Abstract
Phenol is an important petrochemical that is conventionally used as a precursor for synthesizing an array of plastics and fine chemicals. As an emerging alternative to its traditional petrochemical production, multiple enzyme pathways have been engineered to date to enable its renewable biosynthesis from biomass feedstocks, each incorporating unique enzyme chemistries and intermediate molecules. Leveraging all three of the unique phenol biosynthesis pathways reported to date, a series of synthetic ‘metabolic funnels’ was engineered, each with the goal of maximizing net precursor assimilation and flux towards phenol via the parallel co-expression of multiple distinct pathways within the same Escherichia coli host. By constructing and evaluating all possible binary and tertiary pathway combinations, one ‘funnel’ was ultimately identified, which supported enhanced phenol production relative to all three individual pathways by 16 to 69%. Further host engineering to increase endogenous precursor availability then allowed for 26% greater phenol production, reaching a final titer of 554 ± 19 mg/L and 28.8 ± 0.34 mg/g yield on glucose. Lastly, using a diphasic culture including dibutyl phthalate for in situ phenol extraction, final titers were further increased to a maximum of 812 ± 145 mg/L at a yield of 40.6 ± 7.2 mg/g. The demonstrated ‘funneling’ pathway holds similar promise in support of phenol production by other, non-E. coli hosts, while this general approach can be readily extended towards a diversity of other value-added bioproducts of interest.
1. Introduction
As it has been estimated that roughly 40% (by mass) of bulk petrochemicals possess at least one or more aromatic functional groups [1], aromatic chemicals clearly represent an important class of molecules, which predominantly serve as precursors for diverse synthetic applications. In light of this, and in contrast to their conventional production from non-renewable petroleum resources, there has emerged strong and continued interest in developing alternative routes to produce diverse aromatics from renewable biomass feedstocks using microbial biocatalysts [2,3,4,5,6,7,8]. Among aromatic bioproduction targets investigated to date is phenol—a bulk chemical and building-block molecule used in the synthesis of various fine chemicals as well as many plastics and polymers [9,10]. The complete microbial biosynthesis of phenol was first demonstrated and is still commonly achieved from endogenous tyrosine via the expression of heterologous tyrosine phenol lyase (TPL; Pathway 1, Figure 1) [11,12]. In addition to this established route, in efforts to help improve phenol biosynthesis, alternative pathways for phenol biosynthesis have also recently been reported. Specifically, this includes two different pathways derived from endogenous chorismate, each involving different downstream enzyme chemistries and associated intermediates. The first pathway proceeds via salicylate as a key intermediate and involves isochorismate synthase, isochorismate pyruvate lyase, and salicylate decarboxylase (Pathway 2, Figure 1) [13]. The second, meanwhile, employs chorismate pyruvate lyase to produce 4-hydroxybenzoate, which is then decarboxylated to phenol (Pathway 3, Figure 1) [14]. A comparative evaluation of all three phenol pathways was also recently performed by Thompson et al. [15], where it was found that the tyrosine- and salicylate-derived pathways (i.e., Pathways 1 and 2, respectively) provided the best overall and comparable performance, each supporting phenol titers of up to 377 mg/L in simple batch shake flasks.
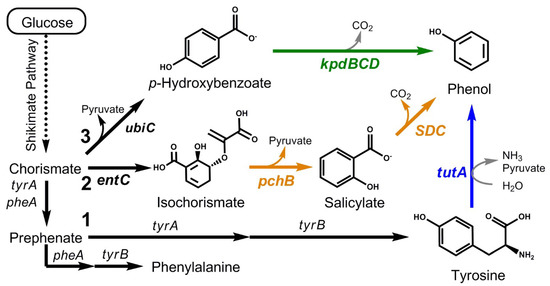
Figure 1.
Phenol biosynthesis from glucose in E. coli via three unique, engineered pathways. Black arrows represent enzyme steps native to E. coli whereas colored arrows are heterologous. Dotted arrows represent multiple enzymatic steps. Individual pathways 1, 2, and 3 were systematically combined to create the four ‘funneling’ pathways F1 (1 + 2), F2 (1 + 3), F3 (2 + 3), and F4 (1 + 2 + 3). Pathways were constructed using the following genes: ubiC from E. coli BW25113, which encodes chorismate pyruvate lyase; entC from E. coli BW25113, which encodes isochorismate synthase; tutA from C. braakii ATCC29063, which encodes tyrosine phenol lyase; pchB from P. aeruginosa PAO1, which encodes isochorismate pyruvate lyase; kpdBCD from K. pneumoniae PZH572, which encodes p-hydroxybenzoate decarboxylase; SDC from T. moniliiforme, which encodes salicylate decarboxylase. Blue, yellow, and green arrows represent the heterologous steps associated with pathways 1, 2, and 3, respectively.
