Abstract
The objective of the present study was to evaluate the potential antioxidant and angiotensin converting enzyme inhibition (ACEI) activity of edible insect flours fermented with Lactococcus lactis strains. For the fermentation, mealworm and grasshoppers flours were dissolved (0.5% w/v) in buffer solution (pH 7.0) and individually inoculated (3%) with Lactococcus lactis strains (NRRL B-50571, NRRL B-50572). The samples were incubated for 72 h at 30 °C, and the pH was recorded. The degree of hydrolysis (DH) and protein content were determined. The total polyphenol compounds, antioxidant activity (ABTS, DPPH, ORAC, and FRAP), and ACEI of the <3 kDa fractions were analyzed. The pH of the fermented samples decreased to 3.5–3.9 (p < 0.05). The fermented grasshopper flour showed an increased DH (0.42%) and overall higher total polyphenol content (8.23 mg Gallic Acid Equivalent/mL). In general, the highest antioxidant activity was for the grasshopper fractions fermented for 24 h by Lactococcus lactis NRRL B-50572, which also showed 23.47% ACEI inhibition with an IC50 of 0.97 mg/mL. The peptide profile obtained increased after fermentation, being higher for the mealworm flour fermented sample. This study presents, for the first time, the use of specific strains of Lactococus lactis for fermenting edible insect-derived products in the production of bioactive compounds with potential antioxidant and antihypertensive activity.
1. Introduction
Different types of insects, raw or processed, have been part of the traditional diet of many cultures around the world [1]. In this sense, their farming, processing, and consumption has increased in the last decade, as an alternative towards food security [2]. Additionally, insects are considered highly nutritional due to their proteins, essential amino acids, fiber, fat, and minerals [3,4]. Moreover, the farming of insects shows a better reproductive capacity and lower environmental impact compared to other traditional farming practices [5,6].
There is a great diversity of insects sought not only for their traditional consumption, and by their high nutritional value; however, they have also been a focus of study as potential sources of bioactive compounds, including peptides, polysaccharides, fatty acids, and polyphenols [7]. For instance, the consumption of cricket powder showed to decrease proinflammatory cytokines in healthy adults [8]. In other studies, the enzymatic hydrolysis of protein from mealworm (Tenebrio molitor), cotton leaf worm (Spodoptera littoralis), locust (Schistocerca gregaria), and cricket (Gryllodes sigillatus; Acheta domesticus), released peptides with antihypertensive, antioxidant, anti-inflammatory, and antidiabetic activity [9,10,11,12].
Similarly, peptides from the locust (S. gregaria) obtained after gastrointestinal conditions showed in vitro inhibition against lipoxygenase and cyclooxygenase-2 [13], while Hall et al. [14] identified antihypertensive, anti-glycemic, and anti-inflammatory cationic peptides (<0.5 kDa) derived from the gastrointestinal digests of cricket (G. sigillatus) peptides that also inhibited the expression of NF-κB in RAW 264.7 macrophage cells.
Fermentation has been commonly used for the release of bioactive compounds [6,15]. For instance, some studies have reported that after 20 days of soy sauce fermentation added with Tenebrio molitor, Bacillus licheniformis, and Aspergillus oryzaep, the amino-nitrogen and aromatic compound content increased, indicating protein degradation [16,17,18]. Although the process of fermentation with lactic acid bacteria (LAB) has been used as an alternative to eliminate undesirable microorganisms or improve sensory characteristics [19], the used of LAB for the modulation of bioactive compounds from edible insects has not been widely reported.
Specifically, Lactococcus lactis NRRLB strains isolated from artisanal fermented dairy products have been reported for their ability to release bioactive peptides from fermented dairy products with Angiotensin Converting Enzyme Inhibitory (ACEI) activity [20] and hypocholesterolemic effects [21]. However, the ability of this strain to ferment insects, such as mealworms and grasshoppers, remains unknown. Therefore, the aim of this study was to evaluate the potential antioxidant and ACEI activity of mealworm and grasshopper flours fermented with two L. lactis strains (NRRLB-50571 and NRRLB-50572).
2. Materials and Methods
2.1. Lactic Acid Bacteria Strains and Insect Flours
The strains of L. lactis (NRRL B-50571 and NRRL B-50572) were obtained from the culture collection of the Dairy Laboratory at the Food Research and Development Center, A. C. (CIAD A.C., Hermosillo, Sonora, México). The mealworm flour (larvae stage) was obtained from OptiProt® (Cuernavaca, Morelos, México), and grasshopper (Sphenarium purpurascens, adult stage) samples were obtained from a public market in Oaxaca City, Mexico. The grasshoppers were washed twice using purified water, and then dried at room temperature for 24 h. Grasshoppers were ground into flour form (Coffee Grinder Hamilton Beach, WI, USA), and both samples (mealworm and grasshopper flours) were defatted (Soxhlet method 31.4.02) [22].
2.2. Conditions for the Growth of L. lactis
The two strains used in this study were propagated (1% v/v) in M17 broth (BD® Difco, St. Louis, MO, USA) supplemented with sterile dextrose solution (5% v/v), and incubated for 18 h (NRRL B-50571), and 12 h (NRRL B-50572) in the first culture. The strains were subsequently cultivated in two successive steps and incubated for 6 and 4 h, at 30 °C. The sterile dextrose solution used was prepared at a concentration of 10% (w/v). Afterward, the M17 broth was modified with two dextrose concentrations (3.5% and 5% v/v) and three insect flour concentrations (0.1%, 0.5%, and 1% w/v) in order to select the best growing conditions for the L. lactis. The last culture of 4 h of both strains was inoculated (1% v/v) in M17 modified broth and incubated 12 h at 30 °C with agitation (500 rpm). A growth kinetic curve was determined, and the dates were adjusted using the modified Gompertz model [23], the lag phase (λ), generation time (G), and maximum growth rate (μmax) were calculated.
