Abstract
Ferulic acid esterases belong to the category of carboxylesterases and possess the capability to enzymatically break down hemicellulose within lignocellulosic substances, thereby liberating ferulic acid. A ferulic acid esterase from Lentinula edodes (LeFae) was expressed using Pichia pastoris, and its characterization and effects on the in vitro fermentation of wheat straw were investigated in this study. The optimal pH and temperature for LeFae were pH 7.0 and 60 °C, respectively. LeFae exhibited a broad temperature and pH adaptability (>60% of the maximum activity at pH 4.0–7.0 and 40–70 °C) and excellent thermal stability. The activity of LeFae was increased by 30.3% with a dosage of Tween 20 at 0.25% (v/v) and exhibited satisfactory resistance to Mn2+ and sodium dodecyl sulfate. LeFae released ferulic acid from wheat straw and exhibited an obvious synergistic effect with cellulase during wheat straw hydrolysis. LeFae severely inhibited the microbial fermentation of wheat straw and reduced the in vitro dry matter digestibility, total volatile fatty acid yield, and 16S rDNA copy numbers of Ruminococcus flavefaciens by 9.6%, 9.9 mM, and 40.1%, respectively. It also increased pH and the concentration of soluble phenols during wheat straw fermentation. Pretreating wheat straw with LeFae did not affect the microbial fermentation of wheat straw but resulted in the leaching of more dissolving sugars. The current results showed that although LeFae can cooperate with cellulase to promote the hydrolysis of wheat straw, its adverse effect on rumen microorganisms when directly fed to ruminants is a problem worthy of consideration.
1. Introduction
As an agricultural by-product, wheat straw has been widely used in animal feed production, biomass conversion, papermaking, and biosynthesis due to its rich biomass content [1]. Wheat straw can be degraded by microorganisms in the rumen into volatile fatty acids such as acetic acid, propionic acid, and butyric acid, providing energy for ruminants [2]. However, despite being a primary source of roughage for ruminants, its ruminal degradation efficiency has not yet attained the desired outcome, typically remaining below 50% [2,3]. This is mainly due to its intricate and stable lignocellulose structure resulting from the complex covalent bonds among cellulose crystals, hemicellulose, and lignin [4]. The hemicellulose of wheat straw contains abundant ferulic acid, which accounts for more than 65% of the total phenolic acid in the plant and about 0.5% of the cell wall [5]. Ferulic acid, in the form of a monomer or dimer, establishes close linkages between arabinoxylan and lignin through ester and ether bonds, respectively. This results in the formation of complexes, such as the “arabinoxylan-ester-ferulic acid-ether-lignin bridge”, which hinders the access of fibrolytic enzymes secreted by rumen microorganisms to the intended location and inhibits the degradation and utilization of fibers [4,6,7].
As an important component of the hemicellulose-degrading enzyme system, ferulic acid esterases (FAEs) are a subclass of carboxylic acid esterases, which can break the ester bonds in ferulic ester, oligosaccharide ferulic ester, polysaccharide ferulic ester, and eventually release ferulic acid [8]. The degradation of branched chains enhances the accessibility of the main chain in heteropolysaccharides to a diverse array of cellulolytic enzymes [7,8], which makes FAEs important for the degradation of hemicellulose. Previous studies proved that the combination of FAEs and other cellulolytic enzymes had better effects on degrading lignocellulose substrates. A feruloyl esterase-1 from termite hindgut bacteria synergistically acted with GH10 xylanases to promote the degradation of arabinoxylans, and the degree of synergy between them was higher than 1 [9]. In a study by Record et al. [10], the delignification of wheat straw was 38% when an Aspergillus niger feruloyl esterase was used alone, but a combined xylanase/feruloyl esterase pretreatment, followed by the laccase treatment, enhanced delignification to 55%.
Although FAEs have been found in various plant-cell-wall-degrading microorganisms, fungi are still the main sources of FAEs used in industry [7]. According to Dilokpimol et al. [7], FAEs from fungi were classified into 13 subfamilies using a novel phylogenetic tree. Fungal FAEs from different subfamilies differ strongly in physical and biochemical properties [7]. Lentinus edodes, as a white rot fungus, can decompose lignocellulose with its enzymatic machinery and improve the nutritional value of low-quality feed including agricultural straws, and consequently, it is a good source of the fibrinolytic enzyme gene pool [11]. A gene coding a ferulic acid esterase (LeFae) from L. edodes was identified by Sakamoto et al. [12] using genome sequencing technology and then deposited into EMBL (accession number GAW07464.1), but there is still little information available on the enzyme. In this study, we assumed that LeFae could hydrolyze the ferulate ester bonds in the cell wall structure, enhance hemicellulose degradation, and improve the rumen fermentation of wheat straw. Therefore, the LeFae was heterologously expressed using Pichia pastoris, and its characteristics and effects on the degradation and in vitro fermentation of wheat straw were investigated in this study. The obtained results provide empirical support and a theoretical basis for the application of LeFae in ruminant production.
2. Materials and Methods
2.1. Analysis of Sequence Features
The amino acid sequence of LeFae from L. edodes (EMBL accession number GAW07464.1) was aligned online by EMBL’s European Bioinformatics Institute (EMBL-EBI) services using the UniProtKB/Swiss-Prot database. Twenty similar sequences in the results were selected to construct the phylogenetic tree for LeFae using Molecular Evolutionary Genetics Analysis (MEGA) 11 software. Multiple alignments of LeFae and several FAEs from Aspergillus oryzae (UniProtKB number Q2UMX6), A. flavus (UniProtKB number B8NPA4), and A. terreus (UniProtKB number Q0CI21) were carried out using ClustalW. The homologous modeling of LeFae was completed with the program Discovery Studio 2016 using A. oryzae esterase (PDB number 6G21) as the template.
