Abstract
Developing an effective method to achieve stability and improve the quality of Tibetan tea has scientific significance. Aspergillus niger K1 isolated and identified from Tibetan tea was inoculated in unsterilized or sterilized tea leaves to develop the bioaugmented fermentation (BF) and normal fermentation (NF) processes of Tibetan tea. The results showed that BF resulted in infusions with a deeper color, a stronger aroma, and a thicker taste compared to NF. The dominant bacterium in BF was Staphylococcus (23.76%), while the dominant fungus was Blastobotrys adeninivorans (50.95%). Moreover, 859 metabolites were identified, and the level of 90 differentially changed metabolites (DCMs) in BF increased significantly (VIP > 1, p < 0.05, FC > 2) compared to those in NF, while the level of 37 DCMs in BF decreased significantly (VIP > 1, p < 0.05, FC < 0.5). Correlation analysis demonstrated that A. niger significantly positively correlated with theabrownins, caffeine, and glutamylisoleucine (p < 0.05, |r| > 0.8). B. adeninivorans showed significant negative correlations with 1-(beta-D-ribofuranosyl)-1,4-dihydronicotinamide and 2-hydroxyacetaminophen sulfate (p < 0.05, |r| > 0.8). Consequently, the inoculation of A. niger for BF has the potential to alter the metabolites in tea through a synergistic interaction with other microorganisms, ultimately improving the sensory quality of Tibetan tea.
1. Introduction
Tibetan tea, a kind of dark tea, is an essential traditional beverage in the daily life of the Tibetan people, and it is a product of geographical indication that is exclusively produced in Ya’an, Sichuan, China [1,2]. It is a traditional Chinese tea with a dark-brown tea infusion, a mellow taste, and a sweet aftertaste flavor [3]. Currently, dark teas have been reported to have various health benefits, such as liver protection, reducing the risk of cardiovascular disease, and neuroprotective activity [4,5]. Meanwhile, Tibetan tea has also been reported to have multiple health benefits, including anti-obesity, anti-hypertensive, anti-hyperlipidemic, anti-inflammatory, lipid-lowering, antioxidation, and gastrointestinal regulation effects [6,7,8,9]. Therefore, the production process of Tibetan tea has received attention from many researchers.
The chemical composition, flavor quality, and health benefits of Tibetan tea are significantly influenced by the origin of raw tea materials as well as by variations in processing techniques and fermenting microorganisms [10]. During the fermentation process of Tibetan tea, a high-temperature and humid environment occurs, and the tea leaves involve microorganisms that modify the phytochemicals of the tea leaves by performing a multitude of reactions, including degradation, oxidation, polymerization, methylation, condensation, and glycosylation [11,12]. Factors such as complex and uncontrollable microbial sources can often lead to problems, such as unstable quality in the traditional fermentation process. Therefore, microbial fermentation is crucial for the formation of the unique features of Tibetan tea, and microorganisms are also a major driver for improving the traditional fermentation process.
At present, based on the dominant microorganisms in the fermentation process, bioaugmented fermentation (BF) is extensively used in traditional fermented food. BF is the inoculation of one or more exogenous microorganisms to an unsterilized raw material in order to improve the quality of fermented products [13]. It was found that BF of Daqu by inoculating Bacillus spp. had a positive effect on the microbiome and metabolome, especially the former, in the process of Baijiu fermentation [14,15]. Both the flavor intensity and the sensory quality of Baijiu have been significantly improved by BF inoculation with Saccharomyces cerevisiae [16]. BF of lignocellulosic material by inoculating Clostridium acetobutylicum and Shigella flexneri str. increased the content of biohydrogen [17]. Penicillium chrysogenum, through cooperation with other fungal species, changed the chemical composition and improved the sensory quality of Pu-erh tea (a kind of dark tea) during the bioaugmented fermentation stage [18]. Moreover, the identification of appropriate microorganisms plays a vital role in the BF of dark tea. The examination of microbial community dynamics at different temperatures during the fermentation of dark tea has demonstrated that Aspergillus is the predominant fungal genus involved in the fermentation process, and its presence is closely associated with the development of key quality attributes in dark tea [19]. Notably, Aspergillus sp. isolated from Pu-erh tea fermentation has been found to effectively convert tea polyphenols into bioactive theabrownins [20]. Meanwhile, the degradation of methylxanthine alkaloids was achieved through biodegradation of theobromine, which, in turn, reduced the bitterness of Pu-erh tea and made it mellow [21,22,23]. Our previous studies found that the phenolic compounds in tea leaves were degraded through the degradation of aromatic compound pathways as well as xenobiotic biodegradation and metabolism pathways by Aspergillus luchuensis [24]. Overall, BF appears to be an effective way of improving the quality of dark tea, especially based on the superior representatives, such as Aspergillus.
Aspergillus niger is one of the dominant microorganisms present in dark tea fermentation, and it can generate great scientific and commercial interest regarding the fermentation of tea and the direct impacts it can have on the quality of tea [25]. It was found that the changes in the composition of flavonoids, glycerophospholipids, organo-oxygen compounds, and fatty acids resulted from BF with Aspergillus niger [26]. Ye et al. investigated the influences of the volatile compounds from Pu-erh tea fermented by four Aspergillus strains, and they confirmed that Aspergillus niger was the most important microorganism for the formation of the aroma quality of Pu-erh tea [27]. However, the effects of Aspergillus niger on the microbiota and the metabolites of Tibetan tea have received little attention in research. Therefore, the interaction and the metabolic function of microbiota during Tibetan tea fermentation through BF with Aspergillus niger have scientific and commercial significance.
We hypothesized that bioaugmented fermentation could improve the quality of Tibetan tea by regulating Aspergillus niger. However, there is little systematic information about how BF affects the microbiota and the metabolites of Tibetan tea. Thus, we investigated the effects of BF and NF on the physicochemical parameters, microbiota, and metabolites during Tibetan tea fermentation. Meanwhile, the biomarkers of microbiota and the core contributors to metabolites were determined. These results may shed new light on microbiota and metabolic regulation during the fermentation processes, and thereby provide new ideas for producing Tibetan tea.