Once the optimal dextrose and protein content conditions were selected, the LAB strains were again cultivated in M17 modified broth in three subcultures, and again the growth kinetic curve was determined. This assay was done with the objective of adapting the new bacteria to a different source of protein.
2.3. Fermentation Conditions
Once the best conditions of growth were selected, the insect flours were dissolved in phosphate buffer salt (PBS, pH 7.0), and a heat treatment was applied (110 °C, 10 min) to eliminate the presence of other microorganisms in the flours, followed by sonication (30 min/50 °C/50 Hz) (VWR Aquasonic™ 50D, Ultrasonic Cleaner, San José, CA, USA). Sonication was applied to yield protein in both flour samples. Then, the flour solutions were supplemented with sterile dextrose (3.5% v/v). For the fermentation, the flour samples were inoculated at 3% (v/v) with each strain from the last subculture of the 5-h incubation (selected in Section 2.2), and these were incubated for 72 h at 30 °C with constant agitation (500 rpm) under aerobic conditions. The pH was measured, and the cellular concentration was determined at 0, 24, 48, and 72 h of fermentation.
The fermented flour samples were centrifuged (3600× g for 30 min at 4 °C), and supernatant (crude extract) was collected and fractionated using a stirred ultrafiltration cell (Model 8050, Amicon, Bedford, MA, USA) with a molecular exclusion membrane (Ultracell 3 kDa, Millipore, Billerica, MA, USA), and stored at −20 °C until further use. The water-soluble < 3 kDa fractions were used for determination of the antioxidant activity, polyphenol content, ACEI, and identification of the peptide profile.
2.4. Determination of Degree of Hydrolysis and Protein Content
In order to determine the degree of hydrolysis (DH), the free amino groups (h) were quantified by spectrometry using the OPA (Fluoraldehyde™ o-Phthaldialdehyde Reagent Solution, Thermo Fisher Scientific, Waltham, MA USA) method [24]. For this assay, 500 μL of crude extracts were mixed with 500 μL of Trichloroacetic acid (24% v/v) (Sigma-Aldrich, Saint Louis, MO, USA) and restored for 10 min. Then, the samples were centrifuged (3600× g for 40 min) and the supernatants were used for the quantification.
For the quantification of free amino groups, 20 μL of sample was mixed with 200 μL of OPA and incubated for 2 min under dark conditions. The fluorescence was measured at 340 nm of excitation and 436 nm of emission. The degree of hydrolysis (DH) was calculated using the formula:
where htot is the total hydrolysis of samples using 6 M HCl at 150 °C for 6 h (Vázquez et al., 1994). The protein contents of the <3 kDa fractions were measured using the Lowry method (DC Protein Assay kit). The results are expressed as mg/mL.
%DH = (h/htot) × 100
2.5. Determination of Total Polyphenol Content in Fermented Insect Flours
The total polyphenol content was determined by the Folin–Ciocalteu method [25] with modifications. Briefly, 20 μL of the water-soluble < 3 kDa fraction of fermented flour was mixed with 200 μL of Folin–Ciocalteu (0.2 n) and incubated for 2 min at 30 °C. Then, 20 μL of sodium carbonate (7.5% v/v) was added and incubated 30 min at 30 °C. The absorbance was measured at 765 nm, and the results are expressed in μg Gallic Acid Equivalents (GAE)/mL.
2.6. Determination of Antioxidant Activity
The antioxidant activities of all water-soluble < 3 kDa fractions of both fermented flours were evaluated using several antioxidant assays, and the results are expressed in mM of Trolox Equivalent (TE). A five-point (0–50 μM in PBS) standard curve of Trolox (6-hydroxy-2,3,7,8-tetramethylchroman-2-carboxylic acid) was established. The 2,2′-azino-bis-(3-ethyl-benzthiazoline-6-sulfonic acid (ABTS•+) (Sigma-Aldrich) radical scavenging activity assay, and the Oxygen Radical Absorbance Capacity (ORAC) were performed following the methodology modified by Zulueta et al. [26]. For the ABTS radical cation, a stock solution (7 mmol/L) was mixed with potassium persulfate (2.45 mM), and kept in the dark for 16 h at 30 °C.
For the reaction, the ABTS radical solution was adjusted to 0.70 Optical Density at 734 nm. Next, the solution (200 μL) and water-soluble < 3 kDa fractions (5 μL) were mixed and recorded after 7 min (SpectraMax M3 microplate reader Molecular Devices, Sunnyvale, CA, USA). For the ORAC assay, 50 μL of Fluorescein (78 nM) (Sigma-Aldrich) was mixed with 50 μL of sample (<3 kDa fractions) or PBS as a control. Next, 25 μL of the peroxyl radical initiator 2,2′-azobis(2-amidinopropane) dihydrochloride was added for to start the reaction. The fluorescence (excitation 485 nm and emission 535 nm) was recorded each 5 s for 5 h. The data were normalized with respect to the control curve.
The free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH•+) (Sigma-Aldrich) was performed according to the methodology reported by Herald et al. [27]; meanwhile, the ferric-reducing antioxidant power (FRAP) assay was performed according to Benzie and Strain [28] with slight modifications. The FRAP solution was prepared mixing 10 mL of acetate buffer (300 mM, pH 3.6 using acetic acid) with 1 mL of TPTZ (2,4,6-tripyridyl-s-triazine; 10 mM in HCl 40 mM) and 1 mL of ferric chloride (20 mM). The mixture was incubated at 37 °C for 30 min. Once incubated, 25 μL of sample was mixed with 175 μL of FRAP solution. The absorbance was measured after 30 min at 595 nm. The sample with the highest antioxidant activity was selected for the evaluation of ACEI, and the fractions were analyzed by reversed-phase high-performance liquid chromatography (HPLC).
