Abstract
The production of cow manure far exceeds the quantity that can be utilized in primary applications such as fertilizer or for the generation of biogas. As a result, alternative value-added applications are being investigated. The purpose of this study is to evaluate the production of lactic acid, using cow manure as the raw material. The methodology involved the implementation of thermochemical pretreatment for the cow manure, followed by simultaneous saccharification and fermentation for lactic acid production. Response surface methodology based on a central composite design was employed to analyze the simultaneous saccharification and fermentation process. The factorial design of the experiments was carried out with three factors, cow manure concentration, temperature, and enzyme concentration, with 80 g·L−1, 50 °C, and 212.5 IU/gCMDry Matter as central point values, respectively. Following the addition of Bacillus coagulans DSM2314 inoculum to enzymatically hydrolyzed cow manure at pH 5.0, after a 24 h period the concentration of lactic acid was recorded at 13.65 g·L−1, with a conversion efficiency of 33.1%. Studies were conducted until 48 h to analyze time impact. Characterization studies for native cow manure and that pretreated using acid reagent were conducted. Sugar content and by-product formation were analyzed, resulting in 23.24 g·L−1 of sugar remaining as the maximum after fermentation, while low values of furfural (1.04 g·L−1), 5-hydroxymethylfurfural (1.35 g·L−1), and acetic acid (1.45 g·L−1) were found. Optimal conditions were calculated at 24 and 48 h with R software, obtaining the lactic acid, with yields of 13.4 g·L−1, 36.28% (for 24 h) and 15.27 g·L−1, 32.76% (for 48 h), respectively. Experimental and statistical studies of enzymatic hydrolysis and fermentation stated that cow manure was a feasible substrate for the production of lactic acid.
1. Introduction
In the culinary, pharmaceutical, cosmetic, and textile industries, lactic acid (LA) is employed as an acidulant and a preservative [1]. In the baking industry, it serves as a precursor in the manufacturing of emulsifiers such as stearoyl-2-lactylates. It performs a wide range of tasks, including flavoring, regulating pH, acting as an acidulant, enhancing the microbiological quality, fortifying minerals, and extending shelf life [2].
Lactic acid can be produced either by chemical synthesis or by the fermentation of renewable carbohydrates. It is possible to produce LA by using biomass as a source of carbohydrates. Lactic acid is an organic acid that occurs naturally and serves as the primary metabolic intermediate in the majority of organisms, including in people and anaerobic prokaryotes [3]. It is classified as Generally Recognized As Safe (GRAS) for general purpose food additives by the United States Food and Drug Administration (USFDA) [4]. Due to the high cost of product recovery and purification, as well as the expensive base materials, production costs are high. So that it can be produced economically, it is essential to find low-cost raw materials for lactic acid fermentation. Typically, lactose, maltose, or glucose are employed in its production [5]. Lactic acid is in high demand as a raw material for the synthesis of poly(lactic acid) (PLA) due to the recent increase in interest in the creation of biodegradable plastic [6]. To produce highly crystalline PLA, which results in the polymer’s high strength and chemical and heat resistant qualities, optically pure lactic acid is required [7].
The most prevalent type of agricultural waste is cow manure, which is also a lignocellulosic substance [8]. Enzymatic hydrolysis into fermentable sugars could successfully disrupt the treated lignocellulosic fraction [9]. Bacillus coagulans DSM 2314 is a fascinating strain to use for manufacturing lactic acid from lignocellulose using a Simultaneous Saccharification and Fermentation (SSAF) technique [10]. With conversion efficiencies above 90 wt.%, it can homoferment glucose and xylose. Furthermore, B. coagulans has a high productivity, from 2.5 to 3 g·L−1·h−1 of lactic acid. It can thrive in surroundings that are slightly acidic and it is a moderate thermophile with an ideal growth temperature of about 50 °C, which is comparable to the ideal circumstances for commercial enzyme combinations like GC220 (Genencor, Denmark) and CTeC2 (Novozymes, Denmark). However, byproducts of processed lignocellulose can hinder the growth of Bacillus coagulans DSM 2314 [11].
Byproducts are produced in every pretreatment techniques. Phenolic compounds, furans, and tiny organic acids have been recognized as the three main groups of byproducts. Based on the quantities present and their inhibitory effects, these byproducts may block the fermentation that leads to the production of biochemicals, reducing productivity, growth, and occasionally the yield of the microorganisms in these processes [12].
The process from cow manure to lactic acid consists of raw material milling, acid pretreatment, and SSAF, as reviewed in [13]. While physical pretreatments involve size reduction and steam explosion, chemical pretreatments involve changing the structure of biomass with solvents that stimulate the breakdown of cellulose, hemicellulose, and lignin [14]. To convert the majority of lignocellulose into dextrose, a fermentable sugar, amylolytic enzymes such as amylase and glucoamylase must first hydrolyze it twice. The first stage is typically rapidly finished at high temperatures (between 90 and 130 °C), and the second stage is typically finished at lower temperatures after an extended saccharification to dextrose process. For many years, this technology has been used on an industrial basis. Industrial enzyme manufacturers like Novozymes and Genencor, for example, offer highly developed, effective, and reasonably priced enzymes for this process. This procedure yields dextrose, which can be used to ferment lactic acid [15].
The bioconversion of carbohydrate materials to lactic acid can be considerably enhanced by combining the microbial fermentation of the resulting sugars and the enzymatic hydrolysis of the carbohydrate substrates into a single phase, known as SSAF [16]. Enzymatic hydrolysis should progress considerably faster when fermentation and enzymatic hydrolysis are combined in an SSAF process, because the microbe can directly absorb the monomerized sugars, reducing product inhibition. Consequently, an SSAF process’ processing time can be significantly reduced [17].
