Abstract
Citric acid finds broad applications in various industrial sectors, such as the pharmaceutical, food, chemical, and cosmetic industries. The bioproduction of citric acid uses various microorganisms, but the most commonly employed ones are filamentous fungi such as Aspergillus niger and yeast Yarrowia lipolytica. This article presents a literature review on the properties of citric acid, the microorganisms and substrates used, different fermentation techniques, its industrial utilization, and the global citric acid market. This review emphasizes that there is still much to explore, both in terms of production process techniques and emerging new applications of citric acid.
1. Introduction
Citric acid (CA), also known as 2-hydroxypropane-1,2,3-tricarboxylic acid, is found in plant and animal tissues such as blood, bone, and muscle. For living organisms, citric acid is one of the essential carboxylic acids in the Krebs cycle, a series of reactions that oxidize glucose into carbon dioxide and water, releasing energy. Due to its harmless nature and chelating and sequestering properties for metal ions, citric acid has applications in the food, pharmaceutical, chemical, and even metallurgical industries [1,2]. The annual global production of citric acid currently reaches approximately 2.8 million tons, and the citric acid market is one of the fastest-growing segments in the food additive industry [3]. The continuous growth in citric acid production is attributed to its wide-ranging applications, not only in the food and pharmaceutical industries but also in biopolymer production, environmental protection, and biomedicine [4,5].
In industrial citric acid production, the dominant method is submerged fermentation involving strains of Aspergillus niger, yeast Yarrowia lipolytica, and some bacterial strains [6]. Aspergillus niger is considered the best among microorganisms in the commercial synthesis of citric acid due to its high production efficiency [7,8]. The development of citric acid production has significantly increased since the last century, thanks to biotechnology, which provides knowledge about fermentation techniques and product recovery; biochemistry, which provides insights into various factors influencing citric acid synthesis and inhibition; and molecular regulatory mechanisms and strategies to enhance citric acid production efficiency [1,9].
In this review, an attempt has been made to gather and update data on the biosynthesis of citric acid by the filamentous fungus Aspergillus niger while also highlighting the differences between it and the yeast Yarrowia lipolytica. The review addresses the progress in citric acid bioproduction, optimal fermentation strategies, and the utilization of conventional and unconventional carbon sources. Additionally, it discusses the prospects and future trends of the global citric acid market.
2. Physical and Chemical Properties
Citric acid [77–92–2], according to IUPAC nomenclature (International Union of Pure and Applied Chemistry), is also known as 2-hydroxypropane-1,2,3-tricarboxylic acid. Citric acid is a polyprotic α-hydroxy acid but can also be classified as a β-hydroxy acid (Figure 1) [8,10]. It is present in plants, animal cells, and physiological fluids. In small quantities, citric acid is found in citrus fruits, especially lemons and limes. In amounts exceeding 1% of the dry weight of the product, it is present in lemons (4–8%), blackberries (1.5–3.0%), grapefruits (1.2–2.1%), as well as oranges, raspberries, and strawberries in the range of 0.6–1.3% [11,12,13].
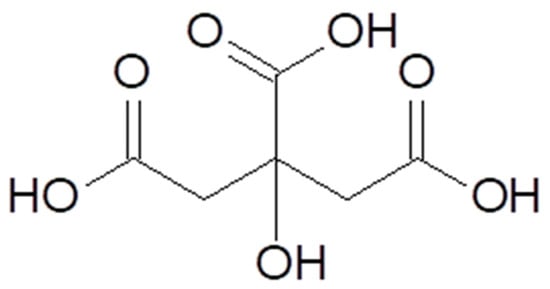
Figure 1.
Chemical structure of citric acid.
Citric acid is an organic compound, a tricarboxylic hydroxy acid, with three carboxylic functional groups. It is a triprotic compound that undergoes three constant dissociations, which allows it to form three types of salts and exhibit buffering properties. The chemical and physical properties of citric acid are presented in Table 1 [14,15]. Citric acid forms crystalline mono-, di-, and tri-basic salts with various cations. From a technological perspective, the most important are calcium citrate, potassium citrate, and sodium citrate [16].
Citric acid is a weak acid in two crystalline forms: Anhydrous citric acid (C6H8O7) and monohydrated citric acid (C6H8O7·H2O). Anhydrous citric acid crystallizes from a hot concentrated solution above 36.6 °C, forming a white crystalline powder. On the other hand, monohydrated citric acid crystallizes from a cold solution at temperatures below 36.6 °C, forming colorless, transparent crystals [16,17]. Anhydrous citric acid absorbs a small amount of water at 25 °C and relative humidity in the 25 to 50% range. If the humidity is between 50% and 75%, it absorbs water significantly, while approaching 75% relative humidity takes the form of a monohydrate. The anhydrous form of citric acid is obtained when the relative humidity is less than 40%. Monohydrated citric acid slightly absorbs moisture at a relative humidity of 65–75% [17].
Citric acid is highly soluble in water and organic solvents such as ethanol, 2-propanol, ether, ethyl acetate, 1.4-dioxane, tetrahydrofuran, acetonitrile, and ethanol-water mixtures [18]. It has a higher solubility in alcohol than in water. Adding alcohol to an aqueous solution significantly increases the solubility of citric acid [19,20]. The solubility of citric acid in different solvents can be ranked as follows: Tetrahydrofuran < 1.4-dioxane < water < 2-propanol < ethanol < acetonitrile [21]. Citric acid does not dissolve in chloroform, toluene, benzene, carbon disulfide, or tetrachloride [17]. Its solubility increases with an increasing temperature of 20.55–60.05 °C [19,20,21].
When heated to 150 °C, citric acid remains stable, losing only its crystalline water. Above 175 °C, it undergoes a melting and decomposition process. Dehydration of citric acid leads to the formation of trans-aconitic acid. It is assumed that further thermal transformations of trans-aconitic acid due to dehydration result in the production of aconitic anhydride or a mixture of both isomers [15,22].
Citric acid can chelate metal ions by forming bonds between the metal, carboxyl, and hydroxyl groups of the citric acid molecule. Citric acid and its salts form complexes with copper, nickel, iron, magnesium, zinc, and tin. This valuable property helps prevent changes in chemical potential, precipitation of solids, or changes in chemical properties [15,23].
Citric acid esterifies with alcohols under typical conditions in the presence of catalysts such as sulfuric acid, p-toluenesulfonic acid, or ion-exchange resin. The esterification reaction of citric acid with alcohols, occurring at temperatures above 150 °C, does not require the presence of a catalyst. Citric acid forms polyesters with polyalcohols such as sorbitol and mannitol. Interrupting the esterification reaction before completion results in the formation of free carboxylic groups, forming salts [15].

Table 1.
Chemical and physical properties of citric acid.
Table 1.
Chemical and physical properties of citric acid.
| Properties | Characteristic | References |
|---|---|---|
| Molar mass | Anhydrous: 192.12 g∙mol−1 Monohydrate: 210.14 g∙mol−1 | [11] |
| Appearance and form | powdery, colorless transparent crystals or white, granular, fine powder Anhydrous: monoclinic holohedral crystals. Monohydrate: rhombic crystals | [14] |
| Melting point | Anhydrous: 153° C Monohydrate: ≈100 °C | [8] |
| Boiling point | None, decomposition into water and CO2 > 175 °C | [24] |
| Vapor pressure | 1.7 × 10−8 mmHg at 25 °C | [18] |
| Density | Anhydrous: 1.665 g∙cm−3 at 20 °C Monohydrate: 1.542 g∙cm−3 at 20 °C | [14] |
| Octanol/water partition coefficient | logPOW = −1.72 ± 0.4 at 20 °C | [10] |
| Partition coefficient | logP = −1.198 ± 0.4 w 25 °C | [14] |
| dissociation constant | pK1 = 3.128 pK2 = 4.761 at 25 °C pK3 = 6.396 | [14] |
| Henry’s constant | KH = 2.3 ×·10−7 Pam3∙mol−1 | [25] |
| Solubility |
| [14] |
3. Citric Acid Biosynthesis
3.1. The Beginning of Citric Acid Production
For the first time, citric acid was isolated from lemon juice in 1784 in England by Carl Scheele, who obtained calcium citrate by adding lime to lemon juice [26]. In 1838, Liebig confirmed the presence of one hydroxyl group and three carboxyl groups in the structure of citric acid. Since 1860, citric acid production from lemons has been carried out in the United Kingdom, France, and Germany. Intensive research was conducted to find an alternative method for obtaining citric acid [27]. In 1893, the German botanist Wehemer observed that citric acid is formed as a byproduct during the production of calcium oxalates by Penicillium glaucum [26]. Industrial-scale production of citric acid involving microorganisms was initiated in 1917 by Currie, who developed a method for obtaining it from filamentous fungi Sterigmatocystis nigra (currently Aspergillus niger) using culture media containing sucrose [28,29]. The significant milestones in the discovery and research of citric acid are shown in Figure 2.