2. Materials and Methods
2.1. Materials and Annotation of Aspergillus niger K1
The raw materials (RM) of tea leaves were “Small and Medium Leaf”, and they were collected from the production company of Tibetan tea (Ya’an, Sichuan, China) in August 2022.
Aspergillus niger K1 was isolated and purified from the fermentation process of Tibetan tea, and it was identified by phylogenetic analysis of fungal internally-transcribed spacer sequencing (ITS). Aspergillus niger K1 was grown on potato dextrose agar (PDA) at 25 °C for 7 d, and its DNA was extracted using the Rapid Fungal Genomic DNA Isolation Kit (Omega Biotech, Norcross, GA, USA) according to the manufacturer’s instructions. The ITS of Aspergillus niger K1 was sequenced by Meiji Biomedical Technology Co., Ltd. (Shanghai, China). The genes were annotated by aligning them with those deposited in the National Center for Biotechnology Information (NCBI) ITS database. Furthermore, barcodes of 18 species within Aspergillus were downloaded from the GenBank of NCBI, and were used for phylogenetic analysis [28]. All sequences were aligned using the MUSCLE algorithm with Molecular Evolutionary Genetics Analysis (MEGA) software ver. 7.0.26, and the evolutionary history was inferred by using the Maximum Likelihood method based on the General Time Reversible model in MEGA software [29]. To evaluate the support for inferred topologies, the percentage of branches in which the associated taxa clustered together was calculated by bootstrapping with 1000 replicates. The bootstrap percentages of the maximum likelihood (ML) analysis were presented at the nodes.
2.2. Fermentation of Tibetan Tea
Aspergillus niger K1 was inoculated onto PDA medium and cultured at 28 °C for 7 d. Then, the spores of Aspergillus niger K1 were collected by washing them with sterilized water, and 1 × 106 spores/mL spores were prepared by the blood cell count method.
BF was performed as follows: 10 kg tea leaves were mixed with 5 kg sterile distilled water and 100 mL of spores’ solution of Aspergillus niger K1. Static fermentation was conducted after evenly mixing and turning every 5 d, fermentation was completed after 30 d, and the finished product was then obtained after heat energy dehydration, ball-breaking, and sifting. Meanwhile, the treatment without adding the spores’ solution of Aspergillus niger K1 was normal fermentations (NF). BF and NF of each group were repeated three times.
2.3. Determination of Characteristic Compositions of Tea Leaves and Sensory Evaluation
The contents of theaflavins (TF), thearubigins (TR), theabrownins (TB), water extracts (WE), tea polyphenols (TP), free amino acids (FAA), and soluble sugars (SS) of Tibetan tea were analyzed by a spectrophotometric method, as described previously [30,31], and the content of caffeine (Ca) of the tea leaves was analyzed by the near-infrared spectroscopy method [32].
The sensory evaluation of Tibetan tea was performed according to the Chinese standard (GB/T 23776—2018), and a randomized double-blind study was employed [33]. Briefly, the appearance of the tea was evaluated by 11 trained sensory testers (26–55 years old, 6 men and 5 women) and then 3 g of dried tea leaves of Tibetan tea were infused in 150 mL boiled water for 5 min, and, after this, the aroma, the taste, and the color of the tea infusion and the brewed leaves were evaluated by the testers.
2.4. Illumina MiSeq Sequencing
High-throughput sequencing analysis of BF and NF were carried out and analyzed after DNA extraction, and PCR amplification by Meiji Biomedical Technology Co., Ltd. (Shanghai, China). The hypervariable regions V3–V4 of the bacterial 16S rRNA gene were amplified with primer pairs 799F (5′-AACMGGATTAAGATACCCKG) and 1193R (5′-ACGTCATCCCCACCTTCC) by T100 Thermal Cycler PCR thermocycler (BIO-RAD, Hercules, CA, USA), while the fungal ITS1 gene were amplified with primer pairs ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and TIS2R (5′-GCTGCGTTCTTCATCGATGC-3′). Chimeric sequences were excluded with default parameters and sequences with similarities > 97% and were clustered into one operational taxonomic unit (OTU) using QIIME (Quantitative Insights Into microbiota, V1.8.0), as described previously [18,34].
2.5. Metabolomics Analysis
Metabolites in the tea leaves of NF and BF were extracted and then analyzed using a liquid chromatography-tandem mass spectrometry (LC-MS/MS) based metabolomics approach, performed by Meiji Biomedical Technology Co., Ltd. (Shanghai, China). Metabolite identification was dependent on the MS/MS data annotated against an in-house database, or the matches of both retention times and MS data to some standard compounds [35]. Variable importance in projection (VIP) was an indicator used to evaluate the correlation between a metabolite and a sample classification, and variables with VIP > 1.0, p < 0.05 (Student’s t test) and fold change (FC) > 2 or <0.5 were considered as differentially changed metabolites (DCMS) [36].
2.6. Statistical Analysis
IBM SPSS Statistics 22.0 software was used for data processing and statistical analysis. The data were expressed as mean ± standard deviation. LSD analysis of variance was used for statistical analysis, and p < 0.05 was considered significant. Correlation network analysis was completed by the Spearman method, and it was plotted using Cytoscape 3.8.2.
2.7. Accession Numbers
The sequencing data of Aspergillus niger K1 was submitted to the National Center for Biotechnology Information (NCBI) Genbank with the accession number OP603895 and the amplicon data of bacterial 16S rRNA and fungal ITS1, which were available at BioProject (https://bigd.big.ac.cn/bioproject/, accessed on 5 October 2022) with the accession numbers PRJNA 886445 and PRJNA 886446, respectively.