Inspired by natural ‘funneling’ mechanisms used by various microbes to degrade aromatic compounds [16,17], we recently developed a synthetic ‘metabolic funnel’ to enhance muconic acid production in E. coli [18]. As achieved via the parallel co-expression of multiple distinct yet converging pathways, this unique biosynthetic approach helps to enhance the bioproduct by maximizing the assimilation of multiple endogenous precursors from different points in metabolism, leading to greater net flux towards the product of interest. This approach is also of potential utility in cases where inherent limitations (e.g., low precursor availability, slow kinetics) control the performance of one or more of the individual pathways composing the ‘funnel’. With these promising features in mind, the goal of this study was to engineer and investigate the utility of a novel ‘metabolic funnel’ for phenol biosynthesis. In addition, the effects of different host mutations known to increase the availability of shikimate pathway precursors as well as in situ solvent extraction to remove phenol from cultures as it is produced were investigated, in both cases with the objective of further amplifying both the final strain and culture performance.
2. Materials and Methods
2.1. Strains, Media, and Culture Conditions
All strains used in this study are listed in Table 1. E. coli NEB10-beta (New England Biolabs (NEB); Ipswich, MA, USA) was used for all cloning and plasmid maintenance. E. coli NST74 (ATCC 31884) was obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) and served as the parent strain in this study. E. coli NST74 ∆pheA [19] and E. coli NST74 ∆pheA ∆pykA ∆pykF ∆crr [18] were constructed as previously described. E. coli BW25113 was obtained from the Coli Genetic Stock Center (CGSC; New Haven, CT, USA) and served as the genetic source of ubiC and entC. Citrobacter braakii (ATCC 29063) was obtained from the ATCC and served as the genetic source for tutA. Pseudomonas aeruginosa PAO1 (DSMZ 22644) was obtained from the Leibniz Institute German Collection of Microorganisms and Cell Cultures and served as the genetic source of pchB. Klebsiella pneumoniae PZH572 (ATCC 25955) was obtained from the ATCC and served as the genetic source of kpdBCD. SDC from T. moniliiforme was synthesized to include codon optimization for E. coli.

Table 1.
Strains, plasmids, and pathways constructed and used for phenol production.
E. coli seed cultures were prepared in Luria-Bertani (LB) broth at 32 °C and supplemented with 100 μg/mL ampicillin, 35 μg/mL kanamycin, and/or 34 μg/mL chloramphenicol, as appropriate. For phenol biosynthesis, strains were grown in phosphate-limited MM1 minimal media supplemented with appropriate antibiotics. MM1 was composed of the following (in g/L): MgSO4·7H2O (0.5), (NH4)2SO4 (4.0), MOPS (24.7), KH2PO4 (0.3), K2HPO4 (0.7), and glucose (20). To accommodate for the deletion of pheA from E. coli NST74, where necessary, L-phenylalanine was supplemented into the medium at an initial concentration of 100 mg/L. Trace elements were also supplemented at the following concentrations (in mg/L): (NH4)6Mo7O24∙4H2O (0.37), H3BO3 (2.5), CoCl2∙6H2O (0.714), CuSO4 (0.16), MnCl2∙4H2O (1.6), ZnSO4∙7H2O (0.288), FeCl3 (0.05). All cultures were performed using 50 mL of aqueous media. For cultures employing in situ phenol extraction, an additional 10 mL dibutyl phthalate was added as a second, immiscible solvent phase. All cultures were seeded using a 1% (vol.) inoculum, after which strains were cultured in 250 mL shake flasks at 32 °C while shaking at 200 rpm (note that 32 °C was selected to minimize possible volatile losses from the cultures). Upon reaching an optical density at 600 nm (OD600) of ~0.7, cultures were induced by the addition of IPTG at a final concentration of 0.4 mM, after which culturing continued for a total period lasting up to 120 h, or until significant sugar consumption was no longer detected. Periodically, samples were drawn to measure cell growth (as OD600) as well as sugar and metabolite levels by HPLC analysis, as described below. Prior to centrifugation, samples for L-tyrosine analysis were diluted 1:10 with 1 N HCl and incubated at 55 °C for 30 min. All samples were then centrifuged at 11,000× g for 5 min before transferring the supernatant to a glass HPLC vial.
