Abstract
About 95% of global hydrogen production is made by fossil fuels using different technologies which are all characterized by high energy consumption and high carbon emissions. Alternatively, more sustainable production methods, such as biological fermentation processes, are under study. Dark fermentation, also called acidogenesis, entails the transformation of a great variety of organic substances into a mixture of organic and inorganic products, as well as gases (H2 and CO2). In this study we tested an exhausted fermentation broth, derived after Clostridium fermentation for H2 production, as a biostimulant via foliar application in an intensive apple orchard. Two different doses were applied upon dilution of the broth in water (100 mL L−1 and 10 mL L−1), evaluating the main fruit quality parameters (fresh weight, fruit diameter, dry matter, firmness, soluble solid content, color lightness, DA index) in addition to macro- and micro-nutrients and heavy metals concentrations. Chemical characterization of the broth showed a high amount of low-MW polypeptides (Trp-Glu-Lys, Ile-Pro-Ile, Phe-Pro-Lys, His-Pro) and organic acids (formic acid, butyric acid, butanedioic acid); moreover, quantitative analyses of inorganic ions showed no heavy metal detection but high concentrations of nitrogen, phosphorus and potassium, compatible with use in agriculture. The fruit quality parameters showed significantly higher mean fruit weight compared to the untreated trees, as well as higher dry matter. No statistical differences were recorded among the treatments for fruit firmness, diameter and yield. Soluble solids content in both treatments were significantly lower than the controls, whereas the DA index mean values were higher in both treatments compared to the controls, indicating a delay in fruit ripening probably due to the high nitrogen broth concentration. Regarding the chemical analyses of fruits, no particular differences were found among the treatments, except for Fe, which showed a significantly higher amount upon treatment with the lower dose. As concerns leaves, no phytotoxic symptoms were detected in both treatments, making the described exhausted broth a candidate for its use as a plant biostimulant. Additional studies are needed to evaluate the ideal application dose, identify further action targets and implement appropriate strategies to concentrate the biostimulant active compounds.
1. Introduction
The current global energy demand is heavily dependent on fossil fuel reserves, which are depleting and cause severe pollution problems due to the emission of greenhouse gases [1]. The scientific community established that increasing CO2 levels are impacting the greenhouse gas effect and global warming [2]. Hydrogen (H2), as a fuel, due to its high energy yield and low heating value, is more efficient than hydrocarbon-based fossil fuels [3]. Hydrogen can also be used in fuel cells as part of an electric power generation system [4]. A fuel cell is an electrochemical low-temperature device in which hydrogen is directly converted into electrical energy [5]. Moreover, hydrogen is a carbon-free molecule whose combustion produces exclusively H2O [6]; it is a colorless, odorless and non-toxic gas. As much as 95% of global hydrogen production is made by fossil fuels through SMR (steam methane reformation) [7], electrolysis and gasification, each a technology with high energy consumption and high carbon emissions. Alternative production methods, eco-friendlier and more sustainable, based on biological fermentation processes, are under study and considered as emerging technologies [8]. Hydrogen produced from biological processes is called biohydrogen and its production is carried out by different microorganisms, such as fermenting bacteria—i.e., photosynthetic bacteria including cyanobacteria and eukaryotic organisms such as algae and fungi—which produce hydrogen with no light requirement via so called “dark fermentation” [9]. Dark fermentation, also called acidogenesis, entails the transformation of a great variety of organic substances into a mixture of organic (e.g., organic acids, peptides) and inorganic products, as well as gases such as H2 and CO2 [10]. In this process, a part of the organic substrate may be oxidized, while a part is reduced. From this oxidation-reduction of organic substrates, microorganisms receive energy, producing different valuable soluble organic compounds [11]. Among the available biological methods, H2 production via dark fermentation is the simplest [12]. Different suitable residual substrates have been tested to this end, including food waste [13], agricultural waste [14] and the organic fraction of municipal solid waste [15], due to their high carbohydrate availability. The best results, in terms of H2 yield, were obtained from substrates rich in oligosaccharides and monosaccharides [13,14]. An innovative biological two-phase technology, referred to as BIH2 technology (Biorenova S.p.A., Montorio al Vomano, Italy), has been developed. The