3. Results and Discussion
3.1. Identification of Isolated Strain K1
The sequence of strain K1 was compared with the NCBI database, the model species were selected for phylogenetic tree analysis using the Maximum Likelihood method, and the results were shown in Figure 1. The results showed that the strain K1 was clustered with NR111348.1. The strain K1 that was isolated and purified from the fermentation process of Tibetan tea was thus identified as Aspergillus niger K1.
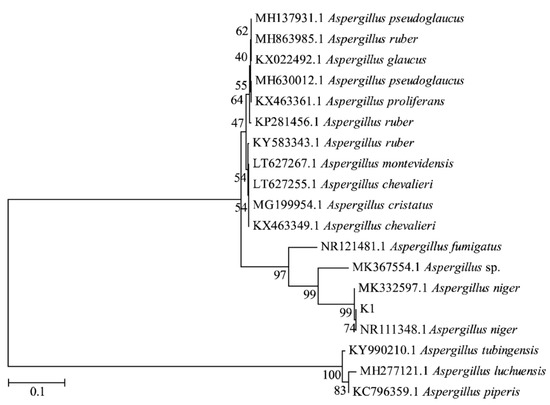
Figure 1.
Molecular phylogenetic analysis by Maximum Likelihood method of DNA sequences of Aspergillus niger K1.
3.2. Sensory Evaluation and Characteristic Compositions of Tea Leaves
Sensory evaluation revealed that the appearance of the NF tea was even, neat, tight, and auburnish black; the color of infusions was bright and deep red; the aroma of the infusions was pure and normal (i.e., a woody aroma); the taste of the infusions was sweet, mellow, and smooth; and the brewed leaves were soft and open, and had a color of auburnish black. However, the tea appearance and the brewed leaves of the BF Tibetan tea by inoculating Aspergillus niger K1 were brownish auburn; the color of infusions was deeper, which was brownish red; the aroma of infusions was strong and lasting (a woody aroma); and the taste of infusions was mellow, thick, and smooth (Table 1). Meanwhile, the sensory scores of the color and the aroma of the infusions in BF of Tibetan tea were significantly higher (p < 0.05) than those in NF (Figure 2A). Therefore, BF improved the sensory quality of Tibetan tea.

Table 1.
Sensory evaluation of Tibetan tea with BF and NF.
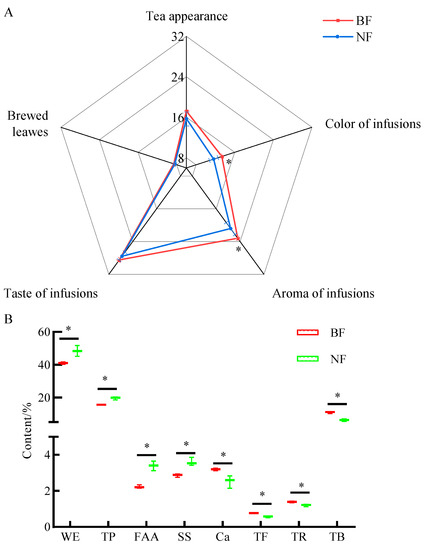
Figure 2.
Sensory score (A) and the contents of characteristic compounds (B) of Tibetan tea in BF and NF. * Indicates significant difference at p < 0.05, WE: water extracts, TP: tea polyphenols, FAA: free amino acids, SS: soluble sugars, Ca: caffeine, TF: theaflavins, TR: thearubigins, TB: theabrownins.
As shown in Figure 2B, the contents of WE, TP, FAA, and SS in BF exhibited a significant decrease when compared to NF (p < 0.05), while the contents of Ca, TF, TR, and TB demonstrated a significant increase (p < 0.05). This observation aligns with the sensory evaluation indicating that BF possesses a deeper color and a more mellow taste. It is hypothesized that these alterations in sensory qualities are closely linked to changes in metabolites, and that they are likely influenced by microbiota. Furthermore, TB has been previously reported as one of the most active and abundant pigments found in dark tea, known for its potential anti-hypercholesterolemia and anti-hyperlipidemia properties [37]. Therefore, the microbiota and the metabolites in the fermentation process were further investigated by Illumina MiSeq Sequencing technology and metabolomic approaches.
3.3. Influence of Aspergillus niger K1 on Microbiota
After filtering out the low-quality reads and chimeras, 36,710 to 41,785 bacterial sequences were obtained, and 1921 OTUs were generated using cluster analysis; meanwhile, 37,116 to 53,600 fungal sequences were obtained, and 353 OTUs were generated.
As shown in the Table 2, the Simpson and Shannon indexes of the bacteria in BF were significantly higher than those of NF (p < 0.05), which indicates that the uniformity and diversity of bacterial species in BF were higher than those of NF, and the Chao1 and ACE indexes of the bacteria in BF were also significantly higher than that of NF (p < 0.05), which indicates that the total number of bacterial species in BF was higher than in NF. In contrast, the Simpson, Shannon, Chao1, and ACE indexes of the fungi in BF were significantly lower than in NF (p < 0.05). Overall, BF inoculated with Aspergillus niger K1 could significantly change (p < 0.05) the microbiota in Tibetan tea.

Table 2.
Comparative analysis of α-diversity indexes of bacteria and fungi in BF and NF.
