Abstract
The rapid growth of aquaculture and the lack of fish meal demand new sustainable ingredients. Although fungal biomass is found to be a promising sustainable fish feed supplementation candidate, the characteristics of this protein-rich source are closely influenced by the quality of the applied growth medium. In this work, the nutritional properties of pure filamentous fungal biomass provided from the cultivation of Aspergillus oryzae, Neurospora intermedia and Rhzopus oryzae were evaluated to assess their potential as alternative novel protein sources in fish feed. In this regard, fungal biomass yields of up to 0.19 ± 0.005 (g dry biomass/g substrate glucose) were obtained during submerged cultivation of fungal strains. The pure fungal biomass acquired could contain significant amounts of protein up to 62.2 ± 1.2% (w/w). The obtained protein had a high quality with notable inclusion of essential amino acids such as lysine, arginine, methionine and threonine with comparable concentrations to those of fish meal. Fungal biomass is mainly considered as protein source, however, entitlement of 6.9 ± 0.5, 4.0 ± 0.7 and 17.2 ± 1.1% (w/w) of lipids and ratio of polyunsaturated fatty acids (PUFA) to saturated fatty acids (SFA) of 1.37:1, 1.74:1 and 1.47:1 in A. oryzae, N. intermedia and R. oryzae, respectively, signal health benefits for the fish. Considering the results, protein-rich pure fungal biomass with amino acid composition is greatly compatible with fish meal, and contains essential nutrients such as fatty acids and minerals. This pure biomass constitutes a promising sustainable alternative supplement to be introduced in fish feed industry.
1. Introduction
World population is increasing dramatically and based on predictions more than 9 billion people will be living of earth by 2050 [1] Consequently, at least 60% more food supply, significantly protein sources, is needed to meet this population’s food demands [2]. In this regard, during the last decades, aquaculture, as one of the most important protein provision sources, had the highest expansion rate, 7.2% annually [2]. However, like other animal farming industries, aquaculture growth is directly challenged by a scarcity of feed ingredients, mainly fish meal as the major protein source in aquatic animal feed [3,4,5]. Limitations of supply, rapid expansion in different farming sectors and increase in the demand has resulted in incredible increases in fishmeal prices. Furthermore, utilization of food-grade protein source ingredient such as fishmeal and other agricultural products, e.g., soybean meal, sunflower meal, etc. for animal feed is not sustainable. Thus, finding a novel animal feed resources, which can be produced through a sustainable pathway, has gained great attentions, recently [4]. Numerous investigations have been conducted to identify potential ingredients that can be used as animal feed components. Among them, protein supplying sources are the most important compounds [4,6,7,8,9].
Microbial protein sources, including bacterial, algal and fungal biomass, have been suggested as a promising feed ingredient to be included in animal diet [10]. Among microbial resources, filamentous fungal biomass contains high protein content (50% w/w) and is a good source of essential fatty acids, vitamins, minerals, antioxidants and immunostimulants that makes it a potential source for fish feed supplementation [11]. Regarding the application of filamentous fungi for feed/food purposes, species such as Aspergillus spp. Neurospora spp. Rhizopus spp. have been categorized as Generally Regarded As Safe (GRAS) microorganisms and they have for long been used in food production [12]. For instance, Aspergillus spp. and Neurospora spp. have been used traditionally in indigenous East Asian foods such as koji, miso and oncom and beverages e.g., sake, shoyu and vinegar [13].
Filamentous fungi are ubiquitous microorganisms capable of degrading and consuming organic substrates such as dead bodies of animals and plants, food leftovers and industrial side streams for growth by using their complex enzymatic systems. This gives filamentous fungi the ability to grow easily on different low cost organic rich sources such as stillage, dairy byproducts, lignocellulosic residues, etc. Information about the nutritional properties and proximate composition of each feed ingredient is essential in order to evaluate its potential to meet animal dietary requirements. The nutritional properties of filamentous fungal biomass for aqua-feed applications have been reviewed by [14]. In a majority of studies, industrial residues have been used as filamentous fungi cultivation substrate, reducing the production cost. Improvement in the different fermentation approaches, such as submerged fermentation, have made it possible to produce high amounts of fungal biomass. However, its production still suffers from high production cost. Utilization of industrial waste, residues and byproducts as cultivation medium, significantly help reduce the production costs. It is proven that the cultivation media have a significant impact on the biomass composition of the cultivated microorganisms. This also applies to the substrates used for fungal cultivation [7,15]. Therefore, in the presence of unwanted chemicals, e.g., xenobiotic compounds, pesticides and polychlorinated biphenyls (PCBs), in the substrate, the fungal biomass composition can change and this may challenge its application as feed. For instance, accumulation of high concentrations of Ca, K and S have been reported in harvested fungal biomass cultivated in vinasse which is a byproduct from ethanol-producing industries [7]. The presence of high concentrations of such compounds in fungal biomass has different negative impacts on the nutritional properties of fungal biomass and can affect its digestibility rate and in some cases interfere with the physiological functions of enzymes and unbalance blood hemostasis which may result in suppressed growth [16]. Therefore, the characterization of fungal biomass grown on different organic-rich industrial side-streams varies broadly in different cases. In order to evaluate the eligibility of a particular fungal biomass, it is necessary to refer to the characterization of pure fungal biomass cultivated on defined media. In this regard, semi-synthetic media rich in essential carbohydrates (e.g., glucose) and nitrogen sources (yeast extract, peptone etc.), which are free of unwanted chemicals, can ensure that cultivation media have no unsavory impacts on the produced fungal biomass.