technology was patented (Patent Cooperation Treaty, 2017) under the Italian Publication Number IT102020000013006 and undergoing international extension PCT/EP2021/063588. BiH2 is an integrated technology aiming at the production of gaseous H2 and valuable fermentation co-products and the conversion of CO2 to cell biomass and organic acids. The technology is based on two distinct and complementary phases: Phase1 (P1) is a dark fermentation process, using sucrose as the carbon source, performed by Clostridium beijerinckii DSM34075 strain, which produces H2 and CO2 and high-value co-products such as organic acids and peptides in the exhausted broth; Phase2 (P2) is a biological process converting CO2 produced from P1 to organic acids with the use of acetogenic bacteria thanks to the Wood–Ljungdahl pathway. At the end of the BiH2 process, an output of pure 100% gaseous hydrogen is obtained as well as high-value co-products and biomass from both phases. Dark fermentation seems to be an important sustainable process in the synthesis of valuable chemical compounds (an alternatively to the petrochemical refinery) [10]. For this reason, the developed process fully fits into the concept of biorefinery, in addition to representing a sustainable alternative to petrochemicals-based technology and fossil resources consumption. Moreover, the recent EU goals defined in the “Farm to Fork” strategy include a reduction of 50% chemical pesticides, 20% fertilizers and 50% nutrient losses by 2030 [16]; further, recent policies invite improvements to soil management and the efficient use of nutrients in agriculture by exploiting wastes and biomass locally, as planned in the Circular Economy Action Plan and the Bioeconomy Strategy [17,18] (EU Commission, 2012–2020). In this study, we characterized the composition of the exhausted fermentation broth derived from the BiH2 technology—Phase 1, the dark fermentation process (P1)—and tested its possible application in the agriculture sector as a plant biostimulant. The fermented broth was used via foliar application in an intensive apple orchard, evaluating seasonal shoot and fruit growth and studying fruit quality parameters at commercial harvest.
2. Materials and Methods
2.1. Exhausted Fermentation Broth Obtained in the H2 Production Process According to the BIH2 Technology
The broth used in this study was obtained after a fermentation process by C. beijerinckii DSM34075. Freeze-dried cells were used to inoculate 20 mL reinforced clostridial medium (RCM) flasks, a commercial broth ideal for cultivation and enumeration of clostridia [19]. Inoculum was cultured anaerobically at 36 °C for 24 h. The cells were then harvested by centrifugation at 10,000 rpm for 8 min (SL 16 R, Thermo Fisher, Waltham, MA, USA) and resuspended in 20 mL of 0.9% NaCl (w/v). Suspended cells were used to inoculate a 4 L bioreactor (Solaris Biotechnology, Porto Mantovano, Italy) containing 2 L mineral medium with sucrose as the carbon source (Patent IT102020000013006), until a cell density of 2.0 g L−1 has been reached. The bioreactor was equipped with pH, E.C. (Electrical Conductibility) and dO2 sensors, total cell density meter Model Dencytee Total Cell Density sensor (Hamilton Company, Reno, NV, USA) and all the necessary inlets/outlets for pH control, pressure measurement, liquid sampling and N2 entry. The inoculated medium was fluxed with filtered N2 until the value of dO2 was 0%. Temperature and stirring were kept at 37 °C and 500 rpm, respectively, and pH was maintained at 6.0 using NaOH 1 M. The fermentation was conducted in fed-batch conditions and the exhausted fermented broth was periodically collected during the steady state periods, chilled to 2 °C and stored at this temperature until use, which was accomplished within a couple of days.
2.2. Chemical Broth Characterization
The organic compounds present in the BiH2-exhausted fermentation broth (BEFB) have been characterized using LC-SACI–MS [19]. The analysis was performed using an Ultimate 3000 HPLC (Thermo Fisher, USA) equipped with a Thermo Hypersil GOLD 50 mm × 4.6 mm column. The analyses were performed using a two-phase gradient: Phase A was 0.2% formic acid in water and Phase B was acetonitrile (CH3CN). Data acquisition was performed with “SANIST” mass spectrometer equipped with an ESI. A nebulizer gas pressure of 30 Psi was used, with a dry gas flow rate of 9.0 L/min. The nebulizer temperature and the dry gas temperature were both 300 °C. The spectrum was acquired in data-dependent scan mode. The raw data were processed in Compound Discoverer 3.1 (CD3.1, Thermo Fisher) for peak alignment, peak picking and quantitation of each metabolite. Peak intensities normalized by total spectral intensity were used to predict the molecular formula based on additive ions, molecular ion peaks and fragment ions. Databases, including mzCloud (https://www.mzcloud.org/; accessed on 7 May 2023), mzVault and MassList, were used to obtain accurate qualitative and relative quantitative results matched with peaks [20].
