Abstract
Olive oil production generates vast quantities of by-products, with olive mill wastewater (OMW) being a particularly challenging effluent. Characterized by its dark color, high acidity, and rich composition of organic matter, phenolic compounds, and residual oils, OMW resists conventional degradation methods and poses significant environmental risks due to its phytotoxicity and microbial inhibition. Addressing this issue requires sustainable solutions that align with circular economy principles. A promising strategy involves the biotechnological valorization of OMW using the non-conventional yeast Yarrowia lipolytica, which thrives on organic-rich substrates and converts them into high-value metabolites. This review provides a comprehensive analysis of recent advances in Y. lipolytica applications for OMW valorization, emphasizing its role in developing eco-friendly industrial processes. It begins by outlining the physicochemical challenges of OMW and the metabolic versatility of Y. lipolytica, including its ability to adapt to acidic, phenolic-rich environments. Subsequent sections critically evaluate the yeast’s capacity to synthesize commercially valuable products such as lipases (used in the food and biofuel industries), citric acid (a food and pharmaceutical additive), and polyols like mannitol and erythritol (low-calorie sweeteners). Strategies to optimize microbial productivity, such as substrate pre-treatment, nutrient supplementation, and process engineering, are also discussed. By synthesizing current research, the review highlights how Y. lipolytica-driven OMW valorization can mitigate environmental harm while creating economic opportunities, bridging the gap between waste management and green chemistry.
1. Introduction
The cultivation of olive trees (Olea europaea L.), one of the first agricultural pursuits in human history, is essential for the production of olive oil. Because of its rich nutritional content and numerous health benefits, olive oil is an essential component of the Mediterranean diet [,]. The Mediterranean region plays a crucial role in olive oil production, contributing more than 98% of the world’s supply, which amounts to approximately 31 million tons [,]. Roughly 75 percent of global production is generated by the European Union, while Turkey, the Maghreb countries (Tunisia, Algeria, Morocco), and Palestine contribute the remaining 7.5 percent, 11.62 percent, and 0.8 percent, respectively []. Between 2016 and 2019, almost 107 million hectares of olive trees were planted; 87% of the harvested olives were used to create oil, while the remaining fraction was used to make table olives [].
Approximately twenty percent of the processed olives are oil, thirty percent is pomace [,], and fifty percent is water, known as olive mill wastewater (OMW) []. OMW is a dark brown liquid with a pH of 3–6, consisting of olive fruit, oil residue, pieces of olive pulp, and a stable emulsion of vegetative water along with water added during processing [,]. The variety of olive, its ripeness, and the processing techniques applied all influence the composition of this water []. Due to their high sulfur content, organic matter, and phenolic compounds, these waters are especially harmful to the environment and adversely affect soil, microbes, plants, and marine species []. This toxicity has been confirmed by several case studies conducted across the Mediterranean region. For example, Mekki and colleagues demonstrated that the land application of olive mill wastewater (OMW) in Tunisia led to the accumulation of phenolic compounds in soils, inhibited the growth of cultivated plants (such as wheat and lettuce), and reduced microbial diversity, indicating significant ecological disturbance []. More recently, El Alami and Fattah reported severe pollution of the Oum Er Rbia River in Morocco caused by OMW discharges. This pollution was characterized by dark water coloration, unpleasant odors, and high toxicity, posing a threat to aquatic life, particularly the Eurasian otter (Lutra lutra) [], in Crete. The prolonged use of OMW on agricultural soils was found to cause heavy metal accumulation (iron and copper), increased salinity, and a reduction in microbial enzymatic activity, ultimately compromising long-term soil fertility [].
Conventional disposal methods, such as burning, are considered very harmful to the environment []. Olive mill wastewater (OMW) has shown potential as a soil spreading agent in agriculture; however, its application is limited by quantitative factors [], which lead to the accumulation of waste in evaporation ponds after each olive oil production season. OMW is particularly resistant to this because phenolic chemicals tend to cluster over time. For example, OMW stored in evaporation ponds had a higher content of phenolic compounds (13.4 g/kg) than fresh OMW (5.14 g/kg), suggesting that the latter was resistant to biodegradation [].
As part of the valorization of olive mill wastewater (OMW), effluents generated from olive processing, various microorganisms have been investigated for their ability to treat these residues, which are rich in organic matter and phenolic compounds—the latter being the primary agents of toxicity. Among them, Yarrowia lipolytica stands out as a particularly promising non-conventional yeast due to its high tolerance to polyphenols and its robustness in complex, poorly or non-sterile environments [,].
Y. lipolytica exhibits remarkable metabolic flexibility, enabling it to efficiently convert a variety of carbon and nitrogen sources into industrially valuable metabolites such as citric acid, fatty acids, and lipid-rich biomass—even under stress or nutrient-limited conditions. This yeast can also grow on numerous industrial wastes, including waste cooking oils (WCOs), olive mill wastewater (OMW), and hydrocarbon-rich effluents, thereby valorizing these low-cost substrates while producing high-value-added compounds such as lipids, enzymes, and polyols [,,]. This capability not only helps reduce production costs but also minimizes the environmental impact of these waste streams, aligning with the principles of the circular economy.
Moreover, Y. lipolytica is known for its ability to grow on lipid-based substrates through the secretion of lipases—enzymes that facilitate the hydrolysis of lipids present in effluents. Finally, this yeast represents a valuable model for studying fungal dimorphism, as it can adopt two distinct morphotypes: a yeast-like form (oval cells) and a filamentous form (pseudohyphae or mycelium). This morphological transition is influenced by the nature of the substrate, with the filamentous form generally being favored on hydrophobic substrates [].
Altogether, these biological and metabolic characteristics position Y. lipolytica as a microorganism of choice for the integrated biovalorization of olive mill liquid effluents, combining pollution mitigation with the sustainable production of biomolecules.
This study aimed to explore key findings from research on the application of the yeast Y. lipolytica in the valorization of olive mill wastewater (OMW). The primary objectives are twofold: (1) to evaluate strategies for mitigating the environmental footprint of OMW by addressing its pollution risks and advancing sustainable waste management practices, and (2) to investigate its potential for driving a circular economy through the conversion of waste into high-value bio-based products. Additionally, the research focuses on optimizing bioprocesses to enhance the production of commercially relevant biocompounds—such as organic acids, lipases, and polyols—while systematically evaluating the efficiency and yields of Y. lipolytica in these processes. To the best of the authors’ knowledge, this is the first review paper to examine the application of Y. lipolytica in valorizing olive mill wastewater. To the best of our knowledge, this review represents the first comprehensive and in-depth analysis of the production of primary metabolites by Y. lipolytica cultivated on olive mill wastewater. It highlights the biotechnological pathways for the valorization of these effluents, providing a critical and comparative synthesis of the available data to date.
2. Olive Mill Wastewater
Olive wastewater, also known as olive mill effluent (OMW) or olive mill wastewater (OMW), is a peculiar byproduct of processing olive oil. It contains approximately 4.1% to 16.4% solids, or about 150 g per liter, according to Gueboudji et al. []. These solids are made up of organic waste, olive pulp, and other by-products of the oil extraction process []. Water makes up the majority of its composition (83% to 94%) [], and its high liquid content is the result of pressing and centrifugation processes []. Furthermore, OMW includes emulsified lipids from oil extraction that can quickly ferment and produce environmental issues if not adequately controlled []. The chemical composition of OMW is presented in Table 1.

Table 1.
Chemical composition of OMW.
Depending on the type of olive and how it is treated, its pH can range from 3 to 6, which is typically acidic []. While the biochemical oxygen demand over five days (BOD5) can reach up to 170 g per liter [], reflecting a high level of biodegradable organic matter, the chemical oxygen demand of OMW ranges from 36.80 to 230 g per liter [], indicating a high load of organic pollutants []. Moreover, treating high concentrations of polyphenols, such as hydroxytyrosol, can be challenging due to their resistance to degradation [].
According to Romeo et al. [], these phenolic compounds are extracted from olives during the olive oil manufacturing process. Typically, OMW holds 98% of the overall phenolic content found in olive fruits, with concentrations ranging from 0.5 to 24 g/L []. Despite their strong antioxidant qualities, high concentrations of them can contaminate soil by preventing the growth of fungi and beneficial bacteria, which are vital to the soil’s fertility and health []. Due to OMW’s high acidity, many plants and soil organisms may find their habitat to be too acidic; this can also alter the pH equilibrium of the soil and reduce the availability of essential nutrients []. The presence of lipids in the OMW forms an impenetrable film on the water’s surface, blocking light (essential for photosynthesis) and oxygen from penetrating. This leads to the total destruction of aquatic fauna and flora, as well as inhibiting the growth of microorganisms []. Another major concern associated with the repeated application of OMW to agricultural soils is the progressive salinization of the land. These effluents contain high concentrations of mineral salts, particularly sodium, which accumulate in the soil over time []. Excess salt prevents plant roots from absorbing water effectively, thereby hindering their growth. Salinity also degrades soil structure, making it compact, dry, or hard, which further impairs crop development. As a result, agricultural yields decrease, and soil fertility deteriorates, potentially leading to the sterilization of specific areas. If this situation persists, once-fertile lands may turn into desert-like zones, incapable of supporting plant or animal life [,]. Furthermore, excessive salinization disrupts soil microbiological activity, which is crucial for the natural degradation of pollutants and the maintenance of soil fertility [].
In reservoirs with anaerobic conditions, methane and other harmful acid gases—such as hydrogen sulfide—are released, greatly exacerbating air pollution []. Additionally, the toxicity of OMW, primarily due to phenols, can lead to extended seed dormancy. This, in turn, may impede plant growth and germination, especially during vital phases like seed germination and seedling development [,,].
3. Yarrowia lipolytica
Y. lipolytica is a member of the Ascomycota phylum and was previously categorized under genera like Candida, Endomycopsis, and Saccharomycopsis. The name “Yarrowia” is a tribute to David Yarrow, a scientist associated with the Delft Microbiology Laboratory in the Netherlands, who played a key role in its taxonomic classification. The species name “lipolytica” highlights this yeast’s key metabolic characteristic, i.e., its ability to hydrolyze lipids [].
Y. lipolytica is a non-pathogenic, ascomycetous yeast classified within the order Saccharomycetales (class: Saccharomycetes, family: Dipodascaceae) []. This yeast species is known for its remarkable lipolytic and proteolytic properties, isolated initially from environments rich in proteins or lipids. It is present in fermented dairy products, meat, poultry, and waste products such as lipid-rich wastewater and hydrocarbon-polluted environments []. Since it was first isolated, Y.lipolytica has been utilized to make a variety of biochemical products, including as fatty acids in the form of lipids [], polyols (erythritol and mannitol) [], organic acids (like citric acid) [] and enzymes (like lipases) []. It also plays a significant role in the bioremediation of contaminated soils and streams [].
