Abstract
This study aims to investigate the difference in the quality of rice wine fermented with different yeasts (Saccharomyces cerevisiae FBKL2.8022 (Sc) and Wickerhamomyces anomalus FBKL2.8023 (Wa)) by adding Dendrobium officinale (D. officinale). The results showed that the addition of D. officinale improved the physicochemical indices and sensory scores in rice wine and promoted the release of active substances from D. officinale. The addition of D. officinale increased the types and contents of flavor substances in Wa-fermented rice wine and inhibited the generation of flavor substances in Sc-fermented rice wine. Untargeted metabolomics analysis showed that the number of differential metabolites was higher before and after fermentation with D. officinale. The main pathways causing the differences were phenylalanine metabolism and alanine, aspartate and glutamate metabolism (which belong to the amino acid metabolism pathways). This study provides a reference to explore the application value of D. officinale in the fermentation and food industry.
1. Introduction
Dendrobium officinale (D. officinale) is a traditional Chinese herbal medicine rich in polysaccharides, phenols, flavonoids, alkaloids, astragalus, bibenzyl, amino acids and other active ingredients [1]. In recent years, D. officinale, as one of the newly added homologous materials of medicine and food, has attracted extensive attention in the field of fermented wine due to its rich active components, anti-inflammatory and anti-apoptotic activities [2], hypoglycemic and lipid-lowering activities and anti-cancer activities [3].
Synergistic effects can occur between medicinal plants and microorganisms, as nutrients present in medicinal plants help microorganisms to divide and grow, further promoting biotransformation [4]. In recent years, medicinal foods have also been used for fermentation [5], and some medicinal fermentation products have been shown to have activities such as antioxidant and antibacterial effects [6], protection against cell damage [7] and anticytotoxic effects [8]. Fermentation not only retains the nutrients in the raw materials but also decomposes large molecules in raw materials into small molecules that can be easily absorbed by the human body under the action of microorganisms, thus increasing the nutritional value [9].
The use of traditional koji to ferment rice wine has problems such as low enzyme activity, insufficient fermentation capacity, incomplete saccharification of raw materials, low utilization of raw material, and high risk of bacterial contamination [10]. Therefore, fermentation with specific strains of inoculated bacteria has become a significant research topic in recent years. Saccharomyces cerevisiae is the strain most commonly used in fermented wine, with the advantages of high temperature resistance, alcohol resistance, fermentation power and other advantages, but its fermented wine is weak in taste and aroma. However, the positive role of non-saccharomyces in fermented wines has been widely reported [11], and it is believed that non-Saccharomyces have some special enzymatic properties that Saccharomyces cerevisiae does not possess [12]. In addition, non-Saccharomyces have been shown to enhance the floral properties of various types of alcoholic beverages [13] and to have a significant impact on the color and appearance of rice wine and the synthesis of volatile flavor substances during the fermentation process [14].
However, there are few studies on D. officinale rice wine, the fermentation conditions and technology are not mature, the effects of D. officinale on rice wine quality have not been reported, and the effects of Saccharomyces cerevisiae and nonsaccharomyces on the leaching of active substances from D. officinale are unknown. Two strains used in this study, Saccharomyces cerevisiae FBKL2.8022 (Sc) and Wickerhamomyces anomalus FBKL2.8023 (Wa) were isolated and screened from traditional Guizhou xiao [15] and stored in our lab (Guizhou Provincial Key Laboratory of Fermentation Engineering and Biopharmaceuticals). They have a low yield of higher alcohols, making them suitable for fermenting rice wine. They can not only increase the biological activity of D. officinale, but also reduce the yield of higher alcohols in the liquor. They have good application value.
In the present study, the effects of D. officinale on the physicochemical index and the content of active substances such as polysaccharides, phenols, flavonoids, free amino acids and volatile flavor components of rice wine were investigated. Based on nontargeted metabolomics analysis, the characteristics of the two strains of fermented rice wine and the effects of D. officinale on the quality of rice wine were compared for the perspective of metabolites. The results of this study demonstrate the potential value of D. officinale, provide theoretical support for the development of new rice wine products and provide ideas for food applications of D. officinale.
2. Materials and Methods
2.1. Chemicals and Reagents
The yeast strains Saccharomyces cerevisiae FBKL2.8022 (Sc) and Wickerhamomyces anomalus FBKL2.8023 (Wa) were obtained from the Key Laboratory of Fermentation Engineering and the Biological Pharmacy of Guizhou Province, CCTCC NO: M2 019406, M2 019412, respectively [15].
D. officinale was obtained from Green Spring Agricultural Development Co., Ltd. (Guiding, China). Organic glutinous rice was purchased from Beijing Hoyatang Food Co., Ltd. (Beijing, China). α-amylase and glucoamylase were purchased from Solarbio Life Sciences Co., (Beijing, China). Gallic acid, rutin, potassium bromide green phenol, feline-phenol and sulfosalicylic acid were purchased from Yuanye Biotechnology Co., (Shanghai, China). The amino acid standards were purchased from Guangmao Biotechnology Co., (Shijiazhuang, China). All other chemicals and reagents were analytical grade.
2.2. Rice Wine Fermentation
The rice wine fermentation process is shown in Figure 1a, and the specific parameters were as follows: The solid–liquid ratio was glutinous rice/water = 1/1.5 (g/V), D. officinale powders accounted for 5% of the glutinous rice, glucoamylase accounted for 0.56% of the glutinous rice, α-amylase accounted for 1.89% of the glutinous rice and the inoculum capacity was 1 × 106 bacteria per milliliter of fermentation broth. The mixture was sealed and placed at 30 °C for incubation. The rice wine was weighed and recorded every 24 h until the weight loss was less than 0.2 g/day. After fermentation, the relevant components of the wine were measured and compared. Each group was set in three parallels. Four rice wines were fermented in different modes: Sc fermented without D. officinale (SC), Sc fermented with D. officinale (DOSC), Wa fermented without D. officinale (WA) and Wa fermented with D. officinale (DOWA).