The aim of this work was to characterize nutritional properties of pure fungal biomass in order to evaluate its potential as an alternative aqua-feed ingredient. Semi-synthetic medium has been used as filamentous fungi cultivation reference medium and for the sake of further comparison and evaluation of the feed supplementation potential, the nutritional value of the produced fungal biomass (A. oryzae, R. oryzae and N. intermedia) including protein, lipid and minerals have been comprehensively evaluated, to assess its potential as alternative source for fish meal in aquaculture.
2. Materials and Methods
2.1. Cultivation Medium
Cultivation was carried out on semi-synthetic medium containing 30 gL−1 glucose and 5 gL−1 yeast extract as major carbon and nitrogen source, respectively. Trace elements including (NH4)2SO4, KH2PO4, CaCl2·2H2O and MgSO4·7H2O with the concentrations of 7.5, 3.5, 1.0 and 0.75 gL−1, respectively, were added to the cultivation medium to support filamentous fungi maximum growth.
2.2. Fungi Species
Spores for three filamentous fungi including Ascomycetes (Aspergillus oryzae CBS 819.72, Neurospora intermedia CBS 131.92) and a Zygomycete (Rhizopus oryzae CCUG 61147) (Culture Collection, University of Gothenburg, Sweden) were collected from the University of Gothenburg microbial culture collection (Gothenburg, Sweden). All these filamentous fungi species were edible food-grade and can be used as food and feed ingredients. Potato dextrose agar (PDA) plates composed of 4 gL−1 potato extract, 20 gL−1 glucose and 15 gL−1 agar has been used in order to preserve the spores; once the plates were inoculated with each individual filamentous fungi spores, they have been incubated at 30 °C for 3–5 days followed by storing in the fridge (4 °C) till the use as cultivation inoculum. For preparation of inoculum solution, 20 mL of sterile distilled water were added to each PDA plate. The spores were then released using gentle agitation and scraping the culture surface. The supernatant mixture containing spores with concentration of 3.9 × 105–3.8 × 106 spore/mL were collected and 500 µL of this mixture was added to a 250 mL shake flask containing 100 mL of cultivation medium. pH was adjusted by adding required amount of H2SO4 (2M) and NaOH (2M) to achieve the required pH.
2.3. Cultivation in Shake-Flasks
Cultivations were carried out in 250 mL Erlenmeyer’s shake-flasks containing 100 mL of synthetic medium for 72 h. Before inoculation, 20 min autoclaving at 121 °C was carried out to prevent the presence of unwanted microorganisms in the cultures. Cultivation was performed in water bath at 35 °C and 125 rpm (9 mm orbital shaking radius). From each flask, 3 mL samples were taken every 24 h (0 h, 24 h, 48 h and 72 h) to detect pH fluctuations, ethanol production and sugar consumption. Then, they were centrifuged at 20 g for 10 min and filtered using 0.2 µm filters and supernatant solution was stored at −18 °C to be analyzed later using HPLC. At the end of the 72 h cultivation period, the fungal biomass was separated carefully by means of a fine mesh sieve (1 mm2) and washed several times with distilled water. Fungal biomass was then kept in an oven at 70 °C for 24 h (reaching a constant weight) for drying.
2.4. Analytical Procedures
Harvested biomass was measured in gL−1 (dry biomass) and biomass yield (g dry biomass/g substrate glucose). The National Renewable Energy Laboratory (NREL) method was used to evaluate ash content [17]. Metabolite production and consumption including ethanol, glucose and sugar-mix, during the cultivation was monitored using HPLC. For this purpose, an analytical hydrogen-based ion exchange column (Aminex HPX-87H, 250 × 4 mm, Bio-Rad, Hercules, CA, USA) was used. The mobile phase consisted of 5 mM H2SO4 with an elution rate of 0.6 mL/min at 60 °C. The HPLC-system consisted of a Waters Alliance separation module 2695 (Waters Corporation, Milford, MA, USA) equipped with a refractive index (RI) detector (Waters 2414) operating at 210 nm wavelength.
The fungal spore concentration was estimated using a Bürker counting chamber under a light microscope (Axiostar plus, Carl Zeiss, Oberkochen, Germany). Minerals content was evaluated according to the method described by [18] using a plasma emission spectrometer (Spectro Analytical Instruments GmbH & Co., Kleve, Germany) with samples extracted with 7M HNO3. The Kjeldahl method was employed to determine total nitrogen (N), and a 2020 digester, and a 2400 Kjeltec Analyser unit (FOSS Analytical A/S, Hilleröd, Denmark) was used for this purpose. The N value from the Kjeldahl assay was multiplied by a factor of 6.25 to measure crude protein content [19]. The amino acid composition in the fungal biomass was determined in accredited laboratory (Eurofins, Lidköping, Sweden) using the SS-EN ISO13903:2005 method. The Official Journal of the European Communities (1984) procedure was used to measure crude fat levels in the fungal biomass. This method was performed using an extraction unit (1047 Hydrolysing Unit and a Soxtec System HT 1043, FOSS Analytical A/S) and then lipids were extracted according to the Folch method with chloroform:MeOH (2:1 v/v) [20]. Methylation of fatty acids methyl esters (FAME) were done by the method described by [21]. The lipids were quantified using methyl 15-methylheptadecanoate (C17:0) as an internal standard (Larodan, Karolinska Institutet Science Park, Solna, Sweden). FAME were analyzed with a GC system (6890, Agilent, Santa clara, Cal, U.S.A.) equipped with a flame ionization detector (FID), split/splitless capillary inlet and 7683 autosampler using a SGE BPX70 capillary column (50 m Ø 0.22 mm/0.25 µ film thickness).
