Innocell Bioreactor: An Open-Source Development to Produce Biomaterials for Food and Packaging Based on Fermentation Processes
Abstract
:1. Introduction
2. Materials and Methods
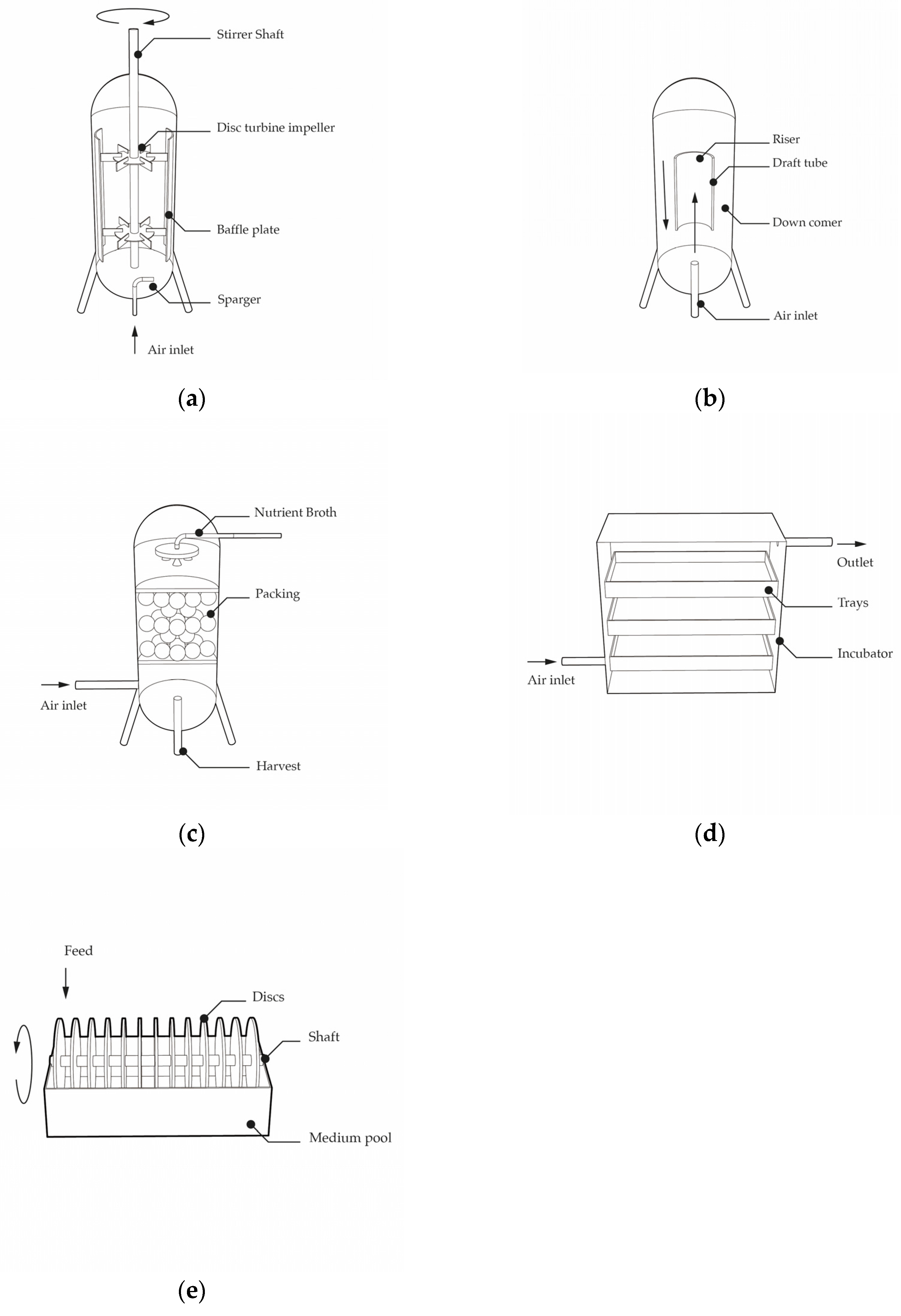
2.1. InnoCell Bioreactor Development
2.2. Initial Prototypes
2.3. From the RDC Prototype to InnoCell Bioreactor Development
3. Results
3.1. Producing SCOBY with the InnoCell Bioreactor
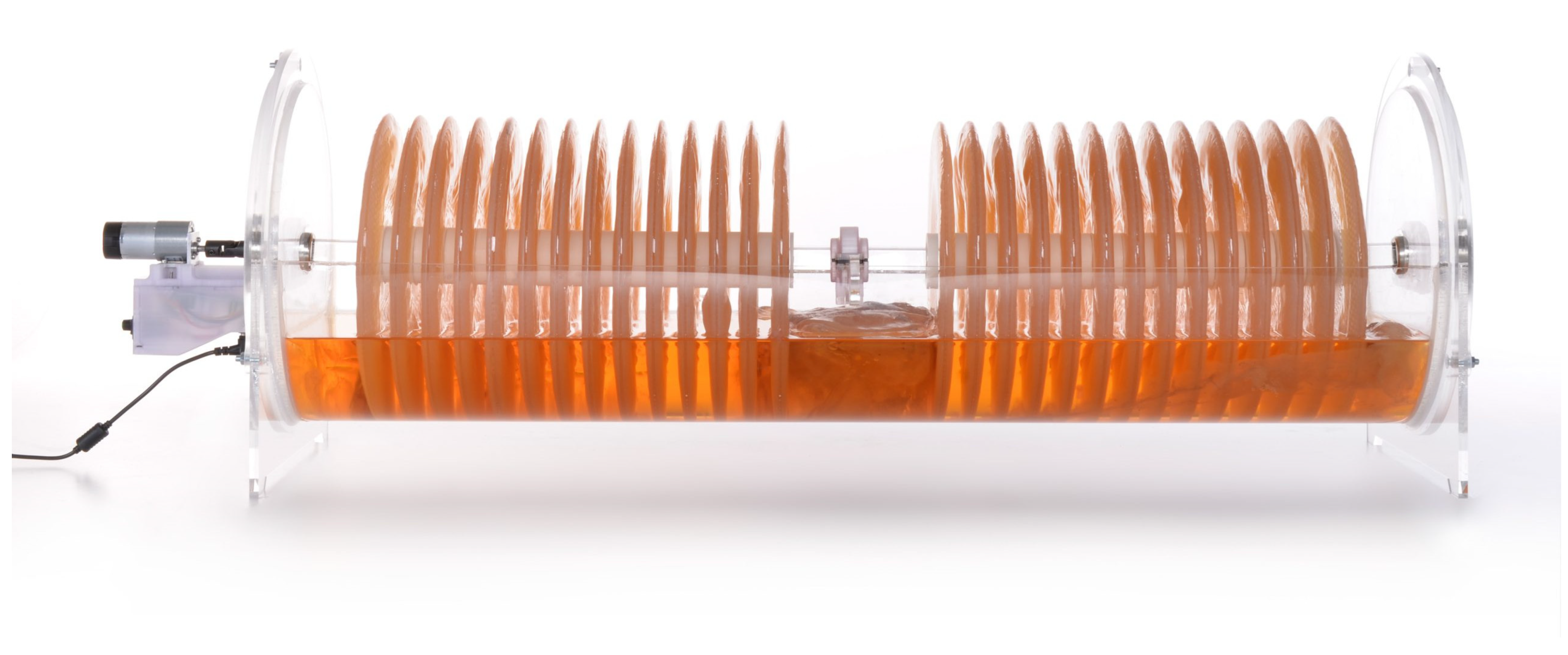
- -
- 25 L capacity (full tank);
- -
