Abstract
Wilted (around 35% DM) or un-wilted (around 35% DM) Italian ryegrass treated with three additives (formic acid, FA; Lactobacillus plantarum, LP; Lactobacillus buchneri, LB) was utilized to evaluate the effects of the dry matter (DM) contents on the microbial community and fermentation characteristics, which was ensiled for 60 days in a laboratory-scale silo, followed by 3 days of aerobic exposure. Significantly lower pH and higher lactic acid (LA) contents were observed in the LP-treated group ensiled at both DM contents (differences were significant when p < 0.05). The contents of LA, acetic acid (AA), numbers of lactic acid bacteria (LAB) and ammonia nitrogen (NH3-N) in the FA-treated group were significantly lower than those in other treatments (p < 0.05). L. buchneri was the dominant bacteria after 60 days fermentation, while Enterobacteria became prevalent after 3 days of aerobic exposure. L. buchneri was found in the LB-treated group with higher acetic acid. Although the best fermentation quality was observed in the LP-treated silages, the aerobic stability was lowest compared to other groups (p < 0.05). In conclusion, our findings suggest that the DM content of Italian ryegrass affected its epiphytic microbial community and the effectiveness of the different type of additives. Formic acid was more suitable for un-wilted Italian ryegrass silage, L. plantarum had a better effect in wilted Italian ryegrass silage, and L. buchneri prolonged the aerobic stability of Italian ryegrass. DM content and purpose of ensiling should be the key factors for choosing different types of additives for Italian ryegrass silage.
1. Introduction
Italian ryegrass (Lolium multiflorum Lam.) is an annual or short-period perennial ryegrass species, widely cultivated in the south of China and known for its rapid growth, high yields, good nutritional quality and palatability [1]. However, Italian ryegrass can be fed as fresh forage, which is periodical, mainly between April and June, and imbalanced along the year in the south of China. Furthermore, Italian ryegrass is always harvested in the rainy season, and the humid climate is not favorable for hay production. Ensiling is a feasible measure to reduce the fresh Italian ryegrass shortage in summer to winter, while it is an effective technique to reduce the loss of nutrients compared with cured hay [2].
Silage production is one of the most effective techniques for ensuring animal feed supply in the winter. Due to the low dry matter (DM) content and high number of undesirable epiphytic microorganisms, with Italian ryegrass, it is difficult to make high-quality silage [3,4]. The investigation regarding effects of dry matter (DM) content and silage additives on the fermentation of bunker-made ryegrass silage suggested that increasing the DM content from 18% to 30% without additives had beneficial effects on fermentation [5]. Other studies also demonstrated that wilting is one of the most effective physical measures to inhibit undesirable microorganisms and reduce nutrient loss [6]. Research has shown that homofermentative lactic acid bacteria (LAB) such as Lactobacillus plantarum affect microflora by increasing the ratio of lactic to acetic acid, positively affect nutrient recovery in alfalfa and wheat silages with over 35%DM contents [7,8,9]. However, homofermentative LAB did not improve the aerobic stability of cereal grain silages [10]. On the contrary, application of heterofermentative LAB, such as for L. buchneri 40788 during the ensiling of alfalfa and barley, can improve the aerobic stability of the ration [11]. Another research study has shown that formic acid, as a common chemical additive, can also effectively improve the utilization of nutrients in alfalfa silage [12]. However, few studies have been conducted to compare the effects of the application of chemical additives and inoculants on fermentation quality and aerobic stability of Italian ryegrass silage ensiled at different dry matter contents.
The fermentation process is accompanied with rapid changes of microorganism compositions from the fresh forage to the terminal aerobic stage of the feeding phase [13]. These changes are directly related to silage quality [14]. In recent years, more reports have focused on the changes in the microbial community in different types of silage [14,15,16]. However, there are limited reports on the effects of different types of additives on the microbial community and quality of ryegrass silage from fermentation to aerobic exposure.
The hypothesis of our study focuses on the additions that could improve the fermentation environment of Italian ryegrass silage with different DM contents so as to effectively promote the growth of beneficial bacteria and extend the aerobic exposure time. Thus, the objectives of the current study were to investigate the influence of L. plantarum, L. buchneri and a formic acid microbial community and fermentation profile of wilted or un-wilted Italian ryegrass silages during ensiling and aerobic exposure.