2.5. Statistical Analysis
The data were analyzed by one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test to compare mean values. R statistical software version 3.6 was used. Significance was at the level of p < 0.05 and all the results are presented as mean ± SD.
3. Results and Discussions
In order to find the optimum cultivation condition and the best choice of fungal strain yielding desirable nutritional value, three different edible filamentous fungi were cultivated on semi-synthetic medium. In this regard, the growth performance of the three fungal species A. oryzae, N. intermedia and R. oryzae on substrate was evaluated and the nutritional potentials of the harvested fungal biomass regarding protein content and quality, fatty acid composition and mineral constituents was investigated.
3.1. Filamentous Fungi Cultivation
The growth behavior of the three fungal species on semi-synthetic medium with initial pH values of 5.0, 5.5, 6.0 and 6.5 was evaluated (Figure 1). As presented in Figure 1, the maximum biomass yields were different based on the fungal species and media pH. The highest biomass yield for A. oryzae (0.19 ± 0.005), N. intermedia (0.10 ± 0.006) and R. oryzae (0.08 ± 0.002) (g dry biomass/g substrate glucose) were obtained at pH 6.5, 5.5 and 5, respectively, which were significantly different from other pH values. Although A. oryzae yielded the highest amount of final biomass, it was the slowest species among all three in glucose consumption. As presented in Figure 2, it took A. oryzae twice the time (48 h) to completely consume the carbon source compared to the two other species (24 h). Regarding metabolite production, the maximum ethanol production on substrate was obtained by N. intermedia and R. oryzae and in the last place A. oryzae (Figure 2).
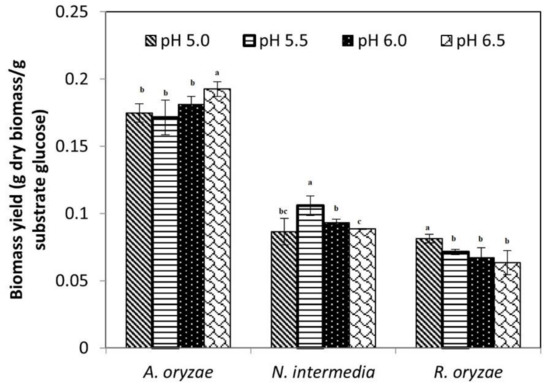
Figure 1.
Biomass yield (g dry biomass/g substrate glucose) of A. oryzae, N. intermedia and R. oryzae on semi-synthetic medium in different initial pH (5.0, 5.5, 6.0 and 6.5). Different letters indicate statistically significant differences among experimental groups (p < 0.05). Values are presented as mean—SD.
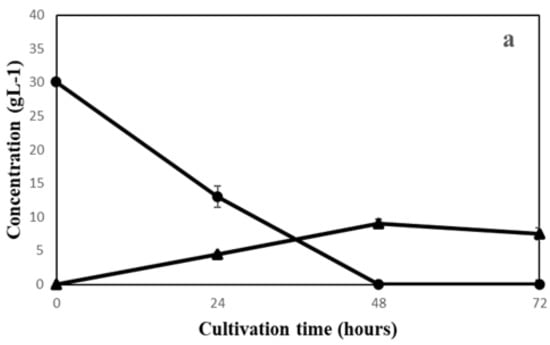
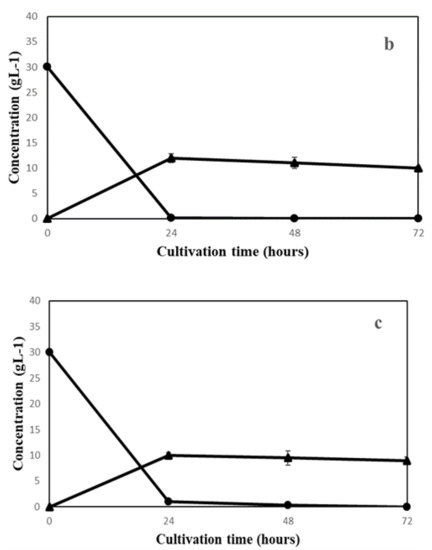
Figure 2.
Sugar utilization and ethanol production of (a) A. oryzae (pH = 6.5), (b) N. intermedia (pH = 5.5) and (c) R. oryzae (pH = 5). (glucose (●) and ethanol (▲)).
The relatively high yield of ethanol can be considered as an important byproduct of the fungal biomass production in e.g., a biorefinery. However, in biomass production for feed applications, low metabolites and high biomass production is the ultimate goal. Even though, it seems that it is not easy to zero side-metabolite production (e.g., ethanol) during the fermentation, optimization of aeration can reduce its synthesize and shift the cultivation toward higher biomass production [12]. In the current study, shake flask cultivation was performed (no external aeration), however, it can be expected that cultivation in the bioreactors with the aeration may result in lesser ethanol production and higher biomass yield.
3.2. Nutritional Properties of Fungal Biomass
Fungal biomass, similar to other single cell proteins (e.g., bacteria and microalgae), contains variable amounts of organic and inorganic constituents such as proteins, lipids, minerals, etc. Evaluation of these micro- and macronutrients is of particular importance to define its supplementation potential as a feed ingredient. In the following sections different quantitative and qualitative analyses of various fungal biomass constituents have been performed with a view to examine the application in fish feed.
