Effect of Seed Spaceflight Storage on Tomato Fruit Quality and Peel/Pulp Mineral and Antioxidant Distribution
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Preparation
2.2. Dry Matter
2.3. Mineral Composition
2.4. Ascorbic Acid
2.5. Total Polyphenols (TP)
2.6. Carotenoids
2.7. Antioxidant Activity (AOA)
2.8. Proline
2.9. Photosynthetic Pigments
2.10. Organic Acids
2.11. Dietary Fiber
2.12. Statistical Analysis
3. Results and Discussion
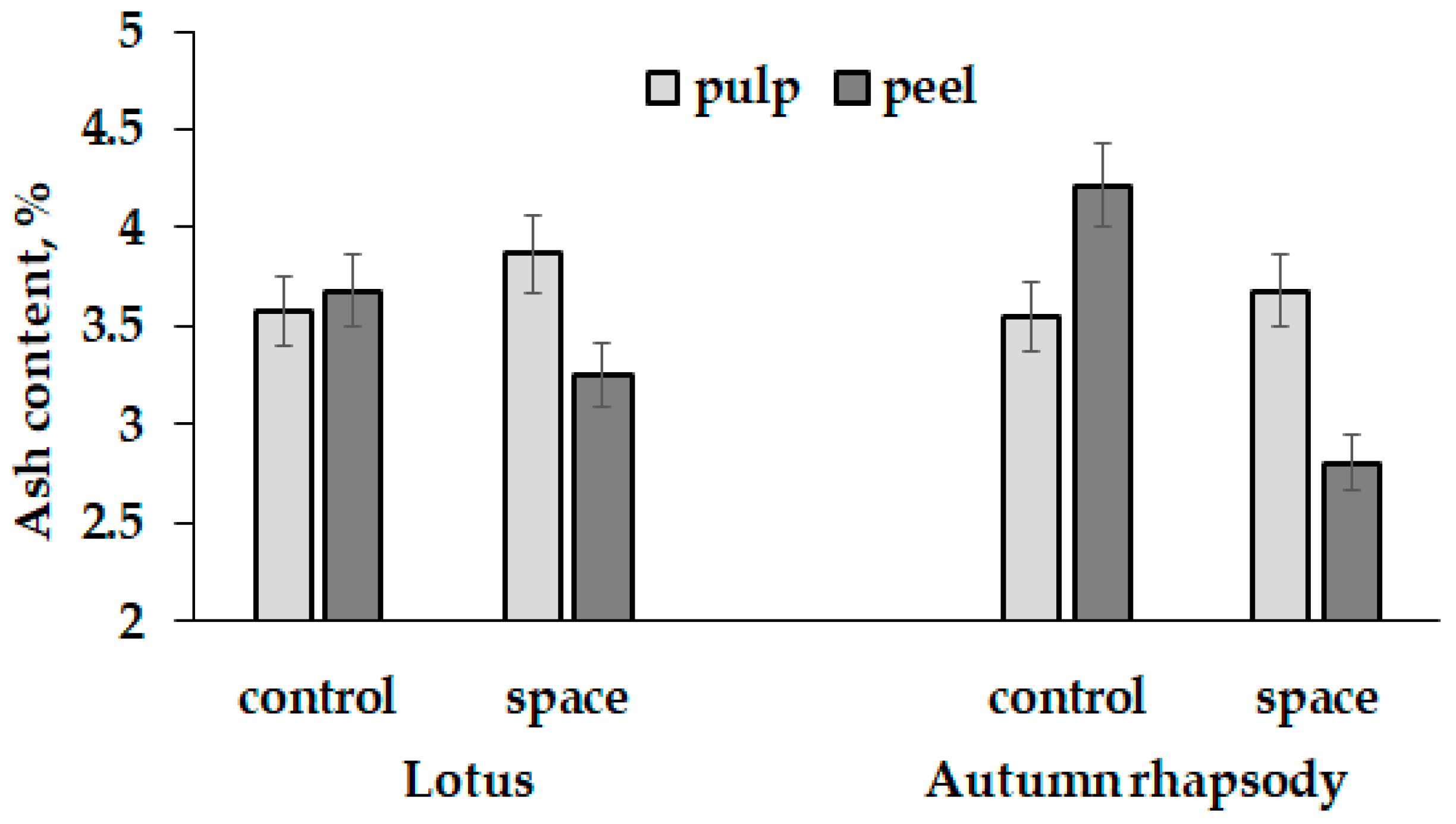
3.1. Biometrical Parameters, Dry Matter and Dietary Fiber
3.2. Photosynthetic Pigments
3.3. Organic Acids
3.4. AOA, TP, Ascorbic Acid and Proline
3.5. Carotenoids
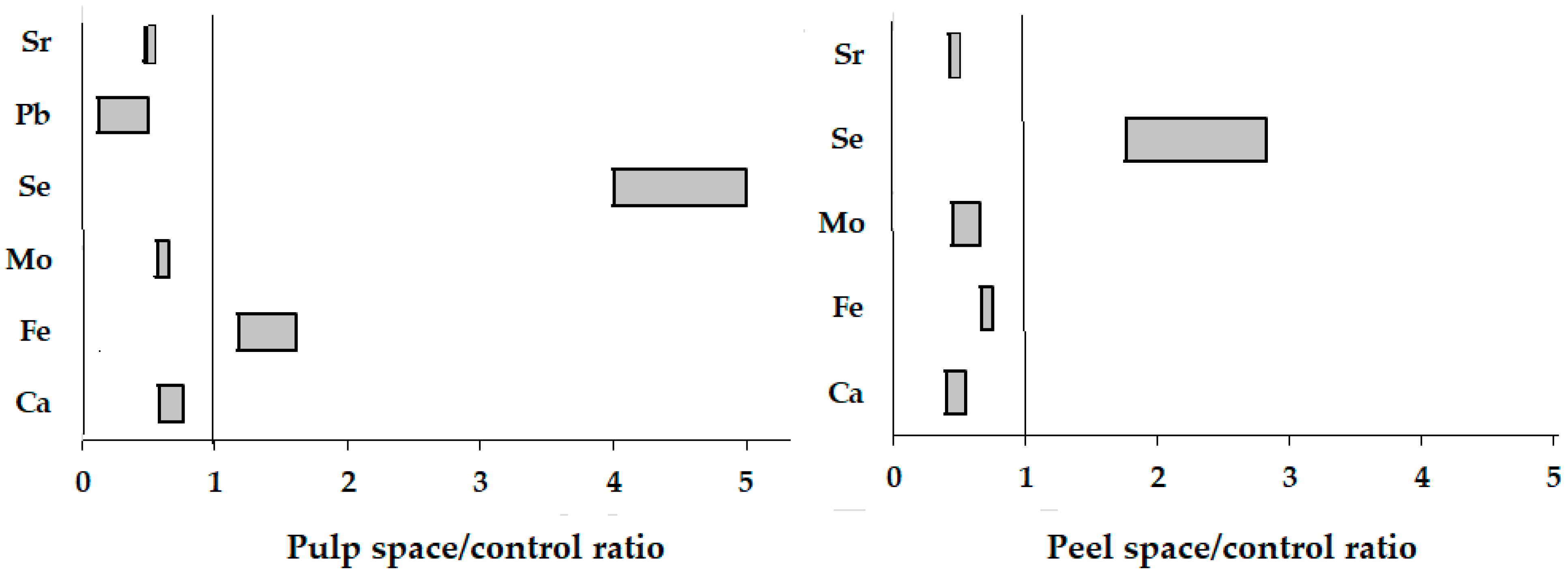
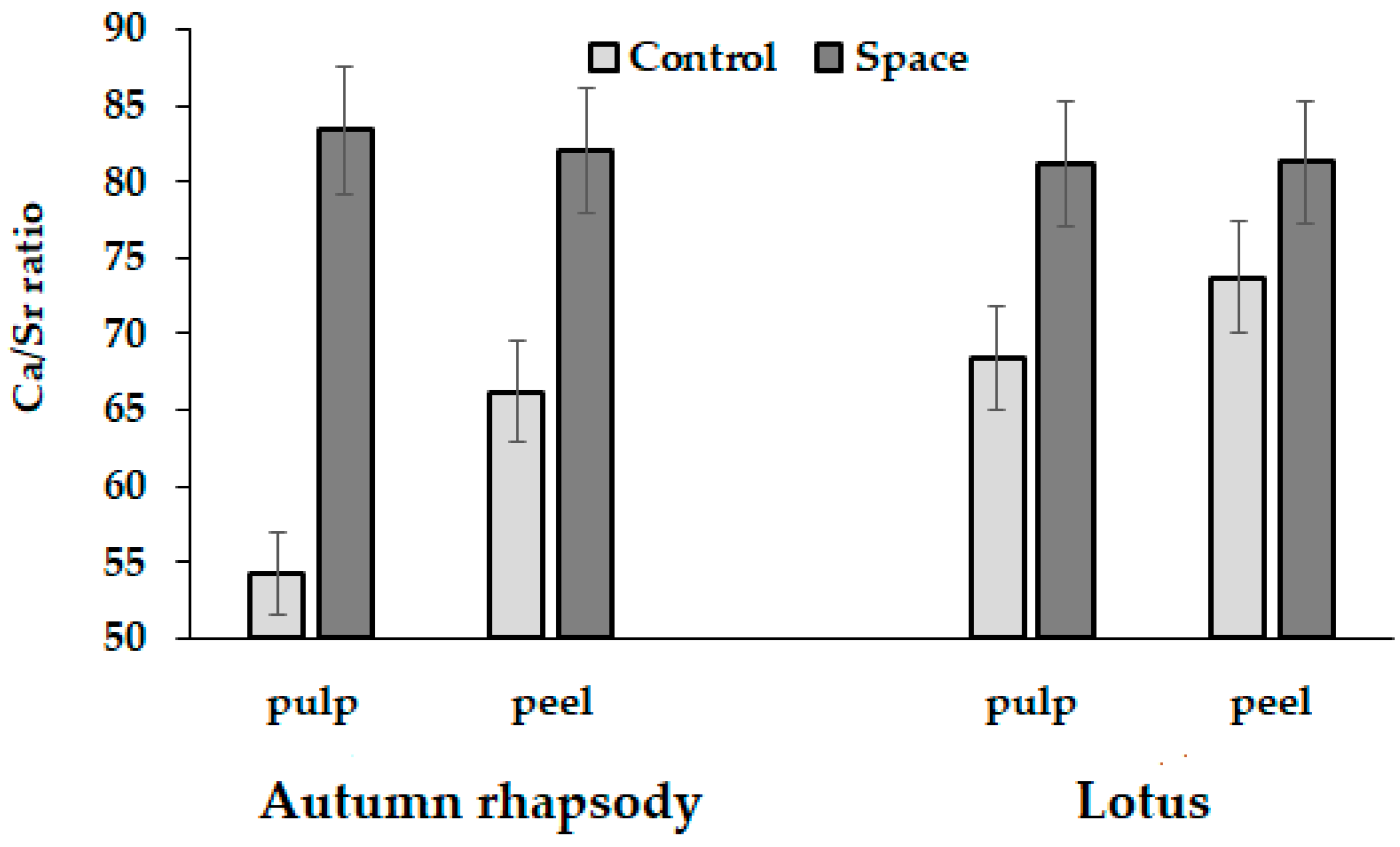
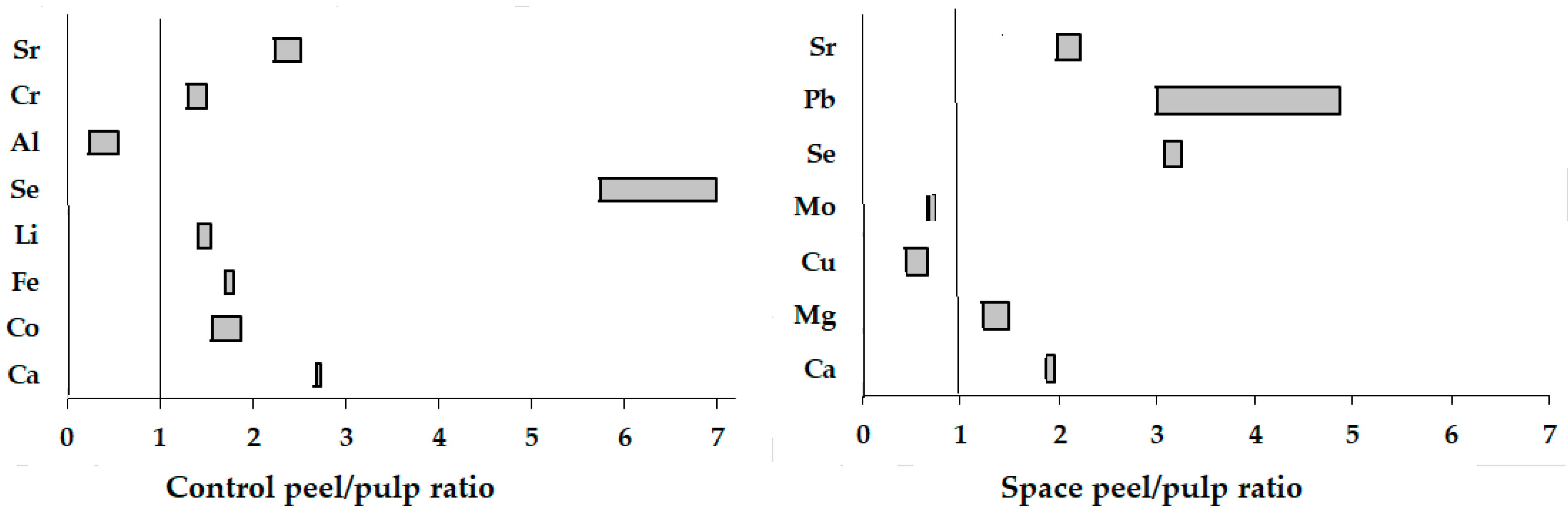
3.6. Elemental Composition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chandler, J.O.; Haas, F.B.; Khan, S.; Bowden, L.; Ignatz, M.; Enfissi, E.M.A.; Gawthrop, F.; Griffiths, A.; Fraser, P.D.; Rensing, S.A.; et al. Rocket Science: The Effect of Spaceflight on Germination Physiology, Ageing, and Transcriptome of Eruca sativa Seeds. Life 2020, 10, 49. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, W.; Zhang, M.; Zhao, L.; Mi, D.; Zhang, B.; Zhou, D.; Zhang, S. Space radiation systems biology research in SJ-10 Satellite. In Research for Development; Duan, E., Long, M., Eds.; Springer: Dordrecht, The Netherlands, 2019; pp. 43–68. [Google Scholar] [CrossRef]
- Zhang, B.-Y.; Wei, X.-L.; Yang, F.-Y.; Zhang, Y.-W. Effects of space flight factors on genetic diversity of Buchloe dactyloides seeds. Afr. J. Biotech. 2011, 10, 12812–12820. [Google Scholar] [CrossRef][Green Version]
- Zeng, D.; Cui, J.; Yin, Y.; Dai, C.; Zhao, H.; Song, C.; Guan, S.; Cheng, D.; Sun, Y.; Lu, W. Combining Proteomics and Metabolomics to Analyze the Effects of Spaceflight on Rice Progeny. Front. Plant Sci. 2022, 13, 900143. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, W.; Sun, Y. Proteomic analysis of high yield rice variety mutated from spaceflight. Adv. Space Res. 2007, 40, 535–539. [Google Scholar] [CrossRef]
- Xiao, W.M.; Yang, Q.Y.; Chen, Z.Q.; Wang, H.; Guo, T.; Liu, Y.Z.; Zhu, X.Y. Blast-resistance inheritance of space induced rice lines and their genomic polymorphism by microsatellite markers. Agr. Sci. China 2009, 8, 387–393. (In Chinese) [Google Scholar] [CrossRef]
- Wei, L.J.; Yang, Q.; Xia, H.M. Analysis of cytogenetic damage in rice seeds induced by energetic heavy ions on-ground and after space flight. J. Rad. Res. 2006, 47, 273–278. [Google Scholar] [CrossRef]
- Cyranoski, D. Satellite will probe mutating seeds in space. Nature 2001, 410, 857. [Google Scholar] [CrossRef] [PubMed]
- Levine, H.G. The Influence of Microgravity on Plants. In Proceedings of the NASA ISS Research Academy and PreApplication Meeting, League City, TX, USA, 3–5 August 2010; Available online: https://ntrs.nasa.gov/api/citations/20110001224/downloads/20110001224.pdf (accessed on 3 May 2021).
