Abstract
Today, there is considerable interest in creating artificial microbial consortia to solve various biotechnological problems. The use of such consortia allows for the improvement of process indicators, namely, increasing the rate of accumulation of target products and enhancing the conversion efficiency of the original substrates. In this work, the prospects for creating artificial consortia based on anaerobic sludge (AS) with cells of different yeasts were confirmed to increase the efficiency of methanogenesis in glucose- and glycerol-containing media and obtain biogas with an increased methane content. Yeasts of the genera Saccharomyces, Candida, Kluyveromyces, and Pachysolen were used to create the artificial consortia. Their concentration in the biomass of consortium cells was 1.5%. Yeast cells were used in an immobilized form, which was obtained by incorporating cells into a cryogel of polyvinyl alcohol. The possibility of increasing the efficiency of methanogenesis by 1.5 times in relation to the control (AS without the addition of yeast cells) was demonstrated. Using a consortium composed of methanogenic sludge and yeast cells of the genus Pachysolen, known for their ability to convert glycerol into ethanol under aerobic conditions, the possibility of highly efficient anaerobic conversion of glycerol into biogas was shown for the first time. Analysis of the metabolic activity of the consortia not only for the main components of the gas phase (CH4, CO2, and H2) and metabolites in the cell culture medium, but also for the concentration of intracellular adenosine triphosphate (ATP), controlled by the method of bioluminescent ATP-metry, showed a high level of functionality and thus, prospects for using such consortia in methanogenesis processes. The advantages and the prospect of using the developed consortia instead of individual AS for the treatment of methanogenic wastewater were confirmed during static tests conducted with several samples of real and model waste.
1. Introduction
Recently, there has been growing interest in the creation of artificial microbial consortia with a heterogeneous composition, especially for bioremediation processes, wastewater treatment, biofuel production, and other processes, analogs of which can be found in natural conditions [1,2,3]. Microbial consortia demonstrate significant advantages in such processes over individual cell strains. This is a result of the emerging possibility of converting a wider range of substrates due to the combination of different microorganisms, catalyzed by their enzymes’ biochemical reactions, which leads to the appearance of intermediate metabolites, the transformation of which ends with an increased accumulation of target products [4,5].
Artificial microbial consortia are, in most cases, created on the basis of isolated, well-characterized cells of microorganisms capable of effectively performing the major stages of biotechnological processes; they are then supplemented with strains possessing catalytic characteristics, thereby improving the process parameters [6,7]. In some cases, artificial consortia are composed of immobilized cells of microorganisms [8], since immobilization of cells allows for their use in the form of intensively functioning and highly concentrated populations in the quorum sensing state, i.e., actively communicating cells [9]. The immobilization of microbial populations by their inclusion in gel matrices imitates the formation of a nature-like stabilized state of cells, similar to what occurs when they are part of heterogeneous biofilms or self-immobilized biosystems.
It is known that consortia involving yeast cells are used to obtain biofuels, various organic compounds, and in winemaking [10,11]. To increase the efficiency of biotechnological processes in terms of the level of accumulation of target products, the possibility of combining cells of different yeast strains with each other and with bacterial cells or mycelial fungi, which often allows for an increase in the level of conversion of the original substrates into the required product, has been shown [12].
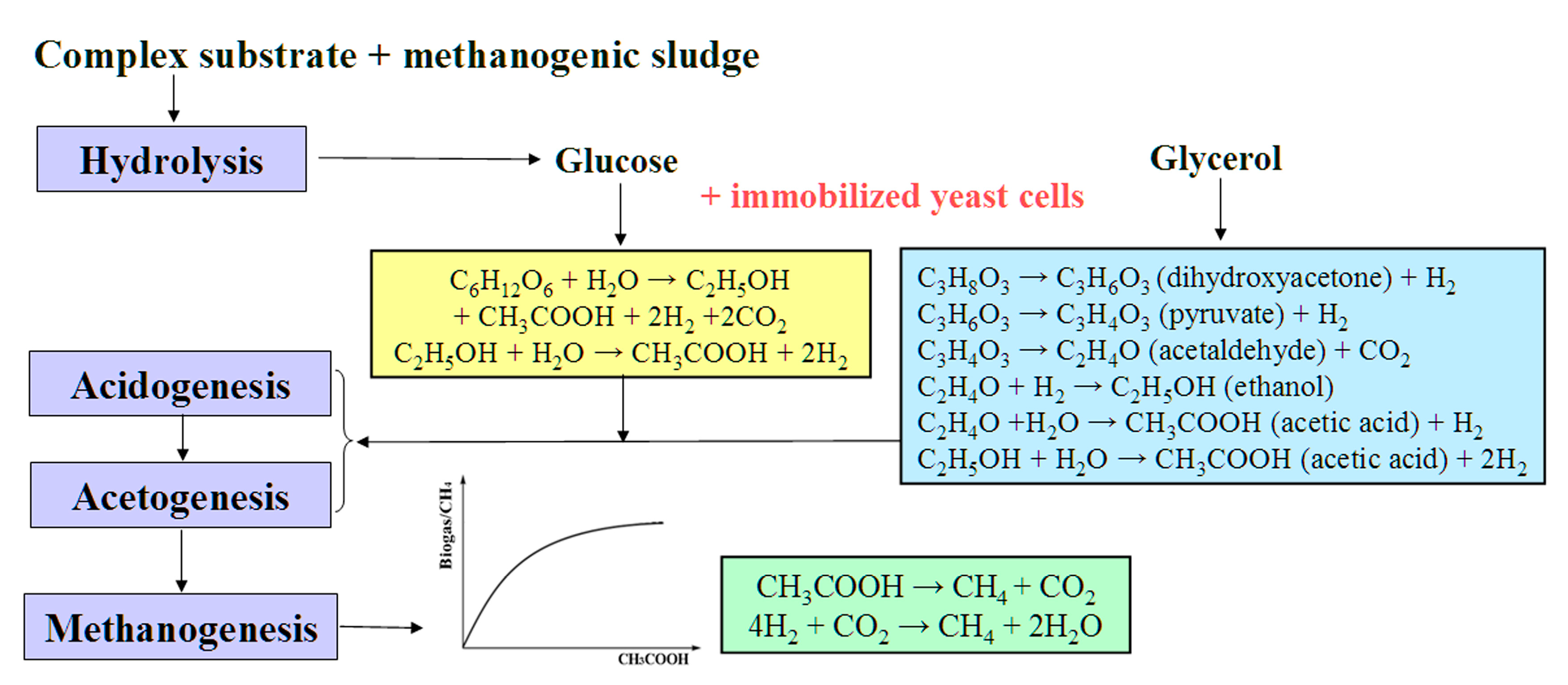
Today, among the urgent tasks of ecology and biotechnology are those related to the study of problems of the accumulation of large volumes of organic waste, in particular, in the food, agriculture, and fuel industries, which require rational and safe disposal [13,14]. The anaerobic conversion of such waste enables the production of biogas with a high methane content [13,14,15,16]. Biogas usually forms as a result of the metabolic activity of anaerobic microbial consortia within four main stages of the methanogenic conversion of complex substrates: hydrolysis, acidogenesis, acetogenesis, and methanogenesis. During the first two stages, substrates (carbohydrates, proteins, and lipids) are converted into small organic compounds, including volatile short-chain acids (propionic, lactic, and butyric), which are further converted into acetic acid, CO2, and H2 (stage three). Then, methanogenic archaea cells transform H2, CO2, and acetate into CH4 (Figure 1).
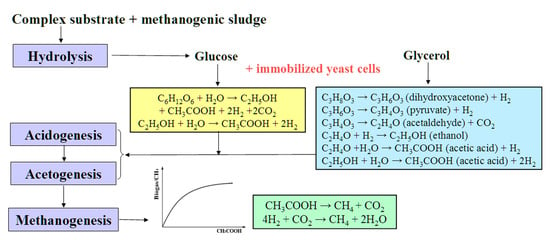
Figure 1.
