Abstract
Currently, there is an urgent need for the growing aquaculture sector to rely on sustainable ingredients which can achieve optimal growth while maintaining fish’s nutritional value (especially omega-3 fatty acid content) for human consumption. Here, C. reinhardtii biomass was substituted for fishmeal in zebrafish (Danio rerio) diets in wild-type and mutant (Casper) strains. Four isonitrogenous (46% cp), isocaloric (19–21 MJ/kg DW) diets were prepared with C. reinhardtii replacing 10% (C10), 20% (C20), and 50% (C50) of the fishmeal component of the diet formulation. Over 8 weeks of feeding trials, the zebrafish showed a significant growth improvement when fed C10, C20, and C50 compared with the control (no C. reinhardtii), with C20 giving the best performance in terms of growth, feed conversion ratio (FCR), and specific growth rate (SGR). Interestingly, C. reinhardtii in the diet increased the levels of linolenic acid (C18:3 n-3) and hexadecatrienoic acid (C16: 4-n-3) (p ≤ 0.05) in the zebrafish. Yellow pigmentation, which was shown to be lutein, was observed in eggs and zebrafish flesh for fish fed a diet containing C. reinhardtii. Moreover, the zebrafish assimilated β-carotene from C. reinhardtii and converted it to vitamin A. Overall, while replacing 20% of fishmen in the zebrafish’s diet with C. reinhardtii biomass offers the best results, replacement with only 10% showed a significant benefit for the zebrafish. Furthermore, replacing fishmeal with 50% C. reinhardtii is still possible and beneficial, and C. reinhardtii whole cells are digestible by zebrafish, thus demonstrating that C. reinhardtii not only has the potential to serve as a feed supplement but that it can also act as a feed substitute once the production cost of microalgae becomes competitive.
Keywords:
aquaculture; microalgae; Chlamydomonas reinhardtii; zebrafish; lutein; β-carotene; fatty acids; fishmeal; fish oil; vitamin A 1. Introduction
Aquaculture produces almost 50% of the fish intended for human consumption [1]. Aquaculture is also one of the fastest-growing food sectors, with an expectation that it will double in capacity by 2050 to cater for the growing global demand for fish, leading to improved nutrition and food security while reducing the burden on wild fisheries [1]. Globally, fish (wild and farmed) provide about 16% of animal protein for the human diet as well as important n-3 fatty acids and essential minerals [2].
The aquafeed industry currently relies heavily on marine ingredients including wild marine forage fish and the by-products of fish processing to provide fishmeal (FM) and fish oil (FO) [3]. Fishmeal supplies aquaculture with high-value protein, essential micronutrients, vitamins, minerals, and lipids, especially long-chain polyunsaturated fatty acids (LC-PUFA); thus, it is considered as the gold standard feed [4].
Currently, the term fish-in-fish-out (FIFO) is used to express the efficiency with which the fishmeal and fish oil from wild fish are converted into farmed fish bodies [5]. This figure has continuously declined from (0.47) in 2000 to (0.19) in 2020, which means that each 1 kg of wild fish gives 5 kg of farmed fish [6]. Keeping this figure in mind, the use of wild fish in aquafeed is more efficient than using them in direct human consumption, although 90% of wild-caught fish are of food grade [5]. However, there are many economic and environmental challenges caused by the use of FM and FO in aquafeed. By 2030, it is expected that the global human consumption of fish will increase by 1.3%, while aquaculture production will provide 57% of the total human consumption compared to 53 % in 2020.On the other hand, it is believed that the growth of wild fish capture will only increase by 3.6% by 2030, added to some fluctuations caused by climate change, such as in the El Niño years (2022–2027) [6,7]. Thus, the availability of both commodities is predicted to slow down compared to the growth in the aquafeed sector, and by 2030, the price of FM is expected to double and for FO, it is expected to increase by over 70% with more competition with omega-3 production for the supplement market [6,7].
Moreover, the supply of FM and FO is not unlimited; at current rates of use, FM and FO are expected to be exhausted by 2040 [8,9]. The reliance of aquaculture on these two commodities raises long-term sustainability issues, including, but not limited to, a reduction in wild fish and a decline in marine ecosystem balance [10]. Finally, industrialisation- and urbanisation-related marine pollution contaminates fish products and affects the safety of FM and FO as feed for farmed fish [10]. Thus, reformulating aquafeed using more sustainable ingredients that can partly or fully replace FM and FO but still maintain fish growth and high nutritional value for human consumption is a prominent challenge and of prime interest to the fish farming industry. In order to achieve this, many plant-based protein sources have been investigated, and some are already included in many fish diets such as pea, soy, and wheat protein. However, higher inclusion of plant-based protein in fish’s diet is generally limited due to poor digestibility and poor nutritional value [11]. This can be due to the high content of anti-nutritional factors or deficiencies in essential amino acids. Moreover, terrestrial crop oils lack the essential n-3 fatty acids, which negatively affect the content of EPA and DHA in fish flesh [11].
Microalgae biomass including eukaryotic organisms and photosynthetic cyanobacteria have recently received greater attention for both human nutrition and animal feed, including terrestrial animals and aquafeed [12]. Several microalgae species have balanced, high nutritional value including lipids with n-3 fatty acids, proteins containing the essential amino acids, carbohydrates, pigments, vitamins, and minerals [13].
In recent years, studies have demonstrated the high nutritional profile of many microalgae species besides the rapid development in microalgae cultivation techniques and biomass processing. Most microalgal species are yet to be tested as animal feed and human supplements. Some of the challenges with microalgae inclusion in both the feed and food sector include the thick and cellulosic cell wall, such as in Chlorella, which hinder the digestibility and thus the release of intracellular nutrients when consumed by animals and humans. This is added to the high levels of inhibitory compounds and anti-nutritional factors, such as the phenolic compounds found in some red microalgae, which can make the algal biomass unusable as a feed ingredient [14,15]. Additionally, there is still a need to reduce the cost of microalgal biomass cultivation and to ensure the consistency of the nutritional quality of microalgae [16]. Thus, it is essential to investigate novel microalgae species using model animals and the processing and formulation of this microalgal-based feed [16].
Chlamydomonas reinhardtii is a green microalga with many biological applications. It has been used extensively as a reference organism in biofuel and bio-product production [17]. The potential of C. reinhardtii biomass as a highly nutrient ingredient has been investigated: C. reinhardtii has a better nutritional profile than commercial algal species such as Chlorella and Spirulina [18]. The cell wall of C. reinhardtii is composed of hydroxyproline-rich glycoprotein [17] and has demonstrated good digestibility when tested in vitro (data not shown). C. reinhardtii is also generally regarded as safe for human consumption by the U.S. Food and Drug Administration (FDA); therefore, it is safe for use as a feed ingredient for animals and for human consumption [19].
Zebrafish belong to the family Cyprinidae. They are warm-water omnivores that naturally consume aquatic insects and phytoplankton [20]. Zebrafish are a well-established vertebrate model for monitoring many biological processes and research in human and animal health as well as aquaculture [21]. They have a number of benefits for preliminary feeding trials: they are small in size, develop quickly, and cost less to feed/keep than large species. They are also easier to handle than rodents [22]. Zebrafish also display a high degree of similarity with humans in terms of their anatomy, genetics (80% of zebrafish gene sequences are similar to human genes), cell development, molecular mechanisms, and organ physiology [23,24]. Moreover, zebrafish and humans share many similarities in intestinal gene function, cell structure, and molecular mechanisms [25]. To the best of our knowledge, the use of C. reinhardtii in aquaculture feed has yet to be reported; however, C. reinhardtii has been used as an oral vaccine delivery platform in zebrafish (Danio rerio) [26].