2.2. Construction of the Recombinant Strain
The coding sequence of the LeFae gene was optimized according to the codon preference in P. pastoris and then synthesized and cloned into a pPICZαA vector using GenScript, and the result was defined as pPICZαA-LeFae. The pPICZαA-LeFae plasmid was transformed into Escherichia coli DH5α using heat shock and then extracted.
The recombinant plasmid pPICZαA-LeFae was linearized with Sac I endonuclease and electrically transformed into P. pastoris X33 competent cells. Preliminary screening of recombinant strains was performed on a YPD solid medium containing Zeocin and then verified with PCR amplification using specific primers: forward prime (ATGGAAGCTTGGTTGCCTCAAAAC) and reverse prime (TCTTTGTGGAGTCCAAGCAG). Finally, gene sequencing of the positive clones was carried out. The screened recombinant strains were grown in buffered glycerol-complex medium (BMGY) on a small scale and then centrifuged and transferred into buffered methanol-complex medium (BMMY) to induce the expression of LeFae, according to our previous report [11]. The LeFae activity was measured using 1% starch-free wheat bran as substrate at pH 5.0 and 50 °C for 1 h [13]. The ferulic acid released was analyzed using spectrophotometry at 310 nm [14]. The active strains were further verified using a Western blot with the antibody against His-tag (66005-1-Ig, Proteintech Group, Inc., Chicago, IL, USA).
2.3. Laboratory-Scale Production of LeFae
The laboratory-scale production of LeFae was performed using a 6 L bench-top bioreactor (Minifors 2, Infors HT, Bottmingen, Switzerland). Shake flask culture was carried out first by inoculating the LeFae strain into 200 mL BMGY media in a 1 L shake flask. Then, the overnight-cultured strains were transferred into the 2 L medium of the bioreactor for high-density culture. The detailed fermentation process followed that detailed in our previous report [15]. The fermentation process was terminated when the yeast biomass did not increase further. The supernatant of the yeast culture was obtained using centrifugation. The LeFae in the supernatant was concentrated and purified with Ni-charged affinity chromatography [15]. The content and purity of LeFae obtained were analyzed using a Bradford Protein Assay Kit (Sangon Biotech, Shanghai, China) and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
2.4. Characterization of LeFae
The temperature dependency of LeFae was analyzed using 0.1 M sodium citrate buffer (pH 5.0) and 1% starch-free wheat bran at 20–90 °C for 1 h, and the LeFae activity was assessed based on the preceding delineation. Similarly, the effect of pH on LeFae activity was determined using 0.1 M sodium citrate buffer (pH 3.0–7.0) or 0.1 M sodium phosphate buffer (pH 7.0–9.0) at 60 °C. The activity obtained was expressed as relative activity, with the maximum activity set at 100%. Thermal stability was determined after pretreating LeFae at 30–90 °C for 1 h, respectively, and then residual activity was analyzed under optimal conditions.
The resistance of LeFae to some metal ions, inhibitors, detergents, and organic solvents was analyzed after adding these additives into the reaction mixture. The reaction was performed at optimal conditions. The activity of the enzyme without any additives was considered as 100%.
2.5. Hydrolysis of Wheat Straw Using LeFae
The hydrolysis of wheat straw using LeFae was performed by incubating 10 mg wheat straw and 25.0–200.0 μg LeFae in sodium citrate buffer (pH 7.0) at 40 °C for 1 h. The ferulic acid released was measured using an HPLC system (model D-7000, Hitachi Ltd., Tokyo, Japan).
2.6. Synergism of LeFae on the Enzymatic Hydrolysis of Wheat Straw
To investigate the synergism of LeFae and cellulolytic enzymes, a reaction mixture containing 10 mg wheat straw and 50 µg LeFae/inactive LeFae (control), was incubated at pH 7.0 and 40 °C for 1 h, and then the liquid portion was discarded. The solid residues were washed twice with distilled water. The residues were re-suspended in 1.0 mL 0.1 M sodium citrate buffer (pH 5.0) containing 0.2 mg cellulase cocktail (C8272, Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) and incubated at 40 °C for 1 h. The reducing sugars released were measured using a 3, 5-dinitrosalicylic acid (DNS) assay.
2.7. In Vitro Fermentation of Wheat Straw
An in vitro fermentation study was performed to investigate the effect of LeFae on the microbial fermentation of wheat straw, according to the reports by Hughes et al. [16], using microbial inocula from bovine feces. Briefly, fresh feces were obtained from healthy beef cattle fed a diet consisting of 700 g/kg rice straw and 300 g/kg concentrates. Four hundred and fifty grams of feces were diluted into 1 L of buffered McDougal’s artificial saliva. The mixture was stirred repeatedly with a glass rod at 39 °C for about 25 min and then filtered through two layers of cheesecloth. Fermentation was carried out in a 120 mL serum bottle containing 200 mg wheat straw and 20 mL fecal inoculum. One milligram of purified LeFae was supplemented into the fermentation bottle as an enzyme treatment, and the same amount of inactivated LeFae was incubated similar to the control. The bottles were incubated in a shaking bath at 39 °C for 48 h in triplicate. The blank only containing fecal inoculum was synchronously incubated to correct the residual dry matter (DM) in the samples. The bottles were placed on ice to terminate the fermentation, and the fermentation mixture was filtered with pre-weighed nylon bags (37 μm aperture) to determine the residual DM weight and in vitro dry matter digestibility (IVDMD) of wheat straw. IVDMD was calculated as the DM that disappeared from the initial weight inserted into the bottle. The filtrate was used for the analysis of pH, volatile fatty acids (VFAs), ammonia-N, total soluble phenols, and 16S rDNA copy numbers of total bacteria and three fibrolytic bacterial species including Ruminococcus flavefaciens, Fibrobacter succinogenes, and Ruminococcus albus.
