Abstract
Consumers’ appreciation of wines is mainly driven by their aroma, which is the most important organoleptic characteristic and key attribute. The volatile bouquet derives from the grape berries and from the processing phases of vinification. In the present study, the volatile emission of six grapevine cultivars has been analysed through four phases of vinification: the headspaces of crushed grapes, fermented must, new wine (2 months old), and wine (7 months old) have been sampled and analysed. This showed the evolution of the volatile compounds based on the chemical and mechanical processes involved in the specific vinification phase. Chemometric tools (hierarchical cluster and principal component analyses) have revealed that samples gather in statistical groups based on the vinification phase they belong to, though they maintain an aroma composition that is typical of the grape berry of origin.
Keywords:
Canaiolo nero; Ciliegiolo; Colorino; Merlot; Montepulciano; Sangiovese; headspace; volatiles 1. Introduction
Red wines are obtained from black grape varieties, whose skin is kept in a must fermenting medium to confer the typical colour to the final product. Red wines undergo higher fermentation temperatures and longer aging, which ultimately produce a product richer in terms of fruity aroma, in comparison with white wines; these typical aromatic notes are due to the bouquet of the volatile esters [1]. Characterization and consumers’ appreciation of wines is, indeed, due mostly to their aroma, which is conferred by the released volatile organic compounds (VOCs). The composition and content of volatiles depend on the vineyard, the fermentation, and the aging processes the wine undergoes [2]. The vineyard influences the primary (or “varietal”) aroma, in which the terroir, together with the used grape variety, plays a major role; grapevines from the same geographical area, indeed, share common traits in their aroma profile. This primary aroma is mainly due to isoprenoids, particularly oxygenated monoterpenes, which confer fruity and floral notes to wine [3,4]. Monoterpenes, as well as other compounds of the aroma of wines, can be distinguished in bound (non-volatile precursor) and free (volatile) forms [2], whose proportion depends on different factors, including the berry ripening [5]. Aroma precursors are of great importance for the quality of wine because, during the fermentation and aging phases, they are converted in odour-contributing free VOCs, thanks to the physical crushing of grapes and the hydrolysis reactions mediated by the glucosidase enzymes released by the yeasts [2,5,6]. The secondary (or “fermentative”) aroma, instead, is due to compounds developed during the fermentation process, such as higher alcohols, esters, and fatty acids [3]. These molecules are produced from yeast substrates, such as sugars, proteins, and lipids, and, as a consequence, they are not dependent on the grape variety and are not responsible for the specific aromas of the final product [4]. Finally, the tertiary aroma is determined by the wine storage and aging, which leads to the loss of VOCs linked to the grape varieties and fermentation [5]. It is characterized by greater amounts of long-chain alcohols and volatile fatty acids, characteristic of older wines, and by a reduction in the volatiles related to fresh and fruity notes, as the ethyl esters of fatty acids, which during the fermentation stage are produced in amounts exceeding their concentration equilibrium [4]. Thus, the complete wine volatile bouquet undergoes several transformations during the whole processing chain [4,5], and in the final product it is composed of various classes of volatile compounds, mainly represented by alcohols, together with their fermentation metabolites, including higher alcohols, branched and non-branched fatty acids, and their ethyl esters [2,7,8]. Different families of aroma compounds play different roles in wine aroma perception and quality [7], even though not all volatiles affect wine aroma to the same extent. Each compound has an olfactory perception threshold, which determines whether its presence in very low amounts can significantly influence the perceived aroma of the wine [2]. Furthermore, an aromatic note is formed not only by the few compounds that dominate it, but also by the simultaneous presence of other odorants that negatively affect its perception [9]. These two phenomena explain the positive attributes of higher alcohols to wine aroma, even though, no matter their purity degree, they contain powerful smelling aldehydes at significant levels [7].
In this study, the variation of the aroma profile throughout the different phases of vinification has been analysed for six black grape cultivars: ‘Canaiolo nero’, ‘Ciliegiolo’, ‘Colorino’, ‘Merlot’, ‘Montepulciano’, and ‘Sangiovese’.