Additional chemical analyses were performed on BEFB to quantify the amount of major inorganic ions and heavy metals to ensure safe use in agriculture. Total organic Carbon (TOC) was determined with the UNI EN 1484:1999 technique; total Nitrogen was estimated with the UNI 11658:2016 method; NH4, NO3, HNO2, PO4, SO4 and Cl were determined following ISO15923-1:2013 protocols; and heavy metals were determined through UNI EN 13657:2004 + UNI EN ISO 11885:2009 methods.
2.3. Experimental Trial in the Orchard
The trial took place at the experimental farm of the University of Bologna, located in Cadriano (Bologna, Italy) (44°32′54.1″ N and 11°24′53.0″ W; 32 m elevation), in an experimental apple orchard (Malus domestica L., cv. Fuji) grafted on M9, in season 2022. The orchard was planted in 2018 on a silty clay loam soil consisting of 4 rows (60 trees in each row). Trees were spaced 3.3 m × 1.0 m with a north–south orientation and trained as slender spindles. Since its plantation, the orchard management followed integrated pest management (IPM) protocols and trees were watered and fertirrigated as needed. Full bloom date was 12 April 2022. Two different application doses of the exhausted broth were used and labeled as BEFB1 and BEFB2, corresponding to 100 mL L−1 and 10 mL L−1 of BiH2-exhausted broth for BEFB1 and BEFB2, respectively, both diluted in water; the control treatment was labeled as CTRL and received only water. Foliar application trial was performed from 1 June 2022 (50 DAFB, i.e., days after full blooming) to 24 August 2022 (125 DAFB), spraying BEFB1 and BEFB2 with a backpack pump on correspondent blocks of trees every 15 days; overall, 6 treatments were conducted. Biometric measurements were carried out before BEFB application and each day before spraying application at 49, 64, 79, 94, 109 and 124 DAFB (corresponding to T0, T1, T2, T3, T4, T5 and T6). Before starting the foliar application trial, 108 fruits and 108 shoots (4 fruits and 4 shoot for each tree) were labeled to evaluate the effect of BEFB1 and BEFB2 on allometry parameters.
Treatments (BEFB1, BEFB2 and CTRL) were assigned according to a randomized blocks design with three replicates (i.e., three trees) for each of the three blocks. Each block was selected in the same three parallel row, with a distance among the blocks of 15 trees (15 m); in each of the three rows inside each block, the three treatments were randomly arranged with a total of 9 trees per block (3 replicates treatment−1 block−1).
2.4. Fruit Quality Assessments
At commercial harvest, all fruits from each tree were calipered and counted and the crop load per each single plant was calculated. The main fruit quality parameters (fresh weight, fruit diameter, dry matter, firmness, soluble solid content) and the three classical parameters for skin fruit color, i.e., color lightness (L), chroma (a*) and chroma (b*), were assessed on 45 fruits per treatment at commercial harvest. Colorimetric analyses were performed using a Minolta CR-400 apparatus (Konica Minolta Sensing Americas, Inc., Ramsey, NJ, USA), “L” showing the lightness, “a” showing the shades from the green (negative value) to the red (positive value) and “b” showing the shades from the blue (negative value) to the yellow (positive value). Fruit firmness was assessed thanks to the 53220 FTA Fruit Texture Analyser (T.R. Turoni srl, Forlì, Italy). Soluble solids content was determined by a digital Brix Refractometer, Model PAL-1 (ATAGO Co., Ltd., Tokyo, Japan). Fruit dry matter content was determined on fruit slices which were dried at 65 °C for several days and weighted with a precision Mettler scale, Model PE3600 (METTLER TOLEDO LLC, Columbus, OH, USA). DA index, a fruit non-destructive ripeness index, was measured by a DA meter, Model 53500 (T.R. Turoni srl, Italy). The same fruits analyzed for fruit quality were checked for macro-micronutrients and heavy metals concentration. Briefly, Ca, Cl, K, Mg, N, Na, P, S, Fe, were determined by Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES, Ametek Spectro Arcos EOP, Kleve, Germany), after digestion with nitric acid (HNO3) by a microwave lab station (Ethos TC-Milestone, Bergamo, Italy), while N was determined by the Kjeldahl method.