- 28 disks (ideal growth per disk 350–600 g);
- -
- 10–15 kg wet pellicle-mass production per cycle.
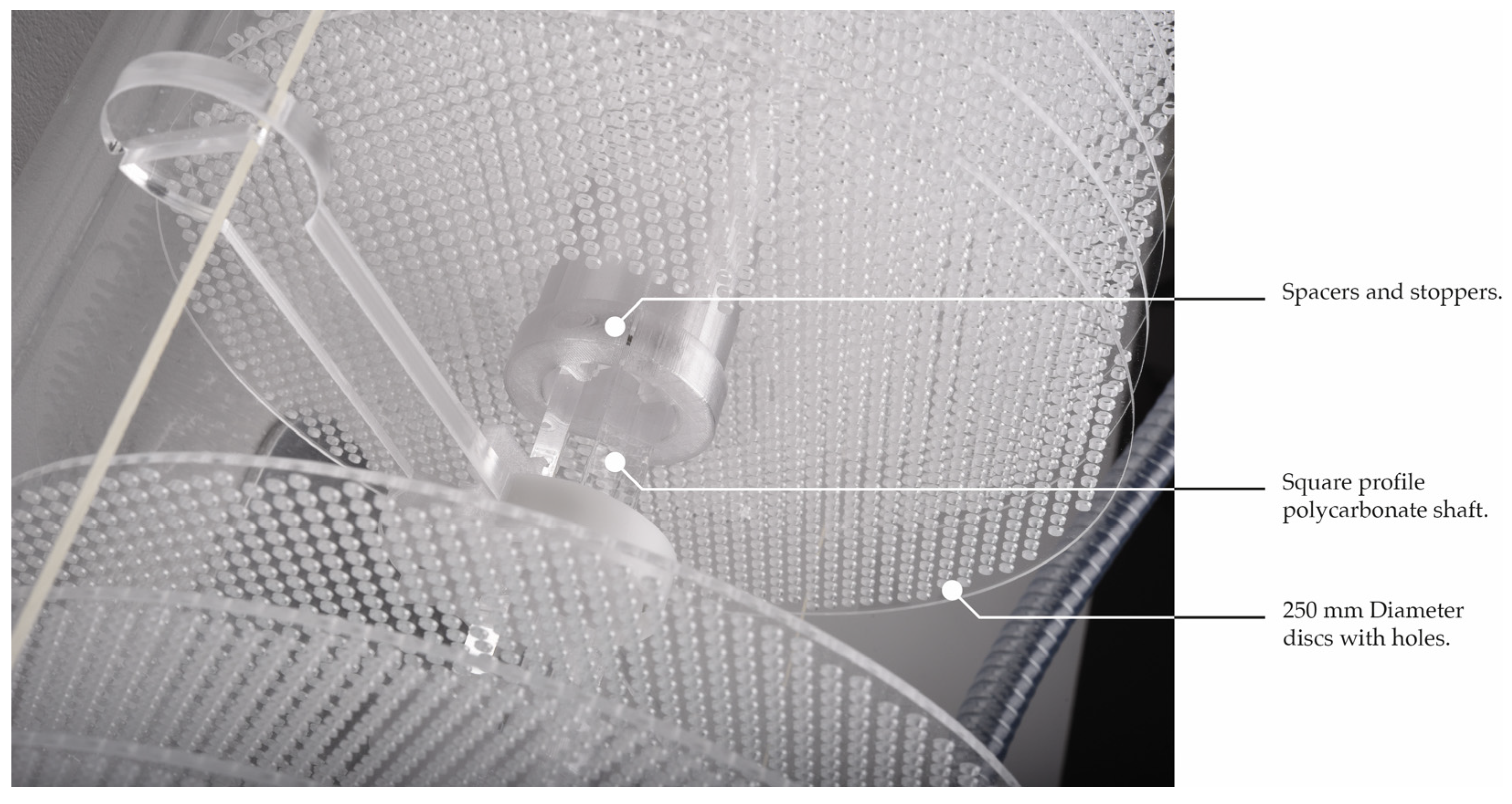
- Tank shape: As the disks are round, it seemed logical to follow their shape for volume optimization. Indeed, a study proved that efficient pellicle growth depends more on the availability of nutrients and oxygen rather than liquid medium volume [49]. Moreover, in the angles of a square-profile tank, undesired elements can concentrate and more easily contaminate