2.7. Determination of Angiotensin Converting Enzyme Inhibition
The ACEI was performed according to the methodology described by Wú et al. [29]. In this assay, the hippuryl-histidyl-leucine as substrate (HHL, 2.17 mM) was in a borate buffer (100 mM with 300 mM of NaCl at pH 8.3) with ACE enzyme (0.2 U/mL) and the inhibitor (<3 kDa fraction). For the enzymatic reaction 50 μL of HHL solution + 10 μL sample were mixed and incubated for 10 min at 37 °C, 450 rpm (Eppendorf ThermoMixer® AG, Hamburg, Germany), and then 10 μL of ACE was added and incubated again under the same conditions. The reaction was stopped by the addition of 85 μL of 1 M HCl and was analyzed by HPLC.
The reversed-phase HPLC was carried out using a ZORBAX eclipse Plus C18 (4.6 × 100 mm, 3.5 μm) column in an Agilent 1260 HPLC system (Agilent Technologies, Waldbronn, Germany) equipped with OpenLAB Chromatography Data System A.02.02 (Agilent Technologies, Waldbronn, Germany). The mobile phase consisted of solvent A (deionized water), and solvent B of acetonitrile into trifluoroacetic acid (0.05%), a gradient elution from 5% to 60% for solvent B over 10 min was used. After 2 min at 60% solvent B, the gradient was inverted to 5% solvent B in 1 min and kept at 5% solvent B for 4 min before the next sample injection. The flow rate was 0.5 mL/min, and detection was at 214 nm. The inhibition percentage of ACE was calculated with the following formula:
where A is the peak area of the hippuric acid (reaction HHL + ACE + buffer), B is the peak area of hippuric acid after the ACEI reaction with substrate HHL in the presence of the sample (inhibitor). The ACE enzyme inhibitory activity by <3 kDa fractions was also expressed as the protein content (mg/mL) necessary to inhibit ACE activity by 50% (IC50).
ACEI (%) = [(A − B)/A)] × 100
2.8. Isolation of Peptide Fractions by Reversed-Phase HPLC
The peptide profiles of the <3 kDa fraction of both fermented flour samples that showed higher antioxidant and ACEI activity were analyzed by reversed-phase HPLC. The conditions were a flow rate of 0.25 mL/min, and solvent A was mixture of deionized water and trifluoroacetic acid (1000:0.4 v/v), and solvent B was acetonitrile and trifluoroacetic acid (1000:0.3 v/v). The peptides were eluted with a linear gradient of solvent B in solvent A from 0.1% to 99% for 40 min, and, for solvent B, it was of 0.1% to 60% in 40 min.
2.9. Statistical Analysis
For the analysis of the growth conditions of the two LAB strains, a 2 × 2 factorial, completely randomized design was performed. An analysis of variance (ANOVA) with p < 0.05 was performed, and the mean differences were analyzed by Tukey–Kramer for the parameters determined during the fermentation. A t-Student’s test was used for the ACE inhibition analysis. The statistical analysis of the data was performed with the NCSS statistical package, 2019 (Kaysville, UT, USA). All tests were performed in triplicate.
3. Results
The results of the kinetic parameters determined in the factorial design are displayed in Table 1. Overall, we observed that treatment B (0.5% insect flour and 3.5% dextrose) for both strains and both insect flours had a shorter lag phase (λ) with 2.38 and 2.27 h as well as a generation time (G) of 0.55 and 0.65 h for mealworm and grasshopper flour, respectively (p < 0.05) using strain NRRL B-50571. For strain NRRL B-50572, the times were 3.43 and 3.96 h for the lag phase; a generation time of 3.96 and 0.65 h for mealworm and grasshopper, respectively. Therefore, these conditions (0.5% of flour and 3.5% dextrose) were selected for the final fermentation process.

Table 1.
The kinetic parameters obtained for the selection of the best growth conditions for L. lactis into edible insect flours.
Table 2 shows the results obtained during the pre-adaptation of L. lactis in three subcultures. The results of the second and third subcultures for L. lactis NRRL B-50572 showed a reduction in the lag phase (p < 0.05) for mealworms and G for L. lactis NRRL B-50571. Although, in general, the kinetic parameters did not show significant changes in the subculture, the cell concentration 109 CFU/mL was considered for fermentation and a second subculture of 5 h of growth in M17 broth was modified for each strain. This time was considered because it was the time when each of the L. lactis strains was at the end of their exponential phase.

Table 2.
The kinetic parameters obtained for the three subcultures of L. lactis.
The parameters evaluated during the fermentation are shown in Table 3. The pH decreased (p < 0.05) ca. 1.98 and 1.66 two-fold at 72 h of fermentation for NRRL B-50571; meanwhile, for NRRL B-50572, the decrease was of 1.92 and 1.73 twist-folder for both flours (mealworm and grasshopper, respectively). The pH decreased and did not change (p > 0.05) after 24 h of fermentation for mealworm flour for both strains; however, a significant decrease was observed between 24 and 48–72 h for grasshopper flour fermented with both strains.

Table 3.
The parameters evaluated during the fermentation process of both insect flours.
On the other hand, the DH increased 0.43% and 0.19% (p < 0.05) at 24 h for mealworm fermented with NRRL B-50571 and B-50572, respectively; meanwhile, for grasshopper flour, it increased around 0.21% and 0.17% (p < 0.05), with the NRRL B-50571 strain as the one with the best proteolytic activity. The highest protein concentration was obtained between at 24 and 48 h fermentation, showing no difference between times (p > 0.05), both for NRRLB-50571 and 50572 using the mealworm flour. On the contrary, the grasshopper flour showed a lower content of protein in the same time frame of 24 and 48 h (p > 0.05) with both strains. The results showed that, by increasing the DH, the protein content also increased, which may suggest that the L. lactis strains partially hydrolyzed the proteins of both insect flours.