According to Gamraoui et al. [], Y. lipolytica is a non-conventional yeast capable of reducing chemical oxygen demand (COD), salinity, and color in wastewater while simultaneously producing single-cell proteins of interest. Studies have demonstrated that exposing this yeast to various sound frequencies, particularly low frequencies (64 Hz to 7500 Hz), can significantly stimulate its growth and metabolite production in glucose-enriched media. Among these, certain low-frequency sounds have been found to be especially effective in promoting cell growth. This acoustic stimulation, including exposure to musical sounds, may enhance the efficiency of Y. lipolytica in wastewater treatment applications. Moreover, this dimorphic yeast exhibits considerable morphological plasticity, ranging from regular spherical cells to septate mycelial structures, which may further influence its treatment performance and adaptability in diverse environmental conditions [,]. It can withstand a wide variety of pH values, low temperatures, and high salinity. Environments that support Y. lipolytica, both natural and processed, encompass high-salinity marine settings [], lipid-rich habitats, and food sources such as cheese and processed meats [].
Yarrowia lipolytica is classified among non-pathogenic yeasts, primarily due to its inability to grow above 32 °C and its strictly aerobic metabolism []. However, similar to other fungal species, it has been reported to cause opportunistic infections in immunocompromised or critically ill individuals [,]. Despite these rare clinical occurrences, Y. lipolytica is widely utilized in industrial, biotechnological, and food-related applications owing to its capacity to synthesize a wide range of high-value products, including flavor compounds, enzymes, microbial biomass, lipids, and other secondary metabolites of industrial interest. It is recognized as safe (“GRAS”—Generally Recognized As Safe) by the U.S. Food and Drug Administration (FDA) for several intended uses, as detailed in notification GRN 000759 []. Moreover, Y. lipolytica processes have also received GRAS status, highlighting its broader safety profile for industrial applications. The yeast is typically cultivated under controlled conditions and has not been associated with pathogenicity in humans or animals under such settings. Long-term in vivo studies involving oral administration of whole yeast cells to rats revealed no toxicological effects, further substantiating its safety for industrial and food-related use []. Within the European Union, Y. lipolytica is approved for use in animal feed and has been assessed as a novel food under Regulation (EU) 2015/2283, confirming its suitability for human consumption [,]. From a biosafety perspective, Y. lipolytica is generally classified as a Biosafety Level 1 (BSL-1) organism, indicating minimal risk to human health or the environment. An exception exists in Switzerland, where it is categorized as a BSL-2 organism as a precautionary measure [].
In summary, based on existing scientific and regulatory evidence, industrial strains of Y. lipolytica, such as W29, demonstrate a favorable biosafety profile and are well suited for use in controlled bioprocesses [,]. Additionally, Y. lipolytica is known for its remarkable ability to degrade hydrophobic substrates, particularly lipids. This capacity is enabled by the secretion of extracellular lipolytic enzymes that hydrolyze fats into simpler compounds. As a result, this yeast can significantly reduce the organic load of wastewaters by metabolizing lipids and other organic constituents as carbon and energy sources [].
Researchers consider several factors when searching for a microorganism that can mitigate the pollution potential of wastewater (OMW), including its limited nutritional requirements, ability to thrive in pathogenic environments, and adaptability to harsh environmental conditions (such as low temperatures, low water activity, and high concentrations of toxic substances). In addition, this microbe ought to be able to flourish under these circumstances and generate intriguing secondary metabolites.
Y. lipolytica is endowed with all of these characteristics. Indeed, this species may thrive at 4 °C even in media with a water activity of 0.93, as shown by Lanciotti []. These capabilities can help cut down on processing and storage expenses. Furthermore, all of the strains utilized in the study were able to grow in undiluted OMW without the need for nutritional supplementation [], even under non-aseptic circumstances. They were also able to produce metabolites of interest []. The various beneficial metabolites produced by different strains of Y. lipolytica when grown on olive pomace substrates under varying conditions—such as supplementation, dilution, and so forth—will be discussed in the following section.
4. Metabolites Produced in the Valorization of Olive Mill Wastewater by Y. lipolytica
The valorization of olive mill wastewater (OMW) by Y. lipolytica leads to the production of various valuable metabolites []. These include lipases [], organic acids (such as citric acid), polyols (like mannitol and erythritol) [,], and biomass []. The synthesis of these metabolites is influenced by culture conditions, including nutrient availability and the addition of compounds [,].
4.1. Lipases
Yarrowia lipolytica is a non-conventional yeast species [] recognized for its ability to convert hydrophobic substrates into microbial lipids via the ex novo synthesis pathway []. This transformation is facilitated through specialized metabolic routes involving lipases—enzymes crucial for the hydrolysis of fatty acid esters and the degradation of triglycerides [,]. These reactions lead to the generation of microbial lipids and are central to the yeast’s capacity to biotransform oils and fats.
Among the various lipases identified in Y. lipolytica, the most prominent is LIP2 (YLLIP2; NCBI accession number CAB91111), which represents over 97% of the extracellular lipase activity []. This enzyme, composed of 301 amino acids with a molecular weight of approximately 38 kDa, is noted for its stability across a wide pH and temperature range, making it well-suited for diverse fermentation conditions. In addition to Lip2p, several other lipases—including intracellular, extracellular, and cell-bound forms—such as Lip7p, Lip8p, Lip9p, Lip11p, Lip12p, Lip14p, Lip18p, YlTgl4, and YlTgl3, have been described over the past two decades []. These enzymes primarily catalyze reactions involving carboxylic ester bonds, including hydrolysis, aminolysis, and (trans)esterification [].
Y. lipolytica strains demonstrate considerable potential for both lipase production and biodegradation of pollutants, such as phenolic compounds and chemical oxygen demand, in OMW []. Certain strains can reduce chemical oxygen demand by 50–80%, significantly contributing to the bioremediation of this agro-industrial waste [,]. For example, Scioli and Vollaro [] reported a decrease in chemical oxygen demand from 146 g/L to 30.3 g/L after 20 h of incubation, accompanied by an increase in biomass from 5% to 22.45%, particularly within the initial 14 h of cultivation. Lipase activity in OMW-rich media varies significantly among Y. lipolytica strains. While strains like Y2, Y12, Y18, Y22, RO2, RO19, and PO10 exhibit limited lipase activity under these conditions [], others—including ATCC 20255, PO1, PO6, PO12, PO18, PO19, PO20, PO23, RO23, RO1, RO5, RO9, RO12, RO15, RO18, RO22, Y4, Y9, Y17, Y21, A1, A10, B7, B16, C11, C12, C23, D2, D5, and D27—demonstrate high enzymatic activity. For instance, the ATCC 20255 strain reaches up to 770 U/L of lipase activity [,,,,,,,,,,,]. In contrast, strains such as PO14, PO22, B4, Y8, Y10, Y16, and Y19 show no detectable lipase activity under similar conditions [].
The optimization of culture media significantly influences lipase production, but the response depends on the specific strain characteristics. For example, the addition of Tween 80 has been shown to double lipase output in Y. lipolytica NRRL Y-1095, reaching up to 850 U/L within four days [], whereas it inhibits production in the W29 strain. Similarly, increasing ammonium sulfate concentration negatively impacts enzyme production [], while pretreatment with 3% NaOH can enhance enzymatic yield tenfold []. Additionally, higher initial cell densities may reduce extracellular lipase activity by approximately 36% []. Overall, lipase production in Y. lipolytica is highly influenced by environmental conditions, strain-specific responses, and the complexity of releasing cell-bound enzymes, as noted by Lanciotti []. Table 2 consolidates key findings from studies investigating specific Y. lipolytica strains grown in OMW-rich media, including experimental outcomes.

Table 2.
Lipase production in the valorization of olive mill wastewater by Y. lipolytica.
The best strain based on the available data is Yarrowia lipolytica NRRL Y-1095, which showed the highest lipase activity (850 IU/L) in liquid fermentation when cultivated in non-diluted olive mill wastewater (OMW) supplemented with Tween 80, ammonium salts, yeast extract, maltose, olive oil, and peptone. Furthermore, lipase production was boosted tenfold when olive cake was pretreated with 3% NaOH, and solid-state fermentation achieved up to 40 IU/g of substrate, demonstrating this strain’s strong adaptability and high enzymatic productivity in OMW-enriched environments.
4.2. Citric Acid
Citric acid is an essential industrial compound used in the food industry (as an acidity regulator), pharmaceuticals, and cosmetics. While its traditional production relies on Aspergillus niger, the limitations of this microorganism have sparked growing interest in the yeast Y. lipolytica. High-yield citric acid production by Y. lipolytica requires nitrogen limitation combined with excess carbon. The co-production of isocitric acid results from the activity of parallel metabolic pathways; however, strategies such as substrate selection, genetic engineering, or the limitation of specific nutrients can minimize isocitric acid formation in favor of citric acid [].
Y. lipolytica is well known for its ability to produce citric acid under appropriate conditions, particularly in nitrogen-limited environments. Several studies have explored the use of olive mill wastewater (OMW) as a substrate or co-substrate for this purpose [,].
It has been observed that cell proliferation is not necessarily correlated with citric acid production. Indeed, while phenolic compound concentration does not always impact the growth of Y. lipolytica strains, it significantly affects citric acid biosynthesis [].
The inhibitory effect of phenolic compounds has been shown to depend strongly on the strain used. For instance, strain ACA-YC 5033 produced 18.9 g/L of citric acid in control medium (without OMW), while production slightly decreased to 18.1 g/L in the presence of 1.1 g/L of phenolics. When the phenolic concentration increased further, citric acid production declined significantly. Strain W29, for example, showed a reduced production of 14.5 g/L under such conditions []. Conversely, other strains demonstrated improved production in the presence of moderate phenolic concentrations: at 2.05 g/L phenolics, strains such as ACA-DC 50109 reached citric acid levels ranging from 27.6 to 28.9 g/L []. Therefore, the metabolic response is highly strain-dependent, with some Y. lipolytica strains being low or even non-producers of citric acid.
Furthermore, enrichment of the culture medium with glucose or glycerol can positively influence citric acid production. The addition of 50 g/L glycerol increased citric acid production from 4.7 to 30.3 g/L under specific conditions []. However, high phenolic concentrations can impair glycerol assimilation and cell growth. Low concentrations of NaCl (≤1%, w/v) have been reported to facilitate glycerol uptake, with a maximum citric acid production of 45.2 g/L observed in medium containing both glycerol and OMW, compared to 32.7 g/L without salt. In contrast, higher NaCl concentrations (3–5%) progressively inhibited production []. Moreover, high glycerol concentrations (up to 70 g/L), combined with OMW containing 3.5 g/L phenolics, allowed citric acid production to reach 37.4 g/L []. Nitrogen-limited conditions are particularly favorable for citric acid biosynthesis; for example, strain ACA-YC 5033 achieved a maximum concentration of 60.2 g/L under nitrogen-restricted conditions [].
