Abstract
γ-Aminobutyric acid (GABA) is a ubiquitous nonprotein amino acid that has multiple physiological functions and has received significant attention in the pharmaceutical and food industries. Although there are many GABA-producing bacteria, the high cost of strain cultivation limits its food additive and pharmaceutical raw material application. In our study, Lactobacillus hilgardii GZ2, a novel GABA-producing strain, was investigated. We attempted to replace nitrogen sources with silkworm pupae, the waste resource of the silk reeling industry, in GYP complex medium. The GABA titer reached 33.2 g/L by using 10 g/L silkworm pupae meal instead of tryptone. Meanwhile, the pH of fermentation was automatically controlled by adjusting the addition of glucose and monosodium glutamate. Finally, the highest GABA yield and productivity were 229.3 g/L and 3.2 g/L/h in L. hilgardii when silkworm pupae meal was replaced with tryptone combined with glucose and monosodium glutamate feeding. By utilizing the waste resource to reduce the cost of the nitrogen source and automatically controlling the pH in L. hilgardii, a hyper titer and productivity of GABA was generated for applications in the food and pharmaceutical industries.
1. Introduction
γ-Aminobutyric acid (GABA) is an amino acid that does not participate in protein building. It is a neurosuppressive transmitter that plays a role in the human/animal central nervous system. It has multiple physiological activities, such as antihypertensive, antidiabetic, antioxidant, anti-inflammatory and antianxiety activities, and improves the functions of the brain, liver, and kidneys [1,2,3,4,5]. In the field of medicine, GABA can be used as a precursor of antiepileptic, sleep-inducing drugs. For the food industry, GABA can be used as an additive or can be enriched by fermentation to develop a variety of functional foods. The European Food Safety (EFSA) and the U.S. Food and Drug Administration (FDA) recognize GABA produced through lactic acid bacteria fermentation as a natural food additive. The EFSA allows the addition of GABA to food, with a maximum recommended dietary intake of 550 mg/day [1]. The FDA states that the addition of GABA to food is safe, and its usage includes beverages, coffee, tea, and chewing gum, but it is not permitted in baby food, meat products, or products containing meat. In 2001, Japan included GABA in its range of food products. In 2009, the Chinese Ministry of Health approved GABA produced through lactic acid fermentation as a new resource food. It stipulates that GABA intake should not exceed 500 mg/day, and its usage includes beverages, cocoa products, chocolate and its products, candy, baked goods, and puffed snacks, but it cannot be added to baby food. The use of the chemical synthesis of GABA in food and medicine is thought to be unhealthy and ineffective. Biocatalytic synthesis of GABA is the main production method used in the food and medicine industries. Therefore, it is crucial to find an efficient biocatalytic synthesis method for GABA.
GABA is formed by glutamate decarboxylase (GAD, EC: 4.1.1.15) catalyzing the decarboxylation of glutamate [6]. This decarboxylase is widely distributed among bacteria, fungi, and yeast [7,8,9,10]. Lactic acid bacteria, such as GRAS (generally considered to be safe) strains, are often used as starters for functional food fermentation and in pharmaceutical fields. Therefore, lactic acid bacteria that produce GABA efficiently are the main focus of GABA-producing bacteria at present [11,12,13,14,15]. The lactic acid bacteria with GABA synthesis identified thus far mainly include Lactobacillus (L.) brevis, L. buchneri, Streptococcus thermophilus, L. paracasei, Lactococcus lactis, and L. plantarum [11,14,16,17,18,19,20]. Lactobacillus sp. is the most common species among the highly efficient GABA-producing strains isolated from traditional fermented foods. After fermentation optimization, the maximum GABA production capacity of L. brevis NCL91 was 103 g/L with 1.43 g/L/h productivity. The strain was isolated from Chinese paocai [21]. L. plantarum EJ2014 was isolated from rice bran, and 19.8 g/L GABA was produced in a simple medium [22]. With xylose as a carbon source, the productivity of GABA produced by L. buchneri WPZ001 was 0.97 g/L/h. This xylose-using strain was isolated from Chinese fermented sausages [11]. Although the GABA yield can be increased by screening high-yielding strains and optimizing the composition of the fermentation medium and culture conditions, the fermentation cost is still a limiting factor in the cultivation of lactic acid bacteria and the industrialization of GABA.
Lactic acid bacteria are commonly found in food and the gastrointestinal tract, and their growth requires rich nutrients, including peptides, amino acids, vitamins, fatty acids, and inorganic salts [23]. The most important source is nitrogen, generally an organic compound nitrogen source. Organic nitrogen sources are commonly extracted substrates from yeast, plants and animals, and peptone hydrolyzed from plant and animal proteins, which are rich in proteins, peptides, and amino acids [24,25]. Commercial MRS and GYP media commonly used for cultivating lactic acid bacteria include 22 g/L and 15 g/L organic nitrogen source mixtures, respectively. These mixtures consist of peptone (protein hydrolysate) and meat and yeast extracts [25,26]. Considering the cost of composite nitrogen sources, finding alternative low-cost nitrogen sources is the key to reducing the production cost of GABA.
Silkworm pupae (Lepidopyera, Bombycidae, Bombyx mori L.) are a byproduct of the silk reeling industry. They are rich in lipids (30%), protein (60%), and ash (10%) [27]. The lipids of silkworm pupae (China) include saturated fatty acids (22.04% C16:0 and 6.84% C18:0), monounsaturated fatty acids (0.92% C16:1 and 33.91% C18:1), and polyunsaturated fatty acids (5.48% C18:2 and 30.81% C18:3) [28]. Silkworm pupae protein is rich in eighteen different amino acids, of which eight are essential amino acids for human beings, accounting for over 42% of the total amino acid content [29]. Silkworm pupae also contain 3–4% chitosan and other necessary trace elements (Cu, Zn, Fe, Se). These trace elements include iron (9.54 mg), zinc (17.75 mg), potassium (1826.59 mg), sodium (274.57 mg), calcium (102.31 mg), phosphorus (1369.94 mg), magnesium (287.96 mg), and manganese (2.49 mg) [30]. Silkworm pupae are abundant in vitamins A, E, D, B1, B2, and B3 [31]. Silkworm pupae are an excellent source for animal feed, crop fertilizer, and raw materials for use in drug development and food rich in nutrients [32,33,34]. Researchers use silkworm pupae meal or defatted silkworm pupae to culture Cordyceps militaris, Phellinus baumii, Yarrowia lipolytica, and so on [35,36,37]. Silkworm pupae can be used as a high-quality nitrogen source for cultured fungi, but GABA production in cultures of lactic acid bacteria is scarce.