3.2.1. Protein
Considering the protein content of the three fungal species grown on the semi-synthetic media, it was observed that N. intermedia contained the highest level of crude protein (62.2 ± 1.2%), followed by R. oryzae and A. oryzae (50.6 ± 2.0% and 45.7 ± 0.8%, respectively). Fish meal and soybean meal, the major protein sources in fish feed contain 62.6% and 43.3% (w/w) crude protein, respectively. From this point of view, with a similar protein level as fish meal, N. intermedia is the most valuable protein source that can be considered as an alternative protein source in fish feed to replace fish meal and soybean meal. The protein content in R. oryzae and A. oryzae are of secondary interest for meeting the dietary protein requirement of fish. In the newly developed feed formulations (high energy feed formulation), higher fat concentrations are used. Accordingly, protein constituents in the feed are not consumed by fish to supply energy and can be retained in the body tissue and support fish growth and health (protein-sparing effect). However, still provision of 26–55% protein in the diet is recommended to ensure optimum growth rates [17].
In addition to crude protein (CP) content, protein quality is of central importance in fish nutrition. The amino acid profiles of the cultivated fungi species (Table 1) showed that filamentous fungal biomass contains considerable levels of indispensable amino acids (IAAs—amino acids that animals cannot synthesize de novo) as well as dispensable amino acids (DAAs—amino acids that animals can synthesize in their body). A. oryzae, N. intermedia and R. oryzae possess 46.8%, 48.1 and 49.0% (w/w) IAAs that determine the fungal protein quality. Glutamine, asparagine, leucine, lysine, alanine and arginine were the six most frequent amino acids in the studied fungal biomass. Only valine was higher than arginine in R. oryzae. Considering the most abundant amino acids in fish meal (glutamine, asparagine, glycine, lysine, leucine and alanine) the only difference lies in the higher concentration of glycine in fish meal compared to the fungal biomass. Each amino acid has its own key functions in the fish body, however, lysine and arginine are commonly considered as the most important amino acids that must be supplied in proper concentrations in fish feed [21]. Lysine is the first limiting amino acid in fish feed, and its deficiency is one of the main reasons limiting the application of plant-based protein sources such as soybean meal in fish feed supplementation. Conversely, fungal biomass contains considerable levels of lysine and arginine. Lysine content in fungal biomass is much higher than in soybean meal and rather comparable to fish meal. The biomass arginine content is comparable to that of fish meal and soybean meal. It was observed that only R. oryzae contains low concentrations of arginine. Lysine has lots of key functions in the body such as acid-base balance maintenance, modification of fat metabolism throughout carnitine synthesis, role in osmoregulation, especially in migrations etc. [22]. Like lysine, arginine plays a role in protein synthesis as well as in anabolic processes by using stimulation of different hormones such as insulin and growth hormone (GH). Furthermore, metabolism of a number of other amino acids such as proline and glutamine is affected by arginine [23]. It has been recently shown that the fish immune system is affected by the arginine content of the feed [24]. Considering concentrations of lysine and arginine in fungal biomass, we concluded that fungal biomass can meet the requirements for these amino acids (3–6% of crude proteins) [21]. Leucine, the other highly concentrated amino acid in fungal biomass, is required in the diet to stimulate muscle protein synthesis and protect muscles from proteolysis [25]. The amount of leucine in fungal biomass is comparable to both fish meal and soybean meal.

Table 1.
Proximate composition and amino acid profile of A. oryzae, N. intermedia and R. oryzae, fish meal and soybean. Fish meal and Soybean meal.
Although, the presence of IAAs in the diet is essential, DAAs also play several key roles in the fish body. As two of most abundant amino acids in feed, glutamine and asparagine are involved in a large number of processes in the body. Glutamine plays a role in protein synthesis, acid-base balance regulation, and both purine and pyrimidine nucleotides synthesis [21,26]. Fungal biomass contains similar levels of both glutamine and asparagine as fish meal, and can thus avoid the symptoms arising from the deficiency of these amino acids such as weight loss, decreased feed intake, intestinal problems and mortality during seasonal and reproductive migrations [27,28] stated that during fish migration, fish that are fed with sufficient asparagine can tolerate changes in environmental factors and salinity. One of the preferences of fungal biomass over fish meal and soybean meal is its higher content of alanine. This issue can result in more energy provision and an increased feeding rate due to the stimulatory effects of alanine [29]. Moreover, as alanine is a major substance required in gluconeogenesis and is a good carrier of nitrogen, it has great impact on amino acid metabolism [30]. Valine together with leucine and isoleucine (branched chain amino acids, BCAAs) are deposited in body proteins, particularly in skeletal muscles, and have key roles in determination of 3D shapes of structural proteins. In addition, valine is involved in the synthesis of myelin that covers nerves [31]. The determined valine content of fungal biomass is higher than those of soybean meal and fish meal.
The amino acid threonine is involved in a variety of immunological responses. This is due to the effect of this amino acid in the maintenance of intestinal and mucosal barriers [32]. Normally, soybean meal, like other plant-based proteins, contains lower concentrations of threonine than the studied filamentous fungal biomass. As a result, fungal biomass has the potential to improve the immunological traits of the fish immunity system. While the methionine and cysteine contents of fungal biomass were comparable to those of fish meal, soybean meal has lower content of these two important sulfur-based amino acids. Methionine is usually one of the major limiting amino acids in many of fish diets, particularly when high concentrations of plant-based proteins are included [33].