the culture. Optimizing the volume allows more cellulose mass with less liquid. The “u” shape (or half-cylindrical) of the bioreactor optimizes the quantity of liquid.
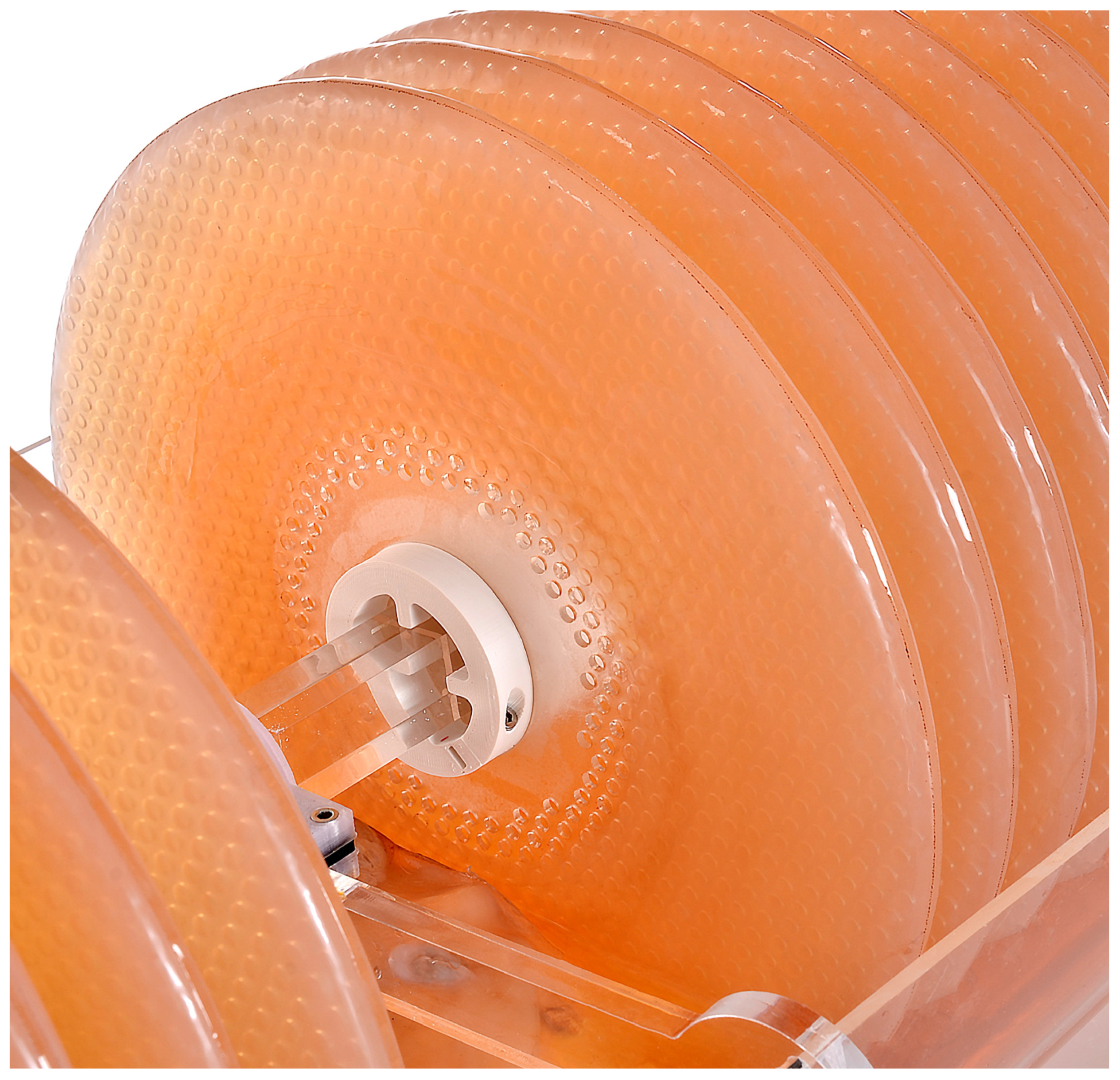
- Shaft: The square profile of the shaft keeps the disks fixed, facilitating a more homogeneous pellicle growth, and prevents them from rolling against each other. This also avoids the discs from spinning loosely by the effect of friction and wear of the axis hole in the long term.