In this study, silkworm pupae were first used as a substitute nitrogen source to attempt to produce culture cells and GABA produced by L. hilgardii. The optimal alternative concentration was optimized to produce GABA. Meanwhile, the best GABA production strategy was obtained by developing a strategy of automatically controlling the pH. The production cost of the cell culture and GABA synthesis was reduced, and a fermentation strategy to produce GABA efficiently was obtained by L. hilgardii. This will help with the reuse of waste resources and the realization of the industrialization expansion of GABA.
2. Materials and Methods
2.1. Materials
Silkworm pupae (B. mori L.) were obtained from the Sericultural Research Institute, Chinese Academy of Agricultural Sciences. The silkworm pupae meal was dried at 60 °C for 4 h and crushed and sifted to make a 1~2 mm powder. Dried silkworm pupae meal is light brown in color with a consistent appearance (Supplementary Figure S1). It does not have any mold and has a distinct odor. It partially dissolves in water, ethanol, chloroform, and ether. The crude protein content of silkworm pupae meal is between 65% and 70%, the crude fat content is between 20% and 30%, the crude ash content is 4% to 5%, the crude fiber content is 4% to 5%, and the moisture content is less than 12%. Silkworm pupae meal can lead to acidification and oxidation at high temperatures or room temperature with prolonged storage. It is recommended that it is stored at low (<4 °C) temperatures.
Yeast extract and tryptone were purchased from OXOID Co., Ltd. (Basingstoke, England), and the catalog numbers were LP0021 and LP0042B. Glucose and monosodium glutamate were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Magnesium sulfate, manganese sulfate, ferrous sulfate, and sodium chloride were purchased from Sangon Biotech Co., Ltd. (Shanghai, China). Standard power (GABA) was purchased from Sigma–Aldrich. O-phthaldialdehyde was purchased from Agilent Technologies Inc. (Santa Clara, CA, USA), and the catalog number was 5061-3335. Boric acid, methanol, and acetonitrile were purchased from Sigma–Aldrich (CA, USA). L. hilgardii was preserved in our laboratory and isolated from the fermented system of the Chinese traditional liquor Baijiu.
2.2. Growth Conditions
The GYP complex medium was composed of the nitrogen source (1% (w/v) yeast extract and 0.5% (w/v) tryptone), carbon source (1% (w/v) glucose), buffer salt (0.2% (w/v) sodium acetate anhydrous), and mineral salt (0.02% (w/v) MgSO4, 0.01% (w/v) MnSO4·H2O, 0.01% (w/v) FeSO4·7H2O and 0.01% (w/v) NaCl). Glucose and other components were separately sterilized and mixed after sterilization to reduce the Maillard reactions. The fermentation medium contained monosodium glutamate in GYP complex medium. The silkworm pupae were used as a nitrogen source by replacing the nitrogen source in GYP complex medium.
A single colony of L. hilgardii was selected from the GYP plate. Then, the colony was inoculated in GYP liquid medium at 37 °C and cultured for 15 h. Finally, this culture solution was used as a seed and inoculated into fresh fermentation medium at a dosage of 10%. The fermentation culture was incubated for 72 h at 37 °C and 200 rpm.
2.3. Fed-Batch Fermentation
The fed-batch fermentation of GABA production by L. hilgardii was performed using the best alternative medium with 5% (w/v) glucose as the carbon source and 5% (w/v) monosodium glutamate. The cultured seeds were inoculated into a 6 L fermenter at a rate of 10% (v/v). The fermentation conditions were 37 °C and 150 rpm without injecting gas for 72 h. Monosodium glutamate was supplemented at 60 mL into the fermenter every hour from 24 h to 54 h by feeding with a solution of monosodium glutamate (300 g/L) for batch fermentation. Glucose (60 mL) was added by feeding with a solution of glucose (20 g/L) every hour from 24 h to 54 h. Each experiment was biologically repeated three times. The final result is expressed as the mean and standard deviation (±SD) of three repetitions.
2.4. Detection of Cell Growth, pH, and GABA Content
The cell growth of L. hilgardii was characterized by the optical density at 600 nm using a multimode reader (Spectra Mas i3 R-3, BUCHI).
The fermentation solution was centrifuged to remove the cells. The pH of the supernatant was detected by a pH meter (METTLER TOLEDO).
The GABA detection method used high-performance liquid chromatography (HPLC) after derivatization by o-phthaldialdehyde [38]. The supernatant was cleared by an 0.22 μm ultrafiltration membrane and then derivatized. The derivatization procedure is shown in Table 1. Ten microliters of the sample was injected after derivatization. Mobile phase A was 4.52 g/L anhydrous sodium acetate at pH 7.2. Mobile phase B was 22.6 g/L anhydrous sodium acetate at pH 7.2, and then 40% acetonitrile and 40% methanol were added. The chromatography column was performed on a C18 column (Agilent, Santa Clara, CA, USA). The analysis temperature was 40 °C, and the analysis time was 30 min. The standard curve of the GABA concentration and peak area was constructed by the GABA standard. The GABA concentration was obtained by calculating the peak area of the sample.

Table 1.
Precolumn derivatization procedure.
2.5. Real-Time Quantitative PCR
The relative gene expression levels (gadR, gadC and gadB) were detected by reverse transcription real-time quantitative PCR.