Main stages of methanogenesis and conversion of substrates used in the processes catalyzed by artificial consortia containing AS and yeast cells. The reactions catalyzed by yeast cells are marked in yellow and blue, depending on the main substrate used.
Based on generally known catalytic dependencies, a higher concentration of substrate is then available for conversion to the product without inhibiting any effect on the biocatalyst, after which a higher velocity of the process and product accumulation are observed.
Despite the prospects of such a process, there are a number of problems that reduce its efficiency, primarily due to the decrease in the pH of the environment resulting from the accumulation of short-chain fatty acids (SCFA) [16]. The addition of yeast to the AS in the process of methanogenesis allows for an increase in the efficiency of biogas production (Table 1 [17,18,19,20,21,22,23,24,25,26]).

Table 1.
The effect of the addition of yeast cells to the AS on the results of methanogenesis.
The authors of such studies associate this effect with a decrease in the concentration of SCFAs accumulating under the influence of AS, due to the conversion by yeast of part of the substrate mainly into ethanol [17], which is then transformed into acetic acid, which is necessary for archaea at the methanogenic stage and the accumulation of biogas (Figure 1).
Preferably, Saccharomyces cerevisiae yeast cells in the form of a suspension, widely available for practical application and most often used in the food industry, are introduced into the anaerobic reactor [27,28]. Immobilized yeast cells have not been studied in similar studies before.
Among the media suitable for anaerobic methanogenic fermentation, the majority are those containing glucose in the composition of various food industry and agriculture waste. So, the existing interest in the cells of S. cerevisiae is understandable. However, there are many other yeasts cells that are also used in biotechnological processes, and in this regard, it was interesting from a scientific and practical point of view to study the possibility of their use for purposes similar to those indicated for S. cerevisiae, and under the same conditions, in order to compare their effect on the methanogenic conversion of glucose.
Another large-tonnage waste product of various processes, including the production of biodiesel fuel, is glycerol [29]. The processing of glycerol into biogas is limited due to the formation of intermediate products, such as long-chain fatty acids and propionic acid [30,31]. In this regard, to increase the efficiency of methanogenesis, glycerol is more often used not as the main substrate, but as a co-substrate in combination with organic sources (waste) rich in nitrogen, while maintaining the optimal C:N ratio [32,33]. Under anaerobic conditions, the conversion of glycerol occurs through the formation of dihydroxyacetone and pyruvate, which are converted into ethanol and acetic acid (Figure 1). Thus, yeasts of the genus Saccharomyces do not directly convert glycerol into ethanol; additional biochemical reactions are required. In this regard, the use of the yeast Pachysolen tannophilus, which has not been previously utilized for these purposes, appears promising in methanogenesis. At the same time, for the P. tannophilus Y-475 yeast cells immobilized in polyvinyl alcohol (PVA) cryogel, it was established that under aerobic conditions, they are capable of providing for the conversion of 25 g/L of glycerol with the formation of 8.3 g/L of ethanol. In addition, the possibility of using these cells in at least 16 working cycles of such a process without reducing the efficiency of operation and the yield of ethanol was established [34].
Since Pachysolen tannophilus cells have not been previously used under anaerobic conditions in immobilized form, such a study seemed very promising as a potential approach to intensifying methanogenesis using glycerol as a substrate. Thus, the aim of this study was to investigate the effect of combining the cultivation of yeasts of different genera (Saccharomyces, Candida, Kluyveromyces, and Pachysolen) in an immobilized form with suspended AS for the conversion of substrates such as glucose and glycerol under methanogenesis conditions.
2. Materials and Methods
2.1. Materials
The components of nutrient media for the accumulation of yeast biomass and their subsequent immobilization (K2HPO4, KH2PO4 × 2H2O, MgSO4 × 7H2O), citric acid, ascorbic acid, glucose, glycerol, peptone, yeast extract, poly(vinyl alcohol) (PVA) (CAS 9002-89-5), ethanol (99.5% CAS 1516-08-1), and acetic acid (CAS 64-19-7), were purchased from Chimmed (Moscow, Russia). For bioluminescent analysis of intracellular adenosine triphosphate (ATP) concentration, the luciferin–luciferase reagent [35] was used. Dimethyl sulfoxide (DMSO) was purchased from Chimmed (Moscow, Russia) and used for the extraction of ATP from the cells.
The yeast strains Saccharomyces cerevisiae Y-185, Candida maltosa Y-2256, Kluyveromyces marxianus Y-5273, and Pachysolen tannophilus Y-475 were purchased from the All-Russian Collection of Industrial Microorganisms, Moscow, Russia (http://www.genetika.ru/vkpm/ has been accessed on 30 April 2025).
Suspended AS was obtained from a food wastewater treatment plant (Kashira, Moscow region, Russia). Sludge was stored at 35 °C with the addition of 1 g COD/L (1 g/L glucose) every month before its use. The characteristics of this consortium were previously determined by the authors of this study [36].
2.2. Yeast Cells Immobilization
To obtain 4 immobilized biocatalysts based on PVA cryogel and yeast cells, a method successfully tested on the example of Pachysolen tannophilus yeast cells was used [34]. Yeast biomass was accumulated as a result of culturing cells on a medium of the following composition (g/L): glucose—20; yeast extract and peptone—5; K2HPO4—3; KH2PO4—2; citric acid—1; ascorbic acid—1; MgSO4—0.5; pH—5.6; at 26 ± 2 °C, 150 ± 10 rpm for 20 ± 1 h. Next, the yeast biomass was separated from the culture broth by centrifugation (Avanti J25, Beckman, Germany) for 15 min at 10,000 rpm and mixed with a 12 wt% PVA solution so that the cell concentration in the suspension was 10% w/w. The resulting suspension was then distributed into the wells of 96-well sterile plates (Paneco Company, Moscow, Russia) and frozen at −20 ± 1 °C to form a polymer cryogel. Before use, the immobilized cells were defrosted at +6 ± 1 °C for 6 ± 1 h.
2.3. Biogas Production by Artificial Consortia
For biogas production, the anaerobic bottles (100 mL) (Mimimed, Bryansk, Russia) with 50 mL of total liquid medium were used. The dose of AS was 10% (v/v) with 2.5 ± 0.1 g of immobilized yeast granules with 10% yeast biomass (Figure 2).

Figure 2.
Bottles with samples of AS and artificial consortia, based on AS with granules of cryogel PVA containing immobilized yeast cells of genus Saccharomyces (S), Candida (C), Pachysolen (P), and Kluyveromyces (K), which were used in experiments.
The ratio of AS to yeast cells was 64:1 (w/w). The ratio of AS to yeast cells was calculated as a ratio of the dry weight of AS to the dry yeast cell biomass in PVA cryogel granules (biomass in granules was 10 ± 0.02% w/w).
The glucose (5 g/L) and glycerol (5 g/L) were used as carbon sources in a 0.2 M phosphate buffer (pH 7.3 ± 0.1) medium. The bottles with the medium and consortia were filled with helium for 20 min to remove oxygen from the gaseous phase.
Methanogenesis was conducted at 36 ± 1 °C for 28 days. The sample with AS cells without yeasts was used as a control.
2.4. Static Test of Artificial Consortia in Methanogenic Treatment of Wastewater
Several artificial consortia were applied for the static methanogenic treatment of the following samples of wastewaters (Table 2): real wastewater from a milk processing plant (OOO Ostankinsky Molochny Kombinat, Moscow, Russia), real wastewater from a meat processing plant OOO “Adria” (Moscow region, Russia), and model wastewater prepared using bagasse hydrolysate. Bagasse hydrolysate was obtained as a result of the enzymatic hydrolysis of 50 g/L dry bagasse pieces by Spezyme CP (Dupont, New York, NY, USA) and Novozyme-188 (Novozymes Corp., Copenhagen, Denmark). Bagasse particles with a size of 100–300 µm were prepared using a Mikrosilema IM-450 impeller mill (Techpribor, Schekino, Russia) and suspended in a 0.1 M citrate buffer (pH 5.0). The enzymes were introduced into the reaction medium at a mass ratio of 3:1. Hydrolysis was carried out at 50 °C with constant mixing (250 rpm) for 24 h. The total carbohydrate, lipid, and protein contents were determined using known procedures [37].