- Motor/bearings/board holders: To minimize alignment issues and weight fatigue for the motor, the various components, discs, and spacers are divided in the middle after the 14th disc by a bearing mounted on a place holder. The motor is mounted on a holding structure that is external to the tank to minimize the contact with the liquid medium. The motor and the power bench should be boxed to minimize the risks of contact between liquid and electricity.
3.2. Considerations for an Effective Fermentation
3.2.1. Capacity and Liquid Parameters
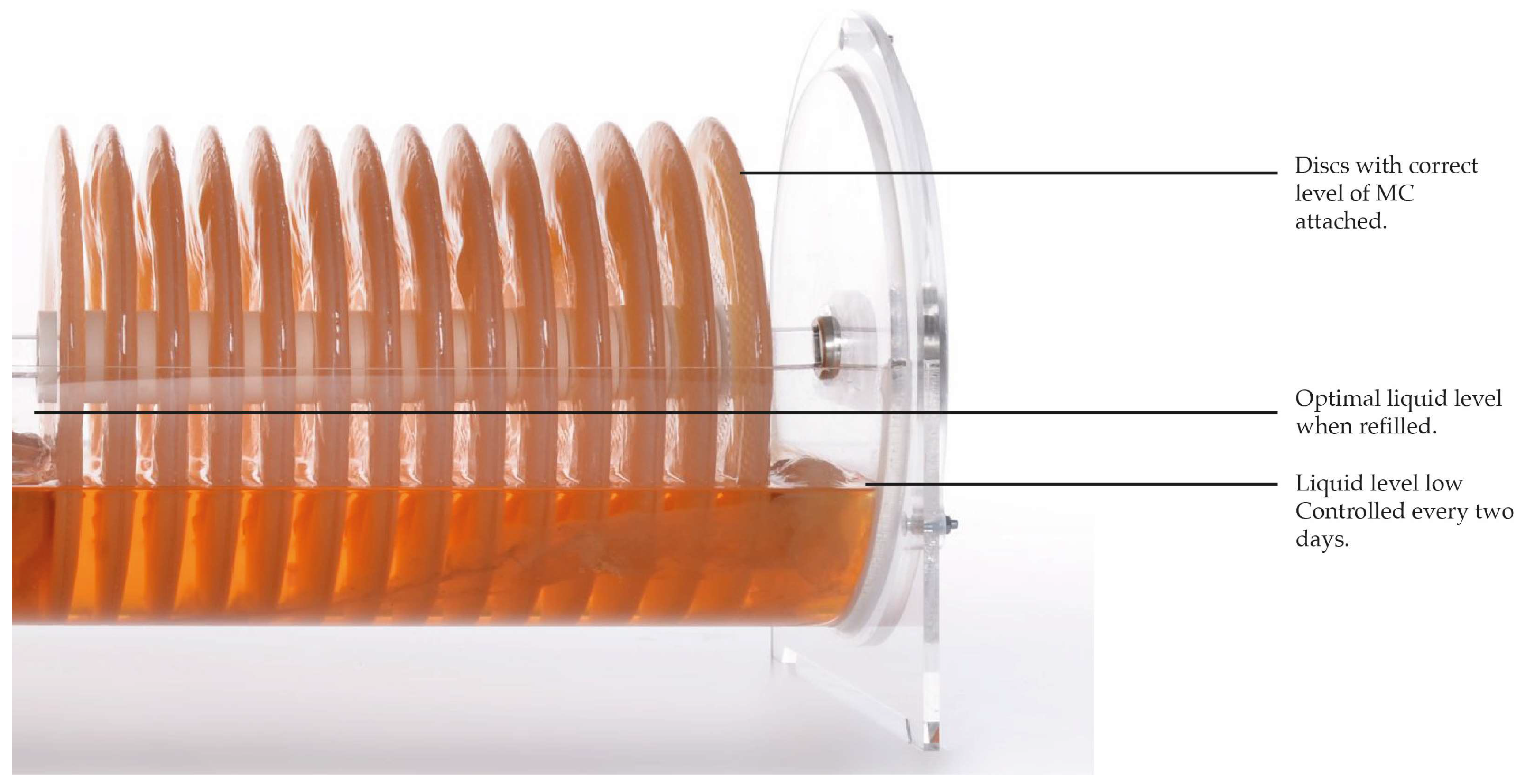
- The InnoCell Bioreactor presented in this study has a capacity of 25 L and yields, depending on conditions (nourishing medium, pH, temperature, time), from 10 to 15 kg over a fermentation time of 14 to 21 days. The liquid medium needs to be refilled on an ongoing basis to ensure growth of the cellulose on as much surface of the discs as possible; the lower the liquid level, the less surface of disc is in contact with nourishment, and no thickening will occur. In our experience, circa 25–30 L of refill is needed throughout one cycle.
