Abstract
Sargassum horneri is a seaweed with antihypertensive properties. However, it is underutilized in some areas, and effective utilization methods are being sought. In this study, we prepared a fermented S. horneri using lactic acid bacteria Lactiplantibacillus pentosus SN001 and investigated its effective utilization by enhancing its antihypertensive effect. The ACE inhibitory activity of S. horneri ranged from 3.6% to a maximum of 63.3% after fermentation. In vivo studies using mice and spontaneously hypertensive rats (SHR) suggested an antihypertensive effect of fermented S. horneri. Purification and NMR analysis of the ACE inhibitory component in fermented S. horneri identified glycerol. Therefore, it is suggested that glycerol is responsible for the strong antihypertensive effect of fermented S. horneri. In conclusion, S. horneri is expected to be used as a dietary ingredient with enhanced antihypertensive effect by fermentation with L. pentosus SN001.
1. Introduction
Seaweeds are divided into green, brown, and red algae based on their coloration [1]. Seaweeds contain bioactive components such as phlorotannins and polysaccharides that differ from land plants and have various functional properties [2]. S. horneri (Turner) C. Agardh is an annual seaweed belonging to the family of brown algae, and is found in shallow water areas of Japan except Hokkaido [3]. S. horneri contains high amounts of fucoidan [4], and fucoidan has been reported to have antihypertensive, anti-inflammatory, and antitumor activities [5,6,7]. However, in Korea, it is either discarded or used as fertilizer or livestock feed, which calls for its effective utilization [8].
Hypertension is defined as systolic blood pressure (SBP) greater than 140 mmHg or diastolic blood pressure greater than 90 mmHg [9]. Hypertension is broadly divided into essential hypertension and secondary hypertension [10]. Essential hypertension is caused by many factors, while secondary hypertension has an identified cause [10]. In the present study, we focused on essential hypertension and used inhibition of the renin-angiotensin system (RAS), which is involved in the hypertensive system, as an index of antihypertensive action, because angiotensin II in the RAS system plays a crucial role in a series of disorders of the cardiovascular system, including hypertension. Inhibition of the RAS system is believed to be effective in lowering blood pressure level [11]. Pharmaceutical ACE inhibitors include captopril, but captopril is known to cause side effects such as cough and taste disorder, and food-derived ACE inhibitors with fewer side effects are sought [12].
Seaweed fermentation is a method developed as a new way to utilize seaweed, focusing on fermentation by microorganisms, considering effective utilization of the entire algae and processing costs [13]. Unlike fermentation techniques for vegetable materials, which have been widely used in the past, marine materials have not been studied, so a technique was developed for lactic acid fermentation of seaweeds using lactic acid bacteria [13]. In seaweed fermentation using lactic acid bacteria, enhancement of the antihypertensive effect of hidaka kelp by Lacticaseibacillus casei 001 fermentation and GABA production by Lactiplantibacillus pentosus SN001 have been reported [14,15]. Phlorotannins, plant polyphenols, have been reported as ACE inhibitory components of brown algae extracts [16], and it has also been reported that Lactiplantibacillus plantarum fermentation increases polyphenol content [17]. In addition, Gln-Val-Glu-Tyr from the red alga Gracilariopsis lemaneiformis [18], and Pro-Ala-Phe-Gly from the green alga Enteromorpha clathrata [19] and D-tryptophan from the brown alga Saccharina angustata [14] have been isolated. Lactic acid bacteria, including L. pentosus SN001, have proteases, which break down proteins and are expected to produce ACE inhibitory components. Therefore, if their antihypertensive effect can be enhanced by fermentation, they can be effectively utilized. In this study, S. horneri was fermented with Lactiplantibacillus pentosus SN001 to investigate the enhancement of ACE inhibitory activity, a parameter of antihypertensive activity. In addition, we evaluated the antihypertensive effect in vivo by conducting in vivo studies using the fermented S. horneri. We attempted to identify the ACE inhibitory components in fermented S. horneri through purification and NMR analysis. Through these experiments, we aimed to present a method for effective utilization of S. horneri.