2.2. Plasmid and Pathway Construction
All plasmids constructed and/or used in this study are also listed in Table 1. All genes were PCR amplified using Q5 High-Fidelity DNA Polymerase (NEB) and custom-designed DNA oligonucleotide primers synthesized by Integrated DNA Technologies (IDT, Coralville, IA, USA). Genomic DNA (gDNA) templates were prepared using the ZR Fungal/Bacterial DNA MiniPrep kit while plasmid DNA was purified using the Zymo Plasmid MiniPrep kit (both Zymo Research, Irvine, CA, USA). Amplified linear DNA fragments were purified using the Zymo DNA Clean and Concentrator MiniPrep kit (Zymo Research). Purified linear and plasmid DNA were digested using appropriate restriction endonucleases (NEB) and subsequently gel purified using the Zymoclean Gel DNA Recovery MiniPrep kit (Zymo Research). Purified digested DNA fragments were ligated using T4 DNA Ligase (NEB). Alternatively, purified linear DNA was subsequently used as template DNA for either circular polymerase extension cloning (CPEC) [20] with Q5 High-Fidelity DNA Polymerase according to manufacturer protocols, or Gibson Assembly [21] using Gibson Assembly Master Mix (NEB) according to manufacturer protocols. Ligation, CPEC, and Gibson Assembly reactions were transformed into chemically competent E. coli NEB10-beta before plating on LB solid agar supplemented with appropriate antibiotics for selection. Transformant pools were screened using colony PCR, restriction digest mapping, and finally confirmed by DNA sequencing.
To evaluate phenol biosynthesis via single and combined pathways, E. coli NST74 ∆pheA and NST74 ∆pheA ∆pykA ∆pykF ∆crr were each co-transformed with the following combinations of plasmids (note the pathway designation provided in parentheses, see Table 1): pTyrAfbr-TutA (1); pSDC-PchB-EntC (2); pUbiC-Kpd (3); pTyrAfbr-TutA and pSDC-PchB-EntC (F1); pTyrAfbr-TutA and pUbiC-Kpd (F2); pSDC-PchB-EntC and pUbiC-Kpd (F3); and pTyrAfbr-TutA, pSDC-PchB-EntC, and pUbiC-Kpd (F4).
2.3. Metabolite Analysis
Aqueous metabolite analysis was performed using an Agilent 1100 series HPLC system. The separation of L-phenylalanine, p-hydroxybenzoate, salicylate, and phenol was achieved using a reverse-phase Hypersil GOLD aQ C18 column (5 μm particle size, 3 mm × 250 mm; Thermo Fisher, Waltham, MA, USA) operated at 45 °C with an isocratic 0.8 mL/min mobile phase consisting of 85% (vol.) 5 mM H2SO4 and 15% (vol.) acetonitrile. The eluent was monitored using a diode array detector (DAD) set at 215 nm for L-phenylalanine and salicylate, 260 nm for p-hydroxybenzoate, and 275 nm for phenol. The separation of L-tyrosine was achieved using the same Hypersil GOLD aQ C18 column, in this case maintained at 30 °C while using a mobile phase consisting of water (A) and methanol plus 0.1% (vol.) formic acid (B) at a constant flow rate of 0.2 mL/min and the following concentration gradients (all by vol.): 5% B from 0 to 8 min, 5% to 40% B from 8 to 13 min, 40% B from 13 to 16 min, 40 to 5% B from 16 to 21 min, and 5% B from 21 to 31 min. The eluent was monitored using a DAD set at 215 nm. Glucose and acetate were separated using an Aminex HPX-87H column (BioRad, Hercules, CA, USA) and detected using a refractive index detector (RID), both operated at 35 °C. The column was eluted with 5 mM H2SO4 at a constant flow rate of 0.55 mL/min. In all cases, external standards were prepared and used to provide calibrations for concentration determination. Phenol levels in dibutyl phthalate were also determined using the same HPLC system, in this case with separation again being performed on a Hypersil GOLD aQ C18 column maintained at 30 °C and using a mobile consisting of 55% methanol in water and flowing at 0.6 mL/min. Phenol was again detected using a DAD set to 275 nm after eluting at 22.6 min. External standards were prepared and used to provide calibrations for concentration determination.