The significant dynamic changes in the microbiota of BF and NF during the fermentation process of Tibetan tea were due to complex and dynamic microbial ecosystems, such as symbiosis, interaction, and communication among bacteria, yeast, and filamentous fungi [38,39]. The compositions of bacteria with BF and NF were statistically analyzed at the phylum and the genus levels, respectively (Figure 3A,B). According to the result of genus annotation, bacterial OTUs of samples were classified into 5 dominant phyla and 18 dominant genera. Compared to NF, there was a decrease in the proportion of Proteobacteria (25.80%), Actinobacteria (12.93%), and Actinobacteria (12.93%), and an increase in the abundance of Firmicutes (31.32%) and Bacteroidetes (6.15%) in BF at the phylum level. Additionally, the dominant bacteria in BF were Staphylococcus (23.76%), Clostridium (10.50%), and Brevibacterium (10.33%) at the genus level, while Staphylococcus (15.47%), Bifidobacterium (13.60%), and Ochrobactrum (13.02%) were the dominant bacteria in NF. In all, inoculation with Aspergillus niger Kl markedly increased the relative abundance of Staphylococcus, Clostridium, Brevibacterium, Sphingobacterium, Brachybacterium, Deinococcus, Pseudomonas, Methylophilus, Bacillus, Camellia, Comamonas, and Klebsiella, and it decreased the relative abundance of Ochrobactrum, Ralstonia, Kocuria, Thermus, Bifidobacterium, and Agrobacterium.
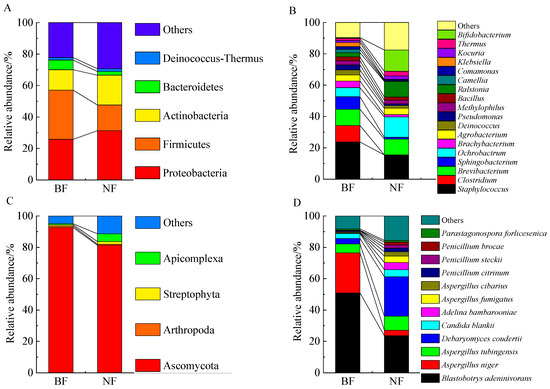
Figure 3.
The relative abundance and composition of dominant bacteria at the phylum level (A) and genus level (B), and dominant fungi at the phylum level (C) and species level (D).
The fungal OTUs were classified into four dominant phyla, including Ascomycota, Arthropoda, Streptophyta, and Apicomplexa. In NF, the abundances of Apicomplexa (4.98%) and Streptophyta (1.7%) were increased and the proportions of Ascomycota (81.34%) and Arthropoda (0.66%) were decreased compared with BF (Figure 3C,D). Additionally, the dominant fungi in BF were Blastobotrys adeninivorans and Aspergillus niger, accounting for 50.95% and 25.58%, respectively. In contrast, Debaryomyces coudertii and Blastobotrys adeninivorans in NF were the dominant fungi, accounting for 25.18% and 23.59%, respectively. In comparison to NF, the abundance of Aspergillus tubingensis, Debaryomyces coudertii, Candida blankii, Adelina bambarooniae, Aspergillus fumigatus, Aspergillus cibarius, Penicillium citrinum, Penicillium steckii, Penicillium brocae, and Parastagonospora forlicesenica was reduced in BF.
Linear discriminant analysis Effect Size (LEfSe) is a possible way to analyze the differences in microbiota between groups an identify the biomarkers between each group [40,41]. As shown in Figure 4, the bacterial and fungal biomarkers were greatly different between BF and NF. Our research results showed that 10 bacterial genera were the biomarkers in BF, including Staphylococcus, Sphingobacterium, Brachybacterium, Peptoniphilus, etc., and that the 15 bacterial biomarkers of NF were Ochrobactrum, Ralstonia, Sphingomonas, Terribacillus, etc. (Figure 4A). For fungi, LEfSe revealed that Penicillium citrinum, Aspergillus niger, Trichomonascus ciferrii, Emmia lacerate, Camptomyia flavocinerea, Phiebia livida, and Poteriospumella sp. were identified as the biomarkers for BF, while Debaryomyces coudertii, Adelina bambarooniae, Aspergillus fumigatus, Aspergillus cibarius, Penicillium steckii, and Candida blankie were identified as the biomarkers for NF (Figure 4B).
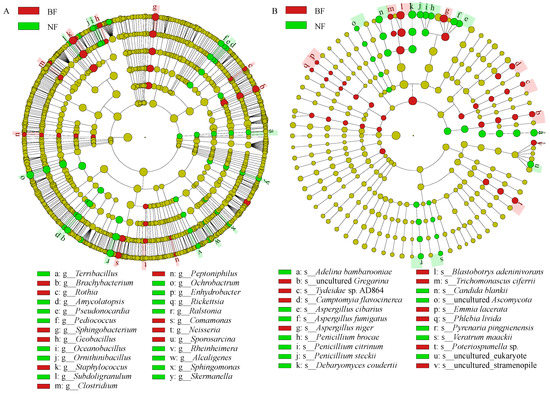
Figure 4.
Linear discriminant analysis of bacterial (A) and fungal (B) communities in tea samples.
Overall, due to changes in the metabolites of tea leaves and the interactions between microorganisms during the fermentation process of Tibetan tea, the microbiota of Tibetan tea undergoes dynamic changes during the fermentation process. These changes in the relative abundance of microorganisms further explain the changes in the characteristic components and sensory characteristics of the tea leaves from BF samples.
3.4. Metabolomic Analysis of Metabolites in BF and NF
Metabolites in tea leaves were extracted from the BF and NF, and analyzed using a LC-MS/MS-based metabolomics approach. A total of 859 metabolites (12 superclasses, 88 classes, and 157 subclasses) were identified (see Figure 5A and Table S1). The major metabolites included lipids and lipid-like molecules (267 metabolites), organic acids and derivatives (200 metabolites), and organic oxygen compounds (100 metabolites), and these were followed by phenylpropanoids and polyketides (74 metabolites); benzenoids (49 metabolites); nucleosides, nucleotides, and analogues (23 metabolites); organic nitrogen compounds (15 metabolites); organoheterocyclic compounds (12 metabolites); alkaloids and derivatives (4 metabolites); hydrocarbons (3 metabolites); lignans, neolignans, and related compounds (1 metabolite); and organosulfur compounds (1 metabolite) (Figure 5A).