Presently, there are no available tests for using cow manure as a feedstock for lactic acid production. This paper describes the conversion of cow manure into lactic acid through pretreatment and efficient enzymatic hydrolysis and fermentation. Furthermore, this study determined the most common composition of cow manure, inhibitors, byproducts, and LA production performance. Cow manure could be effectively disrupted by enzymatic hydrolysis into fermentable sugars to produce lactic acid. Taken together, the research has implications for all cow farms as it is the first attempt to investigate the potential utilization of cow manure for lignocellulosic–lactic acid in combination with lignocellulosic enzyme production, which could serve as a reference for improving bovine waste economics. The aim of this study was to reach lactic acid productivity in SSAF experiments using cow manure, similar to what has been reported for fermentations using high-grade sugars or lignocellulose as feedstock.
2. Materials and Methods
2.1. Raw Material (Cow Manure)
Raw material was collected in cattle fattening stables of a farm located in Lleida (Spain), with straw and compound feeding regime. Collection was carried out inside the stable and in the manure heap, collecting a total of nine samples in each section. Samples were subjected to drying at 55 °C for 72 h in a SELECTA oven (DIGITRONIC-TFT) and ground to an average diameter of 1 mm in a MOULINEX fruit grinder in 50 g portions for 5 s. Dried and milled cow manure was subjected to experimental steps and analysis.
2.2. Cow Manure Analysis
Cellulose, hemicellulose, and lignin content were determined using an Ankrom 200 fiber analyzer. Samples were weighted in a Mettler-Toledo balance, model XS204. Acid Detergent Fiber (ADF) analysis, Neutral Detergent Fiber (NDF), and Crude Fiber Analysis (CFA) were performed. The ADF reagent consisted of 20 g of cetyl trimethylammonium bromide (CTAB) with 1 L 1.00 N H2SO4 previously standardized. The NDF reagent consisted of 30 g sodium dodecyl sulfate, 18.61 g ethylenediaminetetraacetic disodium salt (dehydrate), 6.81 g sodium borate, 4.56 g sodium phosphate dibasic (anhydrous), and 10 mL of triethylene glycol to 1 L distilled water. pH was controlled from 6.9 to 7.1. CFA reagent consisted of sulfuric acid (72% by weight), and reagent grade H2SO4 diluted to a specific gravity of 1634 g·L−1 at 20 °C (24.00 N) by adding 1200 g H2SO4 to 350 mL H2O in a 1 L of mono chloracetic acid (MCA) volumetric flask with cooling. Solution was standardized to 1634 g·L−1 at 20 °C specific gravity by removing solution and adding H2O or H2SO4 as required. ADF and NDF were performed on 0.5 mg of milled and dried samples, sealed in a filter bag F57 from Ankrom. After analysis, samples were rinsed with tap water and dried at 105 °C for 2 h. After that, ADF, NDF, and CFA were performed. Lignin content was determined directly by CFA. Cellulose was determined as ADF minus CFA. Finally, to account for hemicellulose content, NDF minus ADF and CFA was calculated. Ash [18] and humidity [19] values were obtained using gravimetric analysis.
2.3. Microorganism
Bacillus coagulans DSM 2314 was acquired as freeze-dried stock from the German collection of microorganisms and cell cultures (Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany).
Strain was grown in culture medium, TrypticSoy Broth (TSB), Scharlau 02-200-500, batch 132425. The composition was (per liter of medium) 17 g casein peptone, 3 g soy peptone, 5 g sodium chloride, 2.5 g dipotassium phosphate, 2.5 g dextrose, 1000 mL deionized water, and 7.3 ± 0.2 pH ready-to-use. The incubation temperature was 50 °C and time 24 ± 3 h. Optical density (absorbance) was measured by means of culture medium plate count. Plate count growth parameters medium consisted of Tryptic Soy Agar (TSA) and biocardiagnostics BK047HA, batch 0016009. The composition (per liter of medium) was 15 g tryptone, 5 g papaic of digested soybean meal, 5 g sodium chloride, 15 g bacteriological agar, 1000 mL deionized water, and 7.3 ± 0.2 pH ready-to-use. A 300 mL quantity of TSB medium in borosilicate glass bottles with non-hermetic closure was constantly shaken at 150 rpm in an orbital incubator. Peptone saline medium was used to correct dilution. The composition (per liter of medium) was 1 g tryptone, 8.5 g sodium chloride, 1000 mL deionized water, and 6.8 ± 0.2 pH ready-to-use. It was sterilized at 121 °C for 15 min.
2.4. Process Flow
The process of producing acid lactic from cow manure consisted of cow manure collection; cow manure mixing, drying and grinding/sieving; chemical pretreatment; and simultaneous saccharification and co-fermentation process, as shown in Figure 1.
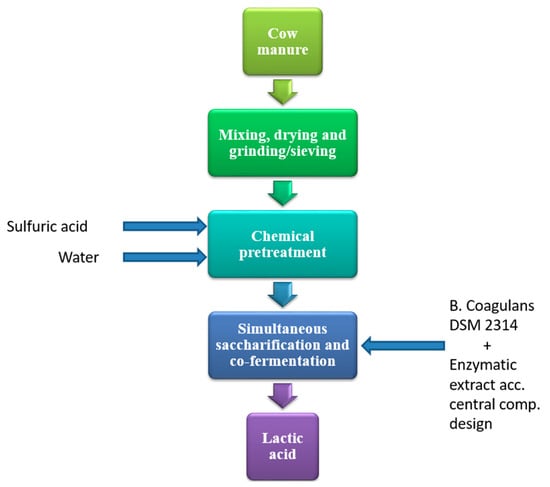
Figure 1.
Process flow for lactic acid production.