2.5.1. Extraction of Whole Cell RNA
L. hilgardii cells were separately grown in GYP complex medium with yeast extract/tryptone and yeast extract/silkworm pupae used as nitrogen sources. Cells were cultured for different times, centrifuged at 8000 rpm and 4 °C for 5 min, and then the cells were obtained and immediately stored in liquid nitrogen. The frozen cells were ground with liquid nitrogen, and 1 mL TRIZOL (Takara, Dalian, China) was added and mixed for 15 s. Two hundred microliters of trichloromethane was added, violently shaken for 15 s, and then placed on ice for 10 min. The colorless liquid from the upper layer was carefully removed after centrifugation at 12,000 rpm and 4 °C for 15 min. Two precooled isopropyl alcohol samples were added to the upper liquid, mixed five times, and precipitated at −20 °C for 30 min. The supernatant was removed after centrifugation at 12,000 rpm and 4 °C for 15 min. One microliter of 75% ethanol was added and gently mixed five times. Finally, the supernatant was removed after centrifugation at 12,000 rpm and 4 °C for 5 min and then dried at room temperature. The precipitate was the cell’s total RNA.
2.5.2. RNA Reverse Transcription and Real-Time Quantitative PCR
One microgram of RNA was taken, and the DNA was removed and converted to cDNA using the PrimeScript TM RT Reagent Kit with the gDNA Eraser (Takara, Dalian, China). cDNA samples were taken and diluted to the appropriate concentration. The real-time PCR system consisted of 2.5 μL cDNA, 0.5 μL primer (Table 2), 5 μL SYBR-Green-I (Takara, Dalian, China) dye, and 1.5 μL ultra-pure water. Real-time quantitative PCR analysis was performed with Applied Biosystem StepOne Plus (Thermo Fisher, Waltham, MA, USA). The PCR conditions were predenaturation at 95 °C for 1 min, denaturation at 95 °C for 10 s, annealing at 50 °C for 30 s, and extension at 72 °C for 30 s for 40 cycles. The phases of the dissolution curve condition were, firstly, 95 °C for 15 s, then 72 °C for 2 min, and then an increase to 95 °C for 15 s with an increasing gradient of 0.5 °C. 16S rDNA was the internal reference gene. The relative expression levels of gadR, gadC, and gadB were analyzed by the 2−ΔΔCT method.

Table 2.
Primers used in this study.
2.6. Statistical Analysis
Each experiment was carried out with three biological replicates. Statistical analysis of the samples was performed at intervals of 12 h. At the same time, an analysis of variance (ANOVA) was conducted with multiple culture conditions as the main factor. This analysis was performed to determine whether the silkworm pupae replaced the nitrogen source, and the concentrations of silkworm pupae, glucose, and monosodium glutamate had significant effects on the cell growth, fermentation pH, and GABA production in L. hilgardii at each time point. The significant differences in gene (gadR, gadC and gadB) expression levels were analyzed by an independent sample T test at the same time. The control group used yeast extract and tryptone as nitrogen sources. The data were analyzed using SPSS software, version 26. ANOVA and T test analyses were conducted with a confidence level of 95%. p < 0.05 was considered a significant difference. Figures were exported from GraphPad Prism 9, and data are expressed as the mean and standard deviation of three biological repetitions. Error bars represent the standard deviation of the mean.
3. Results and Discussion
3.1. Effect of Silkworm Pupae on Cell Growth and GABA Production
Lactic acid bacteria are nutrient-starved microorganisms, and the nutrients needed for cell growth are mainly absorbed from the complex medium. These nutrients include various carbohydrates, peptides, amino acids, vitamins, and mineral salts. The various components must be balanced to achieve the best cell growth in the complex medium [39]. However, the nitrogen sources in this complex medium are generally yeast extract and peptone, and the production cost of lactic acid bacteria and its related products is high because of its nitrogen source cost.
To investigate whether silkworm pupae meal can be used as a nitrogen source to culture lactic acid bacteria and produce GABA, the nitrogen sources in a complex (GYP) medium were replaced by silkworm pupae meal. When silkworm pupae meal replaced peptone, the cell growth of the GABA-producing strain L. hilgardii GZ2 was significantly higher than that in the GYP complex medium (Figure 1a). In particular, the difference between these two samples was extremely significant at 72 h (p = 0.000002 < 0.001, F = 607.439, df = 16). However, cell growth was lower than that in the GYP complex medium when silkworm and tryptone were used as nitrogen sources, especially when silkworm pupae were used to replace all nitrogen sources (Figure 1a). The pH of the fermentation supernatant showed a similar pattern to the cell growth (Figure 1b). When the silkworm pupae replaced tryptone, the pH was higher than that when the silkworm pupae replaced yeast extract or all nitrogen sources (Figure 1b). Moreover, the yield of GABA was also the highest when silkworm pupae meal replaced tryptone in the GYP complex medium (Figure 1c). The maximum GABA concentration was 14.48 ± 0.347 g/L (Figure 1c). The use of silkworm pupae meal as a nitrogen source could inhibit cell growth and GABA production in L. hilgardii GZ2. These results show that yeast extract is more important than tryptone for L. hilgardii cell growth and GABA production, similar to L. plantarum [22]. Therefore, cell growth and GABA production can be improved by using silkworm pupae to replace tryptone in L. hilgardii GZ2.
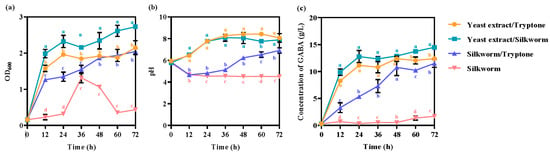
Figure 1.
