.C.; Al-Babili, S. Carotenoid Metabolism: New Insights and Synthetic Approaches. Front Plant Sci 2023, 13, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Du, X.; Yu, J.; Jin, H.; Liu, N. Comparative Metabolomics Study of Flavonoids in the Pericarp of Different Coloured Bitter Gourds (Momordica charantia L.). Physiol. Mol. Biol. Plants 2022, 28, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Yonekura-Sakakibara, K.; Nakabayashi, R.; Higashi, Y.; Yamazaki, M.; Tohge, T.; Fernie, A.R. The Flavonoid Biosynthetic Pathway in Arabidopsis: Structural and Genetic Diversity. Plant Physiol. Biochem. 2013, 72, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, L.; Wei, M.; Li, J.; Yang, F. SmMYB113 Is a Key Transcription Factor Responsible for Compositional Variation of Anthocyanin and Color Diversity Among Eggplant Peels. Front. Plant Sci. 2022, 13, 843996. [Google Scholar] [CrossRef]
- Yan, H.; Pei, X.; Zhang, H.; Li, X.; Zhang, X.; Zhao, M.; Chiang, V.L.; Sederoff, R.R.; Zhao, X. Myb-Mediated Regulation of Anthocyanin Biosynthesis. Int. J. Mol. Sci. 2021, 22, 3103. [Google Scholar] [CrossRef]
- Li, C.; Yu, W.; Xu, J.; Lu, X.; Liu, Y. Anthocyanin Biosynthesis Induced by MYB Transcription Factors in Plants. Int. J. Mol. Sci. 2022, 23, 11701. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, J.; Liu, Z.; Wang, J.; Yang, B.; Chen, W.; Ou, L.; Dai, X.; Zhang, Z.; Zou, X. Integrative Analysis of Metabolome and Transcriptome Reveals the Mechanism of Color Formation in Pepper Fruit (Capsicum annuum L.). Food Chem. 2020, 306, 125629. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, S.; Yang, Y.; Liu, J.; Zhuang, Y. Integrated Metabolome and Transcriptome Analysis Reveals a Regulatory Network of Fruit Peel Pigmentation in Eggplant (Solanum melongena L.). Int. J. Mol. Sci. 2022, 23, 3475. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Li, P.; Sun, C.; Dong, W. Integrative Metabolome and Transcriptome Analyses Reveals the Black Fruit Coloring Mechanism of Crataegus maximowiczii C.K. Schneid. Plant Physiol. Biochem. 2023, 194, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luoa, J. A Novel Integrated Method for Large-Scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Dossou, S.S.K.; Xu, F.; Cui, X.; Sheng, C.; Zhou, R.; You, J.; Tozo, K.; Wang, L. Comparative Metabolomics Analysis of Different Sesame (Sesamum indicum L.) Tissues Reveals a Tissue-Specific Accumulation of Metabolites. BMC Plant Biol. 2021, 21, 352. [Google Scholar] [CrossRef] [PubMed]
- Dossou, S.S.K.; Xu, F.; You, J.; Zhou, R.; Li, D.; Wang, L. Widely Targeted Metabolome Profiling of Different Colored Sesame (Sesamum indicum L.) Seeds Provides New Insight into Their Antioxidant Activities. Food Res. Int. 2022, 151, 110850. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Gu, C.; He, S.; Zhu, D.; Huang, Y.; Zhou, Q. Widely Targeted Metabolomics Analysis Reveals New Biomarkers and Mechanistic Insights on Chestnut (Castanea mollissima Bl.) Calcification Process. Food Res. Int. 2021, 141, 110128. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A Fast Spliced Aligner with Low Memory Requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie Enables Improved Reconstruction of a Transcriptome from RNA-Seq Reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate Transcript Quantification from RNA-Seq Data with or without a Reference Genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Li, S.; Wang, J.; Kang, X.; Weng, Y.; Xing, G. Identification and Validation of Reference Genes for Quantitative Real-Time PCR Studies in Long Yellow Daylily, Hemerocallis citrina Borani. PLoS ONE 2017, 12, e0174933. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Oude Griep, L.M.; Monique Verschuren, W.M.; Kromhout, D.; Ocké, M.C.; Geleijnse, J.M. Colours of Fruit and Vegetables and 10-Year Incidence of CHD. Br. J. Nutr. 2011, 106, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, W.; Jia, K.; Liao, K.; Liu, L.; Fan, G.; Zhang, S.; Wang, Y. Metabolomic and Transcriptomic Analyses of Flavonoid Biosynthesis in Apricot Fruits. Front. Plant Sci. 2023, 14, 1210309. [Google Scholar] [CrossRef]
- Moon, K.M.; Kwon, E.-B.; Lee, B.; Kim, C.Y. Recent Trends in Controlling the Enzymatic Browning of Fruit and Vegetable Products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef]
- Lama, K.; Harlev, G.; Shafran, H.; Peer, R.; Flaishman, M.A. Anthocyanin Accumulation Is Initiated by Abscisic Acid to Enhance Fruit Color during Fig (Ficus carica L.) Ripening. J. Plant Physiol. 2020, 251, 153192. [Google Scholar] [CrossRef]
- Hao, N.; Du, Y.; Li, H.; Wang, C.; Wang, C.; Gong, S.; Zhou, S.; Wu, T. CsMYB36 Is Involved in the Formation of Yellow Green Peel in Cucumber (Cucumis sativus L.). Theor. Appl. Genet. 2018, 131, 1659–1669. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, J.; Rhee, J.; Lee, J.Y.; Lim, S.H. Loss of the R2r3 Myb Transcription Factor Rsmyb1 Shapes Anthocyanin Biosynthesis and Accumulation in Raphanus sativus. Int. J. Mol. Sci. 2021, 22, 10927. [Google Scholar] [CrossRef]
- Morishita, T.; Kojima, Y.; Maruta, T.; Nishizawa-Yokoi, A.; Yabuta, Y.; Shigeoka, S. Arabidopsis NAC Transcription Factor, ANAC078, Regulates Flavonoid Biosynthesis under High-Light. Plant Cell Physiol. 2009, 50, 2210–2222. [Google Scholar] [CrossRef]
- Sun, Q.; Jiang, S.; Zhang, T.; Xu, H.; Fang, H.; Zhang, J.; Su, M.; Wang, Y.; Zhang, Z.; Wang, N.; et al. Apple NAC Transcription Factor MdNAC52 Regulates Biosynthesis of Anthocyanin and Proanthocyanidin through MdMYB9 and MdMYB11. Plant Sci. 2019, 289, 110286. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Li, Z.; Li, J.; Ma, Y.; Miao, M.; Long, H.; Zhou, Y.; Chang, W. Integrative Metabolome and Transcriptome Analyses Reveal the Pericarp Coloration Mechanisms in Bitter Melon (Momordica charantia L.). Horticulturae 2024, 10, 291. https://doi.org/10.3390/horticulturae10030291
Yang L, Li Z, Li J, Ma Y, Miao M, Long H, Zhou Y, Chang W. Integrative Metabolome and Transcriptome Analyses Reveal the Pericarp Coloration Mechanisms in Bitter Melon (Momordica charantia L.). Horticulturae. 2024; 10(3):291. https://doi.org/10.3390/horticulturae10030291
Chicago/Turabian StyleYang, Liang, Zhi Li, Ju Li, Yanqin Ma, Mingjun Miao, Haicheng Long, Yujie Zhou, and Wei Chang. 2024. "Integrative Metabolome and Transcriptome Analyses Reveal the Pericarp Coloration Mechanisms in Bitter Melon (Momordica charantia L.)" Horticulturae 10, no. 3: 291. https://doi.org/10.3390/horticulturae10030291
APA StyleYang, L., Li, Z., Li, J., Ma, Y., Miao, M., Long, H., Zhou, Y., & Chang, W. (2024). Integrative Metabolome and Transcriptome Analyses Reveal the Pericarp Coloration Mechanisms in Bitter Melon (Momordica charantia L.). Horticulturae, 10(3), 291. https://doi.org/10.3390/horticulturae10030291





