Abstract
Arabinoxylans (AXs) enter food processing and fermentation scenarios whenever grain-based ingredients are utilized. Their impacts on process efficiency and food product quality range from strongly negative to clearly beneficial, depending on both the particular food product and the AX structure. This review will focus on two structure-function relationships between AXs and fermented food production: (1) AXs’ native structure in cereal grains and structural changes that arise during production of fermented foods and (2) the impacts of AXs on processing and production of grain-based fermented foods and beverages (bread, beer, and spirits) and how variations in AX structure shift these processing impacts. Results from recently published papers have provided new insights into the connection between AXs’ structure at the molecular level and their effects on fermented food production. The purpose of this article is to review the historical progress in this area and introduce updates from recent years. Current knowledge gaps in the area are highlighted.
1. Introduction
Arabinoxylans (AXs) are the main hemicellulosic polysaccharides in the cell walls of commelinid monocot plants [1]. The commelinid monocot botanical grouping includes grasses, and therefore, all the major true cereal grains used in food and beverage production, such as wheat, corn, barley, and rye. In the plant cell wall, AXs interact extensively with cellulose microfibrils, lignin, and other AX polymers [2,3,4], thus providing mechanical strength and flexibility and helping the plant resist enzymatic attack by microbial pathogens and adapt to various stressors [5]. AXs are ubiquitous throughout the entire plant (leaves, stems, roots, and grains), but their structures vary between tissue types [6]. This review will be restricted to the structure of AXs from the grain tissue of cereals. We will provide a structure-focused discussion of how AXs impact the processing of grain-based foods and will also describe how the native AX structure is altered during the production of common fermented foods.
2. Native Arabinoxylan Structure in Cereal Grains
The structural foundation of AXs is their -1,4-linked xylopyranosyl (Xylp) backbone. The xylan backbone is then substituted with various branching elements, whose quantity and pattern of distribution differ between grain species (see Figure 1). Monomeric arabinofuranose (Araf) is the most abundant branching element and is found as either a mono- and/or di-substituent at the O-3 and/or O-2 positions of xylose. Glucuronic acid, its 4-O-methyl derivative, and acetyl groups are also observed in grains as AX backbone substituents, with particularly high concentrations of these non-arabinose substituents found in maize and sorghum AXs [7,8,9,10,11].
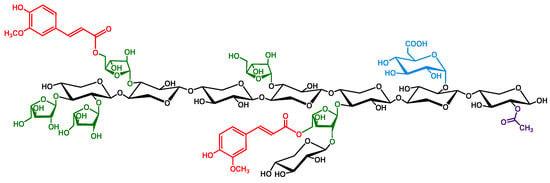
Figure 1.
Model feruloylated arabinoxylan structure from cereal grains. The (1→4)-linked -D-xylopyranosyl backbone (in black) is substituted with side-chains, or branch elements, including O–3 and/or O–2-linked -L-arabinofuranose units (in green), which may be acylated on the O–5 position with ferulic acid (in red) to form feruloylated side-chains. Additional side-chain substituents include glucuronic acid (in blue) and acetyl groups (in purple).
The presence of ester-linked hydroxycinnamic acids is a characteristic feature of graminaceous AXs. Trans-ferulic acid (FA) is the dominant phenolic acid released after alkaline hydrolysis of cereal grain cell wall material, and cis-ferulic, sinapic, and trans- and cis-p-coumaric acids are present in much smaller amounts [12,13]. These hydroxycinnamates O-5-acylate some of the Araf branches, creating feruloylated side-chains. Free radical-induced oxidative coupling of these ferulates produces ferulate dimers and higher oligomers and creates inter- and intramolecular cross-links between AX chains [14,15,16,17]. Ferulates and diferulates also couple with lignin, resulting in AX-lignin cross-linkages [18]. However, on a molar basis, more than half of the ferulates in grains remain in monomeric form and do not form dimers [19]. Although the simplest feruloylated side-chain structure, 5-O-trans-feruloyl-L-arabinofuranose, is the most abundant, cereal grain AXs also contain more complex, feruloylated side-chain oligosaccharides [20].
In the following section, AX structural differences (substitution patterns, ferulate cross-linking, and chain length) will be examined, with a focus on the differences among the most common cereal species used in fermented food and beverage production (wheat, rye, barley, and corn).
2.1. Degree of Arabinoxylan Backbone Substitution Varies between Grain Tissues and Species
The two most important physicochemical and biochemical implications of backbone substitution patterns are water-solubility and enzymatic degradability of the AX polymer. In terms of water-solubility, AXs with extended regions of unsubstituted xylose residues are prone to aggregation and are therefore more likely to be water-unextractable AXs (WU-AXs). Conversely, if other factors are held equal (especially ferulate cross-linking, but also polymer size), AXs with greater backbone substitution density are more likely to be water-extractable AXs (WE-AXs). Increased substitution density reduces AX adsorption to cellulose [21,22], whereas reduced AX branching both increases AX adsorption to cellulose and inhibits enzymatic (cellulase) hydrolysis of cellulose [23]. When considering enzymatic degradability, densely substituted AX regions resist enzymatic hydrolysis with endo-1,4--D-xylanase, which require two to three unsubstituted xylose residues for steric access to the xylan backbone [24]. The pattern of AX substitution is therefore also decisive for degradability with xylanases.
Techniques to assess substitution patterns include calculating the arabinose to xylose ratio (abbreviated as A/X ratio) following monosaccharide composition analysis, creation and analysis of partially methylated alditol acetates, and examination of the AX oligosaccharide profile produced by xylanase catalyzed hydrolysis. These methods have shown that AXs are highly heterogeneous in their substitution patterns. Variations are observed both among cereal species and grain tissue types.
In wheat, the outer pericarp layer contains highly substituted AX polymers (A/X ratio > 1 and a large percentage of di-substituted xylose residues [25]. In contrast, wheat endosperm AXs have a moderate A/X ratio (≈0.55) and contain both WE-AXs and WU-AXs [26]. No substantial differences in arabinose substitution patterns are observed between wheat endosperm WU-AXs and WE-AXs [27]. Rye AXs are comparable to those of wheat, with similar average A/X ratios observed in their endosperm WE-AXs. However, rye tends to have a higher concentration of total AXs in the endosperm than wheat (range of 3.0–3.3% for rye vs. 1.3–2.7% for wheat) and a higher concentration of WE-AXs overall [28,29,30]. Additionally, compared to wheat, WE-AXs from rye contain more AXs at the extremes of substitution density, i.e., a substantial fraction of rye endosperm WE-AXs can have A/X ratios > 1.4 [31]. The average barley endosperm A/X ratio is ≈0.6 [26]. Barley endosperm AX content (1.7–2.2%; [32]) tends to be lower than both wheat and rye. Corn AX content is lower than that of wheat and rye and also has a lower proportion of WE-AXs [33]. Corn AXs have a very complex substitution pattern, including substantial amounts of non-arabinose substituents (acetyl groups, glucuronic and 4-O-methyl-glucuronic acid) and oligosaccharide branches [10,34]. Given their overall higher degree of backbone substitution compared to wheat, rye, and barley, corn’s lower degree of water-solubility is initially surprising, but is easily explained by its extensive ferulate-mediated AX-AX crosslinking, as discussed in the following section.
2.2. Cereal Grain Arabinoxylans Are Feruloylated
Free radical-induced oxidative coupling of ferulates produces dehydrodiferulates (DFAs), which stabilize cereal grains’ cell walls by covalently cross-linking AXs to each other and lignin [16,35]. In addition to DFAs, oxidatively coupled ferulate trimers and tetramers are also ubiquitous in cereal grains [36]. When compared to wheat, rye, and barley, corn AXs have substantially higher levels of ferulate monomers and feruloylated oligosaccharide side-chains [20]. As expected, this leads to higher levels of ferulate oligomers as well. For example, corn contains approximately three times as many DFAs and four times as many ferulate trimers compared to wheat [37].
Although ferulates are a minor component of cereal grains, they exert an outsized effect on AX enzymatic degradability and processing characteristics. Feruloylated oligosaccharide side-chains are recalcitrant to enzymatic digestion [7], and increased ferulate cross-linking in plant materials also enhances their resistance to enzymatic degradation [38]. AX cross-linking drastically reduces the water-solubility of the AX polymer, since the removal of ferulates via alkaline extraction renders the majority of AXs soluble [39]. In their native, cross-linked state, insoluble AXs have considerable water-binding capacities and therefore exert the detrimental effect of immobilizing water in doughs and baked goods [40].
