Abstract
Background: Fermented foods rich in bioactive compounds have been proposed as potential strategy to combat non-communicable diseases. Among them is kombucha, a beverage fermented from sugared Camellia sinensis tea by a symbiotic culture of bacteria and yeasts (SCOBY). Recently, there has been an increased focus on assessing the actual effect of this beverage on human health. In this manner, this systematic review aimed to gather clinical evidence on the impact of kombucha consumption on human health. Methods: The databases Cochrane CENTRAL, MEDLINE/PubMed, and Embase® were searched, and the risk of bias tool used was the Critical Appraisal Tools outlined in the Joanna Briggs Institute. This review followed the PRISMA guidelines and was registered on PROSPERO (CRD42024599464). Results: Eight clinical trials were included (two pre- and post-interventions and six randomized controlled trials) with durations ranging from 10 days to 10 weeks. Two studies reported beneficial effects of kombucha on gastrointestinal symptoms, such as reduced intensity of constipation-related complaints. Two trials observed changes in gut microbiota composition, including increased abundance in Bacteroidota, Akkermansiaceae, Saccharomyces, and Weizmannia coagulans, alongside reductions in Ruminococcus, Dorea, and Rhodotorula. Moreover, five clinical trials evaluated glucose metabolism, evidencing inconsistent results, and other studies identified improvements in salivary microbiota composition and serum metabolomic profile. Conclusion: These findings suggest that kombucha consumption may provide health benefits, particularly in alleviating gastrointestinal symptoms, and demonstrates a modest capacity for modulating gut and salivary microbiota, as well as metabolomic profiles. Although the results are promising, the heterogeneity of the studies and the limited number of available clinical trials highlight the need for further robust research to confirm these effects.
1. Introduction
Non-communicable diseases (NCDs) are currently the leading cause of morbidity and mortality worldwide, with cardiometabolic health being a key contributor to this scenario []. According to the World Obesity Atlas 2025, the number of adults living with obesity is projected to increase by more than 115% between 2010 and 2030, rising from 524 million to 1.13 billion individuals. This alarming trend is closely linked to the growing prevalence of obesity-related conditions, including cardiovascular diseases and type 2 diabetes (T2D) []. In individuals with obesity, there is an elevated production of inflammatory cytokines and reactive species of oxygen (ROS), which exacerbates the low-grade systemic inflammation and oxidative stress []. These alterations contribute to a state of metabolic imbalance that also affects the human microbiome, promoting dysbiosis characterized by reduced microbial diversity, a decline in beneficial species, and an increase abundance of pathogenic bacteria []. Furthermore, intestinal health is compromised by the translocation of lipopolysaccharide (LPS) across the epithelial barrier, contributing to the increased intestinal permeability, which further reinforces metabolic disturbances [].
Despite the high prevalence of these conditions, a significant proportion of affected individuals remain untreated. For example, approximately 30% of men and women with T2D do not receive adequate medical care []. Among the strategies for prevention and management of NCDs, dietary interventions play a central role, particularly the adoption of dietary patterns rich in minimally processed, plant-based foods that are naturally high in bioactive compounds []. In this context, fermented foods have gained attention as potential dietary strategies for improving metabolic health, especially kombucha—a beverage traditionally prepared with sugared Camellia sinensis fermented by a symbiotic culture of bacteria and yeasts (SCOBY) [].
Unlike sugar-sweetened ultra-processed beverages, kombucha contains significantly lower amounts of residual sugar and is rich in bioactive substances [,]. The fermentation process enhances the concentration of phenolic compounds and organic acids, such as gluconic, glucuronic (GlcUA), D-saccharic-1,4-lactone (DSL), and acetic acids—molecules known for their antioxidant and antimicrobial activities []. In addition to these acids, the beverage contains sugars, amino acids, B-complex and C vitamins, minerals, and phenolic compounds. The type and concentration of different phenolic compounds will depend on the tea used. For example, green tea provides a higher content of catechins, while black tea can also ensure other compounds such as theaflavin and thearubigin [].
Additionally, the presence of live microorganisms in kombucha may contribute to overall gut health, supporting intestinal barrier function and potentially counteracting dysbiosis commonly observed in individuals with obesity and related comorbidities [,]. The symbiotic interaction between yeasts and acetic acid bacteria during fermentation is central to the formation of the bioactive compounds. Yeasts hydrolyze sucrose into glucose and fructose, leading to ethanol and CO2 (carbon dioxide) production, while bacteria convert glucose and ethanol into various acids and bacterial cellulose, forming the biofilm of SCOBY [] (Figure 1). Several factors, including time, tea type, addition of flavorings, and even environmental and processing conditions, influence the final composition of kombucha and its potential health benefits [,,,]. These factors will also impact the microbial profile of kombucha. In general, the more prevalent yeast species are Dekkera sp., Saccharomyces cerevisiae, Brettanomyces sp., Zygosaccharomyces sp., Pichia, and Schizosaccharomyces, and the more common bacteria are acetic acid bacteria from the genera Komagataeibacter, Oenococcus, Acetobacter, and Gluconobacter [,].
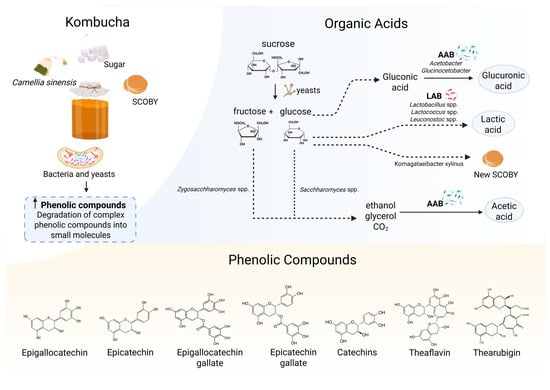
Figure 1.
Mechanisms involved in kombucha fermentation and the final composition rich in phenolic compounds and organic acids. Elaborated using © 2025 BioRender https://www.biorender.com/ (Accessed on 2 May 2025). During the fermentation of sugared Camellia sinensis tea, a symbiotic interaction between yeasts and bacteria is activated. Yeasts hydrolyze sucrose into glucose and fructose, which are further metabolized intro ethanol and CO2 (carbon dioxide). Bacteria subsequently convert glucose and ethanol into various organic acids (e.g., glucuronic, acetic acids) and synthesize bacterial cellulose, which contributes to the formation of a new symbiotic culture of bacteria and yeasts (SCOBY) biofilm. Additionally, complex phenolic compounds present in the tea are broken into smaller molecules (e.g., catechins), leading to an increased concentration of bioavailable phenolic compounds. These biochemical transformations are responsible for the beverage’s antioxidant and anti-inflammatory properties.
Animal models and in vitro studies observed that kombucha may help in cardiometabolic health through inhibition of adipogenesis and promotion of lipolytic activity [], along with attenuation of inflammation [] and modulation of gut microbiota [,,]. Moreover, an antidiabetic effect [] and improvements in lipid profile as well as hepatic and renal health have been observed []. One systematic review focused on fifteen animal model studies concluded that the beverage consumption may help in the prevention and treatment of comorbidities associated with obesity along with gut microbiota modulation []. In this manner, kombucha may represent a plant-based fermented beverage with promising implications for human health. To our knowledge this is the first systematic review to summarize the actual impact of kombucha on human health based on clinical trials. This review aims to provide a comprehensive and critical overview of the available evidence on the effect of kombucha consumption, highlighting current gaps and guiding future research.
2. Materials and Methods
2.1. Protocol, Eligibility Criteria, and Search Strategy
This systematic review was carried out in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement, as detailed in the PRISMA checklist (Supplementary Material Table S1). The review protocol was prospectively registered in the International Prospective Register of Ongoing Systematic Reviews (PROSPERO) under registration number CRD42024599464 (available at: https://www.crd.york.ac.uk/PROSPERO/ (accessed on 30 October 2024).
2.2. Search Strategy and Eligibility Criteria
The primary eligibility question guiding this review was: “How does kombucha consumption influence human health?” To determine study eligibility, we applied the PICOS framework (Population, Intervention, Comparison, Outcomes, and Study design), as outlined in Table 1.

Table 1.
PICOS framework for studies eligibility.
The inclusion criteria for study selection were (1) original research with quasi-experimental design evaluating pre- and post-intervention data or randomized controlled trials (RCT); (2) adults aged 18 or older, either healthy or diagnosed with cardiometabolic disorders, such as diabetes or obesity; and (3) intervention involving original or flavored kombucha intake. The main outcomes were cardiometabolic health (such as parameters associated with glucose and lipid metabolism, hepatic enzymes, and renal health); inflammation markers (e.g., inflammatory cytokines); intestinal health (gastrointestinal symptoms, microbiota modulation) and intestinal markers (short chain fatty acids, tight junction’s proteins and fecal pH); and anthropometry and body composition.
Exclusion criteria comprised (1) studies employing kombucha together with other fermented foods (e.g., kefir, kimchi, etc.); (2) research that did not assess the influence of kombucha intake on the main outcomes described; (3) studies focusing on pediatric populations or individuals with cancer or autoimmune conditions; and (4) case reports, abstracts, reviews, editorials, book chapters, theses, dissertations, letters to the editor, conference proceeding, clinical trial registrations, articles with duplicated data from the same clinical trial, and investigations conducted in animals or in vitro.
Searches for relevant publications were initially conducted on 7 November 2024, and subsequently updated on 4 May 2025. The databases searched were Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE/PubMed, and Embase®. The search strategy was constructed using Medical Subject Headings (MeSH) and Emtree terms in English and combined using Boolean operators (AND/OR/NOT) by two independent reviewers (G.M.F. and D.B.B.) working in parallel. Full search strategies for each database are presented in the Supplementary Material. An additional independent search was made by the same reviewers using the reference lists of the selected articles to identify other potentially relevant studies. No restrictions were applied regarding language or publication date.
2.3. Study Selection and Data Extraction
The identification of eligible studies followed a two-step process, beginning with title and abstract screening and proceeding to full-text analysis. Search outputs from each database were uploaded to Rayyan Platform (https://www.rayyan.ai/) (accessed on 7 November 2024) [], where duplicate records were excluded. Two reviewers (G.M.F. and D.B.B.) independently performed the selection process using a double-blind method. In cases of divergence during the initial screening phase, the articles were advanced to the full-text review for further consideration. Full-text articles were accessed through institutional subscriptions, and disagreements following this stage were resolved through consensus.
Data extraction covered key information, including author names, year, study design and duration, participant characteristics, kombucha type and dosage, control and comparison groups with sample size and assessed outcomes. All extracted data were organized in a standardized Microsoft Excel® spreadsheet (Microsoft 365, version 2408) and summarized in tables (Section 3, Table 2 and Table 3). The initial extraction was conducted by one reviewer (D.B.B.) using a structured data collection form, which was subsequently cross-checked by a second reviewer (G.M.F.). Any inconsistencies were discussed and resolved by mutual agreement.
2.4. Assessment of Risk of Bias
All studies included in the review underwent a comprehensive appraisal conducted by two independent evaluators (R.S.P. and C.M.T.). Any discrepancies in the assessment were resolved by a third researcher (G.M.F). The methodology of quality was based on the Joanna Briggs Institute (JBI) Critical Appraisal Tools for randomized controlled trials and quasi-experimental (non-randomized) designs []. Following the JBI guidance, the reviewers pre-established criteria for rating the methodological rigor of each study. The level of bias was classified according to the proportion of affirmative (“yes”) responses: low risk (≥70%), moderate risk (50–69%), and high risk (<50%) [].