The aim of this study is to investigate the implementation of the green microalgae (C. reinhardtii) as a sustainable aquafeed supplement and substitute in aquaculture as well as probing its ultimate potential in human nutrition. The study focuses on assessing the digestibility and bioavailability of the various intracellular nutrients of C. reinhardtii intact biomass, including proteins, lipids, and pigments, using zebrafish as an animal model. The study will include various levels of fishmeal reduction in the zebrafish’s diet while replacing it with intact C. reinhardtii biomass. This allows for the assessment of the use of C. reinhardtii as a sustainable supplement feed and/or replacement of fishmeal in aquaculture and its optimal replacement level. It also paves the way for more research into the potential impact of C. reinhardtii on human health when utilised in feed indirectly or directly as a food supplement.
2. Materials and Methods
2.1. Diet Formulation and Preparation
Four experimental diets including a control diet were prepared at the University of Liverpool in parallel with our published work [27]. The experimental diets were formulated by substituting fishmeal for C. reinhardtii biomass. The other ingredients were manipulated to achieve an isonitrogenous (46% crude protein), isocaloric (19–21 MJ/kg DM) diet; see Table 1 for the nutritional profile of each diet. The protein level in this study was guided by industry feeds at the time and research on the optimal dietary requirements of zebrafish. Many researchers have suggested a range of protein requirements for the Cyprinidea family with recommended levels of (30–53%) protein in their diet depending on protein quality [28].

Table 1.
Experimental diet compositions showing the ingredient inclusion rates (A) and nutritional profiles of the experimental diets (B).
Briefly, the control diet is referred to as C0 with a 0% reduction in fishmeal (38.74% DW) and 0% inclusion of C. reinhardtii. The C10 diet represents a 10% reduction in fishmeal (34.12% DW) and the inclusion of C. reinhardtii at (19.43% DW). The C20 diet is the result of a 20% reduction in fishmeal (30.98% DW) and the inclusion of (20.50% DW). Likewise, the C50 diet stands for a 50% reduction in fishmeal (19.62% DW) and the inclusion of (25.12% DW) of C. reinhardtii. The composition of C. reinhardtii biomass used in this study is presented in Table 2 as carried out previously in our laboratory [18].

Table 2.
Composition of C. reinhardtii biomass used for zebrafish feeding as in g/100 g DW [18].
Fish oil was not added to any of the four diets, and rapeseed oil was only added in the control (C0) but not the C10, C20, and C50 diets as C. reinhardtii biomass contains lipids (24.62%). Mass balance was achieved by reducing the amount of corn starch. Protein balance was also achieved by varying the amount of wheat gluten.
All of the specified ingredients in Table 1 were mixed thoroughly using a commercial Hobart benchtop food mixer and water was added until the mixture achieved a dough-like consistency. The mixture was spread out on trays and dried for 24 h at 50 °C using a nine-shelf Parallexx Excalibur food dehydrator. The dried diet was crushed and sieved through a series of sieves with apertures of 425 µm and 850 µm, with the desired particle size being collected from between the two.
2.2. Subjects and Husbandry
All of the zebrafish were bred in-house at the University of Liverpool aquarium facility. One trial was conducted to assess their growth and performance using 750 juveniles of D. rerio (AB wild-type strain). The zebrafish were approximately 2 months old at the start of the trial. They were divided into groups of 15 individuals in 50 identical 1.5 L fish tanks (5 cm × 7 cm × 15.5 cm). The tanks were connected to a central system which is maintained by a sump filtration system and 20% weekly water changes. Due to the small size of the fish, the tanks were fitted with a 400 µm fry mesh baffle. Cleaning of the fish tanks was conducted weekly, which took place during the weighing of the fish to prevent further disturbances. The water quality was subsequently kept stable by monitoring and controlling the following parameters: ammonia (NH4), 0 mg/L; nitrite (NO2), 0 mg/L; nitrate (NO3), <50 mg/L; and pH; 7.0. The fish were maintained at 28 ± 1 °C and exposed to a 12/12 h light/dark cycle via cool white (7000K) LED strip lights located above all tanks.
A second trial to visually assess the fish’s bodies and egg pigmentation was conducted using 84 juveniles of D. rerio (Casper strain), which is a transparent mutant of zebrafish. The fish were approximately 7 months old at the start of the trial. The fish were housed in groups of 6 individuals, either all males or all females, in 14 identical tanks, the same 1.5 L tanks as above using the same baffles. The tanks were maintained on the same system as above with the same water quality parameters and environmental conditions. Cleaning of the tanks was carried out while the fish were housed in the breeding tanks.
2.3. Experimental Procedures
2.3.1. Weighing the Fish
Average fish weight was measured weekly and used to assess growth and performance. To minimise disturbance, the fish were weighed while weekly tank cleaning and maintenance were carried out. Average weight was measured using a weighing tank (with the same capacity and dimensions as the housing tanks), filled with system water (covering a 1 cm depth in the tank) and placed on the balance (Fisher Scientific SG-602) and tared. All fish in the housing tank (15 fish) were caught gently in a small net and transferred to the weighing tank on the balance.
2.3.2. Growth Parameters, Calculations, and Assessment
There are several indicators and parameters widely accepted for the assessment of new diet formulations and the incorporation of novel ingredients in fish’s diet. The growth rate was measured using the weekly weight of the fish in each tank over 8 consecutive weeks, and the percentage of weight growth was plotted against time. Other growth parameters were also calculated.
The feed conversion ratio (FCR), which indicates the efficiency at which feed is converted into animal biomass, was also determined:
FCR = feed (Kg)/met aquaculture production (kg)
Total feed intake (TFI) = total feed given–waste output and net production = mass at the end of the study period–mass at the start of the study period [30].
The fishmeal ratio (FMR) indicates the quantity of fishmeal required to produce 1 kg of live fish) [31]:
FMR = FCR × (% fishmeal in feed/100)
Finally, the specific growth rate (SGR) demonstrates the growth achieved per day during the time in which the subjects are fed the test diet:
where ln = natural log; W1 = initial weight; W2 = final weight; t1 = starting time point (day one); and t2 = end time point [32].
SGR (%) = 100 × (ln W2 − ln W1) × (t2 − t1) − 1
For some of these parameters, like the growth rate and the SGR, the higher the value, the better the results. For the FCR, the lower the value the more efficient the diet. The FCR can drop below a value of 1.0 as it is a calculation that uses dry-weight feed and wet weight in animal biomass gain [30,33].