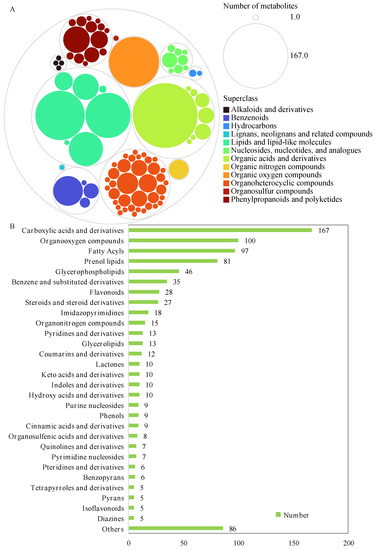
Figure 5.
The classification of identified metabolites in BF and NF. Superclass (A) and class (B) of metabolites.
These metabolites were further grouped into 88 classes (Figure 5B), including carboxylic acids and derivatives (167 metabolites), organooxygen compounds (100 metabolites), fatty acyls (97 metabolites), prenol lipids (81 metabolite), glycerophospholipids (46 metabolites), benzene and substituted derivatives (35 metabolites), flavonoids (28 metabolites), steroids and steroid derivatives (27 metabolites), imidazopyrimidines (18 metabolites), organonitrogen compounds (15 metabolites), glycerolipids (13 metabolites), pyridines and derivatives (13 metabolites), coumarins and derivatives (12 metabolites), hydroxy acids and derivatives (10 metabolites), indoles and derivatives (10 metabolites), keto acids and derivatives (10 metabolites), lactones (10 metabolites), etc.
Volcano plot analysis showed that 127 DCMs were found between BF and NF (Figure 6). The level of 90 DCMs in BF increased significantly (VIP > 1, p < 0.05, FC > 2) compared to that in NF. Interestingly, our results found that 16 DCMs increased more than 10-fold, e.g., prenyl apiosyl-(1->6)-glucoside (263-fold), 1-(beta-D-ribofuranosyl)-1,4-dihydronicotinamide (189-fold), threoninyl-proline (91-fold), thymidine (69-fold), artemoin B (56-fold), 2-hydroxyacetaminophen sulfate (40-fold), 3,5-dihydroxy-4-(sulfooxy)benzoic acid (39-fold), pyrocatechol sulfate (32-fold), hypoxanthine (22-fold), 2-pyrrolidinone (20-fold), xanthine (18-fold), xanthurenic acid (16-fold), 4-hydroxy-3-(sulfooxy)benzoic acid (15-fold), L-alpha-glutamyl-L-hydroxyproline (14-fold), 3-methylene-indolenine (13-fold), and dihydropteroic acid (11-fold). Compared to NF, 37 DCMs decreased significantly in BF (VIP > 1, p < 0.05 and FC < 0.5), including six amino acids, peptides, and analogues; three carbohydrates and carbohydrate conjugates; two terpene glycosides; two monoterpenoids; two glycerophosphates; two fatty acyl glycosides, and others. Among them, kahweol was reduced by more than 92-fold in the results from our research, and yucalexin B5 and glutamylisoleucine were degraded 22-fold and 11-fold, respectively (Figure 6). Benzoic acid is an aromatic acid organic compound with aromaticity and carboxylic acid properties, and the catalysis of enzymes will be converted into benzoic acid during the synthesis of dihydrobutyric acid [42,43]. Lubbers et al. indicated that Aspergillus niger can be engineered to produce protocatechuic acid from plant-derived aromatic compounds [44]. We speculated that these increases appear to result from the metabolites that were mutually fused and transformed, and we confirmed strong and lasting levels of aromas in the fermented tea. Collectively, our findings suggest that the dynamic fluctuations in the relative abundance of various microorganisms during the bioaugmented fermentation process contribute to alterations in the content of metabolites and the overall quality of Tibetan tea.
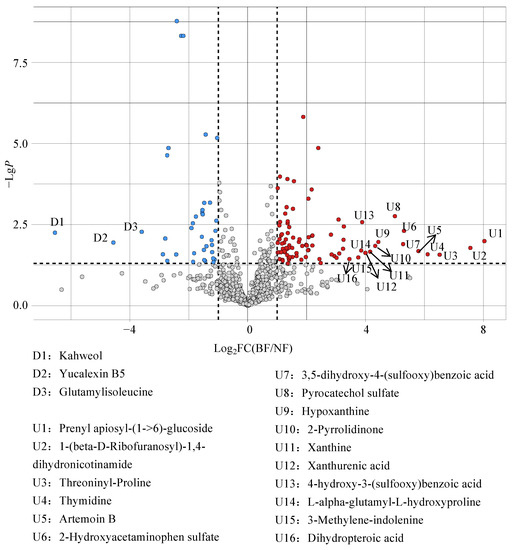
Figure 6.
Volcano plot analysis of DCMs. The blue dots represent significant decreased (VIP > 1, p < 0.05, FC < 0.5) metabolites, while the red dots represent significant increased (VIP > 1, p < 0.05, FC > 2) metabolites.
3.5. Correlation between Microorganisms, Characteristic Compositions and DCMs in BF and NF
The analysis of correlation between microorganisms, characteristic compositions, and DCMs was exhibited (Figure 7). It revealed that Bacillus rigiliprofundi, Brevibacterium sp., Candida blankii, Debaryomyces coudertii, Ralstonia insidiosa, and Staphylococcus kloosii were shown to be significantly positively correlated with the characteristic components, such as TF, TR, TB, FAA, Ca, TP, SS, and WE (p < 0.05, |r| > 0.8). Aspergillus tubingensis, meanwhile, was shown to be significantly positively correlated with TF, TR, TP, SS, and WE (p < 0.05, |r| > 0.8), and Aspergillus niger was shown to be significantly positively correlated with Ca and TB (p < 0.05, |r| > 0.8). This outcome aligns with previous research indicating that Aspergillus niger primarily facilitates the formation of TB in dark tea [20].