2. Materials and Methods
2.1. Samples Preparation
The cultivation and vinification of the six cultivars of red grapevine (Vitis vinifera L.) were performed by Azienda Agricola “Il Grappolo” di Narri Patrizio located in Soiana, Terricciola (PI), Tuscany, Italy. Grapes were collected in September 2021. The company vines are located on the hills of Alta Valdera, at 170 m a.s.l. (45.534283 N, 10.661349 E); they occupy half a hectare and are PGI (Protected Geographical Indication) branded. Vines are arranged with the “archetto toscano” method, a variation of the Guyot theme, in which a bend in the cane limits the vigour of the end shoot; each plant is 90 cm away from the other, with a distance 210 cm between the rows. The six studied cultivars of red grapevine are those cultivated on the farm, namely ‘Sangiovese’, ‘Canaiolo nero’, ‘Ciliegiolo’, ‘Colorino’, ‘Montepulciano’ and ‘Merlot’; the first five are local to Tuscany (Italy), whilst the latter is native to Bordeaux (France). The gathered ripe grapes were destemmed and mechanically pressed: 8 mL of the obtained must, together with marcs, was put in a glass beaker of 20 mL volume and covered with aluminium foil before exposing the polydimethylsiloxane (PDMS) fibre for 45 min in the headspace. The must was left in an open 5 L glass barrel, fermenting for 5 days at 21–25 °C. Then, 8 mL of the obtained wort was put in a glass beaker of 20 mL volume and covered with aluminium foil before exposing the PDMS fibre for 10 min in the headspace. The winemaking was performed by the company in 5 L glass barrels. After the removal of marcs and filtration, 100 mL samples of each cultivar were taken to perform the analysis at different (2-month-old wine and 7-month-old wine) stages of vinification. A volume of 8 mL of wine was put in 20 mL volume glass beakers and covered with aluminium foil, before exposing the PDMS fibre for 12 min in the headspace. For each sample, triplicates were performed.
2.2. Samples Analysis
Supelco (Merck KGaA, Darmstadt, Germany) SPME (Solid Phase Micro-Extraction) devices coated with polydimethylsiloxane (PDMS, 100 μm) were used to sample the headspace. SPME sampling was performed using the same new fibre, preconditioned according to the manufacturer instructions, for all the analyses. Sampling was accomplished in an air-conditioned room (22 ± 1 °C) to guarantee a stable temperature. After the equilibration time, the fibre was exposed to the headspace for a suitable amount of time based on the analysed sample. Once sampling was finished, the fibre was withdrawn into the needle and transferred to the injection port of the GC-MS system. The desorption conditions were identical for all the samples. Furthermore, blanks were performed before each first SPME extraction and randomly repeated during each series. Quantitative comparisons of relative peaks areas were performed between the same chemicals in the different samples.
2.3. GC–MS Analysis
The GC–EI-MS analyses were performed with a Varian CP-3800 (Agilent Technologies Inc., Santa Clara, CA, USA) apparatus equipped with a DB-5 capillary column (30 m × 0.25 mm i.d., film thickness 0.25 μm) and a Varian Saturn 2000 ion-trap mass detector (Agilent Technologies Inc., Santa Clara, CA, USA). The oven temperature was programmed rising from 60° C to 240° C at 3° C/min, with an injector temperature of 220 °C, a transfer-line temperature of 240° C, and the carrier gas, He (1 mL/min).
2.4. Volatiles Analysis
The identification of the constituents was based on the comparison of their retention times (tR) with those of pure reference samples and their linear retention indices (LRIs) determined relative to the tR of a series of n-alkanes. The mass spectra were compared with those listed in the commercial libraries NIST 14 and ADAMS and in a home-made mass-spectral library, built up from pure substances and components of essential oils of known composition, and the MS literature data [10,11,12,13,14,15,16].