3. Results
3.1. Description of Included Studies
A total of 974 articles were retrieved using CENTRAL, MEDLINE/PubMed, and Embase® databases searches. After removing duplicates in Rayyan Platform (https://www.rayyan.ai/) (accessed on 7 November 2024) [], 649 titles remained for evaluation. During title and abstract screening, 624 records were excluded based on the exclusion criteria. Twenty-five records remained for full-text assessment. Of these, ten corresponded to methodological records, five referred to abstracts published in conference proceedings, and one was a dissertation. Consequently, nine articles were assessed in full, of which one was excluded because it was not a clinical trial. In the end, eight articles were included in this systematic review (Figure 2).
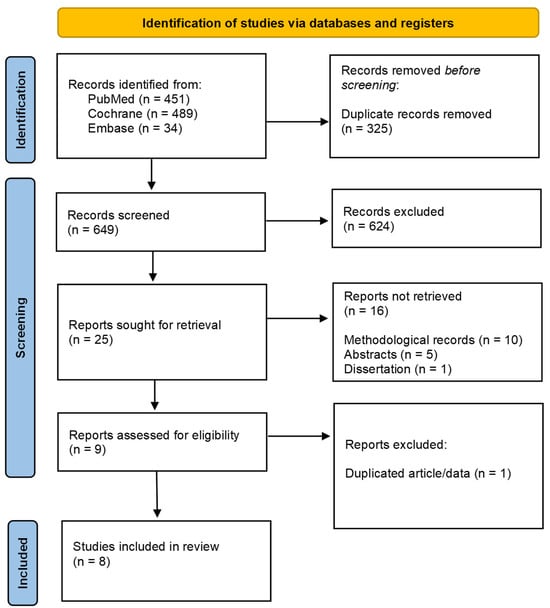
Figure 2.
PRISMA flowchart.
3.2. Characteristics of Included Studies
The eight studies included in this systematic review were published between 2022 and 2024. Of these, three were pre- and post- interventions [,,], and five were randomized clinical trials [,,,,], one of which was double-blind and crossover []. The interventions compromised both an acute study [] and chronic protocols, lasting from 10 days to 10 weeks.
Considering the number of participants in each study, a total of 303 individuals were evaluated in this review, all adults, with the majority aged between 18 and 45 years (n = 225; 74.25%). The participants included individuals with irritable bowel syndrome with a predominance of constipation [], healthy individuals [,], individuals with type 2 diabetes mellitus [], individuals with or without obesity [,], and individuals with excess body weight [,].
The interventions used different types of kombucha: green tea kombucha (n = 3) [,,], black tea kombucha (n = 2) [,], pasteurized black tea kombucha enriched with inulin and B vitamins (n = 1) [], organic green tea and oolong tea kombucha (n = 1) [], and kombucha prepared by mixing green tea with black tea (n = 1) []. The doses administered ranged from 200 mL to 473.18 mL. Control groups did not receive kombucha. In two studies, control group participants consumed water (carbonated or still) [,], while in one study, they were offered a non-fermented carbonated beverage []. In two studies, the kombucha intervention was combined with energy-restricted diet, while the control group received only the dietary intervention [,].
3.3. Kombucha Production and Characterization
Given that the final composition of kombucha can vary significantly depending on factors such as the type of tea, sugar concentration, fermentation time, and temperature. Table 2 in this section aims to describe the production methods and characterization of the kombucha used in each study included in this systematic review.

Table 2.
Kombucha production and characterization in the studies included in this systematic review.
Table 2.
Kombucha production and characterization in the studies included in this systematic review.
| Study | Type of Tea | Fermentation Details | Additional Ingredients | Key Components per Serving |
|---|---|---|---|---|
| Pilipenko et al. (2022) [] | Black tea | Pasteurized non-alcoholic kombucha-based beverage with inulin and B vitamins. Fermentation details are not provided. | Inulin, B1, B2, B3, B6, folic acid, and natural flavor additives such as blackcurrant berries, juniper, strawberry, lemon leaves, mango, and passion fruit | 2.57 g of carbohydrates (sucrose: 0.22 g; glucose: 1.32 g; fructose: 1.03 g), 1.15 g inulin, 0.4 mg of vitamin B1, 0.21 mg of B2, 2.74 mg of B3, 0.48 mg of B6, and 24 mcg of folic acid, and 13 kcal per 100 mL |
| Atkinson et al. (2023) [] | 75% green tea and 25% oolong tea | Commercial organic kombucha (The Good Brew Kombucha Company Pty Ltd., Brunswick, VIC, Australia) | None reported | ~200 probiotic species, high concentration of polyphenols, and 3 g of available carbohydrates (1.7 g sugars) per 330 mL |
| Mendelson et al. (2023) [] | Green tea | Traditional fermentation with SCOBY + second fermentation in barrels. Produced by Craft Kombucha (Washington, DC, USA). | 10% sucrose, 0.6% freeze-dried ginger powder | Not specified |
| Fraiz et al. (2024a) []; Costa et al. (2025) [] | Black tea | Following Brazilian MAPA guidelines. 12 g tea + 50 g sugar/L. 7 days at 25 °C in BOD incubator | None | 2.6 g of sucrose, 138 mg of total phenolic, 0.24 g of theaflavin, and 3.8 g of thearubigin per 200 mL |
| Fraiz et al. (2024b) []; Fraiz et al. (2024c) [] | Green tea | Following MAPA guidelines. 12 g tea + 50 g sugar/L. pH adjusted to 4.2–4.4 with fermented kombucha. 5 days at 25 °C in BOD incubator. | None | 4.5 g of sucrose, 64 mg of total phenolics, and 92 phenolic compounds per 200 mL |
| Ecklu-Mensah et al. (2024) [] | Commercial kombucha with patented probiotic mix: Bacillus coagulans, Saccharomyces boulardii, Lactobacillus sp. | Kiwi and ginger juice | Bacillus coagulans (1 billion), S. boulardii (4 billion), Lactobacillus (1 billion) 100 mg lactic, 75 mg acetic, 1400 mg glucuronic, and 650 mg gluconic acids per 16 oz |
3.4. Dietary Interventions
This section describes the dietary protocols adopted in each clinical trial included in this review. It is important to emphasize that the participants’ dietary patterns during the interventions may influence the outcomes. Across the studies, dietary approaches varied widely, ranging from habitual diet to structured meal plans.
In the study by Pilipenko et al. (2022) [], participants followed a standard diet in accordance with Order No. 330 of the Ministry of Health of the Russian Federation. The addition of the studied beverage to the diet increased the intake of 28.6 kcal and 5.6 g of carbohydrates. Further details about the diet were not provided. In the study by Mendelson et al. (2023) [], participants were instructed to consume 240 mL of the provided kombucha with dinner. In addition, participants performed blood glucose measurements after an overnight fast. Similarly, no further dietary guidelines were reported.
In the interventions by Fraiz et al. (2024a) [] and Costa et al. (2025) [], participants were instructed to maintain their usual diet, and a food frequency questionnaire (FFQ) was administered to participants to assess their habitual food consumption before and after the intervention. Additionally, diet quality indices were also calculated, including the dietary inflammatory index, total dietary antioxidant capacity, and total dietary polyphenols [].
By contrast, there were two clinical trials [,] in which participants from both experimental groups followed a meal plan with an energy deficit of 500 kcal relative to their estimated daily caloric needs, calculated based on the estimated energy requirement (EER) formula. Five different menus were prepared using the IBGE Food Composition Tables (2008–2009) as a reference. The macronutrient distribution followed the guidelines for obesity treatment, with 55% calories from carbohydrates, 30% from fats, and 15% from proteins. Additionally, participants received qualitative nutritional guidance, including explanations about the healthy plate model, the effects of food processing levels, and strategies for more conscious food choices, with practical shopping tips. Materials containing all the recipes from the menus were also provided. Finally, individuals were instructed to strictly adhere to the proposed preparations, avoiding substitutions of foods or meals between the different plans.
Finally, in the study by Ecklu-Mensah et al. (2024) [], all participants followed a diet low in fiber and polyphenols, characteristic of a Western dietary pattern, for four weeks. In the subsequent phase, participants were randomized, and those allocated to the intervention group began consuming kombucha daily without changing their Western diet. Conversely, the control group maintained their usual Western dietary pattern without the addition of the beverage. Adherence to the intervention and dietary restrictions was assessed by self-report, with participants being asked about their regular consumption of the provided kombucha and their ability to limit or avoid foods listed in the provided guidelines.
3.5. Risk of Bias Assessment
The assessment of the risk of bias in the eight studies included in this review indicated that all of them presented a low risk of bias, with more than 70% of the questions answered with “Yes”. Among the five RCTs, two questions were predominantly answered with “No” (60%), both related to blinding: “Were the participants blinded to treatment allocation?” and “Were the professionals responsible for the intervention blinded to treatment allocation?”. In addition, the question “Were the outcome assessors blinded to treatment allocation?” was answered as “Unclear” in all studies evaluated. The remaining questions were mostly answered with “Yes” (Figure 3).
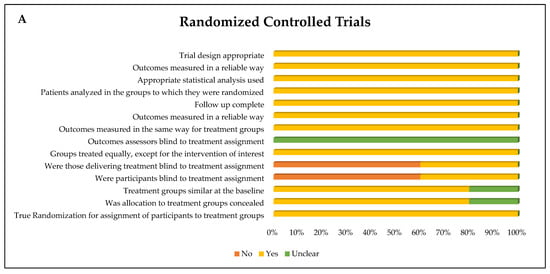
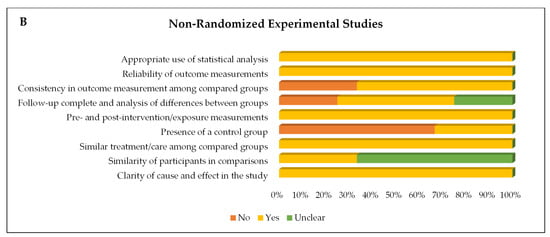
Figure 3.
Risk of bias of (A) randomized controlled trials and (B) quasi-experimental trials (non-randomized) represented by each item assessed and distribution of risk of bias across the studies included in the systematic review.
In the three non-randomized clinical trials, the question regarding the “Presence of a control group” was answered with “No” in two studies (66.7%). Furthermore, in two other studies (66.7%), the question “Similarity between participants in the comparison groups” was classified as “Unclear”. The other questions received a “Yes” response from all studies (Figure 3).
3.6. Evaluated Outcomes
All the analytical procedures used in the evaluated markers of the included clinical trials are described in Appendix A.