- It is recommended to have a starting liquid with a pH of 4–4.5 and a concentration of 5 °Brix degrees (50 g/L) that can be achieved with different sugar sources (with tea-based medium, circa 18.75 L of sweetened tea + 625 L of already fermented tea). If fruit and vegetable secondary products are used, it is important to measure the actual sugar content, as this may strongly influence the fermentation process. In this case, the biomasses chosen needs to be pre-treated to inactivate contaminants by boiling the mass for 30 min if necessary after dilution with water (e.g., in the case of apple pomace, 1:3 ratio—1 part of apple mass in 3 parts of water) or autoclaving at 121 °C for 30 min (heating treatment may, however, denature some valuable substance such as certain vitamins). It is important to start from an organic mass that is not rotten or heavily contaminated by fungi. Various companies already store the secondary products in the forms of dry pellets or concentrated packaged masses, which are more stable, and therefore suitable for this process. However, for such decisions, consultation with food technologists, microbiologists, and scientists is crucial. After the pre-treatment, the liquid needs to be filtered properly, as particles would be otherwise incorporated into the pellicles, decreasing their mechanical properties. Brix degrees were measured using a pocket refractometer (VWR Digital Handheld Refractometer Cat. No. 75997-572 0-54 Brix 1.33-1.42 RI. Manufactured by VWR International, LLC. Radnor, PA 19087 USA).
3.2.2. Speed
3.2.3. Acidity
- In our experience, the starting pH should be between 4 and 4.5. This is more acidic compared to reviews that report an ideal pH of 4 to 6. However, especially in non-controlled environments, starting from a lower pH significantly decreases the contamination risks from certain spores present in the air and, more importantly, from pathogens.
- Such contamination risks increase in warmer seasons due to the more favorable room temperature. In a case of ongoing contamination during the summer of 2020, we started from a pH of 3.8, as suggested by the Food Tech Platform, using fermented liquid and acetic or citric acids, in order to discourage in the early stage the development of contaminants. After the pellicles started to grow, we raised the pH to 4–4.3 through refills. However, for a proper control over spores, an air filtration system should be added.
- During the fermentation, the pH should not drop below 3.5, as this would slow down and even stop the pellicle production. Therefore, the pH should be monitored with a pH meter; when it approaches 3.5, it should be raised to 4–4.5. This works sometimes by solely refilling (in other words, via dilution); however, in case of dramatic acidity, a solution of water and bicarbonate can be used.
3.2.4. Refill
- During the fermentation, part of the liquid evaporates; therefore, the tank should be refilled and adjusted with medium or fermented liquid (which may possibly cause changes in the pH). The fermentation generates acids that protect the culture. Constant monitoring enables the grower to adjust the liquid parameters to ideal conditions. The liquid volume should always reach the level of the holes pattern in the disks (circa the halfway) and be controlled every second day. If the liquid volume is too low, the pellicles may only grow in the external area of the disc and possibly cause drying of the left-over inner areas (Figure 7).
3.2.5. Heating
- A constant temperature of 28–30 °C should be maintained to ensure efficient growth. This can be provided by an external heating system composed of an aquarium pump and vertical heater connected to the tank through flexible plastic tubes. The system provides constant circulation to ensure culture homogenization. The heating system creates a circulation of the liquid inside the tank, which maintains the homogeneity of the medium.
3.2.6. Cover
- A cover based on a breathable cloth and straps was made to protect the fermentation culture from air contamination, and to slow down heat dispersion and liquid evaporation. It was efficient to the scope of the InnoCell research, and could be further adapted and developed.
3.2.7. Collection
3.2.8. Cleaning
- The whole system should be disassembled and cleaned after the end of every cycle. It is crucial to remove the holding structures and the motor, putting them aside. The shaft with the disks should be completely disassembled and cleaned with a sponge and/or brush with dishwashing soap, hydrogen peroxide, or sodium hypochlorite. The same procedure should be carried out for the tank. The system cannot be autoclaved. The fabric used above the tank was washed at 90 °C in a common washing machine before every cycle.
- The biofilm grows everywhere in the liquid medium or passes through; hence, building any heating circulation system would very likely allow it to grow inside and clog it. In order to avoid components being damaged, the system should be checked daily, and if the liquid flow slows down, the heating system should be disassembled and cleaned with the aid of a pipe cleaner and reassembled.
- In our experience, clogs may occur about 2–3 times per cycle. Plastic tubes with a large inner diameter discourage clog formation.