The effects of using silkworm pupae to replace the nitrogen source on (a) cell growth, (b) pH, and (c) GABA production. The strain was grown in GYP complex medium containing 50 g/L monosodium glutamate at 37 °C and 200 rpm. The GYP complex medium contained 10 g/L yeast extract and 5 g/L tryptone as a nitrogen source. The silkworm pupae meal replaced the corresponding nitrogen source. Each experiment was biologically repeated three times. The final results are expressed as the mean and standard deviation of three repetitions. Error bars represent the standard deviation of the mean. Values followed by different small letters at the same time indicate significant differences at p < 0.05.
In L. brevis, there is a positive regulatory factor GadR that regulates the transcription of gadC (encoding glutamate/GABA antiporter) and gadB (encoding glutamate decarboxylase) [26]. We studied the related expression levels of gadR (encoding a potential transcriptional regulator upstream of gadCB), gadC, and gadB in L. hilgardii GZ2 to explore how silkworm pupa promote GABA synthesis. As shown in Figure 2, regardless of whether yeast extract/tryptone or yeast extract/silkworm pupae meal was used as the nitrogen source, the expression levels of gadR, gadC, and gadB increased gradually with the extension of the fermentation time (Figure 2). In particular, the increases in gadC and gadB were more obvious (Figure 2), indicating that GadR may be a potential regulator in L. hilgardii GZ2. The relative expression levels of gadR, gadC, and gadB were significantly higher when yeast extract/silkworm pupae meal was used as the nitrogen source than when yeast extract/tryptone was used as the nitrogen source (Figure 2). These results indicate that silkworm pupae meal could improve the synthesis ability of GABA by promoting the gene transcription levels of gadR, gadC, and gadB in L. hilgardii GZ2. However, how GadR regulates the expression of glutamate decarboxylase-related genes in L. hilgardii remains to be further explored.
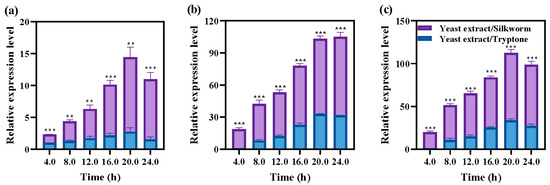
Figure 2.
Gene transcriptional levels of (a) gadR, (b) gadC, and (c) gadB in L. hilgardii GZ2 with yeast extract/tryptone or yeast extract/silkworm pupae used as nitrogen sources. The relative expression levels of genes were compared with 4 h samples of yeast extract and tryptone, which were used as nitrogen sources. The internal reference gene is 16S rDNA. GadR is the gene encoding the potential transcriptional regulator. GadC encodes the glutamate/GABA antiporter. GadB encodes glutamate decarboxylase. The final results are expressed as the mean and standard deviation of the three repetitions. Error bars represent the standard deviation of the mean. The significant differences between these two groups were determined by an independent-samples T test at the same time. **, p < 0.01, ***, p < 0.001. The control group used yeast extract and tryptone as nitrogen sources.
3.2. Effects of the Concentration of Silkworm on Cell Growth and GABA Production
To study the effects of the concentration of silkworm pupae meal on cell growth and GABA production, tryptone in GYP complex medium was replaced by silkworm pupae meal in concentrations of 1, 5, 10, 15, and 20 g/L. The OD600 was improved with the increase in silkworm pupae meal (Figure 3a). Cell growth was inhibited by 20 g/L silkworm pupae meal after 36 h (Figure 3a). High concentrations of silkworm pupae meal were detrimental to the cell viability. The highest cell density (OD600) was 3.5 ± 0.109 when 15 g/L silkworm pupae meal was used (Figure 3a). The trend of the pH was basically consistent at the different concentrations of silkworm pupae meal replacing tryptone (Figure 3b). The pH ultimately increased to approximately 8.6, indicating that hydrogen ions dissociated from organic acids were utilized in the fermentation broth. When the silkworm pupae meal concentration was below 10 g/L, the concentration of GABA increased with an increasing silkworm pupae meal concentration (Figure 3c). However, the specific components of silkworm pupae that enhance GABA synthesis need to be further analyzed. Excessive silkworm pupae meal could inhibit GABA synthesis in L. hilgardii. The optimal concentration for adding silkworm pupae powder was 1% (w/v), while the GABA yield reached 18.8 ± 0.911 g/L after 72 h.
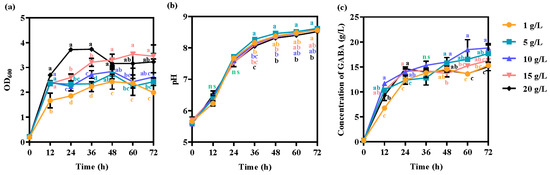
Figure 3.
Effect of the concentration of silkworm pupae meal on (a) cell growth, (b) pH, and (c) GABA production. The strain was grown in GYP medium containing 50 g/L monosodium glutamate with different concentrations of silkworm pupae meal replacing 5 g/L tryptone. Each experiment was biologically repeated three times. The final results are expressed as the mean and standard deviation of the three repetitions. Error bars represent the standard deviation of the mean. Values followed by different small letters at the same time indicate significant differences at p < 0.05. ns, not significant.
3.3. Glucose Control pH Enhances GABA Production
Glucose is a common carbon source for culturing lactic acid bacteria, which can maintain cell viability for a long time [40]. We investigated whether GABA production could be further improved by controlling the availability of a carbon source (glucose). Different concentrations of glucose were used as the carbon source in a fermentation medium with 50 g/L monosodium glutamate and 10 g/L silkworm pupae meal replacing tryptone. By increasing the concentration of glucose, cell growth increased (Figure 4a). The cell density (OD600) reached 4.9 ± 0.311, a 28.6% increase compared with the use of 10 g/L glucose as a carbon source (Figure 3a and Figure 4a). However, the pH of the fermented supernatant significantly changed (Figure 4b). For example, the p value of the significant difference was 1.37 × 10−10 when glucose at concentrations of between 10 g/L and 20 g/L was used as a carbon source at 72 h (F = 128, df = 16). When glucose at concentrations of 10 g/L and 20 g/L was used as a carbon source, the pH was continuously elevated (Figure 4b). The pH reached 8.3, indicating that there was no excessive hydrogen ion concentration after 72 h in the fermentation system (Figure 4b). When glucose (30 g/L) was used as the carbon source, the final pH remained at 6.4 ± 0.056. The pH was maintained at 5.4 ± 0.057 with glucose (40 g/L) as the carbon source, and the pH was maintained at 5.0 ± 0.007 with 50 g/L glucose as the carbon source. After 72 h, the titer of GABA reached 31.2 ± 0.976 g/L, which was 40.0% higher than that of 10 g/L glucose (Figure 3c and Figure 4c). Lactic acid bacteria use glucose to produce organic acids, especially lactic acid [41]. Glutamate decarboxylation generates the same molar amount of GABA, which requires the same molar mass of hydrogen ions [42]. The optimal pH for maintaining the GAD activity of most lactic acid bacteria is between 4.0 and 5.0 [9,10]. Therefore, the pH of the fermentation broth could be controlled to increase GABA production by controlling the glucose concentration.