The chemical hydrolysis pretreatment was carried out with 96% sulfuric acid (Panreac), using 0.5 wt.% of acid on cattle manure on a dry basis, which has been used successfully in similar lignocellulosic raw materials. Dilute acid pretreatment has low requirements, minimizes possible environmental impacts in the proposal, and its effectiveness has been demonstrated in the hydrolysis of cellulose and hemicellulose [11]. The process was carried out in a glass reactor with an effective capacity of 5 L, treating 2.5 L of cow manure suspension in deionized water. It was stirred at 250 min−1 using a propeller stirrer with the geometrical diameter relation of 0.25 reactor diameter. It was operated isothermally at 90 °C for 120 min, by circulating thermal oil P20.275.50 from a HUBERT thermostat through the jacket. The process gases were recovered through a reflux condenser with water circulation. The acid hydrolysate was subjected to vacuum filtration. The amount of glucose, galactose, mannose, xylose and arabinose, acetic acid, furfural and 5-hydroxymethylfurfural (HMF) was determined from the filtered liquid. The fluid was subjected to drying at 55 °C for 72 h in a SELECTA oven (DIGITRONIC-TFT).
The cow manure mixture from the chemical pretreatment was subjected to SSAF in a glass reactor with an effective capacity of 3 L, treating 0.7 L. It was stirred at 150 min−1 using a propeller stirrer with 0.25 diameter agitator/reactor relation. It was operated isothermally. Temperature regulation was carried out by circulating deionized water from a SELECTA thermostat (DIGITERM 200) through the jacket. Pre-saccharification was carried out for 18 h. At the beginning, the acid hydrolysate pH was adjusted to 5 ± 0.1 by adding a 6.25 mol·L−1 NaOH suspension, and 75% of the enzymatic cocktail SAE0020 corresponding to each run was added. The SAE0020 enzyme cocktail with enzyme activity of 1000 IU/g was supplied by SIGMA-ALDRICH. After 18 h of presaccharification, the pH of the medium was adjusted to 5.8 ± 0.1 by adding a suspension of NaOH at 6.25 mol·L−1. The rest of the enzymatic cocktail corresponding to each run was added and supplemented with 1 g·L−1 of KCl, 1 g·L−1 of Na2HPO4, 1.25 g·L−1 of NH4Cl, 3 g·L−1 of yeast extract, 5 g·L−1 of glucose, and 10 g·L−1 of casein. The enriched medium was inoculated with a 5 vol.% suspension of Bacillus coagulans DSM 2314 and non-active aeration was maintained for 48 h of fermentation under isothermal conditions corresponding to the temperature of each experiment.
2.4.1. Experimental Design
Response surface methodology (RSM) is an approach that brings several benefits to traditional one-variable-at-a-time optimization, including the ability to generate a large amount of data from a small number of tests, and the ability to assess how the interaction between factors affects the answer [20]. The application of RSM as an experimental design allows the extraction of complex information, while reducing costs associated with labor, supplies, and time [21].
The experimental design was generated by R language [22] with the RSM package [23]. It consisted of a 2k full factorial design for the factorial portion of a central composite design (CCD), four central points in the cube, and 3 factors (k), with 6 axial points at a rotational distance of α = 1.682, for a total of 18 runs. The quadratic model was selected for predicting the optimal point, and is expressed as Equation (1):
where Y represents response variables (LA, productivity, yield); b0 is the interception coefficient; b1, b2, and b3 are the linear terms; b11, b22, and b33 are the quadratic terms; and X1, X2, and X3 represent the variables studied.
Y = b0 + b1X1 + b2X2 + b3X3 + b11X12 + b22X22 + b33X32 + b12X1X2 + b13X1X3 + b23X2X3
As experimental factors, the solids concentration in the reaction medium subjected to SSAF was considered and expressed as CM (gCM·L−1), the concentration of enzymatic cocktail SAE0020 was expressed as E (IU·gCMDry Matter−1) (DM), and temperature was expressed as T (°C). Table 1 shows a summary of independent variables, ranges, and levels.

Table 1.
Experimental range and levels of independent process variables.
2.4.2. Analysis of Monomeric Sugars, Lactic Acid, and Byproducts
The equipment used to analyze the sugar content [24,25,26,27] was a Waters chromatograph, model Acquity UPLC binary, a CORTECS C18+ 1.6 µm, 2.1 mm × 100 mm column and a Waters Mass spectrometry, Xevo TQS model. To prepare pattern samples, a solution of each aldose (mannose, glucose, galactose, arabinose, and xylose) was separately prepared, together with a 500 ppm (µg/mL) sample of glucose 13C6 (internal standard–IS1), as reference. To analyze it, 100 µL of 3-methyl-1-phenyl-2-pyrazoline-5-one solution and 200 µL of the 25.9% ammonium hydroxide solution were mixed together in each Eppendorf tube. This was stirred and heated at 70 °C for 40 min, then cooled at room temperature and 200 µL of formic acid added to each to neutralize. It was filtered with a 0.22 µ hydrophilic polytetrafluoroethylene (PTFE) sample and injected into the mass spectrometer. The material from the SSAF was subjected to vacuum filtration. Glucose, galactose, mannose, xylose and arabinose, acetic acid and lactic acid content were measured from the filtered liquid. The fluid was subjected to drying at 55 °C for 72 h in a SELECTA oven (DIGITRONIC-TFT). Sugar content was analyzed after chemical pretreatment to check its effectiveness, and after SSAF to check the remaining quantity which was not used by microorganism.