2.3. Arabinoxylan Degree of Polymerization Varies between Grain Species
AX polymer sizes in cereal grains vary widely. Variability appears not only among cereal species (see Table 1), but also when comparing varieties and growing season [41,42]. Measuring WU-AX chain length requires bringing the insoluble AXs into solution, which may be accomplished by alkaline extraction, which hydrolyzes ferulate crosslinks. Ferulate crosslinks are also present in small amounts in WE-AXs, which increases their measured weight average molecular weight [43].

Table 1.
Arabinoxylan (AX) molecular weight in common cereals.
3. Arabinoxylans in Beer and Distilled Spirits Production: Processing Effects and Structural Changes
Beers and grain-based distilled spirits share similar initial processing steps. As a result, beer is a good model overall to study the repercussions of AXs’ presence in alcohol production. The following section will examine AXs’ impacts on the malting, mashing, fermenting, and filtering steps of brewing and structural changes in AXs resulting from these processes.
3.1. Malting
Malting is the controlled germination of cereal grains. The main goal of malting is to produce starch-degrading enzymes within the grain that will later, in the mashing step of beer and grain-based spirits production, break starch down into simpler and more fermentation-friendly sugars. However, during the germination process, additional enzymes are produced, including those that target AXs. The main driving force for AX structural change are xylanases [50], which cleave -(1,4)-glycosidic bonds between Xylp units and shortens the AX backbone length. For barley, there is no significant decrease in overall AX content during malting, but the average degree of polymerization (avDP) is dramatically decreased [51], indicating the activity of xylanases. This results in a partial solubilization of the AXs, i.e., an increase in the WE-AX fraction [46]. The WE-AX content for wheat also increases by 97% during malting [52].
Although arabinofuranosidases are expressed during barley germination [53], the A/X ratio of the total AXs in malted barley grain was comparable or only slightly lower than ungerminated grain [51,54]. Some studies that selectively examined the WE-AX fraction of malted barley found that the A/X ratio of this fraction tends to decrease during germination [53], but others have reported no change [46]. The effects of malting on the A/X ratio of wheat and rye have also been reported: the A/X ratio of WE-AXs decreased from 0.74 to 0.63 for wheat and from 0.86 to 0.67 for rye [55].
Dervilly et al. compared the molar mass distribution and AX backbone substitution patterns of WE-AXs from barley malt vs. ungerminated barley grain [46]. In both materials, the higher molar mass fractions had the highest A/X ratios, but the range of A/X ratio values was greater for barley grain (0.40–0.92) compared to malted barley (0.63–0.87). The barley malt A/X fraction with the highest molecular weight also had the highest concentration of di-substituted xylose units. AXs with a high molar mass also displayed an elevated viscosity. Another study investigated the relationship between a malt’s Kolbach index and the molecular weight of the malt’s WE-AXs by examining wheat malts produced from the same grain source but malted under different conditions to obtain different Kolbach indices. Higher Kolbach indices had no effect on overall WE-AX content or A/X ratio, but did result in WE-AXs with lower molecular weights [56].
3.2. Mashing
Mashing is the process of mixing milled grains, of which all or a portion have first gone through the malting step, with hot water to produce a wort rich in fermentable sugars. Heating in an aqueous system gelatinizes the grain starches, leaving them more accessible to the starch-degrading enzymes produced during malting. This step also distributes the AX-degrading enzymes produced during malting throughout the mash, providing the opportunity for additional AX structural modification.
Langenaeken et al. reported that the mashing step solubilized 18% of the total AXs from malted barley into the wort [51]. This value is slightly higher than the WE-AX content of typical barley malt (10–14%) [52], and the difference could be attributed to the activity of xylanases at the beginning of mashing [57]. The avDP of AXs in the wort AX population decreases slightly during mashing, further pointing toward xylanase activity [51]. Xylanase activity is readily detected in barley wort at low mashing temperatures (40–55 °C) [58], the optimum temperature for xylanases [59]. These enzymes are inactivated rapidly in barley mash at temperatures above 55 °C [52].
Clear evidence for additional AX structural changes, such as debranching, occurring specifically during mashing is lacking. Langenaeken et al. found that the A/X ratio (corrected for free arabinose, an important, but easily overlooked step when calculating the A/X ratio of solubilized AX polymers) of wort AXs unexpectedly increased from 0.56 to 0.74 during mashing [51], which differs from earlier reports of an almost unchanged A/X ratio [60]. Although the precise cause of the increased A/X ratio in the wort (e.g., WE-AX) was not determined, it could simply reflect a shifting population of dissolved AXs during the mashing step and precipitation of AXs with a low backbone substitution rate out of the wort solution. Additionally, xylanases are less active on highly substituted AXs compared to AXs with a low backbone substitution rate [61].
3.3. Fermentation
During fermentation, fermentable sugars in the wort are consumed by yeast and other microorganisms, leading to the accumulation of ethanol and other metabolites. A potential correlation between feruloylated AXs in wort and premature yeast flocculation [62], which leads to incomplete conversion of fermentable sugars to ethanol, has been identified. Xylanases originating from fungal infections in barley grain appear to increase the phenomenon [63], perhaps by having released the specific triggering AX structural elements earlier in the brewing process. WE-AX content decreased by around 25% during the fermentation of barley-based beer [51], although no change in WE-AX content was seen during the fermentation of wheat-based beer [64]. Decreased soluble AX content during fermentation may be explained by aggregation and precipitation of AX polymers as a result of the increased ethanol content [65]. Due to the non-fermentability of AXs by yeast and the degradation of xylanases in the wort-boiling step, fewer structural changes are seen in AXs during fermentation compared to previous steps.
3.4. Filtration
Filtration removes solid particles, yeast cells, and other unwanted substances from the beer. -Glucans negatively affect filterability [66,67], but AXs have an even greater negative effect [68]. Surprisingly, this reduction in filterability is not related to viscosity increases [68]. AX concentration, molecular weight, and membrane pore size all influence the maximum permeable membrane volume [69]. AX polymers with a larger molecular weight are more likely to cause membrane plugging [70]. Gastl et al. observed the same association between larger molecular weight AXs and decreased membrane permeability and also noted an association between membrane permeability and arabinofuranosidase activity [71]. This indicates a need for a deeper examination of the side chain AX branching patterns causing decreased membrane permeability, since decreased branching leads to more AX aggregation [72].
3.5. Viscosity
WE-AXs’ effects on beer viscosity are interesting but controversial. Krahl et al. found no connection between higher WE-AX content and increased viscosity of wort and beer [55], but the molecular weights of the WE-AXs were not determined in this study, and it is possible that the carbohydrate determined as WE-AX was primarily oligosaccharides and free monosaccharides. However, other reports from wort and beer have seen a correlation between WE-AXs and viscosity [73,74]. Certainly, polymeric WE-AXs increase solution viscosity, and, as expected, AXs with higher molecular weight have a greater effect [68]. A significant negative relationship between malt’s xylanase activity and wort viscosity underscores the key role that WE-AXs play in viscosity (without diminishing the importance of -glucan content in beer and wort viscosity) [75].
3.6. Arabinoxylans in Commercial Beer
Commercial beer contains AXs. A profile of 15 different commercial beers from USA and Germany found AX contents ranging from approximately 500 mg/L (USA light beer) to >4000 mg/L (German wheat beer) [76]. AX structures found in beer also vary, although it is clear that the AXs that end up in the final beer products are mostly AX oligosaccharides. Courtin et al. reported that the avDP of AXs from a panel of commercial beers ranged from 19 to 60, with a moderate average degree of substitution (avDS; 0.49–0.66) [77]. However, although the avDP of AXs in beer is low, some larger AXs are also present. For example, graded ethanol precipitation of several regular and non-alcoholic pilsner beers revealed a wide, heterogeneous distribution of AX molecular weights: although the overall avDP in the beers ranged from 18 to 34, a much wider range (4–308) was seen in the avDP of the individual fractions [61]. Similar trends were seen in the AXs’ avDS: the avDS of the overall beers ranged from 0.64 to 0.71, but individual fractions had avDS ranging from 0.43 to 0.88 [61].