- Cheng, Z.L.; Zhang, M.; Hang, X.M. Transcriptomic analysis of space-induced rice mutants with enhanced susceptibility to rice blast. Adv. Space Res. 2007, 40, 540–549. [Google Scholar] [CrossRef]
- Ren, W.B.; Xu, Z.; Cheng, L.B. Cytological changes of root tip cells of alfalfa seeds after space flight. J. Nucl. Agr. Sci. 2008, 22, 566–568. (In Chinese) [Google Scholar]
- Yu, X.; Wu, H.; Wei, L.J.; Cheng, Z.L.; Xin, P.; Huang, C.L.; Zhang, K.P.; Sun, Y.Q. Characteristics of phenotype and genetic mutations in rice after spaceflight. Adv. Space Res. 2007, 40, 528–534. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Q. Space-induced mutations for crop improvement. In China Nuclear Science and Technology Report; CNIC01139/CSNAS-0111; China Nuclear Information Centre, Atomic Energy Press: Beijing, China, 1997. [Google Scholar]
- Musgrave, M.E. Growing plants in space. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2007, 2, 065. [Google Scholar] [CrossRef]
- Wolff, S.A.; Coelho, L.H.; Karoliussen, I.; Jost, A.I. Effects of the Extraterrestrial Environment on Plants: Recommendations for Future Space Experiments for the MELiSSA Higher Plant Compartment. Life 2014, 4, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Vaulina, E.; Anikeeva, I.; Kostina, L. Radiosensibility of higher plant seeds after space flight. Adv. Space Res. 1984, 4, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Sparks, J.A.; Nakashima, J.; Allen, S.N.; Tang, Y.H.; Blancaflor, E.B. Transcriptional response of Arabidopsis seedlings during spaceflight reveals peroxidase and cell wall remodeling genes associated with root hair development. Am. J. Bot. 2015, 102, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.H.; Xie, J.Y.; Zheng, H.Q. Differential protein expression profiling of Arabidopsis thaliana callus under microgravity on board the Chinese SZ-8 spacecraft. Planta 2015, 241, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.B.; Zhang, Y.; Deng, B.; Guo, H.; Cheng, L.; Liu, Y. Effect of space flight factors on alfalfa seeds. Afr. J. Biotechnol. 2010, 9, 7273–7279. [Google Scholar] [CrossRef]
- Xianfang, W.; Long, Z.; Weixu, D.; Chungua, L. Study of space mutation breeding in China. Appl. Life Sci. 2004, 18, 241–246. [Google Scholar]
- He, X.; Liu, M.; Lu, J.; Xue, H.; Pan, Y. Space mutation breeding: A brief introduction of screening. New floricultural, vegetable and medicinal carieties from earth-grown plants returned from China’s satellites and spaceships. In Floriculture, Ornamental and Plant Biotechnology: Advances and Topical Issues, 1st ed.; Teixeira da Silva, J.A., Ed.; Global Sciebce Books: Isleworth, UK, 2006; pp. 266–271. [Google Scholar]
- Prasad, B.; Richter, P.; Vadakedath, N.; Haag, F.W.M.; Strauch, C.M.; Mancinelli, R.; Schwarzwälder, A.; Etcheparre, E.; Gaume, N.; Lebert, M. How the space environment influences organisms: An astrobiological perspective and review. Int. J. Astrobiol. 2021, 20, 159–177. [Google Scholar] [CrossRef]
- Kahn, B.A.; Stoffella, P.J. No evidence of adverse effects on germination, emergence, and fruit yield due to space exposure of tomato seeds. J. Am. Soc. Hortic. Sci. 1996, 121, 414–418. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Yuan, C.; Yang, Q.; Long, C.; Li, Y.; Yang, M. Assessment of genetic diversity and variation of Acer Mono Max seedlings after spaceflight. Pakistan J. Bot. 2015, 47, 197–202. [Google Scholar]
- Liu, L.X.; Guo, H.J.; Zhao, L.; Gu, J.; Zhao, S. Advances in crop improvement by space mutagenesis in China. ICSC 2008, 4, 274. [Google Scholar]
- Dutcher, F.; Hess, E.L.; Halstead, T.W. Progress in plant research in space. Adv. Space Res. 1994, 14, 159–171. [Google Scholar] [CrossRef]
- Caplin, N.M. Developmental, Morphological and Physiological Effects of Chronic Low Doses of Ionizing Radiation on Plants on Earth and in Space. Ph.D. Thesis, University of the West of England, Bristol, UK, 2019. [Google Scholar]
- De Micco, V.; Arena, C.; Pignalosa, D.; Durante, M. Effects of Sparsely and Densely Ionizing Radiation on Plants. Radiat. Environ. Biophys. 2011, 50, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Arena, C.; De Micco, V.; De Maio, A. Growth alteration and leaf biochemical responses in Phaseolus Vulgaris exposed to different doses of ionizing radiation. Plant Biol. 2014, 16 (Suppl. 1), 194–202. [Google Scholar] [CrossRef] [PubMed]
- Arena, C.; De Micco, V.; Aronne, G.; Pugliese, M.G.; Virzo, A.; DeMaio, A. Response of Phaseolus vulgaris L. plants to low-LET ionizing radiation: Growth and oxidative stress. Acta Astronaut. 2014, 91, 107–114. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Li, R.; Ge, Y.; Li, Y.; Li, R. Plants’ Response to Abiotic Stress: Mechanisms and Strategies. Int. J. Mol. Sci. 2023, 24, 10915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Y.; Zhu, J.-K. Thriving under stress: How plants balance growth and the stress response. Dev. Cell. 2020, 55, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Kharchenko, V.; Golubkina, N.; Skrypnik, L.; Murariu, O.C.; Vecchietti, L.; Caruso, G. The Effect of One-year Seed Spaceflight Storage on Biochemical and Mineral Characteristics of Mature Leafy Vegetables Belonging to Brassicaceae, Apiaceae and Asteraceae Families. Horticuturae 2023, 9, 535. [Google Scholar] [CrossRef]
- AOAC Association Official Analytical Chemists. The Official Methods of Analysis of AOAC International; 22 ‘Vitamin C’; AOAC: Rockville, MD, USA, 2012. [Google Scholar]
- Golubkina, N.A.; Kekina, H.G.; Molchanova, A.V.; Antoshkina, M.S.; Nadezhkin, S.; Soldatenko, A.V. Plants Antioxidants and Methods of Their Determination; Infra-M: Moscow, Russia, 2020; (In Russian). [Google Scholar] [CrossRef]
- Ouertani, R.N.; Abid, G.; Karmous, C.; Chikha, M.B.; Boudaya, O.; Mahmoudi, H.; Mejri, S.; Jansen, K.; Ghorbel, A. Evaluating the contribution of osmotic and oxidative stress components on barley growth under salt stress. AoB Plants 2021, 13, plab034. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic bio-membranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Guide for the Evaluation of Quality and Safety of Food Biologically Active Additives; Organic Acids Determination; Health Ministry of RF: Moscow, Russia, 2004; pp. 109–111.
- Guidance on Methods of Quality Control and Safety of Biologically Active Food Supplements, Determination of Soluble and Non Soluble Food Fiber-46-50; Ministry of Health of Russia: Moscow, Russia, 2004; pp. 46–50.