3.6.1. Gastrointestinal Symptoms and Intestinal Health Markers
Two studies evaluated the effect of kombucha intake on gastrointestinal symptoms [,]. Both studies reported beneficial effects, mainly regarding stool consistency and the symptom of incomplete bowel emptying. One of them reported an improvement in stool consistency, along with a reduction in the sensation of incomplete evacuation, compared to the control group []. Additionally, following kombucha consumption, an improvement in bowel movement frequency was also observed compared to baseline. This study also demonstrated that kombucha did not affect symptoms such as dry mouth, bitter taste, heartburn, abdominal pain, nausea, a feeling of heaviness in the stomach, or abdominal distension []. The second study demonstrated positive effects of green tea kombucha supplementation (200 mL) on the Gastrointestinal Symptom Rating Scale (GSRS) questionnaire [] in individuals with excess body weight []. After ten weeks of intervention compared to the control group, there was a decrease on the total GSRS score, reflux, air-filled stomach, stool consistency, and symptoms of incomplete bowel emptying. However, no effects of the beverage on the Bristol Stool Scale (BSS) were observed [].
One study described self-reported gastrointestinal discomforts following a daily intake of 473.18 mL/day (16 oz/day) of kombucha []. The most frequently mentioned symptoms were diarrhea and boating (31.25%) among the kombucha consumers, in contrast to 12.5% in the control group. However, no validated questionnaire was used to assess the effects mentioned in this study, and no statistical analysis was conducted to determine whether the differences between groups were statistically significant. Therefore, these findings should be interpreted cautiously [].
Regarding the intestinal health markers, kombucha consumption did not exert significant effects on intestinal permeability and integrity or on markers of endotoxemia in the studies that evaluated these parameters [,]. Costa et al. (2025) did not identify positive effects on lactulose and mannitol excretion, zonulin, lipopolysaccharide-binding protein (LBP), or short-chain fatty acids (SCFAs) in individuals with and without obesity, compared to baseline values []. Similarly, in a ten-week study with green tea kombucha (200 mL), no changes were observed in fecal pH, butyric acid, acetic acid, propionic acid, LBP, zonulin, or lactulose and mannitol excretion when compared to the control group in individuals with excess body weight after ten weeks of intervention []. Nevertheless, it is important to note that, in the same study, mannitol excretion and butyric acid levels were reduced when compared to baseline values in the kombucha group [].
3.6.2. Changes in Fecal and Salivary Microbiota and Metabolic Functional Pathways
Three studies in this review evaluated the fecal microbiota, and all of them reported beneficial effects of kombucha on shifts in microbial profiles [,,] (Table 3). Costa et al. (2025) reported that the consumption of black tea kombucha (200 mL) for ten weeks promoted effects on bacterial abundance after the intervention (baseline vs. endpoint), such as an increased abundance of Pichia and Dekkera, in addition to fungal diversity (particularly with an increase in Saccharomyces) in eutrophic individuals. In individuals with obesity, the same study identified increases in Bacteroidota, Akkermansiaceae, Subdoligranulum, Pichia, Dekkera, and fungal diversity (with a greater abundance of Saccharomyces), along with reductions in the bacterial genera Ruminococcus and Dorea and in the fungal species Exophiala and Rhodotorula. A decrease in the Bacillota/Bacteroidota ratio was also observed in both groups, with no changes in alpha diversity (Shannon and Chao1) or beta diversity markers []. In the study by Ecklu-Mensah et al. (2024), enrichment in the kombucha-containing bacteria, Weizmannia coagulans, was observed when compared to the control group after the intervention, and differences in beta diversity between the groups were also observed. Alpha diversity (measured by the Shannon index) decreased in the kombucha group when comparing the end of intervention with baseline. Still, there were no significant changes between the control and kombucha groups. The authors also performed a correlation analysis between the species that significantly differentiated after the kombucha intake with biochemical markers, but they did not find any significant Spearman coefficient values [].
Finally, in the third study, the analysis of differential abundance, changes after the consumption of green tea kombucha (200 mL) for ten weeks did not lead to significant microbial shifts between groups after Benjamini–Hochberg correction; however, trends toward variations were observed at family, genus and species levels []. For instance, at the genus level, there was a reducing trend in Alistipes, Odoribacter, and Parabacteroides and an increased abundance of Romboutsia in the kombucha group compared to the control group. No significant changes were observed between groups regarding the Bacillota/Bacteroidota ratio as well as alpha (Chao1 and Shannon indices) and beta diversity based on Bray–Curtis dissimilarity. However, alpha diversity measured using the Chao1 index significantly increased at genus level only in the kombucha group [].
Only one study evaluated the metabolic functional pathways after kombucha consumption []. A total of 37 MetaCyc pathways showed significant differences in relative abundance between the groups, with 22 pathways being more abundant among participants who consumed kombucha. Among the enriched pathways were those related to biosynthesis of nucleotide and nucleoside, as well as small molecules such as electron carriers, vitamins, and sulfur. Other pathways were decreased, such as those involving secondary metabolites biosynthesis, e.g., carotene, and siderophores.
In relation to the changes in salivary microbiota, only one study included in this review was a pioneer in evaluating the effects of kombucha on the salivary microbiota of individuals with excess body weight, and the results were promising []. Compared to the control group, salivary alpha diversity using the Chao1 index increased at the genus level. Moreover, the Bacillota/Bacteroidota ratio decreased in the kombucha group, and beta diversity based on Bray–Curtis dissimilarity differed between groups at the species level after intervention. The authors also found several beneficial shifts in the microbial profile with a decreased abundance of potential pathogenic bacteria such as Veillonella, Eubacterium, and Prevotella pallens [].
3.6.3. Cardiometabolic Health and Inflammation
Five studies included in this review evaluated the effects of kombucha consumption on glucose metabolism, and the results were inconsistent [,,,,]. Atkinson et al. (2023) observed a positive effect of the acute consumption of green and oolong tea kombucha (330 mL) on glycemic and insulin indices in healthy individuals, compared to the control group []. In an 8-week study with black tea kombucha (200 mL), insulin and HOMA-IR were reduced in individuals with obesity, compared to baseline, which was not observed in the group without obesity. Furthermore, in the same study, fasting blood glucose (FBG) and TyG index were not affected by kombucha consumption in the two groups []. Pilipenko et al. (2022) observed no effects on fasting blood glucose compared to baseline [], and Ecklu-Mensah and collaborators (2024) also found no significant differences compared to the control group in insulin, fasting blood glucose, glycated hemoglobin (HbA1c), or homeostatic model assessment of insulin resistance (HOMA-IR) in healthy individuals after four weeks of consumption of a mixture of green tea and black tea kombucha []. However, surprisingly, fasting insulin and HOMA-IR increased within the kombucha group over time (baseline vs. final intervention) []. Finally, Mendelson et al. (2023), after four weeks of intervention, observed no effects of green tea kombucha (240 mL) on fasting blood glucose in individuals with type 2 diabetes mellitus when compared to the control group, although a reduction from baseline was noted. Moreover, when analyzing only participants with baseline glucose levels above 130 mg/dL (n = 5), the kombucha group showed a significantly greater decrease in fasting glucose compared to the placebo group [].
The three studies investigating the effects of kombucha on other markers of metabolic health demonstrated that kombucha consumption does not appear to have effects on lipid metabolism, liver, or kidney markers. In the study by Pilipenko et al. (2022), compared to baseline, the markers ALT, AST, GGT, bilirubin, creatinine, urea, total protein, potassium, sodium, and iron were not affected by the consumption of pasteurized black tea kombucha enriched with inulin and B vitamins (220 mL) for ten days in individuals with constipation-predominant IBS. Similarly, in the study by Ecklu-Mensah et al. (2024), the consumption of kombucha prepared from a mixture of green and black tea (473.18 mL) for eight weeks did not alter the total cholesterol, HDL, LDL, triglyceride (TG), non-HDL cholesterol, creatinine, diastolic and systolic blood pressure, eGFR, potassium, or sodium in healthy individuals, compared to the control group. The third study also identified that the consumption of black tea kombucha for eight weeks did not impact total cholesterol, HDL, LDL, TG, fatty liver index (FLI), ALP, ALT, AST, creatinine, or urea in individuals with and without obesity, compared to baseline []. Besides that, in the group with obesity, a positive effect on the reduction of GGT was observed. Additionally, an increase in total cholesterol and ALP was observed in the eutrophic group. However, when the authors stratified the group considering the diet quality, they observed that the participants who increased the dietary inflammatory index and decreased dietary total antioxidant capacity experienced worsening of these cardiometabolic markers. This may indicate that kombucha alone was not sufficient to counteract the effects of a diet low in antioxidant and anti-inflammatory foods in this population [].
Two studies included in this review evaluated inflammatory markers [,]. Interestingly, in a randomized controlled trial involving individuals with excess body weight following an energy-restricted diet supplemented with green tea kombucha for ten weeks, interleukin (IL)-6 levels increased in both groups; however, this increase reached statistical significance only in the control group. This suggests that the kombucha’s antioxidant and anti-inflammatory properties may have mitigated the rise of this pro-inflammatory cytokine during the dietary intervention in the kombucha group. No differences were observed between the groups regarding the other inflammatory markers analyzed: IL-8, IL-10, IL-1β, TNF, IL-12, and CRP []. In the study by Ecklu-Mensah et al. (2024), the consumption of kombucha prepared from a mixture of green and black tea for eight weeks by healthy individuals did not alter IL-10, IL-6, or CRP compared to the control group [].
3.6.4. Anthropometry and Body Composition
Based on three studies included in this review, kombucha consumption does not appear to affect anthropometry and body composition [,,] (Table 3). In healthy individuals who consumed 473.18 mL of kombucha from a blend of green and black tea, no significant effect on BMI, waist circumference, or body weight was observed []. After consumption of green tea kombucha (200 mL) combined with an energy-restricted diet, individuals with excess body weight presented improvements in anthropometric and body composition markers (BMI, body weight, body fat, waist, hip and neck circumferences, waist-to-height ratio, waist-to-hip ratio, conicity index, and others) compared to baseline; however, these changes were also present in the control group []. No differences between groups at the end of intervention were identified, with the exception of a higher decrease in the lipid accumulation product (LAP), a cardiometabolic risk index, in the kombucha group []. Lastly, a study that evaluated the intake of black tea kombucha in individuals with and without obesity did not observe anthropometric differences between baseline and after eight weeks of intervention [].

Table 3.
Characteristics of included articles that evaluated the impact of kombucha consumption on human health parameters.
Table 3.