2.3.3. Feeding, Sampling, and Preparation for Chemical Analysis
Five replicate tanks were given the same experimental diet, with 75 fish in total per diet. The fish were fed at a rate of 4% of the mean body weight of the fish in each tank daily. At the end of the trial period, the fish were euthanised via a Home-Office-approved schedule 1 method: concussion followed by pithing and destruction of the brain to confirm death. Fish from each of the five tanks fed the same diet were collated together, frozen at −20 °C, transported on dry ice to the Division of Food, Nutrition & Dietetics, University of Nottingham, and stored at −80 °C. The fish were then freeze-dried for one week (Edwards Freeze Dryer, Super Modulyo). During the freeze-drying process, the samples were protected from light exposure. The samples were then ground to a homogeneous powder using a mortar and pestle under dim light and with liquid nitrogen. The dried and homogenised samples were then stored at −20 °C for further analysis.
2.3.4. Zebrafish Spawning and Egg Collection
The zebrafish were divided into a male group and a female group. Five tanks in each group were fed on each experimental diet and the control. Feed was given daily as required until satiation. After three weeks of feeding, the fish were bred by mixing males and females together overnight in 3-L (11.5 cm × 25 cm × 15 cm) breeding tanks. With a 2 mm aperture mesh, used to create a false raised base with a gradient in the tank, the eggs fall through the mesh, preventing cannibalism by the adults. The next morning, as soon as the lights came on, the flow to the tank was switched off and the net was raised slightly to stimulate breeding. After a two-hour period, the fish were removed and returned to all-male and all-female tanks. The eggs were collected by hand, transferred to glass sample jars, then snap-frozen with liquid nitrogen, and then stored at −80 °C until further analysis. Spawning was repeated every three days until a sufficient number of eggs had been collected for the required sample size. The fish were then euthanised using a Home-Office-approved schedule 1 method as previously described. These were also stored at −80 °C until further analysis.
2.4. Ethical Issues
The work carried out here was carried out under the Establishment Licence for the University of Liverpool (X70548BEB), therefore meeting all of the standards required under the Animals (Scientific Procedures) Act 1986. The work was thoroughly reviewed, ethical approval was granted (ref. number AWC0082) via the university’s AWERB (Animal Welfare and Ethical Review Body), and the work was deemed below the threshold for regulation. Throughout the trial, fish welfare was independently monitored by an animal and care welfare officer (NACWO) and was not compromised during this study.
2.5. Total Lipid Extraction
The total lipids of the freeze-dried zebrafish samples fed on (C0, C10, C20, and C50) were extracted using a modified Folch method [32]. Dried fish samples (0.5 g of zebrafish and 0.1 g of eggs) were mixed with a solvent mixture of chloroform: methanol (2:1) (6 mL for zebrafish and 1.2 mL to the eggs) and vortexed for 1 min. To this, 0.9% NaCl (2.5 mL for fish and 0.5 mL for the eggs) was added to the mixture and vortexed for 1 min. The mixture was then centrifuged using a Thermo Jouan CR3i multifunction centrifuge (Thermo Electron Industries SAS, Chateau-Gontier, France) (1300 RCF for 10 min at 4 °C). The lower chloroform layer containing the lipids was collected using a glass Pasteur pipette. The pellets were extracted two more times by repeating the same procedure. The three extracts of each sample were combined and filtered through a 0.45 µm syringe filter into pre-weighed bijoux bottles. The extracts were gently dried under nitrogen gas, then weighed, quantified gravimetrically, and stored at −80 °C until further analysis.
2.6. Fatty Acids (FA) Analysis
The FA composition of the samples was determined using a TRACE GC ultra gas chromatography-mass spectrometer (GC-MS) equipped with a CTS Analytics PAL system autosampler (Thermo Fisher, Loughborough, UK). Initially, the total amount of dried lipid extract produced (see Section 2.5) was dissolved in 2 mL of chloroform and vortexed for 1 min and then divided into two aliquots, 1 mL each. Methyl pentadecanoate (100 µL) was added to the 1 mL aliquots as an internal standard, and 200 µL of Trimethylsulfonium hydroxide was used for methylation and held for 10 min to ensure complete conversion of the fatty acids into fatty acid methyl esters (FAMEs). A 10 μL sample of each sample was then injected into the capillary column (Zebron, ZB-FFAP, 30 m × 0.22 mm internal diameter) (Phenomenex Inc., Macclesfield, UK) using a vaporising injector (split flow of 50 mL/min. The oven temperature was maintained at 120 °C for 1 min and then increased to 250 °C (5 °C/min) for 2 min. The retention time of each peak for the individual fatty acids was compared with the FAME standard. A standard library was also used through the “Thermo Scientific Xcalibur Version 1.4” software (Thermo Fisher Scientific Inc., Loughborough, UK). The percentage content of each FA was calculated, and the concentrations (mg/mL) were determined using the response factor of the internal standard.
2.7. Retinol Analysis
The analysis and detection of all-trans-retinol in the whole zebrafish body of the test trials and the control were based on the method developed by Li et al. (2005) with slight modifications [34]. Briefly, nitrogen-dried lipids obtained from Section 2.5 were re-dissolved with 1 mL acetone and vortexed. The extracts were then vigorously mixed with an equivalent volume of methyl-tetra-butyl ether (MtBE) and 0.5 mL distilled water. The aqueous phase was extracted three more times using the MtBE. The combined MtBE phases were evaporated to dryness under a gentle stream of nitrogen, suspended with 0.5 mL MtBE vortexed and passed to the PTFE membrane filter (0.45 µm), sealed under nitrogen in brown glass vials, and stored in the dark at −80 °C until further analysis.
The analysis was carried out using an Agilent 1100 HPLC equipped with a Diode array with a UV-visible detector (Agilent Technologies, Waldbronn, Germany). A Gemini-NX C18 column (250 mm length, 4.6 mm, 5 µm particle size) and a Phenomenex C18 guard column (4 μm, 3.9 × 20 mm) were used for separation (Phenomenex Inc., Macclesfield, UK). UV-vis absorption spectra were recorded at 325 nm for retinol. The mobile phases consisted of two components: (A) methanol and (B) MtBE. The solvent pump was programmed to inject 90% A from 0–12 min, followed by linear gradients of 90–60% A from 12–13 min, maintained until 22 min followed by a linear gradient back to the 90% A initial conditions until the end of the run at 30 min. The flow rate was 0.8 mL/min.
2.8. Analysis of Carotenoids in Zebrafish and Eggs
The β-carotene and lutein content of the zebrafish and eggs samples was determined with HPLC (Agilent 1100) with photo diode array (PDA) detection using a method slightly modified from Kimura and Rodriguez-Amaya (2002) [35]. The dried lipid extract (see Section 2.5) was dissolved in 10 mL of the acetone solution (containing 0.1% BHT) and syringe filtered (0.45 μm) into an amber HPLC vial.
The flow rate of the mobile phase (acetonitrile: methanol: ethyl acetate) was set at 0.5 mL/min. Two gradient mobile phases were used from 95:5:0 to 60:20:20 in 20 min, with this proportion being maintained until the end of the run. Re-equilibration took 15 min. The samples were injected at a volume of 10 μL through a Sentry guard column (Waters, Nova-Pak C18, 4 μm, 3.9 × 20 mm) and separated using a Waters Spherisorb S3ODS (3 μm, 4.6 × 15 cm) column (Waters limited, Wilmslow, UK), with the temperature set at 22 °C; β-carotene was detected at 454 nm. The concentration of β-carotene and that of lutein were determined using a linear equation created using a calibration curve produced from a range of external (β-carotene) standards (10–100 μg/ mL) dissolved in cold acetone containing 0.1% BHT.