2. Materials and Methods
2.1. Study Site and Silage Preparation
Italian ryegrass (Lolium multiflorum Lam. ver. Chang Jiang II) was grown in the farm of the Chongqing Academy of Animal Sciences (N 29.32°, E 105.59°, 510 m above sea content). The grass was harvested from three different fields at the heading stage on 27 April 2019.All fresh forage was well mixed and separated equally into two parts; half of the fresh grass (around 25% fresh weight) was directly chopped and the other half of fresh grass was dried with natural wind in shadow 24 h until the DM was around 35% of fresh weight (FW). The fresh and wilted grass was chopped into 2~3 cm with straw chopper. The chopped forage of each DM treatment was then assigned to one of the following treatments: (1) untreated (deionized water), (2) formic acid (FA, 99%, 4 mL kg−1 fresh weight, Long Xi chemical company, China) (3) Lactobacillus plantarum MTD/1 (LP, 6.5 × 1010 cfu of viable LAB/g, Ecosyl, Volac International Limited, Hertfordshire, UK), or (4) Lactobacillus buchneri PJB/1 (LB, 2.0 × 1010 cfu of viable LAB/g, Ecosyl, Volac International Limited, Hertfordshire, UK). Each LAB stain was incubated by using De Man, Rogosa, Sharpe agar (MRS) broth (CM 188, Land Bridge, Beijing, China) and was dissolved in sterile distilled water to an equivalent of around 106 colony-forming units (cfu)/mL LAB solution, and every 100 g of chopped forage was sprayed with 3 mL of prepared LAB solution according to inoculant directions, and then mixed thoroughly. For the control treatment, an equal amount of distilled water was applied. Each treatment was prepared in triplicate. A total of 1000 g of forage was packed into nylon-polyethylene bags (35 × 45 cm; Aodeju Brands, Guangdong, China), and vacuum-sealed tightly using a vacuum sealer (DZ-600/2SD, Xinbo Brands, Zhejiang, China). The bags were then stored at an ambient temperature of 25 ± 2 °C for 60 days, followed by 3 days of aerobic exposure.
2.2. Chemical, Microbiological and Fermentation Profile Analysis
After 60 days of ensiling, the bags were opened, with a portion of silage immediately frozen (−20 °C) for further analysis. The DM contents of fresh Italian ryegrass and ensile were measured by drying the samples in a forced-air oven at 60 °C for 72 h and then ground by a grinder (CT293 Cyclotec™, FOSS Analytical A/S, Hillerød, Denmark) and passed through a 1 mm mesh sieve for future chemical analysis. Dry matter recovery (DMR) was calculated by ensiled DM divided to fresh DM. The Ankom 2000 system (Ankom Technology Corporation, Macedon, NY, USA) was used to test the neutral detergent fiber (NDF) and acid detergent fiber (ADF), while the test method was in accordance with the manufacturer’s instructions [17]. Crude protein (CP) was determined by the Kjeldahl method [18]. Water-soluble carbohydrate (WSC) was measured by the thracenone–sulphuric acid method [19].
The microbial population was quantified in the fresh forage and silages. The water extracts from silages and fresh forage samples were prepared by homogenizing a 20 g sample in 180 mL of sterile saline (0.85% NaCl) in an industrial blender for 1 min. Subsequently, the pH of the extracts was measured using a potentiometer (model PHSJ-5; LEICI, Shanghai, China). The water extract was divided into two portions. One portion was subjected to serial dilutions ranging from 10−1 to 10−10. Sterile plates were prepared with De Man, Rogosa and Sharpe agar (Difco, Beijing Land Bridge Co., LTD, Beijing, China) for LAB, and potato dextrose agar (Difco) for yeasts and molds (Y & M) (acidified with 1.5% of tartaric acid solution 10% wt vol−1). Plates of LAB were incubated anaerobically at 37 °C for 48 h, and the Y & M plates were incubated aerobically at 25 °C for 4 d. The method of microbial population was quantified according to the method by Guan et al. [14].
Another portion of the water extract was filtered using filter paper and acidified with meta-phosphoric acid solution (20% wt vol−1) and centrifuged at 12,000× g (centrifuge model 5810R; Eppendoff, Hamburg, Germany) for 15 min at 4 °C to determine the concentration of lactic, acetic, butyric, and propionic acids. Organic acids were then analyzed by high-performance liquid chromatography with a UV detector (210 nm) and a column (KC-811, Shimadzu Co., Ltd., Kyoto, Japan) according to Guan et al. [14]. The mixed standard of four organic acids (purchased from Sigma-Aldrich, Darmstadt, Germany) was used to make the standard curve to calculate the content of four acids in the samples. To determine the NH3-N content, an aliquot of 10 mL (250 g L−1, wt vol−1) TCA was added to 40 mL of the filtrate, and the solution was kept at 4 °C overnight to precipitate the protein. The solution was then centrifuged at 18,000× g for 15 min, and the supernatant was analyzed for NH3-N [20].
Chemical composition and microbial populations of fresh Italian ryegrass before ensiling are shown in Table 1.

Table 1.
Chemical composition and microbial populations of Italian ryegrass before ensiling.
2.3. Aerobic Stability Measurement
After sampling the silages from each bag for fermentative and chemical analysis of 60 days, the silage samples (800 g for each sample) were incubated aerobically in a 2 L clean sterile beaker with two layers of cheesecloth covering. A thermocouple connected to a data logger (MT-X; Shenhua Technology Co., Ltd., Shenzhen, China) was placed in the geometric center of the silage mass to measure its temperature, recorded every 2 h. Aerobic stability was defined as the time starting when the bags were opened until silage temperature increased by 2 °C above the ambient temperature [21].
2.4. Bacterial Community Analysis
2.4.1. DNA Extraction
The method of DNA extraction referred to Guan et al. [14]. A total of 50 g of frozen sample was passed through a 4 mm sieve after freeze-drying and smashing. A subsample (5 g) was ball milled for 1 min at room temperature, and the total DNA was extracted via the TIANamp Bacteria DNA isolation kit (DP302-02, Tiangen, Beijing, China). All samples were purified via purification and recovery of the DNA kit column (DP214-02, Tiangen, Beijing, China) and then eluted in nuclease-free water. NanoDrop2000 was used to detect the purity and concentration of DNA. The qualified DNA samples were stored at 20 ℃ for future analysis.