2.5. Statistical Analysis
Fruit growth was analyzed using a linear mixed model function. Fruit mineral concentration, fruit yield and quality data were compared among treatments using a one-way ANOVA analysis followed by a Tukey HSD test. Statistical significance was established for p < 0.05. Analyses were carried out using R software (www.rproject.org, accessed on 10 May 2023) code.
3. Results and Discussion
3.1. Exhausted Broth Characterization
The presence and amount of potential active compound in BEFB was studied via a metabolomic analysis by LC-SACI–MS. The 15 major peaks were characterized, and the abundance of the relative compounds is presented in Table 1. Peak areas of standard compounds for the 15 metabolites identified (Table 1) by LC-SACI–MS were calculated for comparison. The most abundant metabolites detected by LC-SACI–MS, following the fermentation process, were organic acids; in particular, formic acid (89.5 g L−1), butyric acid (7.45 g L−1) and butanedioic acid (3.58 g L−1). Organic acids are typical metabolites in dark fermentation processes as described by different authors [21,22]. Clostridium spp. Has been demonstrated to be able to produce organic acids due to mixed acids pathways; sugars can be converted to H2, CO2, formate, butyrate and acetate [23]. Furthermore LC-SACI–MS analyses showed the presence of several different peptides at relative lower abundance; in particular Trp-Glu-Lys (3.12 g L−1), Ile-Pro-Ile (2.11 g L−1), Phe-Pro-Lys (2.03 g L−1) and His-Pro (0.80 g L−1). Peptides are produced during fermentation through microbial metabolism [24], and different studies have shown how peptides application has biostimulant effects on plants [25]. In fact, it has been demonstrated that the application of low-molecular-weight peptides has a positive effect on tomato root dry weight and root length [26], promotes vegetative growth and yield in several fruit trees [27], prevents damages from abiotic stresses such as thermal stress [28,29] and improves fruit quality [30,31]. Quantitative analyses on the main inorganic ions and heavy metals were also carried out on BEFB (Table 2); heavy metals were not detected at all, macroelements including nitrogen (N-NO3 1.20 mg L−1; N-NH4 562 mg L−1), phosphorus (PO4 343 mg L−1) and potassium (K 280 mg L−1) have been measured at values compatible with use in agriculture.

Table 1.
LC-SACI–MS quantitative analyses on BEFB.

Table 2.
Chemical analyses on inorganic fraction.
3.2. Experimental Trial in the Orchard and Fruit Quality
Analyses on fruit quality parameters have been carried out at the commercial harvest of the fruits. A total of 45 fruits for each treatment plus the control were analyzed for the different quality parameters, as shown in Table 3. Trees treated with BEFB (BEFB1 and BEFB2) show significantly (p < 0.05) higher mean fruit weight compared to the untreated trees (BEFB1 212 g, BEFB2 215 g, CTRL 201 g), probably due to the presence of low-molecular-weight peptides in BEFB that increase fruit weight, as demonstrated in previous studies [32]. Moreover, an auxin-like activity of low-molecular-weight-peptide-based products has been reported by different authors, probably determined by the tryptophan content in peptides that is the main precursor for the indoleacetic acid auxin in plants [33]. Indeed, it has already been proven that auxins stimulate root growth and apical dominance, delay fruit ripening, stimulate fruit development and the growth of flowering parts and stimulate the stem and cell elongation rate [34,35,36]. No statistical differences were recorded among the treatments for fruit firmness, diameter and yield (Table 3). Even the seasonal pattern of fruit growth was not statistically different among treatments, although BEFB1 trees showed slightly reduced values in fruit diameter in the last part of the season compared to BEFB2 and CTRL (Figure 1). The percentage of dry matter measured on fruits treated with BEFB1 was statistically higher (p < 0.05) than the control and BEFB2 (BEFB1, 40.1%; BEFB2, 32.1%; CTRL, 30.1%, respectively); the higher concentration of low-molecular-weight polypeptides and organic acids in BEFB1 compared to BEFB2 probably influenced this higher accumulation of dry matter in BEFB1 compared to BEFB2. The effect of polypeptides on dry matter percentage have already been investigated in previous studies [27,37]. Soluble solids content (°Brix) in both BEFB treatments were significantly lower (p < 0.05) than the control (BEFB1, 14; BEFB2, 19.9; CTR, 14.4, respectively). Moreover, DA index mean value was higher in both BEFB treatments (BEFB1, 1.1; BEFB2, 1.06; CTRL, 0.98) indicating a ripening delay of the fruit. The decrease in soluble solids content in the fruit is due to a slight delay in fruit ripening as evidenced by higher DA index values. This effect is due to the presence within the BEFB of NO3− and NH4+, as detected by the chemical characterization analyses which favored the vegetative development of the plants (data not shown) at the expense of fruit growth, which was indeed slightly reduced in BEFB1 (Figure 1). furthermore, an auxin-like activity of BEFB1 and BEFB2 may have contributed to a delay in fruit ripening. The ripening delay was also confirmed by the significantly lower values in a* and b* background color compared to the CTRL. In particular, BEFB1 showed lower values in the background redness–greenness component (a*), while BEFB2 had lower values in yellowness–blueness component (b*). As for the surface color, no differences were detected among the treatments (Table 4 and Figure 2).