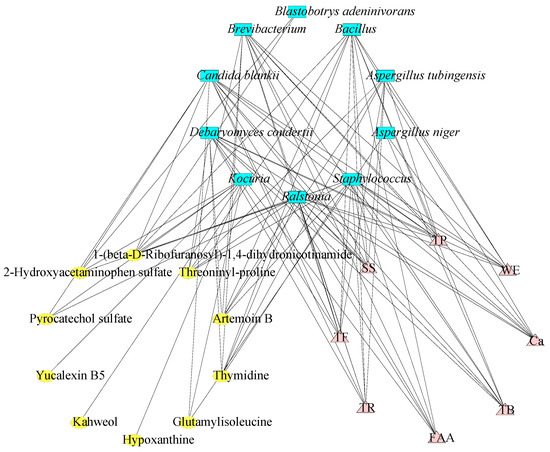
Figure 7.
Interaction of microorganisms, characteristic compositions, and metabolites based on the Spearman correlation analysis (p < 0.05, |r| > 0.8). Red solid lines mean positive correlation, green solid lines mean negative correlation.
Meanwhile, Aspergillus niger showed itself to be significantly positively correlated with DCMs, such as glutamylisoleucine (p < 0.05, |r| > 0.8), and Aspergillus tubingensis was significantly positively correlated with the metabolites of thymidine, artemoin B, and threoninyl-proline (p < 0.05, |r| > 0.8). Furthermore, Kocuria koreensis showed itself to be significantly positively correlated with the metabolites of yucalexin B5, kahweol, and glutamylisoleucine, while it had a significant negative correlation with hypoxanthine, pyrocatechol sulfate, and 2-hydroxyacetaminophen sulfate (p < 0.05, |r| > 0.8). Interestingly, 1-(beta-D-ribofuranosyl)-1,4-dihydronicotinamide showed a negative correlation with the microorganisms of Bacillus rigiliprofundi, Blastobotrys adeninivorans, Candida blankii, Kocuria koreensis, Ralstonia insidiosa, and Staphylococcus kloosii (p < 0.05, |r| > 0.8). Previously, there were similar reports of the correlation between microorganisms and metabolites during the fermentation process of tea [45,46,47]. Put simply, most dominant microorganisms had a significant impact on the characteristic components, and different microorganisms had different positive and negative effects on the metabolites.
4. Conclusions
In summary, Aspergillus niger Kl was inoculated into unsterilized tea leaves to initiate bioaugmented fermentation of Tibetan tea, and the dynamics of microorganisms, characteristic compositions, and metabolites were investigated. The results showed that bioaugmented fermentation promoted the oxidative polymerization of phenolic compounds, regulated the community succession rate, and, thus, significantly increased the content of theabrownins with health benefits, while also making Tibetan tea darker in color, stronger in aroma, and thicker in taste. Therefore, inoculated fermentation with Aspergillus niger Kl can improve the quality of Tibetan tea by a synergistic effect with other microorganisms, and these results demonstrate the application potential of bioaugmented fermentation in the dark tea as well as providing guidance for future research on this subject.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation9070690/s1, Table S1: The metabolites in BF and NF.
Author Contributions
Conceptualization, K.L. and Q.W.; methodology, K.L. and B.J.; software, K.L. and L.H.; resources, X.Z., Y.L. and M.L (Mingyong Li); data curation, K.L. and X.L.; writing—original draft preparation, K.L., L.H. and Q.W.; writing—review and editing, L.Y. and M.L. (Mingli Liu); funding acquisition, K.L. and W.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scientific and Technological Projects of Sichuan Province (No. 2021YFN0091); the Science and Technology Innovation Team Project of Yibin Vocational and Technical College (No. ybzy21cxtd-03); and the Scientific Research Project of Yibin Vocational and Technical College (No. ZRKY21YB-05,06,07).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The experimental data provided in this work are available in articles and Supplementary Materials.
Acknowledgments
The authors gratefully acknowledge Guanzhi Tea Industry Co., Ltd.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xie, H.; Li, X.; Ren, Z.; Qiu, W.; Chen, J.; Jiang, Q.; Chen, B.; Chen, D. Antioxidant and cytoprotective effects of Tibetan tea and its phenolic components. Molecules 2018, 23, 179. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, Z.; He, L.; Lu, X.; Tang, J.; Laghi, L. The longer the storage time, the higher the price, the better the quality? A1H-NMR based metabolomic investigation of aged Ya’an tibetan tea (Camellia sinensis). Foods 2022, 11, 2986. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Ren, H.; Liu, R.; Da Silva, R.R.; Aksenov, A.A.; Melnik, A.V.; Zhao, M.; Le, M.; Ren, Z.; Xu, F.; et al. Microbial and nonvolatile chemical diversities of Chinese dark teas are differed by latitude and pile fermentation. J. Agric. Food Chem. 2022, 70, 5701–5714. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.K.; Foo, K.Y. Recent advances on the beneficial use and health implications of Pu-erh tea. Food Res. Int. 2013, 53, 619–628. [Google Scholar] [CrossRef]
- Lin, F.J.; Wei, X.L.; Liu, H.Y.; Li, H.; Xia, Y.; Wu, D.T.; Zhang, P.Z.; Gandhi, G.R.; Hua, B.L.; Gan, R.Y. State-of-the-art review of dark tea: From chemistry to health benefits. Trends Food Sci. Technol. 2021, 109, 126–138. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, B.; He, J.L.; Wei, T.; Liu, D.J.; Yang, W.J.; Guo, C.Y.; Nie, X.Q. Combinations of Tibetan tea and medicine food homology herbs: A new strategy for obesity prevention. Food Sci. Nutr. 2023, 11, 504–515. [Google Scholar] [CrossRef]