2.8. In Vitro Fermentation of Wheat Straw Pretreated with LeFae
An experiment was performed to investigate the in vitro fermentation of wheat straw pretreated with LeFae. A reaction mixture (150 mL) containing 1.5 g of milled wheat straw (<200 mesh) and 7.5 mg LeFae/inactive LeFae (control) was incubated at pH 7.0 and 40 °C for 24 h. The liquid portion and the solid residues were separated using filtration with a nylon mesh cloth (37 μm aperture). The liquid samples were used for the analysis of leached dissolving sugars. The solid residues were washed thrice with distilled water and then dried in an oven at 65 °C for 72 h. The dried solid residues were used as the substrates for the in vitro microbial fermentation according to the aforementioned descriptions.
2.9. Analytical Procedures
The dry matter in the samples was determined at 65 °C for 72 h. The VFA concentrations in the filtered samples were determined using an HPLC system consisting of a L7100 pump and a UV-Vis L7400 detector, according to Zhao et al. [17], with crotonic acid as an internal standard. Microbial protein in the fermentation liquid (FLMCP) was measured according to a previous report [18]. Total soluble phenols were determined using the Folin–Ciocalteau method [19] with a modification that the reaction solutions were incubated at room temperature in the dark for 2 h. Then, the 16S rDNA copy numbers of the microorganisms selected were determined according to our previous report [11]. Ferulic acid was determined using HPLC with a Hypersil C18 column (4.6 × 150 mm, 10 μm). The flow rate was set to 1 mL/min, and the wavelength for UV detection was 310 nm. The mobile phase was a mixture of water, methanol, and glacial acetic acid in the ratio of 74.8:25:0.2 (v/v). Leached dissolving sugars in the filtered samples were determined using the phenol–sulfuric acid method [20].
2.10. Statistical Analyses
Statistical analyses were carried out using IBM SPSS statistics version 20 (IBM, Chicago, IL, USA). When conducting comparisons among three or more groups, a one-way analysis of variance (ANOVA) was used. A least significant difference (LSD) test was used to conduct multiple comparisons, while an independent samples t-test was utilized for comparisons between two groups. Significance was considered when p ≤ 0.05, and trends were discussed when p ≤ 0.10.
3. Results and Discussion
3.1. Analysis and Production of LeFae
Though the LeFae gene was deposited in the EMBL database (accession number GAW07464.1) by Sakamoto and his coworkers, the sequence analysis was not presented in the previous publication [12]. The predicted theoretical molecular weight and isoelectric point for LeFae were 28 kDa and pH 4.56, respectively, as identified using the ProtParam tool of ExPASy accessed on 18 December 2021 (https://web.expasy.org/protparam/). The phylogenetic tree showed that LeFae exhibited a relatively high genetic relationship with a feruloyl esterase from A. flavus (EMBL-EBI number B8NPT0; Figure 1A). According to Dilokpimol et al. (2016), LeFae may belong to the fungal FAEs 3 subfamily [7]. A multi-alignment between LeFae and several FAEs from A. oryzae (UniProtKB number Q2UMX6, AoFae), A. flavus (UniProtKB number B8NPA4, AfFae), and A. terreuswas (UniProtKB number Q0CI21, AtFae) was carried out. As shown in Figure 1C, LeFae had a high similarity (>60%) to these sequences. The strictly conserved residue, S98 (marked with a pentacle), observed at the transition position between the β-sheet and α-helix in the predicted 3D structure (Figure 1B), proved to be the catalytic site in a previous study [21]. LeFae was heterologously expressed in P. pastoris. Two protein bands at about 62 and 68 kDa were detected using SDS-PAGE and further identified using a Western blot (Figure 2). The two observed molecular weights of LeFae were greater than its theoretical molecular weight (28 kDa), which may be due to the following reasons. First, the LeFae protein has the possibility of being glycosylated because five potential N-glycosylation sites were detected in the LeFae sequence using NetNGlyc-1.0 accessed on 19 December 2021 (https://services.healthtech.dtu.dk/service.php?NetNGlyc-1.0). Second, the LeFae protein possibly existed in the dimer form, which was not untraceable. Dimer proteins consist of two subunits, which may be either identical or different. It is likely that dimer proteins composed of identical subunits demonstrate increased stability in comparison to monomer proteins. Several FAEs with similar structures to LeFae, such as F. oxysporum FAE (PDB number 6FAT) and A. oryzae FAE (PDB number 3WMT), appeared in dimer form during crystal resolution [21,22], while SDS in the SDS-PAGE cannot completely convert the multimers of proteins into monomers [23]. In any case, however, further research is needed to determine the real reason for the current result, although a similar phenomenon was also observed in our previous study [11,15].
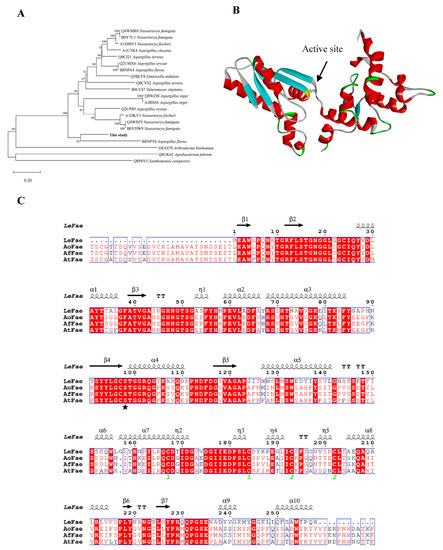
Figure 1.