In comparison to fish meal, the analyzed fungal biomass only lacked a high content of glycine and proline, whereas level of other amino acids are equal and even higher in fungal biomass. In recent years, soybean meal has represented the main alternative protein source for fish meal. Considering the richness of lysine, alanine, glycine, methionine, cysteine, threonine, valine and tyrosine in fungal biomass compared to soybean meal, fungal biomass can be suggested as a more suitable and interesting novel supplementation protein source. Except for glutamine, asparagine and proline (DAAs) that were present at higher amount in soybean meal, the concentration of other amino acids are comparable for fungal biomass and soybean meal.
3.2.2. Lipids
In addition to protein and amino acid content of fungal biomass, the lipid and fatty acid compositions of fungal biomass were evaluated in this study and are summarized in Table 2 and Table 3. R. oryzae contained the highest level of (17.2 ± 1.1% w/w) of lipids, followed by A. oryzae (6.9 ± 0.5%) and N. intermedia (4.0 ± 0.7%). [34] has showed that different species of filamentous fungi can accumulate different amount of intercellular lipids in the biomass. The authors illustrated that the fat accumulation rate can be affected by the substrate, cultivation conditions and different fungal species. Although generally filamentous fungal biomass is considered as potential alternative protein sources for fishmeal, noticeable fat inclusion in fungal biomass, particularly in the case of R. oryzae and A. oryzae, it could be a promising dietary lipid supplement in fish feed. Basically fish and other plant-derived oils provide the lipid requirement in aqua feed, however, like fish meal, fish oil, which is still the major fat ingredient in fish feed, is a limited resource. It must be mentioned that fungal biomass, similar to soybean meal, is not considered a lipid source in fish feed but taking advantage of the good lipid and fatty acid presence in fungal biomass can improve the nutritional quality of fish diets. There are still doubts about the optimum level of lipid inclusion in fish and other aquatic animals feed but it is clear that they are needed to provide energy and reduce protein oxidization as an energy source (protein-sparing effect) [16]. It was reported that up to 20% of lipid sources can be easily taken up by a majority of fish [21]. Complex lipids that are the major part of lipid sources contain fatty acids that serve several key roles in the body [32]. According to the data presented in Table 2, linoleic acid (C18:2) (LA), oleic acid (C18:1) and palmitic acid (C16:0) accounted for the highest percentages of fatty acids in all three studied species. The primitive function of different fatty acids is to provide the energy for the organism via oxidation [21].

Table 2.
Fatty acid profile of A. oryzae, N. intermedia and R. oryzae, fish meal and soybean meal. Fish meal and Soybean meal [35].

Table 3.
Macromineral content of A. oryzae, N. intermedia and R. oryzae, fish meal and soybean meal.
Provision of appropriate concentrations of required fatty acids, particularly polyunsaturated fatty acids (PUFAs), is critically important for optimum fish growth. It has been confirmed that all fish species have their own specific requirements for fatty acids [37]. Recently, the anti-oxidant and feeding stimulatory effects of palmitic acid and oleic acid were confirmed [37]. Furthermore, it is broadly accepted that a number of fatty acids have health beneficial properties [38,39,40,41]. In this regard, PUFAs such as LA, α-linolenic acid (ALA) and arachidonic acid (ARA) are the most important fatty acids. A. oryzae, N. intermedia and R. oryzae contain 24.77, 10.32 and 65.25 (gKg−1) LA and 0.2, 0.77 and 0.82 (gKg−1) ALA, respectively. These data shows that fungal biomass is a very valuable source for ALA comparing with both fish meal and soybean meal that contain only 1.4 and 6.8 LA and 1.3 and 0.9 ALA [35]. LA and ALA represent the two major key substances involve in ω6 and ω3 fatty acid synthesis. It is confirmed that most of fresh water fish species are capable to bio-convert ALA to long chain PUFAs (LC-PUFAs), such as eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic (DHA; 22:4n-6), and LA to ARA [22]. Therefore, regardless of fish life stage, supplementation of 1% of each LA and ALA can meet the requirements for highly unsaturated fatty acids (HUFAs) [16]. Due to the fact that many farmed fish, including marine fish and larvae, are poor in synthesizing LC-PUFA such as DHA and EPA from other PUFAs, similar to other tested alternative protein sources, it cannot be considered as a single fat supplier to the feed, however, possessing other functional nutrient such as EAAs emphasizes its suitability for addition as a protein source in the fish feed [40,42]. Inclusion of the required EFAs and particularly PUFAs have resulted in improved growth in several fish species, including salmonids, channel catfish, halibut etc. [43]. Moreover, this supplementation can improve egg quality parameters such as hatching, fertilization rate and survival status [44,45]. Since all three studied fungal species contained high concentrations of LA, it seems that dietary inclusion of fungal biomass in the diet of fish species that have high capacity to utilize LA to produce ARA can boost the health-related benefits. ARA is a very important PUFA that is critical for producing eicosanoids [46]. Eicosanoids are important substances in immune responses and cell signaling. LC-PUFAs are important in controlling and regulating cell membrane fluidity and also have unique properties to support immune system, growth, health and performance, cell membrane fluidity, brain signaling, motivational processes, stress responses, skeletal muscle development and energy balance [47,48]. Dietary deficiency of these essential fatty acids results in various pathologies, the animal stops growing and reproducing and eventually dies [49]. In conclusion, apart from nutritional quality of fungal biomass from protein standing point, possessing of valuable fatty acids can have beneficial properties for fish.