Table 2.
Characteristics of wastewaters used for the static test of the artificial consortia.
Additionally, the medium containing crude glycerol was used in the experiments as a wastewater contaminated with the by-product of biodiesel production from the lipids of filamentous fungi biomass. It was prepared as published earlier [34]. Glycerol feedstock contained 83% glycerol, 6.5% ash, 0.5% methanol, and 10% water. Glycerol concentration was determined using the glycerol assay kit (Megazyme Ltd., Wicklow, Ireland). Ash content was determined using a muffle furnace at 590 ± 10 °C for 24 h. Ash is expressed as a percentage of the difference in weight before and after ashing.
For biogas production from wastewaters, the anaerobic bottles (1 L) (Mimimed, Bryansk, Russia) with 500 mL of total liquid medium were used. The doses of AS and yeast were the same as in the previous experiment (AS 10% (v/v) with 25 ± 1 g of immobilized yeast). The bagasse hydrolysate and glycerol were previously diluted with a 0.2 M phosphate buffer (pH 7.3 ± 0.1). The pH of all samples was adjusted to 7.3 using 10 M KOH (Chimmed, Moscow, Russia). Methanogenesis was conducted at 36 ± 1 °C for 7 days.
2.5. Analytical Methods
The intracellular ATP concentration was measured using the Microluminometer 3560 (New Horizons Diagnostics Co., Baltimore, MD, USA) and a luciferin–luciferase reagent. Samples were mixed with 800 μL of DMSO and kept for 3 h for intracellular ATP extraction. After extraction, the samples were diluted with distilled water (1:10), and 50 µL of the resulting solution was added to the bioluminometer cuvette with 50 µL of the luciferin–luciferase reagent. For analysis of the obtained results, the calibration curve was used for standard ATP solutions (10–14–10–8 mole/mL).
For potentiometric measurements, the Corning Pinnacle 530 pH meter (Corning Incorporated, Corning, NY, USA) was used. During methanogenesis, the biogas content (H2, CH4, and CO2) was analyzed using a Crystallux-4000M gas chromatograph (RPC ‘Meta-chrom’, Yoshkar-Ola, Russia) equipped with a katharometer. Argon was used as the carrier gas, the flow rate was 20 mL/min, and the column temperature was 51 ± 1 °C. The gas phase sample volume was 0.2 mL. A gas mixture of H2-CH4-CO2 (5%) in argon was used as a standard (PGS-Service, Moscow, Russia). The obtained data was analyzed using NetChrom software (Meta-chrom ver. 2.1, Yoshkar-Ola, Russia).
COD was analyzed using the Merck Cod Test Kit (Merck Life Science LLC (Moscow, Russia).
Methanogenesis efficiency was estimated based on biogas accumulation and the following equations:
where C—concentration of organic matter, g COD/L; V liquid phase—0.05 L; and 0.5—volume of biogas formed from 1 g COD at 273 K.
% = (Biogas experimental/Biogas theoretic) × 100
Biogas theoretic = (C × V liquid phase) × 0.5 × 1000
The accumulation rate of CH4 (mL/day) was calculated as the ratio of the total volume of methane to the duration of the process.
The concentrations of methanol, ethanol, and acetic acid were determined using a Shimadzu GC-15A (Shimadzy Corporation, Kyoto, Japan) gas chromatograph equipped with an FID. Argon was used as the carrier gas. The temperature of the injector, column thermostat, and evaporator was 200, 220, and 240 °C, respectively. The sample volume was 1 μL. The 0.1% (v/v) solutions of methanol, ethanol, and acetic acid were used as standards.
The standard deviation (±SD) of data obtained from three independent experiments conducted in this work was not more than 5%. Statistical analysis was performed using SigmaPlot (ver. 12.5, Systat Software Inc., San Jose, CA, USA).
3. Results
As the main components of various wastes, including food, agricultural, biodiesel production, and palm oil [38,39], glucose and glycerol, which can be utilized by yeast as the main substrate, were chosen as substrates for methanogenesis. Since various components of complex media containing glucose and glycerol can have an ambiguous effect on methanogenesis, it was decided to use as substrates in this work not just any production wastes, but pure substances (glucose and glycerol) for a more objective interpretation of the results obtained.
According to the previously known publications, the increase in the efficiency of methanogenesis when combining yeast cells with AS occurs due to the conversion of the main substrates by these yeasts primarily into ethanol and acetic acid [17].
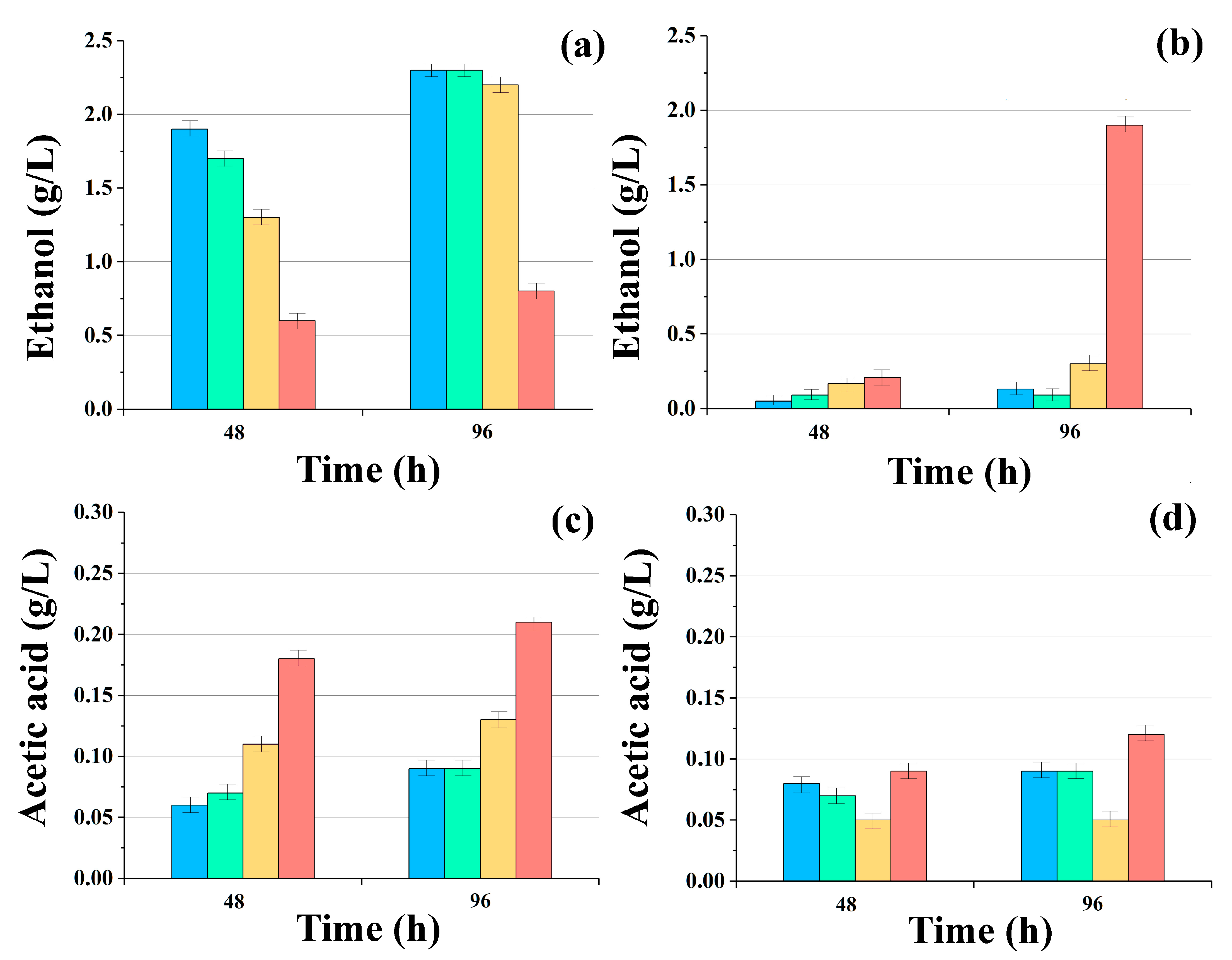
With this in mind, their accumulation in the liquid phase of the reaction methanogenic medium was monitored during the initial period of glucose and glycerol conversion by yeast consortia with AS. Analyses were carried out during the first six days, but only on the second and fourth days of the process was it possible to register changes in the accumulation of these metabolites, since their concentrations only decreased after that as they underwent conversion into biogas (Figure 3).