Both AX content and structure affect beer’s organoleptic characteristics. AXs enhance the stability of protein-stabilized beer foam [78], presumably by increasing the viscosity of the liquid phase. AXs with a greater avDP and A/X ratio are enriched in beer foam compared to the non-foam fraction [79]. AXs also enhance mouthfeel and fullness in beer [80]; the use of a non-malted rye adjunct in beer brewing successfully improved the mouthfeel of no- and low-alcohol beers by increasing overall AX content and avDP [81].
3.7. Ferulic Acid in Brewing
FA is a precursor for 4-vinylguaiacol, a flavor-active volatile in beer generated by FA’s decarboxylation. FA is released from AXs by the action of feruloyl esterase, which is present in barley malt [82], but xylanase activity also correlates strongly with FA release [83], since reducing the AX polymer size creates more suitable esterase substrates. Free FA is released during mashing of both barley and wheat malts, but barley malt mashes had higher levels of free FA compared to wheat malt mashes or wheat-barley mixtures [84]. This could be caused by the xylanase inhibitor proteins in wheat [85,86]. However, grain variety differences also appear to affect free FA levels in wort [83,87], and some wheat varieties produce worts with higher free FA levels than barley [87]. Mashing process parameters can also be tailored to optimize FA release during mashing, thus shaping final phenol levels and aroma potential in beer [88].
Regardless of variety, the majority of FA in wort is not found as free FA, but is still ester-linked to soluble AXs [87]. The bulk (60–90%) of free FA in wheat beers is released during the fermentation step, which indicates that yeast-sourced esterases are cleaving FA [84]. More FA is released during fermentation from wheat-based recipes compared to barley-based batches [84], leading to greater concentrations of 4-vinylguaiacol in wheat beers. 4-Vinylguaiacol is also a key odorant in bourbon whiskey [89], which legally must contain a high percentage of corn in its mash bill (51% or more). As discussed previously, corn AXs are characterized by a very complex branching pattern and a high degree of feruloylation, but to the best of our knowledge, no targeted research exploring the release of FA in bourbon mashing (typically sour mash) and fermentation has been published.
4. Arabinoxylans in Bread: Processing Effects and Structural Changes
In principle, any effect of AXs on bread quality should be traced to molecular interactions between AXs and other molecules in dough and bread. AXs’ relationships with water, gluten, and starch are most impactful for bread quality [90]. These interactions manifest themselves through macroscopic phenomena like gas retention, specific volume, and dough rheology. AXs’ effects on bread and dough structure strongly depend on their structure, e.g., their molecular weight and water extractability. In broad strokes, WU-AXs generally have a negative effect on bread quality, whereas WE-AXs have a positive effect [40,91,92,93,94,95]. However, it is worth mentioning that bread is a complex food system, and any effect of AXs is further shaped by other formula components and processing conditions, meaning that results generated using different processing conditions and recipes are not fully comparable [96].
4.1. Interactions with Water
Interactions between AXs and water are foundational for AXs’ interactions with other complex molecules in bread and dough like gluten and starch [90,97,98].
4.1.1. Water-Holding Capacity
AXs absorb water very effectively, with WU-AXs having a higher water-holding capacity than WE-AXs [99]. A review by Courtin and Delcour reported ranges for WU-AXs and WE-AXs of 6.7–9.9 and 3.5–6.3 g/g, respectively [93]. Even higher water-holding capacity values (13.3–16.1 g of water per gram of material) were reported for the cellulose-rich insoluble fiber fractions remaining after the water and alkaline extraction of wheat bran [100]. The high water-holding capacity of WU-AXs, which limits the water available for gluten hydration, is one of the mechanisms underpinning WU-AXs’ negative effect on dough extensibility and overall bread quality [91,93,101]. Enriching bread formulas with supplemental xylanases that primarily target WU-AXs thus results in water being re-distributed from WU-AXs to other dough polymers, including gluten [90]. Enzymatic solubilization of WU-AXs while limiting degradation of the resulting WE-AXs improves loaf volume [40].
4.1.2. Solubility Behavior of Arabinoxylans: Dynamic Shifts during Bread-Making
During dough mixing and fermentation, the content of WE-AXs in bread dough increases and peaks at the molding stage, while the WU-AXs content decreases [94]. This behavior is thought to be at least partially caused by endogenous xylanases solubilizing a portion of the WU-AXs into WE-AXs [102]. Endogenous xylanase activity is indeed detectable in wheat, but activity levels differ between varieties and are sometimes quite low [94,102,103]. However, the degree of WU-AX solubilization during dough production does not always correlate with the flour’s endogenous xylanase activity, but this could be partly explained by AX structural differences: flour varieties with a higher degree of AX backbone substitution have fewer available sites for xylanase binding and backbone cuts [102].
The proportion of di-substituted xylose residues in the WE-AXs increases during mixing and then remains consistent throughout the rest of the bread-making process [94], which probably reflects the high solubility and resistance of WE-AX polymers rich in this structural component to self-aggregation [104], i.e., once released from WU-AXs, these fragments reliably stay in solution. Another observation supporting endogenous xylanase cleavage of AXs during dough mixing and fermentation is the clear decrease in the average molecular weight of the WE-AX population during these steps [102,105]. Interestingly, although the overall AX population shifts toward smaller AX polymers during dough production, a minority population of WE-AX polymers with an increased molecular weight compared to that of raw flour also appears in doughs from some flours [102,105]. More structural characterization work is necessary to explain these nascent large polymers. It is possible that they result from de novo oxidative cross-linking between two or more ferulates on adjacent AX polymers. Alternatively, they could represent aggregates of WE-AXs with a large molecular weight and less backbone substitution, which tend to become physically entangled with each other in solution and form clusters [104].
Literature data regarding the effects of baking on the solubility behavior of AXs are mixed. Some studies show a clear decrease in WE-AXs after baking [94,106], but others have reported the opposite: a sharp increase in WE-AXs in the baking step [102]. The molecular weights of WE-AXs, after tending to decrease overall during dough production, rise during baking [102,105], which may reflect either oxidative cross-linking at the feruloylated side chains or self-aggregation.
Increasing the WE-AX levels of wheat- and rye-flour-based doughs, either via the addition of purified WE-AXs or by supplementation with xylanases that specifically solubilize WU-AXs without degrading the newly formed WE-AXs [40], tends to improve loaf volume up to a certain WE-AX concentration [92,95,107,108,109]. Excessive degradation of WE-AXs has a negative effect on dough viscosity and loaf volume, so xylanase type and dosage must be chosen carefully [92]. The beneficial effects of WE-AXs come largely from the increased viscosity that they generate in doughs’ liquid fractions [92], which slows the migration of gas bubbles [93]. WE-AXs with a higher molecular weight are thus more potent, and the maximum beneficial effects will be reached at lower concentrations [95,110]. A simple way to increase the molecular weight of WE-AXs is to induce molecular cross-linking of their ferulate moieties using laccase or peroxidase and hydrogen peroxide [111]. However, there is an upper concentration limit to the beneficial effects of increased WE-AXs, and exceeding this concentration harms dough and bread quality [95,110]. In addition to their viscosity-altering effects, WE-AXs may also affect dough and bread stability by interacting with gluten polymers, which will be discussed in the next section.
4.2. Molecular Interactions between Arabinoxylans and Gluten
Gluten is indispensable for the mechanical properties of wheat-based doughs. Gluten consists of two proteinaceous components: gliadin and glutenin. Gliadin contributes viscosity and extensibility, while glutenin contributes strength and elasticity to the dough. Glutenin is a polymer whose subunits are linked by disulfide bonds, whereas gliadins are monomeric. During dough production, a combination of hydration and physical mixing develops the gluten proteins into an inter-meshed, elastic network that can trap gas, and any dough component that either physically blocks gluten proteins from interacting with each other or limits their hydration will weaken the gluten network [112]. WU-AXs have been shown to be detrimental on both fronts [113], and xylanase treatment that targets WU-AXs improves gluten yield [91].