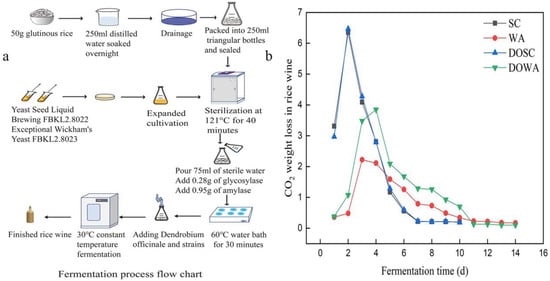
Figure 1.
(a) Flowchart of fermentation process, (b) changes in CO2 weight loss in rice wine.
2.3. Sensory Evaluation and Determination of Physicochemical Indices
At the end of the fermentation, an appropriate amount of fermentation broth was placed in a centrifuge tube and centrifuged at 10,000 rpm for 5 min. The supernatant was filtered through a 0.45 μm filter membrane and left on standby.
The sensory evaluation of the rice wine was carried out in relation to the official method of analysis of Guizhou Province (T/GZSX 017–2020), with slight modifications. Ten food professionals were randomly selected to evaluate rice wine based on four indexes: color and appearance, aroma, taste and style. Each index was worth 25 points out of 100. The evaluation standard is shown in Table S1.
The alcohol content was determined using an S-10 biosensor analyzer (Silman Technology Co., Shenzhen, China). Rice wine was diluted 400 times, and 25 μL was manually injected into the sample to measure the mass of ethanol. The alcohol content was obtained by dividing the mass of ethanol by the density of ethanol at 20 °C. The total sugar content was determined using the phenol-sulfuric acid method [16], and the reduction in sugar content was determined using the DNS method [17]. Total acid was tested according to the Chinese national standard (GB/T 13662–2018), and a precision pH meter PHS-3C (Shanghai Nissima Scientific Instruments Co., Shanghai, China) was used for pH determination.
2.4. Determination of the Content of the Active Substance
A total of 10 mL of rice wine was mixed with 30 mL of anhydrous ethanol and left overnight. Crude polysaccharides were obtained by centrifugation, redissolved, and freeze-dried. The polysaccharide content was then determined using the phenol sulfuric acid method [16]. The content of total phenols and flavonoids was determined by referring to the method of Blanca et al. [18], with slight modifications. For free amino acids, the content and composition were measured according to Chinese standards (GB5009.124–2016).
2.5. Determination of Volatile Flavor Substances in Rice Wine
The volatile components in rice wine were determined using headspace solid-phase microextraction coupled with gas chromatography mass spectrometry (SPME–GC–MS) using a 7890A–5975C gas chromatograph mass spectrometer (Agilent Technologies Inc., Santa Clara, CA, USA). The method was referenced from Yan [19] and Yu [20] with slight modifications. A total of 5.0 mL of the rice wine mixture was added to a 20 mL extraction bottle, followed by the addition of 0.2 g of NaCl and 40 μL of 2-octanol (0.18 mg/mL) as an internal standard. The mixture was shaken well and sealed, and then take subjected to a water bath at 60 °C for 10 min for Hheat preservation. Afterward, headspace extraction was carried out for 30 min, followed by sample injection and desorption for 3 min. The chromatographic conditions included a DB–Wax column (30 m × 0.25 mm, 0.25 μm), split-free mode, column box temperature of 40 °C, inlet temperature of 250 °C and helium (He) flow rate of 1.00 mL/min. The heating program involved maintaining the initial column temperature at 40 °C for 1 min, increasing to 180 °C at 5 °C/min for 1 min and then reaching 230 °C at 8 °C/min for 7 min. For the MS conditions, the ionization mode was EI with an energy of 70 eV, ion source temperature of 250 °C, interface temperature of 250 °C, solvent extension time of 3 min and a quality scanning range of m/z 35~550. Qualitative and quantitative analysis were performed by conducting a preliminary search through the computer spectrum library (NIST 11). Substances with a matching degree greater than 70 were selected for qualitative analysis combined with references, and the relative content of each aroma component was determined using the internal standard method.
2.6. Sample Preparation and Analysis of Metabolites
A total of 50 μL of the sample was transferred to an EP tube. After the addition of 200 μL of extract solution (acetonitrile:methanol = 1:1, containing an isotopically labeled internal standard mixture), the samples were vortexed for 30 s, sonicated for 10 min in an ice-water bath and incubated for 1 h at −40 °C to precipitate the proteins. The sample was then centrifuged at 12,000 rpm (RCF = 13,800× g), R = 8.6 cm) for 15 min at 4 °C. The resulting supernatant was transferred to a fresh glass vial for analysis. The quality control (QC) sample was prepared by mixing equal amounts of supernatants from all the samples.
LC–MS/MS analyses were performed using a UHPLC system (Vanquish, Thermo Fisher Scientific, Waltham, MA, USA) with a UPLC BEH Amide column (2.1 mm × 100 mm, 1.7 μm) coupled to a Q Exactive HFX mass spectrometer (Orbitrap MS, Thermo). Mobile phase A was an aqueous solution of 25 mmol/L ammonium acetate and 25 mmol/L ammonium hydroxide (pH = 9.75), and mobile phase B was acetonitrile. The auto-sampler temperature was 4 °C, and the injection volume was 2 μL.
A QE HFX mass spectrometer was used for its ability to acquire MS/MS spectra on information-dependent acquisition (IDA) mode in the control of the acquisition software (Xcalibur, Thermo). In this mode, the acquisition software continuously evaluates the full MS spectrum scan. The ESI source conditions were established as follows: the sheath gas flow rate was 30 Arb, the Au gas flow rate was 25 Arb, the capillary temperature was 350 °C, full MS resolution was 60,000, MS/MS resolution was 7500, collision energy was 10/30/60 in NCE mode and the spray voltage was 3.6 kV (positive) or −3.2 kV (negative), respectively.