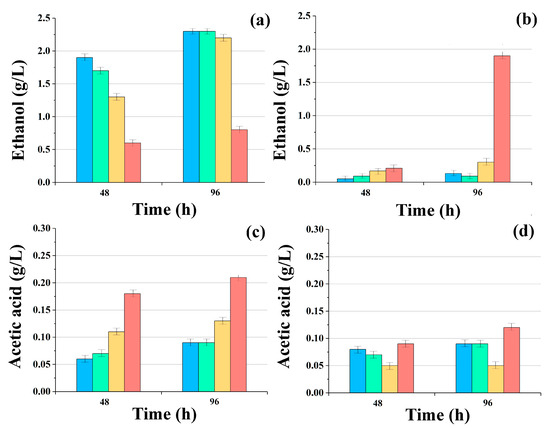
Figure 3.
Accumulation of ethanol (a,b) and acetic acid (c,d) in reaction media during anaerobic conversion of glucose (a,c) and glycerol (b,d) by consortia of AS with immobilized cells of S. cerevisiae ■, C. maltosa ■, K. marxianus ■, and P. tannophilus (P) ■ yeasts. Results are shown by the mean ± SD (n = 3).
When using glucose as the main substrate, the presence of ethanol (in some variants, about 2 g/L) and acetic acid in samples with cells of Saccharomyces, Candida, and Kluyveromyces was noted in the medium after 96 h.
The conversion of glycerol with the accumulation of ethanol in the reaction medium by these same consortia proceeded less dynamically, and in most cases, the current concentration of ethanol did not exceed 0.25 g/L.
However, a very high concentration of ethanol (1.8 ± 0.1 g/L) in comparison with consortia containing different yeast cells was revealed in the variant that contained P. tannophilus yeast. By the 96th hour, the concentration of ethanol in the medium with this consortium was 7.2 times higher than in the others.
This result was undoubtedly due to the fact that these yeasts are capable of highly efficiently converting glycerol into ethanol [34]; however, it was revealed for the first time in this study that these yeasts can carry out such conversion not only under aerobic but also under anaerobic conditions.
The current concentration of acetic acid was comparable with the analogous parameter for consortia with S. cerevisiae and C. maltosa cells, established on the media with glucose. In the medium with the consortium, which contained P. tannophilus cells, the level of acetic acid in the medium with glycerol was 25% higher than in other variants of the consortia, which clearly indicates that this consortium on the medium with glycerol is a clear favorite, and the yeast takes an active part in the processes that occur within the consortium.
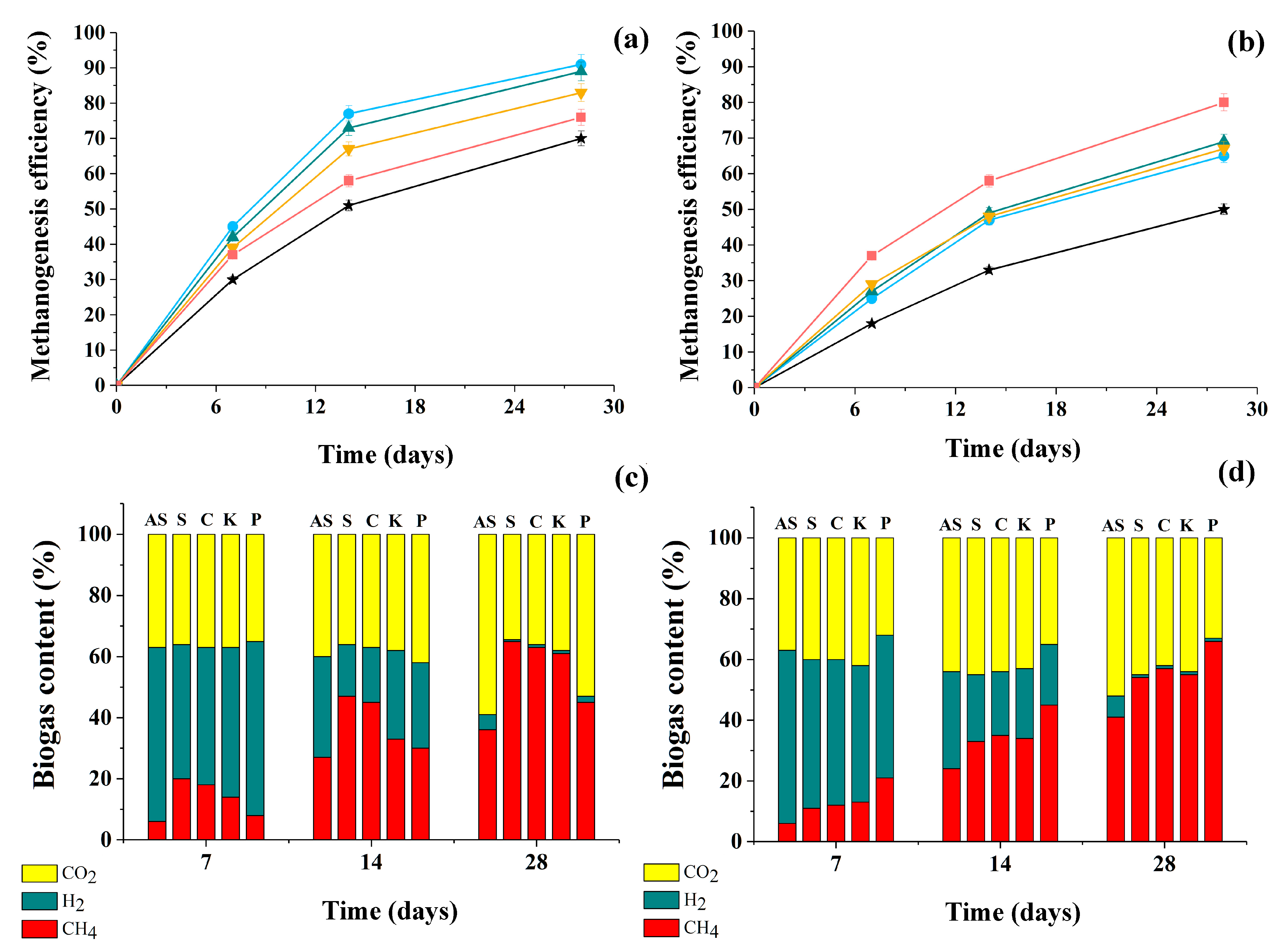
Throughout the entire period of culturing the consortia under anaerobic conditions, the efficiency of methanogenesis was analyzed in each variant, as well as in the control (AS without yeast cells) for biogas yield in relation to the theoretically possible level. An analysis of the current composition of the gas phase in the methanogenic process was also carried out, determining the proportions of gases (CO2, H2, CH4) present in the biogas (Figure 4).