2.9. Statistical Analysis
Five biological experiments were carried out for the zebrafish growth trials. The chemical characterisation of the zebrafish experiments was performed in triplicate. The statistical analysis was performed by the Minitab V. 17 statistical package (Minitab, LLC., State College, PA, USA) using post hoc analysis of variance (ANOVA) and according to Tukey’s test with the statistical significance at p ≤ 0.05.
3. Results and Discussion
3.1. Growth and Palatability Criteria
A set of fish performance parameters (Figure 1) were measured on the zebrafish fed the experimental diets (C10, C20, and C50) and the control (C0). For C10 and C20, the fish growth rates (Figure 1A) were similar and both diets supported a significantly (p ≤ 0.05) better growth rate than both C50 and the control, starting from week 2 to week 8. Although C50 yielded a final higher growth rate than the control, this started only after week 5. Similarly, the specific growth rate (SGR) showed that C10 and C20 gave significantly (p ≤ 0.05) higher values than C50 and the control (Figure 1B). The feed conversion ratio (FCR) revealed that C20 resulted in significantly (p ≤ 0.05) improved feed conversion (values closer to 1) compared to the control and an insignificant improvement (p ≤ 0.05) compared to C10 and C50. Only C20 gave a significantly better FCR than the control (Figure 1C). Improved fish utilisation of feed can reduce feed waste, resulting in cost reductions in aquaculture.
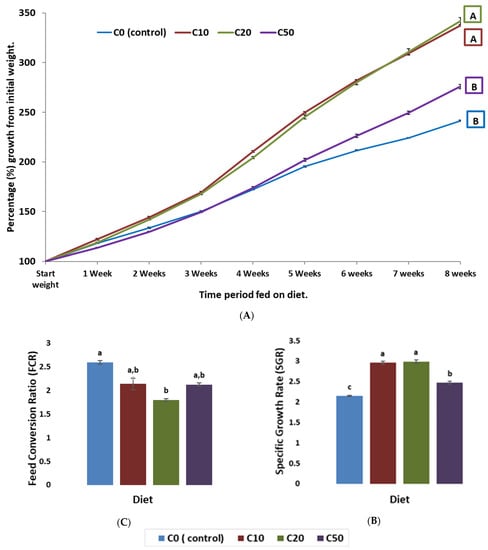
Figure 1.
Growth and performance indicators for zebrafish (wild type) fed experimental diets. (A) Percentage growth from initial weight. (B) Specific growth rate (SGR). (C) Feed conversion ratio (FCR). Within each figure section, diets that do not share a letter are significantly different (p ≤ 0.05).
As shown in Table 1, the amount of wheat gluten was higher in C20 (24.29%) compared to C10 (21.64) and the control (22.91). However, the improved SGR and percentage growth from initial weight cannot be attributed to the increased amount of wheat gluten in C20 because this growth improvement compared to the control was also clearly seen in the C10 diet where wheat gluten was less than the control. Moreover, the wheat gluten amount in C50 is the highest, and yet the growth was less than C10 and C20.
Based on the results in this section, zebrafish can efficiently be fed on a diet with up to a 50% reduction in fishmeal, replaced with C. reinhardtii; however, the reduction levels of 10% and 20% showed the best performance in terms of fish growth. This indicates that C. reinhardtii can be utilised as protein source by zebrafish. Using recombinant proteins as biomarker, Kwon et al. (2019) demonstrated that C. reinhardtii can be digested by zebrafish. The recombinant proteins in the C. reinhardtii chloroplast were dyed with a green fluorescent proteins (GFPs) and fed to the zebrafish. The GFPs were then observed in the intestinal tissues and the blood of zebrafish using a confocal microscope proving that proteins in C.reinhardtii chloroplast can be digested and absorbed by zebrafish [26]
This comes in agreement with the findings of this study; however, this digestibility has a threshold which explains why the zebrafish performed better at the 10% and 20% reduction levels compared to the 50% one.
The threshold of these adverse effects, however, differs greatly depending on many factors including the fish type, algae species, and feed pellet processing conditions. Previous studies about algae inclusion in fish diets have revealed that fish respond differently depending on their species [36,37]. On one hand, the 5% inclusion of Ulva spp. (green algae) in carnivorous fish (European sea bass and rainbow trout) resulted in no effect on growth performance [38]. On the other hand, a higher inclusion level (5–10%) of algae into herbivore and omnivore species (Nile tilapia and common carp) resulted in a significant improvement in growth, nutrient utilisation, and feed efficiency [39,40]. This might be attributed to the higher amylase activity in herbivore and omnivore species which allows for better digestibility of algae cell walls and carbohydrates [41].
Although fish growth performance is a very important indicator of diet viability, it must not compromise the nutritional benefits of the fish for human nutrition, notably as a great source of LC-PUFA [42]. Thus, further analysis was conducted to assess more nutritional parameters of the zebrafish fed different diet formulations.
3.2. The Effect of C. reinhardtii as Fishmeal Replacement on the Protein Content of the Zebrafish
The protein content of the zebrafish samples fed the experimental diets (C10, C20, and C50) compared to the control was calculated by converting the total nitrogen content in the zebrafish carcass into crude protein, using 6.25 as a conversion factor, (Figure 2). The data revealed that the inclusion of C. reinhardtii at levels of C10 and C20 significantly increased the protein content in the zebrafish compared with the control. Fish fed the C50 diet had less protein than both C10 and C20 but still had significantly higher protein than the control. The results indicate the positive impact of C. reinhardtii’s inclusion in the fish’s diets, especially at low and medium fishmeal reduction levels (10% and 20%), on their protein content.
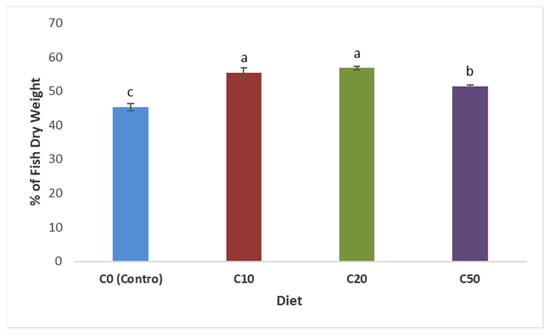
Figure 2.
Protein content (% DW) of zebrafish (wild type) fed different diet formulations (C0, C10, C20, and C50). Diets that do not share a letter are significantly different (p ≤ 0.05).
Although the diets (C0, C10, C20, and C50) were formulated to deliver the same proportion of protein (around 46% DW), C. reinhardtii protein may deliver a more balanced amino acid profile than fishmeal. The replacement of fishmeal protein with C. reinhardtii resulted in significantly (p ≤ 0.05) improved protein content and the growth of fish’s body at C10 and C20 compared to the control. The C. reinhardtii protein assessed in our laboratories showed that it was a high-quality protein containing all of the essential amino acids with a high score, which meets the FAO’s requirements for preschool children [19]. The lower protein content of the carcasses of the zebrafish fed C50 compared to C10 and C20 may be due to the lower digestibility of C. reinhardtii protein by zebrafish at high inclusion levels. It has been reported that fish, generally, cannot digest more than 45–56% protein from algae [10] because of the presence of complex polysaccharides in microalgae, which have an adverse effect on protein hydrolysis [10].