The biochemical foundations of the biosynthesis process of citric acid were elucidated in the 1950s with the discovery of glycolysis and the tricarboxylic acid cycle [30].
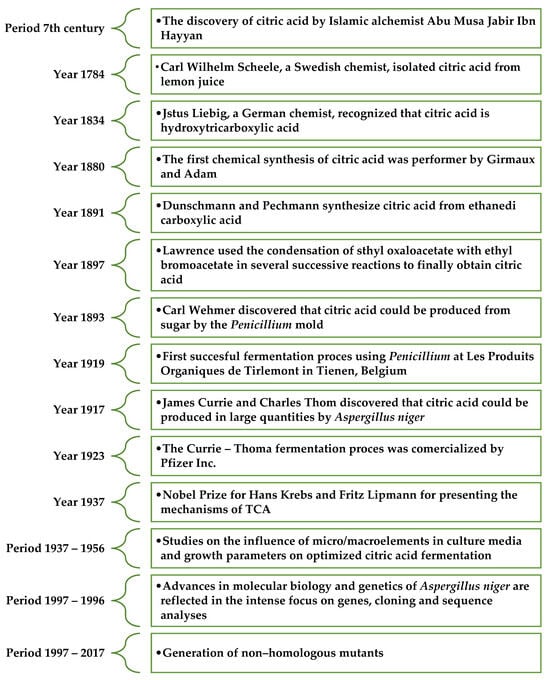
Figure 2.
Major milestones in the discovery and research of citric acid [28,31].
3.2. Microorganisms Producing Citric Acid
Since discovering the potential for microorganisms to produce citric acid, Aspergillus niger strains have remained the preferred microorganisms in the production process [26,29,32,33,34,35]. In addition to filamentous fungi such as Aspergillus niger, various other microorganisms have been used to produce citric acid. These include Aspergillus nidulans, Aspergillus aculeatus, Aspergillus fumaricus, Aspergillus carbonarius, Aspergillus awamori, Aspergillus wentii, Aspergillus saitoi, Aspergillus flavus, Aspergillus foetidus, Aspergillus fonsecaeus, Aspergillus luchensis, Aspergillus phoenicis, Aspergillus saitoi, Aspergillus usumii, Penicillium janthinellum, Penicillium restrictum, Trichoderma viride, Mucor piriformis, Talaromyces sp., Eupenicillium sp., Botrytis sp., Absidia sp., and Ustulina vulgaris. Additionally, promising producers of citric acid include yeasts such as Yarrowia lipolytica, Candida tropicalis, Candida guilliermondii, Candida intermedia, Candida parapsilosis, Candida zeylanoides, Candida fibriae, Candida subtropicalix, Candida oleophila, and bacteria such as Arthrobacter paraffineus, Bacillus licheniformis, and Corynebacterium spp. These microorganisms are all potential sources for citric acid production [36,37,38,39].
3.2.1. Production of Citric Acid Using Aspergillus niger Fungi
In industrial citric acid production, filamentous fungi, mainly Aspergillus niger, are used (Figure 3) [40,41]. These microorganisms offer several advantages, including their ability to quickly adapt and grow on various substrates, regulate and control metabolic pathways, and regulate the secretion of citric acid from both mitochondria and cytosol. This contributes to citric acid accumulation and prevents its degradation in the Krebs cycle. Moreover, cultures using Aspergillus niger are characterized by high production efficiency and homofermentative citric acid biosynthesis [32,42]. Aspergillus niger strains have been recognized as safe, as they do not produce ochratoxin under controlled cultivation conditions and do not elicit strong allergic reactions in humans. In addition to citric acid and other organic acid biosynthesis, Aspergillus niger is also utilized to produce enzymes such as pectinases, proteases, aminoglycosidases, catalases, lipases, and oxidases [43].
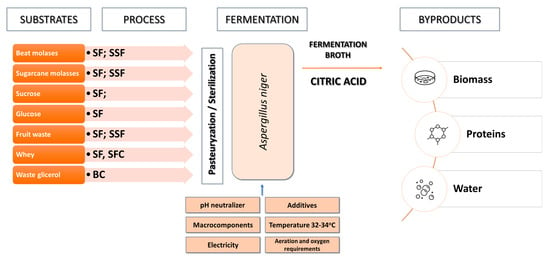
Figure 3.
Citric acid production processes with A. niger.
Until 1980, Aspergillus niger strains used in industrial production were obtained through screening and mutagenesis. Mutagenesis techniques are still in use and continue to yield positive results in improving biosynthesis efficiency. The most commonly used mutagens are physical factors (gamma and UV radiation), chemical factors, and hybrid methods that combine physical and chemical characteristics [44]. The development of genetic engineering has allowed the application of DNA recombination technologies to improve strains, with Aspergillus niger being used as a host for the expression of heterologous proteins. The improvement of Aspergillus niger strains is limited by the relatively small number of available plasmid vectors for these filamentous fungi. Recently, integration and autonomous replication of plasmid vectors have been used to manipulate the genome with targeted gene exchange [1,2,45,46].
In order to enhance the performance of species such as Aspergillus niger, research has been conducted on the application of genome editing technology in filamentous fungi, employing CRISPR (Clustered Regularly Interspaced Palindromic Repeats) elements with associated endonucleases (such as Cas protein family members). The CRISPR/Cas9 system facilitates chromosome engineering in Aspergillus niger, enabling genome manipulations with high efficiency and scalability, thereby increasing the implementation speed of metabolic engineering cycles [46,47]. In experiments involving the production of natural metabolites, genome editing disrupted pyrG, which encodes orotidine-5′-decarboxylase, resulting in a 2.17-fold increase in citric acid production compared to the control, suggesting that inhibiting uridine/pyrimidine synthesis may promote citric acid overproduction [48].
3.2.2. Citric Acid Metabolism in Aspergillus niger
The biochemical mechanism through which Aspergillus niger accumulates citric acid has been the subject of scientific interest since the 1930s, when cultivation conditions were established and the impact of various substrate components was assessed for industrial production needs. Despite numerous models proposed since 1930 to explain Aspergillus niger’s ability to accumulate citric acid, many aspects of the biochemical transformations remain unexplained. Research aimed at understanding the metabolic pathways and properties of enzymes in Aspergillus niger has made it the best-studied filamentous fungus [49].
The ability of certain strains of Aspergillus niger to biosynthesize citric acid is determined genotypically while providing appropriate cultivation conditions, and their control allows for achieving high process yields [50].
In the trophophase, during the growth of Aspergillus niger mycelium, hexoses or other carbohydrates are taken up through glycolysis and the pentose phosphate pathway (Figure 4). The involvement of the pentose phosphate pathway in carbohydrate metabolism is shallow and significantly decreases during citric acid production—the idiophase [51].
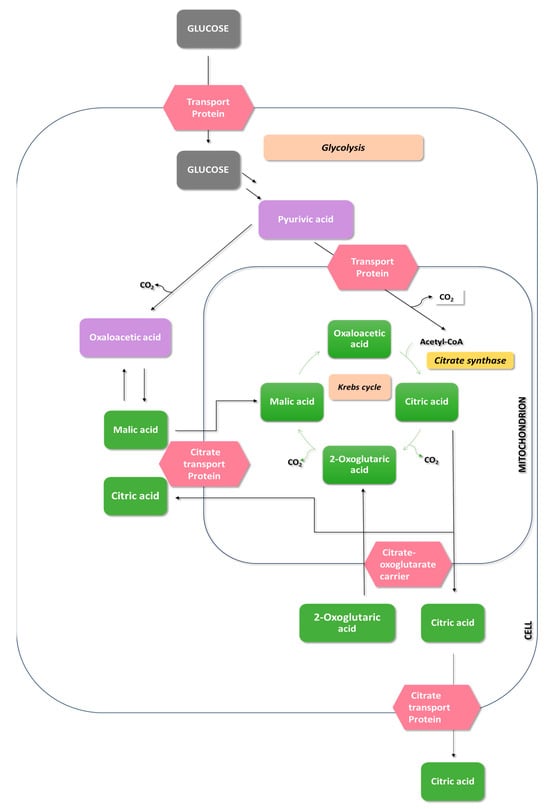
Figure 4.
Overview of pathways leading to citric acid production in A. niger.