3. Results and Discussion
3.1. Developing a ‘Metabolic Funnel’ for Phenol Biosynthesis
Previously, we engineered and compared the performance of three distinct biosynthetic routes to phenol, namely pathways 1, 2, and 3 (Figure 1) [15]. Using E. coli NST74 ΔpheA as the host background, maximal phenol production by these three individual pathways reached 377 ± 23, 377 ± 14, and 259 ± 31 mg/L at glucose yields of 18.7 ± 0.7, 35.7 ± 0.8, and 12.9 ± 1.1 mg/g, respectively (Table 2). Based on these outcomes and motivated by our separate demonstration that muconic acid biosynthesis could be improved via a ‘funneling’ strategy [18], we next sought to construct and investigate the utility of a ‘metabolic funnel’ for improving phenol biosynthesis. Specifically, we explored if and to what extent phenol biosynthesis could be improved as a result of different co-expression strategies using all individual pathways (i.e., 1, 2, and 3) as modular elements. Considering all possible combinations, this resulted in the following set of four ‘funneling’ pathways: F1 (1 and 2), F2 (1 and 3), F3 (2 and 3), and F4 (1, 2, and 3) (Table 1).

Table 2.
Evaluation of phenol production by different engineered pathways and synthetic ‘metabolic funnel’ configurations. In all cases, E. coli NST74 ∆pheA was used as the host background. All strains were initially supplied with 20 g/L glucose and cultured for 120 h. Data for Pathways 1, 2, and 3 are from Thompson et al. (2016). Errors represent one standard deviation from triplicate experiments.
Using E. coli NST74 ∆pheA again as the initial host background, shake flask cultures were performed to assess phenol production by each of the four distinct ‘funneling’ pathways, and compared relative to the three single pathway controls. As seen in Table 2, among both individual and ‘funneling’ pathways, final phenol titers were highest in the case of F1, reaching 439 ± 7 mg/L at a glucose yield of 24.0 ± 0.5 mg/g. This output represented a 16% increase in titer over the best single pathway control (i.e., 1 or 2, the two routes that comprise F1). The yield of phenol by F1, meanwhile, was nearly equivalent to the average of the two individual pathways that comprise it (i.e., 18.7 and 35.7 mg/g for pathways 1 and 2, respectively (for an average of 27.2 mg/g), compared to 24.0 mg/g for pathway F1). All other ‘funneling’ pathways performed no better or even worse than the best-performing individual pathways that comprised them. This is perhaps not surprising since all other ‘funneling’ pathways incorporated pathway 3, the least-productive individual pathway, suggesting that overall performance was diluted by its presence and possibly even further reduced by the increased burden associated with its expression. While the specific reason as to why the ‘funneling’ pathways that incorporate pathway 3 performed the poorest is currently not known, it has been reported that UbiC is feedback inhibited by p-hydroxybenzoate at relatively low levels (~2 μM) [22]. Thus, it is possible that the future performance of pathways 3, F2, and F3 could all be improved by increasing p-hydroxybenzoate decarboxylase activity to thereby enhance the turnover of p-hydroxybenzoate. Of further note, final biomass levels reached by the strain expressing F1 were about half of that of both F2 and F3 (1.2 vs. 2.4 and 2.2 g-DCW/L), a result consistent with greater phenol production by F1 that could be due to increased competition with growth essential amino acid biosynthesis and/or increased accumulation of toxic phenol. Both routes to phenol in pathway F1 stem from chorismate but do so in notably different ways. Whereas pathway 1 extends from the endpoint of a native pathway (i.e., L-tyrosine), pathway 2 directly competes with native aromatic amino acid biosynthesis. We propose that the specific utility of pathway F1 stem both from the balancing of unique, potential conflicts of these routes with native metabolism, as well as due to its ability to maximize total chorismate flux towards phenol. In particular, any chorismate that cannot be shuttled into pathway 2 by EntC ultimately has a second chance at being directed towards phenol via TutA in pathway 1.