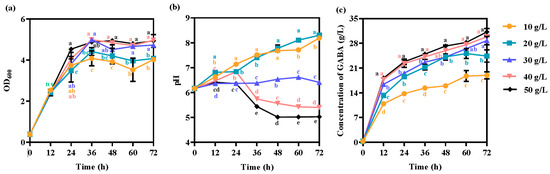
Figure 4.
Effects of the glucose concentration on (a) cell growth, (b) pH, and (c) GABA production. The strain was grown in GYP medium containing 50 g/L monosodium glutamate and 10 g/L silkworm pupae meal with different concentrations of glucose used as carbon sources. Each experiment was biologically repeated three times. The final results are expressed as the mean and standard deviation of the three repetitions. Error bars represent the standard deviation of the mean. Values followed by different small letters at the same time indicate significant differences at p < 0.05.
3.4. Effects of Glucose and Monosodium Glutamate Concentrations on Cell Growth and GABA Production
Monosodium glutamate has been shown to be the most widely used and economical substrate for efficient GABA synthesis in lactic acid bacteria [22,42]. However, the hydrogen ions in the cell are reduced after glutamate decarboxylation, and the intracellular pH is close to neutral, which affects the activity of glutamate decarboxylase [42]. To find a simple and effective way to control intracellular pH, we also evaluated the optimal concentrations of glucose and monosodium glutamate to control the pH and enhance GABA production.
As the concentrations of glucose and monosodium glutamate increased by 10~50 g/L, both cell growth and GABA production increased (Figure 5a,c). After 72 h, the cell density (OD600) increased from 3.3 ± 0.106 to 4.9 ± 0.311, and the GABA titer increased from 6.7 ± 0.976 g/L to 31.2 ± 0. 976 g/L (Figure 5a,c). However, the final pH was maintained at approximately 5.0 after 36 h. Appropriate concentrations of monosodium glutamate and glucose could control the pH. Monosodium glutamate and glucose concentrations ranged from 10 to 50 g/L, and the pH was maintained at between 5.0 ± 0.028 and 5.2 ± 0.021 after 36 h of fermentation (Figure 5b). Thus, to obtain the optical cell density and GABA production, glucose and monosodium glutamate were both used at concentrations of 50 g/L for efficient GABA production by L. hilgardii GZ2.
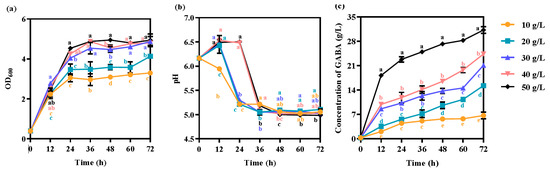
Figure 5.
Effects of glucose and monosodium glutamate concentrations on (a) cell growth, (b) pH, and (c) GABA production. The strain was grown in GYP medium containing 10~50 g/L monosodium glutamate and 10~50 g/L glucose as carbon sources with 10 g/L silkworm pupae meal. Each experiment was biologically repeated three times. The final results are expressed as the mean and standard deviation of the three repetitions. Error bars represent the standard deviation of the mean. Values followed by different small letters at the same time indicate significant differences at p < 0.05.
3.5. Fed-Batch GABA Production via Silkworm Pupae Meal Replaces Tryptone Coupled with Glucose to the Control pH
L. hilgardii GZ2 was fermented for 72 h by fed-batch fermentation for GABA production (Figure 6). GABA production occurs simultaneously with cell growth. After 24 h, by feeding glucose and monosodium glutamate, the pH decreased from 6.2 ± 0.028 to 5.0 ± 0.007 during the growth period, creating a suitable environment for GAD activity (Figure 6). The OD600 of L. hilgardii GZ2 rapidly increased with a pH controlled at 5.0 (Figure 6). Accordingly, the GABA concentration increased within 36 h and then moderately increased after 42 h (Figure 6). Finally, the GABA titer was 229.3 ± 6.846 g/L, and the productivity reached 3.2 g/L/h after 72 h of fermentation (Figure 6), which was 7.3 times higher than that of nonfed-batch fermentation.
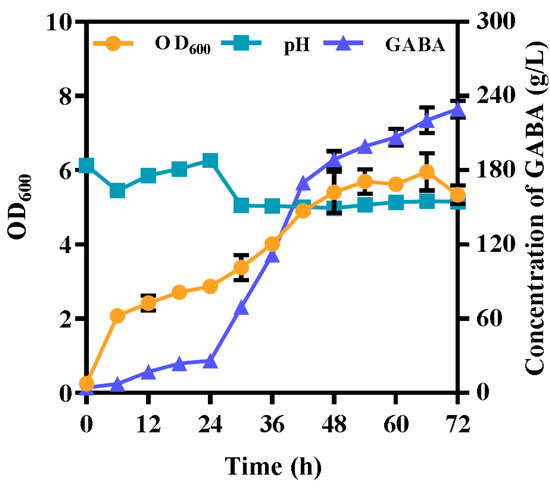
Figure 6.