On the other hand, the concentration of NRRL B-50571 decreased after 48 fermentation to 106 CFU/mL for 72 h for grasshopper flour; meanwhile, for mealworm flour at 48 h, it increased to 109 CFU/mL (p < 0.05) (Table 4). On the contrary, the cell concentration of NRRL B-50572 enhanced an exponential cycle and was sustained at 108 CFU/mL until 72 h for both flours.

Table 4.
The cell concentration of L. lactis strains during the fermentation processes of edible insect flour.
The total polyphenol content is shown in Figure 1. In general, polyphenols increased at 24 h of fermentation to 15.41 and 9.69 mg GAE/mL for NRRL B-50571 as well as 22.08 and 8.23 μg GAE/mL for NRRL B-50572 for mealworm and grasshopper, respectively (p < 0.05) (Figure 1).
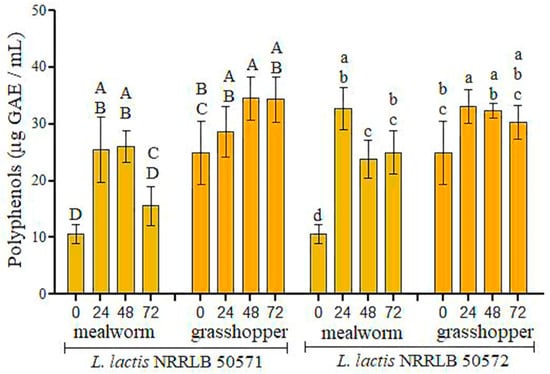
Figure 1.
The total polyphenol contents of the <3 kDa fractions obtained from the two fermented edible insect flours. The values show the mean ± standard deviation (n = 3). Upper case letters (A–D) show significant differences (p < 0.05) among insects fermented for L. lactis NRRL B-50571, and lower case letters (a–c) show a difference (p < 0.05) among insects fermented with L. lactis NRRL B-50572.
The antioxidant activity of the <3 kDa fractions increased during the fermentation process (Figure 2). The major antioxidant activity towards the ABTS• radical (p < 0.05) was observed at 24 h of fermentation for the grasshopper flour samples showing an increase of 164.86 and 267.05 μM TE for NRRL B-50571 and B-50572, respectively (Figure 2A). Specifically, fermented grasshopper and mealworm flour with NRRL B-50572 at 24 h showed the highest antioxidant activity (p < 0.05). This same trend was observed against the DPPH• radical, where the antioxidant activity increased 62.36 and 86.71 μM TE for grasshopper fermented with both strains, and by 68.48 μM TE for mealworm fermented with NRRL B-50572 (p < 0.05) (Figure 2B).
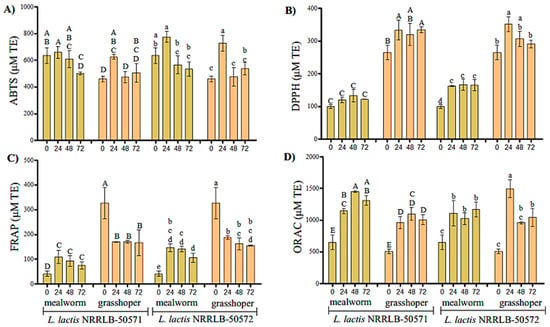
Figure 2.
The antioxidant activity (μM TE/mL) of the <3 kDa fractions obtained from the two insect flours fermented at 0, 24, 48, and 72 h. TEAC (A), DPPH (B), FRAP (C), and ORAC (D). The values shown are the means ± standard deviation (n = 3). Upper case letters (A–D) show a difference (p < 0.05) among insects fermented with NRRL B-50571; lower case letters (a–d) show a difference (p < 0.05) among insects fermented with NRRL B-50572.
The highest antioxidant activity by the ORAC method was recorded in the grasshopper flour fermented for 24 h by NRRL B-50572, which increased to 246.57 μM TE (Figure 2C). Conversely, for the FRAP antioxidant activity, the activity decreased for fermented grasshoppers; however, for mealworm, there was an increase of 68.1 and 105.38 μM Trolox equivalents for NRRL B-50571 and B-50572 (p < 0.05), respectively (Figure 2D).
In brief, the major antioxidant activity was found at 24 h of fermentation with L. lactis NRRLB- 50572, as this was the sample selected to evaluate the ACE inhibition activity. Our results showed that, at 0 h, the grasshopper flour fermented with L. lactis had ACE inhibition; however, for mealworm flour, this effect could not be detected. However, the ACE inhibition activity was enhanced with increasing fermentation time, which may suggest that the addition of fermentative bacteria can favor the release of compounds with this bioactive property. In specific, the ACE inhibition activity for mealworm flour at 24 h was of 17.25% with an IC50 of 1.36 mg/mL; meanwhile, for the fermented grasshopper, it increased (p < 0.05) (Table 5). Although we observed that there was an increase in the percentage of ACE inhibition, the IC50 showed no significant change, and even a protein content similar to the 10% inhibition present at 0 h was considered.

Table 5.
Values of ACEI activity for samples fermented by L. lactis NRRLB-50572.
Figure 3 shows the typical profile of the <3 kDa fraction of both flours fermented by the L. lactis NRRLB- 50,572 strain. The profile showed an increase in the total area between 0 and 24 h of fermentation. At 0 h, the flours showed a total area of 15,785.12 and 16,698.58 mAU (p < 0.05). However, at 24 h of fermentation, there was a 2.2-fold and 1.3-fold increase for the fermented samples of the mealworm and grasshopper flour, respectively.