2.5. Multivariate Statistical Analysis
The statistical analyses were carried out with the JMP software package (SAS Institute, Cary, NC, USA). For the statistical evaluation of the volatile composition, the covariance data matrix was a 161 × 24 matrix (161 individual compounds × 24 samples = 3864 data). The principal component analysis (PCA) was performed selecting the two highest principal components (PCs) obtained by the linear regressions operated on mean-centred, unscaled data; as an unsupervised method, this analysis aimed at reducing the dimensionality of the multivariate data of the matrix, whilst preserving most of the variance (Choi et al., 2004). The chosen PC1 and PC2 cover 77.81 and 9.72% of the variance, respectively, for a total explained variance of 87.53%. The hierarchical cluster analysis (HCA) was performed by the Ward’s method. Both the HCA and the PCA methods can be applied to observe groups of samples even when there are no reference samples that can be used as a training set to establish the model.
3. Results
3.1. Headspace Solid-Phase Micro-Extraction Analyses of the Vinification Phases of All the Cultivars
Table 1 reports the chemical compounds detected in amounts higher than 1% in at least one of the analysed samples’ headspaces. The complete compositions are instead reported in Table S1. The bold numbers in the text refer to the entries in S1.

Table 1.
Headspace compositions of all the analysed vinification phases of the six cultivars, reporting only compounds detected in amounts higher than 1% in at least one sample.
3.1.1. The Crushed and Destemmed Grapes (CG) Headspaces
The crushed and destemmed grapes (CG) of all the cultivars exhibited headspaces rich in non-terpene compounds, mainly alcohols/phenols and aldehydes. The former chemical class was among the three main chemical classes in all the varieties (except ‘Sangiovese’): 1-hexanol (11) was detected in all the CG samples, ranging between 4.7 and 37.7%; ethanol (2), not detected only in ‘Merlot’, showed relative abundances between 0.1 and 4.4%. Among the non-terpene aldehydes, the common ones were hexanal (7), not detected only in ‘Ciliegiolo’, ranging between 1.4 and 15.0%, and (E)-2-hexenal (9; 1.1–13.6%), whose relative amount was lower than 0.1% only in ‘Montepulciano’ grapes. Oxygenated monoterpenes were detected in all the CG samples: the most common were 1,8-cineole (32; 2.3–10.5%), camphor (47; 0.4–2.8%), and isobornyl acetate (76; 0.3–7.7%). ‘Canaiolo’ was the only CG sample in which the oxygenated monoterpenes were present in higher relative abundance than non-terpene compounds. The ‘Ciliegiolo’ cultivar showed a significant non-terpene ester presence, with (E)-2-hexen-1-ol acetate (28) being the most abundant (5.3%) one. ‘Colorino’ headspace showed the highest sesquiterpene hydrocarbons and non-terpene ether abundances, as these chemical classes accounted for 21.6 and 13.8%, respectively. The apocarotenoid methyl-β-ionone (147), accounting for up to 10.0%, constituted the second most abundant chemical class of compounds in the ‘Sangiovese’ CG headspace.
3.1.2. The 5-Day-Old Fermented Must (FM) Headspaces
The 5-day-old fermented must (with marcs) samples (FM) from all the cultivars exhibited qualitatively similar emission profiles, with minor quantitative differences. ‘Canaiolo’, ‘Montepulciano’ and ‘Sangiovese’ FM headspaces exhibited a relative content of non-terpene esters slightly higher than that of non-terpene alcohols/phenols; the opposite was found for ‘Ciliegiolo’, ‘Colorino’ and ‘Merlot’. Non-terpene ethers represented the third most abundant class of compounds in all the cultivars. The ethyl esters of three fatty acids were common to all the FM samples: ethyl octanoate (61; 12.5–17.1%), ethyl hexanoate (23; 6.5–9.2%) and ethyl decanoate (95; 0.4–14.0%). The alcohol compounds common to all the cultivars’ FM headspaces were ethanol (2; 16.6–24.3%), isoamyl alcohol (4; 18.1–24.9%) and phenylethyl alcohol (44; 2.0–3.3%). The only detected non-terpene ether, common to all the FM emission profiles, was butyl methyl ether (3; 8.1–11.4%).