Fermentation profile for GABA production using L. hilgardii GZ2 by fed-batch fermentation. Fermentation was performed at 37 °Cand 150 rpm for 72 h in the optimized GYP medium consisting of 10 g/L silkworm pupae meal to replace tryptone, 50 g/L glucose, and 50 g/L (w/v) monosodium glutamate. Monosodium glutamate was continuously supplemented into the fermenter from 24 h to 54 h at 18 g/h. A solution of glucose (20 g/L) was continuously added at a rate of 1.2 g/h between 24 h and 54 h. Each experiment was biologically repeated three times. The final results are expressed as the mean and standard deviation of three repetitions. Error bars represent the standard deviation of the mean.
The nitrogen source in complex (GYP) medium is expensive. We successfully used silkworm pupae meal (approximately $0.5 per kg) instead of tryptone (over $140 per kg) to reduce GABA production costs. Furthermore, since silkworm pupae are used as a food source enriched in protein (60%), fatty acids (30%), chitosan (3–4%), and trace elements, they can be used as raw materials for food and medicine [43]. However, it is not clear which component enhances GABA synthesis and the regulatory mechanism of silkworm pupae in L. hilgardii. Meanwhile, the production of GABA requires a larger amount of H+, and the optimal reaction conditions for glutamate decarboxylase are acidic [9]. When H+ is restricted, glutamate decarboxylase is not sufficient to guarantee the high conversion rate of GABA. Researchers used glutamic acid ($2.76 per kg) to adjust the fermentation pH in L. brevis [44]. Although the GABA yield was 321.9 g/L in the 3 L fermenter, there was little increase compared to flask fermentation (approximately 320 g/L). The cost of glutamic acid was high, which is not conducive to industrial implementation. In this study, we attempted pH control combined with an inexpensive glucose ($0.08 per kg) and monosodium glutamate ($1.37 per kg) fed-batch fermentation strategy. Indeed, these fermentation control strategies greatly elevated the GABA conversion rate in the L. hilgardii GZ2 strain. However, GABA production was also enhanced by adding pyridoxal-5′-phosphate, a factor of glutamate decarboxylase, to maintain enzyme activity and two-stage pH/temperature control [21,45,46]. Our study indicates that the use of silkworm pupae meal instead of tryptone combined with feeding glucose and monosodium glutamate is sufficient to activate high titers and high productivity for GABA industrial production.
4. Conclusions
L. hilgardii is a common probiotic used in silage and fermented food. It is important to use L. hilgardii to obtain GABA production. To reduce the cost of GABA production, silkworm pupae were used to replace the nitrogen source in the complex medium. By using 10 g/L silkworm pupae meal instead of tryptone in GYP medium combined with feeding glucose and monosodium glutamate to maintain the pH, the highest yield obtained was 229.3 g/L. These results indicate the possibility of using low-cost and safe fermentation strategies to efficiently produce GABA by L. hilgadii, providing an alternative option for the development of GABA industrial expansion.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation9070691/s1. Figure S1: Picture of dried silkworm pupae meal.
Author Contributions
Conceptualization, L.G.; methodology, L.G.; software, L.G.; validation, L.G., T.L., S.L. and X.Z.; formal analysis, L.G.; investigation, L.G. and T.L.; resources, L.G., B.W. and J.W.; data curation, L.G.; writing-original draft preparation, L.G.; writing—review and editing, L.G., B.W. and J.W.; visualization, L.G.; supervision, B.W. and J.W.; project administration, J.W.; funding acquisition, L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of China (32101905) and the Open Project Program of the Key Laboratory of Brewing Molecular Engineering of China Light Industry (BME-202203). We thank Prof. XU Yan and Dr. Ren Cong (Jiangnan University, China) for providing relevant experimental materials.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplemental materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hou, D.; Tang, J.; Feng, Q.; Niu, Z.; Shen, Q.; Wang, L.; Zhou, S. Gamma-aminobutyric acid (GABA): A comprehensive review of dietary sources, enrichment technologies, processing effects, health benefits, and its applications. Crit. Rev. Food Sci. Nutr. 2023, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.H.; Vo, T.S. An updated review on pharmaceutical properties of gamma-aminobutyric acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Wang, J.; Hao, Y.; Zhao, F.; Fu, R.; Yu, Y.; Wang, J.; Niu, R.; Bian, S.; Sun, Z. Exercise ameliorates fluoride-induced anxiety- and depression-like behavior in mice: Role of GABA. Biol. Trace Elem. Res. 2022, 200, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Cheng, P.W.; Ho, W.Y.; Lu, P.J.; Lai, C.C.; Tseng, Y.M.; Fang, H.C.; Sun, G.C.; Hsiao, M.; Liu, C.P.; et al. Renal denervation improves the baroreflex and GABA system in chronic kidney disease-induced hypertension. Sci. Rep. 2016, 6, 38447. [Google Scholar] [CrossRef]