2.4.2. PCR Amplification and DGGE Detection
Because our preliminary experiments demonstrated that triplicate silages exhibited almost the same DGGE profiles, the DNA of three replicates was pooled together as one sample for PCR. As previously described by Li and Nishino [22], the primers used in the amplification system were 357f (5′-CGCCCGCCGCGCGCGGCGGGCGGGGCGGGG CCTACGGGAGGCAGCAG-3′) and 517r (5′-ATTACCGCGGCTGCTGG-3′), and the PCR amplification system and steps were conducted by the same method. An agarose gel at 1.0% (w/v) (120 v, 30 min) was used to detect whether the target DNA was amplified.
The 20 μL amplified products were mixed with 6× loading buffer and detected using DGGE (DCode™ System, Bio-Rad, Hercules, CA, USA) with a 25–55% gradient (100% denaturant is a mixture of 7 M urea and 40% deionized methylphthalamide). After the solution was prepared, it was placed into a gel mixer, and then 18 µL TEMED was added to each tube, 80 µL 10% APS, mixed quickly with a glass rod, and poured into the prepared sandwich clip at a constant speed (the denaturant concentration increases from the top to the bottom of the gel), and the gel polymerized at room temperature for at least 1 h after inserting the comb. Electrophoresis control device was turned on, and the electrophoresis buffer was preheated to 60°C. The gel was run at 200 V for 10 min and then maintained at 150 V for 5 h.
Silver staining and a gel scanner (UV PCDS8000, Bio-Rad, Hercules, California, USA) were used to visualize the bands as previously described [23]. The dominant bands were excised and dissolved in 150 μL sterile water. The 1 μL DNA was re-amplified with GC clamp-free primers, purified using a purification kit (AP-PCR-250, Axygen, Union City, CA, USA), linked to a pMD18-T vector, and transformed into E. coli Top10. Finally, positive clones were outsourced for sequencing (Shengong, Shanghai, China). The sequences were compared and identified using the GenBank database in the BLASTN program from the National Center for Biotechnology Information (NCBI, http://ncbi.nlm.nih.gov/, accessed on 7 January 2022). The images obtained by DGGE were processed by QUANTITY ONE (Bio-Rad, Hercules, California, USA) software, and cluster analysis was performed on the performance of each sample in the map by UPGMA method. At the same time, the abundance (S) and Shannon–Wiener diversity index (Shannon–Wiener index, H), and evenness index (EH) and other indicators were used to compare the diversity of each sample [24].
2.5. Statistical Analysis
Data were analyzed by using the GLM procedure of the Statistical Package for the Social Sciences (SPSS Version 19.0, SPSS Inc., Chicago, IL, USA) according to the model for a 3 × 2 factorial treatment design. The data related to fermentation characteristics, microbial population, chemical composition of silage during ensiling and aerobic exposure were subjected to two-way ANOVA with the model:
where Yij is the dependent variable; μ is the overall mean; αi is the fixed effect of additives; βj is the effect of wilting (high or low DM contents); (α × β)ij is the interaction between additives and wilting (DM contents); and eij is the residual error. Comparisons between additives within each DM content and between DM content were made using Tukey’s test, when at least one of the contrasts of additive ×wilting (DM contents) was significant, and significance was defined as p < 0.05.
Yij = μ + αi + βj + (α × β)ij + eij,
3. Results
3.1. Fermentation Characteristics and Microbial Composition of Italian Ryegrass Ensiled
The fermentation characteristics and microbial composition of Italian ryegrass ensiled for 60 days are shown in Table 2. DM contents, additives and their interaction all had significant effects on pH value, concentration of lactic acid and acetic acid. For both DM contents, groups treated with LP and LB had lower pH and higher lactic acid concentration compared with the control (p < 0.05), while FA had lower pH and lactic acid concentration. The pH values of the FA- and LP-treated groups at 25% DM content were significantly lower than at 35% DM, while the pH values of control and LB-treated group at 25% DM were higher than at 35% DM. The highest concentration of lactic acid was found in LP-treated silages for both DM contents. The groups treated with LB had the highest acetic acid concentration for both DM contents. Compared with inoculated groups and the control, the FA-treated groups had both the lowest concentration of lactic acid and acetic acid for the two DM contents. Moreover, every treatment and control at 25% DM had the highest lactic acid and acetic acid concentrations than at 35% DM. Furthermore, there was no significant difference between the three additives and two DM contents on the concentrations of propionic acid and butyric acid (p > 0.05). The numbers of LAB increased in the untreated groups, the LP-treated and the LB-treated groups at both DM contents after fermentation (the number of LAB of raw materials was 2.63 Log10 cfu g−1 FW). Furthermore, the numbers of LAB were sustained at 10 cfu g−1 in the FA-treated silage of 35% DM, which was significantly (p < 0.05) lower than other treatments, while the numbers of LAB of 35% DM in the untreated groups and LP-treated groups were significantly higher (Table 2) than other treatments (p < 0.05). With yeast, it has been shown that they were inhibited by every group except for the untreated ones at both DM contents. The numbers of mold in all groups remained undetectable.