3.1.3. The Filtered 2-Month-Old New Wine (NW) Headspaces
The 2-month-old new wine (NW) headspaces showed the same behaviour of the FM samples: quantitatively, rather than qualitatively, differences were detected. Moreover, the most abundant chemical classes (and single compounds) were the same as in the FM samples; ‘Ciliegiolo’, ‘Colorino’ and ‘Merlot’ showed the same predominance of non-terpene alcohols/phenols over the non-terpene esters, but, in this case, the same was true for the ‘Sangiovese’ variety, as well. The opposite was found for ‘Canaiolo’ and ‘Montepulciano’. The ethyl esters 61 (17.5–25.4%), 95 (6.8–11.5%) and 23 (4.2–6.2%) were the most abundant non-terpene esters in the NW samples, as well. Moreover, the non-terpene alcohols/phenols 2 (18.2–26.6%), 4 (18.6–25.4%) and 44 (2.1–3.9%) were the most represented. Compound 3 was the only detected non-terpene ether, ranging from 10.1 to 12.1%. Differently from the FM samples, monoterpene hydrocarbons were present in relevant relative abundance in the ‘Ciliegiolo’ (4.3%) and ‘Merlot’ (1.4%) cultivars, with limonene and p-cymene being the most represented.
3.1.4. The Filtered 7-Month-Old Wine (W) Headspaces
The last evaluated samples came from the final vinification phase: the 7-month-old fermented wine (W). For all the cultivars, non-terpene alcohols/phenols dominated the W sample headspaces; their relative abundances ranged from 45.1% in ‘Canaiolo’ to 55.9% in ‘Merlot’. As in the FM and NW samples, the most abundant were compounds 2 (18.9–29.3%), 4 (20.7–26.1%) and 44 (2.4–4.5%). The non-terpene esters followed as the second most relevant class, with relative abundances between 27.3% in ‘Ciliegiolo’ and 39.4% in ‘Canaiolo’. Compounds 61 (13.3–20.9%), 95 (4.9–8.0%) and 23 (4.1–6.8%) were the most abundant, as in the FM and NW samples. Compound 3 was, again, the only detected non-terpene ether, representing the third most represented chemical class of compounds (12.0–17.4%).
3.2. Multivariate Statistical Analysis
The dendrogram of the HCA (Figure 1) shows a sharp tendency of the samples to gather in clusters based on the vinification phase they belong to. In the dendrogram, two macro-clusters, A and B, can be identified. The first one (A) comprises all the samples obtained after the crushing of grapes, once the fermentation has started (FM, NW and W), whilst the second one (B) is composed of the CG samples. In the macro-cluster A, three sub-clusters are evidenced in the dendrogram: A1 groups comprises the W samples, A2 comprises the NW samples and A3 is composed of the FM samples. It is noteworthy that the FM samples, which represent the first fermentation phase at the beginning of the vinification process, are clustered right next to the CG samples of macro-cluster B. The NW samples are grouped between the FM and the W samples. This clustering behaviour sharply reproduces the order in which the vinification process occurred.
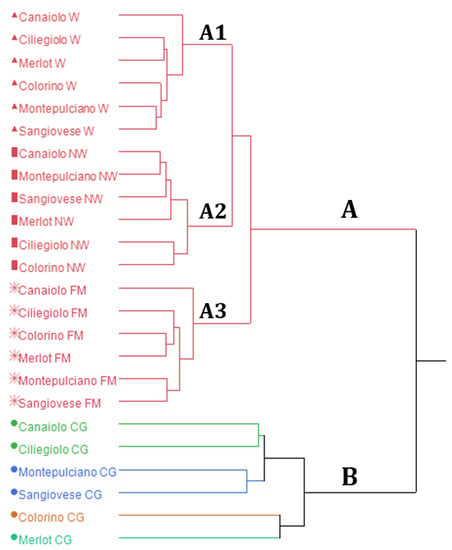
Figure 1.
Hierarchical cluster analysis (HCA) dendrogram.