In addition to simply increasing dough viscosity, AXs also interact molecularly with gluten. The gluten network is created and stabilized with a combination of disulfide bonds, hydrophobic interactions, and hydrogen bonding [112], so the substantial presence of a hydrophilic polymer like WE-AXs will shift the secondary and tertiary structure of gluten to aggregate and bury hydrophobic regions in order to decrease overall surface tension. Supplementation with WE-AXs led to increased beta sheets in a gluten/WE-AX model system [114,115], but the opposite trend (decreased beta sheets) was clearly seen in a dough system [109]. WE-AXs improve the conformational stability of gluten’s disulfide bonds, but the gluten network pore size increases, and the strength and elasticity of the network decrease [109,114,115]. A wheat gluten-AX model system comparing gluten structure when mixed with WU-AXs extracted from wheat bran vs. WE-AXs generated by solubilization of the aforementioned WU-AXs gives us additional insights into AX-gluten interactions at the molecular level [116]: WU-AXs sterically hindered gluten interactions and thus increased the pore size of the gluten network. As also seen by Zhu et al. [115], WE-AXs created a more polar microenvironment and promoted aggregation of glutenins and gliadins. WE-AXs also form hydrogen bonds with tyrosine residues from wheat gluten [114,116]. During heating, WE-AXs promote the partial agglomeration of wheat gluten’s glutenin macropolymer (GMP) particles and a clear shift in the GMP particle size distribution toward higher values [117,118]. Supplementing AXs to high concentrations in rye flour doughs (5–8% AX) reduced the formation of a meshed protein network; instead, proteins tended to agglomerate and dough elasticity was reduced [119,120]. Fluorescent antibody staining and confocal laser scanning microscopy proved experimentally that, in rye doughs, high concentrations of WE-AXs encircle proteins and limit protein network formation [121]. Molecular analysis also indicates that covalent cross-links are formed between ferulic acid from AXs and tyrosine residues in both wheat and rye doughs, which would covalently couple AXs via their feruloylated side-chains into the gluten network [122].
4.3. Interactions with Starch
Starch shapes the final quality of bread by gelatinizing and swelling during baking, which helps turn dough into bread. During storage, starch retrogradation is a key driver of bread staling and crumb hardening, so factors that slow retrogradation enhance bread’s shelf life [112]. AXs have the potential to influence these phenomena both by altering the water distribution in dough and bread and by physically interfering with starch-starch intermolecular associations. Various studies have shown that supplementing wheat bread doughs with WE-AXs has some effect on starch gelatinization, substantially delays retrogradation, and reduces crumb firmness [98,109]. The inhibition of starch retrogradation by AXs is influenced by AX structural differences, with higher molecular weight WE-AXs having a stronger inhibitory effect [123,124].
The mechanism behind WE-AXs’ effects on starch gelatinization and retrogradation and the effects of molecular weight was studied in more detail by Hou et al. [125]. WE-AXs with a lower molecular weight effectively inhibited starch gelatinization and short-term retrogradation, which was attributed to their suppression of amylose leaching and the formation of amylose-lipid complexes. Conversely, WE-AXs with a higher molecular weight tended to interact with amylopectin, leading to a stronger inhibitory impact on the long-term retrogradation of starch [125]. AX branching patterns influence its interactions with starch: more disubstituted Xylp residues correlate with stronger inhibition of amylopectin recrystallization [124].
WE-AXs also reduce starch digestibility by amylases [123,126], which has implications both for the use of amylase as a dough improver and for human nutrition. AXs with a larger molecular weight are the strongest inhibitors and the presence of (1,3)(1,4)--glucan has a synergistic effect [127].
5. Future Research Directions
AXs are complex carbohydrates that exert significant processing effects during the production of fermented cereal-based food and beverages. These processing effects are strongly shaped by variations in the native cereal ingredient’s AX structure like molecular weight, degree of backbone substitution, and feruloylation level. In turn, the structure of AXs is often changed during the fermented food process. It can be seen from the reference list that many research groups have been investigating detailed structure-function relationships for AXs in fermented food production, and we encourage this research direction. The first principle of chemistry is that molecular structures determine macroscopic properties, and we have striven to follow this principle in this review. Although not an exhaustive list, we suggest the following future research directions:
- More structural characterization work on AX structures following food processing is warranted. The bulk of AX structural characterization has targeted AX structures in raw cereal grains and ingredients, leaving many aspects of AX structures in prepared foods unexplored.
- Studies exploring human health effects of AXs should implement AX structures from as-consumed foods instead of from raw cereal grains or extracted AXs. As discussed in this review, AX structures are changed during fermented food processing. Explorations of the human health effects of AX consumption need to use AX structures that are representative of those in prepared foods, not those in raw grains.
- Studies attempting to clarify the effects of native AX structures on fermented food production should be wary of extrapolations from model systems using extracted AXs. The use of alkaline conditions to extract WU-AXs is the most pertinent example. Alkaline conditions will cleave ester-linked ferulates and release AX-AX crosslinks. This dramatically reduces the AX molecular weight and changes the solubility characteristics of the extracted AXs relative to their native states.
- Feruloylation is a consistent component of cereal grain AXs, so the ramifications of feruloylated side-chain branching patterns on AX-linked processing and human health effects should continue to be explored.
Author Contributions
Conceptualization, T.T. and R.R.S.; Software, T.T.; Validation, R.R.S.; Writing—Original Draft Preparation, T.T. and R.R.S.; Writing—Review and Editing, T.T. and R.R.S.; Supervision, R.R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by grant no. 2022-67017-36798 from the National Institute of Food and Agriculture, U.S. Department of Agriculture. Additional financial support was provided by the National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch Program project KY007112 under accession #1021937.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors have no conflict of interest to declare.
Abbreviations
The following abbreviations are used in this manuscript:
| Araf | arabinofuranosyl |
| avDP | average degree of polymerization |
| avDS | average degree of substitution |
| AX | arabinoxylan |
| A/X | arabinose/xylose ratio |
| DFA | dehydrodiferulates |
| FA | ferulic acid |
| GMP | glutenin macropolymer |
| WE-AX | water-extractable arabinoxylan |