Table 2.
Fermentation characteristics and microbial composition of Italian ryegrass ensiled for 60 d.
3.2. Chemical Composition of Italian Ryegrass Ensiled
The effects of different additives on the chemical composition of Italian ryegrass silages at different DM contents are shown in Table 3. Inoculation with LP or LB decreased DM recovery (DMR) of silages compared with untreated or FA-treated silages at 25% DM or 35% DM; silages with added FA at 35% DM had the highest DMR. Compared with the control, additive treatments had higher CP, but CP contents in the different treatments were not significantly different at the two DM contents (p > 0.05), while no effects of DM content on this variable were observed. The FA-treated groups and LP-treated groups had lower NH3-N/TN than those of the untreated or LB-treated groups at both DM contents (p < 0.05), but no significant effect was found in NH3-N/TN between the two DM contents (p > 0.05). FA- or LP-treated groups had higher WSC contents compared with untreated groups, and LB-treated groups had lower WSC content at both DM contents. In addition, all treatments and control ensiled samples with 35% DM had higher WSC content compared with those ensiled with 25% DM. Furthermore, small differences in the concentrations of NDF and ADF were found using the different additives (p > 0.05), but with the increase in DM, the concentration of NDF in every treatment group increased.

Table 3.
Chemical composition of Italian ryegrass ensiled for 60 d.
3.3. Aerobic Stability
The characteristics of pH, WSC and microbial composition of Italian ryegrass silages after 3 days of aerobic exposure are shown in Table 4. Compared with the silages at bag opening ensiled for 60 days (Table 3), the pH of every group at both DM contents increased after aerobic exposure for 3 days. The control groups had higher pH than the treated groups at both DM contents (p < 0.05). pH in the control and in all treatments at 25% DM were higher compared with 35% DM (p < 0.05). In addition, the numbers of LAB in every group increased except for the LP-treated group at 25% DM after the bags had been open for 3 days compared with those at bag opening ensiled for 60 days. All groups except for FA-treated, with 35% DM, had a higher number of LAB than 25% DM (p < 0.05).

Table 4.
Characteristics of pH, WSC and microbial composition of Italian ryegrass silages after 3 days of aerobic exposure.
Untreated groups at both DM contents were started having a fever after 30 h of exposure to air (Figure 1). Treatments with LB significantly improved (p < 0.05) the aerobic stability to more than 170 h for both DM contents, and the LP-treated groups were not bad throughout the 42 h period of monitoring for both DM contents. The FA-treated groups were found to be stable for 54 h.
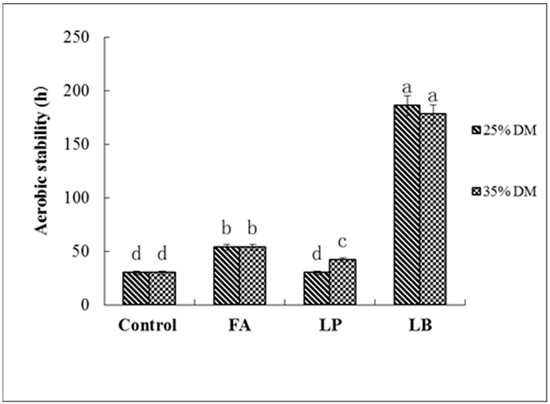
Figure 1.
Effect of different additives on aerobic stability of Italian ryegrass silages ensiled at different DM contents. FA, formic acid; LP, Lactobacillus plantarum; LB, Lactobacillus buchneri. a–d means with different letters differ (p < 0.05). Error bars indicate the standard error of mean values (n = 3).
3.4. Bacterial Community Analysis
As shown in Figure 2, different number of bands were separated, and the intensity and mobility of each band were different for each treatment. The sequence analysis results suggest that bands 1, 4, 6, 7, 9, and 11 all belong to the Lactobacillus genus (Table 5), which accounts for 50% of the total sequenced bands. Furthermore, bands 1, 4, 6, 7 were L. buchneri, especially for band 1, which was present in every lane with strong brightness. However, bands 3, 8, and 10 were shown as weak in Figure 2, with more than 96% similarity to Bacillus cecembensis, Bacillus pumilus, and Lysinibacillus fusiformis, respectively. These bands belong to the family Bacillaceae.
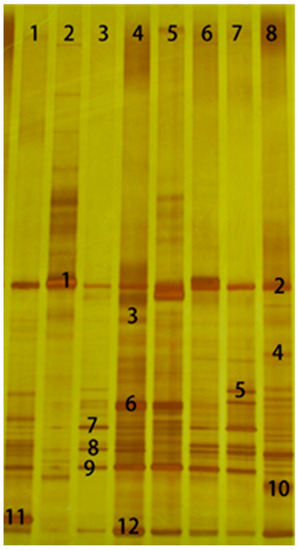
Figure 2.
DGGE analysis of PCR-amplified 16S rDNA fragments of Lolium multiflorum Lam. silage after 60 days fermentation. Lane 1, control group at 35% DM; lane 2, formic acid group at 35% DM; lane 3, LP-treated group at 35% DM; lane 4. LB-treated group at 35% DM; lane 5, control group at 25% DM; lane 6, formic acid group at 25% DM; lane 7, LP-treated group at 25% DM; lane 8, LB-treated group at 25% DM.