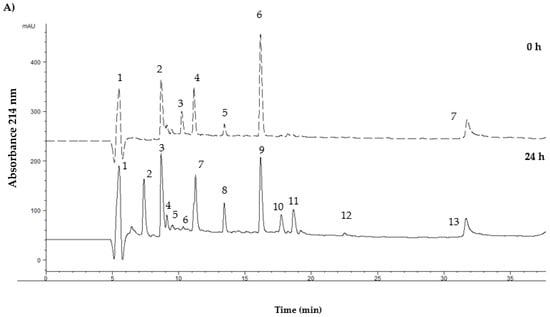
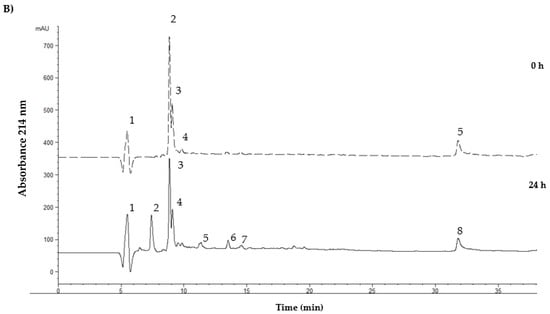
Figure 3.
Peptide profiles of the <3 kDa fraction obtained from the fermented insect flour samples fermented by L. lactis NRRLB- 50572. (A) The peptide profile for mealworm flour; (B) the peptide profile for grasshopper flour.
The fermented mealworm flour showed the presence of peak 2, while peak 3 at 0 h, appeared to fractionate into peaks 5 and 6 at 24 h, in addition to a significant increase in the area of peaks 11 and 12. This could suggest the participation of the bacterial enzymes to hydrolyze the proteins present at 0 h. On the contrary, fermented grasshopper flour at 24 h only showed the presence of peaks 2, 5, 6, and 7 with the area more significantly increased. These results suggest that L. lactis showed a preference for the mealworm protein and was more difficult to hydrolyze for grasshopper protein.
The bioactivity found for both samples could be associated to the presence of phenolic compounds and the release of peptides during fermentation, as both compounds increase, as well as the bioactivities.
4. Discussion
The L. lactis strains showed a better adaptation to the new protein sources after two reactivation times. In this context, the time in the middle of the exponential phase was considered, because, at this stage, the bacteria are metabolically active to improve their growth conditions and decrease their lag phase once fermentation starts.
The results obtained with the protein content during the fermentation processes were higher in the fermented mealworm flour compared with grasshopper flour. In addition, the pH changes were strain dependent and may be explained by differences in the metabolic ability and growth requirements; as well, pH reduction depends on the amount of organic acids released [30]. These results suggest that the strains might hydrolyze the mealworm and grasshopper protein and release peptides with potential biological activity. These results show the potential capacity of the LAB to use both dextrose and the protein from flour insects for their growth.
The results obtained in our study were similar to those obtained by Mouritsen et al. [18], who fermented grasshopper sauces with Aspergillus oryzae and reported a pH of 4.84 to 4.95, also an increase in the free amino acid content. They also improved the sensory characteristics, such as Umami-T and yeasty notes. These characteristics make attractive the incorporation of insects to improve the flavor of the sauces. Another study evaluated the ability to ferment a paste produced from the yellow mealworm (Tenebrio molitor) with different LAB (L. lactis, Lb. curvatus, Lb. farciminis, Lb. plantarum, Lb. sakei, and Pediococcus acidilactici of Chr. Hansen A/S, Hoersholm, Denmark). The pH of the mealworm paste decreased from 6.68 to 4.95 after 72 h of fermentation, and the growth of Lb. sakei was of 0.8 log CFU/g and 2.9 for Pediococcus acidilactici with larvae from mealworm. These results showed the ability of LAB to ferment the larvae paste and inhibit the growth of undesirable microorganisms [31].
The main parameters evaluated that were considered as indicative of the bacterial fermentation (pH, DH, and protein content) showed that, at 24 h, a significant increase was observed; however, after this time, no significant changes were shown in the variables determined. It is possible to consider that the strains were indeed involved in the fermentation, because they have been widely used for milk fermentation, in which similar values have been reported. The strains were able to promote the release of bioactive compounds—in particular, NRRL B-50572 has been deemed the most proteolytic strain in milk [21,32]. This same effect was observed in our study with a different protein source (i.e., edible insects).
In particular, the hydrolyzed protein by LAB is associated to their proteolytic system, which includes proteinases of the cellular envelope, transporters, and peptidases [33], especially for Lb. helveticus, as their proteolytic system was characterized [34]. In a previously study, L. lactis NRRLB showed high proteolytic activity toward milk proteins [21], which could indicate that the proteolytic system of this bacteria is active for the hydrolysis of proteins, due to the increase that was shown by the degree of hydrolysis, mainly with grasshopper proteins. In this sense, during insect flour fermentation, peptides and amino acids are released from insect proteins by the LAB proteolytic system since these compounds are essential for microbial growth.
During the fermentation process, some bacteria remove sugar moieties, hydrolyze galloyl moieties, and release free phenolic compounds. In particular, Lb. brevis 145 metabolizes gallic acid into pyrogallol and hydrolyzes the ester bond linked with gallolyl groups; a similar effect was reported for Lb. plantarum 292 [35,36]. These results could explain the increase in the polyphenol content of the fermented samples.
Due to insects’ herbivore feeding practices, it would be expected for phenolic compounds to be present in flours obtained from insects. Studies have reported the ability of insects to sequester and metabolize plant phenolics from their diet. For example, phenolic compounds have been reported for a variety of insects, such as Lepidopterans (e.g., butterflies and moths), where the main assumption is that these compounds are directly obtained from the insects’ diet [37,38,39,40].
Phenolic compounds and peptides released during the fermentation could be associated with the antioxidant activity. These results have been demonstrated in previous studies using commercially available insect flours that showed antioxidant activity per se, where grasshoppers and mealworms contained the highest levels of total polyphenols, thus, demonstrating edible insects as a potential source of dietary antioxidants [37]. Nevertheless, an over-estimation of phenolic compounds could result from the interference caused by proteins [41,42]. Further research is required to establish a suitable method (e.g., LC-MS/MS) that would allow for better determination of the phenolic content in edible insect flours.