Glucose, upon entering the cell, undergoes phosphorylation, converting into glucose-6-phosphate. This step is catalyzed by hexokinase and glucokinase. Hexokinase is an enzyme that catalyzes the transfer of phosphate groups from ATP to glucose and fructose. On the other hand, glucokinase exhibits high affinity only for glucose [52]. The activity of hexokinase is strongly inhibited by trehalose-6-phosphate. To increase the efficiency of citric acid biosynthesis, the gene tpsA, responsible for the expression of trehalose-6-phosphate synthase, was blocked [53,54].
The next step in glycolysis is the isomerization of glucose-6-phosphate to fructose-6-phosphate. This reaction is catalyzed by phosphoglucose isomerase. Following the isomerization reaction, a phosphorylation reaction catalyzed by phosphofructokinase, a key enzyme in the glycolytic pathway, occurs. As a result of this reaction, fructose-6-phosphate is transformed into fructose-1,6-bisphosphate [55]. Phosphofructokinase activity is inhibited by a high concentration of ATP, leading to a decrease in its affinity for fructose-6-phosphate. This means that the activity of phosphofructokinase increases when the energy charge decreases [56]. Inhibitors of phosphofructokinase also include a high concentration of manganese, citrate, and phosphoenolpyruvate. Stimulating effects on phosphofructokinase activity are exerted by NH4+, Zn2+, Mg2+, and adenosine monophosphate (AMP) [49,57].
The first reaction in the third stage of glycolysis is the conversion of 3-phosphoglyceraldehyde to 1,3-bisphosphoglycerate. This process is catalyzed by 3-phosphoglyceraldehyde dehydrogenase and involves the oxidation of the aldehyde with the participation of NAD+ to form a carboxylic acid and the production of 1,3-bisphosphoglycerate. In the next stage of glycolysis, phosphoglycerate kinase catalyzes the transfer of a phosphate group from 1,3-bisphosphoglycerate, forming ATP and 3-phosphoglycerate. The final stage of glycolysis involves the conversion of 3-phosphoglycerate to pyruvate, accompanied by the production of ATP. In this stage, the critical enzyme is pyruvate kinase, which catalyzes the irreversible transfer of a phosphate group to ATP [58,59].
In the glycolytic pathway, glucose is converted into two molecules of pyruvate. Pyruvate transforms precursors such as citrate, oxaloacetate, and acetyl-CoA. Oxaloacetate is formed through the carboxylation of pyruvate, catalyzed by pyruvate carboxylase. During this reaction, Aspergillus niger consumes CO2 generated during acetyl-CoA formation [50]. Pyruvate is then transported to the mitochondrion, where it undergoes oxidative decarboxylation to form acetyl-CoA in an irreversible reaction catalyzed by pyruvate dehydrogenase. The reaction of acetyl-CoA formation from pyruvate serves as a bridge between the glycolytic pathway and the citric acid cycle. The citric acid cycle occurs in the mitochondrial matrix and begins with oxaloacetate, acetyl-CoA, and H2O condensation to form citrate and CoA. The enzyme catalyzing this reaction is citrate synthase. Subsequently, citrates undergo isomerization to isocitrate through reactions catalyzed by aconitase [13,60,61].
In the past, it was speculated that the accumulation of citric acid in the citric acid cycle occurs due to the inhibition of aconitase, isocitrate dehydrogenase-NADP, and α-ketoglutarate dehydrogenase by external factors (metal ions, pH, Cu2+) or internal factors (glycerol, citrates) [49,52].
The accumulation of citric acid is associated with the activity of tricarboxylate transporters that compete with aconitase for citrates. The affinity of the tricarboxylate transporter for citrate is significantly higher than that of aconitase. Consequently, citrates are released from the mitochondrion without inhibiting the Krebs cycle. The transport of citrates by tricarboxylate carriers operates through exchange with cytosolic malate. Hence, malate can be considered a potential trigger for the accumulation of citric acid, as an increase in its concentration precedes the accumulation of citrates [49].
3.2.3. Production of Citric Acid Using Yarrowia lipolytica Yeast
Yeast, especially Yarrowia lipolytica and Candida strains, have been used in citric acid production since the 1960s [62]. Initially, n-alkanes were used as carbon sources in cultures, but over time, other substrates such as glucose, acetates, molasses, glycerol, inulin, oils, and fatty acids were introduced (Table 2; Figure 5) [63,64,65].
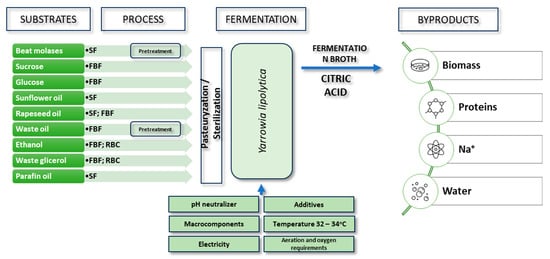
Figure 5.
Citric acid production processes with Y. lipolytica.

Table 2.
Substrates used in citric acid production by Yarrowia lipolytica strains.
Table 2.
Substrates used in citric acid production by Yarrowia lipolytica strains.
| Substrate | Strain | Citric Acid | Cultivation Method | References |
|---|---|---|---|---|
| Glucose | Yarrowia lipolytica | 121–129 g∙dm−3 | SF | [66] |
| Yarrowia lipolytica | 49 g∙dm−3 | SF | [67] | |
| Yarrowia lipolytica VKM Y-2373 | 80–85 g∙dm−3 | FBF | [68] | |
| Yarrowia lipolytica NRRL Y-1094 | 30.31 g∙dm−3 | SF | [69] | |
| Pure glycerol | Yarrowia lipolytica NG40/UV7 | 115 g∙dm−3 | SF | [70] |
| Waste glycerol | Yarrowia lipolytica NG40/UV7 | 112 g∙dm−3 | SF | [70] |
| Yarrowia lipolytica SKY7 | 18.70 g∙dm−3 | FBF | [38] | |
| Yarrowia lipolytica A101 | 75.9 g∙dm−3 | SF | [71] | |
| Inulin | Yarrowia lipolytica SWJ-1b | 85 g∙dm−3 | SF | [72] |
| Yarrowia lipolytica AWG7 INU 8 | 200 g∙dm−3 | RBF | [73] | |
| Waste cooking oil | Yarrowia lipolytica SWJ-1b | 31 g∙dm−3 | SF | [74] |
The main advantages of Yarrowia lipolytica strains compared to filamentous fungi include better tolerance to high carbon source concentrations, lower sensitivity to heavy metal ions, and lower oxygen levels in the growth medium. This allows for the utilization of a wide range of substrates. Moreover, citric acid biosynthesis using Yarrowia lipolytica yeast is characterized by higher efficiency, faster production, and easier control [40,75,76,77].
The accumulation of citric acid in yeast requires a deficiency in a nitrogen source because its production initiates after the depletion of available nitrogen. Citrate synthase, the enzyme converting oxaloacetate and acetyl-CoA into citric acid, is modulated by the provision of ammonium ions in the medium. Therefore, ensuring a high C/N ratio is crucial so that the excess carbon is redirected towards citric acid production in the stationary growth phase (Figure 6) [78,79].
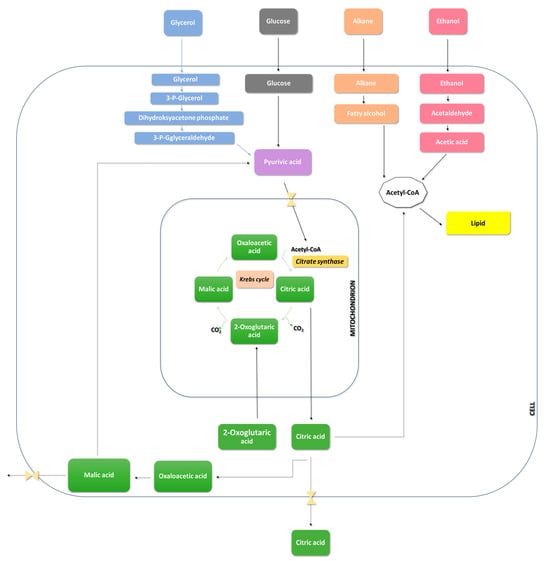
Figure 6.
Metabolic pathways of Y. lipolytica.