The raw data were converted to the mzXML format using ProteoWizard and processed using an internal program which was developed using R and based on XCMS for peak detection, extraction, alignment and integration. Then, an internal MS2 database (BiotreeDB) was applied to the metabolite annotation. The annotation cutoff was set to 0.3.
2.7. Statistical Analysis
All experiments were repeated three times and the results were expressed as the mean ± standard deviation (SD). A one-way analysis of variance (ANOVA) and independent sample t-test (analysis) were used to determine statistically significant differences at the 95% confidence level (p < 0.05). Multiple comparisons were statistically analyzed using SPSS26. Origin 2018 was used for chart drawing and data analysis.
3. Results and Discussion
3.1. Sensory Analysis and Basic Physicochemical Indexes of Rice Wine
Figure 1b shows the variation in the weight loss of CO2 during rice wine fermentation. Rice wine (SC) fermented with Sc lost less than 0.2 g/day of CO2 on day 10, indicating the end of rice wine fermentation, while rice wine (WA) fermented with Wa finished fermentation on day 14. Sc had a faster fermentation rate and shorter fermentation period than Wa. When D. officinale was added for fermentation, it was found that the fermentation period of DOSC and DOWA rice wine did not change compared to SC and WA (fermented without D. officinale). And the interesting finding was that the addition of D. officinale led to a significantly higher weight loss of DOWA, indicating that D. officinale could improve the fermentation performance of Wa.
In terms of the physical state of the rice wine, DOSC and DOWA had cloudy, viscous, light green colors and no aroma before fermentation, while after fermentation, they had a clarified, bright and uniform orange color and the characteristic mellow sweet aroma of rice wine with rice a wine fermentation style. Accordingly, it was tentatively judged that both Sc and Wa could effectively utilize D. officinale for fermentation. According to the sensory scores of rice wine (Table S1), the addition of D. officinale significantly improved the sensory scores of Wa-fermented rice wine.
The basic physicochemical indices of rice wine obtained using different fermentation modes are shown in Table S2. The alcohol content of SC was significantly higher than that of WA, while the reducing sugar, total sugar, and pH contents were low and significantly lower than that of WA, indicating that Sc was able to utilize sugar fermentation to a greater extent to produce ethanol, while the sugar utilization rate by Wa was low, which was consistent with the trend of weight loss of CO2 in both yeasts. The total sugar of DOSC was significantly higher than that of SC after the addition of D. officinale, while there were no significant differences between the two rice wines in the content of other indexes, indicating that the addition of D. officinale had no significant effect on the fermentation performance of Sc. All DOWA physicochemical indices were significantly better than those of WA, especially the alcohol content, which was 1.89 times higher than that of WA, which revealed that the addition of D. officinale could significantly improve the physicochemical quality of fermented Wa rice wine. Thus, it can be seen that the addition of D. officinale had a significant effect on the quality of rice wine fermented by Sc or Wa.
3.2. Analysis of Active Substances
There are many active substances in D. officinale, such as polysaccharides, flavonoids, tannins and phenolics, which are the main active substances [21]. The microbial fermentation process can secrete various biological enzymes [22], which are involved in the breakdown of plant cell walls, allowing the release of active substances from the plant. As shown in Table S3, the polysaccharide contents of SC and WA was 0.44 mg/mL and 1.05 mg/mL, respectively. After fermentation with D. officinale, the polysaccharide content of DOSC and DOWA was 2.40 mg/mL and 2.91 mg/mL, respectively, which increased 5.45 times and 2.77 times, respectively. It was indicated that both Sc and Wa could promote the leaching of D. officinale polysaccharides. Li et al. [23] reported that polysaccharides were easily degraded and utilized by microorganisms during fermentation, and that fermentation promotes leaching of D. officinale polysaccharides and the expression of their functional activities.
The total flavonoid content of SC was 27.62 mg/L, and the total flavonoid content of WA was 87.23 mg/L. After fermentation with D. officinale, the total flavonoid content of DOSC was 137.73 mg/L and the total flavonoid content of DOWA was 157.07 mg/L, which increased 4.98 and 1.80 times, respectively. The trend in the total phenol content in rice wine with different fermentation methods was similar to that of the total flavonoids. The total phenol content of SC was 641.88 mg/L, the total phenol content of WA was 822.47 mg/L, and the total phenol content of DOSC and DOWA increased by 236.15 mg/L and 103.71 mg/L, respectively, compared to the control group (SC and WA). These results suggested that both Sc and Wa can promote the leaching of flavonoids and phenols from D. officinale, and the effect of Sc was stronger.
The acid environment provided by microbial fermentation was conducive to the dissolution of phenolic and flavonoid substances from D. officinale. Flavonoid components such as naringenin, quercetin and rutin in D. officinale have antioxidant, hypoglycemic, cholesterol-lowering, cardiovascular system-protecting and anti-aging activities [24]. D. officinale contained phenols such as 3,4′-Dihydroxy-5-methoxy benzyl, Dendrocandin C and Dendrocandin D [25], which are closely related to antioxidant, immune enhancing and antitumor effects, and the dissolution of these active substances was helpful in improving the function of rice wine.
3.3. Analysis of Free Amino Acids
Table S4 shows that there were significant differences in the content of free amino acids in rice wine fermented using different methods. The total free amino acid content was 64.95 mg/100 g for SC and 158.65 mg/100 g for WA, indicating that Wa was better at synthesizing free amino acids than Sc, in agreement with the findings of Sahin Busra et al. [26]. After the addition of D. officinale, the total free amino acid content of DOSC was 97.08 mg/100 g, which was 32.13 mg/100 g higher than SC. These additional free amino acids might come from the microbial transformation of D. officinale. The amino acids in rice wine were derived mainly from the degradation of proteins in the raw material by the proteases and autolysis of yeast and other microorganisms [27]. The addition of D. officinale increased substrate concentration and facilitated the growth and reproduction of yeast, which may be one of the reasons why the amino acid content in DOSC rice wine was higher than its control (SC). The content of fresh and sweet amino acids increased significantly after the addition of D. officinale (Figure 2b). This increase in amino acids may have been due to the degradation and utilization of raw materials by microorganisms, as D. officinale contains a large amount of fresh and sweet amino acids [28]. However, the high proportion of bitter amino acids in the total free amino acids was detrimental to the flavor and taste of the rice wine. The proportion of bitter amino acids in the DOWA of rice wine was significantly reduced by adding fermentation of D. officinale.