To analyze the lactic acid and byproducts (acetic acid, ethanol, furfural, and 5-HMF), gas chromatography with Flame Ionization Detector (FID) was used. The reactives used were MilliQ water and phosphoric acid. The patterns used were lactic acid, acetic acid, ethanol, furfural, and 5-HMF, respectively. The samples were filtered with a 3 mL aliquot, 1:3 dilutions with 0.35% phosphoric acid solution, using a gas chromatography vial to inject. The equipment for the lactic acid analysis was an HP-5MS UI 30 m × 0.25 mm × 0.25 µm column chromatography. The configuration parameters were an injection split of 1:50; a flux of 1 mL/min He; an injector temperature of 250 °C; an oven ramp with an initial temperature of 65 °C for 1 min, 20 °C/min ramp, ending at 290 °C for 5 min; and the flame ionization detector using an injector temperature of 290 °C. The equipment for the by-products’ analysis was ZB-FFAP 30 m × 0.25 mm × 0.25 µm column chromatography. The configuration parameters were an injection split of 1:10; a flux of 1 mL/min H2; an injector temperature of 230 °C; an oven ramp with an initial temperature of 70 °C for 1 min, 15 °C/min ramp up to 200 °C, and a ramp of 30 °C until 240 °C for 6 min; and an FID injector temperature of 240 °C.
2.5. Experimental Results Calculation
LA production refers to the grams of LA produced per liter of substrate. LA yield (Y) was calculated as the ratio between lignocellulosic material included in CM and LA production. LA productivity (P) was calculated as LA production per hour. Sugar production was determined as the total quantity of sugars (mannose, glucose, galactose, arabinose, and xylose) in diluted acid and SSAF (after 24 and 48 h), minus glucose added.
3. Results and Discussion
3.1. Results
The resulting parameters of the central composite design, specified per experimental set, are summarized in Table 2.

Table 2.
Central composed design parameters.
Homogenized samples were analyzed to determine initial humidity, cellulose, hemicellulose, and lignin content. Results are shown in Table 3.

Table 3.
Cow manure lignocellulosic material analysis.
The components analyzed represent approximately 75% of the total. The remaining percentage was not analyzed, and nutrients and other components were left out of this study, as the focus was on Table 3 components. The average portion of hemicellulose plus cellulose obtained was 51.6%, which means that cow manure has a biodegradable potential for obtaining value-added products. This result, higher than reported in studies to obtain bioethanol from this raw material [8], was associated with a high proportion of lignocellulosic fiber in cow diets. According to the cow manure composition, proximity to other lignocellulosic residues used for same purpose (such as sugarcane bagasse [11] and corn forage [28]) converts this material in a novel and attractive alternative for this (or similar) purpose. A reported average lignin content of only 3.9% was lower than that studied in lignocellulosic materials [29]. Therefore, it does not constitute a significant barrier in the development of these processes. These results allowed the evaluation of the production of lactic acid from cow manure as a raw material, also considering the values obtained of sugars and inhibitory compounds in the stages of the process. Nevertheless, possible barriers should be studied, such as inhibition by product, to improve the current production.
Determinations of sugar concentrations in the liquid fraction of the substrate showed a dependence on the experimental operating conditions. Glucose and xylose were found in high concentrations after chemical pretreatment and also after SSAF. The same behavior was observed in pretreated sugarcane bagasse sugar analysis papers [30]. This means there was a sugar fraction which had not been consumed by the microorganism.
During the pretreatment stage with acid at a high temperature, cow manure content was considered for the central composite design runs. The summary of the analysis of the sugar content after the solubilization of the substrate and the release of sugar oligomeric polymers and monosaccharides is shown in Table 4, as a percentage of lignocellulosic fraction. Xylose and glucose concentrations showed a dependence on the experimental conditions. The highest xylose rating was achieved with 48 h SSAF, high cow manure content, high temperature, and high enzyme value (the same as glucose), as shown in Table 5. Higher sugar concentrations were obtained at higher temperatures, combined with high concentrations of cow manure.

Table 4.
Sugar content per experimental run after chemical pretreatment.

Table 5.
Sugar content per experimental run after SSAF process.
Total sugar concentration reached its maximum value for the upper level of the cow manure factor, while regarding the axial point a decrease in concentration was registered. Lower cow manure values reached a high sugar content, which should be evaluated in future experimentations. The substrate was subjected to SSAF for 48 h, and the variation in cow manure, enzymes, and temperature according to the central composite design was analyzed, showing an increase in the concentration of dissolved monosaccharides, as a result of the depolymerization enzymatic activity. Table 5 shows the resulting sugar content analyzed after the complete process.
Maximum sugar concentration was reached, as for the previous stage, with a cow manure content of 100 gCM·L−1, together with lower values of the enzyme and temperature factors. When the substrate concentration’s extreme point was analyzed after chemical pretreatment, there was a decrease in the reaction mixture’s monosaccharide content, which suggests a limitation of the long-chain cleavage and large-molecule survival for a high concentration of suspended solids [31]. After SSAF, this behavior had the same effect on the enzymatic reaction rate. The reason for the sugars remaining after SSAF should be studied, as a way to increase current production ratios.
Various byproducts are created during chemical preparation. There are variations in byproduct production depending on the lignocellulosic source or the pretreatment technique used to decompose the lignocellulose. Furans, organic acids, and phenolics are the three major subgroups of byproducts produced [32]. Acetic acid, furfural, and 5-HMF were measured after pretreatment. Acetic acid was analyzed again after SSAF.
On substrates high in lignocellulosic byproducts, the addition of furfural to precultures of Bacillus coagulans may improve growth and lactic acid production [11]. Compared to furfural, 5-HMF inhibits dehydrogenase enzymes to a lesser degree [33]. All strains were inhibited by concentrations of furfural and 5-HMF above 5 g·L−1 and 8 g·L−1, respectively, while some strains were already noticeably inhibited at concentrations of 1 to 2 g·L−1 of furans. At concentrations of 15 g·L−1 of acetic acid, growth rates were badly inhibited but productivity was not significantly affected. It should be mentioned that when determining the toxicity of acids, pH is always a crucial consideration. At pH 3.5, 3.5 g·L−1 of acetic acid has the same inhibitory impact as 9 g·L−1 of acetic acid at pH 5 [34]. When determining the toxicity of lignocellulose substrates as a whole, combined effects should be taken into account. Research carried out with pure compounds provides an indication of the inhibitory effect of lignocellulose byproducts. Ethanol was analyzed and no content was found. The content analysis of inhibitors showed low concentrations, as summarized in Table 6.