Finally, in addition to phenol, significant acetate accumulation (reaching as high as 9 g/L) was also observed in all cases (Table 2), suggesting that rates of carbon loss by overflow metabolism were significant. High levels of acetate can negatively affect fitness [23], as is likely reflected here by the incomplete glucose conversion displayed by most strains. Accordingly, host engineering strategies were next investigated in an effort to reduce acetate accumulation as well as to improve precursor availability.
3.2. Host Engineering to Increase Phenol Production via Improved Precursor Availability
To improve strain performance, the host strain was further engineered to increase the intracellular availability of phosphoenolpyruvate (PEP)—a key shikimic acid pathway precursor [24,25]—while also limiting overflow metabolism by reducing glucose uptake rate [26]. During growth on glucose, PEP availability can be increased by blocking its conversion to pyruvate via the deletion of pykA and pykF, both encoding isozymes of pyruvate kinase. Meanwhile, rapid glucose uptake is known to result in the accumulation of acetate [25], which can inhibit cells while reducing aromatic yields [26]. Carbohydrate repression-resistant null mutants (i.e., Δcrr) display lower rates of glucose uptake and thus reduced overflow metabolism—a strategy demonstrated as effective for enhancing phenylalanine production [26]. Accordingly, E coli NST74 ∆pheA ∆pykA ∆pykF ∆crr was constructed and evaluated with respect to its ability to support improved phenol production, in this case focusing just on pathways 1, 2, and F1 as the best-performing individual and ‘funneling’ pathways.
As seen in Table 3, when expressing pathway 1, phenol titers increased by ~16% up to 436 ± 9 mg/L, whereas those resulting from pathway 2 were slightly reduced. The greatest improvement was seen in the case of the ‘funneling’ pathway F1, wherein a ~26% increase in final phenol titer (reaching up to 554 ± 19 mg/L) was realized. In the case of both pathways 1 and F1, acetate accumulation was reduced (now only reaching up to just 3 g/L in each) and glucose utilization increased relative to using E coli NST74 ∆pheA as the host, both of which likely contributed to the increased final titers. However, as significant levels of acetate still remained at the end of each culture, additional host engineering strategies (e.g., deletion of poxB and/or pta-ack) should be investigated to further reduce this wasteful carbon loss.

Table 3.
Comparing phenol production by the best-performing individual and ‘funneling’ pathways using E. coli NST74 ∆pheA ∆pykA ∆pykF ∆crr as the host background, as well as in the presence of a DBP overlay. Strains were cultured for 120 h and supplied with 20 g/L glucose. Error represents one standard deviation from triplicate experiments.
3.3. Enhancing Phenol Production via In Situ Solvent Extraction
As phenol has been reported to inhibit E. coli at final titers as low as 0.8 g/L [27], it is possible that titers achievable by the developed strains have thus far been limited as a result of product inhibition. One effective mechanism by which to circumvent the effects of inhibition caused by the aqueous accumulation of toxic biochemicals is through their in situ extraction into a second, biocompatible solvent phase [28,29,30]. Indeed, such a strategy has previously proven effective in support of enhancing phenol biosynthesis by other engineered microbes [12,14,27]. Accordingly, we lastly sought to investigate whether in situ extraction could be used to enhance the production of phenol when synthesized via the developed ‘funneling’ pathway F1. Dibutyl phthalate (DBP) was chosen as the solvent phase due to its previously characterized biocompatibility with E. coli as well as its prior utility in other extractive fermentation applications [31,32,33]. A series of abiotic extraction experiments were first performed to determine the equilibrium partitioning coefficient (Keq) for phenol between aqueous media and DBP at 32 °C, which, as seen in Figure 2, was estimated as 13.9 ± 0.4 (remaining linear across a range of at least 0 to 7 g/L phenol in DBP). Then, by cultivating the E. coli-expressing pathway F1 in 50 mL MM1 media with a 10 mL DBP overlay, after 120 h, phenol had accumulated up to 3.0 ± 0.5 g/L in the DBP phase, but still only 214 ± 38 mg/L in the aqueous media. This total mass of phenol corresponded to a final effective phenol titer of 812 ± 145 mg/L, which represents a 47% increase relative to the single-phase culture (Table 3). Phenol yield, meanwhile, was similarly increased, reaching up to 40.6 ± 7.2 mg/g.