- Sears, S.M.; Hewett, S.J. Influence of glutamate and GABA transport on brain excitatory/inhibitory balance. Exp. Biol. Med. 2021, 246, 1069–1083. [Google Scholar] [CrossRef]
- Erlander, M.G.; Tobin, A.J. The structural and functional heterogeneity of glutamic acid decarboxylase a review. Neurochem. Res. 1991, 16, 215–226. [Google Scholar] [CrossRef]
- Zhang, L.; Yue, Y.; Wang, X.; Dai, W.; Piao, C.; Yu, H. Optimization of fermentation for gamma-aminobutyric acid (GABA) production by yeast Kluyveromyces marxianus C21 in okara (soybean residue). Bioprocess Biosyst. Eng. 2022, 45, 1111–1123. [Google Scholar] [CrossRef]
- Kadir, S.A.; Wan-Mohtar, W.A.Q.R.; Mohammad, R.; Lim, S.A.H.; Mohammed, A.S.; Saari, N. Evaluation of commercial soy sauce koji strains of Aspergillus oryzae for gamma-aminobutyric acid (GABA) production. J. Ind. Microbiol. Biotechnol. 2016, 43, 1387–1395. [Google Scholar] [CrossRef]
- Li, H.X.; Cao, Y.S. Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amino Acids 2010, 39, 1107–1116. [Google Scholar] [CrossRef]
- Xiao, T.; Shah, N.P. Lactic acid produced by Streptococcus thermophilus activated glutamate decarboxylase (GadA) in c NPS-QW 145 to improve γ-amino butyric acid production during soymilk fermentation. LWT-Food Sci. Technol. 2021, 137, 110474. [Google Scholar] [CrossRef]
- Zhao, A.Q.; Hu, X.Q.; Pan, L.; Wang, X.Y. Isolation and characterization of a gamma-aminobutyric acid producing strain Lactobacillus buchneri WPZ001 that could efficiently utilize xylose and corncob hydrolysate. Appl. Microbiol. Biotechnol. 2015, 99, 3191–3200. [Google Scholar] [CrossRef]
- Pannerchelvan, S.; Rios-Solis, L.; Faizal Wong, F.W.; Zaidan, U.H.; Wasoh, H.; Mohamed, M.S.; Tan, J.S.; Mohamad, R.; Halim, M. Strategies for improvement of gamma-aminobutyric acid (GABA) biosynthesis via lactic acid bacteria (LAB) fermentation. Food Funct. 2023, 14, 3929–3948. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.B.; Rajapuram, D.R.; Jayamanohar, J.; Verma, M.; Kavitake, D.; Meenachi Avany, B.A.; Rani, P.U.; Ravi, R.; Shetty, P.H. Gamma-aminobutyric acid (GABA) production by potential probiotic strains of indigenous fermented foods origin and RSM based production optimization. LWT-Food Sci. Technol. 2023, 176, 114511. [Google Scholar] [CrossRef]
- Amatachaya, A.; Siramolpiwat, S.; Kraisorn, M.; Yasiri, A. Gamma-aminobutyric acid (GABA) producing probiotic Lactiplantibacillus pentosus isolated from fermented spider plant (Pak Sian Dong) in Thailand. J. Pure Appl. Microbiol. 2023, 17, 354–361. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Dong, Y.; Xiang, F.; Zhang, Y.; Zhang, H.; Sun, Y.; Guo, Z. Characterization of two novel pentose-fermenting and GABA-producing species: Levilactobacillus tujiorum sp. nov. and Secundilactobacillus angelensis sp. nov. isolated from a solid-state fermented zha-chili. Syst. Appl. Microbiol. 2022, 45, 126344. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Mazzacane, F.; Rizzello, C.G.; De Angelis, M.; Giuliani, G.; Meloni, M.; De Servi, B.; Gobbetti, M. Synthesis of gamma-aminobutyric acid (GABA) by Lactobacillus plantarum DSM19463: Functional grape must beverage and dermatological applications. Appl. Microbiol. Biotechnol. 2010, 86, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Gao, D.D.; Cao, Y.S.; Xu, H.Y. A high γ-aminobutyric acid-producing Lactobacillus brevis isolated from Chinese traditional paocai. Ann. Microbiol. 2008, 58, 649–653. [Google Scholar] [CrossRef]
- Komatsuzaki, N.; Shima, J.; Kawamoto, S.; Momose, H.; Kimura, T. Production of γ-aminobutyric acid (GABA) by Lactobacillus paracasei isolated from traditional fermented foods. Food Microbiol. 2005, 22, 497–504. [Google Scholar] [CrossRef]
- Lacroix, N.; St-Gelais, D.; Champagne, C.P.; Vuillemard, J.C. Gamma-aminobutyric acid-producing abilities of lactococcal strains isolated from old-style cheese starters. Dairy Sci.Technol. 2013, 93, 315–327. [Google Scholar] [CrossRef]
- Somkuti, G.A.; Renye, J.A., Jr.; Steinberg, D.H. Molecular analysis of the glutamate decarboxylase locus in Streptococcus thermophilus ST110. J. Ind. Microbiol. Biotechnol. 2012, 39, 957–963. [Google Scholar] [CrossRef]
- Li, H.X.; Qiu, T.; Huang, G.D.; Cao, Y.S. Production of gamma-aminobutyric acid by Lactobacillus brevis NCL912 using fed-batch fermentation. Microb. Cell Factories 2010, 9, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, D.H.; Kang, H.J.; Shin, M.; Yang, S.Y.; Yang, J.; Jung, Y.H. Enhanced production of γ-aminobutyric acid (GABA) using Lactobacillus plantarum EJ2014 with simple medium composition. LWT-Food Sci. Technol. 2021, 137, 110443. [Google Scholar] [CrossRef]
- Aspmo, S.I.; Horn, S.J.; Eijsink, V.G. Use of hydrolysates from Atlantic cod (Gadus morhua L.) viscera as a complex nitrogen source for lactic acid bacteria. FEMS Microbiol. Lett. 2005, 248, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Safari, R.; Motamedzadegan, A.; Ovissipour, M.; Regenstein, J.M.; Gildberg, A.; Rasco, B. Use of hydrolysates from Yellowfin Tuna (Thunnus albacares) heads as a complex nitrogen source for lactic acid bacteria. Food Bioprocess Technol. 2009, 5, 73–79. [Google Scholar] [CrossRef]