Yeast also offers the advantage of the ease of genetic modifications using molecular techniques and eliminates the need for prior substrate processing [42]. Tan et al. expressed the pyruvate carboxylase gene cloned from Meyerozyma guilliermondii in Yarrowia lipolytica SWJ-1b to enhance citric acid production. The research resulted in both increased pyruvate carboxylase activity and citric acid production by the obtained recombinant Yarrowia lipolytica [63]. On the other hand, Liu et al. increased the expression of the ICL1 gene and reduced the ACL1 gene of the ATP citrate lyase to enhance citric acid production by Yarrowia lipolytica. The result of these studies was citric acid production reaching 84.0 g∙dm−3 within 214 h [80].
A significant challenge in citric acid production with yeast is the concurrent secretion of isocitric acid, which is undesirable and interferes with crystallization [80]. The amount of accumulated citric acid depends on the yeast strain and carbon source used. In culture media containing vegetable oils or n-alkanes as the carbon source, the proportion of isocitric acid is around 35–45%, while in glycerol-based media, it is about 10–12% [81]. To reduce the presence of isocitric acid in the culture medium, strains have been improved using genetic engineering methods, such as inducing overexpression of isocitrate lyase, resulting in a significant reduction in isocitrate levels, or increasing the activity of pyruvate carboxylase [63,75].
The issue of citric acid production by yeast has been extensively described in the work by Börekçi et al., “Citric Acid Production of Yeasts: An Overview” [69].
4. Production of Citric Acid
4.1. Cultivation Methods and Conditions
Currently, over 90% of the world’s citric acid production is manufactured using three methods: Submerged fermentation (SF), liquid surface fermentation (LSF), and solid-state fermentation (SSF) [37]. The advantages and disadvantages of different cultivation methods used in citric acid biosynthesis are presented in Table 3.

Table 3.
Advantages and disadvantages of different cultivation methods used in the biosynthesis of citric acid by Aspergillus niger.
Table 3.
Advantages and disadvantages of different cultivation methods used in the biosynthesis of citric acid by Aspergillus niger.
| Type of Cultivation | Process Parameters | Process Advantages | Process Disadvantages | References |
|---|---|---|---|---|
| Surface cultivation in liquid substrates (LSF) | Process Duration: 8–12 days Process yield: 70–75% | Ease of operation Energy-efficient Technically simple | Long duration Sensitive to contamination by other microorganisms Requires large production areas Generates large amounts of heat Production on a small and medium industrial scale | [29,32,82] |
| Surface cultivation in solid substrates (SSF) | Process Duration: 4 days | Technically and technologically simple Low substrate cost Energy-efficient Low risk of contamination Low waste generation Low sensitivity to heavy metal pollution | Difficulties in controlling process parameters (pH, humidity, temperature) High product contamination High cost of product acquisition | [36,83,84,85] |
| Submerged fermentation cultures (SF) | Process Duration: 4 days | Ability to control process parameters High process efficiency Low production costs Ease of maintaining sterile conditions | Sensitivity to the inhibitory effects of trace elements A large amount of waste is generated 80% of the world’s citric acid production | [29,50,82] |
4.1.1. Liquid Surface Fermentation Cultures
This method is still used on a small and medium industrial scale due to its simple technology and low production costs [26]. In surface fermentation cultures, Aspergillus niger fungi grow on the surface of the growth medium and form a thick mycelial layer. This process occurs in fermentation chambers on high-quality steel, aluminum, or polyethylene trays (Figure 7). The fermentation chambers are equipped with an aeration system that controls temperature and humidity levels. The air supplied to the fermentation chambers is filtered using bacteriological filters to prevent contamination by Penicillium, other strains of Aspergillus niger, or lactic acid bacteria [4,86].
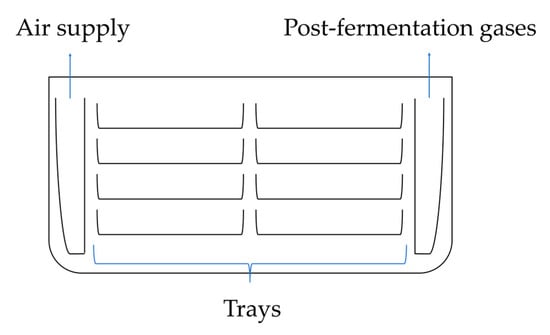
Figure 7.
Schematic of liquid surface fermentation cultures.
Surface cultivation generates significant heat, requiring a high aeration rate to maintain the proper temperature. This process generates significant heat during fermentation, which is controlled by proper aeration. The chamber requires adequate ventilation, and fermentation chambers have ensured efficient air circulation passing over the substrate’s surface through a bacteriological filter to control humidity and temperature through cooling. Carbon dioxide produced during the fermentation process inhibits the production of citric acid at concentrations higher than 10% [4,5].
4.1.2. Solid-State Fermentation Cultures
This cultivation method involves the growth of microorganisms on solid substrates. Initially, the appropriate moisture is provided in the form of humidity in the raw material, and additional moisture is supplied by the air during the process. Solid-state cultivation is considered a reaction in a heterogeneous system with simultaneous multicomponent mass and heat transport [9,83].
On a laboratory scale, SSF devices consist of media such as petri dishes or flasks in which screening tests can be performed. On an industrial scale, various types of bioreactors are used, which differ mainly in the presence or absence of mixing and forced aeration. The simplest type is a shelf bioreactor, in which solid material is placed on trays made of metal or plastic. The trays are placed in a chamber where circulating air regulates temperature and humidity. The second type of culture can take place in packed-bed column bioreactors. The third type is stirred drum bioreactors, which are used in SSFs requiring slow, continuous mixing and no forced aeration (Figure 8) [87,88].
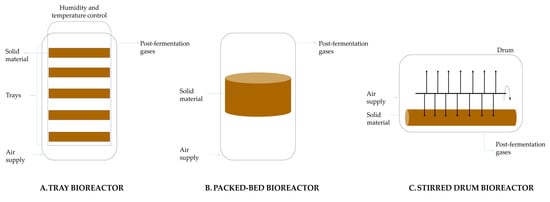
Figure 8.
Schematic of solid-state fermentation cultures.
This method can use waste from agriculture and industry, such as fruit and vegetable processing waste, as substrates. Drum, column, and rotary bioreactors are used for citric acid production on solid-state substrates [89].
One clear advantage of this method is its low energy consumption and minimal waste generation, which is environmentally friendly. Additionally, the process takes about four days under optimal conditions, significantly shorter than submerged and liquid surface fermentation cultures [32,90,91].
Solid-state cultures of Aspergillus niger have gained importance in recent years. Still, due to the low level of process automation and a need for improvements in bioreactor design, they are only marginally used in industrial citric acid production [92].
4.1.3. Submerged Fermentation
Around 80% of the world’s citric acid production is achieved using submerged fermentation. Citric acid production through batch culture is carried out in tank bioreactors of high-quality corrosion-resistant steel equipped with aeration and mixing systems (Figure 9). The most commonly used carbon source for citric acid production is sucrose, as well as by-products of its production, such as molasses [13,32].
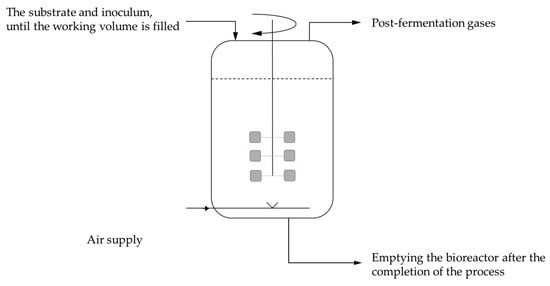
Figure 9.
Schematic of submerged fermentation.
The advantages of submerged culture over surface culture include lower costs, low contamination risk, a high level of automation, and higher process yield. To achieve high citric acid production yields in submerged cultures, control of process parameters and careful substrate selection are crucial [93].
The periodic batch culture is the most frequently used method in industrial citric acid production. Other methods include fed-batch and semicontinuous cultures [94]. In fed-batch fermentation, sterilized nutrients are added to the fermenter during biomass growth (Figure 10). In continuous fermentation, sterilized liquid nutrients are introduced into the fermenter at the same flow rate as the fermenting wort leaving the system. Parameters such as temperature, pH, oxygen consumption, and carbon dioxide production are measured and controlled to optimize the fermentation process [95].
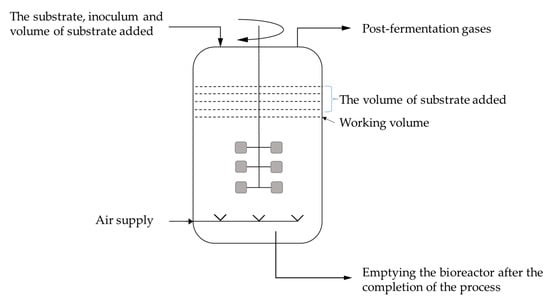
Figure 10.