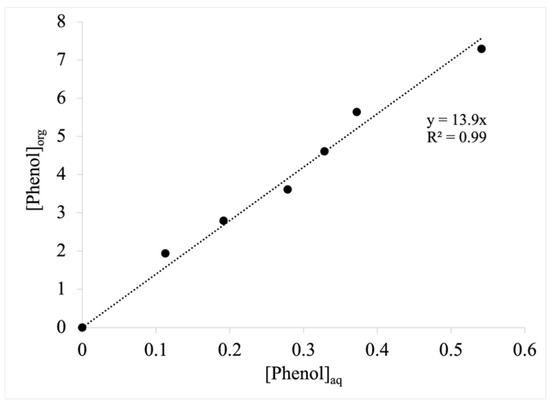
Figure 2.
Aqueous-dibutyl phthalate equilibrium partitioning behavior of phenol as a function of phenol concentration at 32 °C and determination of the equilibrium partitioning coefficient.
3.4. Comparing Strain Performance and Future Prospects
In the case of all individual pathways examined (i.e., 1, 2, or 3), the titers and/or yields achieved here (Table 2) are all notably comparable to those of past studies by other groups when similar culturing methods (e.g., batch shake flasks) were employed [13,14,27]. Compared to our initial strains, meanwhile, others have demonstrated more significant titer improvements as a result of more comprehensively optimizing the host background and/or via alternative culturing strategies. This was true in the case of Noda et al., who demonstrated the production of up to 1100 mg/L phenol by E. coli [31], as well Kim et al., who found that E. coli could produce up to 3.79 g/L phenol under fed-batch conditions using in situ extraction [27] (note that both studies employed just pathway 1). Even in this latter example, however, the final phenol yield (20 mg/g) remained at least 30% lower than what was reported here via pathway F1. Thus, with further optimization of host metabolism and the bioprocess configuration, it is expected that phenol production via pathway F1 in E. coli will ultimately result in even further improvements in phenol production.
Meanwhile, in addition to E. coli, other bacteria have emerged as promising phenol bioproduction platforms, including solvent-tolerant strains of Pseudomonas. For instance, phenol production by a pseudomonad was first reported for P. putida S12, which reached a final titer of 141 mg/L and yield of 35 mg/g in traditional culture, followed by up to 866 mg/L with in situ extraction [12], results similar in magnitude to the present study. More recently, Wynands et al. engineered a strain of P. taiwanensis VLB120 capable of producing phenol from glucose at up to 301 mg/L with a remarkable yield of 82.5 mg/g [32]. That said, since all phenol bioproduction efforts in Pseudomonas have so far relied solely upon pathway 1, it is expected that by adopting the strain-agnostic ‘funneling’ pathway developed here, improved phenol production should also be possible for even these and other non-E. coli hosts.
Lastly, it should be noted that, in addition to muconic acid [18] and now phenol, an analogous ‘funneling’ strategy has also proven useful for enhancing the production of isoprenoids. For example, β-carotene biosynthesis is possible via two distinct pathways: (i) An optimized mevalonate (MVA) pathway and (ii) a hybrid methylerythritol phosphate (MEP) pathway [33]. By simultaneously co-expressing both pathways, Yang and Guo [34] demonstrated that β-carotene production could be increased by as much as 113-fold relative to control strains expressing either individual pathway. Thus, though certainly not compatible for use with each and every biochemical target of interest, future opportunities nevertheless exist by which to apply this same ‘funneling’ approach to enhance the biosynthesis of diverse value-added products.
Author Contributions
Conceptualization, B.T. and D.R.N.; methodology, B.T., M.M. and O.A.; validation, B.T., M.M. and O.A.; formal analysis, B.T., M.M. and O.A.; investigation, B.T., M.M. and O.A.; writing—original draft preparation, B.T., M.M. and D.R.N.; writing—review and editing, D.R.N.; supervision, D.R.N.; project administration, D.R.N.; funding acquisition, D.R.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Science Foundation (CBET-1511637).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data, strain, and materials are available upon request.