2. Materials and Methods
2.1. Materials and Reagents
S. horneri were harvested in Oki, Shimane Prefecture, and delivered frozen. Potassium dihydrogen phosphate, disodium hydrogen phosphate, hydrochloric acid, sodium hydroxide, angiotensin I, special grade methanol, dipotassium hydrogen phosphate, diammonium hydrogen citrate, magnesium sulfate heptahydrate, iron(II) sulfate heptahydrate, manganese(II) sulfate tetrahydrate, Tween-80, L-cysteine, sodium chloride, L-cysteine hydrochloride monohydrate, and tert-butylhydroquinone were purchased from Fujifilm Wako Pure Chemicals Co., (Osaka, Japan). β-cornstarch, milk casein, α-cornstarch, sucrose, soybean oil, cellulose, AIN-76 mineral mixture, and AIN-76A vitamin mixture (without choline bitartrate) were purchased from Oriental Yeast Co., (Tokyo, Japan). Cellulase (ONOZUKA-R10) was purchased from Yakult Pharmaceutical Industries, Ltd. (Tokyo, Japan). Special grade 1-butanol and special grade chloroform were purchased from KOKUSAN CHEMICAL Co., Ltd. (Tokyo, Japan). The ACE Kit-WST was purchased from Dojin Chemical Research Institute (Kumamoto, Japan). Acetonitrile was purchased from Sigma-Aldrich (St. Louis, MO, USA). BCP plate counting agar “Nissui” was purchased from Nissui Pharmaceutical Co., (Tokyo, Japan). Tryptone, yeast extract, and proteose peptone were purchased from Becton Dickinson and Co., (Franklin Lakes, NJ, USA).
2.2. Fermentation of S. horneri with L. pentosus SN001
S. horneri was washed with water, freeze-dried, and crushed using a mixer. After crushing, the powder was sieved through a 500 μm mesh and used for the experiment. The S. horneri powder (5 g) was added to 100 mL of 33 mM phosphate buffer and adjusted to pH 5.0 using 1 N hydrochloric acid. Cellulase (ONOZUKAR-10) was added (50 mg) and enzymatically treated (Bioshaker BR-3000L, Taitec, Co., Ltd., Saitama, Japan, 45 °C, 120 rpm, 24 h). pH 6.8 was adjusted with 10 M sodium hydroxide, and the cells were pressurized and heat sterilized (121 °C, 15 min). The pre-cultured L. pentosus SN001 (1 mL) was inoculated into S. horneri medium and incubated at 30 °C for 7 days. The pre-culture was performed in ILS medium (10 g/L tryptone, 5 g/L yeast extract, 3 g/L proteose peptone, 3 g/L K2HPO4, 3 g/L KH2PO4, 2 g/L diammonium hydrogen citrate, 550 mg/L MgSO4-7H2O, 34 mg/L FeSO4-7H2O, 120 mg/L MnSO4-4H2O, 1 g/L Tween-80, 200 mg/L L-cysteine monohydrochloride, 1.7 g/L CH3COONa-3H2O, 20 g/L glucose). Pre-culture was performed to activate the bacteria that had been stored at −80 °C. The number of viable cell counts and pH changes over time during 7 days of incubation were evaluated, with unfermented as day 0. The viable cell counts were calculated using the BCP plate count agar “Nissui”.
2.3. ACE Inhibitory Activity of Fermented S. horneri
Fermented S. horneri was lyophilized and extracted with 80% ethanol (10 mg/mL, 170 rpm, 3 h). Centrifugation (13,000× g, 5 min) was performed, and the supernatant was lyophilized and used as the sample for assay. The final concentration was 0.83 mg/mL and the ACE inhibition activity was measured according to the ACE-kit WST protocol.
2.4. Blood Pressure Test Using ddY Mice
Experimental animals were ddY mice (Sankyo Lab Service, Tokyo, Japan, 5-weeks-old, male), which were acclimated for 1 week. The mice were kept in a breeding room at 25 ± 0.5 °C and 60 ± 3% humidity. The light-dark cycle was 12 h, and water and feed (MR stock, Nosan Corporation, Yokohama, Japan) were given ad libitum. The mice were randomly divided into three groups of five mice per group, control, unfermented and fermented group. The blood pressure test was a modified version of an existing protocol [20]. Unfermented (1500 mg/kg bw) and fermented (1500 mg/kg bw) extracts dissolved in distilled water were administered directly into the stomach using a sonde, and the mice were placed in a measuring gauge. The mice in the control group were administered distilled water. Blood pressure was measured 30 min after administration. Angiotensin I (AI, 0.1 mL) was administered into the abdominal cavity. Two minutes after AI administration, the blood pressure was kept still and measured. Blood pressure was measured using a non-thermal, non-observational blood pressure monitor for mice and rats (BLOOD PRESSURE MONITOR FOR MICE&RAT Model MK-2000, Muromachi Kikai Co., Tokyo, Japan). Steel-Dwass test was used for significant difference test, and p < 0.05 was used for significant difference.