| WU-AX | water-unextractable arabinoxylan |
| Xylp | xylopyranosyl |
References
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Kang, X.; Kirui, A.; Dickwella Widanage, M.C.; Mentink-Vigier, F.; Cosgrove, D.J.; Wang, T. Lignin-polysaccharide interactions in plant secondary cell walls revealed by solid-state NMR. Nat. Commun. 2019, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, R.D.; Rancour, D.M.; Marita, J.M. Grass cell walls: A story of cross-linking. Front. Plant Sci. 2017, 7, 2056. [Google Scholar] [CrossRef] [PubMed]
- Terrett, O.M.; Dupree, P. Covalent interactions between lignin and hemicelluloses in plant secondary cell walls. Curr. Opin. Biotechnol. 2019, 56, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Buanafina, M.M.d.O.; Morris, P. The impact of cell wall feruloylation on plant growth, responses to environmental stress, plant pathogens and cell wall degradability. Agronomy 2022, 12, 1847. [Google Scholar] [CrossRef]
- Rancour, D.; Marita, J.; Hatfield, R. Cell wall composition throughout development for the model grass Brachypodium distachyon. Front. Plant Sci. 2012, 3, 266. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; de Waard, P.; Kabel, M.A.; Gruppen, H.; Schols, H.A. Enzyme resistant feruloylated xylooligomer analogues from thermochemically treated corn fiber contain large side chains, ethyl glycosides and novel sites of acetylation. Carbohydr. Res. 2013, 381, 33–42. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Kabel, M.A.; Van Eylen, D.; Gruppen, H.; Schols, H.A. Characterization of oligomeric xylan structures from corn fiber resistant to pretreatment and simultaneous saccharification and fermentation. J. Agric. Food Chem. 2010, 58, 11294–11301. [Google Scholar] [CrossRef]
- Verbruggen, M.A.; Beldman, G.; Voragen, A.G.J. The selective extraction of glucuronoarabinoxylans from sorghum endosperm cell walls using barium and potassium hydroxide solutions. J. Cereal Sci. 1995, 21, 271–282. [Google Scholar] [CrossRef]
- Huisman, M.M.H.; Schols, H.A.; Voragen, A.G.J. Glucuronoarabinoxylans from maize kernel cell walls are more complex than those from sorghum kernel cell walls. Carbohydr. Polym. 2000, 43, 269–279. [Google Scholar] [CrossRef]
- Kabel, M.A.; Carvalheiro, F.; Garrote, G.; Avgerinos, E.; Koukios, E.; Parajó, J.C.; Gírio, F.M.; Schols, H.A.; Voragen, A.G.J. Hydrothermally treated xylan rich by-products yield different classes of xylo-oligosaccharides. Carbohydr. Polym. 2002, 50, 47–56. [Google Scholar] [CrossRef]
- Vitaglione, P.; Napolitano, A.; Fogliano, V. Cereal dietary fibre: A natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci. Technol. 2008, 19, 451–463. [Google Scholar] [CrossRef]
- Bunzel, M. Monomere und Dimere PhenolcarbonsäUren als Strukturbildende Elemente in LöSlichen und UnlöSlichen Getreideballaststoffen. Ph.D. Thesis, Staats-Und Universitätsbibliothek Hamburg Carl von Ossietzky, Hamburg, Germany, 2001. [Google Scholar]
- Ralph, J.; Quideau, S.; Grabber, J.H.; Hatfield, R.D. Identification and synthesis of new ferulic acid dehydrodimers present in grass cell walls. J. Chem. Soc. Perkin Trans. 1 1994, 3485–3498. [Google Scholar] [CrossRef]
- Saulnier, L.; Crépeau, M.J.; Lahaye, M.; Thibault, J.F.; Garcia-Conesa, M.T.; Kroon, P.A.; Williamson, G. Isolation and structural determination of two 5,5’-diferuloyl oligosaccharides indicate that maize heteroxylans are covalently cross-linked by oxidatively coupled ferulates. Carbohydr. Res. 1999, 320, 82–92. [Google Scholar] [CrossRef]
- Allerdings, E.; Ralph, J.; Schatz, P.F.; Gniechwitz, D.; Steinhart, H.; Bunzel, M. Isolation and structural identification of diarabinosyl 8-O-4-dehydrodiferulate from maize bran insoluble fibre. Phytochemistry 2005, 66, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Bunzel, M.; Allerdings, E.; Ralph, J.; Steinhart, H. Cross-linking of arabinoxylans via 8-8-coupled diferulates as demonstrated by isolation and identification of diarabinosyl 8-8(cyclic)-dehydrodiferulate from maize bran. J. Cereal Sci. 2008, 47, 29–40. [Google Scholar] [CrossRef]
- Ralph, J.; Grabber, J.H.; Hatfield, R.D. Lignin-ferulate cross-links in grasses: Active incorporation of ferulate polysaccharide esters into ryegrass lignins. Carbohydr. Res. 1995, 275, 167–178. [Google Scholar] [CrossRef]
- Bunzel, M.; Ralph, J.; Marita, J.M.; Hatfield, R.D.; Steinhart, H. Diferulates as structural components in soluble and insoluble cereal dietary fibre. J. Sci. Food Agric. 2001, 81, 653–660. [Google Scholar] [CrossRef]
- Schendel, R.R.; Meyer, M.R.; Bunzel, M. Quantitative profiling of feruloylated arabinoxylan side-chains from graminaceous cell walls. Front. Plant Sci. 2016, 6, 1249. [Google Scholar] [CrossRef]
- Kabel, M.A.; van den Borne, H.; Vincken, J.P.; Voragen, A.G.J.; Schols, H.A. Structural differences of xylans affect their interaction with cellulose. Carbohydr. Polym. 2007, 69, 94–105. [Google Scholar] [CrossRef]
- Köhnke, T.; Pujolras, C.; Roubroeks, J.P.; Gatenholm, P. The effect of barley husk arabinoxylan adsorption on the properties of cellulose fibres. Cellulose 2008, 15, 537–546. [Google Scholar] [CrossRef]
- Selig, M.J.; Thygesen, L.G.; Felby, C.; Master, E.R. Debranching of soluble wheat arabinoxylan dramatically enhances recalcitrant binding to cellulose. Biotechnol. Lett. 2015, 37, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Pollet, A.; Delcour, J.A.; Courtin, C.M. Structural determinants of the substrate specificities of xylanases from different glycoside hydrolase families. Crit. Rev. Biotechnol. 2010, 30, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Brillouet, J.M.; Joseleau, J.P. Investigation of the structure of a heteroxylan from the outer pericarp (beeswing bran) of wheat kernel. Carbohydr. Res. 1987, 159, 109–126. [Google Scholar] [CrossRef]
- Dervilly-Pinel, G.; Rimsten, L.; Saulnier, L.; Andersson, R.; Åman, P. Water-extractable arabinoxylan from pearled flours of wheat, barley, rye and triticale. Evidence for the presence of ferulic acid dimers and their involvement in gel formation. J. Cereal Sci. 2001, 34, 207–214. [Google Scholar] [CrossRef]
- Gruppen, H.; Kormelink, F.J.M.; Voragen, A.G.J. Water-unextractable cell wall material from wheat flour. 3. A structural model for arabinoxylans. J. Cereal Sci. 1993, 18, 111–128. [Google Scholar] [CrossRef]
- Shewry, P.R.; Piironen, V.; Lampi, A.M.; Edelmann, M.; Kariluoto, S.; Nurmi, T.; Fernandez-Orozco, R.; Andersson, A.A.M.; Åman, P.; Fraś, A.; et al. Effects of genotype and environment on the content and composition of phytochemicals and dietary fiber components in rye in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2010, 58, 9372–9383. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hawkesford, M.J.; Piironen, V.; Lampi, A.M.; Gebruers, K.; Boros, D.; Andersson, A.A.M.; Åman, P.; Rakszegi, M.; Bedo, Z.; et al. Natural variation in grain composition of wheat and related cereals. J. Agric. Food Chem. 2013, 61, 8295–8303. [Google Scholar] [CrossRef]
- Gebruers, K.; Dornez, E.; Boros, D.; Fraś, A.; Dynkowska, W.; Bedő, Z.; Rakszegi, M.; Delcour, J.A.; Courtin, C.M. Variation in the content of dietary fiber and components thereof in wheats in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2008, 56, 9740–9749. [Google Scholar] [CrossRef]
- Vinkx, C.; Reynaert, H.; Grobet, P.; Delcour, J. Physicochemical and functional properties of rye nonstarch polysaccharides. V. Variability in the structure of water-soluble arabinoxylans. Cereal Chem. 1993, 70, 311. [Google Scholar]