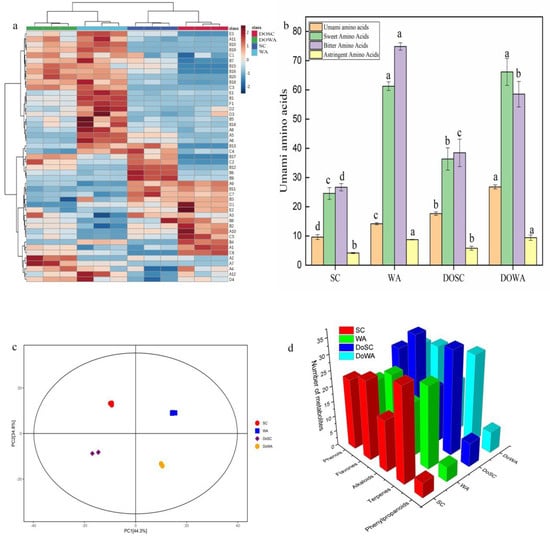
Figure 2.
(a) Heatmap of volatile flavor compounds in rice wine (compound numbers are the same as in Supplementary Table S5). (b) Compositions of flavor amino acids in rice wine. Different lowercase letters indicate significant differences among groups (p < 0.05); (c) PCA of samples with different fermentation methods; (d) metabolite classification statistics chart.
3.4. Analysis of Volatile Flavor Substances in Rice Wine
3.4.1. Comparative Analysis of Volatile Components Classification
A total of 47 volatile components were detected in the four groups of rice wine, including 12 alcohols, 20 esters, 7 acids, 4 aldehydes, 3 ketones and 1 volatile phenol (Table S5). A total of 39 volatile components were found in both SC and WA, with 11 alcohols and 15 esters in SC, and 9 alcohols and 18 esters in WA. It revealed that Sc was good at producing alcohols, while Wa was good at synthesizing aromatic esters. The results were consistent with the fermentation characteristics of the two yeasts reported in the previous study [15]. After adding D. officinale fermentation, 35 volatile components were found in DOSC, and the types and contents of volatile flavor substances in rice wine decreased compared to those of the control group (SC). There were 44 volatile components in rice wine in the DOWA, including 11 alcohols and 19 esters, and the types and contents of volatile flavor substances in rice wine increased compared to those of the control group (WA). Therefore, D. officinale helped improve the flavor of WA rice wine.
3.4.2. Cluster Analysis of Volatile Flavor Substances in Rice Wine
The volatility profiles of the four groups of rice wine were similar, and the HCA model was developed to discuss the volatile flavor substances in rice wine more clearly and to be able to use a more targeted multivariate analysis to differentiate them. As shown in Figure 2a, differences in the 47 volatile compounds in each rice wine were observed.
The content and type of high-grade alcohols in rice wine determine the characteristic aroma substances and taste of fermented wine [29]. An appropriate amount of high-grade alcohol can make the wine soft, rich, thick and long-lasting in flavor, while too little makes the wine taste thin, and too much can easily cause severe headaches and have adverse effects on human health [30]. Therefore, the control of alcohol content is crucial to the quality of rice wine. The alcohols 2-methyl-1-propanol (A3), 3-methyl-1-butanol (A4), 2,3-butanediol (A9) and phenylethyl alcohol (A12) are the most abundant senior alcohols in rice wine. A12 is one of the few high-grade alcohols with a pleasant floral aroma, and 1-hexanol (A6) and A4 also impart strong fruity, floral, whisky and other aromas to fermented wines [31]. The contents of A3, A4, A6 and A12 in DOSC decreased significantly, while the contents of these higher alcohols in DOWA increased significantly, indicating that D. officinale could inhibit the production of higher alcohols by Sc, but promote the production of higher alcohols by Wa.
Esters are a major secondary metabolite produced through yeast metabolism during fermentation and are the main source of the strong fruity aroma of rice wine. A total of 31 esters were identified in a quantitative analysis of aromatic compounds in white wine by Du et al. [32], representing up to 55% of the total. The esters in rice wine were mainly 1-butanol, 3-methyl, acetate (B2), hexanoic acid, ethyl ester (B3), octanoic acid, ethyl ester (B6), decanoic acid, ethyl ester (B9), acetic acid, 2-phenylmethyl ester (B11), dodecanoic acid, ethyl ester (B12), hexadecanoic acid and ethyl ester (B18), similar to the findings of Zhao Jin et al. [33]. The content of esters in SC was higher than in WA, but the types of esters in WA were more abundant. D. officinale inhibited the production of esters in Sc and promoted the production of ester in Wa. Furthermore, the WA and DOWA groups of rice wine contained extremely high levels of ethyl acetate (B1), and the WA and DOWA groups also contained benzoic acid, ethyl ester (B10), ethyl oleate (B19) and linoleic acid ethyl ester (B20), which did not exist in the remaining two groups of rice wine. These esters mostly have typical fruity and floral aromas such as pineapple, pear, coconut, jasmine and iris [34], and make an important contributions to the enrichment of the flavor levels in rice wine.
As an important flavoring substance in rice wine, the content of acids directly influences the taste of rice wine. The highest content in rice wine was acetic acid (C1). The addition of D. officinale was able to promote the production of C1 by Wa and inhibit the production of C1 by Sc. Low levels of octanoic acid (C7) and hexanoic acid (C6) can impart fruity and floral aromas to rice wine, but higher levels produce rancidity and astringency [35]. Therefore, a higher acid content is not better. In this study, the low content of C7 and C6 in rice wine was beneficial for the formation of the flavor of rice wine.