These results were confirmed by the principal component analysis. As shown in Figure 2a, the CG samples for all the cultivars are plotted in the left quadrants, thus showing negative loadings on the PC1 axis. ‘Ciliegiolo’ and ‘Colorino’ CG samples are plotted in the upper left quadrant of the PCA, with positive PC2 loading. 1-hexanol (11) is the reason for this positioning, as it is quite abundant in both the samples, as can be observed in the loadings plot (Figure S1, Supplementary Material). The CG samples of the other cultivars are all plotted in the bottom left quadrant, with negative PC2 loading. ‘Montepulciano’ cultivar is the upper one, due to the quantitatively relevant presence of (E)-3-hexenol (10) and 1,8-cineole (32) in its headspace (Figure S1, Supplementary Material). ‘Canaiolo’, ‘Merlot’ and ‘Sangiovese’ cultivars, instead, all show significant relative abundances of (E)-2-hexenal (9) and hexanal (7), two non-terpene aldehydes which are the reason for this plotting (Figure S1, Supplementary Material). All the other samples for all the cultivars are plotted in the right quadrants, thus with positive loadings on the PC1 axis. In the bottom area of the upper quadrant, with positive loading on the PC2 axis, the samples with a significant relative abundance of ethanol (2) and isoamyl alcohol (4) in their headspace are plotted (Figure S1, Supplementary Material). In the upper section of the bottom right quadrant, thus with negative loading on the PC2 axis, ethyl esters, such as compounds 23 and 61, together with phenyl ethyl alcohol (44), are the reason for the plotting of the other samples in this area (Figure S1, Supplementary Material).
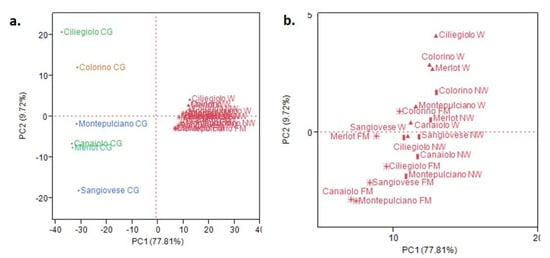
Figure 2.
(a). Principal component analysis (PCA) plot; (b). Magnification of the right quadrants of the plot.
4. Discussion
The CG samples of all the grape cultivars exhibited a larger qualitative variability of the compounds detected in their headspaces, whilst in the following vinification phases, the observed differences were mostly quantitative. This confirms the importance of the raw starting material, in which the variety of the grape defines its volatile emission. The relative abundance of the oxygenated monoterpenes was particularly relevant in the CG samples; except for the ‘Colorino’ variety, this chemical class was among the most important ones in the CG headspaces. In the ‘Colorino’ CG sample, though, the sesquiterpene hydrocarbons were detected in relevant relative concentrations. Once the fermentation began, terpenes appeared only in small quantities in the wine headspace; nevertheless, they are important for the aroma profile because of their low olfactory perception thresholds [3]. They confer wine a positive aroma contribution, as floral and citrus notes [17]. For ‘Ciliegiolo’, ‘Colorino’ and ‘Montepulciano’ varieties, though, non-terpene alcohols/phenols were detected in significant relative abundances in the CG samples, already. In these samples, the most abundant compound of this class is 1-hexanol (11), whose aroma contribution has been described as herbaceous, with a grass-like odour [2,8,18]. As a general pattern evidenced in all the analysed cultivars, terpene compounds decrease with wine aging. Non-terpene aldehydes decreased during the vinification process, as well; the loss of aldehydes in terms of relative abundance with the aging of the material is due to their conversion to the corresponding alcohols during the fermentation phase [8].
Moving forward in the following vinification phases, fusel (>2C) alcohols dominated the emission profiles of all the cultivars. They are secondary products of the yeast’s metabolism; they can be generated by the anabolic pathway from glucose or derive from the catabolic pathway of amino acids, which are different in each grape variety [2,8]. In the red wine fermenting must, ethanol also serves as a co-solvent for fruit pigments of the grape skin, which are important for the development of wine colour.