- Reitz, N.F.; Mitcham, E.J. Lignification of tomato (Solanum lycopersicum) pericarp tissue during blossom-end rot development. Sci. Hortic. 2021, 276, 109759. [Google Scholar] [CrossRef]
- Wang, G.-L.; Wu, J.-Q.; Chen, Y.-Y.; Xu, Y.-J.; Zhou, C.-L.; Hu, Z.-Z.; Ren, X.-Q.; Xiong, A.-S. More or Less: Recent Advances in Lignin Accumulation and Regulation in Horticultural Crops. Agronomy 2023, 13, 2819. [Google Scholar] [CrossRef]
- Shu, F.; Jiang, B.; Yuan, Y.; Li, M.; Wu, W.; Jin, Y.; Xiao, H. Biological Activities and Emerging Roles of Lignin and Lignin-Based Products—A Review. Biomacromolecules 2021, 22, 4905–4918. [Google Scholar] [CrossRef]
- Renault, H.; Werck-Reichhart, D.; Weng, J.-K. Harnessing lignin evolution for biotechnological applications. Curr. Opin. Biotechnol. 2019, 56, 105–111. [Google Scholar] [CrossRef]
- Hu, X.; Gu, T.; Khan, I.; Zada, A.; Jia, T. Research Progress in the Interconversion, Turnover and Degradation of Chlorophyll. Cells 2021, 10, 3134. [Google Scholar] [CrossRef] [PubMed]
- Preedy, V.R.; Watson, R.R. Tomatoes and Tomato Products: Nutritional, Medicinal and Therapeutic Properties; Science Publishers: Enfield, NH, USA, 2008. [Google Scholar]
- Panchal, P.; Miller, A.J.; Giri, J. Organic acids: Versatile stress-response roles in plants. J. Exp. Bot. 2021, 72, 4038–4052. [Google Scholar] [CrossRef]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef] [PubMed]
- El Gamal, R.; Song, C.; Rayan, A.M.; Liu, C.; Al-Rejaie, S.; ElMasry, G. Thermal Degradation of Bioactive Compounds during Drying Process of Horticultural and Agronomic Products: A Comprehensive Overview. Agronomy 2023, 13, 1580. [Google Scholar] [CrossRef]
- Dzhos, E.; Golubkina, N.; Antoshkina, M.; Kondratyeva, I.; Koshevarov, A.; Shkaplerov, A.; Zavarikina, T.; Caruso, G. Effect of Spaceflight on Tomato Seed quality and Biochemical Characteristics of Mature Plants. Horticulturae 2021, 7, 89. [Google Scholar] [CrossRef]
- Golubkina, N.; Skrypnik, L.; Logvinenko, L.; Zayachkovsky, V.; Smirnova, A.; Krivenkov, L.; Romanov, V.; Kharchenko, V.; Poluboyarinov, P.; Sekara, A.; et al. The ‘Edge Effect’ Phenomenon in Plants: Morphological, Biochemical and Mineral Characteristics of Border Tissues. Diversity 2023, 15, 123. [Google Scholar] [CrossRef]
- Nechitailo, G.S.; Lu, J.; Xue, H.; Pan, Y.; Tang, C.; Liu, M. Influence of Long Term Exposure to Space Flight on Tomato Seeds. Adv. Space Res. 2005, 36, 1329–1333. [Google Scholar] [CrossRef]
- Conn, S.; Gilliham, M. Comparative physiology of elemental distributions in plants. Ann. Bot. 2010, 105, 1081–1102. [Google Scholar] [CrossRef] [PubMed]
- Grusak, M.A.; Broadley, M.R.; White, P.J. Plant Macro- and Micronutrient Minerals; John Wiley & Sons: Chichester, UK, 2016. [Google Scholar] [CrossRef]
- Kumari, V.V.; Banerjee, P.; Verma, V.C.; Sukumaran, S.; Chandran, M.A.S.; Gopinath, K.A.; Venkatesh, G.; Yadav, S.K.; Singh, V.K.; Awasthi, N.K. Plant Nutrition: An Effective Way to Alleviate Abiotic Stress in Agricultural Crops. Int. J. Mol. Sci. 2022, 23, 8519. [Google Scholar] [CrossRef]
- Elbadrawy, E.; Sello, A. Evaluation of nutritional value and antioxidant activity of tomato peel extracts. Arab. J. Chem. 2016, 9 (Suppl. 2), S1010–S1018. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Bello, S.K.; Alayafi, A.H.; AL-Solaimani, S.G.; Abo-Elyousr, K.A.M. Mitigating Soil Salinity Stress with Gypsum and Bio-Organic Amendments: A Review. Agronomy 2021, 11, 1735. [Google Scholar] [CrossRef]
- Liu, H.; Xiao, C.; Qiu, T.; Deng, J.; Cheng, H.; Cong, X.; Cheng, S.; Rao, S.; Zhang, Y. Selenium Regulates Antioxidant, Photosynthesis, and Cell Permeability in Plants under Various Abiotic Stresses: A Review. Plants 2023, 12, 44. [Google Scholar] [CrossRef]