Acknowledgments
M.M. was supported by a fellowship from the ARCS Foundation. O.A. was supported by a fellowship from the Master’s Opportunity for Research in Engineering (MORE) program at ASU.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van Haveren, J.; Scott, E.L.; Sanders, J. Bulk chemicals from biomass. Biofuels Bioprod. Biorefin. 2007, 2, 41–57. [Google Scholar] [CrossRef]
- Wang, J.; Shen, X.; Rey, J.; Yuan, Q.; Yan, Y. Recent advances in microbial production of aromatic natural products and their derivatives. Appl. Microbiol. Biotechnol. 2017, 102, 47–61. [Google Scholar] [CrossRef]
- Thompson, B.; Machas, M.; Nielsen, D.R. Creating pathways towards aromatic building blocks and fine chemicals. Curr. Opin. Biotechnol. 2015, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Noda, S.; Kondo, A. Recent Advances in Microbial Production of Aromatic Chemicals and Derivatives. Trends Biotechnol. 2017, 35, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Gosset, G. Production of aromatic compounds in bacteria. Curr. Opin. Biotechnol. 2009, 20, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Martnez, J.A.; Flores, N.; Escalante, A.; Gosset, G.; Bolivar, F. Engineering Escherichia coli to overproduce aromatic amino acids and derived compounds. Microb. Cell Fact. 2014, 13, 1–15. [Google Scholar] [CrossRef]
- Averesch, N.J.H.; Krömer, J. Metabolic Engineering of the Shikimate Pathway for Production of Aromatics and Derived Compounds—Present and Future Strain Construction Strategies. Front. Bioeng. Biotechnol. 2018, 6, 32. [Google Scholar] [CrossRef]
- Machas, M.; Kurgan, G.; Jha, A.K.; Flores, A.; Schneider, A.; Coyle, S.; Varman, A.M.; Wang, X.; Nielsen, D.R. Emerging tools, enabling technologies, and future opportunities for the bioproduction of aromatic chemicals. J. Chem. Technol. Biotechnol. 2018, 94, 38–52. [Google Scholar] [CrossRef]
- Adkins, J.; Pugh, S.; McKenna, R.; Nielsen, D.R. Engineering microbial chemical factories to produce renewable “biomonomers”. Front. Microbiol. 2012, 3, 313. [Google Scholar] [CrossRef]
- Deng, Y.; Ma, L.; Mao, Y. Biological production of adipic acid from renewable substrates: Current and future methods. Biochem. Eng. J. 2016, 105, 16–26. [Google Scholar] [CrossRef]
- Wierckx, N.J.P.; Ballerstedt, H.; de Bont, J.A.M.; de Winde, J.H.; Ruijssenaars, H.J.; Wery, J. Transcriptome Analysis of a Phenol-Producing Pseudomonas putida S12 Construct: Genetic and Physiological Basis for Improved Production. J. Bacteriol. 2008, 190, 2822–2830. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wierckx, N.J.P.; Ballerstedt, H.; de Bont, J.A.M.; Wery, J. Engineering of Solvent-Tolerant Pseudomonas putida S12 for Bioproduction of Phenol from Glucose. Appl. Environ. Microbiol. 2005, 71, 8221–8227. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yang, S.; Yuan, Q.; Sun, X. Microbial production of phenol via salicylate decarboxylation. RSC Adv. 2015, 5, 92685–92689. [Google Scholar] [CrossRef]
- Miao, L.; Li, Q.; Diao, A.; Zhang, X.; Ma, Y. Construction of a novel phenol synthetic pathway in Escherichia coli through 4-hydroxybenzoate decarboxylation. Appl. Microbiol. Biotechnol. 2015, 99, 5163–5173. [Google Scholar] [CrossRef]
- Thompson, B.; Machas, M.; Nielsen, D.R. Engineering and comparison of non-natural pathways for microbial phenol production. Biotechnol. Bioeng. 2016, 113, 1745–1754. [Google Scholar] [CrossRef]
- Bouwer, E.J.; Zehnder, A.J. Bioremediation of organic compounds-putting microbial metabolism to work. Trends Biotechnol. 1993, 11, 360–367. [Google Scholar] [CrossRef]
- Linger, J.G.; Vardon, D.; Guarnieri, M.T.; Karp, E.; Hunsinger, G.B.; Franden, M.A.; Johnson, C.; Chupka, G.; Strathmann, T.J.; Pienkos, P.T.; et al. Lignin valorization through integrated biological funneling and chemical catalysis. Proc. Natl. Acad. Sci. USA 2014, 111, 12013–12018. [Google Scholar] [CrossRef]