Schematic of fed-batch fermentation.
4.2. Factors Influencing Citric Acid Production
The course of Aspergillus niger cultivation and the rate of citric acid biosynthesis in submerged culture are influenced by many factors, including the type of carbon source and its concentration, the type and concentration of metal ions present in the culture media, low molecular weight alcohols, fungal morphology, as well as temperature, pH, aeration rate, and mixing rate. A brief summary of the impact of factors on citric acid biosynthesis in different cultivation methods is presented in Figure 11. The impact of factors that stimulate the citric acid biosynthesis process has been widely researched.
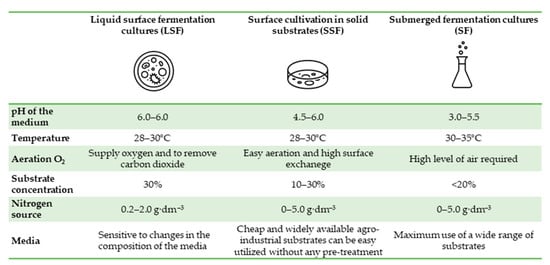
Figure 11.
Summarizing the impact of factors that stimulate the citric acid biosynthesis process.
4.2.1. Nitrogen
The concentration and source of nitrogen have a fundamental impact on the growth of Aspergillus niger and the biosynthesis of citric acid in both submerged and solid-state cultures. The most preferred nitrogen sources are nitrogen salts, including ammonium nitrate, ammonium sulfate, and ammonium chloride. Among other nitrogen sources, urea, peptone, and yeast extract can be distinguished [4]. According to the literature, ammonium nitrate is considered the most favorable nitrogen source [96,97,98,99,100].
Ammonium compounds lead to an advantageous reduction in the pH of the culture medium to a level lower than 2, which is a necessary condition for citric acid production. Limiting the nitrogen source during cultivation inhibits fungal biomass growth and increases citric acid production [50]. The optimal concentration of the nitrogen source should be around 0.2%, as it promotes the biosynthesis of citric acid by Aspergillus niger. The highest citric acid biosynthesis efficiency is achieved when the intracellular concentration of ammonium ions is 2–3 mM∙g−1. However, process efficiency decreases when the intracellular nitrogen ion concentration is 1 mM∙g−1 of biomass [97,98,99]. Culture media are supplied during cultivation to increase the volumetric citric acid biosynthesis rate. In addition to the proper amount of added nitrogen source, the timing of the addition and supplying it at the wrong fermentation phase can reduce the citric acid accumulation rate [26].
In the case of yeast, citric acid biosynthesis begins after the nitrogen source is depleted. Limiting the nitrogen concentration at a high substrate concentration for yeast is crucial because citric acid is released through a specific, energy-dependent transport system induced by intracellular nitrogen restriction [62].
4.2.2. Phosphorus
The presence of phosphorus in the culture medium also influences the efficiency of citric acid biosynthesis. The best sources of phosphorus are KH2PO4 and K2HPO4. Limiting phosphorus concentration in the culture medium, similar to nitrogen, critically impacts citric acid production. Increasing the process efficiency is allowed by a phosphorus concentration in the range of 0.006 to 0.32 g∙dm−3. Fungal mycelium requires the presence of phosphorus at concentrations ranging from 0.01 to 0.02% for proper growth [90,101].
High phosphorus concentrations stimulate mycelial growth, induce secondary enzymatic reactions, and inhibit citric acid production [98].
4.2.3. Trace Elements
Trace elements are a crucial factor affecting the efficiency of citric acid biosynthesis. Manganese, zinc, copper, and iron are of great importance [101]. To achieve high process efficiency, especially in submerged cultures, it is necessary to use culture media with controlled trace element content. This is due to their significant influence on the growth and physiology of Aspergillus niger and the efficiency of citric acid biosynthesis [50].
Magnesium is essential for the proper growth of Aspergillus niger and citric acid production due to its role as a cofactor in enzymatic reactions. Maximum citric acid biosynthesis efficiency is achieved at magnesium sulfate concentrations ranging from 0.020 to 0.025% [29,90].
The addition of manganese to the culture medium plays a significant role in the accumulation of citric acid, cell wall synthesis, sporulation, and the production of secondary metabolites [50]. At a concentration of 10 mg∙dm−3, manganese limits the efficiency of citric acid biosynthesis. However, at concentrations lower than 3 µg∙dm−3, it significantly reduces the process efficiency. Manganese deficiencies contribute to reduced lipid synthesis and increased cell membrane permeability due to decreased concentrations of certain enzymes involved in anabolic processes [102,103,104].
In citric acid production, limiting the presence of iron in the culture medium is crucial. Approximately 1 mg of iron per liter of culture medium is needed to achieve high process efficiency. Higher iron concentrations can lead to the accumulation of oxalic acid [90].
The presence of copper ions reduces the harmful effect of excess iron ions. Furthermore, copper is known to inhibit manganese ions. The optimal concentration of CuSO4∙7H2O in the culture medium should be 78 mg∙dm−3 [101]. The presence of copper at various concentrations affects the morphology of Aspergillus niger mycelium. Therefore, its presence determines the attainment of the appropriate mycelial structure, allowing for high efficiency in citric acid biosynthesis [50,105].
4.2.4. Low-Molecular-Weight Alcohols
Potential stimulators of citric acid fermentation include low-molecular-weight alcohols, such as methanol, ethanol, and n-propyl alcohol [106,107]. Ethanol added to culture media inhibits mycelial growth and sporulation, reduces substrate consumption, increases cell membrane permeability, and influences an increase in citrate synthase activity and a reduction in aconitase activity [108,109,110].
Methyl alcohol, unlike ethanol, is not assimilated by Aspergillus niger and does not undergo conversion to acetyl-CoA, which is a precursor of the Krebs cycle. The mechanism of methanol’s interaction with citric acid biosynthesis in synthetic and natural media has not been fully elucidated. Methyl alcohol in high-purity media disrupts metabolic processes and biomass growth, reducing citric acid production. It also leads to disturbances in the synthesis of cellular proteins in the early stages of cultivation [111,112]. Methyl alcohol affects the permeability of cell membranes, which may be due to changes in the composition of phospholipids and triglycerides [113]. Phospholipids play a significant role in regulating membrane permeability for citric acid. Methyl alcohol may disrupt the formation of mycelial structure through chelation effects on metal ions such as copper (II), which play a significant role in regulating the content of fatty acids in glycolipids and phospholipids [114].
In the study by Maddox et al. [115], methanol in synthetic media containing galactose as a carbon source had a toxic effect by limiting fungal growth and reducing substrate consumption. However, it simultaneously increased the efficiency of citric acid production. Furthermore, methyl alcohol inhibited the activity of 2-oxoglutarate dehydrogenase, resulting in increased citric acid accumulation. Similarly, Yaykaşlı et al. [116] demonstrated that methanol in the biosynthesis process using immobilized conidia of Aspergillus niger in media with sucrose contributed to an increase in citric acid production.
Most of the available literature data report a positive impact of lower concentrations of methyl alcohol on the efficiency of citric acid production by Aspergillus niger in natural media characterized by lower purity, such as molasses-based media [106,111,113,117,118,119,120,121]. The stimulating effect of methyl alcohol added to natural media results from its limitation of the negative impact on the biosynthesis process of metal ions such as manganese, iron, and zinc, which strains of Aspergillus niger are highly sensitive to. Methyl alcohol, on the other hand, increases the tolerance of Aspergillus niger to the content of iron, manganese, and zinc ions present in the media [117]. Methyl alcohol induces changes in the normal carbohydrate metabolism pathway by increasing glycolytic capabilities, leading to citric acid accumulation. In natural media, it stimulates citric acid production by affecting fungal growth and altering the composition of the cell wall lipids [121].
4.2.5. The pH Value
The pH value plays a significant role during spore germination, where the pH should be higher than 5, and during citric acid production, when a low substrate pH is required (pH ≤ 2). Most fungal mycelia grow in the pH range of 3 to 6, and their metabolic activity mainly depends on the culture medium’s pH [122,123,124].
During the production of citric acid by Aspergillus niger in submerged and surface cultures, a pH range of 2 to 6 is utilized. The low pH level reduces the risk of culture contamination by other microorganisms. Furthermore, a pH below 3 prevents the production of oxalic and gluconic acids [124]. Conversely, increasing the pH to 4.5 can lead to a significant reduction in citric acid production efficiency by up to 80% [50,125].