Non-terpene esters and ethers showed an increment along the vinification process in all the cultivars, as well. For the latter, the only detected compound was butyl methyl ether (3). For the former, they are mainly represented by ethyl esters of fatty acids. They are enzymatically synthesized during the yeast’s fermentation or they are derived from the ethanolysis of Acyl-coA, depending on factors such as the used yeast strain, the fermentation temperature and sugar content [2,8]. They have fruity and floral notes, which are sensory criteria positively correlated with consumer preferences [19]. The longer chain esters relative abundance decreases with wine aging, even after only 2 or 3 years, as they undergo hydrolysis during wine maturation. Nevertheless, their characteristic aroma notes linger even at sub-threshold levels through synergistic effects [1,19]. Among the ones detected in the samples of this study, ethyl octanoate (61), ethyl decanoate (95) and ethyl hexanoate (23) are the most abundant. For compound 61, the aroma attributes are described as pineapple- or pear-like, with fresh floral reminiscences [2,17,18,20]. Ethyl decanoate (95) has a fruity and pleasant grape-like aroma [2,17,18]. The aroma attributes of ethyl hexanoate (23) are defined as fruity (green apple) and anise-, brandy- or wine-like [2,17,18,20]. This compound in particular is involved in the red-berry aroma of wine, thus it represents a positive contribution to the wine aroma bouquet [21]. Ethyl esters abundance in red wines is less significant than in white ones, which are, indeed, more fruity and fresh in terms of aroma [19]. From the FM sample on, another quantitatively relevant non-terpene ester is isopentyl acetate (12), deriving from amino acid or carbohydrate degradation [8]; its aroma contribution has been described as fruity (banana-like) and sweet [2,18].
For the ‘Montepulciano’ variety, a published study by Sagratini et al. [22] analysed the headspace of monovarietal ‘Montepulciano’ wines from Abruzzo and Marche (from four different areas of both regions). For the Marche samples, the most abundant chemical class of compounds was the ester one: ethyl octanoate accounted for 38.33% on average, followed by ethyl decanoate (25.06% on average). The most abundant alcohol was 3-methyl-1-butanol (11.39%), but it was not detected in our sample. The same behaviour was reported for the Abruzzo samples, with a slightly higher relative abundance of 3-methyl-1-butanol [22].
5. Conclusions
The volatile bouquet of wine depends on the raw starting material and on the vinification process. Each variety shows a different genetic pattern, which means a different pool of amino acids and sugars available for the fermentation and subsequent winemaking phases, from which volatiles are developed. The biggest qualitative differences in the samples of this study were, indeed, detected among the volatile profiles of the crushed and destemmed grapes, rather than in the following vinification phases.
On the other hand, different processing methods confer different aroma profiles on wines, even when the starting material is the same. As the analysed samples have been subjected to the same vinification stages and they come from the same area, it is reasonable to affirm that the differences evidenced in their volatile emissions are only due to their varietal origin. The overall exhibited behaviour is a decrement of terpenes and non-terpene aldehydes along the vinification process, coupled with an increment in non-terpene alcohols/phenols, esters and ethers. The varieties already exhibiting a higher relative content of non-terpene alcohols/phenols in the grapes showed a stable increment in their content during the vinification. Non-terpene esters and ethers, instead, were developed during the process.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation8120753/s1, Figure S1: Principal component analysis (PCA) loadings plot; Table S1: Complete headspace compositions of all the analysed vinification phases of the six cultivars.
Author Contributions