Under nitrogen-limited conditions, nitrogen is rapidly depleted from the medium, while the carbon source remains in excess. This situation triggers a series of metabolic and physiological shifts, characterized by inhibited cell growth and a redirection of the carbon flux toward the biosynthesis of secondary metabolites. In response to nitrogen depletion, the cell accumulates citrate in the mitochondria, which can either be secreted into the extracellular medium or cleaved in the cytoplasm to initiate de novo lipid synthesis. This complex mechanism, regulated among others by the intracellular concentration of AMP and the inactivation of Krebs cycle enzymes, enables Y. lipolytica to adapt to nutritional imbalances and convert excess carbon into valuable industrial metabolites. The figure below illustrates the main steps involved in this metabolic reprogramming [] (Figure 1).
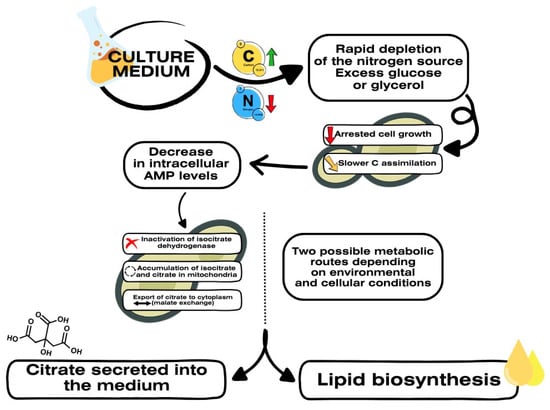
Figure 1.
Metabolic reprogramming of Y. lipolytica under nitrogen limitation. Summary of findings compiled by [].
In conclusion, research demonstrates that citric acid production by Y. lipolytica using olive mill wastewater as a substrate is significantly influenced by the strain employed and the culture conditions—notably the glucose concentration, phenolic content of the OMW, the presence of NaCl, and supplements such as crude glycerol (Table 3).

Table 3.
Citric acid production in the valorization of olive mill wastewater by Y. lipolytica.
The most effectively optimized culture medium for citric acid production by Yarrowia lipolytica involves a combination of stringent nitrogen limitation and carbon excess, alongside careful strain selection and environmental parameter control. Within this framework, strain ACA-YC 5033 demonstrated superior performance, achieving a maximum citric acid concentration of 60.2 g/L. Media enriched with glucose (≥50 g/L) or glycerol (170 g/L), supplemented with low concentrations of NaCl (≤1% w/v), enhanced substrate assimilation and directed metabolism toward citric acid biosynthesis. The incorporation of olive mill wastewater (OMW) as a co-substrate proved compatible with high production levels, provided that phenolic compound concentrations were maintained at moderate levels (≤2 g/L) to avoid metabolic inhibition. Notably, citric acid production was not significantly affected under non-aseptic culture conditions, indicating that strict sterilization may not be essential for efficient biosynthesis in this system. These findings highlight the critical role of the interplay between strain physiology, medium composition, and culture conditions in optimizing citric acid production from renewable and underutilized feedstocks.
4.3. Polyols
Y. lipolytica has become a promising microbial platform for polyol production. This unconventional yeast synthesizes polyols, such as erythritol, arabitol, and mannitol, mainly under osmotic stress or nitrogen-limited conditions []. These polyols function as compatible solutes, helping the organism adapt to high osmotic environments while providing industrial value as low-calorie sweeteners and bio-based additives. The produced polyols serve the food, pharmaceutical, and cosmetic industries. Erythritol, known for its zero glycemic index and dental safety, is perfect for diabetic-friendly products. Mannitol, with 50–60% sweetness of sucrose, is non-hygroscopic, making it ideal for sugar-free coatings (e.g., chewing gum, mint candies) and as a dusting agent to prevent stickiness. Its cooling effect enhances sensory appeal in oral applications [].
Research on the mannitol production by Y. lipolytica varies based on the strains, amounts of crude glycerol, and additions like phenolic compounds and NaCl in OMW. Using 50 g/L of crude glycerol as a medium, the Y. lipolytica A6 strain generated 13.4 g/L of mannitol in 2016 []. According to Sarris et al. [], the ACA-DC 5029 strain exhibited maximum mannitol concentrations in 2019 that ranged from 5.3 g/L to 13.1 g/L. The addition of OMW did not significantly affect the strain’s performance until phenolic compounds reached 2.0 g/L. A different investigation conducted in 2019 using the ACA-YC 5031 strain showed that the addition of NaCl decreased the production of mannitol, which dropped from 8.0 g/L without NaCl to just 1.0 g/L with 5% NaCl []. This highlights the importance of culture conditions on mannitol yields.
Studies on Y. lipolytica’s erythritol production investigated the effects of NaCl concentrations, OMW mixes, and crude glycerol. Without OMW, the ACA-DC 5029 strain produced up to 14.9 g/L of erythritol in 2019; however, with the addition of OMW, the output decreased to only 2.4 g/L when the concentration of phenolic compounds was 3.5 g/L []. The ACA-YC 5031 strain produced a maximum of 4.9 g/L of erythritol with 1% and 3% NaCl; however, production decreased to 2.1 g/L with 5% NaCl, according to another investigation conducted that same year []. These findings demonstrate that erythritol synthesis is adversely affected by phenolic chemicals and NaCl.
The studies by Sarris et al. [,] also revealed the co-production of arabitol alongside the biosynthesis of erythritol and mannitol in glycerol-based media, though in lower quantities compared to the latter. Interestingly, nitrogen-limited conditions and phenolic compounds in OMW influenced metabolic fluxes, favoring citric acid and mannitol over arabitol. Overall, arabitol yields were modest, highlighting the strain- and media-dependent nature of polyol biosynthesis in Y. lipolytica (Table 4).

Table 4.
Polyol production in the valorization of olive mill wastewater by Y. lipolytica.
Regarding the influence of strain and culture conditions among the strains tested, Yarrowia lipolytica ACA-YC 5033 was identified as the most effective for mannitol production. When cultivated in a medium containing crude glycerol (77.9 ± 0.5 g/L) and diluted olive mill wastewater (OMW) with 2.0 g/L of phenolic compounds, supplemented with a nitrogen source and mineral salts, a maximum mannitol concentration of 15.9 g/L was achieved. This yield was the highest recorded across all experimental conditions and exceeded that of other strains tested, including A6, ACA-DC 5029, and LMBF Y-46. The optimal conditions for mannitol biosynthesis involved a high carbon supply via glycerol, a moderate phenolic load, and tailored mineral supplementation. These factors are presumed to have enhanced the metabolic flux toward mannitol by promoting NADPH regeneration and efficient reduction in fructose-6-phosphate. These findings underscore the biotechnological potential of Y. lipolytica ACA-YC 5033 for the valorization of agro-industrial by-products.
For erythritol production, the optimal conditions were obtained using the Y. lipolytica LMBF Y-46 strain cultivated in a medium containing crude glycerol (77.9 ± 0.5 g/L), a nitrogen source, and mineral salts, with water replacing OMW. Under these conditions, a maximum erythritol concentration of 17.3 g/L was reached. The absence of phenolic compounds from OMW was found to be essential, as their presence negatively affected erythritol production, significantly decreasing yields depending on concentration. Therefore, a medium free of OMW, rich in glycerol, and supplemented with nitrogen and minerals was determined to be the most favorable for erythritol biosynthesis by this strain.
Optimal arabitol production was also achieved using Y. lipolytica LMBF Y-46, cultivated in a medium containing crude glycerol (77.9 ± 0.5 g/L), mineral salts, and OMW with 3.0 g/L of phenolic compounds. Under these conditions, a maximum arabitol concentration of 7.9 g/L was recorded, the highest among all tested strains and conditions. Unlike erythritol, arabitol biosynthesis appeared to be positively influenced by higher levels of phenolic compounds in OMW, suggesting a potential inductive effect of these compounds on the metabolic pathway leading to arabitol formation.
In all the studies presented in the three tables regarding the production of lipase, citric acid, and polyols, the authors used wild-type strains of Yarrowia lipolytica. These strains were isolated from diverse environments, such as fermented food products, soils, or industrial effluents, and therefore exhibit specific metabolic characteristics linked to their ecological origin. They have naturally integrated adaptive mechanisms to the specific physicochemical conditions of their native habitats, which strongly influence their fermentative behavior []. Significant differences in metabolic performance among these strains cultivated under identical conditions can thus be attributed to the natural genetic diversity of this species. Indeed, Y. lipolytica shows substantial genomic variability among isolates, directly affecting the expression of genes involved in carbon metabolism as well as in the production of secondary metabolites such as organic acids and lipids [,]. Moreover, certain strains exhibit more efficient regulation of citric acid biosynthetic pathways, particularly through activation of ATP-citrate lyase and repression of isocitrate dehydrogenase, promoting increased citric acid accumulation under nitrogen-limited conditions, as observed with strain ACA-YC 5033 []. Variable tolerance to inhibitory compounds present in complex media, such as olive mill effluents rich in phenolic compounds, also contributes to the contrasting performances observed. Some strains are able to more effectively activate antioxidant defense mechanisms and toxin efflux systems [].
Thus, even in the absence of targeted genetic modifications, wild-type strains can exhibit highly diverse phenotypes due to their ecological origin, natural adaptation to environmental stressors, and intrinsic metabolic profiles. These differences highlight the need for the precise optimization of fermentation conditions to enhance citric acid production or targeted polyol accumulation, depending on the specific goals of the process [,,,,,].
5. Biotechnology Applications of Y. lipolytica
Y. lipolytica has evolved complex mechanisms and approaches for the effective utilization of hydrophobic substrates [], such as those based on olive mill wastewater (OMW) []. Oily substrates are hydrolyzed and then incorporated as an example of these complicated reactions. Some lipases produced by this yeast that can be released into the medium based on the substrate include Lip2p, an extracellular lipase, and Lip7p and Lip8p, which are suggested to be mainly cell-bound []. Extracellular triacylglycerols and lipids can be directly converted by Y. lipolytica into high-value compounds [].
A wide range of commercial products has been developed thanks to the multiple applications of these enzymes, due to the high stability of microorganisms with respect to temperature, detergents, and proteolytic enzymes. Their applications include the agro-food sector (bakery, biscuit making, chocolate production, manufacturing of dairy or fermented products), as well as environmental uses and bioremediation. Additionally, they are used in the detergent industry, in organic synthesis within the cosmetics and fragrance industries, and in the pharmaceutical industry for drug synthesis or the preparation of optically active homochiral intermediates [].
Y. lipolytica also produces glycolipids, which are compounds made up of carbohydrates and lipids, which function as biosurfactants. Nonetheless, this yeast can produce a variety of biosurfactants, the composition of which can change based on the substrates utilized in their synthesis [,]. Compared to their chemical equivalents, microbial surfactants have a number of benefits, including low toxicity, biodegradability, and the ability to be produced from renewable substrates [].