Table 5.
Sequences analysis of DNA recovered from single band in DGGE fingerprints of Lolium multiflorum Lam. silage after 60 days of fermentation.
The diversity index and richness of bacterial populations indicated that each lane of treatment was different (Table 6), while the uniformity was close. Abundance of diversity index shown in lane 4 was the highest, at 3.47 and 32, respectively. In comparison, lane 7 was the lowest, at 2.71 and 15, respectively.

Table 6.
Effects of additives on the Shannon diversity index of DGGE profiles of Lolium multiflorum Lam. silage after 60 days of fermentation.
As shown in Figure 3, the microbial community changed significantly after aerobic exposure for 3 days. As shown in Table 7, the similarities between bands 7 and 9 and Enterobacter aerogenes and Enterobacter lignolyticus were 96% and 98%, respectively, while bands 7 and 9 existed in almost every lane with strong brightness. Lactobacillus still existed after 3 days of aerobic exposure; especially, the brightness for L. buchneri was shown as relatively high in all lanes of treatment except for 2 and 4.
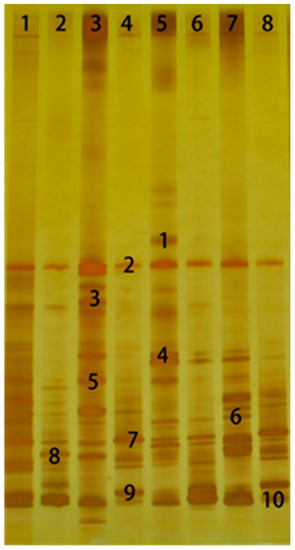
Figure 3.
DGGE analysis of PCR-amplified 16S rDNA fragments of different moisture Lolium multiflorum Lam. silage after aerobic exposure for 3 days. Lane 1, control group at 35% DM; lane 2, formic acid group at 35% DM; lane 3, LP-treated group at 35% DM; lane 4, LB-treated group at 35% DM; lane 5, control group at 25% DM; lane 6, formic acid group at 25% DM; lane7, LP-treated group at 25% DM; lane 8, LB-treated group at 25%DM.

Table 7.
Sequences analysis of DNA recovered from single band in DGGE fingerprints of silage after aerobic exposure for 3 days.
The diversity index and richness of bacterial communities were different in each treatment, while the uniformity was close (Table 8). Lane 5 was the highest (3.00 and 20, respectively) abundance shown in the diversity index, while lane 8 was the lowest, with 2.40 and 11, respectively. This was consistent with the intuitive observation result of the DGGE fingerprints (Figure 3).

Table 8.
Effects of inoculants on the Shannon diversity index of DGGE profiles of different moisture Lolium multiflorum Lam. silage after aerobic exposure for 3 days.
4. Discussion
Generally, it is difficult to produce high-quality silage when WSC content is less than 2% FW. In order to ensure good performance of fermentation, the WSC percentage in the forage should be higher than 6% DM [25]. In our study, the WSC content was 8.23%, which was suitable for producing silage, but the moisture content was as high as 75.07%, and the LAB number was less than 3 Log10 cfu g−1 FW. Under these conditions, the LAB number in the raw materials could not meet the requirement of the minimum number for producing high-quality silage [25]. Thus, adding additives is a common way to prepare Italian ryegrass silage.
It is generally believed that silage with lower pH and NH3-N/TN contents, higher lactic acid content and a higher ratio of lactic acid to total organic acid has better quality [26]. In our study, the pH and NH3-N/TN contents were found lowest in each LP-treated group of two DM contents, while the LA content was the highest after 60 d fermentation. This is due to pH decreasing with the LA produced by a typical homofermentative LAB of L. plantarum in the fermentation process [8]. In addition, the concentration of LA in each treatment with 35% DM was higher than in the 25% DM treatments. This suggested that higher moisture in forage may inhibit the growth and activity of LAB [27]. Under the two DM conditions, the pH content, NH3-N/TN and organic acid content in FA-treated groups were lower than in the others. This is because formic acid is a strong organic acid, which can not only reduce the pH content, but can also inhibit the activity of microorganisms [28]. Furthermore, the pH content of the LB-treated group was shown to be higher compared with the LP-treated group, but lower than untreated groups; NH3-N/TN content and the concentration of acetate increased sharply after ensiling. Our results might be attributed to the fact that L. buchneri is a heterofementative LAB, which consumes more metabolites in the silage process, and also converts LA into AA and 1,2-propanediol under anaerobic conditions [29,30,31].
The numbers of LAB in all except wilted FA-treated groups were found to be higher than raw materials after 60 d of ensiling, whereas the numbers of yeast in all except the un-wilted untreated groups were not found or decreased compared with raw materials. These findings suggest that formic acid could inhibit the growth of LAB; the untreated groups with a lower DM content (25%) could not inhibit the growth of yeast.