Conceptualization, L.P. and G.F.; methodology, B.M., P.N. and R.A.; software, B.M., R.A. and P.L.C.; validation, L.P. and G.F.; formal analysis, B.M., P.L.C., R.A. and Y.P.; investigation, R.A. and B.M.; resources, L.P.; data curation, R.A. and Y.P.; writing—original draft preparation, R.A. and G.F.; writing—review and editing, Y.P., L.P. and B.M.; visualization, R.A., B.M. and Y.P.; supervision, L.P. and G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lytra, G.; Tempere, S.; Le Floch, A.; de Revel, G.; Barbe, J.-C. Study of Sensory Interactions among Red Wine Fruity Esters in a Model Solution. J. Agric. Food Chem. 2013, 61, 8504–8513. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Xi, Z.; Luo, M.; Zhang, Z. Comparison on aroma compounds in Cabernet Sauvignon and Merlot wines from four wine grape-growing regions in China. Food Res. Int. 2013, 51, 482–489. [Google Scholar] [CrossRef]
- Vilanova, M.; Martínez, C. First study of determination of aromatic compounds of red wine from Vitis vinifera cv. Castañal grown in Galicia (NW Spain). Eur. Food Res. Technol. 2007, 224, 431–436. [Google Scholar] [CrossRef]
- Cacho, J.; Ferreira, V. The Aroma of Wine. In Handbook of Fruit and Vegetable Flavors; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 303–317. ISBN 9780470227213. [Google Scholar]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascués, E.; Calderón, F.; Benito, A.; Rauhut, D.; Santos, A.; et al. Effects on varietal aromas during wine making: A review of the impact of varietal aromas on the flavor of wine. Appl. Microbiol. Biotechnol. 2019, 103, 7425–7450. [Google Scholar] [CrossRef] [PubMed]
- de-la-Fuente-Blanco, A.; Sáenz-Navajas, M.-P.; Ferreira, V. On the effects of higher alcohols on red wine aroma. Food Chem. 2016, 210, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Fernandes, A.; Albuquerque, F.F.; Marques, J.C.; Câmara, J.S. Analytical characterization of the aroma of Tinta Negra Mole red wine: Identification of the main odorants compounds. Anal. Chim. Acta 2006, 563, 154–164. [Google Scholar] [CrossRef]
- Aznar, M.; López, R.; Cacho, J.; Ferreira, V. Prediction of Aged Red Wine Aroma Properties from Aroma Chemical Composition. Partial Least Squares Regression Models. J. Agric. Food Chem. 2003, 51, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P.; Zanoni, T.A.; Lara, A.; Barrero, A.F.; Cool, L.G. Comparisons among Cupressus arizonica Greene, C. benthamii Endl., C. lindleyi Klotz, ex Endl. and C. lusitanica Mill, using Leaf Essential Oils and DNA Fingerprinting. J. Essent. Oil Res. 1997, 9, 303–309. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 1995. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on Methyl Silicon and Carbowax 20M phases. J. Chromatogr. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Jennings, W.; Shibamoto, T. Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography; Academic Press: New York, NY, USA; London, UK; Sydney, Australia; Toronto, ON, Canada; San Francisco, CA, USA, 1982; Volume 26. [Google Scholar]
- Masada, Y. Analysis of Essential Oils by Gas Chromatography and Mass Spectrometry; John Wiley & Sons, Inc.: New York, NY, USA, 1976; ISBN 047015019X. [Google Scholar]
- Stenhagen, E.; Abrahamsson, S.; McLafferty, F.W. Registry of Mass Spectral Data; Wiley & Sons: New York, NY, USA, 1974. [Google Scholar]
- Swigar, A.A.; Silverstein, R.M. Monoterpenes; Aldrich Chemical Company: Milwaukee, WI, USA, 1981. [Google Scholar]
- Panighel, A.; Flamini, R. Applications of Solid-Phase Microextraction and Gas Chromatography/Mass Spectrometry (SPME-GC/MS) in the Study of Grape and Wine Volatile Compounds. Molecules 2014, 19, 21291–21309. [Google Scholar] [CrossRef] [PubMed]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Alcaraz Zini, C. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef]
- Antalick, G.; Perello, M.-C.; de Revel, G. Esters in Wines: New Insight through the Establishment of a Database of French Wines. Am. J. Enol. Vitic. 2014, 65, 293–304. [Google Scholar] [CrossRef]
- Ferreira, V.; Ortín, N.; Escudero, A.; López, R.; Cacho, J. Chemical Characterization of the Aroma of Grenache Rosé Wines: Aroma Extract Dilution Analysis, Quantitative Determination, and Sensory Reconstitution Studies. J. Agric. Food Chem. 2002, 50, 4048–4054. [Google Scholar] [CrossRef] [PubMed]
- Pineau, B.; Barbe, J.-C.; Van Leeuwen, C.; Dubourdieu, D. Examples of Perceptive Interactions Involved in Specific “Red-” and “Black-berry” Aromas in Red Wines. J. Agric. Food Chem. 2009, 57, 3702–3708. [Google Scholar] [CrossRef]
- Sagratini, G.; Maggi, F.; Caprioli, G.; Cristalli, G.; Ricciutelli, M.; Torregiani, E.; Vittori, S. Comparative study of aroma profile and phenolic content of Montepulciano monovarietal red wines from the Marches and Abruzzo regions of Italy using HS-SPME–GC–MS and HPLC–MS. Food Chem. 2012, 132, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).