The production of emulsifying agents from yeasts generally requires the presence of substrates that are immiscible in water, which poses a challenge for isolating the produced biosurfactant []. Y. lipolytica can grow on olive mill wastewater (OMW) and has the capacity to degrade organic compounds, including both aliphatic and aromatic hydrocarbons, often resulting in the production of biosurfactants. Additionally, an emulsifier capable of stabilizing water/oil emulsions was identified and isolated from the growth medium of C. lipolytica. This bioemulsifier, referred to as Liposan, has demonstrated promising properties in this field [].
Biosurfactants are surface-active metabolites produced by various microbial species (bacteria, yeasts, and fungi). Their amphiphilic structure gives them excellent surfactant properties, such as emulsification, dispersion, and foaming. Unlike chemical surfactants, they are generally non-toxic, highly biodegradable, stable under extreme environmental conditions (pH, temperature, salinity), and applicable in many fields, including bioremediation, cosmetics, the food industry, and the petroleum sector []. In contrast, synthetic surfactants—although widely used in household and industrial products (with a global market estimated at USD 39.9 billion in 2019 and projected to reach USD 52.4 billion by 2025)—are associated with significant environmental impacts. Their persistence in wastewater and low biodegradability promote their accumulation in aquatic environments, where they can act as vectors for the dispersion of other pollutants, particularly heavy metals. In this context, biosurfactants emerge as a sustainable alternative to chemical surfactants. Produced from natural raw materials by microorganisms, they offer comparable surfactant properties while presenting a better ecological profile. Their growing integration into industrial processes reflects their relevance within a sustainable development perspective. Yeasts, particularly Y. lipolytica, are generating increasing interest as biosynthetic platforms for biosurfactant production. Their GRAS (Generally Recognized As Safe) status especially enhances their attractiveness for applications in the pharmaceutical and food industries [].
Y. lipolytica’s biosurfactants are becoming more well-known due to their numerous applications in the food industry, medical therapies, bioremediation, and cosmetics []. Because of their ability to increase the wettability and solubility of hydrophobic substances, they are used in a wide range of industrial sectors, including foaming, emulsification, detergent, lubrication, solubilization, and phase dispersion. These surfactants are unique because they can change the surface and interfacial properties of liquids by lowering the system’s free energy through the replacement of high-energy molecules at the interface [].
In addition to stimulating the biosynthesis of biosurfactants that are employed as emulsifiers, the adaptation of Y. lipolytica in media enriched with OMW additionally encourages the synthesis of cellular unsaturated fatty acids, primarily as oleic and linoleic acids []. This oleaginous yeast, the level of lipid accumulation and the fatty acid profiles of the produced lipids are influenced by the strain, fermentation conditions (O2, pH, agitation, and temperature), and type of process [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,]. The main fatty acids produced are unsaturated, particularly oleic and linoleic acids, which enhances the nutritional value of this strain []. Using Y. lipolytica, it is possible to produce single-cell oils (SCOs) by utilizing hydrophobic waste as a low-cost culture medium, with potential applications in functional oils or biodiesel []. The transformation of oil waste into SCOs can contribute to bioremediation, contributing to the concept of a circular economy by reducing biotechnology production costs and promoting eco-sustainability [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,].
According to [], the lipids produced by Y. lipolytica are considered a promising source of edible oils as an alternative to the synthesis of polyunsaturated fatty acids (PUFAs), cocoa butter substitutes (CBSs), and structured lipids. It was demonstrated by [] that this yeast could increase the concentration of stearic acid despite its absence in the original substrate, resulting in a lipid profile rich in stearic, palmitic, and oleic acids that approximated cocoa butter.
Additionally, the accumulation of oleic acid in Y. lipolytica allows for the industrial production of ricinoleic acid (RA), a unique hydroxylated fatty acid that is typically derived from castor oil. Through genetic modification, the yeast increases RA accumulation and production while decreasing lipid degradation []. Ledesma-Amaro and Nicaud [] assert that the fact that RA may be processed into a range of economic goods, such as polymers, lubricants, and surfactants, makes it particularly significant.
Microbial lipids made from renewable biomass and biological waste are showing promise as a raw material for biodiesel production in the context of climate change and the pursuit of sustainable energy solutions []. Yarrowia lipids can be utilized to make biodiesel. As a cost-effective and sustainable alternative to conventional vegetable oils, the use of microbial lipids as a second-generation raw material lowers the cost of producing biodiesel and helps to manage biological waste more effectively [,].
Currently, the primary feedstocks for biodiesel production are edible vegetable oils. However, this approach presents several significant drawbacks. On the one hand, global production of edible vegetable oils reaches approximately 30 billion tons per year, constituting a vital food resource for much of the world’s population. On the other hand, large-scale use of these oils for energy purposes could lead to shortages of edible oils, increased food prices, and direct competition between food and fuel. Indeed, feedstock costs account for 60% to 75% of the total biodiesel production cost, representing a significant economic barrier to its broader deployment. Therefore, exploring non-conventional resources capable of providing high-quality oils at a lower cost is essential. Microbial oils offer a promising solution. Oleaginous microorganisms, particularly certain yeasts, can accumulate more than 20% of their dry cell weight as lipids. The amount of recoverable lipids from yeast biomass strongly depends on the substrate used, making it feasible to utilize low-cost carbon sources such as organic residues and wastewater [,].
Oleaginous yeasts can utilize a wide range of carbon sources and compared to microalgae are less affected by seasonal variations or climatic changes. Well-studied genera such as Lipomyces, Yarrowia, Cryptococcus, and Rhodosporidium can store up to 40% of their dry biomass as lipids. Among them, Yarrowia lipolytica is considered a model of non-conventional yeast for lipid production due to its robustness, metabolic versatility, and ability to grow on a variety of substrates, including agro-industrial wastes [].
The study by Sayın et al. [] optimized lipid production from OMW using Y. lipolytica NRRL YB-423 through the Taguchi method, focusing on biomass concentration and lipid yield. Key factors tested included OMW dilution (15–60%), Tween 80 (0–0.6%), and sodium chloride (0–3%), as well as the sterility of the medium. The lipid content in dry biomass varied from 9.97% to 40.88%, while moderate biomass growth ranged from 0.37 to 1.31 g/L. The highest lipid yield was achieved under sterile conditions with 15% diluted OMW, 0.6% Tween 80, and 3% NaCl, demonstrating efficient bioconversion.
Citric acid generation by microorganisms is essential to many businesses, including the food, beverage, detergent, cosmetic, and pharmaceutical sectors. Citric acid (CA), which is highly soluble and has chelating properties, is a safe acidifier. Due to its fragrant and stabilizing properties, it is frequently used in food and drinks. In food packaging, citric acid esters act as non-toxic plasticizers []. Citric acid is in high demand worldwide since it is considered less harmful than other acidifiers. Among its various applications, this acid is used in detergents, cleaning supplies, shampoos, and cosmetics. Y. lipolytica has established itself as a model yeast due to its versatility in production systems [,].
Y. lipolytica is a non-conventional yeast that shows promise for a future study due to its capacity to grow on OMW-based media, which contain high quantities of phenolic compounds, while releasing considerable amounts of citric acid []. Furthermore, Y. lipolytica can use OMW as a substrate to create polyols such as erythritol, mannitol, and arabitol []. The microbial synthesis of polyols has the potential to be more advantageous than chemical synthesis, especially in terms of lower production costs. In the food business, polyols are now indispensable as cooling, texturizing, and sweetening agents. Because of their similarities to sugars, they can be utilized as low-calorie sweeteners in a variety of food products, frequently in conjunction with potent sweeteners [].
Innovative strategies, including microbial valorization, nanoparticle synthesis, and immobilized cell systems, provide sustainable solutions by transforming pollutants and agro-industrial wastewater, such as olive mill wastewater (OMW), into valuable resources while reducing ecological damage caused by their high organic load, salinity, and phenolic content. The three studies below demonstrate innovative and engaging strategies.
The study by Hamimed et al. [] presents a dual approach to valorize olive mill wastewater by biosynthesizing magnesium oxide nanoparticles (MgO NPs) and using the residual effluent for Y. lipolytica biomass production. Recovered polyphenols from OMW facilitated the green synthesis of MgO NPs (average size of 19.4 nm) with cubic/spherical morphology and strong antibacterial activity against pathogens such as Enterobacter aerogenes and Bacillus niacini. After phenol extraction, the treated OMW supported Y. lipolytica growth, resulting in 3.2 g/L biomass in 72 h without nutrient supplementation, while reducing Chemical Oxygen Demand by 73%.
In the subsequent study of this team, the authors explored the synergistic treatment of dephenolated olive mill wastewater (DOMW) and tuna wash processing wastewater (TWPW) using Y. lipolytica, focusing on enhancing biodegradation and desalination. Optimal performance was achieved at a 75:25 DOMW/TWPW ratio, yielding 11.3 g/L biomass and 97.49% Chemical Oxygen Demand reduction, alongside 92% desalination efficiency. FTIR, XRD, and SEM analyses revealed NaCl crystal adsorption on yeast biomass, with cubic structures confirming salt biosorption. The yeast demonstrated significant nutrient removal (100% nitrogen, 98.9% phosphorus) and decolorization (83.06%), highlighting its adaptability to high-salinity conditions.
Hamimed et al. [] confirmed the potential for using immobilized yeast in the biodegradation of OMW. The authors employed a biodegradable PVP/PEG/agar hydrogel for immobilizing Y. lipolytica CLIB40. The hydrogel’s porous structure aided in yeast entrapment, boosting biomass production (7.31 g/L in 7 days) and pollutant removal, achieving an 88.6% reduction in chemical oxygen demand, a 92.64% reduction in turbidity, and complete lipid degradation within 72 h. The immobilized system demonstrated strong reusability over three cycles, maintaining high efficiency (93.33% chemical oxygen demand removal) and storage stability (50% viability after 60 days).
These studies collectively emphasize the promise of combining green chemistry and biotechnology for efficient wastewater treatment. Leveraging the adaptability of yeast and eco-friendly materials, these approaches succeed in removing pollutants, recovering resources, and scaling operations, all of which advance circular economy principles in the management of agro-industrial waste.
6. Bottlenecks and Solutions in the Bioprocessing of Olive Mill Wastewater Valorization
The biotechnological valorization of olive mill wastewater (OMW) by Yarrowia lipolytica represents a promising approach for the production of high-value metabolites such as citric acid, lipids, and polyols. However, several technical and economic challenges still hinder its industrial-scale application. One of the main obstacles is the considerable variability in OMW composition, influenced by factors such as olive variety, agronomic conditions, harvest season, and the extraction method used (two- or three-phase systems). This variability leads to significant fluctuations in the concentrations of phenolic compounds, sugars, fats, and minerals, all of which can directly affect the growth and metabolism of Y. lipolytica. As a result, adapting culture media or pre-treating the effluents becomes necessary, complicating process standardization. This underscores the need to develop more robust strains and simplified protocols, or even adopt semi-continuous or continuous fermentation systems to enhance productivity and reduce costs [,,].