Characteristics of included articles that evaluated the impact of kombucha consumption on human health parameters.
| Author (Year) | Study Design and Duration | Kombucha Type and Dosage | Sample Characteristics | Groups | Main Health Outcomes |
|---|---|---|---|---|---|
| Pilipenko et al. (2022) [] | Clinical trial 10 days | Pasteurized black tea kombucha, enriched with inulin and B vitamins (220 mL) | Individuals with constipation-predominant IBS Age: 18 to 80 years | G1: Kombucha; n = 20 G2: Control (water); n = 20 | G1 vs. G2: improves stool consistency and ↓ sensation of incomplete evacuation G1 vs. baseline: ↑ stool frequency G1 vs. baseline: ↔ bilirubin, creatinine, urea, ALT, AST, GGT, FBG, total protein, potassium, sodium, iron |
| Atkinson et al. (2023) [] | Randomized, placebo-controlled, crossover study Acute | Organic green tea and oolong kombucha (330 mL) | Healthy individuals Age: 18 to 45 years | G1: Kombucha; n = 11 G2: Control (sparkling water); n = 11 G3: Placebo (diet lemonade soda); n = 11 | G1 vs. G2 and G3: ↓ glycemic index G1 vs. G2: ↓ insulin index |
| Mendelson et al. (2023) [] | Randomized, double-blind, crossover clinical trial 4 weeks | Green tea kombucha (240 mL) | Individuals with type 2 diabetes mellitus Age: >18 years | G1: Kombucha; n = 12 G2: Placebo (unfermented carbonated beverage); n = 12 | G1 vs. baseline: ↓ FBG G1 vs. G2: ↔ FBG |
| Fraiz et al. (2024a) [] | Clinical trial 8 weeks | Black tea kombucha (200 mL) | Individuals with or without obesity Age: 18 to 45 years | G1: Kombucha—without obesity; n = 20 G2: Kombucha—with obesity; n = 16 | G1 vs. baseline: ↑ total cholesterol, ALP G1 vs. baseline: ↔ ALT, AST, FBG, FLI, GGT, HDL, HOMA-IR, LDL, TG, TyG, insulin, creatinine, urea G2 vs. baseline: ↓ insulin, HOMA-IR, GGT G2 vs. baseline: ↔ ALP, ALT, AST, TC, FLI, FBG, HDL, LDL, TG, TyG, creatinine, urea |
| Fraiz et al. (2024b) [] | Randomized clinical trial 10 weeks | Green tea kombucha (200 mL) | Individuals with excess body weight Age: 18 to 45 years | G1: Kombucha + energy-restricted diet; n = 30 G2: Control (energy-restricted diet); n = 29 | G1 vs. G2: ↑ salivary alpha and beta diversity G1 vs. G2: ↓ IL-6, Bacillota/Bacteroidota ratio, and bacterial species (e.g., Schaalia odontolytica, Lachnoanaerobaculum umeaense, Veillonella dispar, Prevotella pallens) G1 vs. baseline: ↓ weight, BMI, WC, HC, NC, waist-to-height ratio, conicity-index, ABSI, AVI, BRI, LAP, trunk BF, android BF, gynoid BF, total BF, android MM, gynoid MM, total MM, trunk BF, android BF, gynoid BF, total BF, IL-1β, IL-8 |
| Fraiz et al. (2024c) [] | Randomized clinical trial 10 weeks | Green tea kombucha (200 mL) | Individuals with excess body weight Age: 18 to 45 years | G1: Kombucha (+energy-restricted diet); n = 30 G2: Control (energy-restricted diet); n = 29 | G1 vs. G2: ↓ gastrointestinal symptoms (total score, reflux, having a stomach full of air, hard stools, and not completely emptying the intestine), ↑ putative metabolites G1 vs. baseline: ↑ total score of quality of life and domains (general health, vitality, and role of emotional), alpha diversity (Chao1), ↓ mannitol excretion and butyric acid G1 vs. G2: ↔ quality of life, intestinal permeability markers, fecal microbiota abundance, Bacillota/Bacteroidota ratio, alpha and beta diversity |
| Costa et al. (2025) [] | Clinical trial 8 weeks | Black tea kombucha (200 mL) | Healthy individuals or those with excess body weight Age: 18 to 45 years | G1: Kombucha—without obesity; n = 21 G2: Kombucha—with obesity; n = 17 | G1 vs. baseline: ↑ fungal diversity and bacterial and fungal abundance (↑ Pichia, Dekkera, Saccharomyces) G2 vs. baseline: ↑ fungal diversity and bacterial and fungal abundance (↑ Bacteroidota, Akkermanciaceae, Subdoligranulum, Pichia, Dekkera, Saccharomyces;↓ Ruminococcus, Dorea, Exophiala, Rhodotorula) G1 and G2 vs. baseline: ↓ Bacillota/Bacteroidota ratio G1 and G2 vs. baseline: ↔ lactulose and mannitol excretion, zonulin, LBP, SFCA, alpha and beta diversity |
| Ecklu-Mensah et al. (2024) [] | Randomized clinical trial 8 weeks | Kombucha from a mixture of green tea and black tea (473.18 mL) | Healthy individuals Age: 21 to 55 years | G1: Kombucha; n = 16 G2: Control (without kombucha); n = 8 | G1 vs. baseline: ↑ insulin, HOMA-IR, and bacterial abundance (Weizmannia coagulans), ↓ Shannon index G1 vs. G2 and vs. baseline: ≠ beta diversity G1 vs. G2: ↔ anthropometric/body composition parameters, cardiometabolic and inflammation markers, alpha diversity and OGUs |
↔ indicates no difference, ↓ indicates a reduction, ↑ indicates an increase in the score. ABSI = A Body Shape Index; ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate aminotransferase; AVI = abdominal volume index; BF = body fat; BMI = body mass index; BRI = body roundness index; BP = blood pressure; BSS = Bristol Stool Scale; CG = control group; CRP = C-reactive protein; EGFR = estimated glomerular filtration rate; FBG = fasting blood glucose; FLI = Liver Fat Index; G1, 2, 3 = Group 1, 2, 3; GGT = gamma-glutamyl transferase; GSRS = Gastrointestinal Symptom Rating Scale; HbA1c = hemoglobin glycated; HC = hip circumference; HDL = high-density lipoprotein; HOMA-IR = homeostasis model assessment-insulin resistance; IBS = irritable bowel syndrome; IL = interleukin; KG = kombucha group; LAP = lipid accumulation product; LBP = lipopolysaccharide-binding protein; L/M = lactulose/mannitol ratio; LDL = low-density lipoprotein; MM = muscle mass; NC = neck circumference; OGUs = operational genomic units; SF-36 = 36-Item Short Form Health Survey; SFCA = short-chain fatty acids; TC = total cholesterol; TG = triglycerides; TyG = triglyceride/glucose index; WC = waist circumference.
3.6.5. Quality of Life and Metabolomics
One of the studies included in this review was the first to investigate the effects of green tea kombucha (200 mL) on quality of life and serum metabolomics (Table 3). The study lasted ten weeks and was conducted with individuals with excess body weight. The authors used the SF-36 questionnaire, an instrument that evaluates multiple domains of health-related quality of life. Kombucha consumption was associated with within-group improvements in the total SF-36 score, as well as in specific domains such as general health general health, vitality, and role-emotional. However, no statistically significant differences were found between the kombucha and control groups at the end of the intervention [].
Regarding serum untargeted metabolomics, the authors found 14 putative metabolites in the positive polarity and 15 in the negative polarity after exclusion of toxic and drugs metabolites []. The majority of compounds found were metabolites related to fungi, and the others were derived from amino acids and fatty acid metabolism, as well as vitamins and phenolic compounds. Among them, there was diethyl malonate, a compound produced by a common yeast found in kombucha, Saccharomyces cerevisiae. This compound presented a positive correlation with the genus Romboutsia in the kombucha group. Another interesting metabolite that increased after kombucha consumption was taurine, which showed a negative correlation with pathogens, such as Clostridium sensu stricto [].
4. Discussion
To our knowledge, this is the first systematic review that raised evidence on the impact of kombucha intake on human health. A total of eight clinical trials were included in this review. Among the main findings, kombucha consumption was associated with improvements in gastrointestinal symptoms, particularly in enhancing stool consistency and reducing the sensation of incomplete bowel evacuation. Regarding a probiotic effect, kombucha showed modest and heterogeneous impacts on gut microbiota composition, with some studies reporting within-group shifts in specific bacterial and fungal taxa, but few significant differences between intervention and control groups. Findings related to the effect of the beverage on glucose metabolism were inconsistent, with some studies reporting modest benefits, particularly in individuals with metabolic alterations. Lipid profiles and liver and kidney function parameters were not significantly affected by kombucha across the studies. Although most inflammatory markers remained unchanged, one study suggested that kombucha might attenuate increases in IL-6 during an energy-restricted diet intervention, possibly due to its antioxidant properties. No significant effects were observed on anthropometry or body composition when compared to control groups, with exception to one study that reported a significant decreased in the LAP index when compared to the control group. Additionally, kombucha intake positively influenced the serum metabolome and salivary microbiota of individuals with excess body weight, which may potentially promote metabolic improvements in this population.
Kombucha is a complex beverage, and its health effects are attributed mainly to its rich chemical and microbial composition []. This makes kombucha an appealing product in the health food market, often promoted as a probiotic beverage on commercial labels [,]. However, this probiotic health claim is not well-established, particularly in considering the current clinical evidence in this systematic review. One possible mechanism involved in the modulation of gut microbiota is through the phenolic compounds present in kombucha exerting a prebiotic effect [,]. Since a small proportion (5–10%) of these molecules are absorbed in the small intestine, the other percentage reaches the colon where the gut microbiota metabolizes it, stimulating the growth of beneficial bacteria [] and enhancing the production of SCFAs (e.g., butyrate, acetate, and propionate) []. These SCFAs are crucial in maintaining microbial community structure, improving intestinal barrier function, and regulating host metabolic and immune responses (Figure 4). Indeed, animal experimental studies demonstrated modulation of gut microbiota and decreased endotoxemia with a higher production of propionate and acetic acids [,,].
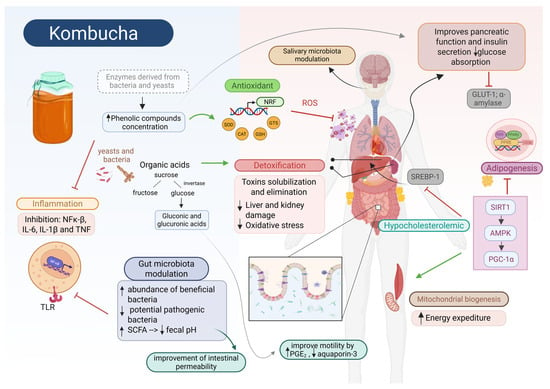
Figure 4.
Potential molecular mechanisms involved in promising health benefits of kombucha consumption. The rich composition of kombucha, including organic acids and phenolic compounds, provides potent antioxidant and anti-inflammatory properties that influence multiple molecular pathways across organs. Phenolic compounds exert prebiotic effects by modulating salivary and fecal microbiota, lowering fecal pH, stimulating beneficial bacteria, and increasing short-chain fatty acid (SCFA) production. This enhances gut barrier function and regulates metabolic, immune, and inflammatory responses. Additionally, kombucha’s bioactive compounds show antimicrobial action, reducing oral pathogens linked to periodontitis and cavities. In the gut, organic acids improve motility by stimulating prostaglandin E2 secretion, enhancing peristalsis, and downregulating aquaporin-3, leading to softer stools and greater volume. Kombucha also activates SIRT-1 and AMPK, suppressing chronic inflammation via NF-κB inhibition, boosting mitochondrial biogenesis and ATP catabolism, while reducing lipogenesis and hepatic cholesterol synthesis—mechanisms associated with improved energy expenditure and anti-obesity effects. Furthermore, it mitigates hepatic fat accumulation by downregulating lipid-related genes (CD36, PPAR-γ, SREBP-1C) and enhancing β-oxidation. Regarding glucose metabolism, kombucha supports pancreatic function, improves insulin secretion, and reduces glucose absorption by inhibiting α-amylase and GLUT1, mediated by its low pH and microbial activity. Its high organic acid content also promotes hepatic and renal detoxification via glucuronidation, enhancing toxin elimination and protecting against oxidative stress. Additionally, kombucha activates NRF2 signaling, boosting antioxidant defenses through increased expression of enzymes like SOD, CAT, and GSH. ↑: increased; ↓: decreased.