In summary, Sc-fermented rice wine had fewer types and a higher total content of volatile flavor substances, and D. officinale had an inhibitory effect on the types and content of volatile flavor substances in rice wine. When Wa was used for fermentation, there were many types of volatile flavor substances, and the total content was low. The effect of D. officinale on the types and content of volatile flavor substances was generally promoted in rice wine.
3.5. Untargeted Metabolomics Analysis of Rice Wine
3.5.1. Metabolite Classification Statistics and PCA Analysis
The secondary profiles of the metabolites were matched using the secondary profiles of the public database standards, the substances identified in the secondary profiles were subjected to principal component analysis based on the HMDB database classification information, and the PCA plots of the relationships between the total samples of positive and negative ion patterns are shown in Figure 2c. Each group of samples had good clustering, indicating that the intragroup differences of the samples were small and reproducible. SC was on the negative half-axis and WA was on the positive half-axis of the first principal component (PC1), indicating that there was a significant difference in the first principal component between fermented rice wine with two different yeast strains. For rice wine fermented with D. officinale, both rice wines were on the negative half-axis of the second principal component (PC2), and rice wines fermented without D. officinale were on the positive half-axis, indicating that the effect of D. officinale on the metabolites of the two yeast strains was different.
Based on the classification information in the HMDB database (URL), the metabolites in each group of rice wine were further identified and analyzed using the secondary spectrum of standard substances in the public database for matching. A total of 621 substances were identified. A total of 128 phenols, flavonoids, terpenoids, alkaloids and phenylpropanoids identified in the four groups of rice wines were analyzed for relative quantification, and 3D bar graphs of the number of each type of active metabolite are shown in Figure 2d.
Compared to WA rice wine, SC had more types of active substances, with 102 SC and 93 WA, and Sc and Wa were able to synthesize active substances on their own during the fermentation of the rice wine. The relative content of phenolics was the highest among the total active substances in both groups of rice wines, and the relative content of flavonoids was lower, which was consistent with previous results in the determination of the active substance content in rice wine. The relative content of phenols in WA was higher than that of SC. The previous results on the metabolic pathway of rice wine found that the differences in the amino acid metabolic pathway between WA and SC were mainly upregulated. The free amino acid content in the rice wine was high, and the ability of Wa to use these amino acids in large quantities to produce phenols during fermentation may have been one of the reasons for the higher relative content of phenols in the WA rice wine.
When comparing DOSC and DOWA with rice wine without D. officinale (SC and WA), DOSC increased by 18 active substances and DOWA increased by 10 active substances. In terms of the relative content of active substances, most of the active substances in rice wine after the addition of D. officinale increased. For example, the relative percentage of phenolic substances in DOSC increased by 4.43%, and DOWA increased by 1.52%. Phenols and flavonoids usually have anti-aging and antioxidant effects and contribute more to the bioactivity of rice wine [32]. Alkaloids are one of the active substances in D. officinale, which have antipyretic and immune regulatory effects [36]. Studies have shown that lignans exhibit inhibitory activity against the tyrosinase enzyme and have anti-aging potential [37]. These data show that the addition of D. officinale fermentation can effectively promote the leaching of beneficial substances and increase the quality of rice wine. Furthermore, yeast fermentation was shown to contribute to the release of active substances, especially phenolic substances, in the raw materials of D. officinale.
3.5.2. Analysis of the Enrichment of the KEGG Metabolite Pathway
The metabolites identified from the different fermentation methods were submitted to the KEGG website for enrichment analysis of metabolic pathways and listing of the most divergent metabolic pathways, which were presented in a differential abundance score (DA Score) graph or a bubble graph for pathway analysis.
The metabolites in rice wine fermented without D. officinale were compared. Both positive and negative ion modes showed that fermentation with single strains of Sc and Wa produced a higher number of differential metabolites. The difference analysis of WA–SC metabolic pathways is shown in Figure 3. The overall expression of all metabolic pathways in the positive ion mode was upregulated, indicating that the metabolites produced through these pathways were more in WA and less in SC, with significant differences between the two rice wines. Among them, D-amino acid metabolism, arginine and proline metabolism, phenylalanine metabolism, amino acid biosynthesis and cyanoamino acid metabolism belonged to the amino acid metabolism pathways. The metabolites generated by amino acid metabolism in WA were higher than in SC. The above analysis of free amino acids in fermented rice wine revealed that the content of free amino acids in WA was higher than in SC, which was consistent with the study of metabolic pathways here. Amino sugar and nucleotide sugar metabolism, galactose metabolism belonging to the sugar metabolism pathway and sugar metabolites were upregulated in WA rice wine relative to SC.
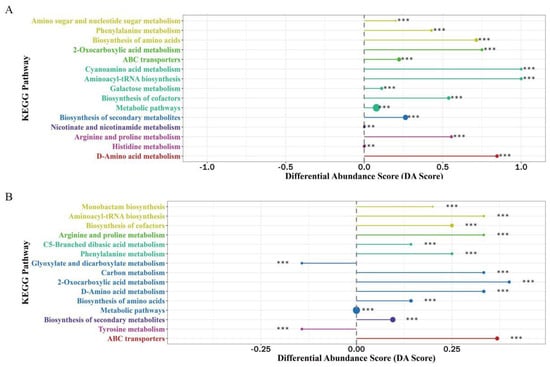
Figure 3.
Differential abundance score (DA Score) plot for group WA–SC. (A) Positive ion mode, (B) negative ion mode. DA score reflects the overall changes of all metabolites in the metabolic pathway. The size of the dots represents the number of differential metabolites annotated in the pathway, and the distribution of dots on the central axis represents the overall expression of the pathway (upregulated or downregulated). *** means p (<0.001) value.