3.3. The Effect of C. reinhardtii as Fishmeal Replacement on the Fatty Acid Profile of the Zebrafish
The fatty acid composition of the zebrafish fed the control and experimental diets is depicted in Table 3. Generally, there were differences in the proportions of most of the fatty acids in the different fish diets as the fatty acid metabolism of fish strongly correlates with the fatty acid quality and quantity of their diet [38,43]. The detailed fatty acid composition of C. reinhardtii used in this study was reported by Darwish et al. (2020) [19]. The fatty acids which were not present in C. reinhardtii, such as C14:0, or found in small amounts compared to the control diet, such as C18:2n (n-6) and C18:1) (n-9), are lower in the fish fed C. reinhardtii compared to the ones fed the control (Table 3). In contrast, some fatty acids, such as C16:4 (n-3), were not present in the fish fed the control but were found in the fish fed diets containing C. reinhardtii. The presence of these fatty acids indicates that zebrafish can efficiently absorb and assimilate fatty acids from C. reinhardtii at different inclusion levels in their diet. The content of α-linolenic acid C18:3 (n-3) was also higher in the fish fed diets containing C. reinhardtii. The amount was significantly higher in the fish fed on C50 than C10 and C20. Both C18:3 and C16:4 are the most abundant fatty acids (67%) of the monogalactosyldiacylglycerol (MGDG) lipids, which are found in the thylakoid membranes of C. reinhardtii chloroplast [44]. This demonstrates that the thylakoid membranes of C. reinhardtii have been efficiently digested and that zebrafish have the ability to digest MGDG and other galactolipids.

Table 3.
Fatty acid (FA) profile of zebrafish (wild type) fed C0, C10, C20, and C50 diets.
The C16:4 acyl group found in C. reinhardtii lipids is also present in the membranes of other terrestrial plants and microalgae but only in trace amounts [45]. C16:4 n-3 can, therefore, be used as an indicative marker of feed uptake into the zebrafish’s bodies (or any other animal model) for diets containing C. reinhardtii.
Similarly, DHA was significantly higher in the zebrafish fed on C10 compared with the control. Overall, the highest levels of total n-3 fatty acids were seen in the fish fed the C50 C. reinhardtii diet. Accordingly, C. reinhardtii contains the essential fatty acids required for LCPUFA biosynthesis by zebrafish, and these fatty acids are bioavailable.
3.4. The Effect of C. reinhardtii as Fishmeal Replacement on the Carotenoid Content in the Zebrafish
The Lutein content of the zebrafish fed the C. reinhardtii-containing diet (C10, C20, and C50) was significantly (p ≤ 0.05) higher than the control (<0.1 mg/kg DW). Zebrafish fed on the C20-containing diet displayed the lutein content of 4.5 mg/kg DW, which is higher, although not significantly, than the zebrafish fed on the C10 and C50 diets (Table 4). Although both β-carotene and lutein are the main carotenoids in C. reinhardtii biomass fed to zebrafish in this study (Table 2), only lutein was detected in the body and eggs of zebrafish fed with experimental diets while β-carotene was not detected.

Table 4.
Lutein and retinol concentrations in the whole body of zebrafish (wild type) and their eggs (mg/kg DW).
Carotenoids are absorbed in the enterocyte brush border inside a fish’s body, transported across the body as low-density lipoprotein cholesterol, and accumulate in a fish’s integuments, gonads, and skin (especially in small fish). Fish differ greatly in their ability to ingest and transform different carotenoids [46]. The Salmonidae family can accumulate astaxanthin in their muscles, giving them a favourable pink colour [47]. Generally, marine and freshwater fish can be divided into three groups in terms of their ability to assimilate and store astaxanthin [48]. The first group can convert zeaxanthin or lutein to astaxanthin, and they can absorb and accumulate astaxanthin from their diet. The second group can convert β-carotene and zeaxanthin to astaxanthin, while the third group cannot convert β-carotene, lutein, or zeaxanthin to astaxanthin so must obtain it directly from their diet and store it in their body as pigment esters [48]. In this study, astaxanthin was not detected either in the zebrafish’s bodies or in their eggs. We assume that zebrafish belong to this third group that cannot convert β-carotene or lutein to astaxanthin; instead, lutein accumulated in both.
Besides their role as colourant agents, carotenoids possess several favorable biological effects in many aquatic species. They can improve growth performance by enhancing fish immunity, fertilisation, and egg survival as well as protecting fish from UV light due to their antioxidant activity [49]. Currently, the use of carotenoids including synthetic astaxanthin in aquafeed represents 6–8% of the total production cost, and they are mostly obtained from synthetic sources with an estimated price of USD 300–USD 3000 per kg [50].
Therefore, the production of carotenoids from natural sources, such as microalgae, and the increase in lutein and β-carotene use in aquafeed are potential alternatives to replace the current practice of using synthetic astaxanthin [46]. Regarding the microalgae carotenoids in the fish-feeding sector, many studies have targeted the use of Haematococcus microalgae species as a rich source of natural astaxanthin and found a positive impact, with it enhancing flesh colour. However, studies suggested that Haematococcus consists of high levels of esterified astaxanthin with poor digestibility, due to its cell wall, which might affect fish growth [49,51]. Within this scenario, C. reinhardtii could act as an important alternative source of natural carotenoids, namely lutein and β-carotene.
3.5. The Effect of C. reinhardtii as Fishmeal Replacement on the Carotenoid Content in the Fish Eggs
The eggs from the zebrafish fed on the diets containing a range of C. reinhardtii concentrations all looked yellow compared with the control diet where the eggs were colourless. The pigment was extracted and analysed and identified as lutein (see Table 4 for the concentration values).
The zebrafish fed on the C20 diet produced eggs with the highest lutein content (45.4 µg/g DW) followed by the eggs from the zebrafish fed on the C10 diet (43.3 µg/g DW), and both were significantly higher than the lutein content in the eggs from the zebrafish fed on the C50 diet (29 µg/g DW) and the control (23 µg/g DW). This observation led us to extend the study to assess the effect of feeding the zebrafish on a diet that included C. reinhardtii on their flesh colour using a transparent mutant of zebrafish (Casper strain).
Carotenoids generally accumulate in the external parts of small fish, particularly in structures called chromophores in the skin, giving them a different colour [52]. The AB wild-type zebrafish pigmentation is produced by three chromophore cells: black melanophores, reflective iridophores, and yellow or orange xanthophores [53]. Casper strain zebrafish only have yellow xanthophores which gives them a transparent appearance and enables the eggs to be seen within the females [54].
Digital images of males and females, as well as their eggs, are presented in Figure 3. The eggs produced by the zebrafish fed on the (C10, C20, and C50) diets had an intense yellow-to-orange colour in contrast with the fish fed on the control diet which had white eggs. Although all fish, male and female, fed on the C. reinhardtii-containing diet displayed an intense yellow colour, the males exhibited a denser colour compared to the females with the yellow colour extended to the males’ tails.