The pH of the culture medium can also affect the morphology of Aspergillus niger mycelia. At pH values of 2.0 to 2.2, the mycelia assume the desired form of small aggregates and short hyphae, which is most desirable for citric acid production. At a pH of 1.6, the morphological development of mycelia is disrupted, and process efficiency decreases significantly. In a medium with a pH of 3.0, mycelia form larger aggregates, favoring oxalic acid biosynthesis [50].
4.2.6. Aeration and Mixing Rate
The appropriate dissolved oxygen concentration in the cultivation medium is a critical factor influencing the efficiency of citric acid biosynthesis [26,113]. The aeration rate significantly affects citric acid biosynthesis, especially in submerged cultivation methods. In submerged cultivations, the process efficiency increases with higher aeration rates and pressures (0.10–0.17 MPa) [90]. In cases of insufficient oxygen in the medium or interruptions in oxygen supply, citric acid production may be inhibited, and fungal growth may be affected [29,126,127]. The proper concentration of dissolved oxygen in the cultivation medium influences the rate of glycolysis and the respiratory chain, leading to high ATP levels and increased citrate secretion [57].
In industrial production, the aeration rate is adjusted according to the current fungal demand. Initially, the aeration rate is around 0.1 vvm, and during the phase of intensive fungal growth, it is increased to approximately 0.5 vvm [26]. To enhance the efficiency of citric acid biosynthesis in submerged cultivations, aeration rates ranging from 0.9 to 1.3 vvm have been applied [39]. Inappropriate aeration rates can have an adverse impact on the efficiency of the bioprocess [50].
Proper aeration, combined with high dissolved oxygen levels, contributes to the reduced activity of cytochrome-dependent enzymes and the increased activity of alternative oxidase, consequently favoring the alternative respiratory pathway. This process conserves energy by bypassing the need to generate ATP. As a result, citric acid fermentation requires continuous cooling because the free electron transport generates heat [49].
An essential parameter associated with aeration is the mixing rate, which affects fungal dispersion, dissolved oxygen concentration in the medium, and even enzyme activity, such as citrate synthase, aconitase, and isocitrate dehydrogenase. The activity of citrate synthase decreases with increasing mixing rates, while the activity of aconitase and isocitrate dehydrogenase increases with higher mixing rates. The optimal mixing rate in laboratory bioreactors is 300 to 1000 rpm [57].
Intense mixing also leads to the development of fungal mycelia in short, branched hyphae characterized by high citric acid overproduction. It also contributes to mycelial fragmentation and regrowth. This phenomenon can be beneficial because metabolically inactive and highly vacuolated mycelial fragments are the most susceptible to fragmentation, and this process generates new Aspergillus niger mycelial fibers [105].
4.2.7. Temperature
Temperature is another parameter that affects enzymatic activity, microbial transport systems, and consequently, the efficiency of citric acid biosynthesis. In the production of citric acid, the optimal temperature falls within the range of 28 °C to 30 °C. Numerous studies have shown that the highest process efficiency was achieved at a temperature of 30 °C [122,128,129,130,131]. Temperatures above 30 °C lead to the denaturation of citrate synthase, limiting citric acid accumulation and biomass growth in the medium while promoting oxalic acid production. Cultures at lower temperatures decrease enzymatic activity [122,131].
4.3. Substrate
The search for citric acid biosynthesis substrates involving Aspergillus niger has been the subject of extensive research [54,123,132,133,134,135,136,137,138,139,140,141]. High-purity substrates such as sucrose, glucose, fructose, and maltose can be utilized in citric acid production. Examples of using high-purity raw materials in the citric acid production process involving Aspergillus niger are presented in Table 4.
Sucrose is the most favorable carbon source due to its low molecular weight, facilitating transport across microbial cell membranes, and rapid hydrolysis by invertase activated in low-pH environments [101,142]. High-purity substrates allow for the control of the citric acid production process with a yield greater than 70% [26,90]. However, using pure sugar in industrial citric acid production is associated with high costs, as the raw material cost often exceeds the obtained product’s price [143]. Therefore, in industrial citric acid production involving Aspergillus niger, cheap and renewable carbon sources such as agro-industrial waste materials are employed [2,94].
Examples of using agro-industrial waste materials in the citric acid production process with Aspergillus niger are presented in Table 5 and Table 6. Environmental concerns and the high pollution control costs drive the interest in utilizing waste and industrial effluents in citric acid biosynthesis. Industrial by-products used in citric acid production include beet molasses [144,145,146], sugarcane molasses [147], cellulose [148], lipids [137,149], whey, fruit pomaces [92,150,151], inulin [152], starch materials [121,134,153,154], sweet potatoes [155,156], cassava [4,136], seaweed [122], and glycerol [157,158]. Industrial waste is considered the best carbon source in solid-state cultures [32].
The primary raw materials in citric acid production involving Aspergillus niger are cane and beet molasses, primarily due to their low cost [50,93]. Molasses is characterized by a high carbohydrate content, approximately 50%, mainly in sucrose, glucose, and fructose. However, its chemical composition is variable and heterogeneous, which could hinder the biosynthesis process. To enhance the quality of the culture medium, molasses undergoes various treatments involving ferrocyanide, hydrochloric acid, tricalcium phosphate with hydrochloric acid, ammonium oxalate, ammonium dihydrogen phosphate, and lime, as well as fractionation [119,159,160,161].

Table 4.
High-purity substrates used in citric acid production by Aspergillus niger strains.
Table 4.
High-purity substrates used in citric acid production by Aspergillus niger strains.
| Substrate | Strain | Substrate Concentration | Cultivation Method | Yield | References |
|---|---|---|---|---|---|
| Sucrose | Aspergillus niger C–12 | 150 g∙dm−3 | SF | 77.7% (m/m) | [26] |
| Aspergillus niger C–12 | 150 g∙dm−3 | SF | 81.2% (m/m) | ||
| Aspergillus niger NCIM705 | 60 g∙dm−3 | SF | 30.7 g∙dm−3 | [162] | |
| Glucose | Aspergillus niger PM–1 | 150 kg∙m−3 | SF | 121 kg∙m−3 | [86] |
| Aspergillus niger Yang no. 2. | 0.12 mg∙dm−3 | SF | 15.4 mg∙mL−1 | [112] | |
| Galactose | Aspergillus niger ATCC 12846 | 100 g∙dm−3 | SF | 0.3% | [115] |
| Aspergillus niger ATCC 26036 | 100 g∙dm−3 | SF | 0.4% | ||
| Aspergillus niger ATCC 26550 | 100 g∙dm−3 | SF | 0.1% | ||
| Aspergillus niger IMI 31821 | 100 g∙dm−3 | SF | 2.3% | ||
| Aspergillus niger IMI 83856 | 100 g∙dm−3 | SF | 1.5% | ||
| Starch hydrolysates | Aspergillus niger UE–1 | 15% (glucose equivalent) | LSF | 490 g∙kg−1 | [163] |
| Starch | Aspergillus niger GCB–47 | 150 g∙dm−3 | SF | 45.1 g∙dm−3 | [164] |
| Aspergillus niger GCMC | 150 g∙dm−3 | SF | 69.5 g∙dm−3 | ||
| Anhydrous glycerol | Aspergillus niger W78B | 150 g∙dm−3 | SF | 59.0 g dm−3 | [165] |
| Anhydrous glycerol | Aspergillus niger PD66 | 100 g∙dm−3 | SF | 64.2% (m/m) | [166] |
| Anhydrous glycerol + sucrose | Aspergillus niger PD66 | 135 g∙dm−3 +15 g∙dm−3 | SF | 95.80% (m/m) | [167] |

Table 5.
Agricultural and industrial wastes used in citric acid production by Aspergillus niger strains.
Table 5.