3.3. Fermentation Process with the InnoCell Bioreactor
Instructions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leroi-Gourhan, A. L’homme et la Matière: Évolution et Techniques; Albin Michel: Paris, France, 1943; Volume I, p. 367. [Google Scholar]
- Hudson, P. The Industrial Revolution; Bloomsbury: London, UK, 1992; Volume 3, pp. 166–171. [Google Scholar]
- Riccini, R. History from Things: Notes on the History of Industrial Design. Des. Issues 1998, 14, 43. [Google Scholar] [CrossRef]
- Welch, R.W.; Mitchell, P.C. Food processing: A century of change. Br. Med. Bull. 2000, 56, 1–17. [Google Scholar] [CrossRef]
- Dai, S.; Duan, X.; Zhang, W. Knowledge map of environmental crisis management based on keywords network and co-word analysis, 2005–2018. J. Clean. Prod. 2020, 262, 121168. [Google Scholar] [CrossRef]
- Tregidga, H.; Laine, M. On crisis and emergency: Is it time to rethink long-term environmental accounting? Crit. Perspect. Account. 2022, 82, 102311. [Google Scholar] [CrossRef]
- Meyer, M.W.; Norman, D. Changing Design Education for the 21st Century. She Ji J. Des. Econ. Innov. 2020, 6, 13–49. [Google Scholar] [CrossRef]
- Vezzoli, C. Design for Environmental Sustainability: Life Cycle Design of Products. Second Edition; Springer: Cham, Switzerland, 2018; p. 25. [Google Scholar]
- Karana, E.; Rognoli, V.; Jacob-Dazarola, R. La función del diseño en el desarrollo de nuevos materiales: Entrevista con Elvin Karana. Diseña 2020, 17, 46–55. [Google Scholar] [CrossRef]
- Rognoli, V.; Bianchini, M.; Maffei, S.; Karana, E. DIY materials. Mater. Des. 2015, 86, 692–702. [Google Scholar] [CrossRef]
- Rognoli, V.; Ayala-Garcia, C. Defining the DIY-Materials approach. In Materials Experience 2; Butterworth-Heinemann: London, UK, 2021; pp. 227–258. [Google Scholar] [CrossRef]
- Zhou, J.; Barati, B.; Wu, J.; Scherer, D.; Karana, E. Digital biofabrication to realize the potentials of plant roots for product design. Bio-Design Manuf. 2021, 4, 111–122. [Google Scholar] [CrossRef]
- Cohen, N.; Sicher, E.; Yavuz, S.U. Designing with microbial cellulose to feed new biological cycles. Int. J. Food Des. 2020, 4, 155–171. [Google Scholar] [CrossRef]
- Hair Highway. Studio Swine. Available online: https://studioswine.com/work/hair-highway/ (accessed on 6 September 2023).
- Microbes Are “the Factories of the Future”. Suzanne Lee, Dezeen. Available online: https://www.dezeen.com/2014/02/12/movie-biocouture-microbes-clothing-wearable-futures/ (accessed on 6 September 2023).
- Can City. Studio Swine. Available online: https://studioswine.com/work/can-city/ (accessed on 6 September 2023).
- Ahmad, M.; Cantarella, G.; Angeli, M.A.C.; Madagalam, M.; Ebner, C.; Ciocca, M.; Riaz, R.; Ibba, P.; Petrelli, M.; Merino, I.; et al. 2.4 GHz Microstrip Patch Antenna Fabricated by Means of Laser Induced Graphitization of a Cellulose-based Paper Substrate. In Proceedings of the 2021 IEEE International Flexible Electronics Technology Conference (IFETC), Columbus, OH, USA, 8–11 August 2021; pp. 0044–0046. [Google Scholar] [CrossRef]
- D’olivo, P.; Karana, E. Materials Framing: A Case Study of Biodesign Companies’ Web Communications. She Ji J. Des. Econ. Innov. 2021, 7, 403–434. [Google Scholar] [CrossRef]
- Llorach, P. Circular Design and Circular Material Design in: MaDe Material Designers. 2021. Available online: http://materialdesigners.org/book (accessed on 6 September 2023).
- Gershenfeld, N. How to Make Almost Anything. The Digital fabrication revolution. Foreign Aff. 2012, 91, 43. [Google Scholar]
- Bettiol, M.; Micelli, S. The Hidden Side of Design: The Relevance of Artisanship. Des. Issues 2014, 30, 7–18. [Google Scholar] [CrossRef]
- Tanenbaum, J.G.; Williams, A.M.; Desjardins, A.; Tanenbaum, K. Democratizing technology: Pleasure, utility and expressive-ness in DIY and maker practice. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, Paris, France, 27 April–2 May 2013; pp. 2603–2612. [Google Scholar]
- Sennett, R. The Craftsman; Yale University Press: New Haven, CT, USA, 2008. [Google Scholar]
- Anderson, C. Makers: The New Industrial Revolution; Crown Business: New York, NY, USA, 2012. [Google Scholar]
- Manzini, E.; Vezzoli, C. A strategic design approach to develop sustainable product service systems: Examples taken from the ‘environmentally friendly innovation’ Italian prize. J. Clean. Prod. 2003, 11, 851–857. [Google Scholar] [CrossRef]
- Coelho, R.M.D.; de Almeida, A.L.; Amaral, R.Q.G.D.; da Mota, R.N.; de Sousa, P.H.M. Kombucha: Review. Int. J. Gastron. Food Sci. 2020, 22, 100272. [Google Scholar] [CrossRef]
- De Oliveira, P.V.; Júnior, A.H.d.S.; de Oliveira, C.R.S.; Assumpção, C.F.; Ogeda, C.H. Kombucha benefits, risks and regulatory frameworks: A review. Food Chem. Adv. 2023, 2, 100288. [Google Scholar] [CrossRef]