Table 6.
Inhibitors content.
The results of this research, including the dependent (or response) variables lactic acid content, yield, and productivity, are given in Table 7.

Table 7.
Experimental design results obtained after 24 and 48 h.
The highest values of lactic acid concentration and productivity were reported for a substrate concentration of 80 g/L and a temperature of 50 °C, which is optimal for the growth of Bacillus coagulans DSM 2314. In a period of 24 h of SSAF, the highest values of lactic acid content and productivity were reported for substrate concentrations of 60 g·L−1 and 80 g·L−1, and temperatures corresponding to the central point (50 °C). The enzyme concentration, with a variable behavior, maximizes both responses for its central point value. If the SSAF time is increased to 48 h, a considerable increase in product formation is also highlighted for cow manure concentrations of 100 g·L−1. On the other hand, the yield increases for substrate concentrations corresponding to the lower limit and the lower axial point, according to the lactic acid content measured. The maximum lactic acid value of 13.65 g·L−1 was obtained with 212.5 IU·gCMDM−1 of enzyme, also achieving the maximum yield (33%) and maximum productivity of 0.57. At 48 h of SSAF, the same conditions showed better results for LA production and yield, as shown in Table 7. The 16th run achieved 15.09 g·L−1 of LA at 48 h, while the 10th run achieved a yield of 49.91%. The maximum productivity was achieved at 24 h, obtaining a value of 0.57 g LA·(g lignocellulosic portion)−1. These effects would suggest carrying out an analysis to establish a compromise between the values of the study factors that maximize the responses evaluated.
The reported yield values of the product, taking into account the stoichiometry of the consumed sugars, xylose and glucose, were between 30% and 99%, registered after the use of Bacillus coagulans strains, from lignocellulosic raw materials in different operational configurations [35]. The above evidences the potential of cow manure for its use for this purpose. However, the conversion rate of total sugar into lactic acid depends on the conditions, according to the levels established for the experimental factors [36], where elevated xylose and glucose concentrations were reported after SSAF (Table 5). These effects on enzymatic catalysis and fermentation must be evaluated through kinetic studies such as the one developed by [37], which allows the visualization of the growth phases of the microorganism as well as the possible existence of inhibition by substrate or byproduct formation.
3.2. Statistical Evaluation
A regression analysis was performed to fit response functions and experimental data. During the statistical evaluation, the analysis of variance indicated that the adjusted models were significant (p-value < 0.05), while not all the terms had significant results. In addition, determination coefficients (R2) higher than 80% were verified in all cases. A quadratic model was obtained, excluding those non-significant terms. This procedure was carried out for all models. Two Way Interaction (TWI) for cow manure content against enzyme and temperature versus enzyme was not significant in any case. A summary can be found in Table 8.

Table 8.
Model values at 24 h and 48 h for lactic acid, productivity, and yield.
Based on the projected regression model, Figure 2 shows the response surfaces to estimate the lactic acid production relative to the independent variables: cow manure, enzyme, and temperature at 24 h of SSAF. Figure 2a shows how LA increases significantly with enzyme and cow manure, until it reaches its maximum at 13.65 g·L−1, with cow manure and enzyme values around the central point. In Figure 2b, the maximum is obtained with a lower value of temperature and cow manure. In Figure 2c, the curved graphic shows the maximum lactic acid production with the lower temperature value, and the central point for the enzyme. Figure 2d shows the productivity reaching its maximum of 0.57 g·L−1·h−1 around the central point for temperature and cow manure, to decrease again at high values of both variables. Figure 2e shows a linear relationship of the yield against cow manure, while the enzyme maximizes the yield around the central point, decreasing it at high or low values. In Figure 2f, the maximum yield is produced at low values of cow manure and temperature, decreasing when both variables increase. Figure 2g shows the maximum yield at the enzyme’s central point and lower temperatures. It decreases when the temperature increases, or if the enzyme is away from the central value.
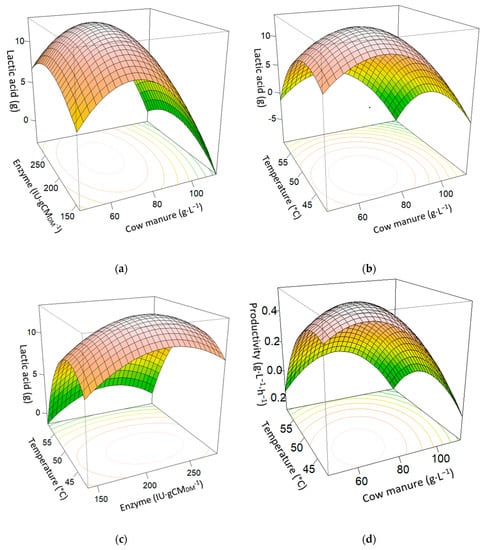
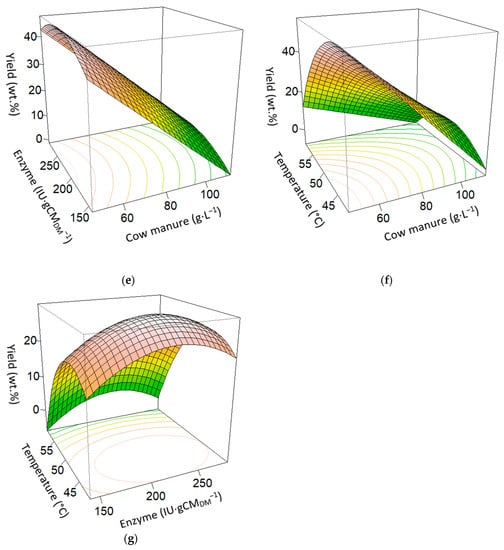
Figure 2.