The overall expression of most of the metabolic pathways in the negative ion mode was upregulated, similar to the results in the positive ion mode. It should be noted that the overall expression of glyoxylate and dicarboxylate metabolism and tyrosine metabolism were negatively regulated, and the main metabolites in these two pathways were amino acids and fatty acids, indicating that more metabolites such as amino acids were produced through these two pathways than WA in SC after fermentation, and the difference between them was significant. Tyrosine metabolism, as one of the key metabolic pathways for phenolic and flavonoid metabolites in rice wine [38], has a greater impact on active metabolites such as phenolic and flavonoids in rice wine. Sc probably used the amino acids produced through the tyrosine metabolism pathway to eventually synthesize active substances such as phenols and flavonoids.
3.5.3. Effect of D. officinale on the Enrichment of the Metabolite Pathway of Fermented Rice Wine
A comparison of metabolites in rice wine fermented with and without D. officinale revealed a significant effect of D. officinale on the metabolites rice wine, producing more differential metabolites and involving complex metabolic pathways. The pathways with greater differentials are shown in Figure 4. The most significant metabolic pathways that differed between DOSC–SC were phenylalanine metabolism and alanine, aspartate and glutamate metabolism, both of which are amino acid metabolic pathways. During the fermentation process, microorganisms can use the free amino acids in the raw material to produce active substances such as phenols and flavonoids through these amino acid metabolic pathways. Phenylalanine metabolism was the main pathway for phenol and flavonoids metabolism in rice wine, such as cinnamic acid, ferulic acid and erucic acid [39]. Therefore, the elevated content of free amino acids and active substances in rice wine after the addition of D. officinale may be related to these amino acid metabolic pathways. The most significant metabolic pathways that differed between DOWA and WA were phenylalanine metabolism, beta-alanine metabolism and nicotinate and nicotinamide metabolism, which to some extent contributed to the production of amino acids in DOWA rice wine. This influenced the synthesis of metabolites such as phenolic flavonoids in rice wine. However, in general, the differences between DOSC and SC in these amino acid metabolic pathways were more significant than those between DOWA and WA, which further demonstrated that Sc was more favorable than Wa for the leaching of active substances from D. officinale raw materials.
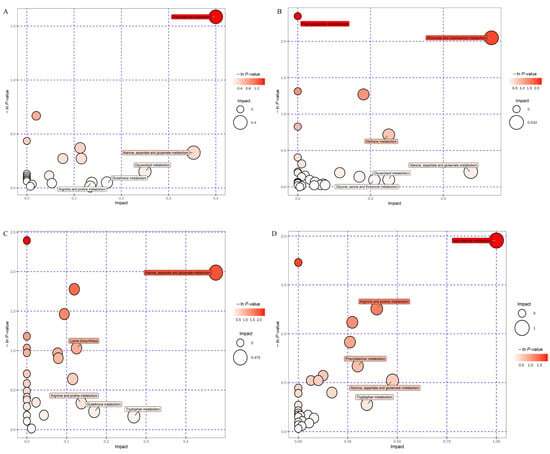
Figure 4.
Bubble plots of differential pathway analysis for rice wine. (A) Pathway analysis diagram of DOSC–SC in positive ion mode, (B) pathway analysis diagram of DOSC–SC in negative ion mode, (C) pathway analysis diagram of DOWA–WA in positive ion mode, (D) pathway analysis diagram of DOWA–WA in negative ion mode. Each bubble in the bubble diagram represents a metabolic pathway. The horizontal coordinate of the bubble and the size of the bubble indicate the influence factor of the pathway in the topological analysis. The larger the value, the greater the influence factor. The vertical coordinate of the bubble and the color of the bubble indicate the p value of the enrichment analysis (taking the negative natural logarithm, −ln (p)). The darker the color, the smaller the p value and the more significant the enrichment degree.
4. Conclusions
The effects of different fermentation methods on rice wine quality were compared in terms of sensory evaluation, physicochemical indexes, the content of active substances (polysaccharides, total phenols, total flavonoids, etc.), free amino acids and the content of volatile flavor components. The addition of D. officinale significantly improved the sensory score, physicochemical index and the content of active substances in rice wine. Furthermore, the effects of Sc fermentation on the types and total contents of volatile flavor substances were generally inhibited. However, the effects of Wa fermentation on the types and total contents of volatile flavor substances in rice wine were generally promoted. Based on the results of the untargeted metabolomics, the number of differential metabolites and the metabolic pathways involved in DOSC–SC and DOWA–WA were higher, indicating that D. officinale had a different effect on the two strains. The main pathways that caused the differences were phenylalanine metabolism and alanine, aspartate, and glutamate metabolism, which significantly increased the content of active substances in rice wine with D. officinale. A total of 621 types of major differential active metabolites were identified in rice wine of the four groups, among which phenols, flavonoids, terpenoids, alkaloids and phenylpropanoids were the most abundant. The types and relative contents of most of the active substances in rice wine increased after the addition of D. officinale, further demonstrating both yeast fermentation and the release of active substances in D. officinale.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation9070627/s1, Table S1: Sensory evaluation of rice wine; Table S2: Basic physical and chemical indices of rice wine; Table S3: Active substance contents in rice wine; Table S4: Compositions of free amino acids in rice wine; Table S5: Volatile Components contents of Rice Wine.