Despite these limitations, one key advantage of this approach lies in the potential to reduce fermentation costs. OMW is an abundant agro-industrial waste, typically available at little or no cost, significantly lowering the expense of raw materials. However, to ensure optimal performance, the addition of glucose is often necessary to achieve a sufficient concentration of fermentable sugars—an added cost when no cheap alternative substrates are available. Moreover, due to their low content of assimilable nitrogen, OMW-based media require supplementation with ammonium sulfate and yeast extract to support cell growth and metabolite production [].
A simple and effective pretreatment protocol can help standardize the raw material before fermentation. This protocol includes an initial high-speed centrifugation (e.g., 9000 rpm for 15 min at room temperature) to remove suspended solids and decomposing matter. Next, the wastewater is diluted with sterile water to adjust the concentration of phenolic compounds to 2 g/L—the optimal concentration for most strains—and the concentration of fermentable sugars, thereby reducing toxicity and promoting microbial growth. A mild heat treatment, such as pasteurization at 70–80 °C for 30 min, is also recommended to decrease the indigenous microbial load without significantly altering the available nutrients. After this pretreatment, supplementation with glucose, ammonium sulfate, and yeast extract is performed to compensate for the low content of assimilable nitrogen and vitamins. These steps result in a more homogeneous medium that is better suited for fermentation by Yarrowia lipolytica (Figure 2).
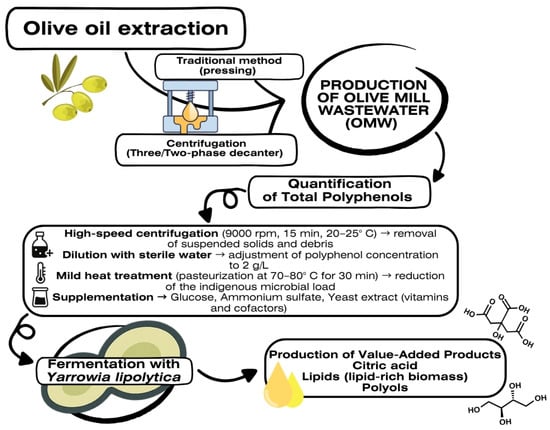
Figure 2.
Pretreatment and fermentation workflow of olive mill wastewater using Y. lipolytica.
Another limiting factor is the relatively high energy demand associated with cultivating Y. lipolytica. This yeast requires strictly aerobic conditions, including high aeration rates, continuous pH regulation, and stable temperature control—factors that increase energy consumption. Additionally, OMW often needs to undergo pre-treatment steps such as centrifugation, dilution, or pasteurization to remove solids and reduce toxicity, further complicating the operational workflow []. A further challenge is the requirement for aseptic conditions in fermentation systems, which complicates scale-up due to the high natural microbial load of OMW.
Solutions being explored include the use of robust strains capable of thriving under non-sterile conditions or in the presence of elevated phenolic concentrations. However, these approaches still require thorough validation under semi-industrial conditions [,,]. Downstream processing is another critical bottleneck. Fermentation broths derived from OMW are particularly complex, containing pigments, residual phenolics, colloids, and various organic compounds that interfere with product purification. The recovery of target metabolites such as citric acid, lipids, or polyols often necessitates additional steps—clarification, filtration, and extraction—that significantly increase production costs. It is well established that downstream processing can account for up to 70% of total bioprocessing costs [,,]. Nonetheless, biotechnological processes remain economically competitive compared to conventional methods. Traditional industrial production of high-value molecules, such as polyols, relies on highly purified substrates and sophisticated technologies like chromatography, which are expensive at scale. In contrast, bioprocesses that utilize low-cost substrates (such as OMW) can achieve high yields while minimizing raw material costs. This balance can offset the higher expenses associated with downstream operations, making the overall process economically viable [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,].
The global polyol market illustrates this potential. In 2019, it was valued at USD 26 billion and is expected to reach USD 34 billion by 2024, driven by increasing demand in the food, pharmaceutical, chemical, and polymer industries, particularly for polyurethane production. Traditionally, polyols are synthesized via catalytic hydrogenation of pure sugars under high temperature and pressure, requiring high-purity raw materials and extensive purification steps—an energy- and cost-intensive process []. As a result, current research is focused on more sustainable biotechnological alternatives that use carbohydrate-rich, non-conventional substrates such as lignocellulosic hydrolysates (corn cobs, rapeseed straw), oilseed cakes, fruit juices, molasses, sugarcane bagasse, soybean residues, or crude glycerol. Robust microorganisms, including lactic acid bacteria and non-conventional yeasts like Y. lipolytica, can efficiently metabolize these substrates, which can convert them into polyols or lipids [,].
The choice of fermentation method also strongly influences metabolite production by Yarrowia lipolytica. To efficiently valorize olive mill wastewater (OMW), it is essential to identify the most suitable strategy (batch, fed-batch, or solid-state fermentation), taking into account the available nutrients, inhibitory compounds, and production objectives. Table 5 presents a summary of the advantages and disadvantages of these approaches for optimizing OMW valorization.

Table 5.
Comparative characteristics of the fermentation methods used for Y. lipolytica in the valorization of olive wastewater (OMW).
Despite the inherent challenges of cultivating Y. lipolytica on olive mill wastewater (OMW), pilot-scale proof-of-concept studies have extended these findings under conditions closer to industrial settings. For instance, Sarris et al. [] demonstrated that Y. lipolytica could efficiently co-metabolize crude glycerol and OMW, simultaneously producing citric acid, arabitol, mannitol, erythritol, and cellular lipids. These pilot fermentations not only revealed a strong tolerance to complex substrates but also showed metabolic reprogramming depending on the medium composition, underscoring the potential for adaptable and tunable industrial applications based on available resources. Similarly, Gonçalves et al. [] confirmed in fed-batch mode at a 2 L bioreactor scale the potential for producing value-added metabolites from olive mill effluents. A lipase activity of 5 U/mL was achieved, accompanied by a 50% reduction in chemical oxygen demand (COD), indicating effective degradation of extractable organic matter (EOM). Additionally, 54% of sugars were consumed and 55% of lipids metabolized without microbial growth inhibition, demonstrating the strain’s robustness towards substrate complexity.
More recently, studies focused on optimizing culture parameters have reinforced the technological feasibility of the process. Sayın [] applied the Taguchi method to optimize Y. lipolytica growth on OMW, obtaining protein-rich biomass with concentrations exceeding 11 g/L. These works highlight the relevance of OMW as a low-cost raw material for biomolecule production and illustrate academia’s ability to develop concrete, industrially applicable solutions. Collectively, these results, combined with pilot-scale feasibility tests, contribute to bridging the gap between fundamental research and industrial transfer in the field of sustainable bioprocesses, thereby confirming the feasibility of viable scale-up despite initial constraints.
7. Conclusions
Many publications emphasize the significance of valorizing by-products from liquid olive oil, especially olive mill wastewater (OMW), which is recognized for its detrimental effects on soil, water, and air quality. This valorization aims to create high-value products while minimizing environmental impact. While producing microbial metabolites shows promise, it is crucial to pinpoint the factors that affect this process.
This article highlights the detrimental effects of OMW and investigates the potential of Y. lipolytica for its valorization. It covers the application of this yeast in producing lipases, citric acid, and polyols while reviewing research focused on enhancing production yields, valorization approaches, and the selection of high-performing strains. Furthermore, the metabolites generated by Y. lipolytica during the fermentation of olive pomace hold noteworthy biotechnological potential; however, extensive research is required to assess this valorization capability thoroughly.
Several challenges, however, remain to be addressed. These include the variability in the composition of OMW depending on the olive cultivar and extraction method, the high energy costs associated with purification steps, and the difficulties in scaling up processes from laboratory to industrial levels. Moreover, specific research directions, such as engineering Y. lipolytica to enhance its tolerance to inhibitory phenolic compounds and broaden its substrate assimilation capabilities, deserve particular attention. The development of hybrid or co-culture systems, combining Y. lipolytica with specialized microorganisms capable of degrading phenolics, is another promising avenue. Additionally, adaptive evolution strategies to select strains better suited to prolonged OMW exposure, along with the use of omics approaches (genomics, transcriptomics, metabolomics) to understand tolerance and biosynthesis mechanisms at the molecular level, are crucial.