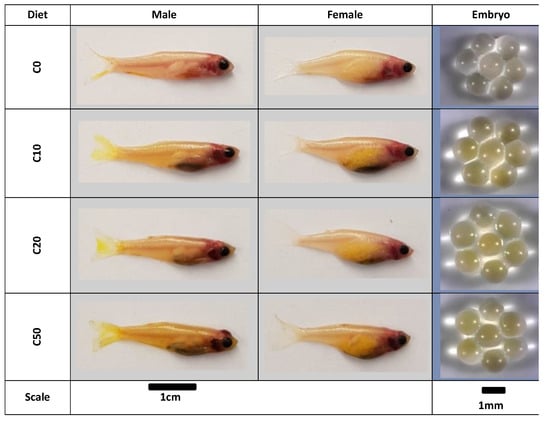
Figure 3.
Zebrafish (Casper strain) egg pigmentation and colour variation due to carotenoid accumulation in response to the C. reinhardtii-containing diets. These images were taken with a Samsung Galaxy S7.
These images show that some pigments were absorbed from the C. reinhardtii biomass, assimilated, and stored in the zebrafish’s bodies for the male and eggs for the females. Bjerkeng (2008) established that the amount of carotenoids, represented by the visual quality that ultimately reaches the target tissues, is a convenient indicator of carotenoids’ bioavailability in the diet [55]. Although adult male and female zebrafish do not have different colour patterns (they are not sexually dimorphic), upon maturity, male zebrafish take on a brighter, more orange colouration compared with females [56]. The colour of xanthophores in both males and females (although more intense in the males) could be attributed to the lutein content [56], which is the case in this study as well.
3.6. The Effect of C. reinhardtii as Fishmeal Replacement on the Retinol Content in the Zebrafish
All trans-retinol (as a form of vitamin A) was measured in the whole body of the zebrafish (but not in the eggs because of the limited sample available) fed on the experimental diets and the control (Table 4). There was significant (p ≤ 0.05) enrichment of trans-retinol in the zebrafish fed on diets including C. reinhardtii, with the highest value of (3.36 µg/g DW) for the C20 diet. This suggests that the provitamin A carotenoids from the C. reinhardtii were converted into retinol in vivo by the zebrafish. Furthermore, β-carotene was not detected in the zebrafish’s bodies nor in their eggs; only lutein was found, demonstrating the conversion of β-carotene (from C. reinhardtii biomass) into retinol.
In mammals, the absorption of β-carotene starts with incorporation into mixed micelles in the intestinal lumen. These then travel into the enterocyte of the small intestine and are absorbed by a passive, non-saturable mechanism. Carotenoids are then packaged in chylomicrons and move, via the lymph, to the liver [57]. It has been agreed that the extent of β-carotene absorption by intestinal enterocytes is species dependent; humans, for example, absorb significant amounts of intact β-carotene compared to rodents [58]. Green et al. (2016) stated that fish generally cannot absorb intact β-carotene into their bloodstream, while xanthophylls are absorbed preferentially, like lutein in this study [58]. Similarly, our findings indicate that zebrafish cannot absorb intact β- carotene. Instead, oxidative cleavage of β-carotene to vitamin A occurs mainly at the brush border membrane of the zebrafish’s intestine, and presumably, the key enzyme that cleaves the chain is a β-carotene-15,15’ oxygenase (BCO) [59]. The gene which encodes the BCO enzyme is found naturally in zebrafish [60]. Furthermore, a study by Lampert et al. (2003) demonstrated that knocking out this gene during embryonic development resulted in vitamin A deficiency symptoms, such as malformation of the eyes, pectoral fin, and craniofacial skeleton [61].
4. Conclusions
Reducing fishmeal by 10%, 20%, and 50% and replacing it with C. reinhardtii (C10, C20, and C50, diets respectively) has achieved promising results. The C20 diet (closely followed by the C10 diet in most of the studied parameters) resulted in the best growth performance, feed conversion ratio, and protein content in the zebrafish’s bodies. This suggests that C. reinhardtii protein contains the essential amino acids that can be efficiently utilised by zebrafish to achieve better growth and better protein content. The fatty acid profile of the zebrafish fed on the C. reinhardtii-containing diet, for the three diets, showed a significant improvement in terms of total n-3 fatty acid content, especially C18:3-n- 3 (ALA) compared to the control. Carotenoid accumulation in the zebrafish was reflected by colour changes in their bodies and eggs, whereby the zebrafish fed C. reinhardtii exhibited a brighter yellow-to-orange colour. This was due to lutein accumulation in the whole body and eggs. β-carotene is another C. reinhardtii intracellular component that proved to be readily bioavailable to zebrafish to convert it to vitamin A by means of enzymatic cleavage believed to be in the zebrafish’s intestines. The findings presented in this study show that many nutrients (proteins, carotenoids, and fatty acids) were released from the C. reinhardtii cells and were, at least in part, absorbed and assimilated by the fish. Currently, as the costs of microalgae production remain higher than that of fishmeal and fish oil, C. reinhardtii can be used as a supplement in omnivores fishby up to 10%. However, as ongoing research and development in microalgae cultivation continue, it is anticipated that economically and sustainably viable microalgae production will become commercially accessible. Consequently, C. reinhardtii could potentially substitute up to 50% of the fishmeal in zebrafish’s diets, with extra, individual addition of some essential amino acids such as lysine and arginine.
As zebrafish are a well-known biological model for other omnivorous fish species, vertebrates, and humans, these results constitute a solid base to propose C. reinhardtii for human supplementation studies, assuming that it is digestible and that its beneficial intracellular components will be released in the gastrointestinal tract of human.
Author Contributions
R.M.D.: conceptualisation, methodology, data curation, writing—original draft, preparation, and visualisation. M.A.G.: visualisation, investigation, methodology, and formal analysis. K.J.M.: visualisation, investigation, methodology, and formal analysis. I.Y.: resources, supervision, conceptualisation, and writing—review and edit. A.F.: writing—review and editing and visualisation, A.S.Z.: writing—review and editing and visualisation. D.A.G.: supervision, funding acquisition, project administration, conceptualisation, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly funded by the University of Nottingham, School of Biosciences and partly funded by Mahan Charitable Trust (reference RL3514).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank our colleagues at the lab who facilitated the use of HPLC and GC-MS (Robert Linforth and Mui Lim). We also thank the team at the University of Liverpool for offering the zebrafish facility and raising the fish, collecting the fish and their eggs, and sending them to us (Kieran Magee and Ian Young). This work was supported by a grant from the University of Nottingham. I also extend my thanks to the Aga Khan Education Board (AKEB), which provided my maintenance funding during the time of this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sarker, P.K.; Kapuscinski, A.R.; Bae, A.Y.; Donaldson, E.; Sitek, A.J.; Fitzgerald, D.S.; Edelson, O.F. Towards sustainable aquafeeds: Evaluating substitution of fishmeal with lipid-extracted microalgal co-product (Nannochloropsis oculata) in diets of juvenile nile tilapia (Oreochromis niloticus). PLoS ONE 2018, 13, e0201315. [Google Scholar] [CrossRef]
- Pradeepkiran, J.A. Aquaculture role in global food security with nutritional value: A review. Transl. Anim. Sci. 2019, 3, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Kok, B.; Malcorps, W.; Tlusty, M.F.; Eltholth, M.M.; Auchterlonie, N.A.; Little, D.C.; Harmsen, R.; Newton, R.W.; Davies, S.J. Fish as feed: Using economic allocation to quantify the fish in: Fish out ratio of major fed aquaculture species. Aquaculture 2020, 528, 735474. [Google Scholar] [CrossRef]
- Hua, K.; Cobcroft, J.M.; Cole, A.; Condon, K.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vucko, M.J.; Zeng, C.; Zenger, K.; et al. The future of aquatic protein: Implications for protein sources in aquaculture diets. One Earth 2019, 1, 316–329. [Google Scholar] [CrossRef]
- Cashion, T.; Le Manach, F.; Zeller, D.; Pauly, D. Most fish destined for fishmeal production are food-grade fish. Fish Fish. 2017, 18, 837–844. [Google Scholar] [CrossRef]
- OECD/FAO. OECD-FAO Agricultural Outlook 2021–2030; OECD Publishing: Paris, France, 2021. [Google Scholar] [CrossRef]
- Black, K.; Hughes, A. Future of the Sea: Trends in Aquaculture; Government Office for Science: London, UK, 2017. Available online: https://www.gov.uk/government/publications/future-of-the-sea-trends-in-aquaculture (accessed on 6 July 2023).