Table 3.
Effect of the BEFB treatment on fruit quality parameters: diameter, weight, SSC, DA index, dry matter, flesh firmness and yield.
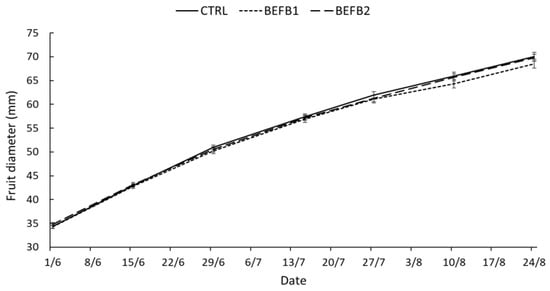
Figure 1.
Seasonal pattern of fruit growth (mm fruit-1) of BEFB1 (short-dashed line), BEFB2 (long-dashed line) and CTRL (continuous line) (Avg. ± SE).

Table 4.
Effect of the BEFB treatment on fruit skin lightness (L*), a* and b* in over and background color.
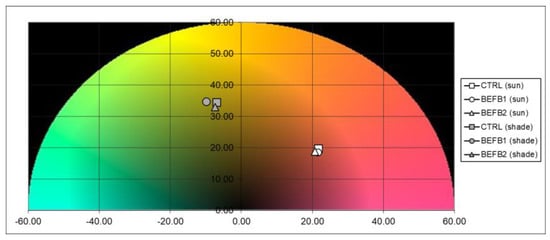
Figure 2.
Over (white symbol) and background (grey symbol) colors representation for BEFB1 (circle symbol), BEFB2 (triangle symbol) and CTRL (square symbol).
The same fruits were analyzed for macro and micronutrients concentration, and the results are expressed in Table 5. No particular differences were found among the treatments except for Fe, which was found in BEFB2 (18.1 µg g−1) in significantly higher (p < 0.05) quantities compared to untreated plants (9.75 µg g−1) and BEFB1 (9.20 µg g−1). The effect of organic acids contained in BEFB—in particular, formic acid—likely improved iron absorption as previously demonstrated by other authors [38]. As for Na, the BEFB treatments showed higher concentrations compared to the control (Table 5). This is likely related to the high concentration of Na in the broth (Table 5). Indeed, the more concentrated treatment (BEFB1) had the highest fruit Na values, followed by BEFB2 (ten times lower) and then by the control (Table 5). Furthermore, as concerns leaves, no phytotoxic symptoms were detected upon both BEFB1 and BEFB2 treatment.

Table 5.
Effect of the BEFB application on fruit macro and micronutrient concentration.
4. Conclusions
In this study, an exhausted fermentation broth (BEFB), coming from a dark fermentation process via a C. beijerinckii strain and aimed at the production of biohydrogen, was used at two different concentrations as a biostimulant on apple trees with a foliar application. The broth contained a good amount of low-molecular-weight peptides (Trp-Glu-Lys, Ile-Pro-Ile, Phe-Pro-Lys, His-Pro) and organic acids (formic acid, butyric acid, butanedioic acids). The application determined significantly higher mean fruit weight and soluble solids content compared to the untreated trees, regardless of the concentration of the broth, and probably because of the presence of peptides. Moreover, the fruit DA index was higher in both BEFB treatments, and a decreased soluble solids content was observed, indicating a slight delay in fruit ripening. No phytotoxic effects on leaves and chemical risks due to heavy metals in the fruit were highlighted in this study. This preliminary study shows a biostimulating effect of BEFB, which is a valuable by-product. Therefore, the whole process fits the biorefinery concept and is fully in accordance with the recent EU goals defined in the Farm to Fork strategy, the Circular Economy Action Plan and the Bioeconomy Strategy (EU Commission, 2012–2020). Further studies are needed to evaluate the ideal application dose, identify further action targets and implement appropriate strategies for the concentration of the biostimulants active compounds.