Today, the presence of antioxidants in food has an important role in the prevention of some diseases such as cancer, aging, and inflammation [43]. In this sense, some studies have reported the release of antioxidant peptides from insects, obtained by enzymatic hydrolysis or under in vitro gastrointestinal conditions [44]. For example, an increased scavenging activity of ABTS and DPPH was observed after the simulated gastrointestinal digestion of cricket (G. sigillatus) peptides compared to non-digested cricket protein [11]. The strong reducing potential of the peptides was attributed to the availability of hydrogen protons and electrons caused by cleavage of the peptide bond. In other studies, raw mealworm hydrolysate with digestive enzymes showed the highest activity against ABTS, and baked G. sigillatus and S. gregaria protein showed the highest activity against DPPH [44], indicating that heat treatment promoted the release of peptides with antioxidant activity [45].
In particular, extracts from mealworm contain certain amino acids that could be responsible for its antioxidant activity [46]. In addition, the exoskeleton of grasshoppers is made of chitin, which has showed antioxidant activity in DPPH and FRAP assays [47]. This could explain, in part, why the antioxidant activity of the fermented grasshopper flour presented greater antioxidant activity, due to the fact that chitin was not removed, and some oligosaccharides could still be present. A recent study showed that some Lactobacillus strains selected from different sources had a chitinase coding gene but did not have chitinolytic activity [48]. Therefore, it will be necessary to explore if the LAB strains used in this study can present chitinases, which, once the bacteria are exposed to stress conditions, can express these enzymes and show activity.
The ACE has an important role in the regulation of blood pressure, and its inhibition by bioactive peptides is a common method used in determining the biological activity of these peptides [49]. In this study, the <3 kDa fractions of the fermented insect flours showed ACE inhibitory activity. Other studies showed that hydrolyzed cricket protein (5 mg/mL) inhibited > 70% ACE activity, which might be enhanced by gastrointestinal proteases [10].
Several studies have identified peptide sequences from B. mori pupae (Lys-His-Leu, Ala-Ser-Leu, and Gly-Asn-Pro-Trp-Met) obtained from enzymatic hydrolysis [10,50,51]. For example, one study reported that the tripeptide Tyr-Ala-Asn from Tenebrio molitor hydrolysate showed good hypertensive activity, decreasing the systolic blood pressure in spontaneously hypertensive rats [52]. ACE-inhibitory peptides for insects fermented by LAB have been less documented; however, the action mechanisms for the ACE-inhibition could be influenced by several factors, such as the presence of C-terminal tripeptide residues, and the presence of Trp, Tyr, Phe, or Pro in the peptide structure [53].
In brief, an increase in the extent of hydrolysis, amine concentration, cell growth, and low pH indicates that the L. lactis strains were able to ferment the mealworm and grasshopper flours. In addition, the fermented flours presented bioactivity by means of antioxidant and ACE-inhibition activities, which may also be associated with the presence of phenolic compounds, chitin, and <3 kDa peptide fractions.
The search for new foods with high protein contents have been studied because of the potential they may have for obtaining bioactive peptides. In this sense, edible insects, which are widely consumed in several countries, have been of great interest in recent years [54]. The results obtained in the study showed the potential to use lactic acid bacteria to ferment insects and, thus, be able to obtain compounds with biological activity.
5. Conclusions
To the best of our knowledge, this is the first study that involves the fermentation of edible insects for the production of bioactive compounds with antioxidant and ACE inhibition. The process involves a sustainable raw material since producing edible insects has benefits for the environment due to its high feed conversion ratio and fewer greenhouse gas emissions. Additionally, the production of insects as food increases the livelihood opportunities for people in developing and developed countries. Finally, insects are often consumed whole, but including a sustainable food processing step, such as fermentation with specific lactic acid bacteria, may decrease the disgust factor and, at the same time, increase the consumption of insect-based food products with added health benefits.
Author Contributions
A.M.-S. Methodology, formal analysis and writing—original draft; L.S.-L., formal analysis and writing—review and editing original draft; M.J.T.-L., software and formal analysis of HPLC; A.M.L. review and editing the manuscript; A.H.-M., B.V.-C., A.M.L. and A.F.G.-C., Conceptualization, Review, Editing, and Supervision of the experiments. A.F.G.-C. revised the manuscript and had primary responsibility for the final content of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank the National Council for Science and Technology (CONACyT) of Mexico for the graduate scholarship of author A.M.-S.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Imathiu, S. Benefits and food safety concerns associated with consumption of edible insects. NFS J. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A Review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef] [Green Version]