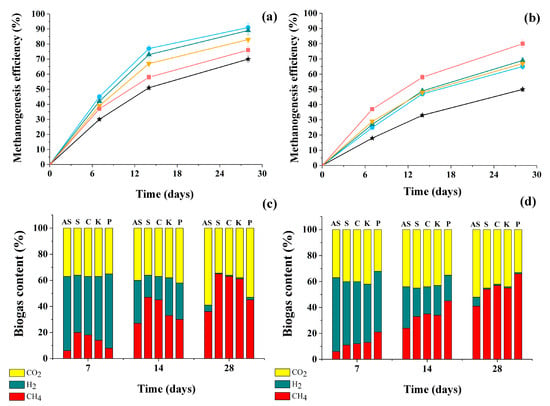
Figure 4.
The efficiency of methanogenesis (a,b) and the composition of the resulting biogas (c,d) as a result of anaerobic conversion of glucose (a,c) and glycerol (b,d) under the action of consortia of AS with yeast cells of different strains for 28 days. AS itself ★, AS with yeast cells of S. cerevisiae (S) ●, C. maltosa (C) ▲, K. marxianus (K) ▼, and P. tannophilus (P) ■. Results are shown by the mean ± SD (n = 3).
It was found that the addition of any of the four yeast strains used in the study to AS resulted in an increase in the efficiency of methanogenesis by 110–155% (biogas accumulation) during the conversion of both substrates compared to the AS in the control (without yeast). The efficiency of methanogenesis during the conversion of glucose was higher than that of glycerol. This was probably due to the faster conversion of glucose consortia by cells (Figure 1) and a higher COD level in the medium with glycerol (6.15 g/L) compared to the COD level in the glucose-containing medium (5.35 g/L).
In this work, the efficiency of methanogenesis during glycerol conversion in the consortium of AS with Pachysolen yeast was the highest among other studied samples of consortia and the anaerobic methanogenic sludge itself. It should be noted that in the first 7 days, CO2 and H2 predominated in the composition of the resulting biogas, and they composed up to 90% of the total gaseous phase volume. Then, a gradual increase in the proportion of CH4 in the biogas was observed. At the same time, the methane content in the biogas produced by the consortia of AS with yeast was significantly higher than in the gas phase with sludge without yeast.
During glucose conversion, the highest amount of CH4 was observed in samples with a consortium of sludge and S. cerevisiae yeast cells, followed by consortia obtained by combining methanogenic sludge and C. maltose or K. marxianus yeasts at almost the same level across all parameters (methane concentration in biogas and its accumulation rate) (Table 3).

Table 3.
Accumulation rate of CH4 in methanogenesis carried out for 28 h in glucose and glycerol media using different variants of AS-based consortia.
During glycerol methanogenesis, the maximum in terms of methane accumulation rate in biogas and its accumulation level in the composition was observed when studying the functioning of a consortium of sludge with P. tannophilus yeast cells. Moreover, in media with glucose, this consortium with P. tannophilus yeast exceeded the control level (sludge without yeast) but was not as successful as other variants of the consortia.
Thus, it is obvious that the presence of immobilized yeast cells in the consortia with AS was exclusively positive in media containing both substrates (glucose and glycerol), but at the same time, obvious preferences were revealed among the consortia due to the presence of different yeast cells in them in relation to these substrates.
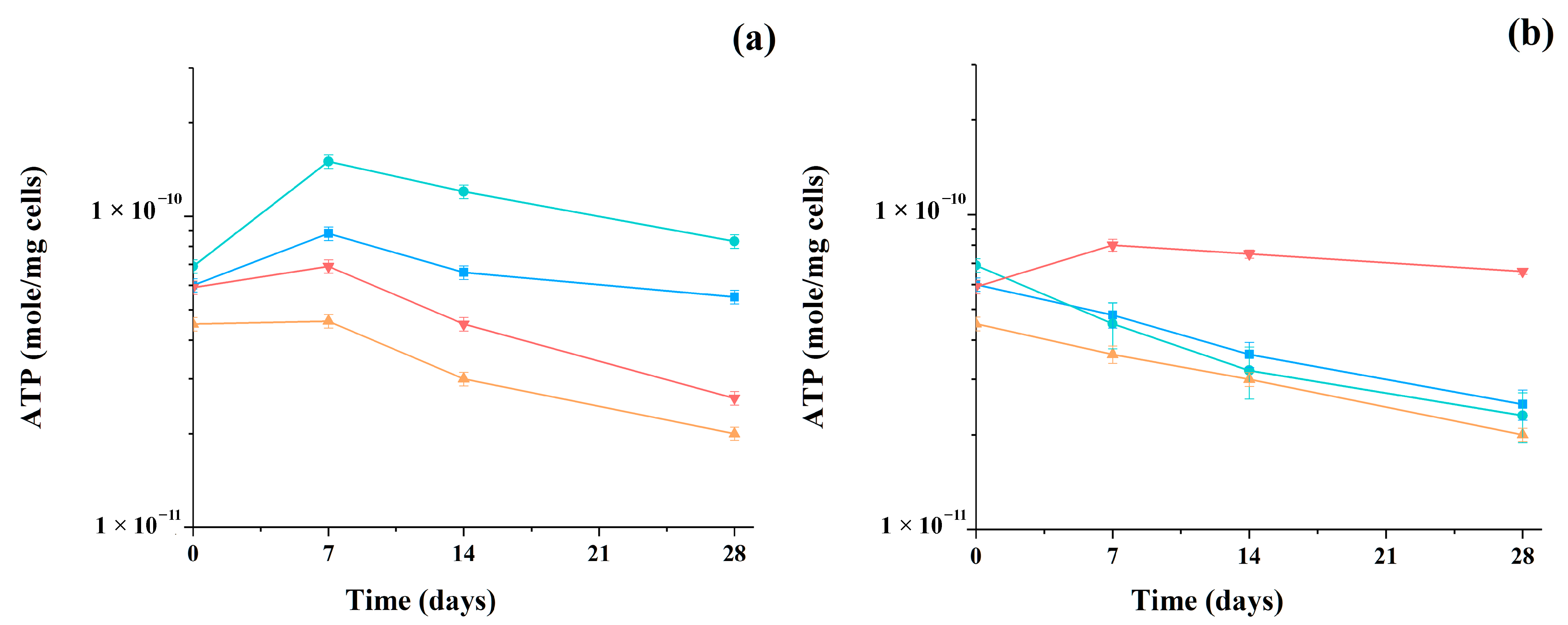
During the study of methanogenesis, the metabolic activity of microorganisms in the consortia was also monitored by assessing the change in the concentration of intracellular ATP in the samples of mixed cells (Figure 5).
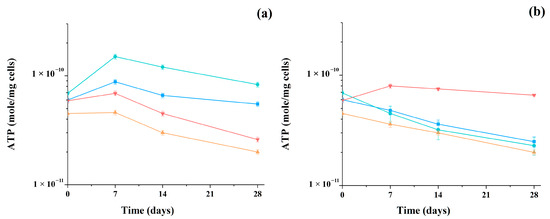
Figure 5.
Changing of intracellular ATP concentration during methanogenesis catalyzed by artificial consortia containing AS and yeast cells (S. cerevisiae ■, C. maltosa ●, K. marxianus ▲, and P. tannophilus ▼) using glucose (a) and glycerol (b) as the main substrates. Results are shown by the mean ± SD (n = 3).
When using glucose as a substrate, an increase in the level of intracellular ATP was observed in all consortia during the first seven days of the process, and then it decreased slightly, which was associated with a gradual decrease in the concentration of the main substrate in the reaction medium. Thus, in consortia with K. marxianus and P. tannophilus yeast cells, the ATP concentration in the analyzed samples, cultured in a medium with glucose, decreased below the initial level by an average of 40% during the process itself.
In the medium with glycerol, in samples of three consortia with the yeasts S. cerevisiae, C. maltosa, and K. marxianus, a similar decrease in ATP from the initial level was recorded during the entire process of methanogenesis. At the same time, the consortium with P. tannophilus yeast cells demonstrated a very high level of ATP in the medium with glycerol, which was predetermined by the known efficiency of these cells in the utilization of glycerol as the main substrate.