5. Patents
BIH2 technology has been patented (Patent Cooperation Treaty, 2017) under the Italian Publication Number IT102020000013006 (Ufficio Italiano Brevetti), applied for PCT/EP2021/063588.
Author Contributions
Conceptualization, M.G. and G.D.P.; methodology, M.G., D.D.G., G.D.P. and B.M.; software, M.G. and G.D.P.; validation, M.G., D.D.G., G.D.P. and B.M; formal analysis, M.G. and G.D.P.; investigation, M.G. and G.D.P.; resources, D.D.G. and B.M.; data curation, M.G. and G.D.P.; writing—original draft preparation. M.G. and G.D.P.; writing—review and editing, M.G., D.D.G., G.D.P. and B.M.; visualization, M.G. and G.D.P.; supervision, D.D.G. and B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Biorenova S.p.A., https://www.biorenovatech.com/.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Marbán, G.; Valdés-Solís, T. Towards the hydrogen economy? Int. J. Hydrogen Energy 2007, 32, 1625–1637. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Ferraren-De Cagalitan, D.D.T.; Abundo, M.L.S. A review of biohydrogen production technology for application towards hydrogen fuel cells. Renew. Sustain. Energy Rev. 2021, 151, 111413. [Google Scholar] [CrossRef]
- Singla, M.K.; Nijhawan, P.; Oberoi, A.S. Hydrogen fuel and fuel cell technology for cleaner future: A review. Environ. Sci. Pollut. Res. 2021, 28, 15607–15626. [Google Scholar] [CrossRef]
- Lucia, U. Overview on fuel cells. Renew. Sustain. Energy Rev. 2014, 30, 164–169. [Google Scholar] [CrossRef]
- Hren, R.; Vujanović, A.; Van Fan, Y.; Klemeš, J.J.; Krajnc, D.; Čuček, L. Hydrogen production, storage and transport for renewable energy and chemicals: An environmental footprint assessment. Renew. Sustain. Energy Rev. 2023, 173, 113113. [Google Scholar] [CrossRef]
- Riera, J.A.; Lima, R.M.; Knio, O.M. A review of hydrogen production and supply chain modeling and optimization. Int. J. Hydrogen Energy 2023, 48, 13731–13755. [Google Scholar] [CrossRef]
- Maroušek, J. Nanoparticles can change (bio) hydrogen competitiveness. Fuel 2022, 328, 125318. [Google Scholar] [CrossRef]
- Islam, A.K.; Dunlop, P.S.; Hewitt, N.J.; Lenihan, R.; Brandoni, C. Bio-hydrogen production from wastewater: A comparative study of low energy intensive production processes. Clean Technol. 2021, 3, 156–182. [Google Scholar] [CrossRef]
- Bastidas-Oyanedel, J.R.; Bonk, F.; Thomsen, M.H.; Schmidt, J.E. Dark fermentation biorefinery in the present and future (bio) chemical industry. Rev. Environ. Sci. Bio/Technol. 2015, 14, 473–498. [Google Scholar] [CrossRef]
- Gerardi, M.H. The Microbiology of Anaerobic Digesters; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Cappai, G.; De Gioannis, G.; Friargiu, M.; Massi, E.; Muntoni, A.; Polettini, A.; Pomi, R.; Spiga, D. An experimental study on fermentative H2 production from food waste as affected by pH. Waste Manag. 2014, 34, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, S.H.; Shin, H.S. Hydrogen fermentation of food waste without inoculum addition. Enzym. Microb. Technol. 2009, 45, 181–187. [Google Scholar] [CrossRef]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrère, H.; Steyer, J.P. Hydrogen production from agricultural waste by dark fermentation: A review. Int. J. Hydrogen Energy 2010, 35, 10660–10673. [Google Scholar] [CrossRef]
- Alibardi, L.; Cossu, R. Composition variability of the organic fraction of municipal solid waste and effects on hydrogen and methane production potentials. Waste Manag. 2015, 36, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Montanarella, L.; Panagos, P. The relevance of sustainable soil management within the European Green Deal. Land Use Policy 2021, 100, 104950. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. A New Circular Economy Action Plan. For a Cleaner and more Competitive Europe. Brussels, 11.03.2020. COM (2020) 98 Final; 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1583933814386&uri=COM:2020:98:FIN (accessed on 10 February 2023).