- Govorushko, S. Global status of insects as food and feed source: A review. Trends Food Sci. Technol. 2019, 91, 436–445. [Google Scholar] [CrossRef]
- Tang, C.; Yang, D.; Liao, H.; Liu, C.; Wei, L.; Li, F. Edible insects as a food source: A review. Food Prod. Process. Nutr. 2019, 1, 8. [Google Scholar] [CrossRef] [Green Version]
- Oonincx, D.G.; van Itterbeeck, J.; Heetkamp, M.J.; van den Brand, H.; van Loon, J.J.; van Huis, A. An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS ONE 2010, 5, e14445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-López, C.; Santiago-López, L.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Liceaga, A.M.; García, H.S.; Hernández-Mendoza, A. An insight to fermented edible insects: A global perspective and prospective. Food Res. Int. 2020, 137, 109750. [Google Scholar] [CrossRef] [PubMed]
- da Silva Lucas, A.J.; de Oliveira, L.M.; da Rocha, M.; Prentice, C. Edible insects: An alternative of nutritional, functional and bioactive Compounds. Food Chem. 2020, 311, 126022. [Google Scholar] [CrossRef]
- Stull, V.J.; Finer, E.; Bergmans, R.S.; Febvre, H.P.; Longhurst, C.; Manter, D.K. Impact of edible cricket consumption on gut microbiota in healthy adults, a double-blind, randomized crossover trial. Sci. Rep. 2018, 8, 10762. [Google Scholar] [CrossRef] [Green Version]
- Vercruysse, L.; Smagghe, G.; Beckers, T.; Van Camp, J. Antioxidative and ACE inhibitory activities in enzymatic hydrolysates of the cotton leafworm, Spodoptera littoralis. Food Chem. 2009, 114, 38–43. [Google Scholar] [CrossRef]
- Tao, M.; Wang, C.; Liao, D.; Liu, H.; Zhao, Z.; Zhao, Z. Purification, modification and inhibition mechanism of angiotensin I-converting enzyme inhibitory peptide from silkworm pupa (Bombyx mori) protein hydrolysate. Process. Biochem. 2017, 54, 172–179. [Google Scholar] [CrossRef]
- Hall, F.; Johnson, P.E.; Liceaga, A. Effect of enzymatic hydrolysis on bioactive properties and allergenicity of cricket (Gryllodes sigillatus) protein. Food Chem. 2018, 262, 39–47. [Google Scholar] [CrossRef]
- Mishyna, M.; Martinez, J.J.I.; Chen, J.; Benjamin, O. Extraction, characterization and functional properties of soluble proteins from edible grasshopper (Schistocerca gregaria) and honey bee (Apis mellifera). Food Res. Int. 2019, 116, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, E.; Karaś, M.; Jakubczyk, A.; Zieliński, D.; Baraniak, B. Edible Insects as Source of Proteins. Bioact. Mol. Food 2018, 1–53. [Google Scholar]
- Hall, F.; Reddivary, L.; Liceaga, A.M. Identification and characterization of edible cricket peptides on hypertensive and glycemic in vitro inhibition and their anti-inflammatory activity on RAW 264.7 macrophage cells. Nutrients 2020, 12, 3588. [Google Scholar] [CrossRef] [PubMed]
- de Castro, R.J.S.; Ohara, A.; dos Santos Aguilar, J.G.; Domingues, M.A.F. Nutritional, functional and biological properties of insect proteins: Processes for obtaining, consumption and future challenges. Trends Food Sci. Technol. 2018, 76, 82–89. [Google Scholar] [CrossRef]
- Cho, J.H.; Zhao, H.L.; Kim, J.S.; Kim, S.H.; Chung, C.H. Characteristics of fermented seasoning sauces using Tenebrio molitor larvae. Innov. Food Sci. Emerg. Technol. 2018, 45, 186–195. [Google Scholar] [CrossRef]
- Yi, L.; Van Boekel, M.A.; Boeren, S.; Lakemond, C.M. Protein identification and in vitro digestion of fractions from Tenebrio molitor. Eur. Food Res. Technol. 2016, 242, 1285–1297. [Google Scholar] [CrossRef] [Green Version]
- Mouritsen, O.G.; Duelund, L.; Calleja, G.; Frøst, M.B. Flavour of fermented fish, insect, game, and pea sauces: Garum revisited. Int. J. Gastron. Food Sci. 2017, 9, 16–28. [Google Scholar] [CrossRef]
- Kewuyemi, Y.O.; Kesa, H.; Chinma, C.E.; Adebo, O.A. Fermented edible insects for promoting food security in Africa. Insects 2020, 11, 283. [Google Scholar] [CrossRef]
- Beltrán-Barrientos, L.M.; González-Córdova, A.F.; Hernández-Mendoza, A.; Torres-Inguanzo, E.H.; Astiazarán-García, H.; Esparza-Romero, J.; Vallejo-Cordoba, B. Randomized double-blind controlled clinical trial of the blood pressure–lowering effect of fermented milk with Lactococcus lactis: A pilot study. J. Dairy Sci. 2018, 101, 2819–2825. [Google Scholar] [CrossRef] [Green Version]
- Rendon-Rosales, M.Á.; Torres-Llanez, M.J.; González-Córdova, A.F.; Hernández-Mendoza, A.; Mazorra-Manzano, M.A.; Vallejo-Cordoba, B. In Vitro Antithrombotic and Hypocholesterolemic Activities of Milk Fermented with Specific Strains of Lactococcus lactis. Nutrients 2019, 11, 2150. [Google Scholar] [CrossRef] [Green Version]
- Association of Official Analytical Chemists International (AOAC). Official Methods pf Analysis of A.O.A.C. International: Agricultural Chemicals, Contaminants, Drugs; 17 Ed. Dr. George W. Latimer; AOAC: Washington, DC, USA, 2000. [Google Scholar]
- Chowdhury, B.R.; Chakraborty, R.; Chaudhuri, U.R. Validity of modified Gompertz and Logistic models in predicting cell growth of Pediococcus acidilacticiH during the production of bacteriocin pediocin AcH. J. Food Eng. 2007, 80, 1171–1175. [Google Scholar] [CrossRef]
- Nielse, P.M.; Petersen, D.; Dambmann, C. Improve method for determining Food protein degree of hydrolysis. J. Food Sci. 2001, 66, 641–646. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteau reagent. Methods Enzym. 1999, 299, 152–178. [Google Scholar]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Aluko, R.E.; Muir, A.D. Improved method for direct high-performance liquid chromatography Assay of angiotensin-converting enzyme-catalyzed reactions. J. Chromatogr. A 2002, 950, 125–130. [Google Scholar] [CrossRef]