Phylogenetic tree (A), homology model (B), and multialignment analysis (C) of LeFae. (A) The phylogenetic tree for LeFae was analyzed using the neighbor-joining (NJ) method. Putative catalytic residues (pentacle).
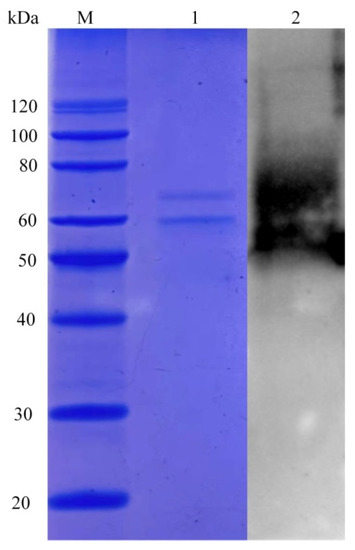
Figure 2.
Analysis of recombinant LeFae using SDS-PAGE and Western blot. M, protein marker; 1, purified LeFae; 2, Western blot analysis of LeFae.
3.2. Characterization of LeFae
The maximum activity of LeFae was observed at 60 °C. When the temperature was between 40 °C and 80 °C, the enzyme could still maintain more than 50% of its maximum activity (Figure 3A). LeFae exhibited its maximum activity at pH 7.0 and could maintain more than 58% of the maximum activity between pH 4.0 and 8.0 (Figure 3B). The temperature stability results showed that after being pretreated at 40–70 °C for 1 h, LeFae could still retain more than 85% of its original activity (Figure 3A). These results indicate that LeFae has a broad temperature and pH range and excellent temperature stability. Similar to the current result, an FAE from A. aculeatus showed its maximum activity at pH 7.0 and 60 °C [24].
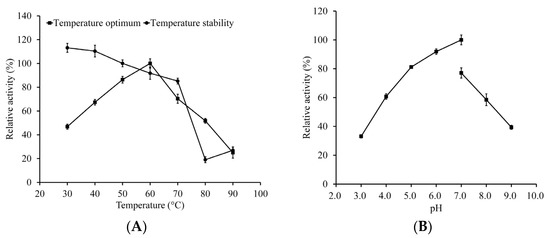
Figure 3.
Analysis of dependence on temperature (A) and pH (B) and the temperature stability (A) of LeFae. Activity was obtained by determining pH and temperature dependence and is expressed as relative activity, where the highest activity is 100%. For temperature stability, activity for untreated LeFae was defined as 100%. Each value represents the mean ± SD.
Except for Mn2+, other metal ions more or less inhibited the activity of LeFae (Figure 4A). The inhibitory effect of Zn2+, Cu2+, and Ni2+ on the activity of LeFae was observed to be substantial. The three ions inhibited LeFae activity by 43–48% at the concentration of 1 mM, but as the added concentration increased to 5 mM, more than 60% of the activity of LeFae was inhibited, and especially, Cu2+ almost completely inactivated the enzyme. The influence of metal ions on FAEs is related to the source of FAEs. In a study by Kanauchi et al. [25], the presence of 1 mM of Zn2+, Cu2+, and Ni2+ had a minimal inhibitory impact on the activity of FAE from A. awamori, as evidenced by maintaining 100%, 80%, and 95% of the original activity, respectively. However, the activity of FEA from A. aculeatus was reduced by about 40% and 60% with 5 mM of Zn2+ and Cu2+, respectively [24]. Donaghy and McKay [26] observed that 5 mM of Cu2+ repressed 95% of the initial activity of FAE from Penicillium expansum. The current results were partially consistent with the preceding research. Metal ions may inhibit enzyme reactions by combining with the active group of the enzymes, causing oxidative stress and damage to proteins, or by reacting with the enzyme–substrate complex [27].
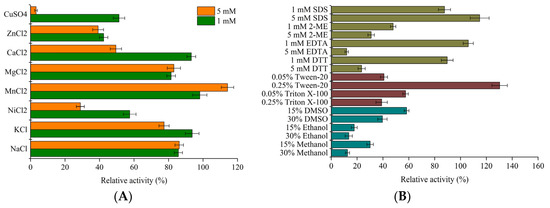
Figure 4.
Effects of metal ions (A), inhibitors, detergents, and organic solvents (B) on LeFae activity. Activity without additives was considered as 100%. Each value represents the mean ± SD.
The resistance of LeFae to some inhibitors, detergents, and organic solvents was investigated in the current study (Figure 4B). Regardless of the concentration, Triton-X100 and 2-mercaptoethanol both significantly inhibited the activity of LeFae. At a concentration of 1 mM, dithiothreitol and EDTA did not exert a significant inhibitory influence on the activity of LeFae. However, at a concentration of 5 mM, these substances demonstrated substantial inhibitory effects on LeFae. These results were partially consistent with the FAE from Lactobacillus plantarum [28], but the latter was not significantly inhibited with Triton X-100. The inhibitory effect of dithiothreitol and 2-mercaptoethanol may be related to the disulfide bonds in LeFae. Three potential disulfide bonds (Cys24-Cys184, Cys97-Cys167, and Cys193-Cys202) were predicted using an online website (https://predictprotein.org/ accessed on 18 December 2021). Disulfide bonds play an important role in the three-dimensional structure and catalytic activity of enzyme proteins, and a reducing agent such as dithiothreitol or 2-mercaptoethanol could disrepute disulfide bonds, resulting in an enzymatic conformational change and activity loss [29]. LeFae resisted a certain concentration of SDS, which was similar to the results for A. awamori [25]. The impact of Tween-20 on LeFae appeared to exhibit concentration-dependent behavior. Specifically, at a concentration of 0.05%, Tween-20 demonstrated significant repression of LeFae activity, resulting in a 60% decrease. Conversely, at a concentration of 0.25%, Tween-20 exhibited a notable increase in LeFae activity, leading to a 30% rise. Similarly, the enhancement of FAEs using Tween-20 has been observed in many reports [28,30]. However, we did not find the reason why low concentration detergents inhibited the enzyme and high concentration detergents promoted the enzyme, though this phenomenon was also observed in other studies [31]. The organic solvents used in the present study all significantly inhibited the activity of LeFae, regardless of the concentration.