2.5. Blood Pressure Test Using SHR
Experimental animals were SHR (Sankyo Lab Service, Tokyo, Japan, 14-week-old, male), which were acclimated for 1 week. The rats were kept in a breeding room at a temperature of 25 ± 0.5 °C and 60 ± 10% humidity. The light-dark cycle was 12 h. The rats were divided into groups of 6 rats per group with equal blood pressure values, and the two groups control and fermented group, received different diets ad libitum. The composition of each experimental diet is shown in Table 1. In the fermentation group, NaCl level was adjusted to 1%, the same as in the control group. Fermented S. horneri was adjusted to 5%. The diets were administred for 65 days. Blood pressure was measured using a non-thermal, non-observational blood pressure monitor for mice and rats (BLOOD PRESSURE MONITOR FOR MICE&RAT Model MK-2000). All blood pressure measurements were taken in the morning after 8:00 a.m. Blood pressure readings were rejected for outliers by Smirnoff’s rejection test. A t-test was used for significant tests, with p < 0.05 for significant differences.

Table 1.
Diet composition.
2.6. Purification and Structure Determination of ACE Inhibitor Components
Ethanol extraction was performed on fermented S. horneri. Liquid-liquid partitioning of the aqueous layer with ethyl acetate followed by liquid-liquid partitioning of the resulting aqueous layer with water:1-butanol was performed to obtain the ACE inhibitory component in the aqueous layer. The aqueous layer was further fractionated into four fractions (<3 kDa, 3–10 kDa, 10–30 kDa, and >30 kDa) using a Vivaspin 20 column (Sartorius Stedim Lab Ltd., Goettingen, Germany). Nuclear magnetic resonance (NMR) was used to identify ACE inhibitory components.
3. Results
3.1. Fermentation of S. horneri with L. pentosus SN001 and ACE Inhibitory Activity
Figure 1A shows the daily changes in viable cell counts and pH of the fermented S. horneri. The viable cell counts increased from 1.0 × 107 to 2.0 × 108 within 1 day of incubation. The pH decreased from 6.47 to 3.99. Figure 1B shows the daily changes ACE inhibitory activity. The ACE inhibition activity of the fermented S. horneri extracted with 80% ethanol was 57% on the first day of incubation, and the maximum ACE inhibition activity was 63% on the fourth day of incubation. Thereafter, the 4-day incubated S. horneri was used as fermented.
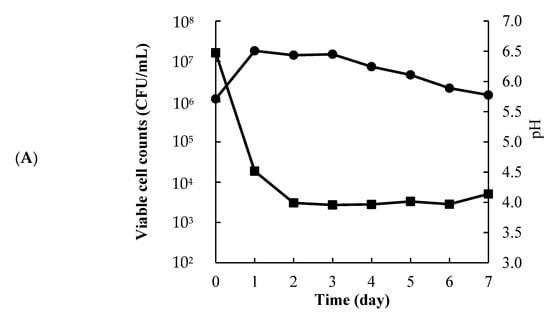
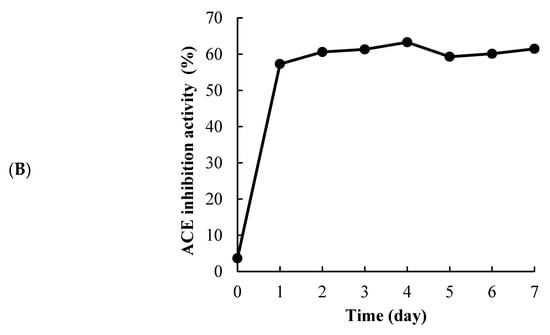
Figure 1.
Viable cell counts (●) and pH (■) of L. pentosus SN001 fermented S. horneri (A), ACE inhibition activity (final concentration: 0.83 mg/mL) (B) The data represented the mean values ± standard error (n = 4).
3.2. Blood Pressure Test Using ddY Mice
Systolic blood pressure levels before and after AI treatment on the ddY mice are shown in Figure 2. The SBP levels after AI injection were 46 mmHg, 25 mmHg, and 38 mmHg higher in the control, fermented, and unfermented groups, respectively, than before. The systolic blood pressure levels in the fermented group were significantly lower than those of the control group.