Despite this promising mechanism, the clinical trials included in this systematic review showed limited and heterogeneous findings related to gut microbiota modulation, along with no effects related to intestinal permeability and SCFA production. The effects appeared to vary depending on the tea type, study population, and microbiota analytical parameters, including the 16S rRNA region sequenced, the platform used, and the bioinformatics methods applied [,]. Costa et al. (2024) reported within-group shifts in microbial abundance and fungal diversity after black tea kombucha intake, especially among individuals with obesity, including an increase in Akkermansiaceae and Subdoligranulum and a decrease in Ruminococcus and Dorea []. Similarly, Ecklu-Mensah et al. (2024) observed an enrichment of Weizmannia coagulans, a bacteria present in kombucha fermentation, and alterations in beta diversity following kombucha consumption, as well as changes in microbial metabolic pathways, such as biosynthesis of nucleotides, vitamins, and electron carriers. However, the authors also observed a reduced microbial evenness and lower overall diversity based on the decreased Shannon index within the kombucha group []. In contrast, another clinical trial found an increase in alpha diversity measured using the Chao1 index within the group that took the beverage. Moreover, there was a reducing trend of Alistipes, Odoribacter, and Parabacteroides and an increasing trend of Romboutsia in the kombucha group compared to control group [].
Despite the lack of significant effects on intestinal biomarkers and the heterogeneous results regarding microbiota composition, kombucha showed promising effects in alleviating gastrointestinal symptoms, particularly those related to constipation in individuals with excess body weight and with irritable bowel syndrome [,]. This benefit appears to be mediated by the presence of organic acids commonly found in the beverage (e.g., acetic, citric, malic acids) that may positively influence intestinal motility by increasing secretion of prostaglandin E2 (PGE2) that binds to EP3 receptors on intestinal smooth muscle cells, enhancing the frequency and amplitude of peristaltic contractions. Moreover, the expression of aquaporin-3 can be downregulated. This limits water absorption from the intestinal lumen, resulting in increased stool volume and softer consistency [,,].
Only one study explored the impact of kombucha consumption on the salivary microbiota, demonstrating a great potential for modulatory effects in individuals with excess body weight []. Other recent studies have investigated the effect of diet and certain foods, such as fermented foods [] and teas [], on the salivary microbiota since there are mechanisms linking the metabolic imbalance with oral dysbiosis [,]. This association may be partly explained by two main pathways: the oral–gut axis, characterized by the translocation of oral pathobionts to the gut, disrupting the intestinal homeostasis [], and the oral–blood axis, through which bacteria or their components, such as LPS, enter the bloodstream, mainly when periodontitis and cavities are present, triggering systemic inflammation and metabolic dysfunction [] (Figure 4). In this context, green tea kombucha reduced the abundance of salivary bacteria associated with chronic periodontitis, dental cavities, and diabetes []. The authors suggested that the antimicrobial activity of the kombucha bioactive compounds, such as (−)-epigallocatechin-3-gallate, may have influenced these results, reinforcing previous findings in which green tea exhibits therapeutic potential as an adjunctive treatment for gingival inflammation and dental cavities [].
Another relevant beneficial effect after kombucha consumption was related to metabolomic changes. Although only one study assessed serum metabolites, it reported noteworthy findings, including an increase in anti-inflammatory and antioxidant properties [,], such as compounds derived from fungi and yeasts (e.g., diethyl malonate) and medicinal plants (e.g., asperuloside) []. Additionally, the authors reported higher levels of taurine, an amino acid associated with a reduced risk of metabolic syndrome []. They also observed a negative correlation between taurine levels and an abundance of pathogenic bacteria such as Clostridium sensu stricto [].
Evidence from experimental studies and a systematic review based on animal models suggest that kombucha may exert therapeutic effects against conditions associated with low-grade inflammation and oxidative stress, including obesity, diabetes, dyslipidemia, and metabolic dysfunction-associated steatotic liver disease (MASLD), due to its antioxidant and anti-inflammatory properties [,,]. Unexpectedly, the human clinical trials included in this systematic review did not demonstrate significant improvements in lipid profiles or renal or hepatic biomarkers following kombucha consumption, except for one study that reported a reduction in GGT levels in individuals with obesity []. This discrepancy may be partly explained by the highly controlled conditions of animal studies, which often do not reflect the complexity and variability of human clinical settings. Nevertheless, the biological mechanisms proposed to explain the benefits observed in preclinical studies are mainly attributed to its rich phenolic profile, which may stimulate sirtuin-1 (SIRT-1), a NAD+-dependent histone deacetylase involved in regulating metabolic homeostasis. Activation of SIRT-1, along with AMP-activated protein kinase (AMPK) signaling, suppresses chronic inflammation via nuclear factor kappa B (NF-κB) inhibition, enhances mitochondrial biogenesis and ATP catabolism, and suppresses lipogenesis and hepatic cholesterol synthesis []. These pathways contribute to improved energy expenditure and metabolic balance, which are strongly linked to anti-obesity effects []. However, despite these mechanisms, only three clinical trials assessed weight loss and body composition, and none of them reported significant differences after kombucha consumption [,,]. Additionally, only one study detected attenuation in IL-6 elevation compared to control group, suggesting a modest anti-inflammatory effect in individuals with excess body weight [].
Moreover, in animals, kombucha has been demonstrated to mitigate hepatic fat accumulation by downregulating lipid genes associated with fatty acid uptake and lipogenesis, such as cluster of differentiation 36 (CD36), peroxisome proliferator-activated receptor gamma (PPAR-γ), and sterol regulatory element-binding protein 1c (SREBP-1C), while simultaneously upregulating key mediators of β-oxidation, including fatty acid-binding protein 1 (FABP1), acyl-CoA oxidase 1 (ACOX1), carnitine palmitoyltransferase 1 (CPT1), PPAR-α, and medium-chain acyl-CoA dehydrogenase (MCAD) [,,]. Additionally, kombucha influences the inhibition of enzymes like diacylglycerol acyltransferase 2 (DGAT2), contributing to better management of triglyceride (TG) synthesis and preventing lipotoxic damage [,]. An in vitro study using murine OP9 preadipocyte cells and enzymatic assays demonstrated that yellow and oolong tea kombucha tea decreased pancreatic lipase activity and adipocyte differentiation by downregulating the expression of adipogenic markers (e.g., PPARγ and CCAAT/enhancer-binding protein-α), alongside decreasing intracellular lipid accumulation []. These findings suggest a potential anti-obesogenic effect through modulation of lipid metabolism and adipogenesis pathways. No articles included in this systematic review evaluated gene expression, but one observed a higher reduction in the LAP in the kombucha group compared to control group []. LAP is a cardiometabolic index that combines TG and waist circumference to evaluate the cardiometabolic risk associated with lipid dysfunction and central adiposity [].
Most clinical trials included in this systematic review assessed the impact of kombucha intake on glucose metabolism, showing heterogeneous results. One trial reported improvements in postprandial glycemic and insulin responses following acute kombucha consumption in healthy individuals compared to the control group []. At the same time, another study observed a reduction in fasting insulin and HOMA-IR in individuals with obesity compared to baseline []. A third study demonstrated that a subset of participants with diabetes (n = 5) with FBG higher than 110 mg/dL presented a greater decreased in FBG than the control group after kombucha intake []. Conversely, other studies did not find significant changes between groups [,]; however, one noted a significant increase within the kombucha group in fasting insulin and HOMA-IR of free-living adults on a Western diet [].
Kombucha has been linked to improved glucose metabolism in preclinical studies, demonstrating enhanced pancreatic architecture, improved insulin secretion and function, and reduced glucose absorption in the small intestine [,]. One possible mechanism by which kombucha reduced the postprandial glycemia and insulin responses may involve the inhibition of the pancreatic enzyme α-amylase, likely influenced by its low pH and the metabolic activity of live microorganisms. In addition, the phenolic compounds present in kombucha may further contribute to this effect by reducing glucose absorption through the inhibition of the glucose transporter glucose transporter 1 (GLUT1) []. Additionally, a study with diabetic mice showed that the anti-hyperglycemic effect of kombucha is associated with gut microbiota modulation, with decreased circulating LPS levels and systematic inflammation contributing to insulin sensitivity. Furthermore, the increased abundance of SCFA-producing bacteria stimulated the production of SCFA, and, consequently, increased secretion of glucagon-like peptide-1 (GLP-1) and YY (peptide PYY) which enhance pancreatic B function and regulate blood sugar [].
The high content of organic acids, such as GlcUA and DSL, are related to the hepatic and renal protective effects of kombucha []. GlcUA plays a crucial role in detoxification through glucuronidation reactions that convert lipophilic and potentially toxic compounds into water-soluble glucuronides, facilitating their excretion via bile and urine [] (Figure 4). DSL, a compound produced by Gluconacetobacter sp. during fermentation, inhibits the activity of beta-glucuronidase—an enzyme responsible for breaking glucuronide bonds in the intestinal lumen, which can release toxic aglycones. By inhibiting this enzyme, DSL contributes to intestinal and hepatic protection and also exhibits antioxidant properties that mitigate oxidative stress-related renal damage []. Moreover, the restoration of antioxidant defenses, such as normalization of glutathione and nitric oxide levels, along with the reduction of creatinine and malondialdehyde, contributes to the kombucha’s beneficial impact on kidney function [] (Figure 4). Additionally, phenolic compounds in kombucha have the potential to decrease the oxidative stress by scavenging reactive oxygen species, up-regulating NRF2 responsive genes, consequently enhancing the expression of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH), and glutathione s-transferase (GST) [] (Figure 4). No improvements in renal function were reported, and only one study demonstrated a reduction in the hepatic marker GGT in the clinical trials. However, it is important to highlight that none of the participants presented pre-existing liver or kidney dysfunction, which may have impacted the absence of significant changes in these biomarkers.
This systematic review has several strengths. To our knowledge, it is the first study to comprehensively synthesize the current evidence from human clinical trials evaluating the effects of kombucha consumption on metabolic health. Furthermore, it was conducted following rigorous methodological standards, including a comprehensive search strategy across multiple databases, strict eligibility criteria, and a critical assessment of study quality. However, some limitations should be acknowledged. The small number of available clinical trials as well as the considerable heterogeneity in study designs, intervention durations, kombucha compositions, and health conditions of participants limit the generalizability of the findings. Additionally, due to the heterogeneity observed among the included studies, particularly in terms of intervention protocols, outcome measures, and follow-up durations, a meta-analysis was deemed inappropriate. In such cases, statistical pooling may lead to misleading conclusions and compromise the validity of the synthesis. Therefore, a qualitative synthesis was conducted in accordance with current methodological guidance for systematic reviews, which recommends narrative approaches when data are not sufficiently comparable to support a quantitative combination. This strategy ensures a more accurate and context-sensitive interpretation of the available evidence [].
5. Conclusions
This systematic review provides a comprehensive and novel synthesis of clinical evidence regarding the effects of kombucha consumption on human health. Regular consumption of kombucha has shown promising effects on reducing gastrointestinal symptoms such as the sensation of incomplete evacuation and hard stools, supporting its potential as an adjunct strategy for managing constipation and improving bowel regularity. In addition, the beverage showed modest capacity to modulate gut microbiota, reinforcing the need for further randomized clinical trials to determine whether kombucha indeed exerts probiotic functions.