3.3. Hydrolysis of Wheat Straw Using LeFae
In the current investigation, LeFae demonstrated an ability to liberate ferulic acid from wheat straw. The maximum ferulic acid yield was observed at a LeFae loading of 5 µg/mg substrate, but further increasing LeFae concentration reduced ferulic acid yield (Figure 5A). Similarly, ferulic acid release from wheat straw using other FAEs has also been observed in many studies [32]. The mechanism underlying the observed behavior that ferulic acid yield decreases at high LeFae loading in enzymatic hydrolysis of wheat straw is still unknown. It might be due to the lower adsorption efficiency for higher enzyme loading than for diluted ones [33].
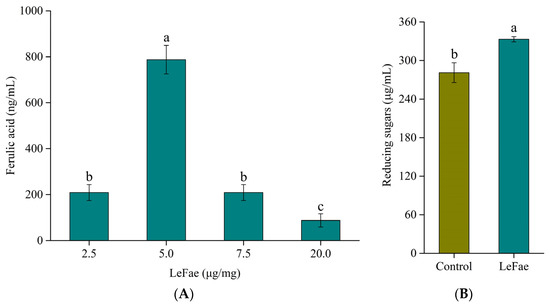
Figure 5.
The hydrolysis of wheat straw using LeFae and the synergism between LeFae and cellulase. (A) The hydrolysis of wheat straw using LeFae. (B) The synergism between LeFae and cellulase on wheat straw hydrolysis. An equivalent quantity of inactivated LeFae was used in the control group. Different lowercase letters in the figure indicate significant differences (p ≤ 0.05). Each value represents the mean ± SD.
3.4. Synergism between LeFae and Cellulase
Previous studies have shown that the complexes formed by ferulic acid and other hemicellulose components through ester bonds block the accessibility of fibrolytic enzymes to fiber [4,6]. Therefore, the current study investigated the effect of removing ferulic acid using LeFae on the cellulase hydrolysis of wheat straw. Compared with the control, LeFae pretreatment significantly improved the following hydrolysis of wheat straw by cellulase, resulting in an increase in reducing sugars by 18.5% (p < 0.05, Figure 5B). This suggested that there was a synergistic effect between LeFae and cellulase on the hydrolysis of wheat straw.
3.5. In Vitro Fermentation of Wheat Straw
An experiment was carried out to investigate the effects of LeFae on the in vitro fermentation of wheat straw. The results showed that LeFae obviously affected the fermentation parameters of wheat straw (Table 1). After incubation for 48 h, LeFae significantly reduced the IVDMD (31.7% vs. 22.1%, p = 0.01) and the production of total VFA (71.4 vs. 61.5 mM, p < 0.01), acetate (42.7 vs. 35.2 mM, p < 0.01), and propionate (14.0 vs. 11.1 mM, p < 0.01). Simultaneously, adding LeFae significantly increased or tended to increase the pH (5.8 vs. 6.1, p < 0.01) and the concentration of ammonia-N (6.4 vs. 7.1 mM, p = 0.03), ferulic acid (35.0 vs. 63.2 ng/mL, p = 0.02), and total soluble phenols (24.3 vs. 26.7 μg-GAE/mL, P = 0.10) during the fermentation of wheat straw. LeFae did not affect total bacteria, i.e., F. succinogenes and R. albus, but tremendously reduced the 16S rDNA copy numbers of R. flavefaciens by 40.1% (p < 0.01). The reduced IVDMD echoed and coincided with the reduced VFA and increased pH. The current results indicated that the presence of LeFae severely hindered the fermentation of wheat straw by inhibiting the growth of some microorganisms. Although these results were unexpected, they also had patterns to consider. Many previous studies proved that phenolic acids, such as ferulic acid, p-coumaric acid, and sinapic acid, have a very strong toxic effect on rumen microorganisms, including rumen bacteria, protozoa, and fungi, consequently reducing the digestion and utilization of cell walls in forage [34,35,36]. Although some ruminal bacteria, such as R. albus, R. flavefaciens, and Wolinella succinogenes, can catabolize and/or hydrogenate ferulic acid into less toxic forms, their FA-reducing ability was possibly inhibited to some extent when the concentration of ferulic acid was higher [35,37]. The addition of phenolic compounds could result in fewer bacteria associated with forage fiber [36]. Marvin et al. [38] reported that ferulic acid, p-coumaric acid, and syringic acid released from maize cell walls by rumen microorganisms during in vitro incubations negatively correlated with organic matter digestibility. Increased soluble phenols and inhibited microbial fermentation of wheat straw with LeFae in the current study confirmed the reports described above. However, unlike the current studies, FAEs did not inhibit rumen microbial fermentation in some other studies [39,40], which may be due to the discrepancy in phenolic acids produced by different FAEs and substrates, and different phenolic acids and their metabolites have different toxicity to rumen microorganisms [34,35]. However, in any case, the current results suggest that when LeFae is directly fed to rumen microorganisms, the phenolic acids produced and their adverse effects on microorganisms are a problem that has to be considered.

Table 1.
Effects of LeFae on the fermentation of wheat straw during in vitro incubation.