Author Contributions
L.C.: data curation, formal analysis, writing—review and editing. X.S.: data curation, investigation, methodology, software, writing—original draft. L.Z.: investigation, visualization. S.Q., investigation, validation. X.Z., investigation, validation. Y.D., investigation, validation. C.W. (Chunxiao Wang), validation, supervision. C.W. (Chaoyang Wei), data curation, formal analysis, funding acquisition, methodology, supervision, project administration, writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 32160566) and the National Natural Science Foundation of China (No. 32060518), Natural Science Special Project (Special Post) Scientific Research Foundation of Guizhou University (No. 07, 2020), Guizhou Provincial Science and Technology Projects (ZK [2022] General 114), Key Laboratory of Wuliangye-flavor Liquor Solid-state Fermentation, and China National Light Industry (2021JJ014).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, X.; Zhang, C.; Wang, N.; Xu, Y.; Tang, G.; Xu, L.; Feng, Y. Bioactivities and Mechanism of Actions of Dendrobium officinale: A Comprehensive Review. Oxidative Med. Cell. Longev. 2022, 22, 6293355. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.S.; Ruan, W.F.; Wang, Z.Q. Dendrobium officinale polysaccharide inhibits vascular calcification via anti-inflammatory and anti-apoptotic effects in chronic kidney disease. FASEB J. 2022, 36, e22504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhou, X.T.; Wang, J.Q.; Zhou, Y.J.; Qi, W.C.; Chen, H.H.; Nie, S.P.; Xie, M.Y. Dendrobium officinale polysaccharide triggers mitochondrial disorder to induce colon cancer cell death via ROS-AMPK-autophagy pathway. Carbohydr. Polym. 2021, 264, 118018. [Google Scholar] [CrossRef] [PubMed]
- Sauer, S.; Dlugosch, L.; Milke, F.; Brinkhoff, T.; Kammerer, D.R.; Stintzing, F.C.; Simon, M. Succession of Bacterial and Fungal Communities during Fermentation of Medicinal Plants. Fermentation 2022, 8, 383. [Google Scholar] [CrossRef]
- Wang, Y.; Jung, Y.J.; Kim, K.H.; Kwon, Y.; Kim, Y.J.; Zhang, Z.; Kang, H.S.; Wang, B.Z.; Quan, F.S.; Kang, S.M. Antiviral Activity of Fermented Ginseng Extracts against a Broad Range of Influenza Viruses. Viruses 2018, 10, 471. [Google Scholar] [CrossRef]
- Eom, S.J.; Hwang, J.E.; Jung, J.; Jee, H.-S.; Kim, K.-T.; Paik, H.-D. Short communication: Antioxidative and antibacterial activities on Staphylococcus aureus and Escherichia coli O157:H4 in milk with added ginseng marc extract fermented by Lactobacillus plantarum KCCM 11613P. J. Dairy Sci. 2017, 100, 7788–7792. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.; Dong, M. Flavonoids of Kudzu Root Fermented by Eurtotium cristatum Protected Rat Pheochromocytoma Line 12 (PC12) Cells against H2O2-Induced Apoptosis. Int. J. Mol. Sci. 2017, 18, 2754. [Google Scholar] [CrossRef]
- Xiao, Y.; Han, F.; Lee, I.-S. Biotransformation of the Phenolic Constituents from Licorice and Cytotoxicity Evaluation of Their Metabolites. Int. J. Mol. Sci. 2021, 22, 10109. [Google Scholar] [CrossRef]
- Zhao, C.; Su, W.; Mu, Y.C.; Jiang, L.; Mu, Y. Correlations between microbiota with physicochemical properties and volatile flavor components in black glutinous rice wine fermentation. Food Res. Int. 2020, 138, 109800. [Google Scholar] [CrossRef]
- Yang, H.Y.; Peng, Q.; Zhang, H.B.; Sun, J.Q.; Shen, C.; Han, X.Y. The volatile profiles and microbiota structures of the wheat Qus used as traditional fermentation starters of Chinese rice wine from Shaoxing region. LWT 2022, 154, 112649. [Google Scholar] [CrossRef]
- Borren, E.; Tian, B. The Important Contribution of Non-Saccharomyces Yeasts to the Aroma Complexity of Wine: A Review. Foods 2021, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Shiqi, L.; Pengfei, B.; Nan, S.; Zhiyi, G.; Xiaowen, C.; Jing, G. Characterization of different non-Saccharomyces yeasts via mono-fermentation to produce polyphenol-enriched and fragrant kiwi wine. Food Microbiol. 2021, 103, 103867. [Google Scholar]
- Chua, J.Y.; Lu, Y.; Liu, S.Q. Evaluation of five commercial non-Saccharomyces yeasts in fermentation of soy (tofu) whey into an alcoholic beverage. Food Microbiol. 2018, 76, 533–542. [Google Scholar] [CrossRef]
- Del Fresno, J.M.; Morata, A.; Loira, I.; Bañuelos, M.A.; Escott, C.; Benito, S.; González Chamorro, C.; Suárez-Lepe, J.A. Use of non-Saccharomyces in single-culture, mixed and sequential fermentation to improve red wine quality. Eur. Food Res. Technol. 2017, 243, 2175–2185. [Google Scholar] [CrossRef]
- Chunxiao, W.; Jiadai, T.; Shuyi, Q. Profiling of Fungal Diversity and Fermentative Yeasts in Traditional Chinese Xiaoqu. Front. Microbiol. 2020, 11, 2103. [Google Scholar]
- Chen, F.; Huang, G. Antioxidant activity of polysaccharides from different sources of ginseng. Int. J. Biol. Macromol. 2018, 125, 906–908. [Google Scholar] [CrossRef] [PubMed]
- Juan, M.-P.; Yuranis, J.-A.; Eduardo, S.-T.; Dario, G.-D.Á.; Karina, A.O. Variables Affecting Delignification of Corn Wastes Using Urea for Total Reducing Sugars Production. ACS Omega 2020, 5, 12196–12201. [Google Scholar]
- Blanca, E.-L.; Isabel, C.; Griselda, H.-M.; Damaso, H.-M.; Angel, G.-I.; Sonia, M.; Federico, F.; Genoveva, B.; Francisco, M.; Maria-Soledad, F.-P. Fermented orange juice: Source of higher carotenoid and flavanone contents. J. Agric. Food Chem. 2013, 61, 8773–8782. [Google Scholar]