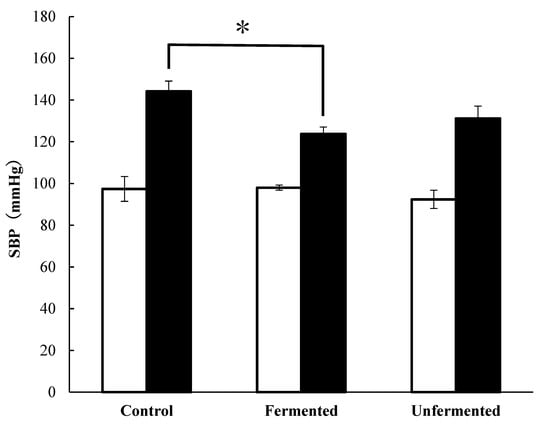
Figure 2.
Systolic blood pressure results before and after AI administration in ddY mice loaded with AI, p < 0.05 Fermented vs. Control (*); The data represented the mean values ± standard error (n = 5).
3.3. Blood Pressure Test Using SHR
The daily changes in blood pressure levels on the SHR are shown in Figure 3. No significant differences in body weight or food intake were observed among the study segments. The SBP values of the fermentation group were always lower than those of the control group after 7 days. Significant differences were also observed at 15, 18, 22, 29, 33, 40, and 65 days.
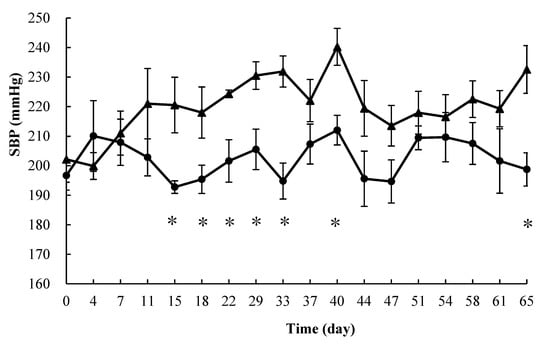
Figure 3.
Systolic blood pressure of spontaneously hypertensive rats (SBP) treated with different products: Control (▲) and Fermented (●), p < 0.05 (*), The data represented the mean values ± standard error (n = 6).
3.4. Purification and Identification of ACE Inhibitory Components
Vivaspin fractionation resulted in high ACE inhibitory activity and weight in the <3 kDa fraction. Next, high ACE inhibitory activity was found in the 3–10 kDa and >30 kDa fractions, with little activity in the 10–30 kDa fractions. Thus, the major ACE inhibitor was found to be in the <3 kDa molecular size fraction. Therefore, NMR analysis of the <3 kDa fraction was performed.
3.5. Purification and Structure Determination of ACE Inhibitor Components
Nuclear magnetic resonance (NMR) was used for identification and glycerol was identified; NMR results are shown in Figure 4 and Table 2. The structure of glycerol is also shown in Figure 5. In addition, ACE inhibitory activity was measured using the identified glycerol standard and is shown in Figure 6. The approximation curve revealed that glycerol caused 50% ACE inhibition at a volume concentration of 1.78%, indicating that glycerol has ACE inhibitory activity.
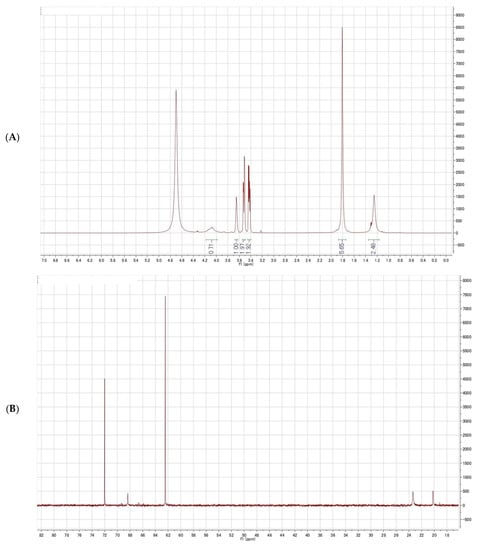
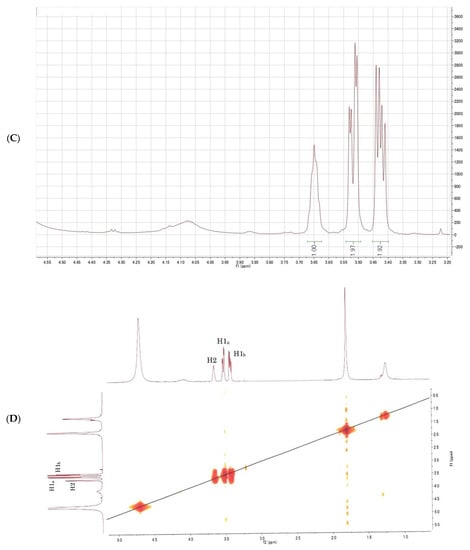
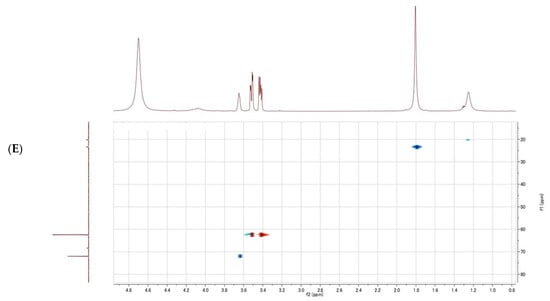
Figure 4.