- Utami, T.; Kusuma, E.N.; Satiti, R.; Rahayu, E.S.; Cahyanto, M.N. Hydrolyses of meat and soybean proteins using crude bromelain to produce halal peptone as a complex nitrogen source for the growth of lactic acid bacteria. Int. Food Res. J. 2019, 26, 117–122. [Google Scholar]
- Gong, L.C.; Ren, C.; Xu, Y. Deciphering the crucial roles of transcriptional regulator GadR on gamma-aminobutyric acid production and acid resistance in Lactobacillus brevis. Microb. Cell Factories 2019, 18, 108. [Google Scholar] [CrossRef]
- Bian, Y.; Xu, Y.; Qi, Z.; Wang, J.; Wu, F. Effects of fermented silkworm pupa meal on grow th performance of Micropterus salmonides. Sci. Sericul. 2021, 47, 575–580. [Google Scholar]
- Wang, J.Z.; Liu, X.; Li, W.J.; Song, W.M.; Herman, R.A.; Sheng, S.; Wu, F.A.; Wang, J. One hour enzymatic synthesis of structure lipids enriched unsaturated fatty acids from silkworm pupae oil under microwave irradiation. J. Chem. Technol. Biotechnol. 2019, 95, 363–372. [Google Scholar] [CrossRef]
- Hu, M.B.; Wang, J.L.; Liu, Y.J.; Yuan, X.; Li, J.H.; Wu, C.J.; Li, L. Structure characterization and antioxidant properties of proteins extracted from the larva of Bombyx mori L. Trop. J. Pharm. Res. 2019, 17, 2177. [Google Scholar] [CrossRef]
- Ray, M.; Gangopadhyay, D. Effect of maturation stage and sex on proximate, fatty acid and mineral composition of eri silkworm (Samia ricini) from India. J. Food Compost. Anal. 2021, 100, 103898. [Google Scholar] [CrossRef]
- Wu, X.; He, K.; Velickovic, T.C.; Liu, Z. Nutritional, functional, and allergenic properties of silkworm pupae. Food Sci. Nutr. 2021, 9, 4655–4665. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Zhang, L.L.; Chen, M.; Dai, J.J.; Wu, C.H.; Liu, J.; Fan, T. Research progress of value, extraction and application of silkworm chrysalis protein. Anim. Husb. Feed Sci. 2018, 10, 241–245. [Google Scholar] [CrossRef]
- Wang, W.; Wang, N.; Liu, C.; Jin, J. Effect of silkworm pupae peptide on the fermentation and quality of yogurt. J. Food Process. Preserv. 2017, 41, e12893. [Google Scholar] [CrossRef]
- Miah, M.Y.; Singh, Y.; Cullere, M.; Tenti, S.; Dalle Zotte, A. Effect of dietary supplementation with full-fat silkworm (Bombyx mori L.) chrysalis meal on growth performance and meat quality of Rhode Island Red × Fayoumi crossbred chickens. Ital. J. Anim. Sci. 2020, 19, 447–456. [Google Scholar] [CrossRef]
- Shi, X.Y.; Li, T.Y.; Wang, M.; Wu, W.W.; Li, W.J.; Wu, Q.Y.; Wu, F.A.; Wang, J. Converting defatted silkworm pupae by Yarrowia lipolytica for enhanced lipid production. Eur. J. Lipid Sci. Technol. 2016, 119, 1600120. [Google Scholar] [CrossRef]
- Li, Z.N.; Li, W.J.; Wang JZ You, S.; Wang, J.; Wu, F.A. Defatted silkworm pupae hydrolysates as a nitrogen source to produce polysaccharides and flavonoids using Phellinus baumii. Biomass Convers. Biorefin. 2020, 11, 527–537. [Google Scholar] [CrossRef]
- Guo, L.; Li, K.; Kang, J.S.; Kang, N.J.; Son, B.G.; Choi, Y.W. Strawberry fermentation with Cordyceps militaris has anti-adipogenesis activity. Food Biosci. 2020, 35, 100576. [Google Scholar] [CrossRef]
- Rea, K.; Cremers, T.I.F.H.; Westerink, B.H.C. HPLC conditions are critical for the detection of GABA by microdialysis. J. Neurochem. 2005, 94, 672–679. [Google Scholar] [CrossRef]
- Miranda, M.H.; Nader-Macias, M.E.F. Low-cost culture media designed for biomass production of beneficial lactic acid bacteria for their inclusion in a formula to treat bovine reproductive infections. FEMS Microbiol. Lett. 2023, 370, fnad033. [Google Scholar] [CrossRef]
- Hussin, F.S.; Chay, S.Y.; Hussin, A.S.M.; Wan Ibadullah, W.Z.; Muhialdin, B.J.; Abd Ghani, M.S.; Saari, N. GABA enhancement by simple carbohydrates in yoghurt fermented using novel, self-cloned Lactobacillus plantarum Taj-Apis362 and metabolomics profiling. Sci. Rep. 2021, 11, 9417. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; de Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress physiology of lactic acid bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef] [PubMed]
- Capitani, G.; De Biase, D.; Aurizi, C.; Gut, H.; Bossa, F.; Grütter, M.G. Crystal structure and functional analysis of Escherichia coli glutamate decarboxylase. EMBO J. 2003, 22, 4027–4037. [Google Scholar] [CrossRef] [PubMed]
- Hăbeanu, M.; Gheorghe, A.; Mihalcea, T. Nutritional value of silkworm pupae (Bombyx mori) with emphases on fatty acids profile and their potential applications for humans and animals. Insects 2023, 14, 254. [Google Scholar] [CrossRef]
- Jia, M.; Zhu, Y.; Wang, L.; Sun, T.; Pan, H.; Li, H. pH auto-sustain-based fermentation supports efficient gamma-aminobutyric acid production by Lactobacillus brevis CD0817. Fermentation 2022, 8, 208. [Google Scholar] [CrossRef]
- Lyu, C.; Zhao, W.; Peng, C.; Hu, S.; Fang, H.; Hua, Y.; Yao, S.; Huang, J.; Mei, L. Exploring the contributions of two glutamate decarboxylase isozymes in Lactobacillus brevis to acid resistance and γ-aminobutyric acid production. Microb. Cell Factories 2018, 17, 180. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Kim, S.-K.; Ra, C.H. Evaluation of gamma-aminobutyric acid (GABA) production by Lactobacillus plantarum using two-step fermentation. Bioprocess Biosyst. Eng. 2021, 44, 2099–2108. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).