- Duarte, C.M.; Marbá, N.; Holmer, M. Rapid domestication of marine species. Sci.-N. Y. Then Wash. 2007, 316, 382. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, U.K.; Dubey, V.K.; Jena, J.K. Retracted article: Freshwater fish biodiversity of India: Pattern, utilization, importance, threats and challenges. Rev. Fish Biol. Fish. 2013, 23, 555. [Google Scholar] [CrossRef]
- Norambuena, F.; Hermon, K.; Skrzypczyk, V.; Emery, J.A.; Sharon, Y.; Beard, A.; Turchini, G.M. Algae in fish feed: Performances and fatty acid metabolism in juvenile atlantic salmon. PLoS ONE 2015, 10, e0124042. [Google Scholar] [CrossRef]
- Turchini, G.M.; Torstensen, B.E.; Ng, W.K. Fish oil replacement in finfish nutrition. Rev. Aquac. 2009, 1, 10–57. [Google Scholar] [CrossRef]
- Wang, Y.; Tibbetts, S.M.; McGinn, P.J. Microalgae as sources of high-quality protein for human food and protein supplements. Foods 2021, 10, 3002. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef]
- Soler-Vila, A.; Coughlan, S.; Guiry, M.D.; Kraan, S. The red alga Porphyra dioica as a fish-feed ingredient for rainbow trout (Oncorhynchus mykiss): Effects on growth, feed efficiency, and carcass composition. J. Appl. Phycol. 2009, 21, 617–624. [Google Scholar] [CrossRef]
- Oliveira, M.N.D.; Freitas, A.L.P.; Carvalho, A.F.U.; Sampaio, T.M.T.; Farias, D.F.; Teixeira, D.I.A.; Gouveia, S.T.; Pereira, J.G.; Sena, M.M.D.C.C.D. Nutritive and non-nutritive attributes of washed-up seaweeds from the coast of Ceará, Brazil. Food Chem. 2009, 115, 254–259. [Google Scholar] [CrossRef]
- Ansari, F.A.; Guldhe, A.; Gupta, S.K.; Rawat, I.; Bux, F. Improving the feasibility of aquaculture feed by using microalgae. Environ. Sci. Pollut. Res. 2021, 28, 43234–43257. [Google Scholar] [CrossRef] [PubMed]
- Scranton, M.A.; Ostrand, J.T.; Fields, F.J.; Mayfield, S.P. Chlamydomonas as a model for biofuels and bio-products production. Plant J. 2015, 82, 523–531. [Google Scholar] [CrossRef]
- Darwish, R.; Gedi, M.A.; Akepach, P.; Assaye, H.; Zaky, A.S.; Gray, D.A. Chlamydomonas reinhardtii is a potential food supplement with the capacity to outperform chlorella and spirulina. Appl. Sci. 2020, 10, 6736. [Google Scholar] [CrossRef]
- Wang, X. Green Algae as a Platform for Protein Production: Food, Feed, and Nutritional Supplements; Triton Algae Innovations: Montreal, QC, Canada, 2017. [Google Scholar]
- Spence, R.; Gerlach, G.; Lawrence, C.; Smith, C. The behaviour and ecology of the zebrafish, Danio rerio. Biol. Rev. Camb. Philos. Soc. 2008, 83, 13–34. [Google Scholar] [CrossRef]
- Bailone, R.L.; Fukushima, H.C.S.; Fernandes, B.H.V.; De Aguiar, L.K.; Corrêa, T.; Janke, H.; Setti, P.G.; Roça, R.D.O.; Borra, R.C. Zebrafish as an alternative animal model in human and animal vaccination research. Lab. Anim. Res. 2020, 36, 13. [Google Scholar] [CrossRef]
- Carneiro, W.F.; Castro, T.F.D.; Orlando, T.M.; Meurer, F.; Paula, D.A.D.J.; Virote, B.D.C.R.; Vianna, A.R.D.C.B.; Murgas, L.D.S. Replacing fish meal by Chlorella sp. Meal: Effects on zebrafish growth, reproductive performance, biochemical parameters and digestive enzymes. Aquaculture 2020, 528, 735612. [Google Scholar] [CrossRef]
- M Kent, L.; Harper, C.; Wolf, J.C. Documented and potential research impacts of subclinical diseases in zebrafish. ILAR J. 2012, 53, 126–134. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Maddison, L.A.; Chen, W. Zebrafish as a model for obesity and diabetes. Front. Cell Dev. Biol. 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.-C.; Lamb, A.; Fox, D.; Jegathese, S.J.P. An evaluation of microalgae as a recombinant protein oral delivery platform for fish using green fluorescent protein (gfp). Fish Shellfish Immunol. 2019, 87, 414–420. [Google Scholar] [CrossRef]
- Gedi, M.A.; Magee, K.J.; Darwish, R.; Eakpetch, P.; Young, I.; Gray, D.A. Impact of the partial replacement of fish meal with a chloroplast rich fraction on the growth and selected nutrient profile of zebrafish (Danio rerio). Food Funct. 2019, 10, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Watts, S.A.; Powell, M.; D’Abramo, L.R. Fundamental approaches to the study of zebrafish nutrition. Ilar J. 2012, 53, 144–160. [Google Scholar] [CrossRef]
- Kaushik, S.; Georga, I.; Koumoundouros, G. Growth and body composition of zebrafish (Danio rerio) larvae fed a compound feed from first feeding onward: Toward implications on nutrient requirements. Zebrafish 2011, 8, 87–95. [Google Scholar] [CrossRef]
- Boyd, C.E.; Tucker, C.; McNevin, A.; Bostick, K.; Clay, J. Indicators of resource use efficiency and environmental performance in fish and crustacean aquaculture. Rev. Fish. Sci. 2007, 15, 327–360. [Google Scholar] [CrossRef]
- Jackson, A. Fish in—Fish out ratios explained. Aquac. Eur. 2009, 34, 5–10. [Google Scholar]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Korkmaz, A.S.; Cakirogullari, G.C. Effects of partial replacement of fish meal by dried baker’s yeast (Saccharomyces cerevisiae) on growth performance, feed utilization and digestibility in koi carp (Cyprinus carpio L., 1758) fingerlings. J. Anim. Vet. Adv. 2011, 10, 346–351. [Google Scholar] [CrossRef]
- Li, H.; Tyndale, S.T.; Heath, D.D.; Letcher, R.J. Determination of carotenoids and all-trans-retinol in fish eggs by liquid chromatography–electrospray ionization–tandem mass spectrometry. J. Chromatogr. B 2005, 816, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Rodriguez-Amaya, D.B. A scheme for obtaining standards and HPLC quantification of leafy vegetable carotenoids. Food Chem. 2002, 78, 389–398. [Google Scholar] [CrossRef]