Nuclear magnetic resonance (NMR) spectrum of glycerol; (A) 1H-NMR spectrum of the purified fraction; (B) 1H-NMR spectrum of the purified fraction (enlarged); (C) 1H-1H COSY spectrum of the purified fraction; (D) 13C-NMR spectrum of the purified fraction; (E) 1H-13C HSQC spectrum of the purified fraction.

Table 2.
NMR data for the compound.
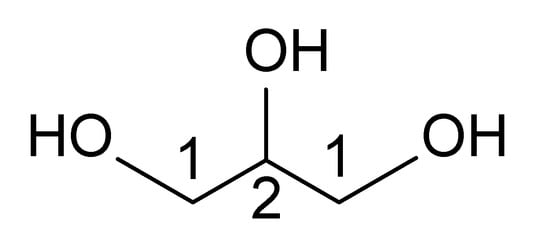
Figure 5.
The structure of glycerol.
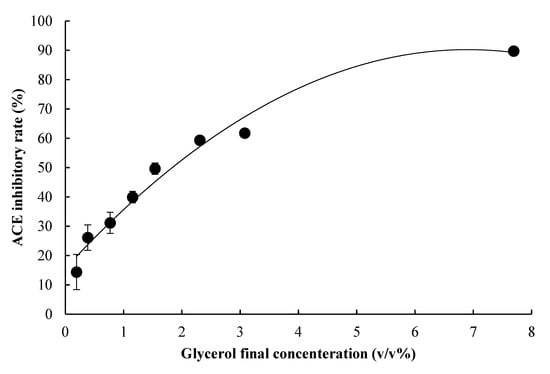
Figure 6.
ACE inhibitory rate at each concentration of glycerol; The data represented the mean values ± standard error (n = 3).
4. Discussion
In this study, S. horneri was fermented with L. pentosus SN001 to investigate the enhancement of ACE inhibitory activity, a parameter of antihypertensive activity. Fermentation of S. horneri by L. pentosus SN001 was found to enhance ACE inhibitory activity. The SBP levels after AI injection were 46 mmHg, 25 mmHg, and 38 mmHg higher in the control, fermented, and unfermented groups, respectively, than before. In the SHR test, the blood pressure levels in the fermented group showed lower compared to those of the control group. In abalone viscera, L. casei 001 fermentation has been reported to reduce blood pressure levels in AI-injected ddY mice and SHR, and homalin has been identified as a component involved in blood pressure reduction [20]. Therefore, in this study, we isolated and purified similarly fermented S. horneri and performed NMR measurements to identify components with ACE inhibitory activity. The fermented S. horneri was isolated, purified, and subjected to NMR to identify components with ACE inhibitory activity. As the result, glycerol was identified. The absence of glycerol in the S. horneri and pre-cultures suggested that glycerol was first produced when S. horneri was fermented with L. pentosus SN001. Glycerol is 1,2,3-propantriol, a simple alcohol with many uses as a raw material in the manufacture of foods and chemicals [21]. Previous studies related to blood pressure have reported the antihypertensive effects of a combination of uracil and glycerol from Lactobacillus plantarum strain TWK10 [22]. The mechanism has been identified as vasodilatation due to renin inhibitors, ACE inhibitors, and enhanced NO production [22]. In the previous study, uracil was identified in the fermentation product using the same L. pentosus SN001 [23]. Furthermore, it was reported that the content of glycerol and uracil increased in fermented vegetable juices from L. plantarum HY7712 and L. helveticus HY7801 fermentations, respectively [24]. Therefore, it may be suggested that fermented S. horneri exhibit ACE inhibitory activity due to the action of both glycerol identified in this study and uracil produced by the fermentation with L. pentosus SN001.