- Yan, S.; Xiangsong, C.; Xiang, X. Improvement of the aroma of lily rice wine by using aroma-producing yeast strain Wickerhamomyces anomalus HN006. AMB Express 2019, 9, 89. [Google Scholar] [CrossRef]
- Yu, H.; Xie, T.; Xie, J.; Ai, L.; Tian, H. Characterization of key aroma compounds in Chinese rice wine using gas chromatography-mass spectrometry and gas chromatography-olfactometry. Food Chem. 2019, 293, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Ren, Z.; Zhang, J.; Song, X.; Gao, Z.; Jing, H.; Li, S.; Wang, S.; Jia, L. Antioxidant and anti-hyperlipidemic effects of mycelia zinc polysaccharides by Pleurotus eryngii var. tuoliensis. Int. J. Biol. Macromol. 2017, 95, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Gaensly, F.; Agustini, B.C.; Silva, G.A.d.; Picheth, G.; Bonfim, T.M.B. Autochthonous yeasts with β-glucosidase activity increase resveratrol concentration during the alcoholic fermentation of Vitis labrusca grape must. J. Funct. Foods 2015, 19, 288–295. [Google Scholar] [CrossRef]
- Lixia, W.; ChiYu, L.; Chen, H.; PiSen, G.; MengZong, L.; ShaoHua, Z. Purification and Structural Characterization of Dendrobium officinale Polysaccharides and Its Activities. Chem. Biodivers. 2021, 18, e2001023. [Google Scholar]
- Li, Y.Y.; Lyu, C.H.; Wu, G.; Zheng, Z.B.; Luo, Y.B.; Qin, S. Research progress on molecular mechanism of Dendrobium officinale and its active components to metabolic syndrome. China J. Chin. Mater. Med. 2019, 44, 5102–5108. [Google Scholar]
- Wang, Y.-H. Traditional Uses and Pharmacologically Active Constituents of Dendrobium Plants for Dermatological Disorders: A Review. Nat. Prod. Bioprospect. 2021, 11, 465–487. [Google Scholar] [CrossRef] [PubMed]
- Busra, S.; Isleten, H.M.; Onur, G.; Yonca, K.-Y. Fermented Spirulina products with Saccharomyces and non-Saccharomyces yeasts: Special reference to their microbial, physico-chemical and sensory characterizations. Food Biosci. 2022, 47, 101691. [Google Scholar]
- Yang, Y.; Xia, Y.; Wang, G.; Tao, L.; Yu, J.; Ai, L. Effects of boiling, ultra-high temperature and high hydrostatic pressure on free amino acids, flavor characteristics and sensory profiles in Chinese rice wine. Food Chem. 2019, 275, 407–416. [Google Scholar] [CrossRef]
- Hu, J.; Huang, W.X.; Zhang, F.T.; Luo, X.D.; Chen, Y.L.; Xie, J.K. Variability of Volatile Compounds in the Medicinal Plant Dendrobium officinale from Different Regions. Molecules 2020, 25, 5046. [Google Scholar] [CrossRef]
- Rocco, L.; Anna, C.; Samantha, S.; Belinda, K.; Fiona, K. A review on the aroma composition of Vitis vinifera L. Pinot noir wines: Origins and influencing factors. Crit. Rev. Food Sci. Nutr. 2020, 61, 1762535. [Google Scholar]
- Zhong, X.; Wang, A.; Zhang, Y.; Wu, Z.; Li, B.; Lou, H.; Huang, G.; Wen, H. Reducing higher alcohols by nitrogen compensation during fermentation of Chinese rice wine. Food Sci. Biotechnol. 2020, 29, 805–816. [Google Scholar]
- Evangelos, K.; Anastasios, N.; Yiannis, K.; Panagiotis, K. Evaluation of Yeast Strains for Pomegranate Alcoholic Beverage Production: Effect on Physicochemical Characteristics, Antioxidant Activity, and Aroma Compounds. Microorganisms 2020, 8, 1583. [Google Scholar]
- Du, J.; Li, Y.; Xu, J.; Huang, M.; Ye, H. Characterization of key odorants in Langyatai Baijiu with Jian flavour by sensory-directed analysis. Food Chem. 2021, 352, 129363. [Google Scholar] [CrossRef]
- Jin, Z.; Cai, G.; Wu, C.; Hu, Z.; Xu, X.; Xie, G.; Wu, D.; Lu, J. Profiling the key metabolites produced during the modern brewing process of Chinese rice wine. Food Res. Int. 2021, 139, 109955. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.C.; Chen, F.; Wang, L.Y.; Niu, Y.W.; Shu, C.; Chen, H.X.; Xiao, Z.B. Comparison of aroma-active compounds and sensory characteristics of durian (Durio zibethinus L.) wines using strains of Saccharomyces cerevisiae with odor activity values and partial least-squares regression. J. Agric. Food Chem. 2015, 63, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.L.; Shi, Y.; Jiang, R.; Yang, Q.; Wang, Y.Q.; Liu, P.T.; Duan, C.Q.; Yan, G.L. Effects of adding unsaturated fatty acids on fatty acid composition of saccharomyces cerevisiae and major volatile compounds in wine. S. Afr. J. Enol. Vitic. 2016, 36, 285–295. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Lyu, P.; Chen, L.; Shen, C.; Sun, C. Comparative transcriptomic analysis reveal the regulation mechanism underlying MeJA-induced accumulation of alkaloids in Dendrobium officinale. J. Plant Res. 2019, 132, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Munsch, T.; Lanoue, A.; Garros, L.; Tungmunnithum, D.; Messaili, S.; Destandau, E.; Billet, K.; St-Pierre, B.; Clastre, M.; et al. UPLC-HRMS Analysis Revealed the Differential Accumulation of Antioxidant and Anti-Aging Lignans and Neolignans in In Vitro Cultures of Linum usitatissimum L. Front. Plant Sci. 2020, 11, 508658. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Lin, J.B.; Wang, W.Y.; Zou, H.; Dai, Y.M. Analysis of Related Genes in Phytosterol Biosynthesis in Dendrobium officinale Based on Transcriptome Sequencing Technology. J. Trop. Subtrop. Bot. 2019, 27, 693–701. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).