Agricultural and industrial wastes used in citric acid production by Aspergillus niger strains.
| Substrate | Strain Aspergillus niger | Cultivation Method | Yield | References |
|---|---|---|---|---|
| Sugarcane bagasse | Aspergillus niger ATCC 9142 | SSF | 97.81 g∙kg−1 | [168] |
| Aspergillus niger 14/20 | SSF | 50.01 μg∙g−1 | [147] | |
| Aspergillus niger DS 1 | SSF | 31.8% | [169] | |
| Sugarcane molasses | Aspergillus niger ATCC 9142 | SF | 106.65 g dm−3 | [170] |
| Aspergillus niger EB–3 | SSF | 0.112 mg∙dm−3 | [171] | |
| Aspergillus niger GCMC–7 | SF | 96.1 g dm−3 | [161] | |
| Beet molasses | Aspergillus niger A20 | SLF | 29.7 g dm−3 | [172] |
| Aspergillus niger A20 | SF | 8.6 g dm−3 | ||
| Aspergillus niger W78B | SF | 110 g dm−3 | [173] | |
| Cassava | Aspergillus niger FUO–2 | SF | 88.73 g dm−3 | [136] |
| Aspergillus niger NRRL 2001 | SSF | 88.0 g∙kg−1 | [36] | |
| Pineapple waste | Aspergillus niger DS 1 | SSF | 54.2% | [174] |
| Apple waste | Aspergillus niger NRRL 567 | SSF | 65.6 | [151] |
| Aspergillus niger NRRL 567 | SF | 8.3 g dm−3 | ||
| Fruit waste– Parkia biglobosa | Aspergillus niger | SF | 1.15 g dm−3 | [123] |
| Palm oil | Aspergillus niger IBO–103MNB | SSF | 337.94 g∙kg−1 | [149] |
| Starch | Aspergillus niger ATCC 9142 | SF | 2.7 g dm−3 | [139] |
| Whey | Aspergillus niger ATCC 9642 | SFC | 2.43 g dm−3 | [175] |
| Date syrup | Aspergillus niger J4 | SF | 56.7 g dm−3 | [176] |
| Peat | Aspergillus niger NRRL 567 | SF | 82.0 g∙kg−1 | [177] |
| Distillery stillage | Aspergillus niger ATCC 9142 | SSF | 6.15 g∙kg−1 | [178] |
| Aspergillus niger ATCC 201122 | SF | 71.63% | [179] | |
| Molasses (14%) + corn starch (14%) + sucrose (5%) | Aspergillus niger NCIM 1055 | SF | 0.13 mg∙dm−3 | [121] |
| Corn starch + sucrose (15%) | Aspergillus niger | SSF | 138.24 g∙kg−1 | [134] |
| Date waste + whey | Aspergillus niger ATCC 6275 | SF | 32.4 g dm−3 | [180] |
| Orange waste + cane molasses | Aspergillus niger von Tiegh 1867 | SF | 640 g∙kg−1 | [181] |
| Grape waste + sucrose (15%) | Aspergillus niger | SSF | 34.4 g∙kg−1 | [182] |
| Lime waste + sucrose (15%) | 28.6 g∙kg−1 |
Apart from cane and beet molasses, starch-based and lignocellulosic products are considered inexpensive and readily available carbon sources due to their high carbohydrate content [106,121,183,184]. However, these resources are limited due to contamination with heavy metals, amino acids, or proteins and the need for hydrolysis. Citric acid biosynthesis from non-hydrolyzed starch-based materials can be conducted using amylolytic strains of Aspergillus niger. This process, however, is characterized by low efficiency [90,106]. For starch-based materials, enzymatic hydrolysis includes treatment with α-amylase, amyloglucosidase, isoamylase, or pullulanase [90]. For cellulose hydrolysis, β-endoglucanase, β-exoglucanase, and β-glucosidase are used [183].
There are reports in the literature on using Aspergillus niger strains for citric acid biosynthesis using glycerol as the sole carbon source, but this issue has not been widely studied [107,157,158,160,165]. The use of pure glycerol as the primary carbon source was investigated by Foryś et al. Their studies obtained 59.0 g∙dm−3 of citric acid produced with a yield of 0.39 g∙g−1 [136].
In studies using glycerol as a carbon source in Aspergillus niger cultures, it was mainly used with other substrates. Schneider et al. used glycerol as an additive in concentrations ranging from 0 to 40% in solid substrates composed of waste from tung oil production as the primary carbon source. They achieved the highest yield (350.0 g∙kg−1) after seven days of cultivation with a 20% glycerol addition [158].
Bauwelers and Grosenker, in surface cultures using molasses as the primary carbon source with a 30% addition of waste glycerol, which had been previously treated with CaO at a concentration of 3.0 g∙kg−1, achieved a 95% yield in citric acid biosynthesis. In their submerged cultures, using substrates containing 70% cassava flour, 20% cornmeal, and 10% waste glycerol, they obtained an 88% yield in citric acid biosynthesis. However, in substrates containing 60% cassava flour, 20% cornmeal, and 20% waste glycerol, the process yield was slightly lower, at 85% (Table 6) [157].

Table 6.
Examples of using glycerol for citric acid biosynthesis by strains of Aspergillus niger.
Table 6.
Examples of using glycerol for citric acid biosynthesis by strains of Aspergillus niger.
| Substrate | Strain Aspergillus niger | Cultivation Method | Yield | References |
|---|---|---|---|---|
| Molasses (70%) + waste glycerol (30%) | Aspergillus niger | SLF | 95% | [157] |
| Cassava flour (70%) + corn flour (20%) + waste glycerol (10%) | SF | 88% | ||
| Cassava flour (60%) + corn flour (20%) + waste glycerol (20%) | SF | 85% | ||
| Glucose (80%) + waste glycerol (20%) | SF | 90% | ||
| Waste glycerol | Aspergillus niger PD66 | SF | 6.2% (m/m) | [166] |
| Waste glycerol | Aspergillus niger PD66 | SF | 114.14 g dm−3 | [185] |
There is limited scientific literature on the biosynthesis of citric acid by Aspergillus niger strains on glycerol-based substrates, even though they are considered the best acid producers and are used in industrial production. The lack of interest is likely due to the belief that glycerol slows down the growth rate of filamentous fungi and is not conducive to citric acid production by Aspergillus niger [186].
The intensive growth of research into glycerol biotransformation by microorganisms is driven by the challenge of managing the glycerol phase resulting from biodiesel production and the increasing demand for industrially valuable products, such as citric acid, docosahexaenoic acid, propionic acid, lactic acid, and dihydroxyacetone. Glycerol metabolism is of great importance due to the production of double the amount of reducing equivalents compared to glucose metabolism, indicating that glycerol provides more energy for further conversions [187].
In addition to the type of carbon source, its concentration also plays a significant role in the process of citric acid biosynthesis. High efficiency and rapid citric acid biosynthesis are achieved with carbohydrates rapidly taken up and metabolized by filamentous fungi. The effect of carbon source concentration and type on citric acid accumulation depends on the properties of phosphofructokinase-1. Under physiological conditions, its activity is inhibited by citric acid at concentrations of 1–5 mM. However, this inhibition of enzyme activity does not occur during fermentation. This is because high concentrations of sucrose or glucose are used, which leads to an increase in fructose-2,6-bisphosphate, a potent activator of phosphofructokinase-1. High carbohydrate concentrations also induce premeases, allowing for the rapid uptake of carbon and, as a result, glycolysis. Also, high carbon source concentrations significantly regulate pyruvate carboxylase activity [49,188,189]. Carbohydrates in concentrations exceeding 200.0 g∙dm−3 lead to a reduction in the rate of citric acid biosynthesis. This reduction may be due to an increase in fungal biomass concentration, elevated medium viscosity, and the synthesis of polyalcohols. On the other hand, carbohydrate concentrations below 50.0 g∙dm−3 result in low citric acid biosynthesis efficiency and the accumulation of oxalic acid [50,190,191].
5. Application of Citric Acid in the Food Industry
From a health quality perspective, citric acid, when used as a food additive, has been approved as generally recognized as safe (GRAS) by the FAO/WHO Expert Committee on Food Additives, and its Acceptable Daily Intake (ADI) does not require limitation [54]. Derivatives of citric acid, such as calcium citrate, iron citrate, manganese citrate, potassium citrate, sodium citrate, diammonium citrate, isopropyl citrate, and stearyl citrate, have also received GRAS status as food additives [13].
Citric acid is characterized by its low production cost, easy accessibility, non-toxicity, biocompatibility, universality, and the safety of its decomposition products. It finds wide applications in the food, pharmaceutical, biomedical, chemical, agricultural, and environmental protection industries [92]. Examples of citric acid applications in the food industry and other sectors are presented in Table 7 and Table 8. In food products, citric acid serves various functions, including acidity regulation, preservation, antioxidant properties, emulsification, flavor and aroma enhancement, buffering, and antibacterial activity. Citric acid’s ability to chelate metal ions and its buffering properties, when combined with citrates, make it an ideal additive in food and nutraceutical production [42,192,193].
Citric acid plays a significant role as an antioxidant in oil production and in limiting the oxidation of lipids in meat processing. It inhibits lipid oxidation by forming bonds between pro-oxidative metal ions and the carboxyl or hydroxyl groups of the acid. The antioxidant activity of citric acid in food depends on the dose applied and increases with higher acid concentrations [194].
In meat processing, citric acid reduces the pink color and increases the brightness of heat-treated meat. In cooked meat, citric acid limits the endogenous pink color and the color induced by sodium nitrite and nicotinamide. Reduction of the pink color in meat may also result from the chelation of heme iron by citric acid, preventing heme from binding with ligands that cause a pink color [195,196].