- Thompson, B.; Pugh, S.; Machas, M.; Nielsen, D.R. Muconic Acid Production via Alternative Pathways and a Synthetic “Metabolic Funnel”. ACS Synth. Biol. 2017, 7, 565–575. [Google Scholar] [CrossRef]
- Pugh, S.; McKenna, R.; Osman, M.; Thompson, B.; Nielsen, D.R. Rational engineering of a novel pathway for producing the aromatic compounds p-hydroxybenzoate, protocatechuate, and catechol in Escherichia coli. Process. Biochem. 2014, 49, 1843–1850. [Google Scholar] [CrossRef]
- Quan, J.; Tian, J. Circular polymerase extension cloning for high-throughput cloning of complex and combinatorial DNA libraries. Nat. Protoc. 2011, 6, 242–251. [Google Scholar] [CrossRef]
- Gibson, D.G.; Young, L.; Chuang, R.-Y.; Venter, J.C.; Hutchison, C.A.; Smith, H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Meth. 2009, 6, 343–345. [Google Scholar] [CrossRef]
- Holden, M.J.; Mayhew, M.P.; Gallagher, D.T.; Vilker, V.L. Chorismate lyase: Kinetics and engineering for stability. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 2002, 1594, 160–167. [Google Scholar] [CrossRef]
- Shiloach, J.; Kaufman, J.; Guillard, A.S.; Fass, R. Effect of glucose supply strategy on acetate accumulation, growth, and recombinant protein production by Escherichia coli BL21 (lambdaDE3) and Escherichia coli JM109. Biotechnol. Bioeng. 1996, 49, 421–428. [Google Scholar] [CrossRef]
- Postma, P.W.; Lengeler, J.W.; Jacobson, G.R. Phosphoenolpyruvate: Carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 1993, 57, 543–594. [Google Scholar] [CrossRef] [PubMed]
- Gosset, G. Improvement of Escherichia coli production strains by modification of the phosphoenolpyruvate: Sugar phosphotransferase system. Microb. Cell Fact. 2005, 4, 14. [Google Scholar] [CrossRef]
- Liu, S.P.; Liu, R.X.; Xiao, M.R.; Zhang, L.; Ding, Z.Y.; Gu, Z.H.; Shi, G.Y. A systems level engineered E. coli capable of efficiently producing L-phenylalanine. Proc. Biochem. 2014, 49, 751–757. [Google Scholar] [CrossRef]
- Kim, B.; Park, H.; Na, D.; Lee, S.Y. Metabolic engineering of Escherichia coli for the production of phenol from glucose. Biotechnol. J. 2013, 9, 621–629. [Google Scholar] [CrossRef]
- Flores, A.; Wang, X.; Nielsen, D.R. Recent trends in integrated bioprocesses: Aiding and expanding microbial biofuel/biochemical production. Curr. Opin. Biotechnol. 2019, 57, 82–87. [Google Scholar] [CrossRef]
- Brennan, T.C.R.; Turner, C.D.; Kromer, J.O.; Nielsen, L.K. Alleviating monoterpene toxicity using a two-phase extractive fermentation for the bioproduction of jet fuel mixtures in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2012, 109, 2513–2522. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Q.; Hou, H.; Wu, J.; Zheng, Z.; Ouyang, J. Efficient biosynthesis of cinnamyl alcohol by engineered Escherichia coli overexpressing carboxylic acid reductase in a biphasic system. Microb. Cell Fact. 2020, 19, 163. [Google Scholar] [CrossRef]
- Noda, S.; Shirai, T.; Oyama, S.; Kondo, A. Metabolic design of a platform Escherichia coli strain producing various chorismate derivatives. Metab. Eng. 2016, 33, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Wynands, B.; Lenzen, C.; Otto, M.; Koch, F.; Blank, L.M.; Wierckx, N. Metabolic engineering of Pseudomonas taiwanensis VLB120 with minimal genomic modifications for high-yield phenol production. Metab. Eng. 2018, 47, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gil, J.; Rodríguez-Concepción, M. Metabolic plasticity for isoprenoid biosynthesis in bacteria. Biochemical. J. 2013, 452, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, L. Biosynthesis of β-carotene in engineered E. coli using the MEP and MVA pathways. Microb. Cell Fact. 2014, 13, 160. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).