3.2.3. Minerals
The minerals contents of fungal biomass, including Ca, K, Mg, Na, P and S, are presented in Table 3. The minerals with the highest concentration in fungal biomass were P, K and S, respectively. Dietary mineral requirement is highly variable among fish and crustacean species. From a nutritional point of view, supplementation of minerals is very important especially in crustacean rearing and breeding practices. P and K are considered as macronutrients in fish nutrition and according to the [22], the P and K dietary requirement of fish and shrimp range from 5–13 and 2–12 gKg−1 of feed, respectively. Fungal biomass contains high amounts of these minerals (P range 12.4–21.2, K range 9.3–11.3 except for R. oryzae, that contains 1.3 and S range 3.1–4.6 (gKg−1)) and can satisfy the dietary requirements of fish and shrimps. Macro-minerals have vital functions in the animal body such as being a component of skeletal and other hard tissues such as the exoskeleton, teeth, fin rays etc., electron transfer, acid:base balance regulation, cell membrane properties and osmoregulation [50]. In cases where these minerals are not supplied in sufficient levels in the diet, the organism would be susceptible to different pathological problems [51]. For example, deficiency in P will lead to reduction in growth capacity and feed conversion, skeletal malformation, intermediary metabolism impairment, reduction in tissue hardness, reduction in antibody production and reduced weight gain etc. [52,53].
Phosphorous is one of major mineral components of a complete diet. Phosphorous is a component of nucleotides, skeletal tissues, phospholipids, coenzymes, DNA, RNA and special enzymes involved in energy production [53,54]. Moreover, P has a buffering effect and helps organisms maintain a normal pH [52]. Potassium is an essential macromineral required for a balanced acid:base equilibrium, osmoregulation, and maintaining muscle and nerve activity [55]. Compared to fish meal, A. oryzae and N. intermedia biomass have higher K content. However, fish meal is richer in other mineral elements. On the other hand, fungal biomass is superior to soybean meal considering the higher content of Na and P.
4. Conclusions
The nutritional potential of filamentous fungal biomass as a dietary supplement of fish feed has been investigated in the current study. Protein-rich fungal biomass has been produced throughout submerged cultivation of three edible filamentous fungi on a semi-synthetic medium. Considering the high protein content (range 45–62% (w/w)) and suitable amino acid profile, filamentous fungal biomass can be a promising protein source supplement. Along with the high protein content and desirable amino acid profile that were strongly compatible with fish meal, the fatty acid composition and mineral content of the obtained fungal biomass confirm the feed dietary potential of fungal biomass. Therefore, it is a great possibility that supplementation with fungal biomass can address the challenges arising from the current fish meal provision bottleneck and help achieve sustainable aquaculture growth.
Author Contributions
Conceptualization, M.J.T., N.M.S.; methodology, A.M., S.K., T.L., J.A.F.; Data curation, S.K., T.L., M.J.T.; validation, S.K., M.J.T., N.M.S.; formal analysis, T.L., S.K.; resources, M.J.T., N.M.S., T.L.; writing—originaldraft preparation, S.K., A.M.; writing—review and editing, M.J.T., N.M.S., T.L., A.K., J.A.F.; supervision, M.J.T., N.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data availability is according to MDPI Research Data Policies.
Acknowledgments
The authors would like to express their gratitude to Swedish Economic and Regional Growth (Tillväxtverket) through a European Regional Development Fund “Ways2Taste,” Lantmännen Agroetanol (Sweden), Swedish University of Agricultural Sciences (SLU, Sweden) and Isfahan University of Technology (Iran) for their technical and financial support of this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- United Nations. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables; United Nations: New York, NY, USA, 2017. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2018. [Google Scholar]
- Oliva-Teles, A.; Enes, P.; Peres, H. Replacing fishmeal and fish oil in industrial aquafeeds for carnivorous fish. In Feed and Feeding Practices in Aquaculture; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 203–233. [Google Scholar]
- Randazzo, B.; Zarantoniello, M.; Cardinaletti, G.; Cerri, R.; Giorgini, E.; Belloni, A.; Contò, M.; Tibaldi, E.; Olivotto, I. Hermetia illucens and poultry by-product meals as alternatives to plant protein sources in gilthead seabream (Sparus aurata) diet: A multidisciplinary study on fish gut status. Animals 2021, 11, 677. [Google Scholar] [CrossRef] [PubMed]
- Tacon, A.G.; Metian, M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Karimi, S.; Ferreira, J.A.; Taherzadeh, M.J. The application of fungal biomass as feed. In Encyclopedia of Mycology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 601–612. [Google Scholar] [CrossRef]