ATP plays a central role in metabolism and provides energy for many biochemical reactions in all living cells. The synthesis and hydrolysis of ATP are used by microorganisms to generate energy during various biochemical transformations and physiological processes; therefore, changes in the concentration of ATP in cells confirm their viability and the high level of metabolic activity. The concentration of ATP can be quickly and easily determined using the bioluminescent method of ATP-metry [36,40].
In general, all cells of all consortia were characterized by a high intracellular level of ATP after the end of the process, which allows us to expect their at least repeated use in similar processes. According to known data [40], the absence of significant (more than 3–4 times) fluctuations in the controlled level of ATP during the functioning of the consortia indicates a favorable “working” state of the cells in the studied processes.
Various samples of real and model wastewater from industrial and agricultural enterprises were processed under the action of artificial anaerobic consortia formed for this study, based on AS and immobilized yeast cells under conditions of a static methanogenic process (Table 4).

Table 4.
Static methanogenic tests of various wastewaters using developed anaerobic consortia for seven days.
To compare the results obtained in similar experiments, AS was used without the addition of yeast cells. Since the task of the study was precisely to obtain comparative estimates of the functioning of AS itself and its consortia with yeast in wastewaters, the testing was carried out for only seven days. According to the data obtained from the analysis of intracellular ATP concentration at the previous stage of the study (Figure 5), this time period was favorable for consortium cells, and their metabolic activity during this time period was presentable.
According to the literature data [41], such static methanogenic testing is indicative for evaluating the prospects of the proposed process. It should be noted that in this study, additional nutrients and trace elements were not introduced into the wastewater, but the composition of the media components was used, which was predetermined by the conditions of their production (real samples) or preparation (model samples). It was found that the use of consortia of AS with immobilized yeast cells provided stable increased COD removal (12–22%) and efficiency of biogas production (16–25%). This effect was found in all samples of the wastewaters studied.
4. Discussion
The information presented in Table 1 clearly confirms the relevance of the existing scientific and practical interest in the processes of methanogenic treatment of various wastes from the food industry and agriculture, focusing on the production of biogas by using combined biocatalysts consisting of anaerobic activated sludge and yeast cells.
It should be noted that the authors of the conducted studies (Table 1) clearly note improved indicators for the yield of accumulating biogas with an increased content of methane. This is despite the differences in the compositions of the processed substrates, the variability of the conditions for methanogenesis, and the ratio of components in the composition of the used biocatalyst combining AS and yeast.
At the same time, the analysis of the data in Table 1 also shows that, in fact, only S. cerevisiae yeast cells are used to create artificial consortia for the purpose of converting most protein–carbohydrate substrates into biogas for methanogenic sludge. Only in the case of processing fat-containing waste (waste frying fat), the possibility of using other yeasts, such as Yarrowia lipolytica, capable of converting lipid-containing substrates, was shown [12]. In this regard, the results achieved in this work are new, since they demonstrate the success of using yeast cells of different genera (Candida, Kluyveromyces, and Pachysolen) in artificial consortia with methanogenic sludge to obtain an increased yield of biogas with an improved rate of methane accumulation and enhanced methanogenesis efficiency in comparison with the same parameters typical of AS itself.
Thus, significantly improved indicators for the conversion of both glucose- and glycerol-containing media under the action of different variants of artificial consortia with yeast cells have been demonstrated. It should be noted that among those media that are subject to methanogenic treatment, especially in the food industry and agriculture, there are many such media; therefore, the potential prospects of the results shown in this work for their practical application can be assumed. Of particular importance are those preferences identified for the use of yeast cells in consortia that were identified when carrying out the process in media with glucose and glycerol.
The results shown with the use of Pachysolen cells in a consortium with sludge are outstanding in terms of the parameters achieved (the rates and levels of methane accumulation in biogas and the degrees of glycerol conversion into biogas), and, to the best of our knowledge, have no comparable analogs, since, as noted above, glycerol is a substrate that is difficult to utilize for methanogenic sludge and is therefore introduced into the environment for obtaining biogas not as the main substrate, but as a co-substrate [42].
It should also be noted that this work demonstrates for the first time the possibility of combining Pachysolen yeasts with methanogenic sludge for co-cultivation and conversion of glycerol under anaerobic conditions, whereas it was previously shown that it is under aerobic conditions that these yeasts are effective producers of ethanol from such an unusual substrate as glycerol.
The reason for this is that anaerobic conversion of glycerol into biogas under the action of methanogenic sludge proceeds slowly through the formation of dihydroxyacetone and pyruvate, which are converted into acetic acid and ethanol, as well as other metabolites. It is obvious that the presence of Pachysolen yeasts in artificial consortia significantly accelerated the overall process of glycerol conversion into biogas, although the proportion of yeast cells in the overall consortium was insignificant, since the ratio of AS to yeast cells was 64:1 (w/w).
In this work, the immobilized form of different yeasts introduced into the consortia together with AS demonstrated clear advantages in comparison with the results obtained in similar studies conducted earlier by us using bacterial cells as components of artificial consortia created on the basis of the same methanogenic sludge.
Such experiments with anaerobic consortia based on sludge and individual bacterial cultures of Clostridium acetobutylicum, Pseudomonas sp., or Enterococcus faecalis were carried out by us earlier, with the introduction of bacterial cells in a ratio of 9:1 (w/w) to methanogenic sludge. Bacterial cells were also used in a form immobilized in PVA cryogel, which led, as in the case of yeast, to an improvement in the characteristics of methanogenesis. The use of the same support for the immobilization of different cells (bacteria and yeast) was dictated by the well-known positive characteristics of this polymer carrier [43]. In the work with bacterial participants in the methanogenic consortium, enzymatic hydrolysates of Jerusalem artichoke stems and sugar beet pulp with a significantly higher level of COD in the medium (10.5 g COD/L) were used instead of pure glucose [44], which ensured intensive methanogenesis, leading to an increase in the level of accumulated methane in the biogas by 1.7 times in relation to the control.
When discussing the potential practical application of the results obtained, based on an easily implemented technical solution consisting of combining methanogenic sludge and yeast cells in an anaerobic process, it is necessary to emphasize that large volumes of wastewater with high concentrations of organic pollutants are regularly neutralized during the process of methanogenesis. In this regard, the construction of artificial consortia based on AS and yeast can be an effective approach to improving the performance of wastewater treatment-oriented processes at various enterprises [18,26,45].
As noted earlier, S. cerevisiae yeast cells were mainly added to the AS in various methanogenic processes [17,18,19,20,21,22,23,24,25], while yeast cells of other genera were practically not considered as participants. Although judging by the results of this study, they may have a clear technological potential. In this regard, the prospects of this work lie in expanding the range of different types of yeast introduced into the composition of AS, which may be a by-product of various industries, for example, K. marxianus [46].
The resistance of immobilized cells to the possible presence of various toxicants (heavy metals, antimicrobial agents, etc.) in wastewater certainly provides advantages in using yeast as part of an artificial consortium in this form. This allows for the methanogenic treatment of toxic effluents, which include, for example, effluents from oil desulfurization that are subject to methanogenic treatment [47]. The use of artificial consortia with yeast cells may be extremely interesting here from the point of view of improving the characteristics of the process. Since there is a practical interest in the use of artificial consortia based on AS for the methanogenic conversion of various lignocellulose wastes containing antibiotics and pesticides [44], in this case, for the treatment of wastewater containing substrates hydrolysable to glucose, the obtained artificial consortia may be of undoubted practical interest.
However, this result was achieved with a completely different ratio of cells participating in the consortium. Thus, yeast produced an increase in methane yield by 1.5 times in this work on a glucose-containing medium, when yeast cells made up only 1.5% of the biomass of all cells in the consortium, while the bacteria, with which the comparison is being conducted, made up 10% of the consortium.