- Li, L.; Shi, M.; Salerno, S.; Tang, M.; Guo, F.; Liu, J.; Feng, Y.; Fu, M.; Huang, Q.; Ma, L.; et al. Microbial and metabolomic remodeling by a formula of Sichuan dark tea improves hyperlipidemia in apoE-deficient mice. PLoS ONE 2019, 14, e219010. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, W.; Zhang, C.; Li, C.; Fang, Z.; Zeng, Z.; Hu, B.; Chen, H.; Wu, W.; Wang, T.; et al. Targeted and untargeted metabolomic analyses and biological activity of Tibetan tea. Food Chem. 2022, 384, 132517. [Google Scholar] [CrossRef]
- Wang, N.; Wu, T.; Du, D.; Mei, J.; Luo, H.; Liu, Z.; Saleemi, M.K.; Zhang, R.; Chang, C.; Mehmood, M.A.; et al. Transcriptome and gut microbiota profiling revealed the protective effect of Tibetan tea on ulcerative colitis in mice. Front. Microbiol. 2022, 12, 748594. [Google Scholar] [CrossRef]
- Cheng, L.; Yang, Q.; Chen, Z.; Zhang, J.; Chen, Q.; Wang, Y.; Wei, X. Distinct changes of metabolic profile and sensory quality during Qingzhuan tea processing revealed by LC-MS-Based metabolomics. J. Agric. Food Chem. 2020, 68, 4955–4965. [Google Scholar] [CrossRef]
- Gong, Z.; Ouyang, J.; Wu, X.; Zhou, F.; Lu, D.; Zhao, C.; Liu, C.; Zhu, W.; Zhang, J.; Li, N.; et al. Dark tea extracts: Chemical constituents and modulatory effect on gastrointestinal function. Biomed. Pharmacother. 2020, 130, 110514. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, J.; Li, Z.; Li, Q.; Lai, X.; Sun, L.; Chen, R.; Wen, S.; Sun, S.; Lai, Z. HS-SPME and GC/MS volatile component analysis of Yinghong No. 9 dark tea during the pile fermentation process. Food Chem. 2021, 357, 129654. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zeng, H.; Qin, L. Physicochemical, taste, and functional changes during the enhanced fermentation of low-salt Sufu paste, a Chinese fermented soybean food. Int. J. Food Prop. 2018, 21, 2714–2729. [Google Scholar] [CrossRef]
- Mu, Y.; Huang, J.; Zhou, R.; Zhang, S.; Qin, H.; Tang, H.; Pan, Q.; Tang, H. Bioaugmented Daqu-induced variation in community succession rate strengthens the interaction and metabolic function of microbiota during strong-flavor Baijiu fermentation. LWT 2023, 182, 114806. [Google Scholar] [CrossRef]
- Mu, Y.; Huang, J.; Zhou, R.; Zhang, S.; Qin, H.; Dong, Y.; Wang, C.; Wang, X.; Pan, Q.; Tang, H. Comprehensive analysis for the bioturbation effect of space mutation and biofortification on strong-flavor Daqu by high-throughput sequencing, volatile analysis and metabolomics. Food Chem. 2023, 403, 134440. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Zhang, Y.; Lu, N.; Shi, C.; Yan, S. Yeasts from Chinese strong flavour Daqu samples: Isolation and evaluation of their potential for fortified Daqu production. AMB Express 2021, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, V.; Gao, L.; Sheng, T.; Liu, C.; Chen, C.; Liu, W.; Wang, A. Bioaugmented hydrogen production from lignocellulosic substrates using co-cultures of Shigella flexneri str. G3 and Clostridium acetobutylicum X9. J. Technol. Innov. Renew. Energy 2014, 3, 36–43. [Google Scholar]
- Liu, K.; Wang, L.; Jiang, B.; An, J.; Nian, B.; Wang, D.; Chen, L.; Ma, Y.; Wang, X.; Fan, J.; et al. Effect of inoculation with Penicillium chrysogenum on chemical components and fungal communities in fermentation of Pu-erh tea. Food Res. Int. 2021, 150, 110748. [Google Scholar] [CrossRef]
- Wu, S.; Wang, W.; Zhu, W.; Chen, W.; Xu, W.; Sui, M.; Jiang, G.; Xiao, J.; Ning, Y.; Ma, C.; et al. Microbial community succession in the fermentation of Qingzhuan tea at various temperatures and their correlations with the quality formation. Int. J. Food Microbiol. 2022, 382, 109937. [Google Scholar] [CrossRef]
- Wang, Q.; Gong, J.; Chisti, Y.; Sirisansaneeyakul, S. Fungal isolates from a Pu-erh type tea fermentation and their ability to convert tea polyphenols to theabrownins. J. Food Sci. 2015, 80, M809–M817. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, C.; Ren, X.; Xia, T.; Li, X.; Wu, Y. Production of theophylline via aerobic fermentation of Pu-erh tea using tea-derived fungi. BMC Microbiol. 2019, 19, 261. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, C.; Wang, H.; Xia, T. Biodegradation of caffeine by whole cells of tea-derived fungi Aspergillus sydowii, Aspergillus niger and optimization for caffeine degradation. BMC Microbiol. 2018, 18, 53. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, C.; Zheng, C.; Xia, T.; Ma, B.; Liu, X. 3-Methylxanthine production through biodegradation of theobromine by Aspergillus sydowii PT-2. BMC Microbiol. 2020, 20, 269. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Jiang, B.; Liu, K.; Li, R.; Chen, L.; Liu, Z.; Xiang, G.; An, J.; Luo, H.; Wu, J.; et al. Multi-omics analysis of the metabolism of phenolic compounds in tea leaves by Aspergillus luchuensis during fermentation of Pu-erh tea. Food Res. Int. 2022, 162, 111981. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, M.; An, T.; Zu, Z.; Song, P.; Chen, M.; Yue, P.; Gao, X. Preparation of instant dark tea by liquid-state fermentation using sequential inoculation with Eurotium cristatum and Aspergillus niger: Processes optimization, physiochemical characteristics and antioxidant activity. LWT 2022, 162, 113379. [Google Scholar] [CrossRef]