3.6. In Vitro Fermentation of Wheat Straw Pretreated with LeFae
In order to mitigate the inhibition of phenolic acids on microorganisms, we further investigated the in vitro fermentation of wheat straw pretreated with LeFae. The results showed that all fermentation parameters listed for wheat straw residues were not different between the LeFae and control groups (Table 2). This indicated that, compared to the results in Section 3.5, the differences between the effects of control and LeFae on wheat straw fermentation were shortened after phenolic acid removal. However, this still did not meet our expectation that LeFae would be able to improve the utilization of wheat straw. We speculate that this may be related to a change in the chemical composition of wheat straw residues after pretreatment with LeFae. Our previous results showed that changing the structure of the straw substrate could affect the leaching of dissolving sugars from straw [41]. Therefore, we detected the total soluble sugars leached from wheat straw during the pretreatment. The result showed that the soluble sugars released from the wheat straw treated with LeFae were significantly increased compared with the control, suggesting that the degradation of ferulic acid ester in the wheat straw enhanced the leaching of soluble sugars (Figure 6). The wheat straw residues in the LeFae group contained less soluble sugars than those in the control group, which may partly explain why there were no significant differences in the in vitro fermentation parameters between the control and LeFae groups, though LeFae may improve fiber degradation in wheat straw residues. These results indicate that pretreating wheat straw with LeFae may be not a good approach to utilize LeFae due to the loss of soluble sugar during the pretreatment.

Table 2.
The fermentation of wheat straw pretreated with LeFae during in vitro incubation.
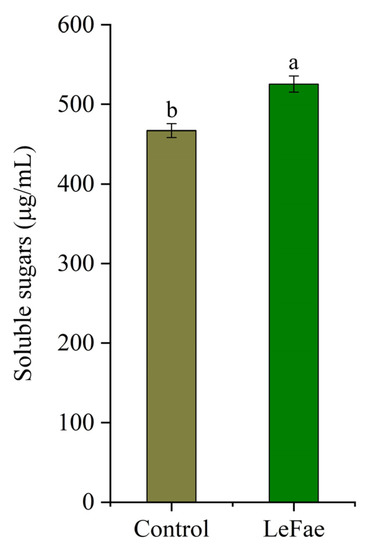
Figure 6.
Effects of LeFae on the leached soluble sugars from wheat straw. Different lowercase letters in the figure indicate significant differences (p ≤ 0.05). Each value represents the mean ± SD.
4. Conclusions
A ferulic acid esterase from L. edodes was expressed in P. pastoris. The optimal condition for LeFae was pH 7.0 and 60 °C. LeFae had a broad temperature and pH range and excellent temperature stability. The LeFae activity was significantly enhanced with Tween-20. LeFae demonstrated the ability to perform hydrolysis on wheat straw, resulting in the liberation of ferulic acid. Additionally, a noticeable synergistic effect was observed when cellulase was used in conjunction with LeFae for the hydrolysis of wheat straw. LeFae severely inhibited the fermentation of wheat straw by suppressing the growth of some microorganisms. Pretreating wheat straw with LeFae resulted in the leaching of more soluble sugars. The current results indicated that LeFae can be used to produce ferulic acid from wheat straw, but when it is fed to ruminants, its adverse effect on rumen microorganisms is a problem worthy of consideration.
Author Contributions
Conceptualization, X.Z. (Xianghui Zhao); methodology, X.Z. (Xianghui Zhao) and W.Z.; software, X.Z. (Xiangyu Zhang); validation, X.Z. (Xiangyu Zhang); formal analysis, X.Z. (Xiangyu Zhang); investigation, K.P., C.L., Q.Q., Y.L. and Y.Z.; resources, K.P.; data curation, X.Z. (Xiangyu Zhang); writing—original draft preparation, X.Z. (Xianghui Zhao) and X.Z. (Xiangyu Zhang); writing—review and editing, X.Z. (Xianghui Zhao); visualization, X.Z. (Xiangyu Zhang); supervision, X.Z. (Xianghui Zhao); project administration, X.Z. (Xianghui Zhao); funding acquisition, X.Z. (Xianghui Zhao), X.L., K.O. and M.Q. All authors have read and agreed to the published version of the manuscript.