- Sarraf, M.; Janeeshma, E.; Arif, N.; Farooqi, M.Q.U.; Kumar, V.; Ansari, N.A.; Ghani, M.I.; Ahanger, M.A.; Hasanuzzaman, M. Understanding the role of beneficial elements in developing plant stress resilience: Signalling and crosstalk with phytohormones and microbes. Plant Stress 2023, 10, 100224. [Google Scholar] [CrossRef]
- Golubkina, N. Selenium biorhythms and hormonal regulation Chapter 2 in Selenium. In Sources, Functions and Health Effects; Aomori, C., Hokkaido, M., Eds.; Novapublishers: Hauppauge, NY, USA, 2012; pp. 33–74. [Google Scholar]
- Riaz, N.; Guerinot, M.L. All together now: Regulation of the iron deficiency response. J. Exp. Bot. 2021, 72, 2045–2055. [Google Scholar] [CrossRef]
- Kaiser, B.N.; Gridley, K.L.; Brady, J.N.; Phillips, T.; Tyerman, S.D. The role of molybdenum in agricultural plant production. Ann. Bot. 2005, 96, 745–754. [Google Scholar] [CrossRef]
- Ahmed, D.A.E.A.; Slima, D.F.; Al-Yasi, H.M.; Hassan, L.M.; Galal, T.M. Risk assessment of trace metals in Solanum lycopersicum L. (tomato) grown under wastewater irrigation conditions. Environ. Sci. Pollut. Res. 2023, 30, 42255–42266. [Google Scholar] [CrossRef]
- Heraldy, E.; Lestari, W.W.; Permatasari, D.; Arimurti, D.D. Biosorbent from tomato waste and apple juice residue for lead removal. J. Environ. Chem. Eng. 2018, 6, 1201–1208. [Google Scholar] [CrossRef]
- Kosiorek, M.; Wyszkowski, M. Macroelement content in plants after amendment application to cobalt-contaminated soil. J. Soils Sediments 2021, 21, 1769–1784. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B. Effect of selenium on selected macronutrients in maize plants. J. Elementol. 2008, 13, 513–519. [Google Scholar]
- Golubkina, N.; Antoshkina, M.; Bondareva, L.; Sekara, A.; Campagna, E.; Caruso, G. Effect of Foliar Application of Sodium Selenate on Mineral Relationships in Brassicaceae Crops. Horticulturae 2023, 9, 535. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, J.; Huang, B.; Ying, H.; He, J.; Jiang, L. Selenium metabolism and selenoproteins in prokaryotes: A bioinformatics perspective. Biomolecules 2022, 12, 917. [Google Scholar] [CrossRef] [PubMed]
- Heshmat, K.; Lajayer, B.A.; Shakiba, M.R.; Astatkie, T. Assessment of physiological traits of common bean cultivars in response to water stress and molybdenum levels. J. Plant Nutr. 2020, 44, 366–372. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, C.; Zhao, X.; Tan, Q.; Sun, X.; Li, N. Impact of molybdenum on Chinese cabbage response to selenium in solution culture. Soil Sci. Plant Nutr. 2012, 58, 595–603. [Google Scholar] [CrossRef]


| Parameter | Lotus | Autumn Rhapsody | ||
|---|---|---|---|---|
| Control | Space | Control | Space | |
| Plant height (cm) | 99 ± 10 a | 97 ± 10 a | 105 ± 12 a | 100 ± 11 a |
| Fruit weight (g) | 84 ± 8 b | 79 ± 8 b | 143 ± 14 a | 138 ± 14 a |
| Fruit number per plant | 36 ± 3 a | 39 ± 4 a | 14 ± 2 b | 16 ± 3 b |
| Early fruit yield (kg m−2) | 2.53 ± 0.24 a | 2.76 ± 0.30 a | 1.81 ± 0.20 b | 2.02 ± 0.20 b |
| Total fruit yield (kg m−2) | 9.18 ± 0.95 a | 9.04 ± 0.90 a | 9.25 ± 0.91 a | 9.13 ± 0.90 a |
| Fruit diameter (cm) | 58.4 ± 6 b | 54.8 ± 5 b | 73.9 ± 7 a | 61.7 ± 6 a |
| Marketability (%) | 80 ± 5 a | 79 ± 5 a | 84 ± 5 a | 85 ± 5 a |
| Leaf dry matter (%) | 10.5 ± 1.0 b | 12.9 ± 1.1 a | 11.5 ± 1.0 ab | 13.5 ± 1.1 a |
| Fruit pulp dry matter (%) | 5.3 ± 0.5 c | 8.0 ± 0.8 a | 6.0 ± 0.6 bc | 6.8 ± 0.7 ab |
| Fruit peel dry matter (%) | 9.9 ± 0.9 b | 11.9 ± 1.1 ab | 9.8 ± 0.9 b | 13.2 ± 1.2 a |
| Dietary fiber (% f.w.) | 5.4 ± 0.3 c | 6.2 ± 0.4 b | 6.1 ± 0.5 b | 8.6 ± 0.7 a |
| Photosynthetic Pigments (mg g−1 d.w.) | Lotus | Autumn Rhapsody | ||
|---|---|---|---|---|
| Control | Space | Control | Space | |
| Chlorophyll a | 12.08 ± 1.0 ab | 12.40 ± 1.20 ab | 10.52 ± 1.00 b | 13.70 ± 1.21 a |
| Chlorophyll b | 6.86 ± 0.61 ab | 6.05 ± 0.60 b | 8.96 ± 0.90 a | 7.41 ± 0.71 a |
| Total chlorophyll | 18.94 ± 1.71 a | 18.45 ± 1.70 a | 19.48 ± 1.88 a | 21.11 ± 2.01 a |
| Carotene | 2.38 ± 0.20 a | 2.71 ± 0.22 a | 2.35 ± 0.20 a | 2.52 ± 0.21 a |