In summary, the valorization of OMW using Y. lipolytica offers a sustainable and innovative pathway that bridges industrial innovation and environmental responsibility. Achieving this potential requires a systemic approach combining molecular biology, process engineering, and sustainability assessment, ultimately paving the way for a circular and zero-waste olive oil industry.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
I would like to express my sincere gratitude to Frères Mentouri University of Constantine 1 for their continuous support and for granting me a generous scholarship that enabled me to travel to the Warsaw University of Life Sciences-SGGW in Poland. This opportunity greatly contributed to the advancement of my research and the broadening of my academic horizons.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| OMW | Olive Mill Wastewater |
| TOC | Total Organic Carbon |
| TN | Total Nitrogen |
| COD | Chemical Oxygen Demand |
| BOD5 | Biochemical Oxygen Demand |
| OME | Olive Mill Effluent |
| GRAS | Generally Recognized As Safe |
| U.S. FDA | United States Food and Drug Administration |
| Phen | Phenolic Compound |
| Cit or Citmax | Citric Acid |
| Glc0 | Initial Glucose Concentration |
| Manmax | Maximum Mannitol Concentration |
| Erymax | Maximum Erythritol Concentration (g/L) |
| Ph0 | Initial Concentration of Phenolic Compounds |
References
- Shabir, S.; Ilyas, N.; Saeed, M.; Bibi, F.; Sayyed, R.Z.; Almalki, W.H. Treatment Technologies for Olive Mill Wastewater with Impacts on Plants. Environ. Res. 2023, 216, 114399. [Google Scholar] [CrossRef] [PubMed]
- Sygouni, V.; Pantziaros, A.G.; Iakovides, I.C.; Sfetsa, E.; Bogdou, P.I.; Christoforou, E.A.; Paraskeva, C.A. Treatment of Two-Phase Olive Mill Wastewater and Recovery of Phenolic Compounds Using Membrane Technology. Membranes 2019, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Alaoui, S.B.; Lamy, E.; Achak, M. Assessment of the Impact of Diluted and Pretreated Olive Mill Wastewater on the Treatment Efficiency by Infiltration-Percolation Using Natural Bio-Adsorbents. Environ. Sci. Pollut. Res. 2023, 30, 16305–16320. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Valorization of By-Products from Olive Oil Industry and Added-Value Applications for Innovative Functional Foods. Food Res. Int. 2020, 137, 109683. [Google Scholar] [CrossRef]
- Hamimed, S.; Landoulsi, A.; Chatti, A. The Bright Side of Olive Mill Wastewater: Valuables Bioproducts after Bioremediation. Int. J. Environ. Sci. Technol. 2021, 18, 4053–4074. [Google Scholar] [CrossRef]
- Blanco, I.; De Bellis, L.; Luvisi, A. Bibliometric Mapping of Research on Life Cycle Assessment of Olive Oil Supply Chain. Sustainability 2022, 14, 3747. [Google Scholar] [CrossRef]
- Achak, M.; Hafidi, A.; Ouazzani, N.; Sayadi, S.; Mandi, L. Low Cost Biosorbent “Banana Peel” for the Removal of Phenolic Compounds from Olive Mill Wastewater: Kinetic and Equilibrium Studies. J. Hazard. Mater. 2009, 166, 117–125. [Google Scholar] [CrossRef]
- Aktas, E.S.; Imre, S.; Ersoy, L. Characterization and Lime Treatment of Olive Mill Wastewater. Water Res. 2001, 35, 2336–2340. [Google Scholar] [CrossRef]
- Laconi, S.; Molle, G.; Cabiddu, A.; Pompei, R. Bioremediation of Olive Oil Mill Wastewater and Production of Microbial Biomass. Biodegradation 2007, 18, 559–566. [Google Scholar] [CrossRef]
- Fleyfel, L.M.; Leitner, N.K.V.; Deborde, M.; Matta, J.; El Najjar, N.H. Olive Oil Liquid Wastes–Characteristics and Treatments: A Literature Review. Process Saf. Environ. Prot. 2022, 168, 1031–1048. [Google Scholar] [CrossRef]
- El Hassani, F.Z.; El Karkouri, A.; Errachidi, F.; Merzouki, M.; Benlemlih, M. The Impact of Olive Mill Wastewater Spreading on Soil and Plant in Arid and Semi-Arid Areas. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100798. [Google Scholar] [CrossRef]
- Mekki, A.; Dhouib, A.; Sayadi, S. Polyphenols Dynamics and Phytotoxicity in a Soil Amended by Olive Mill Wastewaters. J. Environ. Manag. 2007, 84, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Alami, A.E.; Fattah, A. Olive Mill Wastewater Causing Pollution in the Oum Er Rbia River and Potential Environmental Effects and Impact on the Eurasian Otter. J. Anal. Sci. Appl. Biotechnol. 2020, 2, 115. [Google Scholar] [CrossRef]
- Doula, M.K.; Papadopoulos, A.; Kolovos, C.; Lamnatou, O.; Zorpas, A.A. Evaluation of the influence of olive mill waste on soils: The case study of disposal areas in Crete, Greece. Comptes Rendus Chim. 2020, 23, 705–720. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Zoubi, H.; Hudaib, B.; Omar, W.; Soleimani, M.; Abu-Romman, S.; Frontistis, Z. Sustainable vs. Conventional Approach for Olive Oil Wastewater Management: A Review of the State of the Art. Water 2022, 14, 1695. [Google Scholar] [CrossRef]
- Ayoub, S.; Al-Absi, K.; Al-Shdiefat, S.; Al-Majali, D.; Hijazean, D. Effect of Olive Mill Wastewater Land-Spreading on Soil Properties, Olive Tree Performance and Oil Quality. Sci. Hortic. 2014, 175, 160–166. [Google Scholar] [CrossRef]
- Lakhtar, H.; Kharbouch, B.; Askarne, L.; Ait Hamza, M.; El Mousadik, A. Effect of Thermophilic Composting Duration on Vermiconversion of Olive Mill Wastewater Using Eisenia Andrei. Bioresour. Technol. Rep. 2023, 23, 101560. [Google Scholar] [CrossRef]
- Crognale, S.; D’Annibale, A.; Federici, F.; Fenice, M.; Quaratino, D.; Petruccioli, M. Olive Oil Mill Wastewater Valorisation by Fungi. J. Chem. Technol. Biotechnol. 2006, 81, 1547–1555. [Google Scholar] [CrossRef]
- Sarris, D.; Stoforos, N.G.; Mallouchos, A.; Kookos, I.K.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Production of Added-value Metabolites by Yarrowia lipolytica Growing in Olive Mill Wastewater-based Media under Aseptic and Non-aseptic Conditions. Eng. Life Sci. 2017, 17, 695–709. [Google Scholar] [CrossRef]
- Lopes, M.; Miranda, S.M.; Costa, A.R.; Pereira, A.S.; Belo, I. Yarrowia lipolytica as a Biorefinery Platform for Effluents and Solid Wastes Valorization—Challenges and Opportunities. Crit. Rev. Biotechnol. 2022, 42, 163–183. [Google Scholar] [CrossRef]
- Gueboudji, Z.; Kadi, K.; Nagaz, K.; Addad, D.; Secrafi, M.; Yahya, L.B.; Lachehib, B. Phenolic Compounds and Biological Activities of Phenolic Extract of Olive Oil Mill Wastewater Issue from the Cold Extraction of Olive Oil from Khenchela (Algeria). Res. Sq. 2021. [Google Scholar] [CrossRef]
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. The Use of Elemental Sulphur as Organic Alternative to Control pH during Composting of Olive Mill Wastes. Chemosphere 2004, 57, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Morillo-Pérez, J.A.; Antizar-Ladislao, B.; Monteoliva-Sánchez, M.; Ramos-Cormenzana, A.; Russell, N. Bioremediation and Biovalorisation of Olive-Mill Wastes. Appl. Microbiol. Biotechnol. 2009, 82, 25–39. [Google Scholar] [CrossRef]
- Gueboudji, Z.; Kadi, K.; Nagaz, K. Extraction and Quantification of Polyphenols of Olive Oil Mill Wastewater from the Cold Extraction of Olive Oil in the Region of Khenchela-Algeria. Genet. Biodivers. J. 2021, 5, 116–122. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive Mill Wastes: Biochemical Characterizations and Valorization Strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Prambudy, H.; Supriyatin, T.; Setiawan, F. The Testing of Chemical Oxygen Demand (COD) and Biological Oxygen Demand (BOD) of River Water in Cipager Cirebon. J. Phys. Conf. Ser. 2019, 1360, 012010. [Google Scholar] [CrossRef]
- Feki, M.; Allouche, N.; Bouaziz, M.; Gargoubi, A.; Sayadi, S. Effect of Storage of Olive Mill Wastewaters on Hydroxytyrosol Concentration. Eur. J. Lipid Sci. Technol. 2006, 108, 1021–1027. [Google Scholar] [CrossRef]
- Romeo, R.; De Bruno, A.; Imeneo, V.; Piscopo, A.; Poiana, M. Impact of Stability of Enriched Oil with Phenolic Extract from Olive Mill Wastewaters. Foods 2020, 9, 856. [Google Scholar] [CrossRef]
- Leouifoudi, I.; Zyad, A.; Amechrouq, A.; Oukerrou, M.A.; Mouse, H.A.; Mbarki, M. Identification and Characterisation of Phenolic Compounds Extracted from Moroccan Olive Mill Wastewater. Food Sci. Technol. 2014, 34, 249–257. [Google Scholar] [CrossRef]
- Keskin, A.; Ünlü, A.E.; Takaç, S. Utilization of Olive Mill Wastewater for Selective Production of Lipids and Carotenoids by Rhodotorula Glutinis. Appl. Microbiol. Biotechnol. 2023, 107, 4973–4985. [Google Scholar] [CrossRef]
- Aly, A.A.; Alashgar, K.N.S.; Al-Farraj, A.S.; Ibrahim, H.M. Contaminants and Salinity Removal of Olive Mill Wastewater Using Zeolite Nanoparticles. Sep. Sci. Technol. 2018, 53, 1638–1653. [Google Scholar] [CrossRef]
- Sahbeni, G.; Ngabire, M.; Musyimi, P.K.; Székely, B. Challenges and Opportunities in Remote Sensing for Soil Salinization Mapping and Monitoring: A Review. Remote Sens. 2023, 15, 2540. [Google Scholar] [CrossRef]
- Fanari, F.; Carboni, G.; Grosso, M.; Desogus, F. Thermogravimetric Analysis of Different Semolina Doughs: Effect of Mixing Time and Gluten Content. Chem. Eng. Trans. 2019, 75, 343–348. [Google Scholar] [CrossRef]
- Kavvadias, V.; Doula, M.; Papadopoulou, M.; Theocharopoulos, S. Long-Term Application of Olive-Mill Wastewater Affects Soil Chemical and Microbial Properties. Soil Res. 2015, 53, 461–473. [Google Scholar] [CrossRef]