- Ahmad, A.; Hassan, S.W.; Banat, F. An overview of microalgae biomass as a sustainable aquaculture feed ingredient: Food security and circular economy. Bioengineered 2022, 13, 9521–9547. [Google Scholar] [CrossRef] [PubMed]
- Valente, L.M.P.; Gouveia, A.; Rema, P.; Matos, J.; Gomes, E.F.; Pinto, I.S. Evaluation of three seaweeds Gracilaria bursa-pastoris, Ulva rigida and Gracilaria cornea as dietary ingredients in european sea bass (Dicentrarchus labrax) juveniles. Aquaculture 2006, 252, 85–91. [Google Scholar] [CrossRef]
- Guroy, D.; Guroy, B.; Merrifield, D.L.; Ergun, S.; Tekinay, A.A.; Yigit, M. Effect of dietary Ulva and Spirulina on weight loss and body composition of rainbow trout, Oncorhynchus mykiss (walbaum), during a starvation period. J. Anim. Physiol. Anim. Nutr. 2011, 95, 320–327. [Google Scholar] [CrossRef]
- Diler, I.; Tekinay, A.A.; Guroy, D.; Guroy, B.K.; Soyuturk, M. Effects of Ulva rigida on the growth, feed intake and body composition of common carp, Cyprinus carpio L. J. Biol. Sci. 2007, 7, 305–308. [Google Scholar] [CrossRef]
- Wassef, E.A.; El-Sayed, A.F.M.; Kandeel, K.M.; Sakr, E.M. Evaluation of pterocladia (Rhodophyta) and Ulva (Chlorophyta) meals as additives to gilthead seabream Sparus aurata diets. Egypt. J. Aquat. Res. 2005, 31, 321–332. [Google Scholar]
- Montgomery, W.L.; Gerking, S.D. Marine macroalgae as foods for fishes: An evaluation of potential food quality. Environ. Biol. Fishes 1980, 5, 143–153. [Google Scholar] [CrossRef]
- Tocher, D.R. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac. Res. 2010, 41, 717–732. [Google Scholar] [CrossRef]
- Watanabe, T.; Takeuchi, T.; Saito, M.; Nishimura, K. Effect of low protein-high calory or essential fatty acid deficiency diet on reproduction of rainbow trout [Salmo gairdnerii]. Bull. Jpn. Soc. Sci. Fish. 1984, 50, 1207–1215. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Cuiné, S.; Beyly-Adriano, A.; Légeret, B.; Billon, E.; Auroy, P.; Beisson, F.; Peltier, G.; Li-Beisson, Y. The green microalga Chlamydomonas reinhardtii has a single ω-3 fatty acid desaturase that localizes to the chloroplast and impacts both plastidic and extraplastidic membrane lipids. Plant Physiol. 2013, 163, 914–928. [Google Scholar] [CrossRef] [PubMed]
- Zäuner, S.; Jochum, W.; Bigorowski, T.; Benning, C. A cytochrome b(5)-containing plastid-located fatty acid desaturase from Chlamydomonas reinhardtii. Eukaryot. Cell 2012, 11, 856–863. [Google Scholar] [CrossRef]
- Yuangsoi, B.; Jintasataporn, O.; Areechon, N.; Tabthipwon, P. The pigmenting effect of different carotenoids on fancy carp (Cyprinus carpio). Aquac. Nutr. 2011, 17, e306–e316. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R.; Caramujo, M.J. Carotenoids in aquatic ecosystems and aquaculture: A colorful business with implications for human health. Front. Mar. Sci. 2017, 4, 93. [Google Scholar] [CrossRef]
- Stewart, G.F.; Schweigert, B.S.; Hawthorn, J.; Bauernfeind, J.C. Carotenoids as colorants and vitamin A precursors. In Technological and Nutritional Applications; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Sommer, T.R.; Potts, W.T.; Morrissy, N.M. Utilization of microalgal astaxanthin by rainbow trout (Oncorhynchus mykiss). Aquaculture 1991, 94, 79–88. [Google Scholar] [CrossRef]
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Bioaccessibility of marine carotenoids. Mar. Drugs 2018, 16, 397. [Google Scholar] [CrossRef]
- Lagocki, S. Evaluation of Haematococcus pluvialis as a Natural Dietary Source of the Carotenoid Astaxanthin for Rainbow Trout Flesh Pigmentation; University of Plymouth: Plymouth, UK, 2001. [Google Scholar]
- Goodwin, T.W. The Comparative Biochemistry of the Carotenoids; Chapman and Hall Ltd.: London, UK, 1952. [Google Scholar]
- Rawls, J.F.; Mellgren, E.M.; Johnson, S.L. How the zebrafish gets its stripes. Dev. Biol. 2001, 240, 301–314. [Google Scholar] [CrossRef]
- White, R.M.; Sessa, A.; Burke, C.; Bowman, T.; LeBlanc, J.; Ceol, C.; Bourque, C.; Dovey, M.; Goessling, W.; Burns, C.E.; et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2008, 2, 183–189. [Google Scholar] [CrossRef]
- Bjerkeng, B. Carotenoids in aquaculture: Fish and crustaceans. In Carotenoids: Volume 4: Natural Functions; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Basel: Basel, Switzerland, 2008; pp. 237–254. [Google Scholar]
- Nüsslein-Volhard, C.; Singh, A.P. How fish color their skin: A paradigm for development and evolution of adult patterns. BioEssays 2017, 39, 1600231. [Google Scholar] [CrossRef]
- Reboul, E. Absorption of vitamin A and carotenoids by the enterocyte: Focus on transport proteins. Nutrients 2013, 5, 3563–3581. [Google Scholar] [CrossRef]
- Green, A.S.; Fascetti, A.J. Meeting the vitamin A requirement: The efficacy and importance of β-carotene in animal species. Sci. World J. 2016, 2016, 7393620. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, T.W. Metabolism, nutrition, and function of carotenoids. Annu. Rev. Nutr. 1986, 6, 273–297. [Google Scholar] [CrossRef] [PubMed]
- von Lintig, J.; Vogt, K. Vitamin a formation in animals: Molecular identification and functional characterization of carotene cleaving enzymes. J. Nutr. 2004, 134, 251s–256s. [Google Scholar] [CrossRef] [PubMed]
- Lampert, J.M.; Holzschuh, J.; Hessel, S.; Driever, W.; Vogt, K.; von Lintig, J. Provitamin a conversion to retinal via theβ, β-carotene-15, 15′-oxygenase (bcox) is essential for pattern formation and differentiation during zebrafish embryogenesis. Development 2003, 130, 2173–2186. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).