- Ma, Y.; Ling, T.; Su, X.; Jiang, B.; Nian, B.; Chen, L.; Liu, M.; Zhang, Z.; Wang, D.; Mu, Y.; et al. Integrated proteomics and metabolomics analysis of tea leaves fermented by Aspergillus niger, Aspergillus tamarii and Aspergillus fumigatus. Food Chem. 2021, 334, 127560. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wang, W.; Li, J.; Guo, X.; Zhao, M.; Jiang, Y.; Tu, P. Influence of the post-fermentation by four Aspergillus strains on the aroma of Pu-erh tea. J. Chin. Pharm. Sci. 2016, 25, 284–290. [Google Scholar]
- Edgar, R.C. Muscle: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Sudhir, K.; Glen, S.; Koichiro, T. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar]
- Wang, Q.; Peng, C.; Gong, J. Effects of enzymatic action on the formation of theabrownin during solid state fermentation of Pu-erh tea. J. Sci. Food Agric. 2011, 91, 2412–2418. [Google Scholar] [CrossRef]
- Wang, Q.; Gong, J.; Chisti, Y.; Sirisansaneeyakul, S. Production of theabrownins using a crude fungal enzyme concentrate. J. Biotechnol. 2016, 231, 250–259. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, J.; Huang, X.; Zhang, H.; Liu, M. Simultaneous determination of total polyphenols and caffeine contents of green tea by near-infrared reflectance spectroscopy. Microchem. J. 2006, 83, 42–47. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Jiang, R.; Ouyang, J.; Liu, Q.; Li, J.; Wen, H.; Li, Q.; Chen, J.; Xiong, L.; et al. Characterization of aroma differences on three drying treatments in Rucheng Baimao (Camellia pubescens) white tea. LWT 2023, 179, 114659. [Google Scholar] [CrossRef]
- Li, P.; Lin, W.; Liu, X.; Wang, X.; Luo, L. Environmental factors affecting microbiota dynamics during traditional solid-state fermentation of Chinese Daqu starter. Front. Microbiol. 2016, 7, 1237. [Google Scholar] [CrossRef]
- Li, P.; Su, R.; Wang, Q.; Liu, K.; Yang, H.; Du, W.; Li, Z.; Chen, S.; Xu, B.; Yang, W. Comparison of fungal communities and nonvolatile flavor components in black Huangjiu formed using different inoculation fermentation methods. Front. Microbiol. 2022, 13, 955825. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Q.; Luo, H.; Li, R.; Chen, L.; Jiang, B.; Liang, Z.; Wang, T.; Ma, Y.; Zhao, M. An in Vitro catalysis of tea polyphenols by polyphenol oxidase. Molecules 2023, 28, 1722. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zheng, X.; Ma, X.; Jiang, R.; Zhou, W.; Zhou, S.; Zhang, Y.; Lei, S.; Wang, S.; Kuang, J.; et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 2019, 10, 4971. [Google Scholar] [CrossRef] [PubMed]
- van Hijum, S.A.; Vaughan, E.E.; Vogel, R.F. Application of state-of-art sequencing technologies to indigenous food fermentations. Curr. Opin. Biotechnol. 2013, 24, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, B.E.; Dutton, R.J. Fermented foods as experimentally tractable microbial ecosystems. Cell 2015, 161, 49–55. [Google Scholar] [CrossRef]
- Nicola, S.; Sahar, A.; Johannes, G.; Alyxandria, M.S.; Jacques, I.; Brandi, L.C.; Beltran, R.; Levi, W.; Jeremy, Z.; Mathangi, T.; et al. Microbial community function and biomarker discovery in the human microbiome. Genome Biol. 2011, 12, 47. [Google Scholar]
- Kennedy, R.; Lappin, D.F.; Dixon, P.M.; Buijs, M.J.; Zaura, E.; Crielaard, W.; O’Donnell, L.; Bennett, D.; Brandt, B.W.; Riggio, M.P. The microbiome associated with equine periodontitis and oral health. Vet. Res. 2016, 47, 49. [Google Scholar] [CrossRef] [PubMed]
- Arsano, I.; Talapatra, S.; Ma, X.; Tsige, M. Adsorptive structure and mobility on carbon nanotube exteriors using benzoic acid as a molecular probe of amphiphilic water contaminants. J. Phys. Chem. B 2022, 126, 4956–4966. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wan, X.; He, X.; Rong, C.; Liu, S. Impacts of external fields on aromaticity and acidity of benzoic acid: A density functional theory, conceptual density functional theory and information-theoretic approach study. Phys. Chem. Chem. Phys. 2023, 25, 2595–2605. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, R.; de Vries, R.P. Production of protocatechuic acid from p-hydroxyphenyl (H) units and related aromatic compounds using an Aspergillus niger cell factory. MBio 2021, 12, e39121. [Google Scholar] [CrossRef]
- Li, J.; Xu, R.; Zong, L.; Brake, J.; Cheng, L.; Wu, J.; Wu, X. Dynamic evolution and correlation between metabolites and microorganisms during manufacturing process and storage of Fu Brick tea. Metabolites 2021, 11, 703. [Google Scholar] [CrossRef]
- Li, Q.; Huang, J.; Li, Y.; Zhang, Y.; Luo, Y.; Chen, Y.; Lin, H.; Wang, K.; Liu, Z. Fungal community succession and major components change during manufacturing process of Fu brick tea. Sci. Rep. 2017, 7, 6947. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.; Xu, N.; Yu, Y.; Brake, J.; Xu, R.; Wu, X. Dynamic evolution and correlation between microorganisms and metabolites during manufacturing process and storage of Pu-erh tea. LWT 2022, 158, 113128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).