- Andersson, A.A.M.; Lampi, A.M.; Nyström, L.; Piironen, V.; Li, L.; Ward, J.L.; Gebruers, K.; Courtin, C.M.; Delcour, J.A.; Boros, D.; et al. Phytochemical and dietary fiber components in barley varieties in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2008, 56, 9767–9776. [Google Scholar] [CrossRef] [PubMed]
- Collins, H.M.; Burton, R.A.; Topping, D.L.; Liao, M.; Bacic, A.; Fincher, G.B. Variability in fine structures of noncellulosic cell wall polysaccharides from cereal grains: Potential importance in human health and nutrition. Cereal Chem. 2010, 87, 272–282. [Google Scholar] [CrossRef]
- Xu, H.; Reuhs, B.L.; Cantu-Jungles, T.M.; Tuncil, Y.E.; Kaur, A.; Terekhov, A.; Martens, E.C.; Hamaker, B.R. Corn arabinoxylan has a repeating structure of subunits of high branch complexity with slow gut microbiota fermentation. Carbohydr. Polym. 2022, 289, 119435. [Google Scholar] [CrossRef]
- Bunzel, M. Chemistry and occurrence of hydroxycinnamate oligomers. Phytochem. Rev. 2010, 9, 47–64. [Google Scholar] [CrossRef]
- Jilek, M.L.; Bunzel, M. Dehydrotriferulic and dehydrodiferulic acid profiles of cereal and pseudocereal flours. Cereal Chem. 2013, 90, 507–514. [Google Scholar] [CrossRef]
- Waterstraat, M.; Bunzel, M. A stable isotope dilution approach to analyze ferulic acid oligomers in plant cell walls using liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 5047–5062. [Google Scholar] [CrossRef]
- Grabber, J.; Hatfield, R.; Ralph, J. Diferulate cross-links impede the enzymatic degradation of non-lignified maize walls. J. Sci. Food Agric. 1998, 77, 193–200. [Google Scholar] [CrossRef]
- Gruppen, H.; Hamer, R.; Voragen, A. Water-unextractable cell wall material from wheat flour. 1. Extraction of polymers with alkali. J. Cereal Sci. 1992, 16, 41–51. [Google Scholar] [CrossRef]
- Courtin, C.M.; Roelants, A.; Delcour, J.A. Fractionation–reconstitution experiments provide insight into the role of endoxylanases in bread-making. J. Agric. Food Chem. 1999, 47, 1870–1877. [Google Scholar] [CrossRef]
- Buksa, K.; Nowotna, A.; Ziobro, R.; Praznik, W. Molecular properties of arabinoxylan fractions isolated from rye grain of different quality. J. Cereal Sci. 2014, 60, 368–373. [Google Scholar] [CrossRef]
- Izydorczyk, M.; Biliaderis, C. Structural heterogeneity of wheat endosperm arabinoxylans. Cereal Chem. 1993, 70, 641–646. [Google Scholar]
- Dervilly, G.; Saulnier, L.; Roger, P.; Thibault, J.F. Isolation of homogeneous fractions from wheat water-soluble arabinoxylans. Influence of the structure on their macromolecular characteristics. J. Agric. Food Chem. 2000, 48, 270–278. [Google Scholar] [CrossRef]
- Dervilly-Pinel, G.; Thibault, J.F.; Saulnier, L. Experimental evidence for a semi-flexible conformation for arabinoxylans. Carbohydr. Res. 2001, 330, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Delcour, J.A. Structural characterisation of water-extractable and water-unextractable arabinoxylans in wheat bran. J. Cereal Sci. 2002, 35, 315–326. [Google Scholar] [CrossRef]
- Dervilly, G.; Leclercq, C.; Zimmermann, D.; Roue, C.; Thibault, J.F.; Saulnier, L. Isolation and characterization of high molar mass water-soluble arabinoxylans from barley and barley malt. Carbohydr. Polym. 2002, 47, 143–149. [Google Scholar] [CrossRef]
- Verwimp, T.; Van Craeyveld, V.; Courtin, C.M.; Delcour, J.A. Variability in the structure of rye flour alkali-extractable arabinoxylans. J. Agric. Food Chem. 2007, 55, 1985–1992. [Google Scholar] [CrossRef]
- Andersson, R.; Fransson, G.; Tietjen, M.; Åman, P. Content and molecular-weight distribution of dietary fiber components in whole-grain rye flour and bread. J. Agric. Food Chem. 2009, 57, 2004–2008. [Google Scholar] [CrossRef]
- Chanliaud, E.; Saulnier, L.; Thibault, J.F. Alkaline extraction and characterisation of heteroxylans from maize bran. J. Cereal Sci. 1995, 21, 195–203. [Google Scholar] [CrossRef]
- Mendis, M.; Simsek, S. Production of structurally diverse wheat arabinoxylan hydrolyzates using combinations of xylanase and arabinofuranosidase. Carbohydr. Polym. 2015, 132, 452–459. [Google Scholar] [CrossRef]
- Langenaeken, N.A.; De Schepper, C.F.; De Schutter, D.P.; Courtin, C.M. Carbohydrate content and structure during malting and brewing: A mass balance study. J. Inst. Brew. 2020, 126, 253–262. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J.; Gu, G.; Shi, Z.; Mao, Z. Studies on water-extractable arabinoxylans during malting and brewing. Food Chem. 2005, 93, 33–38. [Google Scholar] [CrossRef]
- Sungurtas, J.; Swanston, J.; Davies, H.; McDougall, G. Xylan-degrading enzymes and arabinoxylan solubilisation in barley cultivars of differing malting quality. J. Cereal Sci. 2004, 39, 273–281. [Google Scholar] [CrossRef]
- Betts, N.S.; Wilkinson, L.G.; Khor, S.F.; Shirley, N.J.; Lok, F.; Skadhauge, B.; Burton, R.A.; Fincher, G.B.; Collins, H.M. Morphology, carbohydrate distribution, gene expression, and enzymatic activities related to cell wall hydrolysis in four barley varieties during simulated malting. Front. Plant Sci. 2017, 8, 1872. [Google Scholar] [CrossRef]
- Krahl, M.; Müller, S.; Zarnkow, M.; Back, W.; Becker, T. Arabinoxylan and fructan in the malting and brewing process. Qual. Assur. Saf. Crop. Foods 2009, 1, 246–255. [Google Scholar] [CrossRef]
- Guo, M.; Du, J.; Zhang, K.; Jin, Y. Content and molecular weight of water-extractable arabinoxylans in wheat malt and wheat malt-based wort with different Kolbach indices. J. Sci. Food Agric. 2014, 94, 2794–2800. [Google Scholar] [CrossRef] [PubMed]
- Debyser, W.; Derdelinckx, G.; Delcour, J.A. Arabinoxylan and arabinoxylan hydrolyzing activities in barley malts and worts derived from them. J. Cereal Sci. 1997, 26, 67–74. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J.; Gu, G. Control of arabinoxylan solubilization and hydrolysis in mashing. Food Chem. 2005, 90, 101–108. [Google Scholar] [CrossRef]
- Peng, Z.; Jin, Y.; Du, J. Enzymatic properties of endo-1,4-β-xylanase from wheat malt. Protein Pept. Lett. 2019, 26, 332–338. [Google Scholar] [CrossRef]
- Viëtor, R.J.; Voragen, A.G.J.; Angelino, S.A.G.F. Composition of non-starch polysaccharides in wort and spent grain from brewing trials with malt from a good malting quality barley and a feed barley. J. Inst. Brew. 1993, 99, 243–248. [Google Scholar] [CrossRef]
- Michiels, P.; Delputte, N.; Debyser, W.; Langenaeken, N.A.; Courtin, C.M. The occurrence and structural heterogeneity of arabinoxylan in commercial pilsner beers and their non-alcoholic counterparts. Carbohydr. Polym. 2023, 306, 120597. [Google Scholar] [CrossRef]
- Shang, Y.L.; Li, X.M.; Cai, G.L.; Lu, J. The role of ferulic acid and arabinoxylan in inducing premature yeast flocculation. J. Inst. Brew. 2015, 121, 49–54. [Google Scholar] [CrossRef]
- Xie, Y.; Cai, G.; Xu, M.; Han, B.; Li, C.; Lu, J. The effect of barley infected with xylanase-producing filamentous fungi on premature yeast flocculation. J. Inst. Brew. 2022, 128, 162–170. [Google Scholar] [CrossRef]
- Li, J.; Du, J.; Wu, X.; Zhang, Z.; Zhang, K. Changes in crude arabinoxylan during cloudy wheat beer brewing on a production scale. J. Inst. Brew. 2017, 123, 192–198. [Google Scholar] [CrossRef]
- Martinez Amezaga, N.M.J.; Lataza Rovaletti, M.M.; Benitez, E.I. Particle size distribution of polysaccharides in beer before the filtration process. Int. J. Food Res. 2017, 5, 13–19. [Google Scholar]