As silage is exposed to air, the anaerobic environment changes to aerobic. In this case, dormant microorganisms, especially some species of yeast with acid tolerance, surviving under a low pH condition, start to proliferate when they are in contact with oxygen, which leads to instability of the silage [32]. Filya et al. found that adding homofermentative LAB can effectively improve the quality of silage, but will harm silage aerobic stability [30]. Conversely, heterofermentative LAB could significantly improve the aerobic stability. In the current study, the amount of yeast in every group increased sharply, except for the LB-treated groups. This suggested that inoculation with L. buchneri could improve the aerobic stability of Italian ryegrass at both DM contents. Other specific reasons for spoilage of silage are a rise in temperature and pH and a sharp decrease in WSC content [28]. In our study, FA-treated groups at higher WSC contents became spoiled quickly, which showed that with increased exposure to air, the inhibition of undesirable bacteria by formic acid continued to decrease, and higher WSC content provided sugar for large numbers of undesirable bacteria, which caused the silage to deteriorate rapidly. This is consistent with the findings of Hill and Leaver [33]. WSC loss and low pH contents did not increase significantly under LB treatment, indicating that the AA produced by L. buchneri could still inhibit the activity of undesirable bacteria and keep the quality of the silage [31]. The pH of the LP-treated groups significantly increased, while the WSC content decreased rapidly, and the silage quality deterioration, this may due to L. plantarum producing LA, which provided the substrate for the growth and reproduction of the yeast [34].
Since there are many types of microorganisms involved in the silage process, fermentation is a dynamic process of microbial activity, as the diversity of microbial communities in silage is complex and constantly changing [35]. Although next-generation sequencing (NGS) methods have been widely used in silage microbial ecology [35], it is difficult to annotate microbial communities to the species level without the expensive single molecule real-time (SMRT) technology. Therefore, DGGE technology can be used as a supplement to the NGS technology to annotate microorganisms to the species level. The number of bands of Lactobacillus spp. accounted for more than 50% of the total sequenced bands after 60 days of fermentation, especially for L. buchneri, which appeared in large numbers in each lane, suggesting that L. buchneri gradually occupied the dominant position in the late stage of ensiling. Our study showed that the number of bands under 35% DM conditions (lanes 1, 2, 3, 4) was higher than that under 25% DM conditions (lanes 5, 6, 7, 8), which indicated that much higher moisture content forage is not conducive to the growth and reproduction of microorganisms. Compared to the results by Ni et al., although the pH of wilted Italian ryegrass silage was higher than 4.9 in both studies, the dominant microorganisms were different at the end of fermentation. Hetero-fermentative Weissella spp. was detected as distinctive LAB species in Ni’s study, while Lactobacillus fabifermentans prevailed in the current study [36]. These bacteria with a low acid-producing effect may also be the main reason for the high pH of Italian ryegrass ensiling. The low numbers of LAB and other microorganisms in high DM forage lend themselves to the use of inoculants because of the lower competitive pressure [37]. Hu et al. (2009) found that inoculation with Lactobacillus buchneri led to greater improvements in silage aerobic stability in whole-plant corn ensiled at a moderately high DM content (41%) than at a normal DM content (33%) [38]. However, the effect of moisture on the microbial population was opposite in silage samples after 3 days of aerobic exposure. It may be that under aerobic conditions, the greater availability of water and substrates in high-moisture silage reduced the inoculant effectiveness directly, by stimulating the development of microorganisms that cause aerobic deterioration, which was then unable to control the development of aerobic microorganisms [37].
The DGGE results showed that the number of bands in the formic acid group (lanes 2, 5) was less than that of other treatment groups, indicating that formic acid inhibited the growth of bacteria [39,40]. In the LP-treated group (lanes 3, 7), the bacterial population was stable. Although the presence of L. plantarum was still detected, the brightness and the number of bands were lower than those of L. buchneri. This may be because L. buchneri usually begins to grow fast at the late stage of ensiling, which may also inhibit the growth of L. plantarum. The microbial community dramatically changed with the appearance of Enterobacter and the disappearance of Bacillus after 3 days of aerobic exposure. L. buchneri was still dominant in the LB-treated group (lanes 4 and 8), indicating that L. buchneri still played a significant role after 3 days of aerobic exposure. Some studies have shown that L. plantarum can not only inhibit the activities of undesirable microorganisms during aerobic exposure, but it can also accelerate the deterioration of the silage [41], which is consistent with the current results. The LP-treated group (lanes 3 and 7) had more bands and greater brightness, indicating that the aerobic bacteria multiplied. pH, WSC content and aerobic stability also proved that L. plantarum accelerated the deterioration of wilted and un-wilted Italian ryegrass silage.
5. Conclusions
All the additives used in the present study improved the fermentation quality of Italian ryegrass at both DM contents. The highest fermentation quality was observed in LP-treated silages with 35% DM. L. buchneri was the dominant bacteria after 60 days of fermentation, while Enterobacteria became prevalent after 3 days of aerobic exposure. L. buchneri was found in the LB-treated group with higher acetic acid. Formic acid was suitable for both un-wilted and wilted Italian ryegrass silage, L. plantarum had a better effect in wilted Italian ryegrass silage, and L. buchneri prolonged the aerobic stability of Italian ryegrass.