Surface plot effects of enzyme, temperature, and cow manure interactions at 24 h SSAF process. (a) Cow manure (g·L−1) and enzyme (IU·gCMDM−1) on lactic acid (g). (b) Cow manure (g·L−1) and temperature (°C) on lactic acid (g). (c) Temperature (°C) and enzyme (IU·gCMDM−1) on lactic acid (g). (d) Cow manure (g·L−1) and temperature (°C) on productivity (g·L−1·h−1). (e) Cow manure (g·L−1) and enzyme (IU·gCMDM−1) on yield (wt.%). (f) Cow manure (g·L−1) and temperature (°C) on yield (wt.%). (g) Enzyme (IU·gCMDM−1) and temperature (°C) on yield (wt.%).
In Figure 3, the response surfaces estimation at 48 h are presented. Figure 3a shows the lactic acid response against enzymes and cow manure, with a curved relation. The maximum was reached at lower or central values of both cow manure and temperature. In Figure 3b, maximum productivity was also reached at lower or central values of both cow manure and temperature. Figure 3c reaches the maximum yield at lower values of cow manure and temperature.
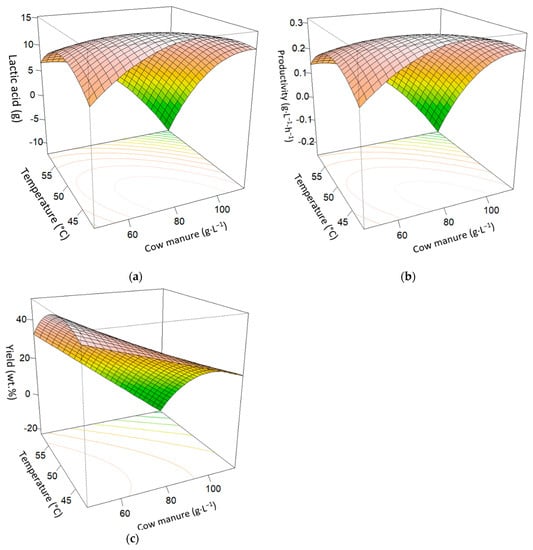
Figure 3.
Surface plot effects of enzyme, temperature, and cow manure interactions at 48 h SSAF process. (a) Cow manure (g·L−1) and temperature (°C) on lactic acid (g). (b) Cow manure (g·L−1) and temperature (°C) on productivity (g·L−1·h−1). (c) Cow manure (g·L−1) and temperature (°C) on yield (wt.%).
Based on the model, a numerical optimization according to RSM results was carried out with the software R. The optimal working conditions with a combined desirability bigger than 0.9, based on the three variables, were obtained. Results are summarized in Table 9.

Table 9.
Numerical optimization calculated with R.
Comparing 24 with 48 h of SSAF process, there was no considerable increase in lactic acid production. Therefore, yield and productivity decreased. The results for 24 h were lower than those achieved with the use of the same microbial strain on sugarcane bagasse in the same operational strategy [11].
Studies using pretreated bagasse, with same microorganism, and enzyme cocktail GC220, reported a yield of 74%, producing 55.6–59.3 g·L−1. Productivities during SSAF of 0.78–1.14 g·L−1 were achieved, which were lower compared to the productivities of 2.5–3 g·L−1 reached in research carried out with high-grade sugars [17].
However, the yield was close to values obtained with the same microorganism on wheat straw with basic pretreatment [10], and improved values reported using Bacillus coagulans LA-15-2 on rice straw hydrolysate in a batch process [38]. On the other hand, lactic acid values and productivity obtained under these conditions were higher than other studies utilizing different lignocellulosic materials, pretreatments, microorganisms, and operational strategies. Such is the case in the study to obtain this product with Lactobacillus brevis ATCC 8287 on spent coffee ground hydrolysate [39] and Saccharomyces cerevisiae on spent coffee grounds [40]. In the first case, the reported performance was slightly higher than results obtained in this study, while in the second one it was lower.
4. Conclusions
The production of lactic acid from cow manure was performed using an SSAF process. Lactic acid productivity experimentation showed similar values to fermentations using high-grade sugars or lignocellulose as feedstock. The data collected through the central composite design (24 h and 48 h) fit well to a quadratic model with interactions of all variables. TWI was only significant for cow manure and temperature. The least significant of the three factors analyzed was the enzyme.
The cellulose and hemicellulose composition of cow manure (similar to commonly used lignocellulosic materials, also with low lignin content) makes it a biodegradable material with great potential for obtaining lactic acid.
The maximum lactic acid production conditions were reached at 48 h of fermentation consisting of 15.09 gLA·L−1, productivity of 0.31 gLA·L−1 h, and a yield of lactic acid based on cellulose and hemicellulose of 36.56% wt.%. Concerning the 24 h SSAF analysis, higher LA values were obtained around the central point or with low values of temperature and cow manure, achieving values from 11 to 13.5 g·L−1. The enzyme quantity was not so decisive. At high temperatures and/or high cow manure concentrations, LA production was lower. The best yield values were obtained together with higher LA production. With the 48 h SSAF process, moderate increases in the LA product were obtained, reaching 13.5 to 15 g·L−1. The trend continues to reach best values around the central point or for lower temperatures and cow manure. Additionally, the enzyme quantity was not determinative.