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. Innovating for Sustainable Growth: A Bioeconomy for Europe, SWD(2012) 11 Final. Brussels, 13.2.2012. COM (2012) 60 Final; 2012. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52012DC0060 (accessed on 10 February 2023).
- Sandoval-Espinola, W.J.; Chinn, M.; Bruno-Barcena, J.M. Inoculum optimization of Clostridium beijerinckii for reproducible growth. FEMS Microbiol. Lett. 2015, 362, fnv164. [Google Scholar] [CrossRef]
- Piccirella, S.; Uberti, D.; Xiong, C.; Fowler, C.; Doecke, J.; Fagan, A.; Frisoni, G.; Kinnon, P. Performance of a non-invasive blood test for a conformational variant of p53 to predict Alzheimer’s disease within 6 years of clinical diagnosis. Preprints.org 2021, 2021050267. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Li, X.; Zhang, J.; Li, Y.; Wu, X.M.; Yang, Y.Z.; Zhang, X.F.; Ma, L.Z.; Liu, Y.D.; Wang, Z.; et al. Plasma metabolomic characterization of premature ovarian insufficiency. J. Ovarian Res. 2023, 16, 2. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Mota, V.T.; de Oliveira, V.M.; Zaiat, M. Hydrogen and organic acid production from dark fermentation of cheese whey without buffers under mesophilic condition. J. Environ. Manag. 2022, 304, 114253. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Nanda, S. Biohydrogen production through dark fermentation. Chem. Eng. Technol. 2020, 43, 601–612. [Google Scholar] [CrossRef]
- Penning, H.; Conrad, R. Carbon isotope effects associated with mixed-acid fermentation of saccharides by Clostridium papyrosolvens. Geochim. Cosmochim. Acta 2006, 70, 2283–2297. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial fermentation and its role in quality improvement of fermented foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef] [PubMed]
- Morales-Payan, J.P.; Stall, W. Passion fruit (Passiflora edulis) transplant production is affected by selected biostimulants. In Proceedings of the Florida State Horticultural Society; Florida State Horticultural Society: Gainesville, FL, USA, 2004; Volume 117, pp. 224–227. [Google Scholar]
- Marfà, O.; Cáceres, R.; Polo, J.; Ródenas, J. Animal protein hydrolysate as a biostimulant for transplanted strawberry plants subjected to cold stress. In VI International Strawberry Symposium 842; ISHS: Bierbeek, Belgium, 2008; pp. 315–318. [Google Scholar]
- Botta, A. Enhancing plant tolerance to temperature stress with amino acids: An approach to their mode of action. In I World Congress on the Use of Biostimulants in Agriculture 1009; ISHS: Bierbeek, Belgium, 2012; pp. 29–35. [Google Scholar]
- Gurav, R.G.; Jadhav, J.P. A novel source of biofertilizer from feather biomass for banana cultivation. Environ. Sci. Pollut. Res. 2013, 20, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil 2013, 364, 145–158. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. J. Plant Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Cohen, J.D.; Bandurski, R.S. Chemistry and physiology of the bound auxins. Annu. Rev. Plant Physiol. 1982, 33, 403–430. [Google Scholar] [CrossRef]
- Ohmiya, A. Effects of auxin on growth and ripening of mesocarp discs of peach fruit. Sci. Hortic. 2000, 84, 309–319. [Google Scholar] [CrossRef]
- Parrado, J.; Bautista, J.; Romero, E.J.; García-Martínez, A.M.; Friaza, V.; Tejada, M. Production of a carob enzymatic extract: Potential use as a biofertilizer. Bioresour. Technol. 2008, 99, 2312–2318. [Google Scholar] [CrossRef] [PubMed]
- Gillooly, M.; Bothwell, T.H.; Torrance, J.D.; MacPhail, A.P.; Derman, D.P.; Bezwoda, W.R.; Mills, W.; Charlton, R.W.; Mayet, F. The effects of organic acids, phytates and polyphenols on the absorption of iron from vegetables. Br. J. Nutr. 1983, 49, 331–342. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).