- El Hassani, F.Z.; Zinedine, A.; Amraoui, M.B.; Errachidi, F.; Alaoui, S.M.; Aissam, H.; Merzouki, M.; Benlemlih, M. Characterization of the Harmful Effect of Olive Mill Wastewater on Spearmint. J. Hazard. Mater. 2009, 170, 779–785. [Google Scholar] [CrossRef]
- Zieniuk, B.; Fabiszewska, A. Yarrowia lipolytica: A Beneficious Yeast in Biotechnology as a Rare Opportunistic Fungal Pathogen: A Minireview. World J. Microbiol. Biotechnol. 2018, 35, 10. [Google Scholar] [CrossRef]
- Georgiadis, I.; Tsiligkaki, C.; Patavou, V.; Orfanidou, M.; Tsoureki, A.; Andreadelli, A.; Theodosiou, E.; Makris, A.M. Identification and Construction of Strong Promoters in Yarrowia lipolytica Suitable for Glycerol-Based Bioprocesses. Microorganisms 2023, 11, 1152. [Google Scholar] [CrossRef]
- Gamraoui, A.; Hamimed, S.; Landoulsi, A.; Chatti, A. Musico-Bioremediation of Seafood Canning Wastewater by Yarrowia lipolytica. World J. Microbiol. Biotechnol. 2023, 39, 303. [Google Scholar] [CrossRef]
- Liu, H.; Song, Y.; Fan, X.; Wang, C.; Lu, X.; Tian, Y. Yarrowia lipolytica as an Oleaginous Platform for the Production of Value-Added Fatty Acid-Based Bioproducts. Front. Microbiol. 2021, 11, 608662. [Google Scholar] [CrossRef]
- Vastaroucha, E.-S.; Stoforos, N.G.; Aggelis, G.; Papanikolaou, S. Studies of Polyol Production by the Yeast Yarrowia lipolytica Growing on Crude Glycerol under Stressful Conditions. Carbon Resour. Convers. 2024, 7, 100210. [Google Scholar] [CrossRef]
- Lazar, Z.; Walczak, E.; Robak, M. Simultaneous Production of Citric Acid and Invertase by Yarrowia lipolytica SUC+ Transformants. Bioresour. Technol. 2011, 102, 6982–6989. [Google Scholar] [CrossRef] [PubMed]
- Brígida, A.I.S.; Amaral, P.F.F.; Coelho, M.A.Z.; Gonçalves, L.R.B. Lipase from Yarrowia lipolytica: Production, Characterization and Application as an Industrial Biocatalyst. J. Mol. Catal. B Enzym. 2014, 101, 148–158. [Google Scholar] [CrossRef]
- Darvishi Harzevili, F. Yarrowia lipolytica in Biotechnological Applications. In Biotechnological Applications of the Yeast Yarrowia lipolytica; Darvishi Harzevili, F., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 17–74. [Google Scholar] [CrossRef]
- Wierzchowska, K.; Zieniuk, B.; Fabiszewska, A. Use of Non-Conventional Yeast Yarrowia lipolytica in Treatment or Upgradation of Hydrophobic Industry Wastes. Waste Biomass Valorization 2022, 13, 757–779. [Google Scholar] [CrossRef]
- Gamraoui, A.; Vasilakis, G.; Ouaer, M.E.; Karayannis, D.; Papanikolaou, S.; Landoulsi, A.; Chatti, A. Innovative Combined Method for Tuna Wastewater Treatment Using Yarrowia lipolytica, TiO2 Nanoparticles and Sounds. Waste Biomass Valorization 2025, 16, 1687–1699. [Google Scholar] [CrossRef]
- Colacicco, M.; Ciliberti, C.; Biundo, A.; Agrimi, G.; Pisano, I. Study of Lipase Production by Yarrowia lipolytica Grown in High Concentration of Hydrophobic Carbon Sources. Chem. Eng. Trans. 2022, 93, 247–252. [Google Scholar] [CrossRef]
- al Mualad, W.; Bouchedja, N.; Selmania, A.; Maadadi, R.; Ikhlef, A.; Kabouche, Z.; Elmechta, L.; Boudjellal, A. Recycling Pollutants and Used Oils as Substrates for Producing Useful Lipids in the Form of Single-Cell Oil by the Aerobic Yeast Yarrowia lipolytica. Int. J. Environ. Res. 2022, 16, 97. [Google Scholar] [CrossRef]
- Groenewald, M.; Boekhout, T.; Neuveglise, C.; Gaillardin, C.; Van Dijck, P.W.M.; Wyss, M. Yarrowia lipolytica: Safety Assessment of an Oleaginous Yeast with a Great Industrial Potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.-I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Yarrowia lipolytica Yeast Biomass as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2019, 17, e05594. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Esteban, M.Á.; Angulo, C. Yarrowia lipolytica, Health Benefits for Animals. Appl. Microbiol. Biotechnol. 2021, 105, 7577–7592. [Google Scholar] [CrossRef]
- da Silva, R.M.; da Silva, C.N.; de Faria-Júnior, C.S.; Buarque, F.S.; Ribeiro, B.D.; Lemes, A.C.; Coelho, M.A.Z. Extraction and Characterization of High-Value Compounds from Yarrowia lipolytica W29 Using Sequential Hydrolysis. Processes 2025, 13, 615. [Google Scholar] [CrossRef]
- Dunoyer, A.T.; Cuello, R.E.G.; Salinas, R.P. Biodegradation of Dairy Wastes Using Crude Enzymatic Extract of Yarrowia lipolytica ATCC 9773. Rev. Ambiente Água 2020, 15, e2448. [Google Scholar] [CrossRef]
- Lanciotti, R. Use of Yarrowia lipolytica Strains for the Treatment of Olive Mill Wastewater. Bioresour. Technol. 2005, 96, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Sarris, D.; Rapti, A.; Papafotis, N.; Koutinas, A.A.; Papanikolaou, S. Production of Added-Value Chemical Compounds through Bioconversions of Olive-Mill Wastewaters Blended with Crude Glycerol by a Yarrowia lipolytica Strain. Molecules 2019, 24, 222. [Google Scholar] [CrossRef] [PubMed]
- Tzirita, M.; Kremmyda, M.; Sarris, D.; Koutinas, A.A.; Papanikolaou, S. Effect of Salt Addition upon the Production of Metabolic Compounds by Yarrowia lipolytica Cultivated on Biodiesel-Derived Glycerol Diluted with Olive-Mill Wastewaters. Energies 2019, 12, 3649. [Google Scholar] [CrossRef]
- Sarris, D.; Galiotou-Panayotou, M.; Koutinas, A.A.; Komaitis, M.; Papanikolaou, S. Citric Acid, Biomass and Cellular Lipid Production by Yarrowia lipolytica Strains Cultivated on Olive Mill Wastewater-based Media. J. Chem. Technol. Biotechnol. 2011, 86, 1439–1448. [Google Scholar] [CrossRef]
- Carvalho, A.S.S.; Sales, J.C.S.; do Nascimento, F.V.; Ribeiro, B.D.; Souza, C.E.C.D.; Lemes, A.C.; Coelho, M.A.Z. Lipase Production by Yarrowia lipolytica in Solid-State Fermentation Using Amazon Fruit By-Products and Soybean Meal as Substrate. Catalysts 2023, 13, 289. [Google Scholar] [CrossRef]
- Colacicco, M.; Ciliberti, C.; Agrimi, G.; Biundo, A.; Pisano, I. Towards the Physiological Understanding of Yarrowia lipolytica Growth and Lipase Production Using Waste Cooking Oils. Energies 2022, 15, 5217. [Google Scholar] [CrossRef]
- Moujehed, E.; Zarai, Z.; Khemir, H.; Miled, N.; Bchir, M.S.; Gablin, C.; Bessueille, F.; Bonhommé, A.; Leonard, D.; Carrière, F.; et al. Cleaner Degreasing of Sheepskins by the Yarrowia lipolytica LIP2 Lipase as a Chemical-Free Alternative in the Leather Industry. Colloids Surf. B Biointerfaces 2022, 211, 112292. [Google Scholar] [CrossRef]
- da Silva, J.L.; Sales, M.B.; de Castro Bizerra, V.; Nobre, M.M.R.; de Sousa Braz, A.K.; da Silva Sousa, P.; Cavalcante, A.L.G.; Melo, R.L.F.; Gonçalves De Sousa Junior, P.; Neto, F.S.; et al. Lipase from Yarrowia lipolytica: Prospects as an Industrial Biocatalyst for Biotechnological Applications. Fermentation 2023, 9, 581. [Google Scholar] [CrossRef]
- Zieniuk, B.; Jasińska, K.; Wierzchowska, K.; Uğur, Ş.; Fabiszewska, A. Yarrowia lipolytica Yeast: A Treasure Trove of Enzymes for Biocatalytic Applications—A Review. Fermentation 2024, 10, 263. [Google Scholar] [CrossRef]
- do Nascimento, F.V.; Lemes, A.C.; de Castro, A.M.; Secchi, A.R.; Zarur Coelho, M.A. A Temporal Evolution Perspective of Lipase Production by Yarrowia lipolytica in Solid-State Fermentation. Processes 2022, 10, 381. [Google Scholar] [CrossRef]
- Gonçalves, C.; Oliveira, F.; Pereira, C.; Belo, I. Fed-Batch Fermentation of Olive Mill Wastewaters for Lipase Production. J. Chem. Technol. Biotechnol. 2012, 87, 1215–1218. [Google Scholar] [CrossRef]
- Scioli, C.; Vollaro, L. The Use of Yarrowia lipolytica to Reduce Pollution in Olive Mill Wastewaters. Water Res. 1997, 31, 2520–2524. [Google Scholar] [CrossRef]
- Moftah, O.A.S.; Grbavcic, S.Z.; Moftah, W.A.; Luković, N.D.; Prodanović, O.; Jakovetic, S.M.; Knezevic-Jugović, Z.D. Lipase Production by Yarrowia lipolytica Using Olive Oil Processing Wastes as Substrates. J. Serbian Chem. Soc. 2013, 78, 781–794. [Google Scholar] [CrossRef]
- Lopes, M.; Araújo, C.; Aguedo, M.; Gomes, N.; Gonçalves, C.; Teixeira, J.A.; Belo, I. The Use of Olive Mill Wastewater by Wild Type Yarrowia lipolytica Strains: Medium Supplementation and Surfactant Presence Effect. J. Chem. Technol. Biotechnol. 2009, 84, 533–537. [Google Scholar] [CrossRef]
- Barros, F.; Awika, J.M.; Rooney, L.W. Interaction of Tannins and Other Sorghum Phenolic Compounds with Starch and Effects on in Vitro Starch Digestibility. J. Agric. Food Chem. 2012, 60, 11609–11617. [Google Scholar] [CrossRef]
- Kamzolova, S.V. A Review on Citric Acid Production by Yarrowia lipolytica Yeast: Past and Present Challenges and Developments. Processes 2023, 11, 3435. [Google Scholar] [CrossRef]
- Dourou, M.; Kancelista, A.; Juszczyk, P.; Sarris, D.; Bellou, S.; Triantaphyllidou, I.-E.; Rywinska, A.; Papanikolaou, S.; Aggelis, G. Bioconversion of Olive Mill Wastewater into High-Added Value Products. J. Clean. Prod. 2016, 139, 957–969. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Galiotou-Panayotou, M.; Fakas, S.; Komaitis, M.; Aggelis, G. Citric Acid Production by Yarrowia lipolytica Cultivated on Olive-Mill Wastewater-Based Media. Bioresour. Technol. 2008, 99, 2419–2428. [Google Scholar] [CrossRef]