- Jin, Y.L.; Speers, R.A.; Paulson, A.T.; Stewart, R.J. Effect of β-glucans and process conditions on the membrane filtration performance of beer. J. Am. Soc. Brew. Chem. 2004, 62, 117–124. [Google Scholar] [CrossRef]
- Jin, Y.L.; Speers, A.; Paulson, A.T.; Stewart, R.J. Effects of β-glucans and environmental factors on the viscosities of wort and beer. J. Inst. Brew. 2004, 110, 104–116. [Google Scholar] [CrossRef]
- Sadosky, P.; Schwarz, P.B.; Horsley, R.D. Effect of arabinoxylans, β-glucans, and dextrins on the viscosity and membrane filterability of a beer model solution. J. Am. Soc. Brew. Chem. 2002, 60, 153–162. [Google Scholar] [CrossRef]
- Egi, A.; Speers, R.; Paulson, A. The physical behavior of arabinoxylans in model brewing solutions. Tech. Q.-Master Brew. Assoc. Am. 2004, 41, 268–276. [Google Scholar]
- Lu, J.; Li, Y.; Gu, G.; Mao, Z. Effects of molecular weight and concentration of arabinoxylans on the membrane plugging. J. Agric. Food Chem. 2005, 53, 4996–5002. [Google Scholar] [CrossRef]
- Gastl, M.; Kupetz, M.; Becker, T. Determination of cytolytic malt modification – Part II: Impact on wort separation. J. Am. Soc. Brew. Chem. 2021, 79, 66–74. [Google Scholar] [CrossRef]
- Shrestha, U.R.; Smith, S.; Pingali, S.V.; Yang, H.; Zahran, M.; Breunig, L.; Wilson, L.A.; Kowali, M.; Kubicki, J.D.; Cosgrove, D.J.; et al. Arabinose substitution effect on xylan rigidity and self-aggregation. Cellulose 2019, 26, 2267–2278. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y. Effects of arabinoxylan solubilization on wort viscosity and filtration when mashing with grist containing wheat and wheat malt. Food Chem. 2006, 98, 164–170. [Google Scholar] [CrossRef]
- Marconi, O.; Alfeo, V.; Tomasi, I.; Maranghi, S.; De Francesco, G.; Sileoni, V.; Perretti, G. Effects of malting process on molecular weight distribution and content of total and water-extractable arabinoxylan in barley. J. Cereal Sci. 2022, 107. [Google Scholar] [CrossRef]
- Mangan, D.; Cornaggia, C.; Liadova, A.; Draga, A.; Ivory, R.; Evans, D.E.; McCleary, B.V. Development of an automatable method for the measurement of endo-1,4-β-xylanase activity in barley malt and initial investigation into the relationship between endo-1,4-β-xylanase activity and wort viscosity. J. Cereal Sci. 2018, 84, 90–94. [Google Scholar] [CrossRef]
- Schwarz, P.B.; Han, J.Y. Arabinoxylan content of commercial beers. J. Am. Soc. Brew. Chem. 1995, 53, 157–159. [Google Scholar] [CrossRef]
- Courtin, C.M.; Broekaert, W.F.; Swennen, K.; Aerts, G.; Van Craeyveld, V.; Delcour, J.A. Occurrence of arabinoxylo-oligosaccharides and arabinogalactan peptides in beer. J. Am. Soc. Brew. Chem. 2009, 67, 112–117. [Google Scholar] [CrossRef]
- Evans, D.; Sheehan, M.; Stewart, D. The impact of malt derived proteins on beer foam quality. Part II: The influence of malt foam-positive proteins and non-starch polysaccharides on beer foam quality. J. Inst. Brew. 1999, 105, 171–178. [Google Scholar] [CrossRef]
- Li, J.; Du, J. Molecular characterization of arabinoxylan from wheat beer, beer foam and defoamed beer. Molecules 2019, 24, 1230. [Google Scholar] [CrossRef]
- Sohrabvandi, S.; Mousavi, S.M.; Razavi, S.H.; Mortazavian, A.M.; Rezaei, K. Alcohol-free beer: Methods of production, sensorial defects, and healthful effects. Food Rev. Int. 2010, 26, 335–352. [Google Scholar] [CrossRef]
- Langenaeken, N.A.; De Schutter, D.P.; Courtin, C.M. Arabinoxylan from non-malted cereals can act as mouthfeel contributor in beer. Carbohydr. Polym. 2020, 239, 116257. [Google Scholar] [CrossRef]
- Sancho, A.I.; Faulds, C.B.; Bartolomé, B.; Williamson, G. Characterisation of feruloyl esterase activity in barley. J. Sci. Food Agric. 1999, 79, 447–449. [Google Scholar] [CrossRef]
- Vanbeneden, N.; Gils, F.; Delvaux, F.; Delvaux, F.R. Variability in the release of free and bound hydroxycinnamic acids from diverse malted barley (Hordeum vulgare L.) Cultiv. Wort Prod. J. Agric. Food Chem. 2007, 55, 11002–11010. [Google Scholar] [CrossRef]
- Coghe, S.; Benoot, K.; Delvaux, F.; Vanderhaegen, B.; Delvaux, F.R. Ferulic acid release and 4-vinylguaiacol formation during brewing and fermentation: Indications for feruloyl esterase activity in Saccharomyces cerevisiae. J. Agric. Food Chem. 2004, 52, 602–608. [Google Scholar] [CrossRef]
- McLauchlan, W.R.; Garcia-Conesa, M.T.; Williamson, G.; Roza, M.; Ravestein, P.; Maat, J. A novel class of protein from wheat which inhibits xylanases. Biochem. J. 1999, 338, 441–446. [Google Scholar] [CrossRef]
- Fierens, E.; Rombouts, S.; Gebruers, K.; Goesaert, H.; Brijs, K.; Beaugrand, J.; Volckaert, G.; Van Campenhout, S.; Proost, P.; Courtin, C.; et al. TLXI, a novel type of xylanase inhibitor from wheat (Triticum aestivum) Belong. Thaum. Fam. Biochem. J. 2007, 403, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Kalb, V.; Seewald, T.; Hofmann, T.; Granvogl, M. Studies on the impact of malting and mashing on the free, soluble ester-bound, and insoluble ester-bound forms of desired and undesired phenolic acids aiming at styrene mitigation during wheat beer brewing. J. Agric. Food Chem. 2020, 68, 12421–12432. [Google Scholar] [CrossRef]
- Vanbeneden, N.; Van Roey, T.; Willems, F.; Delvaux, F.; Delvaux, F.R. Release of phenolic flavour precursors during wort production: Influence of process parameters and grist composition on ferulic acid release during brewing. Food Chem. 2008, 111, 83–91. [Google Scholar] [CrossRef]
- Poisson, L.; Schieberle, P. Characterization of the most odor-active compounds in an American bourbon whisky by application of the aroma extract dilution analysis. J. Agric. Food Chem. 2008, 56, 5813–5819. [Google Scholar] [CrossRef]
- Leys, S.; De Bondt, Y.; Bosmans, G.; Courtin, C.M. Assessing the impact of xylanase activity on the water distribution in wheat dough: A 1H NMR study. Food Chem. 2020, 325, 126828. [Google Scholar] [CrossRef]
- Wang, M.; Hamer, R.J.; van Vliet, T.; Gruppen, H.; Marseille, H.; Weegels, P.L. Effect of water-unextractable solids on gluten formation and properties: Mechanistic considerations. J. Cereal Sci. 2003, 37, 55–64. [Google Scholar] [CrossRef]
- Courtin, C.M.; Gelders, G.G.; Delcour, J.A. Use of two endoxylanases with different substrate selectivity for understanding arabinoxylan functionality in wheat flour breadmaking. Cereal Chem. 2001, 78, 564–571. [Google Scholar] [CrossRef]
- Courtin, C.M.; Delcour, J.A. Arabinoxylans and endoxylanases in wheat flour bread-making. J. Cereal Sci. 2002, 35, 225–243. [Google Scholar] [CrossRef]
- Nishitsuji, Y.; Whitney, K.; Nakamura, K.; Hayakawa, K.; Simsek, S. Changes in structure and solubility of wheat arabinoxylan during the breadmaking process. Food Hydrocoll. 2020, 109, 106129. [Google Scholar] [CrossRef]
- Biliaderis, C.G.; Izydorczyk, M.S.; Rattan, O. Effect of arabinoxylans on bread-making quality of wheat flours. Food Chem. 1995, 53, 165–171. [Google Scholar] [CrossRef]
- Pietiäinen, S.; Moldin, A.; Ström, A.; Malmberg, C.; Langton, M. Effect of physicochemical properties, pre-processing, and extraction on the functionality of wheat bran arabinoxylans in breadmaking—A review. Food Chem. 2022, 383, 132584. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, Z.; Niu, M.; Zhao, S.; Jia, C.; Wu, Y. Thermomechanical behaviors and protein polymerization in bread dough modified by bran components and transglutaminase. LWT 2020, 133, 109894. [Google Scholar] [CrossRef]