- Widyastuti, Y.; Rohmatussolihat; Febrisiantosa, A. The role of lactic acid bacteria in milk fermentation. Food Nutr. Sci. 2014, 5, 435–442. [Google Scholar] [CrossRef] [Green Version]
- An, B.; Sam, C.; Dries, V.; Ruben, S.; Christel, V.; Borght Mik, V.D.; Bart, L.; Leen, V.C. Comparison of Six Commercial Meat Starter Cultures for the Fermentation of Yellow Mealworm (Tenebrio molitor) Paste. Microorganisms 2019, 7, 540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beltrán-Barrientos, L.M.; Garcia, H.S.; Reyes-Díaz, R.; Estrada-Montoya, M.C.; Torres-Llanez, M.J.; Hernández-Mendoza, A.; González-Córdova, A.F.; Vallejo-Cordoba, B. Cooperation between Lactococcus lactis NRRL B-50571 and NRRL B-50572 for Aroma Formation in Fermented Milk. Foods 2019, 8, 645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Bayjanov, J.R.; Renckens, B.; Nauta, A.; Siezen, R.J. The proteolytic system of lactic acid bacteria revisited: A genomic comparison. BMC Genom. 2010, 11, 36. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, M.W.; Tellez, A.M. Lactobacillus helveticus: The proteolytic system. Front. Microbiol. 2013, 4, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkar, S.G.; Trower, T.M.; Stevenson, D.E. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe 2013, 23, 12–19. [Google Scholar] [CrossRef]
- Zhao, D.; Shah, N.P. Lactic acid bacterial fermentation modified phenolic composition in tea extracts and enhanced their antioxidant activity and cellular uptake of phenolic compounds following in vitro digestion. J. Funct. Foods 2016, 20, 182–194. [Google Scholar] [CrossRef]
- Di Mattia, C.; Battista, N.; Sacchetti, G.; Serafini, M. Antioxidant Activities in vitro of Water and Liposoluble Extracts Obtained by Different Species of Edible Insects and Invertebrates. Front. Nutr. 2019, 6, 106. [Google Scholar] [CrossRef] [Green Version]
- Nino, M.; Reddivari, L.; Osorio, C.; Kaplan, I.; Liceaga, A. Insects as a source of phenolic compounds and potential health benefits. J. Insects Food Feed. 2021, in press. [Google Scholar] [CrossRef]
- Burghardt, F.; Fiedlert, K.; Proksch, P. Uptake of flavonoids from Vicia villosa (Fabaceae) by the lycaenid butterfly, Polyommatus icarus (Lepidoptera: Lycaenidae). Biochem. Syst. Ecol. 1997, 25, 527–536. [Google Scholar] [CrossRef]
- Wiesen, B.; Krug, E.; Fiedler, K.; Wray, V.; Proksch, P. Sequestration of host-plant-derived flavonoids by lycaenid butterfly Polyommatus icarus. J. Chem. Ecol. 1994, 20, 2523–2538. [Google Scholar] [CrossRef] [PubMed]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. A thorough of reactivity of various compounds classes towards the Folin-Ciocalteun reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikawa, M.; Schafer, T.; Dollard, C.; Sasner, J. Utilization of Folin-Ciocalteu reagent for the detection of certain nitrogen compounds. J. Agric. Food Chem. 2003, 51, 1811–1815. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh-Fokou, P.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Zielińska, E.; Karaś, M.; Jakubczyk, A. Antioxidant activity of predigested protein obtained from a range of farmed edible insects. Int. J. Food Sci. 2017, 52, 306–312. [Google Scholar] [CrossRef]
- You, L.; Zheng, L.; Regenstein, J.M.; Zhao, M.; Liu, D. Effect of thermal treatment on the characteristic properties of loach peptide. Int. J. Food Sci. Technol. 2012, 47, 2574–2581. [Google Scholar] [CrossRef]
- Navarro del Hierro, J.; Gutiérrez-Docio, A.; Otero, P.; Reglero, G.; Martin, D. Characterization, antioxidant activity, and inhibitory effect on pancreatic lipase of extracts from the edible insects Acheta domesticus and Tenebrio molitor. Food Chem. 2019, 309, 125742. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Baran, T.; Asan-Ozusaglam, M.; Cakmak, Y.S.; Tozak, K.O.; Mol, A.; Mentes, A.; Sezen, G. Extraction and characterization of chitin and chitosan with antimicrobial and antioxidant activities from cosmopolitan orthoptera species (Insecta). Biotechnol. Bioproc. E. 2015, 20, 168–179. [Google Scholar] [CrossRef]
- Horvath-Szanics, E.; Perjéssy, J.; Klupács, A.; Takács, K.; Nagy, A.; Koppány-Szabó, E.; Hegyi, F.; Németh-Szerdahelyi, E.; Du, M.Y.; Wang, Z.R.; et al. Study of chinitase and chitinolytics activity of Lactobacillus strains. Acta Aliment. 2020, 49, 214–224. [Google Scholar] [CrossRef]
- Daskaya-Dikmen, C.; Yucetepe, A.; Karbancioglu-Guler, F.; Daskaya, H.; Ozcelik, B. Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Plants. Nutrients 2017, 9, 316. [Google Scholar] [CrossRef]
- Jia, J.; Wu, Q.; Yan, H.; Gui, Z. Purification and molecular docking study of a novel angiotensin-I converting enzyme (ACE) inhibitory peptide from alcalase hydrolysate of ultrasonic-pretreated silkworm pupa (Bombyx mori) protein. Process. Biochem. 2015, 50, 876–883. [Google Scholar] [CrossRef]
- Wu, Q.; Jia, J.; Yan, H.; Du, J.; Gui, Z. A novel angiotensin-I converting enzyme (ACE) inhibitory peptide from gastrointestinal protease hydrolysate of silkworm pupa (Bombyx mori) protein: Biochemical characterization and molecular docking study. Peptides 2015, 68, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Ma, H.; Luo, L.; Yin, X. Angiotensin I-converting enzyme (ACE) inhibitory peptide derived from Tenebrio molitor (L.) larva protein hydrolysate. Eur. Food Res. Technol. 2013, 236, 681–689. [Google Scholar] [CrossRef]
- Sanchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Karaś, M.; Rybczyńska-Tkaczyk, K.; Zielińska, E.; Zieliński, D. Current Trends of Bioactive Peptides—New Sources and Therapeutic Effect. Foods 2020, 9, 846. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).