- Karimi, S.; Soofiani, N.M.; Lundh, T.; Mahboubi, A.; Kiessling, A.; Taherzadeh, M.J. Evaluation of filamentous fungal biomass cultivated on vinasse as an alternative nutrient source of fish feed: Protein, lipid, and mineral composition. Fermentation 2019, 5, 99. [Google Scholar] [CrossRef] [Green Version]
- Øverland, M.; Karlsson, A.; Mydland, L.T.; Romarheim, O.H.; Skrede, A. Evaluation of Candida utilis, Kluyveromyces marxianus and Saccharomyces cerevisiae yeasts as protein sources in diets for Atlantic salmon (Salmo salar). Aquaculture 2013, 402, 1–7. [Google Scholar] [CrossRef]
- Gasco, L.; Gai, F.; Maricchiolo, G.; Genovese, L.; Ragonese, S.; Bottari, T.; Caruso, G. Feeds for the Aquaculture Sector—Current Situation and Alternative Sources; Springer International Publishing: New York, NY, USA, 2018. [Google Scholar]
- Nalage, D.; Khedkar, G.; Kalyankar, A.; Sarkate, A.; Ghodke, S.; Bedre, V. Single cell proteins. In Encyclopedia of Food and Health, 1st ed.; Oxford Academic Press: London, UK, 2016; pp. 790–794. [Google Scholar]
- Karimi, S.; Soofiani, N.M.; Mahboubi, A.; Taherzadeh, M.J. Use of organic wastes and industrial by-products to produce filamentous fungi with potential as aqua-feed ingredients. Sustainability 2018, 10, 3296. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, J.A.; Mahboubi, A.; Lennartsson, P.R.; Taherzadeh, M.J. Waste biorefineries using filamentous ascomycetes fungi: Present status and future prospects. Bioresour. Technol. 2016, 215, 334–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lennartsson, P.R. Zygomycetes and Cellulose Residuals: Hydrolysis, Cultivation and Applications. Ph.D. Thesis, Chalmers Tekniska Högskola, Göteborg, Sweden, 2012. [Google Scholar]
- Cavka, A.; Jönsson, L.J. Comparison of the growth of filamentous fungi and yeasts in lignocellulose-derived media. Biocatal. Agric. Biotechnol. 2014, 3, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Mahboubi, A.; Ferreira, J.A.; Taherzadeh, M.J.; Lennartsson, P.R. Production of fungal biomass for feed, fatty acids, and glycerol by Aspergillus oryzae from fat-rich dairy substrates. Fermentation 2017, 3, 48. [Google Scholar] [CrossRef]
- Halver, J.E.; Hardy, R.W. Fish Nutrition; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. In Laboratory Analytical Procedure (LAP); NREL: Golden, CO, USA, 2008. [Google Scholar]
- Langeland, M.; Vidakovic, A.; Vielma, J.; Lindberg, J.E.; Kiessling, A.; Lundh, T. Digestibility of microbial and mussel meal for Arctic charr (Salvelinus alpinus) and Eurasian perch (Perca fluviatilis). Aquac. Nutr. 2016, 22, 485–495. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Appelqvist, L.-Å. Rapid methods of lipid extraction and fatty acid methyl ester preparation for seed and leaf tissue with special remarks on preventing the accumulation of lipid contaminants. Ark. Kemi 1968, 28, 551–570. [Google Scholar]
- NRC. Nutrient Requirements of Fish and Shrimp; 978-0-309-47322-4; The National Academies Press: Washington, DC, USA, 2011; p. 392. [Google Scholar]
- Ovie, S.; Eze, S. Lysine requirement and its effect on the body composition of Oreochromis niloticus fingerlings. J. Fish. Aquat. Sci. 2012, 8, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Zhao, H.; Huang, Y.; Cao, J.; Wang, G.; Sun, Y.; Li, Y. Effects of dietary arginine levels on growth performance, body composition, serum biochemical indices and resistance ability against ammonia-nitrogen stress in juvenile yellow catfish (Pelteobagrus fulvidraco). Anim. Nutr. 2016, 2, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, N.; Qiu, X.; Zhao, M.; Jin, L. Arginine requirement and effect of arginine intake on immunity in largemouth bass, Micropterus salmoides. Aquac. Nutr. 2011, 18, 107–116. [Google Scholar] [CrossRef]
- Azizi, S.; Nematollahi, M.A.; Mojazi Amiri, B.; Vélez, E.; Lutfi Royo, E.; Navarro, I.; Gutierrez, J. Lysine and leucine deficiencies affect myocytes development and IGF signaling in gilthead sea bream (Sparus aurata). PLoS ONE 2016, 11, e0147618. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Mai, K.; Xu, W.; Zhang, Y.; Zhou, H.; Ai, Q. Effects of dietary glutamine on survival, growth performance, activities of digestive enzme, antioxidant status and hypoxia stress resistance of half-smooth tongue sole (Cynoglossus semilaevis Günther) post larvae. Aquaculture 2015, 446, 48–56. [Google Scholar] [CrossRef]
- Mohanty, B.; Mahanty, A.; Ganguly, S.; Sankar, T.V.; Chakraborty, K.; Rangasamy, A.; Sharma, A. Amino acid compositions of 27 food fishes and their importance in clinical nutrition. J. Amino Acids 2014, 2014. [Google Scholar] [CrossRef]
- Bystriansky, J.S.; Frick, N.T.; Ballantyne, J.S. Intermediary metabolism of Arctic char Salvelinus alpinus during short-term salinity exposure. J. Exp. Biol. 2007, 210, 1971–1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, T.; Matsunari, H.; Sugita, T.; Furuita, H.; Masumoto, T.; Iwashita, Y.; Amano, S.; Suzuki, N. Optimization of the supplemental essential amino acids to a fish meal-free diet based on fermented soybean meal for rainbow trout Oncorhynchus mykiss. Fish. Sci. 2012, 78, 359–366. [Google Scholar] [CrossRef]