Beyond intestinal outcomes, kombucha also showed promising effects on salivary microbiota modulation and metabolomic profiles, which may contribute to improvements in metabolic health. However, only one study has analyzed salivary microbiota. Regarding cardiometabolic and body composition, the beverage exhibited minimal effects, including inconsistent findings on glucose metabolism. Therefore, while preclinical evidence is promising, the current clinical data remain inconclusive, highlighting the need for further well-designed randomized controlled trials with larger populations, standardized kombucha formulations, and longer follow-up periods to fully elucidate the health effects of this beverage and its potential role in human nutrition.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation11060353/s1, Table S1: Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Checklist; Table S2: Detailed search strategy used for each database.
Author Contributions
G.M.F. designed and conceptualized the study. G.M.F. and D.B.B. conducted literature searches, data screening, selection, and extraction. R.S.d.P. and C.M.T. performed the quality assessment. G.M.F. and D.B.B. wrote the first draft of the manuscript. J.B. and F.I.M. supervised the work. All authors (G.M.F., D.B.B., R.S.d.P., C.M.T. H.S.D.M., F.A.R.d.B., J.B., and F.I.M.) interpreted the results and revised and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Coordination for the Improvement of Higher Educational Personnel (CAPES) Foundation (Ministry of Education, Brazil, Financial code 001); the National Council of Technological and Scientific Development—CNPq (Ministry of Science, Technology and Innovation, Brazil, process number 404770/2021-5); Fapemig (Minas Gerais, Brazil, CDS-APQ-01808-22); and CIBEROBN (CB12/03/30002—Spain). J. Bressan is a CNPq Productivity Research Fellow.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACOX1 | Acyl-CoA oxidase 1 |
| AMPK | AMP-activated protein kinase |
| BSS | Bristol Stool Scale |
| CAT | Catalase |
| CD36 | Cluster of differentiation 36 |
| CRP | C-reactive protein |
| CPT1 | Carnitine palmitoyltransferase 1 |
| DGAT2 | Diacylglycerol acyltransferase 2 |
| DSL | D-saccharic-1,4-lactone |
| FABP1 | Fatty acid-binding protein 1 |
| FBG | Fasting blood glucose |
| FFQ | Food Frequency Questionnaire |
| GlcUA | Glucuronic acid |
| GGT | Gamma-glutamyl transferase |
| GLP-1 | Glucagon-like peptide-1 |
| GLUT-1 | Glucose transporter 1 |
| HOMA-IR | Homeostasis model assessment for insulin resistance |
| HbA1c | Hemoglobin A1c |
| IBS | Irritable bowel syndrome |
| IL | Interleukin |
| LBP | LPS-binding protein |
| LPS | Lipopolysaccharide |
| MAPA | Ministry of Agriculture, Livestock and Food Supply (Brazil) |
| MCAD | Medium-chain acyl-CoA dehydrogenase |
| NCDs | Non-communicable diseases |
| NF-κB | Nuclear factor kappa B |
| PGE2 | Prostaglandin E2 |
| PPAR | Peroxisome proliferator-activated receptor |
| GSH | Reduced glutathione |
| GST | Glutathione S-transferase |
| ROS | Reactive oxygen species |
| SCFA | Short-chain fatty acids |
| SCOBY | Symbiotic culture of bacteria and yeasts |
| SOD | Superoxide dismutase |
| SREBP-1C | Sterol regulatory element-binding protein-1C |
| T2D | Type 2 diabetes |
| TNF | Tumor necrosis factor |
| TyG | Triglyceride-glucose index |
| eGFR | Estimated glomerular filtration rate |
Appendix A
A description of all the analytical procedures used in the evaluated markers of the included clinical trials are described in Appendix A.
Appendix A.1. Gastrointestinal Health
Pilipenko et al. (2022) [] assessed gastrointestinal symptoms such as dryness, bitterness in the mouth, belching, heartburn, stomach heaviness, abdominal pain, nausea, flatulence, and the frequency and characteristics of stools using a complaint dynamics method, without further details. Meanwhile, Fraiz et al. (2024c) [] applied a GSRS, validated in Brazil, with 15 questions across five domains (diarrhea, constipation, bloating, abdominal pain, reflux/indigestion) using a seven-point Likert scale. Both studies also applied the BSS to assess stool consistency and shape. In the study by Ecklu-Mensah et al. (2024) [], self-reported gastrointestinal discomfort was assessed in percentages, but without comparisons between groups or with baseline data.
Fraiz et al. (2024c) [] and Costa et al. (2025) [] evaluated intestinal permeability through the ingestion of a solution containing lactulose (10 g), mannitol (5 g), and sucrose (20 g), ingested after fasting, with urine collected postprandially and analyzed by high-performance liquid chromatography (HPLC). Likewise, both studies also evaluated zonulin and LBP using enzyme-linked immunosorbent assay (ELISA) kits. In Fraiz et al., stool samples were also analyzed for SCFA using HPLC with a refractive index detector. In Costa et al. (2025) [], fecal material was analyzed using gas chromatography (GC). Additionally, Fraiz et al. (2024c) [] performed fecal pH analysis using a pH meter.
Appendix A.2. Fecal and Salivary Microbiota
Fraiz et al. (2024c) [] used the QIAamp® DNA Mini kit (Qiagen, Hilden, Bundesland, Germany), with DNA purity assessed by A260/A280 ratio using a NanoDrop 7000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), and integrity verified using agarose gel electrophoresis. Similarly, Costa et al. (2025) [] applied the QIAamp® PowerFecal® Pro kit (Qiagen), which included an additional mechanical cell lysis step using microspheres, and performed automated extraction on QIAcube Connect® equipment(Hilden, Germany), assessing purity by the A260/280 and A260/230 ratios on the NanoDrop. Ecklu-Mensah et al. (2024) [] adopted the MagMAX Microbiome Ultra Nucleic Acid Isolation Kit (Thermo Fisher Scientific, Waltham, MA, USA) with automation using KingFisher Flex robots, quantifying the purified DNA by PicoGreen fluorescence assay. Fraiz et al. (2024c) [] focused on the V4 region of the 16S rRNA gene (primers 515F/806R), while Costa et al. (2025) [] targeted the V3-V4 regions of the 16S rRNA gene (primers 341F/806R) as well as the ITS1/ITS2 regions for fungal rRNA analysis. Ecklu-Mensah et al. (2024) [] conducted whole-genome metagenomic sequencing. Sequence processing employed established tools and pipelines. Fraiz et al. (2024) used QIIME2 with cutadapt trimming, ASV generation via DADA2, and taxonomic classification based on the RDP database. Costa et al. (2025) [] performed metataxonomic analysis combining QIIME2 with specialized R packages for statistical analysis and visualization. Ecklu-Mensah et al. (2024) [] applied fastp for quality filtering, minimap2 to remove human reads, Bowtie2 for alignment against the Web of Life database, and the Woltka pipeline for classification into Operational Genomic Units (OGUs).
Alpha diversity was evaluated using Chao1 and Shannon indices, employing nonparametric statistical tests (Wilcoxon, Kruskal–Wallis) as per Fraiz et al. (2024c) [] and Costa et al. (2025) [], or mixed linear models adjusted for demographic variables, including sex and age, according to Ecklu-Mensah et al. (2024) []. Beta diversity analyses applied Bray–Curtis and UniFrac (weighted and unweighted) metrics, assessed using permutational multivariate analysis of variance (PERMANOVA, adonis2), with results visualized by principal coordinates analysis (PCoA), as detailed by Costa et al. (2025) [] and Ecklu-Mensah et al. (2024) [].
Differential analyses of microbial composition were performed using different approaches. Fraiz et al. (2024c) [] used CLR transformations followed by mixed models with false discovery rate (FDR) adjustment. Costa et al. (2025) [] applied the LEfSe tool with an LDA threshold ≥ 2 and p < 0.05. Ecklu-Mensah et al. (2024) [] conducted analyses using ANCOMBC, including covariates such as sex, body mass index, and age. Finally, associations between microorganisms and metabolic or clinical variables were investigated using Spearman correlation (Fraiz et al., 2024c) [] or linear multivariable models via MaAsLin2 (Costa et al., 2025), both with corrections for multiple comparisons via FDR.
In the study by Fraiz et al. (2024b) [], unstimulated saliva (5 mL) was collected by spontaneous spitting, after fasting for at least eight hours, and stored at −80 °C for later analysis. Genomic DNA was extracted using the QIAamp® DNA Mini Kit, with purity assessed using a NanoDrop spectrophotometer and integrity by 1% agarose gel electrophoresis. PCR amplification of the V4 region of the 16S rRNA gene was performed with universal primers and sequenced on the Illumina NovaSeq 6000 platform. The quality of the PCR products was verified by Bioanalyzer.
Sequencing data processing followed the QIIME2 pipeline, including sequence trimming, removal of low-quality and chimeric reads, and identification of amplicon sequence variants (ASVs). Taxonomy was assigned using the Ribosomal Database Project (RDP) classifier. For the analysis of microbial diversity, alpha (Chao1 and Simpson) and beta (Bray–Curtis index with PCoA) indices were calculated with statistical tests such as Student’s t and PERMANOVA. Differential analysis was performed using the ALDEx2 package in R, with multiple testing (FDR) correction, including assessment of the Bacillota/Bacteroidota ratio. The association between taxonomic abundance and metadata (group, visit, age, and sex) was performed using a generalized linear model.
Appendix A.3. Cardiometabolic and Inflammation Markers
Plasma glucose was assessed by Atkinson et al. (2023) [] using a hexokinase assay on an automated spectrophotometric analyzer, by Fraiz et al. (2024a) [] with a Mindray/BS-200® analyzer, and by Ecklu-Mensah et al. (2024) [] using a Roche Cobas® 8000 analyzer(Basel, Switzerland). Mendelson et al. (2023) [] recorded daily fasting blood glucose levels using portable meters. Plasma insulin was measured by Atkinson et al. (2023) [] using sandwich ELISA and by Fraiz et al. (2024a) [] and Ecklu-Mensah et al. (2024) [] using automated methods. Fraiz et al. (2024a) [] and Ecklu-Mensah et al. (2024) [] also calculated the HOMA index, in addition to the TyG index (Fraiz et al., 2024a) [].
Fraiz et al. (2024a) [] performed analyses of other metabolic markers using the Mindray/BS-200® Chemistry Analyzer (Shenzhen, China), following protocols from commercial kits from Bioclin Bioquímica. Ecklu-Mensah et al. (2024) [] used the Roche Cobas® 8000 modular analyzer, adopting standard procedures for quantifying metabolic markers. The estimated glomerular filtration rate was calculated without further details. Additionally, blood pressure, pulse, and respiratory rate were determined using the Mindray Accutorr 7 equipment(Shenzhen, China). Other biochemical parameters, such as bilirubin, total protein, potassium, sodium, and iron, were analyzed by Pilipenko et al. (2022) [] using standard biochemical methods, according to laboratory protocols.