Along with statistical evaluation, the adjusted quadratic models turned out to be significant, where the optimal values obtained in the study range showed that if time was increased up to 48 h, LA production increased by 1.87 gLA·L−1 and the enzyme amount was reduced, but performance and productivity decreased compared to a 24 h time period. The remaining sugar quantity increased after SSAF, and there is potential to increase LA production in this step.
The experimentally determined composition of byproducts did not impair bacterial activity, so the process does not require additional pretreatment. Nevertheless, the significant presence of sugars in the medium after the SSAF process suggests the possibility of inhibitory effects, which should be further evaluated by means of a kinetic study.
The best operational conditions should be determined based on an economical and environmental function, based on maximizing productivity, lower economic cost, or environmental benefits by using as much cow manure as possible for the process. Possible barriers for product formation should be studied, such as the inhibition of byproducts, to improve the current production, taking into account that not all sugars are consumed after SSAF. Future research on new operational conditions is recommended.
Author Contributions
Conceptualization, V.F. and L.F.C.; methodology, O.P.N. and V.F.; formal analysis, L.F.C. and V.F.; investigation, R.G., O.P.N. and A.A.S.; resources, L.F.C.; data curation, L.F.C.; writing—original draft preparation, R.G., O.P.N. and A.A.S.; writing—review and editing, V.F. and L.F.C.; visualization, R.G.; supervision, V.F. and L.F.C.; project administration, L.F.C.; funding acquisition, L.F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work received financial support from the Doctorat Industrial grant (2021 DI 22) from the AGAUR through the Secretariat of Universities and Research of the Department of Business and Knowledge of the Generalitat de Catalunya.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request to the correspondence author.
Acknowledgments
The authors at the University of Lleida would like to thank the Catalan Government for the quality accreditation given to their research group: GREiA (2021 SGR 01615). GREiA is a certified agent TECNIO in the category of technology developers from the Government of Catalonia. This work is partially supported by ICREA under the ICREA Academia programme.
Conflicts of Interest
Author Víctor Falguera was employed by the company AKIS International. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Wee, Y.J.; Kim, J.N.; Ryu, H.W. Biotechnological production of lactic acid and its recent applications. Food Technol. Biotechnol. 2006, 44, 163–172. [Google Scholar]
- Datta, R.; Henry, M. Lactic acid: Recent advances in products, processes and technologies—A review. J. Chem. Technol. Biotechnol. 2006, 81, 1119–1129. [Google Scholar] [CrossRef]
- Datta, R. Technological and economic potential of poly(lactic acid) and lactic acid derivatives. FEMS Microbiol. Rev. 1995, 16, 221–231. Available online: https://www.academia.edu/25459908/Technological_and_economic_potential_of_poly_lactic_acid_and_lactic_acid_derivatives (accessed on 7 January 2021). [CrossRef]
- Timbuntam, W.; Sriroth, K.; Tokiwa, Y. Lactic acid production from sugar-cane juice by a newly isolated Lactobacillus sp. Biotechnol. Lett. 2006, 28, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Garrido, R.; Cabeza, L.F.; Falguera, V. An Overview of Bioplastic Research on Its Relation to National Policies. Sustainability 2021, 13, 7848. [Google Scholar] [CrossRef]
- Södegard, A.; Stolt, M. Properties of polylactic acid fiber based polymers and their correlation with composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, X.; Wang, Y.; Li, H.; Li, Z.; Zhou, L.; Qu, Y.; Li, Z.; Bao, X. Cow manure as a lignocellulosic substrate for fungal cellulase expression and bioethanol production. AMB Express 2018, 8, 190. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Zi, L.H.; Bai, F.W.; Lin, H.L.; Hao, X.M.; Yue, G.J.; Ho, N.W. Bioethanol from Lignocellulosic Biomass; Bai, F.-W., Liu, C.-G., Huang, H., Tsao, G.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 25–51. [Google Scholar]
- Maas, R.H.; Bakker, R.R.; Jansen, M.L.; Visser, D.; De Jong, E.; Eggink, G.; Weusthuis, R.A. Lactic acid production from lime-treated wheat straw by Bacillus coagulans: Neutralization of acid by fed-batch addition of alkaline substrate. Appl. Microbiol. Biotechnol. 2008, 78, 751–758. [Google Scholar] [CrossRef]
- van der Pol, E.C.; Eggink, G.; Weusthuis, R.A. Production of l(+)-lactic acid from acid pretreated sugarcane bagasse using Bacillus coagulans DSM2314 in a simultaneous saccharification and fermentation strategy. Biotechnol. Biofuels 2016, 9, 248. [Google Scholar] [CrossRef]
- van der Pol, E.C.; Bakker, R.R.; Baets, P.; Eggink, G. By-products resulting from lignocellulose pretreatment and their inhibitory effect on fermentations for (bio)chemicals and fuels. Appl. Microbiol. Biotechnol. 2014, 98, 9579–9593. [Google Scholar] [CrossRef]
- Garrido, R.; Cabeza, L.F.; Falguera, V.; Navarro, O.P. Potential Use of Cow Manure for Poly(Lactic Acid) Production. Sustainability 2022, 14, 16753. [Google Scholar] [CrossRef]
- Castro, Y.P. Aprovechamiento de Biomasa Lignocelulósica: Algunas Experiencias de Investigación en Colombia; UTadeo: Bogotá, Colombia, 2014. [Google Scholar]
- Miller, C.; Fosmer, A.; Rush, B.; McMullin, T.; Beacom, D.; Suominen, P. Industrial Production of Lactic Acid, 2nd ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2011; Volume 3. [Google Scholar]
- Singhvi, M.; Gokhale, D. Biomass to biodegradable polymer (PLA). RSC Adv. 2013, 3, 13558. [Google Scholar] [CrossRef]
- Van Der Pol, E.C. Development of a Lactic Acid Production Process Using Lignocellulosic Biomass as Feedstock. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 2016. [Google Scholar]
- Sluiter, J.S.A.; Hames, B.; Ruiz, R.; Scarlata, C.; Templeton, D. Determination of Ash in Biomass, 2005th ed.; NREL/TP-510-42622; NREL: Golden, CO, USA, 2005; Available online: https://www.nrel.gov/docs/gen/fy08/42622.pdf (accessed on 3 January 2021).