5. Conclusions
In this study, the fermentation of underutilized resource S. horneri with L. pentosus SN001 was found to enhance ACE inhibitory activity and antihypertensive effects in vivo. Furthermore, glycerol was identified as a result of the identification of ACE inhibitory components in the fermented product. Therefore, S. horneri fermented with L. pentosus SN001 is expected to be utilized as a functional material with antihypertensive activity. This achievement is expected to lead to effective utilization of S. horneri, and the identification of glycerol is expected to provide important knowledge in the field of medicine and pharmaceuticals.
Author Contributions
Conceptualization, N.H.-S.; methodology, N.H.-S.; formal analysis, M.T. and S.S.; investigation, N.H.-S.; resources, N.H.-S.; data curation, M.T.; writing—original draft preparation, M.T.; writing—review and editing, H.N., O.Y. and N.H.-S.; supervision, N.H.-S.; project administration, N.H.-S.; funding acquisition, N.H.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All experiments using rats in this study were conducted in accordance with the Tokyo University of Marine Science and Technology Animal Experiment Guidelines (Approval No. R2-4). This study was conducted in accordance with the ARRIVE guidelines, the UK Animals (Chemical Procedures) Act 1986 and related guidelines, Directive 2010/63/EU on animal experimentation, or the UK National Institute for Health Research Guide for the Care and Use of Laboratory Animals (NIH Publication No. 8023, revised 1978).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Belghit, I.; Rasinger, J.D.; Heesch, S.; Biancarosa, I.; Liland, N.S.; Torstensen, B.; Waagbo, R.; Lock, E.-J.; Bruckner, C.G. In-depth metabolic profiling of marine macroalgae confirms strong biochemical differences between brown, red and green algae. Algal Res. 2017, 26, 240–249. [Google Scholar] [CrossRef]
- Brown, E.; Allsopp, P.J.; Magee, P.; Gill, C.I.R.; Nitecki, S.; Strain, C.R.; McSorley, E.M. Seaweed and human health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Preeprame, S.; Hayashi, K.; Lee, J.-B.; Sankawa, U.; Hayashi, T. A Novel Antivirally Active Fucan Sulfate Derived from an Edible Brown Alga, Sargassum horneri. Chem. Pharm. Bull. 2001, 49, 484–485. [Google Scholar] [CrossRef] [PubMed]
- Skriptsova, A.V. Seasonal variations in the fucoidan content of brown algae from Peter the Great Bay, Sea of Japan. Russ. J. Mar. Biol. 2016, 42, 351–356. [Google Scholar] [CrossRef]
- Corona, D.M.H.; Martínez-Abundis, E.; González-Ortiz, M. Effect of Fucoidan Administration on Insulin Secretion and Insulin Resistance in Overweight or Obese Adults. J. Med. Food 2014, 17, 830–832. [Google Scholar] [CrossRef]
- Wen, Z.-S.; Xiang, X.-W.; Jin, H.-X.; Guo, X.-Y.; Liu, L.-J.; Huang, Y.-N.; Ouyang, X.; Qu, Y.-L. Composition and anti-inflammatory effect of polysaccharides from Sargassum horneri in RAW264.7 macrophages. Int. J. Biol. Macromol. 2016, 88, 403–413. [Google Scholar] [CrossRef]
- Shao, P.; Chen, X.; Sun, P. Chemical characterization, antioxidant and antitumor activity of sulfated polysaccharide from Sargassum horneri. Carbohydr. Polym. 2014, 105, 260–269. [Google Scholar] [CrossRef]
- Lee, G.; Midorikawa, Y.; Kuda, T.; Harada, M.; Fujita, S.; Takahashi, H.; Kimura, B. In vitro antioxidant and anti-glycation properties of Sargassum horneri from golden tides on the South Korean coast and the effect on gut microbiota of mice fed a high-sucrose and low-fibre diet. J. Appl. Phycol. 2022, 34, 1–12. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Lee, B.H.; Oh, D.H. Current Perspectives on Antihypertensive Probiotics. Probiot. Antimicrob. Proteins 2016, 9, 91–101. [Google Scholar] [CrossRef]