- Martínez Leal, J.; Valenzuela Suárez, L.; Jayabalan, R.; Huerta Oros, J.; Escalante-Aburto, A. A review on health benefits of kombucha nutritional compounds and metabolites. CYTA J. Food 2018, 16, 390–399. [Google Scholar] [CrossRef]
- Chen, C.; Liu, B. Changes in major components of tea fungus metabolites during prolonged fermentation. J. Appl. Microbiol. 2000, 89, 834–839. [Google Scholar] [CrossRef]
- Esa, F.; Tasirin, S.M.; Rahman, N.A. Overview of Bacterial Cellulose Production and Application. Agric. Agric. Sci. Procedia 2014, 2, 113–119. [Google Scholar] [CrossRef]
- Andriani, D.; Apriyana, A.Y.; Karina, M. The optimization of bacterial cellulose production and its applications: A review. Cellulose 2020, 27, 6747–6766. [Google Scholar] [CrossRef]
- Avcioglu, N.H. Bacterial cellulose: Recent progress in production and industrial applications. World J. Microbiol. Biotechnol. 2022, 38, 86. [Google Scholar] [CrossRef]
- Czaja, W.; Romanovicz, D.; Brown, R.M. Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose 2004, 11, 403–411. [Google Scholar] [CrossRef]
- Chawla, P.R.; Bajaj, I.B.; Shrikant, A.S.; Singhal, R.S. Microbial Cellulose: Fermentative Production and Applications. Food Technol. Biotechnol. 2009, 47, 107–124. [Google Scholar]
- Cohen, N.; Sicher, E.; Merino, I.; Yavuz, S.U. An Open-Source Bioreactor Enhancing Microbial Cellulose Production and Novel Sustainable substances. In Sustainable Design and Manufacturing, Proceedings of the 8th International Conference on Sustainable Design and Manufacturing (KES-SDM 2021), Split, Croatia, 15–17 September 2021; Scholz, S.G., Howlett, R.J., Setchi, R., Eds.; Springer: Singapore, 2022; Volume 262, pp. 77–86. [Google Scholar] [CrossRef]
- Kozyrovska, N.O.; Reva, O.M.; Goginyan, V.B.; de Vera, J.-P. Kombucha microbiome as a probiotic: A view from the perspective of post-genomics and synthetic ecology. Biopolym. Cell 2012, 28, 103–113. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of bacterial cellulose in food. Food Hydrocoll. 2013, 35, 539–545. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial Cellulose as a Raw Material for Food and Food Packaging Applications. Front. Sustain. Food Syst. 2019, 3, 7. [Google Scholar] [CrossRef]
- Chisti, Y.; Moo-Young, M. Bioreactors. In Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 247–271. [Google Scholar] [CrossRef]
- Oslan, S.N.H.; Tan, J.S.; Abbasiliasi, S.; Sulaiman, A.Z.; Saad, M.Z.; Halim, M.; Ariff, A.B. Integrated Stirred-Tank Bioreactor with Internal Adsorption for the Removal of Ammonium to Enhance the Cultivation Performance of gdhA Derivative Pasteurella multocida B:2. Microorganisms 2020, 8, 1654. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; KarimiDermani, B.; Razmi, E.; Kasmuri, N. Anaerobic Membrane Bioreactor (AnMBR) for the Removal of Dyes from Water and Wastewater: Progress, Challenges, and Future Perspectives. Processes 2023, 11, 855. [Google Scholar] [CrossRef]
- Gutiérrez, C.F.; Rodríguez-Romero, N.; Egan, S.; Holmes, E.; Sanabria, J. Exploiting the Potential of Bioreactors for Creating Spatial Organization in the Soil Microbiome: A Strategy for Increasing Sustainable Agricultural Practices. Microorganisms 2022, 10, 1464. [Google Scholar] [CrossRef]
- Ge, X.; Vasco-Correa, J.; Li, Y. Solid-State Fermentation Bioreactors and Fundamentals. In Current Developments in Biotechnology and Bioengineering; Larroche, C., Ángeles Sanromán, M., Du, G., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 381–402. [Google Scholar] [CrossRef]
- Kuure-Kinsey, M.; Weber, D.; Bungay, H.; Plawsky, J.; Bequette, B. Modeling and predictive control of a rotating disk bioreactor. In Proceedings of the 2005, American Control Conference, Portland, OR, USA, 8 June 2005; Volume 5, pp. 3259–3264. [Google Scholar] [CrossRef]
- Pa’e, N. Rotary Discs Reactor for Enhanced Production of Microbial Cellulose. Master’s Thesis, University of Technology Malaysia, Skudai, Malaysia, 2009. [Google Scholar]
- Zahan, K.; Fadhrullah, M.; Pa’e, N.; Ng, C.; Muhamad, I. Designing economical production of microbial cellulose from waste using modified bioreactor. In Proceedings of the ICPE 2010, San Jose, CA, USA, 28–30 January 2010; Association for Computing Machinery: New York, NY, USA, 2014. [Google Scholar]
- Bungay, R.H.; Serafica, G.; Mormino, R. Environmental implications of microbial cellulose. Glob. Environ. Biotechnol. 1997, 66, 691–701. [Google Scholar]
- InnoCell Bioreactor (Version 1_v-3) Production Manual. Available online: https://designfrictionlab.com/project/bioreactor/ (accessed on 6 September 2023).