5. Conclusions
The creation of artificial consortia comprising anaerobic methanogenic sludge and various types of yeast in immobilized form is aimed at stimulating biogas production by improving the quality of biocatalysts for this process. Methanogenesis, with the addition of yeast, showed a higher rate of CH4 accumulation due to the formation of ethanol and acetic acid, which can be easily used by methanogens, thereby increasing biogas production and ensuring stable operation of the entire consortium. The maximum efficiency of methanogenesis was over 90%, while the methane content in biogas reached 60–65% during glucose conversion by the consortium of AS with S. cerevisiae, C. maltose, and K. marxianus yeasts.
The addition of yeast cells of the genus Pachysolen proved to be a very effective approach to solving the problem of converting glycerol-containing substrates. The consistently high level of intracellular ATP in the consortium cells confirmed their high metabolic activity and the potential for reusing artificial consortia as biocatalysts. In general, the approach to creating such artificial methanogenic consortia is relevant and promising for obtaining biogas from a wide variety of substrates. The additional immobilization of cells makes it easy to regulate their composition and metabolic activity, helping to maintain their intensive functioning.
A demonstration of the work of the obtained consortia in different wastewaters confirmed the identified advantages of combining AS with different yeast cells and the prospects for their practical application in the methanogenic treatment of sewage of various origins.
Author Contributions
Conceptualization, E.E.; investigation, N.S., O.S., A.A., O.M. and E.E.; data curation, N.S. and E.E.; writing—original draft preparation, N.S., O.S., A.A. and E.E.; writing—review and editing, N.S. and E.E.; supervision, E.E. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Russian Science Foundation (23-14-00092).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AS | Anaerobic sludge |
| ATP | Adenosine triphosphate |
| COD | Chemical oxygen demand |
| DMSO | Dimethyl sulfoxide |
| SCFA | Short-chain fatty acids |
| VS | Volatile solids |
References
- Lawson, C.E.; Harcombe, W.R.; Hatzenpichler, R.; Lindemann, S.R.; Löffler, F.E.; O’Malley, M.A.; Martín, H.G.; Pfleger, B.F.; Raskin, L.; Venturelli, O.S.; et al. Common principles and best practices for engineering microbiomes. Nat. Rev. Microbiol. 2019, 17, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Lü, H.; Wei, J.-L.; Tang, G.-X.; Chen, Y.-S.; Huang, Y.-H.; Hu, R.; Mo, C.; Zhao, H.-M.; Xiang, L.; Li, Y.-W.; et al. Microbial consortium degrading of organic pollutants: Source, degradation efficiency, pathway, mechanism and application. J. Clean. Prod. 2024, 451, 141913. [Google Scholar] [CrossRef]
- Adamu, K.S.; Bichi, Y.H.; Nasiru, A.Y.; Babangida, A.M.; Umar, M.M.; Usman, G.; Muhammad, R. Synthetic microbial consortia in bioremediation and biodegradation. Int. J. Res. Sci. Innov. Appl. Sci. 2023, 8, 232–241. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Kumar, A. Enhanced degradation of anthraquinone dyes by microbial monoculture and developed consortium through the production of specific enzymes. Sci. Rep. 2021, 11, 7678. [Google Scholar] [CrossRef]
- Elumalai, P.; Parthipan, P.; Huang, M.; Muthukumar, B.; Cheng, L.; Govarthanan, M.; Rajasekar, A. Enhanced biodegradation of hydrophobic organic pollutants by the bacterial consortium: Impact of enzymes and biosurfactants. Environ. Pollut. 2021, 289, 117956. [Google Scholar] [CrossRef] [PubMed]
- Tondro, H.; Musivand, S.; Zilouei, H.; Bazarganipour, M.; Zargoosh, K. Biological production of hydrogen and acetone- butanol-ethanol from sugarcane bagasse and rice straw using co-culture of Enterobacter aerogenes and Clostridium acetobutylicum. Biomass Bioenergy 2020, 142, 105818. [Google Scholar] [CrossRef]
- Cao, Z.; Yan, W.; Ding, M.; Yuan, Y. Construction of microbial consortia for microbial degradation of complex compounds. Front. Bioeng. Biotechnol. 2022, 10, 1051233. [Google Scholar] [CrossRef]
- Efremenko, E.; Stepanov, N.; Maslova, O.; Senko, O.; Aslanli, A.; Lyagin, I. “Unity and Struggle of Opposites” as a basis for the functioning of synthetic bacterial immobilized consortium that continuously degrades organophosphorus pesticides. Microorganisms 2022, 10, 1394. [Google Scholar] [CrossRef]
- Efremenko, E.; Senko, O.; Stepanov, N.; Aslanli, A.; Maslova, O.; Lyagin, I. Quorum sensing as a trigger that improves characteristics of microbial biocatalysts. Microorganism 2023, 11, 1395. [Google Scholar] [CrossRef]
- Pourcelot, E.; Vigna, A.; Marlin, T.; Galeote, V.; Nidelet, T. Design of a new model yeast consortium for ecological studies of enological fermentation. Peer Community J. 2025, 5, e5. [Google Scholar] [CrossRef]
- Darvishi, F.; Rafatiyan, S.; Abbaspour Motlagh Moghaddam, M.H.; Atkinson, E.; Ledesma-Amaro, R. Applications of synthetic yeast consortia for the production of native and non-native chemicals. Crit. Rev. Biotechnol. 2024, 44, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Mittermeier, F.; Bäumler, M.; Arulrajah, P.; García Lima, J.D.J.; Hauke, S.; Stock, A.; Weuster-Botz, D. Artificial microbial consortia for bioproduction processes. Eng. Life Sci. 2023, 23, e2100152. [Google Scholar] [CrossRef] [PubMed]
- Malik, K.; Salama, E.-S.; El-Dalatony, M.M.; Jalalah, M.; Harraz, F.A.; Al-Assiri, M.S.; Zheng, Y.; Sharma, P.; Li, X. Co-fermentation of immobilized yeasts boosted bioethanol production from pretreated cotton stalk lignocellulosic biomass: Long-term investigation. Ind. Crops Prod. 2021, 159, 113122. [Google Scholar] [CrossRef]
- Kong, Z.; Li, L.; Kurihara, R.; Kubota, K.; Li, Y.-Y. Anaerobic treatment of N, N-dimethylformamide-containing wastewater by co-culturing two sources of inoculum. Water Res. 2018, 139, 228–239. [Google Scholar] [CrossRef]
- Alengebawy, A.; Ran, Y.; Osman, A.I.; Jin, K.; Samer, M.; Ai, P. Anaerobic digestion of agricultural waste for biogas production and sustainable bioenergy recovery: A review. Environ. Chem. Lett. 2024, 22, 2641–2668. [Google Scholar] [CrossRef]
- Chew, K.R.; Leong, H.Y.; Khoo, K.S.; Vo, D.V.N.; Anjum, H.; Chang, C.K.; Show, P.L. Effects of anaerobic digestion of food waste on biogas production and environmental impacts: A review. Environ. Chem. Lett. 2021, 19, 2921–2939. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, S.; Ma, X.; Guan, W.; Song, N.; Wang, Q.; Wu, C. Effect of yeast addition on the biogas production performance of a food waste anaerobic digestion system. R. Soc. Open Sci. 2020, 7, 200443. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, G.D.; Škrjanec, I.; Logar, R.M. Anaerobic co-digestion of excess brewery yeast in a granular biomass reactor to enhance the production of biomethane. Bioresour. Technol. 2012, 124, 328–337. [Google Scholar] [CrossRef]