| Chl a/Chl b ratio | 1.80 | 2.05 | 1.17 | 1.84 |
| Parameter | Lotus | Autumn Rhapsody | ||
|---|---|---|---|---|
| Control | Space | Control | Space | |
| Citric acid (mg 100 g−1 d.w.) | 7079 ± 395 b | 8921 ± 480 a | 7030 ± 480 b | 8333 ± 430 a |
| Malic acid (mg 100 g−1 d.w.) | 1015 ± 57 c | 1261 ± 61 b | 1248 ± 63 b | 1447 ± 67 a |
| Oxalic acid (mg 100 g−1 d.w.) | 43.4 ± 2.5 a | 10.0 ± 0.6 c | 33.3 ± 1.7 b | 5.9 ± 0.4 d |
| Total (mg 100 g−1 d.w.) | 8137.4 ± 422 b | 10,192 ± 499 a | 8311 ± 405 b | 9785.9 ± 512 a |
| Citric/malic ratio | 6.97 | 7.07 | 5.63 | 5.76 |
| Monosaccharides (g 100 g−1 d.w.) | 50.8 ± 4.8 a | 56.8 ± 5.2 a | 36.7 ± 3.2 b | 45.6 ± 4.2 a |
| Nitrates (mg kg−1 d.w.) | 10,774 ± 780 a | 7662.5 ± 540 b | 9283 ± 650 a | 6956 ± 430 b |
| Parameter | Plant Part | Lotus | Autumn Rhapsody | ||
|---|---|---|---|---|---|
| Control | Space | Control | Space | ||
| AOA (mg GAE g−1 d.w.) | Pulp | 43.9 ± 3.9 b | 41.9 ± 4.0 b | 56.2 ± 5.2 a | 56.7 ± 5.3 a |
| Peel | 48.9 ± 4.2 ab | 50.2 ± 4.9 a | 57.9 ± 5.2 a | 54.6 ± 5.2 a | |
| Peel/pulp | 1.114 | 1.198 | 1.030 | 0.963 | |
| Polyphenols (TP) (mg GAE g−1 d.w.) | Pulp | 18.0 ± 1.7 c | 17.3 ± 1.6 c | 18.7 ± 1.7 c | 18.7 ± 1.7 c |
| Peel | 19.8 ± 1.8 bc | 24.2 ± 2.1 a | 22.6 ± 1.8 ab | 22.4 ± 1.8 ab | |
| Peel/pulp | 1.100 | 1.399 | 1.209 | 1.198 | |
| Ascorbic acid (mg 100 g−1 d.w.) | Pulp | 232 ± 23 b | 272 ± 27 ab | 278 ± 27 ab | 328 ± 32 a |
| Proline (mg g−1 d.w.) | 8.0 ± 0.7 a | 9.3 ± 0.9 a | 9.1 ± 0.9 a | 9.6 ± 0.9 a | |
| Parameter | Plant Part | Lotus | Autumn Rhapsody | ||
|---|---|---|---|---|---|
| Control | Space | Control | Space | ||
| Total Carotenoids (mg 100 g−1 d.w.) | Pulp | 117.1 ± 10.1 a | 68.8 ± 6.2 b | 81.5 ± 8.0 b | 56.7 ± 5.1 c |
| β-carotene (mg 100 g−1 d.w.) | Trace | Trace | 62.7 ± 6.0 a | 35.1 ± 3.1 b | |
| Lutein (mg 100 g−1 d.w.) | 31.1 ± 2.9 a | 25.9 ± 2.2 ab | 18.8 ± 1.7 b | 21.6 ± 2.0 b | |
| Lycopene (mg 100 g−1 d.w.) | 86 ± 8 a | 42.9 ± 4.0 b | traces | traces | |
| Total Carotenoids (mg 100 g−1 d.w.) | Peel | 245.3 ± 23.5 b | 299.1 ± 28.2 a | 92.9 ± 9.1 d | 136.4 ± 12.9 c |
| β-carotene (mg 100 g−1 d.w.) | Trace | Trace | 54.1 ± 5.0 b | 103.8 ± 9.3 a | |
| Lutein (mg 100 g−1 d.w.) | 49.5 ± 4.3 a | 49.6 ± 4.4 a | 38.8 ± 3.5 b | 32.6 ± 2.9 b | |
| Lycopene (mg 100 g−1 d.w.) | 195.8 ± 19.0 b | 249.5 ± 24.2 a | Trace | Trace | |
| Total carotenoids | Peel/ /pulp ratio | 2.09 | 4.30 | 1.14 | 2.41 |
| Lutein | 1.592 | 1.915 | 2.064 | 1.509 | |
| Lycopene | 2.28 | 5.82 | - | - | |
| β-carotene | - | - | 0.86 | 2.96 | |
| Autumn Rhapsody | Lotus | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Space Treatment | Control | Space Treatment | |||||
| Pulp | Peel | Pulp | Peel | Pulp | Peel | Pulp | Peel | |
| Ca | 1127 ± 113 c | 3084 ± 302 a | 851 ± 85 d | 1658 ± 164 b | 1313 ± 130 bc | 3569 ± 360 a | 766 ± 76 d | 1432 ± 143 b |
| K | 27,254 ± 2700 ab | 27,805 ± 2700 an | 27,722 ± 2700 ab | 19,515 ± 1900 c | 27,595 ± 2700 ab | 27,187 ± 2700 ab | 30,939 ± 3000 a | 23,839 ± 2300 b |
| Mg | 1061 ± 103 bcd | 1531 ± 150 a | 1254 ± 124 ab | 845 ± 84 d | 1071 ± 105 abc | 860 ± 86 d | 1204 ± 120 bc | 990 ± 98 cd |
| Na | 275 ± 27 b | 363 ± 36 a | 328 ± 33 a | 298 ± 30 ab | 248 ± 24 bc | 219 ± 22 c | 268 ± 27 bc | 268 ± 26 bc |
| P | 5594 ± 560 bc | 9125 ± 910 a | 6321 ± 630 b | 5522 ± 550 bc | 5226 ± 520 bc | 4735 ± 470 c | 5309 ± 530 bc | 5780 ± 570 b |
| B | 8.41 ± 0.80 bc | 11.4 ± 1.0 ac | 7.19 ± 0.70 c | 8.14 ± 0.80 bc | 10.7 ± 1.0 a | 9.71 ± 0.95 ab | 10.4 ± 1.0 a | 9.92 ± 1.00 ab |
| Co | 0.023 ± 0.002 cd | 0.043 ± 0.04 a | 0.026 ± 0.002 bcd | 0.031 ± 0.003 b | 0.021 ± 0.002 d | 0.033 ± 0.003 b | 0.022 ± 0.002 cd | 0.028 ± 0.002 b |
| Cu | 5.9 ± 0.6 b | 4.78 ± 0.4 c | 8.06 ± 0.80 a | 3.54 ± 0.33 d | 7.48 ± 0.75 a | 2.94 ± 0.30 d | 5.08 ± 0.50 bc | 3.35 ± 0.33 d |
| Fe | 30.7 ± 3.0 e | 55.4 ± 5.2 a | 49.3 ± 4.9 ab | 37.2 ± 3.6 cde | 32.2 ± 3.2 de | 54.7 ± 5.4 a | 38.0 ± 3.7 cd | 41.0 ± 4.0 bc |