- Jones, N.R. The free amino acids of fish. 1-Methylhistidine and β-alanine liberation by skeletal muscle anserinase of codling (Gadus callarias). Biochem. J. 1955, 60, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Tan, X.-H.; Zhou, C.-P.; Yang, Y.-K.; Qi, C.-L.; Zhao, S.-Y.; Lin, H.-Z. Effect of dietary valine levels on the growth performance, feed utilization and immune function of juvenile golden pompano, Trachinotus ovatus. Aquac. Nutr. 2018, 24, 74–82. [Google Scholar] [CrossRef]
- Cowey, C.; Sargent, J. Lipid nutrition in fish. Comp. Biochem. Physiol. Part B Comp. Biochem. 1977, 57, 269–273. [Google Scholar] [CrossRef]
- Wu, P.; Jiang, W.; Hu, K.; Liu, Y.; Jiang, J.; Kuang, S.; Tang, L.; Tang, W.; Zhang, Y.; Zhou, X.; et al. The relationship between dietary methionine and growth, digestion, absorption, and antioxidant status in intestinal and hepatopancreatic tissues of sub-adult grass carp (Ctenopharyngodon idella). J. Anim. Sci. Biotechnol. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meletiadis, J.; Meis, J.F.G.M.; Mouton, J.W.; Verweij, P.E. Analysis of growth characteristics of filamentous fungi in different nutrient media. J. Clin. Microbiol. 2001, 39, 478. [Google Scholar] [CrossRef] [Green Version]
- Sauvant, D.; Perez, J.-M.; Tran, G. Tables of Composition and Nutritional Value of Feed Materials: Pigs, Poultry, Cattle, Sheep, Goats, Rabbits, Horses and Fish; Wageningen Academic Publishers: Wageningen, The Netherlands, 2004. [Google Scholar]
- Randazzo, B.; Zarantoniello, M.; Gioacchini, G.; Giorgini, E.; Truzzi, C.; Notarstefano, V.; Cardinaletti, G.; Huyen, K.T.; Carnevali, O.; Olivotto, I. Can insect-based diets affect zebrafish (Danio rerio) reproduction? A Multidisciplinary Study. Zebrafish 2020, 17, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Elagbar, Z.A.; Naik, R.R.; Shakya, A.K.; Bardaweel, S.K. Fatty Acids Analysis, Antioxidant and Biological Activity of Fixed Oil of Annona muricata L. Seeds. J. Chem. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Izquierdo, M.S. Essential fatty acid requirements of cultured marine fish larvae. Aquac. Nutr. 1996, 2, 183–191. [Google Scholar] [CrossRef]
- Sargent, J.; Bell, G.; McEvoy, L.; Tocher, D.; Estévez, A. Recent developments in the essential fatty acid nutrition of fish. Aquaculture 1999, 177, 191–199. [Google Scholar] [CrossRef]
- Tocher, D. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac. Res. 2010, 41, 717–732. [Google Scholar] [CrossRef]
- Watanabe, T. Lipid nutrition in fish. Comp. Biochem. Physiol. Part B Comp. Biochem. 1982, 73, 3–15. [Google Scholar] [CrossRef]
- Zarantoniello, M.; Randazzo, B.; Nozzi, V.; Truzzi, C.; Giorgini, E.; Cardinaletti, G.; Freddi, L.; Ratti, S.; Girolametti, F.; Osimani, A.; et al. Physiological responses of Siberian sturgeon (Acipenser baerii) juveniles fed on full-fat insect-based diet in an aquaponic system. Sci. Rep. 2021, 11, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.; Sargent, J.R. Arachidonic acid in aquaculture feeds: Current status and future opportunities. Aquaculture 2003, 218, 491–499. [Google Scholar] [CrossRef]
- Rodriguez, C.; Cejas, J.; Martín, M.V.; Badia, P.; Samper, M.; Lorenzo, A. Influence of n-3 highly unsaturated fatty acid deficiency on the lipid composition of broodstock gilthead seabream (Sparus aurata L.) and on egg quality. Fish. Physiol. Biochem. 1998, 18, 177–187. [Google Scholar] [CrossRef]
- Salze, G.; Tocher, D.R.; Roy, W.J.; A Robertson, D. Egg quality determinants in cod (Gadus morhua L.): Egg performance and lipids in eggs from farmed and wild broodstock. Aquac. Res. 2005, 36, 1488–1499. [Google Scholar] [CrossRef] [Green Version]
- Hamre, K.; Yúfera, M.; Ronnestad, I.; Boglione, C.; Conceicao, L.; Izquierdo, M. Fish larval nutrition and feed formulation: Knowledge gaps and bottlenecks for advances in larval rearing. Rev. Aquac. 2013, 5, S26–S58. [Google Scholar] [CrossRef] [Green Version]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods—A review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norambuena, F.; Morais, S.; Emery, J.A.; Turchini, G. Arachidonic acid and eicosapentaenoic acid metabolism in juvenile atlantic salmon as affected by water temperature. PLoS ONE 2015, 10, e0143622. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, M.; Socorro, J.; Arantzamendi, L.; Hernandez-Cruz, C.M. Recent advances in lipid nutrition in fish larvae. Fish Physiol. Biochem. 2000, 22, 97–107. [Google Scholar] [CrossRef]
- Joint FAO/World Health Organization. Vitamin and Mineral Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- He, F.J.; MacGregor, G.A. Beneficial effects of potassium on human health. Physiol. Plant. 2008, 133, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Cashman, K. Minerals and trace elements. Nutr. Prim. Care Provid. 2015, 111, 45–52. [Google Scholar] [CrossRef]
- Yamauchi, M.; Anderson, J.J.B.; Garner, S.C. Calcium and Phosphorus in Health and Disease; CRC Press: New York, NY, USA, 1996. [Google Scholar]
- Takeda, E.; Yamamoto, H.; Yamanaka-Okumura, H.; Taketani, Y. Dietary phosphorus in bone health and quality of life. Nutr. Rev. 2012, 70, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.; Gatlin, D.M., III. Dietary mineral requirement of fish and marine crustaceans. Rev. Fisher. Sci. 1996, 4, 75–99. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).