Funding
The current study was funded by the Central Leading Local Science and Technology Development Special Project (20221ZDF03017), Natural Science Foundation of Jiangxi (20224ACB205007), Jiangxi Provincial Cattle and Sheep Industry Technology & System (JXARS-13), Earmarked Fund for the Innovation Team of Jiangxi Agricultural University (JXAUCXTD008), and National Beef Cattle Industry Technology & System (CARS-Beef Cattle System: CARS-37).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tufail, T.; Saeed, F.; Afzaal, M.; Ain, H.B.U.; Gilani, S.A.; Hussain, M.; Anjum, F.M. Wheat straw: A natural remedy against different maladies. Food Sci. Nutr. 2021, 9, 2335–2344. [Google Scholar] [CrossRef]
- Togtokhbayar, N.; Cerrillo, M.A.; Rodríguez, G.B.; Elghandour, M.M.; Salem, A.Z.; Urankhaich, C.; Jigjidpurev, S.; Odongo, N.E.; Kholif, A.E.J.A.S.J. Effect of exogenous xylanase on rumen in vitro gas production and degradability of wheat straw. Anim. Sci. J. 2015, 86, 765–771. [Google Scholar] [CrossRef]
- Haddad, S.; Grant, R.; Kachman, S. Effect of wheat straw treated with alkali on ruminal function and lactational performance of dairy cows. J. Dairy Sci. 1998, 81, 1956–1965. [Google Scholar] [CrossRef]
- Jung, H.; Allen, M. Characteristics of plant cell walls affecting intake and digestibility of forages by ruminants. J. Anim. Sci. 1995, 73, 2774–2790. [Google Scholar] [CrossRef]
- Pan, G.X.; Bolton, J.L.; Leary, G.J. Determination of ferulic and p-coumaric acids in wheat straw and the amounts released by mild acid and alkaline peroxide treatment. J. Agric. Food Chem. 1998, 46, 5283–5288. [Google Scholar] [CrossRef]
- Mnich, E.; Bjarnholt, N.; Eudes, A.; Harholt, J.; Holland, C.; Jørgensen, B.; Larsen, F.H.; Liu, M.; Manat, R.; Meyer, A.S.; et al. Phenolic cross-links: Building and de-constructing the plant cell wall. Nat. Prod. Rep. 2020, 37, 919–961. [Google Scholar] [CrossRef]
- Dilokpimol, A.; Mäkelä, M.R.; Aguilar-Pontes, M.V.; Benoit-Gelber, I.; Hildén, K.S.; de Vries, R.P. Diversity of fungal feruloyl esterases: Updated phylogenetic classification, properties, and industrial applications. Biotechnol. Biofuels 2016, 9, 231. [Google Scholar] [CrossRef]
- Li, J.S.; Lau, Y.Q.; Sun, T.Y.; Chen, C.S. Purification and biochemical characterization of an alkaline feruloyl esterase from Penicillium sumatrense NCH-S2 using rice bran as substrate. CyTA J. Food 2020, 19, 1–10. [Google Scholar] [CrossRef]
- Mafa, M.S.; Malgas, S.; Pletschke, B.I. Feruloyl esterase (FAE-1) sourced from a termite hindgut and GH10 xylanases synergy improves degradation of arabinoxylan. AMB Express 2021, 11, 21. [Google Scholar] [CrossRef]
- Record, E.; Asther, M.; Sigoillot, C.; Pagès, S.; Punt, P.J.; Delattre, M.; Haon, M.; Hondel, C.A.M.J.J.V.D.; Sigoillot, J.-C.; Lesage-Meessen, L. Overproduction of the Aspergillus niger feruloyl esterase for pulp bleaching application. Appl. Microbiol. Biotechnol. 2003, 62, 349–355. [Google Scholar] [CrossRef]
- Li, L.; Qu, M.; Liu, C.; Xu, L.; Pan, K.; Song, X.; OuYang, K.; Li, Y.; Zhao, X. Expression of a Recombinant Lentinula edodes Xylanase by Pichia pastoris and Its Effects on Ruminal Fermentation and Microbial Community in in vitro Incubation of Agricultural Straws. Front. Microbiol. 2018, 9, 2944. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Nakade, K.; Sato, S.; Yoshida, K.; Miyazaki, K.; Natsume, S.; Konno, N. Lentinula edodes Genome Survey and Postharvest Transcriptome Analysis. Appl. Environ. Microbiol. 2017, 83, e02990-16. [Google Scholar] [CrossRef]
- Johnson, K.; Harrison, B.; Schneider, H.; MacKenzie, C.; Fontana, J. Xylan-hydrolysing enzymes from Streptomyces spp. Enzym. Microb. Technol. 1988, 10, 403–409. [Google Scholar] [CrossRef]
- Siew, C.K.; Williams, P.A. Role of Protein and Ferulic Acid in the Emulsification Properties of Sugar Beet Pectin. J. Agric. Food Chem. 2008, 56, 4164–4171. [Google Scholar] [CrossRef]
- Li, L.; Qu, M.; Liu, C.; Pan, K.; Xu, L.; OuYang, K.; Song, X.; Li, Y.; Zhao, X. Expression of a recombinant Lentinula edodes cellobiohydrolase by Pichia pastoris and its effects on in vitro ruminal fermentation of agricultural straws. Int. J. Biol. Macromol. 2019, 134, 146–155. [Google Scholar] [CrossRef]
- Hughes, M.; Mlambo, V.; Lallo, C.H.O.; Jennings, P.G.A. Potency of microbial inocula from bovine faeces and rumen fluid for in vitro digestion of different tropical forage substrates. Grass Forage Sci. 2012, 67, 263–273. [Google Scholar] [CrossRef]
- Zhao, X.H.; Liu, C.J.; Liu, Y.; Li, C.Y.; Yao, J.H. Effects of replacing dietary starch with neutral detergent-soluble fibre on ruminal fermentation, microbial synthesis and populations of ruminal cellulolytic bacteria using the rumen simulation technique (RUSITEC). J. Anim. Physiol. Anim. Nutr. 2012, 97, 1161–1169. [Google Scholar] [CrossRef]
- Makkar, H.; Sharma, O.; Dawra, R.; Negi, S. Simple Determination of Microbial Protein in Rumen Liquor. J. Dairy Sci. 1982, 65, 2170–2173. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Cheng, C.-W.; Liang, J.-Y. Effect of esterification condensation on the Folin–Ciocalteu method for the quantitative measurement of total phenols. Food Chem. 2015, 170, 10–15. [Google Scholar] [CrossRef]