- Pickering, E.Y. Essential Hypertension. BMJ 1962, 1, 945. [Google Scholar] [CrossRef][Green Version]
- Unger, T. The role of the renin-angiotensin system in the development of cardiovascular disease. Am. J. Cardiol. 2002, 89, 3–9. [Google Scholar] [CrossRef]
- Wijesekara, I.; Kim, S.-K. Angiotensin-I-Converting Enzyme (ACE) Inhibitors from Marine Resources: Prospects in the Pharmaceutical Industry. Mar. Drugs 2010, 8, 1080–1093. [Google Scholar] [CrossRef]
- Uchida, M.; Miyoshi, T. Algal fermentation—The seed for a new fermentation industry of foods and related products. Jpn. Agric. Res. Q. JARQ 2013, 1, 53–63. [Google Scholar] [CrossRef]
- Sekine, T.; Nagai, H.; Hamada-Sato, N. Antihypertensive and Probiotic Effects of Hidakakombu (Saccharina angustata) Fermented by Lacticaseibacillus casei 001. Foods 2021, 10, 2048. [Google Scholar] [CrossRef]
- Sekine, T.; Yamanushi, M.; Hamada-Sato, N. GABA production and probiotic addition in Saccharina angustata (Hidakakombu) by fermentation of Lactiplantibacillus pentosus SN001. Fish. Sci. 2022, 88, 429–435. [Google Scholar] [CrossRef]
- Olivares-Molina, A.; Fernández, K. Comparison of different extraction techniques for obtaining extracts from brown seaweeds and their potential effects as angiotensin I-converting enzyme (ACE) inhibitors. J. Appl. Phycol. 2015, 28, 1295–1302. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Shah, N.P.; Wang, M.-F.; Lui, W.-Y.; Corke, H. Lactobacillus plantarum WCFS1 Fermentation Differentially Affects Antioxidant Capacity and Polyphenol Content in Mung bean (Vigna radiata) and Soya Bean (Glycine max) Milks. J. Food Process. Preserv. 2016, 41, e12944. [Google Scholar] [CrossRef]
- Cao, D.; Lv, X.; Xu, X.; Yu, H.; Sun, X.; Xu, N. Purification and identification of a novel ACE inhibitory peptide from marine alga Gracilariopsis lemaneiformis protein hydrolysate. Eur. Food Res. Technol. 2017, 243, 1829–1837. [Google Scholar] [CrossRef]
- Pan, S.; Wang, S.; Jing, L.; Yao, D. Purification and characterisation of a novel angiotensin-I converting enzyme (ACE)-inhibitory peptide derived from the enzymatic hydrolysate of Enteromorpha clathrata protein. Food Chem. 2016, 211, 423–430. [Google Scholar] [CrossRef]
- Fujimura, Y.; Shimura, M.; Nagai, H.; Hamada-Sato, N. Evaluation of angiotensin-converting enzyme-inhibitory activity in abalone viscera fermented by Lactobacillus casei 001. J. Funct. Foods 2021, 82, 104474. [Google Scholar] [CrossRef]
- Wang, Z.; Zhuge, J.; Fang, H.; Prior, B.A. Glycerol production by microbial fermentation: A review. Biotechnol. Adv. 2001, 19, 201–223. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Zeng, S.-Y.; Leu, Y.-L.; Tsai, T.-Y. Antihypertensive Effect of a Combination of Uracil and Glycerol Derived from Lactobacillus plantarum Strain TWK10-Fermented Soy Milk. J. Agric. Food Chem. 2015, 63, 7333–7342. [Google Scholar] [CrossRef]
- Yamanushi, M.; Shimura, M.; Nagai, H.; Hamada-Sato, N. Antihypertensive effects of abalone viscera fermented with Lactiplantibacillus pentosus SN001 via angiotensin-converting enzyme inhibition. Food Chem. X 2022, 13, 100239. [Google Scholar] [CrossRef]
- Chung, H.-J.; Lee, H.; Na, G.; Jung, H.; Kim, D.-G.; Shin, S.-I.; Jung, S.-E.; Choi, I.-D.; Lee, J.-H.; Sim, J.-H.; et al. Metabolic and Lipidomic Profiling of Vegetable Juices Fermented with Various Probiotics. Biomolecules 2020, 10, 725. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).