- Sahito, A.R.; Mahar, R.; Siddiqui, Z.; Brohi, K.M. Estimating Calorific Values of Lignocellulosic Biomass from Volatile and Fixed Solids. Int. J. Biomass Renew. 2013, 2, 1–6. [Google Scholar]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Kalavathy, M.H.; Regupathi, I.; Pillai, M.G.; Miranda, L.R. Modelling, analysis and optimization of adsorption parameters for H3PO4 activated rubber wood sawdust using response surface methodology (RSM). Colloids Surfaces B Biointerfaces 2009, 70, 35–45. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022; Available online: https://www.r-project.org (accessed on 3 January 2021).
- Lenth, R.V. Response-Surface Methods in R, Using rsm. J. Stat. Softw. 2009, 32, 1–17. [Google Scholar] [CrossRef]
- Fan, B.; Li, T.; Song, X.; Wu, C.; Qian, C. A rapid, accurate and sensitive method for determination of monosaccharides in different varieties of Osmanthus fragrans Lour by pre-column derivatization with HPLC-MS/MS. Int. J. Biol. Macromol. 2019, 125, 221–231. [Google Scholar] [CrossRef]
- Xia, Y.-G.; Wang, T.-L.; Sun, L.-M.; Liang, J.; Yang, B.-Y.; Kuang, H.-X. A New UPLC-MS/MS Method for the Characterization and Discrimination of Polysaccharides from Genus Ephedra Based on Enzymatic Digestions. Molecules 2017, 22, 1992. [Google Scholar] [CrossRef]
- Sun, X.; Wang, H.; Han, X.; Chen, S.; Zhu, S.; Dai, J. Fingerprint analysis of polysaccharides from different Ganoderma by HPLC combined with chemometrics methods. Carbohydr. Polym. 2014, 114, 432–439. [Google Scholar] [CrossRef]
- Gao, Y.-Y.; Jiang, Y.; Chen, G.-C.; Li, S.-S.; Yang, F.; Ma, Q. A Sensitive and Rapid UPLC-MS/MS Method for Determination of Monosaccharides and Anti-Allergic Effect of the Polysaccharides Extracted from Saposhnikoviae Radix. Molecules 2018, 23, 1924. [Google Scholar] [CrossRef]
- Cheng, Q.; Shi, X.; Liu, Y.; Liu, X.; Dou, S.; Ning, C.; Liu, Z.Q.; Sun, S.; Chen, X.; Ren, X. Production of nisin and lactic acid from corn stover through simultaneous saccharification and fermentation. Biotechnol. Biotechnol. Equip. 2018, 32, 420–426. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, E.; Bakker, R.; van Zeeland, A.; Garcia, D.S.; Punt, A.; Eggink, G. Analysis of by-product formation and sugar monomerization in sugarcane bagasse pretreated at pilot plant scale: Differences between autohydrolysis, alkaline and acid pretreatment. Bioresour. Technol. 2015, 181, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Zou, Y.; Gu, Z.; Li, Z.; Jiang, Z.; Cheng, L.; Hong, Y.; Li, C. Liquefaction concentration impacts the fine structure of maltodextrin. Ind. Crops Prod. 2018, 123, 687–697. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Modig, T.; Lidén, G.; Taherzadeh, M.J. Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem. J. 2002, 363, 769–776. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Niklasson, C.; Lidén, G. Acetic acid-friend or foe in anaerobic batch conversion of glucose to ethanol by Saccharomyces cerevisiae? Chem. Eng. Sci. 1997, 52, 2653–2659. [Google Scholar] [CrossRef]
- Yankov, D. Fermentative Lactic Acid Production From Lignocellulosic Feedstocks: From Source to Purified Product. Front. Chem. 2022, 10, 823005. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M.; Aroca, R. Plant Responses to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- González-Leos, A.; Bustos-Vázquez, M.G.; Rodríguez-Castillejos, G.C.; Rodríguez-Durán, L.V.; Del Ángel-Del Ángel, A. Kinetics of Lactic Acid Fermentation from Sugarcane Bagasse by Lactobacillus Pentosus. Rev. Mex. Ing. Quim. 2019, 19, 377–386. [Google Scholar] [CrossRef]
- Chen, H.; Huo, W.; Wang, B.; Wang, Y.; Wen, H.; Cai, D.; Zhang, C.; Wu, Y.; Qin, P. L-lactic acid production by simultaneous saccharification and fermentation of dilute ethylediamine pre-treated rice straw. Ind. Crops Prod. 2019, 141, 111749. [Google Scholar] [CrossRef]
- Lee, K.H.; Jang, Y.W.; Lee, J.; Kim, S.; Park, C.; Yoo, H.Y. Statistical Optimization of Alkali Pretreatment to Improve Sugars Recovery from Spent Coffee Grounds and Utilization in Lactic Acid Fermentation. Processes 2021, 9, 494. [Google Scholar] [CrossRef]
- Kim, J.; Jang, J.H.; Yeo, H.J.; Seol, J.; Kim, S.R.; Jung, Y.H. Lactic Acid Production from a Whole Slurry of Acid-Pretreated Spent Coffee Grounds by Engineered Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 2019, 189, 206–216. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).