- Zupančič, G.D.; Panjičko, M.; Zelić, B. Biogas production from brewer’s yeast using an anaerobic sequencing batch reactor. FTB 2017, 55, 187–196. [Google Scholar] [CrossRef]
- Primaloka, A.D.; Ardhannari, L.; Matin, H.H.; Sumardiono, S. Study of biogas production from cassava industrial waste by anaerobic process. MATEC Web Conf. 2018, 156, 03052. [Google Scholar] [CrossRef]
- Tang, X.; Liao, C.; Zhou, S.; Chen, C.; Li, L.; Lu, G.; Xuang, X.; Zhang, M.; Chen, C.; Li, P. Potential of perennial sorghum for biogas production: Pretreatment with yeast-contained inoculants during anaerobic storage. Fuel 2024, 359, 130365. [Google Scholar] [CrossRef]
- Syaichurrozi, I.; Rusdi, R.; Hidayat, T.; Bustomi, A. Kinetics studies impact of initial pH and addition of yeast Saccharomyces cerevisiae on biogas production from tofu wastewater in Indonesia. Int. J. Eng. Trans. B Appl. 2016, 29, 1037–1046. [Google Scholar]
- Islas-Espinoza, M.; de las Heras, A.; Vázquez-Chagoyán, J.C.; Salem, A. Anaerobic cometabolism of fruit and vegetable wastes using mammalian fecal inoculums: Fast assessment of biomethane production. J. Clean. Prod. 2017, 141, 1411–1418. [Google Scholar] [CrossRef]
- Syaichurrozi, I.; Basyir, M.F.; Farraz, R.M.; Rusdi, R. A preliminary study: Effect of initial pH and Saccharomyces cerevisiae addition on biogas production from acid-pretreated Salvinia molesta and kinetics. Energy 2020, 207, 118226. [Google Scholar] [CrossRef]
- Fang, H.; Shi, Y.; Li, D.; Song, L.; Li, Y.Y.; Liu, R.; Yuan, D.; Niu, Q. Synergistic co-digestion of waste commercial yeast and chicken manure: Kinetic simulation, DOM variation and microbial community assessment. Renew. Energy 2020, 162, 2272–2284. [Google Scholar] [CrossRef]
- Moeller, L.; Bauer, A.; Zehnsdorf, A.; Lee, M.Y.; Müller, R.A. Anaerobic co-digestion of waste yeast biomass from citric acid production and waste frying fat. Eng. Life Sci. 2018, 18, 425–433. [Google Scholar] [CrossRef]
- Zhao, S.; Li, P.; Fang, H.; Song, L.; Li, D.; Liu, R.; Niu, Q. Enhancement methane fermentation of Enteromorpha prolifera waste by Saccharomyces cerevisiae, batch kinetic investigation, dissolved organic matter characterization, and synergistic mechanism. Environ. Sci. Pollut. Res. 2020, 27, 16254–16267. [Google Scholar] [CrossRef] [PubMed]
- Jojoa-Unigarro, G.D.; González-Martínez, S. OFMSW Fermentation with different inocula and its effects on methane production. Waste Biomass Valor. 2023, 14, 1461–1476. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dębowski, M.; Zieliński, M.; Kasiński, S.; Cruz Sanchez, J. Biotechnological valorization of waste glycerol into gaseous biofuels—A Review. Energies 2024, 17, 338. [Google Scholar] [CrossRef]
- de Mello, B.S.; Pozzi, A.; Rodrigues, B.C.G.; Costa, M.A.M.; Sarti, A. Anaerobic digestion of crude glycerol from biodiesel production for biogas generation: Process optimization and pilot scale operation. Environ. Res. 2024, 244, 117938. [Google Scholar] [CrossRef]
- Vikromvarasiri, N.; Koyama, M.; Kurniawan, W.; Pisutpaisal, N.; Nakasaki, K. Enhancing methane recovery by intermittent substrate feeding and microbial community response in anaerobic digestion of glycerol. Renew. Energy 2023, 204, 106–113. [Google Scholar] [CrossRef]
- Bułkowska, K.; Mikucka, W.; Pokój, T. Enhancement of biogas production from cattle manure using glycerine phase as a co-substrate in anaerobic digestion. Fuel 2022, 317, 123456. [Google Scholar] [CrossRef]
- Gebreegziabher, B.W.; Dubale, A.A.; Adaramola, M.S.; Morken, J. Advancing anaerobic digestion of biodiesel byproducts: A comprehensive review. Bioenerg. Res. 2025, 18, 15. [Google Scholar] [CrossRef]
- Stepanov, N.; Efremenko, E. Immobilised cells of Pachysolen tannophilus yeast for ethanol production from crude glycerol. N. Biotechnol. 2017, 34, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Ugarova, N.; Koksharov, M.; Lomakina, G. Reagent for Adenosine-5-Triphosphate Determination. Patent RU 2420594, 20 May 2009. [Google Scholar]
- Stepanov, N.; Senko, O.; Perminova, I.; Efremenko, E. A new approach to assess the effect of various humic compounds on the metabolic activity of cells participating in methanogenesis. Sustainability 2019, 11, e3158. [Google Scholar] [CrossRef]
- Sisman-Aydin, G.; Simsek, K. Municipal wastewater effects on the performance of nutrient removal, and lipid, carbohydrate, and protein productivity of blue-green algae Chroococcus turgidus. Sustainability 2022, 14, 17021. [Google Scholar] [CrossRef]
- Nwokolo, N.; Mukumba, P.; Obileke, K.; Enebe, M. Waste to Energy: A focus on the impact of substrate type in biogas production. Processes 2020, 8, 1224. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dębowski, M.; Zieliński, M.; Ignaciuk, A.; Mlonek, S.; Cruz Sanchez, J. The biosynthesis of liquid fuels and other value-added products based on waste glycerol—A comprehensive review and bibliometric analysis. Energies 2024, 17, 3035. [Google Scholar] [CrossRef]
- Efremenko, E.; Senko, O.; Stepanov, N.; Maslova, O.; Lomakina, G.Y.; Ugarova, N. Luminescent analysis of ATP: Modern objects and processes for sensing. Chemosensors 2022, 10, 493. [Google Scholar] [CrossRef]
- Strotmann, U.J.; Eismann, F.; Hauth, B.; Bias, W.R. An integrated test strategy for the assessment of anaerobic biodegradability of wastewaters. Chemosphere 1993, 26, 2241–2254. [Google Scholar] [CrossRef]
- Alves, I.R.; Mahler, C.F.; Oliveira, L.B.; Reis, M.M.; Bassin, J.P. Assessing the use of crude glycerol from biodiesel production as an alternative to boost methane generation by anaerobic co-digestion of sewage sludge. Biomass Bioenergy 2020, 143, 105831. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryostructuring of polymeric systems. 55. Retrospective view on the more than 40 years of studies performed in the A.N. Nesmeyanov Institute of organoelement compounds with respect of the cryostructuring processes in polymeric systems. Gels 2020, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Senko, O.; Gladchenko, M.; Maslova, O.; Efremenko, E. Long-term storage and use of artificially immobilized anaerobic sludge as a powerful biocatalyst for conversion of various wastes including those containing xenobiotics to biogas. Catalysts 2019, 9, 326. [Google Scholar] [CrossRef]
- Kallistova, A.; Goel, G.; Nozhevnikova, A. Microbial diversity of methanogenic communities in the systems for anaerobic treatment of organic waste. Microbiology 2014, 83, 462–483. [Google Scholar] [CrossRef]
- Karim, A.; Gerliani, N.; Aïder, M. Kluyveromyces marxianus: An emerging yeast cell factory for applications in food and biotechnology. Int. J. Food Microbiol. 2020, 333, 108818. [Google Scholar] [CrossRef]
- Maslova, O.; Senko, O.; Stepanov, N.; Gladchenko, M.; Gaydamaka, S.; Akopyan, A.; Polikarpova, P.; Lysenko, S.; Anisimov, A.; Efremenko, E. Formation and use of anaerobic consortia for the biotransformation of sulfur-containing extracts from pre-oxidized crude oil and oil fractions. Bioresour. Technol. 2021, 319, 124248. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).