Author Contributions
Conceptualization, Q.R. and Y.X.; methodology, H.G. and H.L.; software, H.G.; validation, Q.R. and H.G.; formal analysis, W.H.; investigation, Y.H. and L.Z.; resources, Y.F. and Y.X.; data curation, R.Z. and L.Z.; writing—original draft preparation, Q.R and H.L.; writing—review and editing, H.G and Q.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Southwest Minzu University Research Startup Funds (grant bo. RQD2022032), the Chongqing financial fund special project (no. 22538C), Chongqing performance incentive guide special project (22532J), and by the China Agriculture Research System of MOF and MARA (CARS-34).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Zhu, Y.; Nishino, N.; Kishida, Y.; Uchida, S. Ensiling characteristics and ruminal degradation of Italian ryegrass and lucerne silages treated with cell wall-degrading enzymes. J. Sci. Food Agric. 1999, 79, 1987–1992. [Google Scholar] [CrossRef]
- Shao, T.; Ohba, N.; Shimojo, M.; Masuda, Y. Dynamics of Early Fermentation of Italian Ryegrass (Lolium multiflorum Lam.) Silage. Asian-Australas. J. Anim. Sci. 2002, 15, 1606–1610. [Google Scholar] [CrossRef]
- Li, Y.; Nishino, N. Bacterial and fungal communities of wilted Italian ryegrass silage inoculated with and without Lactobacillus rhamnosus or Lactobacillus buchneri. Lett. Appl. Microbiol. 2011, 52, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Cai, Y.; Uegaki, R.; Shimizu, M.; Nakajima, M.; Kanaya, C.; Okajima, T.; Takada, R. Microorganism composition of high moisture Italian ryegrass (Lolium multiflorum Lam.) and its fermentation characteristics of silage inoculated with lactic acid bacteria. Jpn. J. Grassland Sci. 2010, 56, 39–46. [Google Scholar]
- Haigh, P.M. The effect of wilting and silage additives on the fermentation of autumn made grass silage ensiled in bunkers on commercial farms in South Wales 1983-85. Grass Forage Sci. 1988, 43, 337–345. [Google Scholar] [CrossRef]
- Conaghan, P.; O’Kiely, P.; O’Mara, F. Conservation characteristics of wilted perennial ryegrass silage made using biological or chemical additives. J. Dairy Sci. 2010, 93, 628–643. [Google Scholar] [CrossRef]
- Jones, B.A.; Satter, L.D.; Muck, R.E. Influence of bacterial inoculant and substrate addition to lucerne ensiled at different dry matter contents. Grass Forage Sci. 1992, 47, 19–27. [Google Scholar] [CrossRef]
- Whiter, A.G.; Kung, L., Jr. The Effect of a Dry or Liquid Application of Lactobacillus plantarum MTD1 on the Fermentation of Alfalfa Silage. J. Dairy Sci. 2001, 84, 2195–2202. [Google Scholar] [CrossRef]
- Ely, L.O.; Sudweeks, E.M.; Moon, N.J. Inoculation with Lactobacillus plantarum of Alfalfa, Corn, Sorghum, and Wheat Silages. J. Dairy Sci. 1981, 64, 2378–2387. [Google Scholar] [CrossRef]
- Weinberg, Z.; Ashbell, G.; Hen, Y.; Azrieli, A. The effect of applying lactic acid bacteria at ensiling on the aerobic stability of silages. J. Appl. Bacteriol. 1993, 75, 512–518. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Ranjit, N. The effect of Lactobacillus buchneri and other additives on the fermentation and aerobic stability of barley silage. J. Dairy Sci. 2001, 84, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Davidson, T.R.; Stevenson, K.R. Influence of formic acid and formalin on quality of direct-cut alfalfa silage. Can. J. Plant Sci. 1973, 53, 75–79. [Google Scholar] [CrossRef]
- Muck, R. Recent advances in silage microbiology. Agric. Food Sci. 2013, 22, 3–15. [Google Scholar] [CrossRef]
- Guan, H.; Yan, Y.; Li, X.; Li, X.; Shuai, Y.; Feng, G.; Ran, Q.; Cai, Y.; Li, Y.; Zhang, X. Microbial communities and natural fermentation of corn silages prepared with farm bunker-silo in Southwest China. Bioresour. Technol. 2018, 265, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Duniere, L.; Xu, S.; Long, J.; Elekwachi, C.; Wang, Y.; Turkington, K.; Forster, R.; McAllister, T.A. Bacterial and fungal core microbiomes associated with small grain silages during ensiling and aerobic spoilage. BMC Microbiol. 2017, 17, 1–16. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Guan, H.; Huang, L.; Ma, X.; Peng, Y.; Li, Z.; Nie, G.; Zhou, J.; Yang, W.; et al. Microbial community and fermentation characteristic of Italian ryegrass silage prepared with corn stover and lactic acid bacteria. Bioresour. Technol. 2019, 279, 166–173. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated Simultaneous Determination of Ammonia and Total Amino Acids in Ruminal Fluid and In Vitro Media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Teller, R.; Schmidt, R.; Whitlow, L.; Kung, L. Effect of physical damage to ears of corn before harvest and treatment with various additives on the concentration of mycotoxins, silage fermentation, and aerobic stability of corn silage. J. Dairy Sci. 2012, 95, 1428–1436. [Google Scholar] [CrossRef]