- Sarris, D.; Tsouko, E.; Photiades, A.; Tchakouteu, S.S.; Diamantopoulou, P.; Papanikolaou, S. Growth Response of Non-Conventional Yeasts on Sugar-Rich Media: Part 2: Citric Acid Production and Circular-Oriented Valorization of Glucose-Enriched Olive Mill Wastewaters Using Novel Yarrowia lipolytica Strains. Microorganisms 2023, 11, 2243. [Google Scholar] [CrossRef]
- Carsanba, E.; Papanikolaou, S.; Fickers, P.; Agirman, B.; Erten, H. Citric Acid Production by Yarrowia lipolytica. In Non-Conventional Yeasts: From Basic Research to Application; Sibirny, A., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 91–117. [Google Scholar] [CrossRef]
- Sarris, D.; Tsouko, E.; Kothri, M.; Anagnostou, M.; Karageorgiou, E.; Papanikolaou, S. Upgrading Major Waste Streams Derived from the Biodiesel Industry and Olive Mills via Microbial Bioprocessing with Non-Conventional Yarrowia lipolytica Strains. Fermentation 2023, 9, 251. [Google Scholar] [CrossRef]
- Bilal, M.; Xu, S.; Iqbal, H.M.N.; Cheng, H. Yarrowia lipolytica as an Emerging Biotechnological Chassis for Functional Sugars Biosynthesis. Crit. Rev. Food Sci. Nutr. 2021, 61, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.; Zannini, E.; Arendt, E.K.; Coffey, A. A Review of Polyols—Biotechnological Production, Food Applications, Regulation, Labeling and Health Effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 2034–2051. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, Y.-W.; Chen, X.-Y.; Wang, Y.-T.; Ye, C.; Shi, T.-Q. Application of Adaptive Laboratory Evolution for Yarrowia lipolytica: A Comprehensive Review. Bioresour. Technol. 2024, 391, 129893. [Google Scholar] [CrossRef]
- Hamé, A.K.K.; Aboubacar, K.; Ousmane, Z.M.; Bondaba, M. Connaissances paysannes sur la conservation du sorgho et du mil dans le département d’Aguié au Niger. J. Des Sci. De L’ Environ. 2013, 2, 10–18. [Google Scholar]
- Barbosa, S.; Coelho, D.; Silveira, E.; Tambourgi, E.; Souza, R. Biosurfactant Production by Pseudomonas Aeruginosa and Yarrowia lipolytica and Its Use for Detergent Formulations. J. Chem. Chem. Eng. 2013, 7, 1934–7375. [Google Scholar]
- Fickers, P.; Benetti, P.; Wache, Y.; Marty, A.; Mauersberger, S.; Smit, M.; Nicaud, J. Hydrophobic Substrate Utilisation by the Yeast, and Its Potential Applications. FEMS Yeast Res. 2005, 5, 527–543. [Google Scholar] [CrossRef]
- Soong, Y.V.; Liu, N.; Yoon, S.; Lawton, C.; Xie, D. Cellular and Metabolic Engineering of Oleaginous Yeast Yarrowia lipolytica for Bioconversion of Hydrophobic Substrates into High-value Products. Eng. Life Sci. 2019, 19, 423–443. [Google Scholar] [CrossRef]
- Fontes, G.C.; Fonseca Amaral, P.F.; Nele, M.; Zarur Coelho, M.A. Factorial Design to Optimize Biosurfactant Production by Yarrowia lipolytica. BioMed Res. Int. 2010, 2010, 821306. [Google Scholar] [CrossRef]
- Fontes, G.C.; Ramos, N.M.; Amaral, P.F.F.; Nele, M.; Coelho, M.a.Z. Renewable Resources for Biosurfactant Production by Yarrowia lipolytica. Braz. J. Chem. Eng. 2012, 29, 483–494. [Google Scholar] [CrossRef]
- Amaral, P.F.F.; Da Silva, J.M.; Lehocky, M.; Barros-Timmons, A.M.V.; Coelho, M.A.Z.; Marrucho, I.M.; Coutinho, J.A.P. Production and Characterization of a Bioemulsifier from Yarrowia lipolytica. Process Biochem. 2006, 41, 1894–1898. [Google Scholar] [CrossRef]
- Coelho, M.A.Z.; Amaral, P.F.F.; Belo, I. Yarrowia lipolytica: An Industrial Workhorse. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 2, 930–940. [Google Scholar]
- Shatila, F.; Uyar, E.; Yalçın, H.T. Screening of Biosurfactant Production by Yarrowia lipolytica Strains and Evaluation of Their Antibiofilm and Anti-Adhesive Activities against Salmonella Enterica Ser. Enteritidis Biofilms. Microbiology 2021, 90, 839–847. [Google Scholar] [CrossRef]
- Fernandes, N.D.A.T.; Simões, L.A.; Dias, D.R. Biosurfactants Produced by Yeasts: Fermentation, Screening, Recovery, Purification, Characterization, and Applications. Fermentation 2023, 9, 207. [Google Scholar] [CrossRef]
- Csutak, O.; Corbu, V.; Stoica, I.; Ionescu, R.; Vassu, T. Biotechnological Applications of Yarrowia lipolytica CMGB32. Agric. Agric. Sci. Procedia 2015, 6, 545–553. [Google Scholar] [CrossRef]
- Mulligan, C.N. Environmental Applications for Biosurfactants. Environ. Pollut. 2005, 133, 183–198. [Google Scholar] [CrossRef]
- Gonçalves, C.; Lopes, M.; Ferreira, J.P.; Belo, I. Biological Treatment of Olive Mill Wastewater by Non-Conventional Yeasts. Bioresour. Technol. 2009, 100, 3759–3763. [Google Scholar] [CrossRef]
- Bouchedja, D.N.; Danthine, S.; Kar, T.; Fickers, P.; Sassi, H.; Boudjellal, A.; Blecker, C.; Delvigne, F. pH Level Has a Strong Impact on Population Dynamics of the Yeast Yarrowia lipolytica and Oil Micro-Droplets in Multiphasic Bioreactor. FEMS Microbiol. Lett. 2018, 365, fny173. [Google Scholar] [CrossRef]
- Fabiszewska, A.; Paplińska-Goryca, M.; Misiukiewicz-Stępień, P.; Wołoszynowska, M.; Nowak, D.; Zieniuk, B. Expression Profile of Selected Genes Involved in Storage Lipid Synthesis in a Model Oleaginous Yeast Species Yarrowia lipolytica. Int. J. Mol. Sci. 2022, 23, 1041. [Google Scholar] [CrossRef]
- Ledesma-Amaro, R.; Nicaud, J.-M. Yarrowia lipolytica as a Biotechnological Chassis to Produce Usual and Unusual Fatty Acids. Prog. Lipid Res. 2016, 61, 40–50. [Google Scholar] [CrossRef]
- Muniraj, I.K.; Uthandi, S.K.; Hu, Z.; Xiao, L.; Zhan, X. Microbial Lipid Production from Renewable and Waste Materials for Second-Generation Biodiesel Feedstock. Environ. Technol. Rev. 2015, 4, 1–16. [Google Scholar] [CrossRef]
- Poli, J.S.; Da Silva, M.A.N.; Siqueira, E.P.; Pasa, V.M.D.; Rosa, C.A.; Valente, P. Microbial Lipid Produced by Yarrowia lipolytica QU21 Using Industrial Waste: A Potential Feedstock for Biodiesel Production. Bioresour. Technol. 2014, 161, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Vasaki, M.; Sithan, M.; Ravindran, G.; Paramasivan, B.; Ekambaram, G.; Karri, R.R. Biodiesel Production from Lignocellulosic Biomass Using Yarrowia lipolytica. Energy Convers. Manag. X 2022, 13, 100167. [Google Scholar] [CrossRef]
- Yu, A.; Zhao, Y.; Li, J.; Li, S.; Pang, Y.; Zhao, Y.; Zhang, C.; Xiao, D. Sustainable Production of FAEE Biodiesel Using the Oleaginous Yeast Yarrowia lipolytica. MicrobiologyOpen 2020, 9, e1051. [Google Scholar] [CrossRef]
- Sayın, B.; Polat, Z.; Kaban, G. Enhanced Lipid Yield from Olive-Mill Wastewater by Yarrowia lipolytica NRRL YB-423. J. Agric. Prod. 2025, 6, 32–40. [Google Scholar] [CrossRef]
- Behera, B.C.; Mishra, R.; Mohapatra, S. Microbial Citric Acid: Production, Properties, Application, and Future Perspectives. Food Front. 2021, 2, 62–76. [Google Scholar] [CrossRef]
- Książek, E. Citric Acid: Properties, Microbial Production, and Applications in Industries. Available online: https://www.mdpi.com/1420-3049/29/1/22 (accessed on 5 November 2024).
- Cavallo, E.; Charreau, H.; Cerrutti, P.; Foresti, M.L. Yarrowia lipolytica: A Model Yeast for Citric Acid Production. FEMS Yeast Res. 2017, 17, fox084. [Google Scholar] [CrossRef]
- Hamimed, S.; Jebli, N.; Sellami, H.; Landoulsi, A.; Chatti, A. Dual Valorization of Olive Mill Wastewater by Bio-Nanosynthesis of Magnesium Oxide and Yarrowia lipolytica Biomass Production. Chem. Biodivers. 2020, 17, e1900608. [Google Scholar] [CrossRef]
- Hamimed, S.; Ben Ammar, N.E.; Slimi, H.; Asses, N.; Hamzaoui, A.H.; Chatti, A. Innovative Entrapped Yarrowia lipolytica within Polyvinylpyrrolidone (PVP)/Polyethylene Glycol (PEG)/Agar for Improving Olive Mill Wastewater Bioremediation. J. Clean. Prod. 2024, 449, 141828. [Google Scholar] [CrossRef]
- Vuppala, S.; Paulista, L.O.; Morais, D.F.S.; Pinho, I.L.; Martins, R.J.E.; Gomes, A.I.; Moreira, F.C.; Vilar, V.J.P. Multistage Treatment for Olive Mill Wastewater: Assessing Legal Compliance and Operational Costs. J. Environ. Chem. Eng. 2022, 10, 107442. [Google Scholar] [CrossRef]
- Börekçi, B.S.; Kaban, G.; Kaya, M. Citric Acid Production of Yeasts: An Overview. EuroBiotech J. 2021, 5, 79–91. [Google Scholar] [CrossRef]
- Vaz, T.; Quina, M.M.J.; Martins, R.C.; Gomes, J. Olive Mill Wastewater Treatment Strategies to Obtain Quality Water for Irrigation: A Review. Sci. Total Environ. 2024, 931, 172676. [Google Scholar] [CrossRef] [PubMed]
- Wierzchowska, K.; Pakulska, A.; Derewiaka, D.; Piasecka, I.; Zieniuk, B.; Nowak, D.; Fabiszewska, A. Concept of Batch and Fed-Batch Cultures of Yarrowia lipolytica as a Valuable Source of Sterols with Simultaneous Valorization of Molasses and Post-Frying Rapeseed Oil. Appl. Sci. 2022, 12, 12877. [Google Scholar] [CrossRef]
- Bondarenko, P.Y.; Fedorov, A.S.; Sineoky, S.P. Optimization of Repeated-Batch Fermentation of a Recombinant Strain of the Yeast Yarrowia lipolytica for Succinic Acid Production at Low pH. Appl. Biochem. Microbiol. 2017, 53, 882–887. [Google Scholar] [CrossRef]
- Fed-Batch Fermentation of Yarrowia lipolytica Using Defatted Silkworm Pupae Hydrolysate: A Dynamic Model-Based Approach for High Yield of Lipid Production|Waste and Biomass Valorization. Available online: https://link.springer.com/article/10.1007/s12649-017-0180-y (accessed on 22 May 2025).
- Vong, W.C.; Hua, X.Y.; Liu, S.-Q. Solid-State Fermentation with Rhizopus Oligosporus and Yarrowia lipolytica Improved Nutritional and Flavour Properties of Okara. LWT 2018, 90, 316–322. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).