Fraiz et al. (2024b) [] quantified plasma concentrations of cytokines (IL-6, IL-10, IL-8, TNF-α, IL-1β, IL-12p70) using flow cytometry with a BD Accuri™ C6 Plus cytometer (BD Biosciences, San Jose, CA, USA) and a specific commercial kit (Cytometric Bead Array CBA Human Th1/Th2/Th17 Kit, BD Biosciences), with data analysis performed using FCAP Array v3.0 software. CRP was determined with a chemical method using a Mindray/BS-200® analyzer (Shenzhen, China), according to the protocol of the Bioclin Bioquímica commercial kit. Ecklu-Mensah et al. (2024) [] quantified serum IL-6, IL-10, and CRP using LEGENDplex bead-based immunoassays (BioLegend, San Diego, CA, USA) following the manufacturer’s protocols, with readings performed on a BD FACSCanto™ II cytometer (BD Biosciences) and data analysis performed using LEGENDplex software and the R environment (version 4.1.1).
Appendix A.4. Anthropometry and Body Composition
In Fraiz et al. (2024b) [], participants were weighed using a digital electronic scale (InBody®, model 230, Biospace Corp., Seoul, Republic of Korea) while wearing light clothing, and height was measured with a wall-mounted stadiometer (SECA®, model 206, Hamburg, Germany). BMI was calculated using the formula weight (kg) divided by height squared (m2). WC and HC were obtained using a flexible and inelastic tape measure, according to specific protocols. Body composition was assessed by bone densitometry (DEXA), with analysis of total fat mass and fat of the android, gynoid, and trunk regions performed after a 10-hour fast. Several indices related to abdominal fat and body shape were also calculated. Ecklu-Mensah et al. (2024) [] used a wall-mounted stadiometer and an electronic scale to measure height and weight, respectively, calculating BMI using the same method described previously. WC was measured at the midpoint between the last rib and the iliac crest, using a non-elastic anthropometric tape.
Appendix A.5. Quality of Life and Metabolomics
The quality of life of participants was assessed by Fraiz et al. (2024c) [] using the Brazilian version of the SF-36 questionnaire []. For metabolomic analysis, serum samples were collected and subjected to an untargeted analysis of the blood metabolome at the University of Navarra (Spain) using liquid chromatography coupled to mass spectrometry (LC-MS) with a time of flight (TOF) detector. The samples were prepared with methanol, centrifuged, and evaporated before chromatographic analysis using a Zorbax SB-C18 column, with an aqueous formic acid and methanol formic acid eluent gradient. The detector was configured for electrospray ionization (ESI) in both positive and negative modes, collecting spectra in the m/z range 100–2000. Quality controls were applied to ensure data reliability. Chromatogram analysis was performed using MassHunter and XCMS Online software (Santa Clara, CA, USA) for alignment and identification of the detected metabolites.
References
- Lobstein, T.; Jackson-Leach, R.; Powis, J.; Thompson, R.; Jackson-Leach, R. World Obesity Atlas 2025; World Obesity Federation: London, UK, 2025; Available online: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2025 (accessed on 9 January 2025).
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Physiol.-Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Gasmi Benahmed, A.; Gasmi, A.; Doşa, A.; Chirumbolo, S.; Mujawdiya, P.K.; Aaseth, J.; Dadar, M.; Bjørklund, G. Association between the Gut and Oral Microbiome with Obesity. Anaerobe 2021, 70, 102248. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zou, Z.-P.; Ye, B.-C.; Zhou, Y. Gut Microbiota and Associated Metabolites: Key Players in High-Fat Diet-Induced Chronic Diseases. Gut Microbes 2025, 17, 2494703. [Google Scholar] [CrossRef] [PubMed]
- D’Almeida, A.P.; Neta, A.A.I.; de Andrade-Lima, M.; de Albuquerque, T.L. Plant-Based Probiotic Foods: Current State and Future Trends. Food Sci. Biotechnol. 2024, 33, 3401–3422. [Google Scholar] [CrossRef]
- Andrade, D.K.A.; Wang, B.; Lima, E.M.F.; Shebeko, S.K.; Ermakov, A.M.; Khramova, V.N.; Ivanova, I.V.; Rocha, R.d.S.; Vaz-Velho, M.; Mutukumira, A.N.; et al. Kombucha: An Old Tradition into a New Concept of a Beneficial, Health-Promoting Beverage. Foods 2025, 14, 1547. [Google Scholar] [CrossRef]
- Shi, S.; Wei, Y.; Lin, X.; Liang, H.; Zhang, S.; Chen, Y.; Dong, L.; Ji, C. Microbial Metabolic Transformation and Antioxidant Activity Evaluation of Polyphenols in Kombucha. Food Biosci. 2023, 51, 102287. [Google Scholar] [CrossRef]
- de Noronha, M.C.; Cardoso, R.R.; dos Santos D’Almeida, C.T.; Vieira do Carmo, M.A.; Azevedo, L.; Maltarollo, V.G.; Júnior, J.I.R.; Eller, M.R.; Cameron, L.C.; Ferreira, M.S.L.; et al. Black Tea Kombucha: Physicochemical, Microbiological and Comprehensive Phenolic Profile Changes during Fermentation, and Antimalarial Activity. Food Chem. 2022, 384, 132515. [Google Scholar] [CrossRef]
- Zailani, N.S.; Adnan, A. Substrates and metabolic pathways in symbiotic culture of bacteria and yeast (SCOBY) fermentation: A mini review. J. Teknol. 2022, 84, 155–165. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Sarikaya Aydin, S.; Gültekin Subasi, B.; Erskine, E.; Gök, R.; Ibrahim, S.A.; Yilmaz, B.; Özogul, F.; Capanoglu, E. Additional Advances Related to the Health Benefits Associated with Kombucha Consumption. Crit. Rev. Food Sci. Nutr. 2022, 64, 6102–6119. [Google Scholar] [CrossRef]
- Chong, A.Q.; Lau, S.W.; Chin, N.L.; Talib, R.A.; Basha, R.K. Fermented Beverage Benefits: A Comprehensive Review and Comparison of Kombucha and Kefir Microbiome. Microorganisms 2023, 11, 1344. [Google Scholar] [CrossRef]
- Vargas, B.K.; Fabricio, M.F.; Záchia Ayub, M.A. Health Effects and Probiotic and Prebiotic Potential of Kombucha: A Bibliometric and Systematic Review. Food Biosci. 2021, 44, 101332. [Google Scholar] [CrossRef]
- Antolak, H.; Piechota, D.; Kucharska, A. Kombucha Tea—A Double Power of Bioactive Compounds from Tea and Symbiotic Culture of Bacteria and Yeasts (SCOBY). Antioxidants 2021, 10, 1541. [Google Scholar] [CrossRef] [PubMed]
- Chaluvadi, S.; Hotchkiss, A.T.; Smith, B.; McVaugh, B.; White, A.K.; Guron, G.K.P.; Renye, J.A.; Yam, K.L. Key Kombucha Process Parameters for Optimal Bioactive Compounds and Flavor Quality. Fermentation 2024, 10, 605. [Google Scholar] [CrossRef]
- Sogin, J.H.; Worobo, R.W. Primary Metabolites and Microbial Diversity in Commercial Kombucha Products. Fermentation 2024, 10, 385. [Google Scholar] [CrossRef]
- Alves, R.O.; de Oliveira, R.L.; de Moraes, M.M.; Santos, W.W.V.; Gomes da Câmara, C.A.; da Silva, S.P.; Porto, C.S.; Porto, T.S. Evaluation of the Impact of Fermentation Conditions, Scale Up and Stirring on Physicochemical Parameters, Antioxidant Capacity and Volatile Compounds of Green Tea Kombucha. Fermentation 2025, 11, 201. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Kupnicka, P.; Melkis, K.; Mielczarek, O.; Walczyńska, J.; Chlubek, D.; Janda-Milczarek, K. Effects of Fermentation Time and Type of Tea on the Content of Micronutrients in Kombucha Fermented Tea. Nutrients 2022, 14, 4828. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Kałduńska, J.; Kochman, J.; Janda, K. Chemical Profile and Antioxidant Activity of the Kombucha Beverage Derived from White, Green, Black and Red Tea. Antioxidants 2020, 9, 447. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Wang, W.; Wang, Z.; Han, S.; Zhou, P. Kombucha Enables to Inhibit Digestive Enzymes Activity and Adipocyte Differentiation of OP9 Cells. J. Food Sci. 2024, 89, 10053–10063. [Google Scholar] [CrossRef]
- Wang, P.; Feng, Z.; Sang, X.; Chen, W.; Zhang, X.; Xiao, J.; Chen, Y.; Chen, Q.; Yang, M.; Su, J. Kombucha Ameliorates LPS-Induced Sepsis in a Mouse Model. Food Funct. 2021, 12, 10263–10280. [Google Scholar] [CrossRef]
- Costa, M.A.D.C.; Dias Moreira, L.D.P.; Duarte, V.D.S.; Cardoso, R.R.; São José, V.P.B.D.; Silva, B.P.D.; Grancieri, M.; Corich, V.; Giacomini, A.; Bressan, J.; et al. Kombuchas from Green and Black Tea Modulate the Gut Microbiota and Improve the Intestinal Health of Wistar Rats Fed a High-Fat High-Fructose Diet. Nutrients 2022, 14, 5234. [Google Scholar] [CrossRef]
- Permatasari, H.K.; Nurkolis, F.; Ben Gunawan, W.; Yusuf, V.M.; Yusuf, M.; Kusuma, R.J.; Sabrina, N.; Muharram, F.R.; Taslim, N.A.; Mayulu, N.; et al. Modulation of Gut Microbiota and Markers of Metabolic Syndrome in Mice on Cholesterol and Fat Enriched Diet by Butterfly Pea Flower Kombucha. Curr. Res. Food Sci. 2022, 5, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Sasaki, G.Y.; Wei, P.; Li, J.; Wang, L.; Zhu, J.; McTigue, D.; Yu, Z.; Bruno, R.S. Green Tea Extract Prevents Obesity in Male Mice by Alleviating Gut Dysbiosis in Association with Improved Intestinal Barrier Function That Limits Endotoxin Translocation and Adipose Inflammation. J. Nutr. Biochem. 2019, 67, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.R.; Moreira, L.D.P.D.; de Campos Costa, M.A.; Toledo, R.C.L.; Grancieri, M.; do Nascimento, T.P.; Ferreira, M.S.L.; da Matta, S.L.P.; Eller, M.R.; Duarte Martino, H.S.; et al. Kombuchas from Green and Black Teas Reduce Oxidative Stress, Liver Steatosis and Inflammation, and Improve Glucose Metabolism in Wistar Rats Fed a High-Fat High-Fructose Diet. Food Funct. 2021, 12, 10813–10827. [Google Scholar] [CrossRef] [PubMed]
- Moreira, G.V.; Araujo, L.C.C.; Murata, G.M.; Matos, S.L.; Carvalho, C.R.O. Kombucha Tea Improves Glucose Tolerance and Reduces Hepatic Steatosis in Obese Mice. Biomed. Pharmacother. 2022, 155, 113660. [Google Scholar] [CrossRef]
- Costa, M.A.d.C.; Vilela, D.L.d.S.; Fraiz, G.M.; Lopes, I.L.; Coelho, A.I.M.; Castro, L.C.V.; Martin, J.G.P. Effect of Kombucha Intake on the Gut Microbiota and Obesity-Related Comorbidities: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 3851–3866. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Aromataris, E.; Munn, Z. (Eds.) JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2024; Available online: https://synthesismanual.jbi.global (accessed on 17 February 2025).