| Li | 0.033 ± 0.003 d | 0.051 ± 0.004 ab | 0.019 ± 0.002 e | 0.054 ± 0.005 a | 0.033 ± 0.003 d | 0.047 ± 0.004 abc | 0.043 ± 0.004 bc | 0.042 ± 0.004 c |
| Mn | 5.22 ± 0.50 c | 9.28 ± 0.91 a | 8.76 ± 0.85 a | 6.03 ± 0.60 b | 5.25 ± 0.51 c | 6.22 ± 0.61 b c | 6.01 ± 0.60 bc | 6.92 ± 0.70 b |
| Mo | 1.62 ± 0.13 a | 1.59 ± 0.15 a | 1.07 ± 0.10 b | 0.72 ± 0.07 cd | 1.56 ± 0.15 a | 0.95 ± 0.09 b | 0.89 ± 0.08 bc | 0.62 ± 0.06 d |
| Se | 0.04 ± 0.01 e | 0.23 ± 0.02 c | 0.20 ± 0.02 c | 0.65 ± 0.06 a | 0.03 ± 0.01 e | 0.21 ± 0.02 c | 0.12 ± 0.01 d | 0.37 ± 0.03 b |
| Zn | 13.8 ± 1.3 b | 19.2 ± 1.9 a | 17.7 ± 1.8 a | 17.7 ± 1.7 a | 13.8 ± 1.3 b | 13.9 ± 1.3 b | 16.0 ± 1.6 ab | 17.3 ± 1.7 a |
| Al | 143 ± 14 b | 81.5 ± 8.0 d | 178 ± 17.1 a | 133 ± 13 b | 212 ± 21 a | 51.2 ± 5.0 e | 105 ± 10 c | 57.7 ± 5.6 e |
| As | 0.046 ± 0.005 bc | 0.066 ± 0.006 a | 0.048 ± 0.05 bc | 0.043 ± 0.04 c | 0.051 ± 0.005 b | 0.025 ± 0.002 b | 0.057 ± 0.006 ab | 0.037 ± 0.003 c |
| Cd | 0.057 ± 0.005 d | 0.095 ± 0.01 ab | 0.063 ± 0.006 d | 0.11 ± 0.01 a | 0.078 ± 0.007 c | 0.092 ± 0.009 ab | 0.11 ± 0.01 a | 0.086 ± 0.008 bc |
| Cr | 0.10 ± 0.01 d | 0.15 ± 0.01 b | 0.12 ± 0.01 cd | 0.15 ± 0.01 b | 0.15 ± 0.01 b | 0.19 ± 0.02 a | 0.12 ± 0.01 cd | 0.14 ± 0.01 bc |
| Ni | 0.13 ± 0.01 ef | 0.23 ± 0.02 a | 0.14 ± 0.01 de | 0.18 ± 0.01 b | 0.17 ± 0.01 bc | 0.12 ± 0.01 f | 0.14 ± 0.01 de | 0.15 ± 0.01 cd |
| Pb | 1.12 ± 0.11 b | 0.75 ± 0.07 c | 0.15 ± 0.01 f | 0.73 ± 0.07 c | 0.20 ± 0.02 e | 1.82 ± 0.2 a | 0.10 ± 0.01 h | 0.30 ± 0.03 d |
| Sr | 20.8 ± 0.2 b | 46.6 ± 4.4 a | 10.2 ± 1.0 c | 20.2 ± 2.0 b | 19.2 ± 1.9 b | 48.4 ± 4.8 a | 9.43 ± 0.92 c | 20.9 ± 2.0 b |
| V | 0.073 ± 0.007 d | 0.11 ± 0.01 ac | 0.086 ± 0.008 cd | 0.07 ± 0.01 b | 0.13 ± 0.01 a | 0.071 ± 0.007 d | 0.099 ± 0.01 bc | 0.080 ± 0.008 d |
| Ca | Fe | Mo | Se | Sr | Co | Cr | Carotenoids | Dry Matter | |
|---|---|---|---|---|---|---|---|---|---|
| K | −0.080 | 0.149 | 0.454 | −0.875 a | −0.022 | −0.124 | −0.239 | 0.417 | −0.696 d |
| Ca | 0.717 d | 0.096 | 0.133 | 0.983 a | 0.803 b | 0.420 | 0.647 | 0.376 | |
| Fe | −0.104 | 0.076 | 0.664 | 0.756 c | 0.189 | 0.337 | 0.403 | ||
| Mo | −0.680 d | 0.231 | 0.180 | −0.544 | −0.437 | −0.693 d | |||
| Se | 0.050 | 0.315 | 0.359 | 0.271 | 0.843 a | ||||
| Sr | 0.794 c | 0.381 d | 0.584 | 0.299 | |||||
| Co | −0.023 | 0.146 | 0.366 | ||||||
| Cr | 0.591 | 0.482 | |||||||
| Carotenoids | 0.550 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubkina, N.; Dzhos, E.; Bogachuk, M.; Antoshkina, M.; Verba, O.; Zavarykina, T.; Nechitailo, G.; Murariu, O.C.; Tallarita, A.V.; Caruso, G. Effect of Seed Spaceflight Storage on Tomato Fruit Quality and Peel/Pulp Mineral and Antioxidant Distribution. Horticulturae 2024, 10, 289. https://doi.org/10.3390/horticulturae10030289
Golubkina N, Dzhos E, Bogachuk M, Antoshkina M, Verba O, Zavarykina T, Nechitailo G, Murariu OC, Tallarita AV, Caruso G. Effect of Seed Spaceflight Storage on Tomato Fruit Quality and Peel/Pulp Mineral and Antioxidant Distribution. Horticulturae. 2024; 10(3):289. https://doi.org/10.3390/horticulturae10030289
Chicago/Turabian StyleGolubkina, Nadezhda, Elena Dzhos, Maria Bogachuk, Marina Antoshkina, Olga Verba, Tatiana Zavarykina, Galina Nechitailo, Otilia Cristina Murariu, Alessio Vincenzo Tallarita, and Gianluca Caruso. 2024. "Effect of Seed Spaceflight Storage on Tomato Fruit Quality and Peel/Pulp Mineral and Antioxidant Distribution" Horticulturae 10, no. 3: 289. https://doi.org/10.3390/horticulturae10030289
APA StyleGolubkina, N., Dzhos, E., Bogachuk, M., Antoshkina, M., Verba, O., Zavarykina, T., Nechitailo, G., Murariu, O. C., Tallarita, A. V., & Caruso, G. (2024). Effect of Seed Spaceflight Storage on Tomato Fruit Quality and Peel/Pulp Mineral and Antioxidant Distribution. Horticulturae, 10(3), 289. https://doi.org/10.3390/horticulturae10030289