- Nielsen, S.S. Total Carbohydrate by Phenol-Sulfuric Acid Method. In Food Analysis Laboratory Manual. Food Science Text Series; Springer: Cham, Switzerland, 2017; pp. 137–141. [Google Scholar] [CrossRef]
- Dimarogona, M.; Topakas, E.; Christakopoulos, P.; Chrysina, E.D. The crystal structure of a Fusarium oxysporum feruloyl esterase that belongs to the tannase family. FEBS Lett. 2020, 594, 1738–1749. [Google Scholar] [CrossRef]
- Suzuki, K.; Hori, A.; Kawamoto, K.; Thangudu, R.R.; Ishida, T.; Igarashi, K.; Samejima, M.; Yamada, C.; Arakawa, T.; Wakagi, T.; et al. Crystal structure of a feruloyl esterase belonging to the tannase family: A disulfide bond near a catalytic triad. Proteins Struct. Funct. Bioinform. 2014, 82, 2857–2867. [Google Scholar] [CrossRef]
- Li, L.; Shaw, P.E. A STAT3 dimer formed by inter-chain disulphide bridging during oxidative stress. Biochem. Biophys. Res. Commun. 2004, 322, 1005–1011. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Liu, Y.; Leng, J. Heterologous Expression and Characterization of a Ferulic Acid Esterase from Aspergillus aculeatus with Potential Use in Sunflower Seed Processing. Appl. Sci. 2021, 11, 4453. [Google Scholar] [CrossRef]
- Kanauchi, M.; Watanabe, S.; Tsukada, T.; Atta, K.; Kakuta, T.; Koizumi, T. Purification and Characteristics of Feruloyl Esterase from Aspergillus awamori G-2 Strain. J. Food Sci. 2008, 73, C458–C463. [Google Scholar] [CrossRef]
- Donaghy, J.; McKay, A.M. Purification and characterization of a feruloyl esterase from the fungus Penicilliumexpansum. J. Appl. Microbiol. 1997, 83, 718–726. [Google Scholar] [CrossRef]
- Hemida, S.K.; Omar, S.A.; Abdel-Mallek, A.Y. Microbial populations and enzyme activity in soil treated with heavy metals. Water Air Soil Pollut. 1997, 95, 13–22. [Google Scholar] [CrossRef]
- Esteban-Torres, M.; Reverón, I.; Mancheño, J.M.; Rivas, B.d.L.; Muñoz, R. Characterization of a Feruloyl Esterase from Lactobacillus plantarum. Appl. Environ. Microbiol. 2013, 79, 5130–5136. [Google Scholar] [CrossRef]
- Akira, Y.; Hiroshi, M.; Yutaka, T.; Naomi, W.; Akira, M.; Hajime, N.; Takashi, I.; Kazue, O.; Junji, Y. Lymphocyte transformation and thiol compounds: The role of ADF/thioredoxin as an endogenous reducing agent. Mol. Immunol. 1992, 29, 263–270. [Google Scholar] [CrossRef]
- Fu, Z.; Zhu, Y.; Teng, C.; Fan, G.; Li, X. Biochemical characterization of a novel feruloyl esterase from Burkholderia pyrrocinia B1213 and its application for hydrolyzing wheat bran. 3 Biotech 2021, 12, 24. [Google Scholar] [CrossRef]
- Fazary, A.E.; Ismadji, S.; Ju, Y.-H. Biochemical studies on native and cross-linked aggregates of Aspergillus awamori feruloyl esterase. Int. J. Biol. Macromol. 2009, 44, 240–248. [Google Scholar] [CrossRef]
- Cheng, F.; Sheng, J.; Cai, T.; Jin, J.; Liu, W.; Lin, Y.; Du, Y.; Zhang, M.; Shen, L. A Protease-Insensitive Feruloyl Esterase from China Holstein Cow Rumen Metagenomic Library: Expression, Characterization, and Utilization in Ferulic Acid Release from Wheat Straw. J. Agric. Food Chem. 2012, 60, 2546–2553. [Google Scholar] [CrossRef]
- Ghazali, N.F.; Makhtar, N.A. Enzymatic hydrolysis of oil palm empty fruit bunch and its kinetics. Malays. J. Anal. Sci. 2018, 22, 715–722. [Google Scholar] [CrossRef]
- Martin, S. Effects of p-coumaric acid and ferulic acid on methane production and fibre digestion by mixed rumen micro-organisms in vitro. Lett. Appl. Microbiol. 1988, 7, 113–114. [Google Scholar] [CrossRef]
- Chesson, A.; Stewart, C.S.; Wallace, R.J. Influence of Plant Phenolic Acids on Growth and Cellulolytic Activity of Rumen Bacteria. Appl. Environ. Microbiol. 1982, 44, 597–603. [Google Scholar] [CrossRef]
- Akin, D.; Rigsby, L.; Theodorou, M.; Hartley, R. Population changes of fibrolytic rumen bacteria in the presence of phenolic acids and plant extracts. Anim. Feed. Sci. Technol. 1988, 19, 261–275. [Google Scholar] [CrossRef]
- Soberon, M.; Cherney, D.; Cherney, J. Free ferulic acid uptake in ram lambs1. J. Anim. Sci. 2012, 90, 1885–1891. [Google Scholar] [CrossRef]
- Marvin, H.J.P.; Krechting, C.F.; Van Loo, E.N.; Snijders, C.H.A.; Lommen, A.; Dolstra, O. Relationship between Phenolic Acids Formed During Rumen Degradation of Maize Samples and in vitro Digestibility. J. Sci. Food Agric. 1996, 71, 111–118. [Google Scholar] [CrossRef]
- Kanelias, K.; Mould, F. The effect of ferulic acid esterase on the in vitro degradability of wheat straw. Proc. Br. Soc. Anim. Sci. 2006, 189. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Wang, W.-K.; Wu, Q.-C.; Yang, H.-J. The release and catabolism of ferulic acid in plant cell wall by rumen microbes: A review. Anim. Nutr. 2022, 9, 335–344. [Google Scholar] [CrossRef]
- Gu, S.; Liu, C.; Zhang, W.; Qu, M.; Li, Y.; Zang, Y.; Xiong, X.; Pan, K.; Zhao, X. Characteristics of a recombinant Fusarium verticillioides cutinase and its effects on enzymatic hydrolysis of rice straw. Int. J. Biol. Macromol. 2021, 171, 382–388. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).