- Pilipenko, V.I.; Isakov, V.A.; Morozov, S.V.; Vlasova, A.V.; Kochetkova, A.A. Efficacy of Newly Developed Kombucha-Based Specialized Food Product for Treatment of Constipation-Predominant Irritable Bowel Syndrome. Probl. Nutr. 2022, 91, 95–104. [Google Scholar] [CrossRef]
- Costa, M.A.d.C.; da Silva Duarte, V.; Fraiz, G.M.; Cardoso, R.R.; da Silva, A.; Martino, H.S.D.; dos Santos D’Almeida, C.T.; Ferreira, M.S.L.; Corich, V.; Hamaker, B.R.; et al. Regular Consumption of Black Tea Kombucha Modulates the Gut Microbiota in Individuals with and without Obesity. J. Nutr. 2025, 155, 1331–1349. [Google Scholar] [CrossRef]
- Fraiz, G.M.; Costa, M.A.C.; Cardoso, R.R.; Hébert, J.R.; Zhao, L.; Corich, V.; Giacomini, A.; Milagro, F.I.; Barros, F.A.R.; Bressan, J. Black Tea Kombucha Consumption: Effect on Cardiometabolic Parameters and Diet Quality of Individuals with and without Obesity. Fermentation 2024, 10, 384. [Google Scholar] [CrossRef]
- Fraiz, G.M.; Bonifácio, D.B.; Lacerda, U.V.; Cardoso, R.R.; Corich, V.; Giacomini, A.; Martino, H.S.D.; Echeverría, S.E.; de Barros, F.A.R.; Milagro, F.I.; et al. Green Tea Kombucha Impacts Inflammation and Salivary Microbiota in Individuals with Excess Body Weight: A Randomized Controlled Trial. Nutrients 2024, 16, 3186. [Google Scholar] [CrossRef]
- Fraiz, G.M.; Bonifácio, D.B.; Lacerda, U.V.; Cardoso, R.R.; Corich, V.; Giacomini, A.; Martino, H.S.D.; Esteban-Echeverría, S.; Romo-Hualde, A.; Muñoz-Prieto, D.; et al. The Impact of Green Tea Kombucha on the Intestinal Health, Gut Microbiota, and Serum Metabolome of Individuals with Excess Body Weight in a Weight Loss Intervention: A Randomized Controlled Trial. Foods 2024, 13, 3635. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, F.S.; Cohen, M.; Lau, K.; Brand-Miller, J.C. Glycemic Index and Insulin Index after a Standard Carbohydrate Meal Consumed with Live Kombucha: A Randomised, Placebo-Controlled, Crossover Trial. Front. Nutr. 2023, 10, 1036717. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, C.; Sparkes, S.; Merenstein, D.J.; Christensen, C.; Sharma, V.; Desale, S.; Auchtung, J.M.; Kok, C.R.; Hallen-Adams, H.E.; Hutkins, R. Kombucha Tea as an Anti-Hyperglycemic Agent in Humans with Diabetes—A Randomized Controlled Pilot Investigation. Front. Nutr. 2023, 10, 1190248. [Google Scholar] [CrossRef] [PubMed]
- Ecklu-Mensah, G.; Miller, R.; Maseng, M.G.; Hawes, V.; Hinz, D.; Kim, C.; Gilbert, J.A. Modulating the Human Gut Microbiome and Health Markers through Kombucha Consumption: A Controlled Clinical Study. Sci. Rep. 2024, 14, 31647. [Google Scholar] [CrossRef]
- Souza, G.S.; Sardá, F.A.H.; Giuntini, E.B.; Gumbrevicius, I.; de Morais, M.B.; de Menezes, E.W. Translation and validation of the brazilian portuguese version of the gastrointestinal symptom rating scale (GSRS) questionnaire. Arq. Gastroenterol. 2016, 53, 146–151. [Google Scholar] [CrossRef]
- Bortolomedi, B.M.; Paglarini, C.S.; Brod, F.C.A. Bioactive Compounds in Kombucha: A Review of Substrate Effect and Fermentation Conditions. Food Chem. 2022, 385, 132719. [Google Scholar] [CrossRef]
- Soares, M.G.; de Lima, M.; Reolon Schmidt, V.C. Technological Aspects of Kombucha, Its Applications and the Symbiotic Culture (SCOBY), and Extraction of Compounds of Interest: A Literature Review. Trends Food Sci. Technol. 2021, 110, 539–550. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary Polyphenol Impact on Gut Health and Microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef]
- de Freitas, P.L.; Miranda, J.P.N.; França, L.M.; Paes, A.M. de A. Plant-Derived (Poly)Phenols and Their Metabolic Outcomes: The Pursuit of a Role for the Gut Microbiota. Nutrients 2022, 14, 3510. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.; Wang, J.; Geng, W. Kombucha Reduces Hyperglycemia in Type 2 Diabetes of Mice by Regulating Gut Microbiota and Its Metabolites. Foods 2022, 11, 754. [Google Scholar] [CrossRef]
- Xu, Z.; Yeoh, Y.K.; Tun, H.M.; Fei, N.; Zhang, J.; Morrison, M.; Kamm, M.A.; Yu, J.; Chan, F.K.L.; Ng, S.C. Variation in the Metagenomic Analysis of Fecal Microbiome Composition Calls for a Standardized Operating Approach. Microbiol. Spectr. 2024, 12, e01516-24. [Google Scholar] [CrossRef] [PubMed]
- Na, J.-R.; Kim, E.; Na, C.-S.; Kim, S. Citric Acid-Enriched Extract of Ripe Prunus Mume (Siebold) Siebold & Zucc. Induces Laxative Effects by Regulating the Expression of Aquaporin 3 and Prostaglandin E2 in Rats with Loperamide-Induced Constipation. J. Med. Food 2022, 25, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cen, S.; Wang, G.; Lee, Y.; Zhao, J.; Zhang, H.; Chen, W. Acetic Acid and Butyric Acid Released in Large Intestine Play Different Roles in the Alleviation of Constipation. J. Funct. Foods 2020, 69, 103953. [Google Scholar] [CrossRef]
- Ibarlucea-Jerez, M.; Monnoye, M.; Chambon, C.; Gérard, P.; Licandro, H.; Neyraud, E. Fermented Food Consumption Modulates the Oral Microbiota. NPJ Sci. Food 2024, 8, 55. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, H.; Zhang, W.; Ni, L. Salivary Microbiota Shifts under Sustained Consumption of Oolong Tea in Healthy Adults. Nutrients 2020, 12, 966. [Google Scholar] [CrossRef]
- Schamarek, I.; Anders, L.; Chakaroun, R.M.; Kovacs, P.; Rohde-Zimmermann, K. The Role of the Oral Microbiome in Obesity and Metabolic Disease: Potential Systemic Implications and Effects on Taste Perception. Nutr. J. 2023, 22, 28. [Google Scholar] [CrossRef]
- Gupta, U.; Dey, P. The Oral Microbial Odyssey Influencing Chronic Metabolic Disease. Arch. Physiol. Biochem. 2024, 130, 831–847. [Google Scholar] [CrossRef]
- Yamazaki, K.; Kamada, N. Exploring the Oral-Gut Linkage: Interrelationship between Oral and Systemic Diseases. Mucosal Immunol. 2024, 17, 147–153. [Google Scholar] [CrossRef]
- Vyas, T.; Nagi, R.; Bhatia, A.; Bains, S. Therapeutic Effects of Green Tea as an Antioxidant on Oral Health—A Review. J. Fam. Med. Prim. Care 2021, 10, 3998. [Google Scholar] [CrossRef]
- Manzione, M.G.; Martorell, M.; Sharopov, F.; Bhat, N.G.; Kumar, N.V.A.; Fokou, P.V.T.; Pezzani, R. Phytochemical and Pharmacological Properties of Asperuloside, a Systematic Review. Eur. J. Pharmacol. 2020, 883, 173344. [Google Scholar] [CrossRef]
- Surai, P.F.; Earle-Payne, K.; Kidd, M.T. Taurine as a Natural Antioxidant: From Direct Antioxidant Effects to Protective Action in Various Toxicological Models. Antioxidants 2021, 10, 1876. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.; Penas, M.R.; Pintado, M.; Oliveira-Silva, P. Review Kombucha: Perceptions and Future Prospects. Foods 2022, 11, 1977. [Google Scholar] [CrossRef] [PubMed]
- Wanyo, P.; Chamsai, T.; Toontom, N.; Nghiep, L.K.; Tudpor, K. Evaluation of In Vitro Digested Mulberry Leaf Tea Kombucha: A Functional Fermented Beverage with Antioxidant, Anti-Inflammatory, Antihyperglycemic, and Antihypertensive Potentials. Fermentation 2025, 11, 258. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, S.; Wei, X.; Zhang, M.; Chen, Y.; Mao, X.; Chen, G.; Liu, C. Short-Term Moderate Caloric Restriction in a High-Fat Diet Alleviates Obesity via AMPK/SIRT1 Signaling in White Adipocytes and Liver. Food Nutr. Res. 2022, 66. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.; Wang, S.; Sung, S.; Kim, N.; Lee, H.-H.; Seo, Y.-S.; Jung, Y. Hepatoprotective Effect of Kombucha Tea in Rodent Model of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Int. J. Mol. Sci. 2019, 20, 2369. [Google Scholar] [CrossRef]
- Hyun, J.; Lee, Y.; Wang, S.; Kim, J.; Kim, J.; Cha, J.; Seo, Y.-S.; Jung, Y. Kombucha Tea Prevents Obese Mice from Developing Hepatic Steatosis and Liver Damage. Food Sci. Biotechnol. 2016, 25, 861–866. [Google Scholar] [CrossRef]
- Takhttavous, A.; Saberi-Karimian, M.; Hafezi, S.G.; Esmaily, H.; Hosseini, M.; Ferns, G.A.; Amirfakhrian, E.; Ghamsary, M.; Ghayour-Mobarhan, M.; Alinezhad-Namaghi, M. Predicting the 10-Year Incidence of Dyslipidemia Based on Novel Anthropometric Indices, Using Data Mining. Lipids Health Dis. 2024, 23, 33. [Google Scholar] [CrossRef]
- Zubaidah, E.; Afgani, C.A.; Kalsum, U.; Srianta, I.; Blanc, P.J. Comparison of in Vivo Antidiabetes Activity of Snake Fruit Kombucha, Black Tea Kombucha and Metformin. Biocatal. Agric. Biotechnol. 2019, 17, 465–469. [Google Scholar] [CrossRef]
- Martínez-Leal, J.; Ponce-García, N.; Escalante-Aburto, A. Recent Evidence of the Beneficial Effects Associated with Glucuronic Acid Contained in Kombucha Beverages. Curr. Nutr. Rep. 2020, 9, 163–170. [Google Scholar] [CrossRef]
- Massoud, R.; Jafari, R.; Khosravi-Darani, K. Kombucha as a Health-Beneficial Drink for Human Health. Plant Foods Hum. Nutr. 2024, 79, 251–259. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.5 (Updated August 2024); Cochrane: Oxford, UK, 2024; Available online: www.training.cochrane.org/handbook (accessed on 17 February 2025).
- Ciconelli, R.M.; Ferraz, M.B.; Santos, W.; Meinão, I.; Quaresma, M.R. Brazilian-Portuguese Version of the SF-36. A Reliable and Valid Quality of Life Outcome Measure. Rev. Bras. Reumatol. 1999, 39, 143–150. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).