- Gudmundsson, M.; Eliasson, A.C.; Bengtsson, S.; Aman, P. The effects of water-soluble arabinoxylan on gelatinization and retrogradation of starch. Starch—Stärke 1991, 43, 5–10. [Google Scholar] [CrossRef]
- Zhang, L.; van Boven, A.; Mulder, J.; Grandia, J.; Chen, X.D.; Boom, R.M.; Schutyser, M.A.I. Arabinoxylans-enriched fractions: From dry fractionation of wheat bran to the investigation on bread baking performance. J. Cereal Sci. 2019, 87, 1–8. [Google Scholar] [CrossRef]
- Kaur, A.; Singh, B.; Yadav, M.P.; Bhinder, S.; Singh, N. Isolation of arabinoxylan and cellulose-rich arabinoxylan from wheat bran of different varieties and their functionalities. Food Hydrocoll. 2021, 112, 106287. [Google Scholar] [CrossRef]
- Li, J.; Kang, J.; Wang, L.; Li, Z.; Wang, R.; Chen, Z.X.; Hou, G.G. Effect of water migration between arabinoxylans and gluten on baking quality of whole wheat bread detected by magnetic resonance imaging (MRI). J. Agric. Food Chem. 2012, 60, 6507–6514. [Google Scholar] [CrossRef]
- Cleemput, G.; Booij, C.; Hessing, M.; Gruppen, H.; Delcour, J. Solubilisation and changes in molecular weight distribution of arabinoxylans and protein in wheat flours during bread-making, and the effects of endogenous arabinoxylan hydrolysing enzymes. J. Cereal Sci. 1997, 26, 55–66. [Google Scholar] [CrossRef]
- Zhang, D.; Rudjito, R.C.; Pietiäinen, S.; Chang, S.C.; Idström, A.; Evenäs, L.; Vilaplana, F.; Jiménez-Quero, A. Arabinoxylan supplemented bread: From extraction of fibers to effect of baking, digestion, and fermentation. Food Chem. 2023, 413, 135660. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, R.; Wu, T.; Zhang, M. Aggregation and rheological behavior of soluble dietary fibers from wheat bran. Food Res. Int. 2017, 102, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Nishitsuji, Y.; Whitney, K.; Nakamura, K.; Hayakawa, K.; Simsek, S. Analysis of molecular weight and structural changes in water-extractable arabinoxylans during the breadmaking process. Food Chem. 2022, 386, 132772. [Google Scholar] [CrossRef] [PubMed]
- Kiszonas, A.M.; Fuerst, E.P.; Luthria, D.; Morris, C.F. Arabinoxylan content and characterisation throughout the bread-baking process. Int. J. Food Sci. Technol. 2015, 50, 1911–1921. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Yang, M.; Ou, Z.; Lin, Y.; Zhao, F.; Han, S. Characterization and application of a novel xylanase from Halolactibacillus miurensis Wholewheat Bread Making. Front. Bioeng. Biotechnol. 2022, 10, 1018476. [Google Scholar] [CrossRef]
- Wang, P.; Hou, C.; Zhao, X.; Tian, M.; Gu, Z.; Yang, R. Molecular characterization of water-extractable arabinoxylan from wheat bran and its effect on the heat-induced polymerization of gluten and steamed bread quality. Food Hydrocoll. 2019, 87, 570–581. [Google Scholar] [CrossRef]
- Zhu, X.F.; Tao, H.; Wang, H.L.; Xu, X.M. Impact of water soluble arabinoxylan on starch-gluten interactions in dough. LWT 2023, 173, 114289. [Google Scholar] [CrossRef]
- Buksa, K.; Nowotna, A.; Ziobro, R. Application of cross-linked and hydrolyzed arabinoxylans in baking of model rye bread. Food Chem. 2016, 192, 991–996. [Google Scholar] [CrossRef]
- Figueroa-Espinoza, M.C.; Rouau, X. Oxidative cross-linking of pentosans by a fungal laccase and horseradish peroxidase: Mechanism of linkage between feruloylated arabinoxylans. Cereal Chem. 1998, 75, 259–265. [Google Scholar] [CrossRef]
- Goesaert, H.; Brijs, K.; Veraverbeke, W.S.; Courtin, C.M.; Gebruers, K.; Delcour, J.A. Wheat flour constituents: How they impact bread quality, and how to impact their functionality. Trends Food Sci. Technol. 2005, 16, 12–30. [Google Scholar] [CrossRef]
- Si, X.; Li, T.; Zhang, Y.; Zhang, W.; Qian, H.; Li, Y.; Zhang, H.; Qi, X.; Wang, L. Interactions between gluten and water-unextractable arabinoxylan during the thermal treatment. Food Chem. 2021, 345, 128785. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.; Li, J.; Li, F.; Teng, C.; Li, X. Effects of water-extractable arabinoxylan on the physicochemical properties and structure of wheat gluten by thermal treatment. J. Agric. Food Chem. 2017, 65, 4728–4735. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, F.; Wang, Y.; Li, J.; Teng, C.; Wang, C.; Li, X. Effects of different molecular weight water-extractable arabinoxylans on the physicochemical properties and structure of wheat gluten. J. Food Sci. Technol. 2019, 56, 340–349. [Google Scholar] [CrossRef]
- Sun, J.; Si, X.; Li, T.; Zhao, J.; Qian, H.; Li, Y.; Zhang, H.; Qi, X.; Wang, L. The influence of water-unextractable arabinoxylan and its hydrolysates on the aggregation and structure of gluten proteins. Front. Nutr. 2022, 9, 877135. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Tao, H.; Jin, Z.; Xu, X. Impact of water extractable arabinoxylan from rye bran on the frozen steamed bread dough quality. Food Chem. 2016, 200, 117–124. [Google Scholar] [CrossRef]
- Zhao, X.; Hou, C.; Tian, M.; Zhou, Y.; Yang, R.; Wang, X.; Gu, Z.; Wang, P. Effect of water-extractable arabinoxylan with different molecular weight on the heat-induced aggregation behavior of gluten. Food Hydrocoll. 2020, 99, 105318. [Google Scholar] [CrossRef]
- Beck, M.; Jekle, M.; Selmair, P.L.; Koehler, P.; Becker, T. Rheological properties and baking performance of rye dough as affected by transglutaminase. J. Cereal Sci. 2011, 54, 29–36. [Google Scholar] [CrossRef]
- Döring, C.; Nuber, C.; Stukenborg, F.; Jekle, M.; Becker, T. Impact of arabinoxylan addition on protein microstructure formation in wheat and rye dough. J. Food Eng. 2015, 154, 10–16. [Google Scholar] [CrossRef]
- Döring, C.; Hussein, M.A.; Jekle, M.; Becker, T. On the assessments of arabinoxylan localization and enzymatic modifications for enhanced protein networking and its structural impact on rye dough and bread. Food Chem. 2017, 229, 178–187. [Google Scholar] [CrossRef]
- Piber, M.; Koehler, P. Identification of dehydro-ferulic acid-tyrosine in rye and wheat: Evidence for a covalent cross-link between arabinoxylans and proteins. J. Agric. Food Chem. 2005, 53, 5276–5284. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Kang, J.; Wang, N.; Xiao, M.; Li, Z.; Wang, C.; Guo, Q.; Hu, X. Arabinoxylan from wheat bran: Molecular degradation and functional investigation. Food Hydrocoll. 2020, 107, 105914. [Google Scholar] [CrossRef]
- Wang, P.; Li, D.; Hou, C.; Yang, T.; Yang, R.; Gu, Z.; Jiang, D. Tailormade wheat arabinoxylan reveals the role of substitution in regulating gelatinization and retrogradation behavior of wheat starch. J. Agric. Food Chem. 2022, 70, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Zhao, X.; Tian, M.; Zhou, Y.; Yang, R.; Gu, Z.; Wang, P. Impact of water extractable arabinoxylan with different molecular weight on the gelatinization and retrogradation behavior of wheat starch. Food Chem. 2020, 318, 126477. [Google Scholar] [CrossRef]
- Rosicka-Kaczmarek, J.; Tkaczyk, M.; Makowski, B.; Komisarczyk, A.; Nebesny, E. The influence of non-starch polysaccharide on thermodynamic properties of starches from facultative wheat varieties. Eur. Food Res. Technol. 2017, 243, 2243–2253. [Google Scholar] [CrossRef]
- Ying, R.; Zhou, T.; Xie, H.; Huang, M. Synergistic effect of arabinoxylan and (1,3)(1,4)-β-glucan reduces the starch hydrolysis rate in wheat flour. Food Hydrocoll. 2023, 141, 108668. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).