- Li, Y.; Nishino, N. Monitoring the bacterial community of maize silage stored in a bunker silo inoculated with Enterococcus faecium, Lactobacillus plantarum and Lactobacillus buchneri. J. Appl. Microbiol. 2011, 110, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, C.J.; Dias-Neto, E.; Simpson, A.J. Rapid silver staining and recovery of PCR products separated on polyacrylamide gels. BioTechniques 1994, 17, 914–921. [Google Scholar] [PubMed]
- Luo, H.; Qi, H.; Zhang, H. Assessment of the Bacterial Diversity in Fenvalerate-Treated Soil. World J. Microbiol. Biotechnol. 2004, 20, 509–515. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A.; Heron, S.J.E. The Biochemistry of Silage; Chalcombe Publications: Marlow, UK, 1991. [Google Scholar]
- Harrison, J.; Blauwiekel, R.; Stokes, M. Fermentation and Utilization of Grass Silage. J. Dairy Sci. 1994, 77, 3209–3235. [Google Scholar] [CrossRef]
- Luchini, N.; Broderick, G.; Muck, R.; Makoni, N.; Vetter, R. Effect of Storage System and Dry Matter Content on the Composition of Alfalfa Silage. J. Dairy Sci. 1997, 80, 1827–1832. [Google Scholar] [CrossRef]
- Johnson, L.; Harrison, J.; Davidson, D.; Mahanna, W.; Shinners, K.; Linder, D. Corn Silage Management: Effects of Maturity, Inoculation, and Mechanical Processing on Pack Density and Aerobic Stability. J. Dairy Sci. 2002, 85, 434–444. [Google Scholar] [CrossRef]
- Elferink, S.J.W.H.O.; Krooneman, J.; Gottschal, J.C.; Spoelstra, S.F.; Faber, F.; Driehuis, F. Anaerobic Conversion of Lactic Acid to Acetic Acid and 1,2-Propanediol by Lactobacillus buchneri. Appl. Environ. Microbiol. 2001, 67, 125–132. [Google Scholar] [CrossRef]
- Filya, I.; Sucu, E.; Karabulut, A. The effect of Lactobacillus buchneri on the fermentation, aerobic stability and ruminal degradability of maize silage. J. Appl. Microbiol. 2006, 101, 1216–1223. [Google Scholar] [CrossRef]
- Schmidt, R.; Hu, W.; Mills, J.; Kung, L. The development of lactic acid bacteria and Lactobacillus buchneri and their effects on the fermentation of alfalfa silage. J. Dairy Sci. 2009, 92, 5005–5010. [Google Scholar] [CrossRef]
- Woolford, M. The detrimental effects of air on silage. J. Appl. Bacteriol. 1990, 68, 101–116. [Google Scholar] [CrossRef]
- Hill, J.; Leaver, J. Changes in chemical composition and nutritive value of urea treated whole crop wheat during exposure to air. Anim. Feed Sci. Technol. 2002, 102, 181–195. [Google Scholar] [CrossRef]
- Nishino, N.; Hattori, H. Resistance to aerobic deterioration of total mixed ration silage inoculated with and without homofermentative or heterofermentative lactic acid bacteria. J. Sci. Food Agric. 2007, 87, 2420–2426. [Google Scholar] [CrossRef]
- McAllister, T.; Dunière, L.; Drouin, P.; Xu, S.; Wang, Y.; Munns, K.; Zaheer, R. Silage review: Using molecular approaches to define the microbial ecology of silage. J. Dairy Sci. 2018, 101, 4060–4074. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Minh, T.T.; Tu, T.T.M.; Tsuruta, T.; Pang, H.; Nishino, N. Comparative microbiota assessment of wilted Italian ryegrass, whole crop corn, and wilted alfalfa silage using denaturing gradient gel electrophoresis and next-generation sequencing. Appl. Microbiol. Biotechnol. 2017, 101, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- da Silva, É.B.; Liu, X.; Mellinger, C.; Gressley, T.F.; Stypinski, J.D.; Moyer, N.A.; Kung, L. Effect of dry matter content on the microbial community and on the effectiveness of a microbial inoculant to improve the aerobic stability of corn silage. J. Dairy Sci. 2022, 105, 5024–5043. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Schmidt, R.J.; Mcdonell, E.E.; Klingerman, C.M.; Kung, L., Jr. The effect of Lactobacillus buchneri 40788 or Lactobacillus plantarum MTD-1 on the fermentation and aerobic stability of corn silages ensiled at two dry matter contents. J. Dairy Sci. 2009, 92, 3907–3914. [Google Scholar] [CrossRef]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef]
- Takano, N.; Manda, T.; Masoka, Y. Formic acid as an additive of high-moisture silage. III. Silage quality and characteristics of seepage. Bull. Natl. Grassl. Res. Inst. 1975, 7, 71–80. [Google Scholar]
- Guan, H.; Ke, W.; Yan, Y.; Shuai, Y.; Li, X.; Ran, Q.; Yang, Z.; Wang, X.; Cai, Y.; Zhang, X. Screening of natural lactic acid bacteria with potential effect on silage fermentation, aerobic stability and aflatoxin B1 in hot and humid area. J. Appl. Microbiol. 2020, 128, 1301–1311. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).