- Hsieh, J.-T.; Wang, M.-J.; Lai, J.-T.; Liu, H.-S. A novel static cultivation of bacterial cellulose production by intermittent feeding strategy. J. Taiwan Inst. Chem. Eng. 2016, 63, 46–51. [Google Scholar] [CrossRef]
- Laavanya, D.; Shirkole, S.; Balasubramanian, P. Current challenges, applications and future perspectives of SCOBY cellulose of Kombucha fermentation. J. Clean. Prod. 2021, 295, 126454. [Google Scholar] [CrossRef]
- De Oliveira, A.C.L.; Rolim, V.A.d.O.; Gaspar, R.P.L.; Rossini, D.Q.; de Souza, R.; Bogsan, C.S.B. The Technological Perspectives of Kombucha and Its Implications for Production. Fermentation 2022, 8, 185. [Google Scholar] [CrossRef]
- Ellen Macarthur Foundation. What Is a Circular Economy? 2023. Available online: https://ellenmacarthurfoundation.org/topics/circular-economy-introduction/overview (accessed on 6 September 2023).
- Koskinen, I.; Zimmerman, J.; Binder, T.; Redstrom, J.; Wensveen, S. Design research through practice: From the lab, field, and showroom. IEEE Trans. Prof. Commun. 2013, 56, 262–263. [Google Scholar] [CrossRef]





| Rotating Disk Culture Bioreactor (RDC) | Static Culture Tank (SCT) | |
|---|---|---|
| Culture ingredients | 12 L Tea. 4 L Fermented liquid (25%). 500 g of Sugar—Sucrose (3 °Brix). 185 g of Kombucha starter (SCOBY and liquid). | 12 L Tea. 4 L Fermented liquid (25%). 500 g of Sugar—sucrose (3° Brix). 185 g of Kombucha starter (SCOBY and liquid). |
| Rotating disk system elements | 11 disks laser-engraved and sanded with different grain patterns. 1 DC geared motor with encoder. 1 PVC tube. 2 MDF holders. 2 plastic bearings with inox spheres. 1 holder for the motor and speed regulation controller. 1 cloth-holding structure. 1 synthetic breathable cloth. | 4 plastic clamps. 1 cloth-holding structure. 1 synthetic breathable cloth. |
| Fermentation Time | Rotating Disk Culture Bioreactor (RDC) | Static Culture Tank Bioreactor (SCT) |
|---|---|---|
| Day 1 | Assembly. | Assembly. |
| Day 5 | Dots/little amounts of cellulose were seen attached to some disks. | Mold was spotted on the liquid medium. |
| Day 9 | Uniform layers of microbial cellulose grew homogeneously on some disks. | A thin molded layer of microbial cellulose grew slowly. |
| Day 14 | Uniform layers of microbial cellulose grew homogeneously on some disks. -pH of the liquid: Bottom left angle—pH 2.91 Bottom right angle—pH 2.85 Top right angle—pH 2.80 Top left angle—pH 2.83 Average pH: 2.85 | A thin molded layer of microbial cellulose grew slowly. -pH of the liquid: Bottom left angle—pH 3.10 Bottom right angle—pH 3.09 Top right angle—pH 3.07 Top left angle—pH 3.11 Average pH: 3.09 |
| Day 16 | Adjustment of the pH from 2.85 to 4.52 with a solution of water and circa 100 g of bicarbonate 1. | A thin molded layer of microbial cellulose continued to grow slowly. |
| Day 21 | Microbial cellulose successfully grew on some disks. The system was disassembled, and the most efficient disk was weighed: the disk had a gross weight of 195 g. | A thin molded layer of microbial cellulose continued to grow slowly. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, N.; Sicher, E.; Ayala-Garcia, C.; Sanchez-Fayos, I.M.; Conterno, L.; Ugur Yavuz, S. Innocell Bioreactor: An Open-Source Development to Produce Biomaterials for Food and Packaging Based on Fermentation Processes. Fermentation 2023, 9, 915. https://doi.org/10.3390/fermentation9100915
Cohen N, Sicher E, Ayala-Garcia C, Sanchez-Fayos IM, Conterno L, Ugur Yavuz S. Innocell Bioreactor: An Open-Source Development to Produce Biomaterials for Food and Packaging Based on Fermentation Processes. Fermentation. 2023; 9(10):915. https://doi.org/10.3390/fermentation9100915
Chicago/Turabian StyleCohen, Nitzan, Emma Sicher, Camilo Ayala-Garcia, Ignacio Merino Sanchez-Fayos, Lorenza Conterno, and Secil Ugur Yavuz. 2023. "Innocell Bioreactor: An Open-Source Development to Produce Biomaterials for Food and Packaging Based on Fermentation Processes" Fermentation 9, no. 10: 915. https://doi.org/10.3390/fermentation9100915
APA StyleCohen, N., Sicher, E., Ayala-Garcia, C., Sanchez-Fayos, I. M., Conterno, L., & Ugur Yavuz, S. (2023). Innocell Bioreactor: An Open-Source Development to Produce Biomaterials for Food and Packaging Based on Fermentation Processes. Fermentation, 9(10), 915. https://doi.org/10.3390/fermentation9100915