Table 7.
Examples of citric acid applications in the food industry.
Table 7.
Examples of citric acid applications in the food industry.
| Industry | Application | References |
|---|---|---|
| Beverages—wines, juices, non-alcoholic beverages, syrups | Used as an acidity regulator in carbonated and non-carbonated beverages, a buffering agent, pH regulator | [12,197] |
| Sweets—jams, jellies, candies | Used as an antioxidant, antibacterial agent, controlling sugar inversion and product pH for optimal gelling, preservative, providing a bitter taste and enhancing flavor | [192] |
| Dairy products | Sodium citrate is used in cream production to stabilize casein, prevent the formation of creams during hot milk beverage production, and act as an emulsifier to stabilize the water and oil phases in cheese production. Aqueous solutions of citric acid are used for milkstone removal from equipment | [14] |
| Meat products | It acts as a chelating agent, helping maintain the natural color and prevent discoloration of preserved meats; acts as an antioxidant and synergist for antibacterial agents; Sodium citrate is used in slaughterhouses to prevent coagulation or clotting of fresh blood | [32] |
| Fruit and vegetable industry | Citric acid, along with ascorbic acid, inhibits enzyme activity and oxidation reactions that may deteriorate colors and flavors | [192] |
| Oils | Used in the deodorization and hydrogenation of oil to chelate metal ions, catalyze the rancidity of fats, interrupt the formation of peroxides and other oxidation products in the auto-oxidation of oils | [37] |
| Seafood | Prevents discoloration and the development of unwanted odors by chelating metals | [12] |
Citric acid and its salts are widely used in the food industry to prevent enzymatic browning [198]. Enzymatic browning of fruits and vegetables is a phenomenon that reduces shelf life and influences consumer decisions [199,200].
Citric acid is used as an additive in rinse water before deep freezing and in fruit syrups [201]. Adding citric acid to stored fruits and vegetables positively affects color retention and organoleptic quality and extends their shelf life. It can also be combined with other anti-browning agents, such as ascorbic acid [202].
The inhibition of citric acid’s polyphenol oxidase (PPO) activity is due to its pH-lowering capacity. PPO activity gradually decreases with increasing citric acid concentrations. However, the citric acid concentration needed to inhibit PPO activity varies depending on the PPO activity and buffer solutions used [198]. Radish slices immersed in a 0.3% aqueous citric acid solution showed no browning during storage. Querioz et al. found that a citric acid concentration of 100 mM inhibited the PPO activity of cashew apples [199]. However, a 10 mM citric acid solution inhibited banana PPO [148].
Citric acid also contributes to a decrease in the thermodynamic parameters of polyphenol oxidase. This is believed to be due to a reduction in the stability of PPO and the number of non-covalent bonds in the enzyme’s structure, leading to changes in the protein’s secondary and tertiary structure [198].
Citric acid can be considered a substance capable of controlling cellular respiration and contributing to the better preservation of fruits and vegetables during storage [200,203].

Table 8.
Examples of citric acid applications in various industries.
Table 8.
Examples of citric acid applications in various industries.
| Industry | Application | References | |
|---|---|---|---|
| Pharmaceutical industry | Medicines, pharmaceutical preparations, blood banks | It is used as an anticoagulant, effervescent in combination with bicarbonates or carbonates, a flavoring agent, and a stabilizer. It imparts the desired sour taste, which helps mask medicinal flavors | [8,14,204,205] |
| Cosmetics industry | Detergents, cosmetics | It is added to hair care products, cosmetics, and detergents for pH regulation and used as a stabilizer, buffering agent, and chelating agent to prevent discoloration | [14,206] |
| Agriculture | Animal feeds | Enhances the bioavailability of mineral nutrient chelates, improves taste, regulates stomach pH, and enhances the effectiveness of animal feeds; used as a flavor enhancer in pet food | [207] |
| Fertilizers | Forms chelate with Fe, Cu, Mg, and Zn, used for soil correction, increase phosphorus availability to plants, are employed to remove lead from contaminated soils, and are used for copper chelation in algaecides for water reservoirs | [208] | |
| Other applications in industry | Textile industry | It is used for pH regulation, as a buffer, and as a chelating agent in the dyeing process | [37] |
| Metallurgical industry | Cleans steam boiler from metal oxides and purifies iron and copper oxides used in nuclear reactor welding | ||
| Electroplating | It is used as a chelating agent to control the metal deposition rate on substrates | ||
| Biomedical engineering | Utilized as a copolymer in nanomaterials to encapsulate biologically active compounds | [209] | |
| Water purification | Solutions of citric acid are used to remove iron, calcium, and other cations that damage cellulose acetate membranes used in reverse osmosis systems | [210] | |
5.1. Newly Emerging Applications of Citric Acid
Research into new applications of citric acid in various industries is currently the subject of many studies [211,212,213,214,215,216]. One of the new applications of citric acid is the production of household detergents. Citric acid chelates Mg2+ and Ca2+ ions responsible for water hardness and does not contribute to the eutrophication of aquatic systems, unlike phosphates used in detergents [217]. New and innovative applications of citric acid in the food industry and beyond are expected to lead to increased production.
5.1.1. Cross-linking Agent and Plasticizer
Citric acid can successfully be used in the process of cross-linking proteins [218], polysaccharides [219], and hydroxyapatite [220]. A breakthrough in using citric acid as a cross-linking agent came with the discovery by Rothenberg and Alberts from the University of Amsterdam. They demonstrated that glycerol and citric acid can polymerize, creating a water-soluble, biodegradable, and thermosetting resin. The combination of citric acid and glycerol at temperatures above 100 °C and below 130 °C under normal conditions leads to the formation of polyester resins through the Fischer esterification reaction [221].
The use of citric acid as a compatibilizer for various polysaccharides, including starch, thermoplastic starch, cotton, chitosan, and cellulose, is justified by its multi-carboxyl structure [222]. This allows it to be used as a cross-linking agent, plasticizer, and hydrolyzing agent [223].
The mechanism of the cross-linking reaction is based on the well-known Fischer esterification reaction between the carboxyl groups of citric acid and the hydroxyl groups of starch [224]. Citric acid can react with all three hydroxyl groups of starch. Esterification between starch and citric acid leads to mono-, di-, and tri-esters forming. Esterification primarily occurs within the branching points of amylopectin [225]. The formation of ester bonds can be catalyzed by reducing the pH or adding Lewis acids, which are chemical compounds capable of accepting an electron pair from a base [224]. In many previous studies on starch cross-linking, temperatures above 100 °C have initiated the cross-linking process [226]. Heating citric acid causes dehydration and the formation of an anhydride, which can react with starch to form starch citrate. With further heating, the citrate undergoes dehydration, and cross-linking can occur [227,228].
It is possible to conduct cross-linking reactions at lower temperatures (around 70 °C) using higher concentrations of citric acid. However, the efficiency of the reaction under these conditions is low because only a tiny amount of added citric acid participates in the cross-linking reaction. Furthermore, citric acid that has not reacted can act as a plasticizer [225]. As a plasticizer, citric acid increases the tensile strength of starch films. The improvement in tensile strength is more significant than when glycerol is used as a plasticizer [229]. Excessive citric acid concentration does not interact with starch molecules but can react with water, disrupting hydrogen bonds and reducing the matrix’s cohesion. This results in increased water solubility, susceptibility to deformation, and reduced thermal resistance [153].
FTIR spectroscopy and X-ray diffraction of starch films with citric acid have shown that citric acid can effectively inhibit starch recrystallization or retrogradation due to strong hydrogen bonding between starch and citric acid [224]. Additionally, citric acid-cross-linked starch films exhibit significantly higher tensile strength, up to 150% more than non-cross-linked films. However, achieving the appropriate increase in material strength requires an optimal amount of citric acid [230]. Citric acid concentrations below 5% act as a cross-linking agent and enhance the tensile strength of starch films. When the concentration increases from 5% to 30%, tensile strength decreases, but flexibility and material adhesiveness increase. This suggests that excess free citric acid is a plasticizer [231].
The main disadvantage of citric acid as a cross-linking and plasticizing agent in starch barrier films is starch degradation due to acid hydrolysis. Acid hydrolysis of starch glycosidic bonds involves the cleavage of these bonds, resulting in the protonation of oxygen and the addition of a water molecule, leading to the formation of a reducing sugar group. Effectively preventing starch hydrolysis during cross-linking in the presence of citric acid can be achieved by maintaining a pH of 4 or lower and a temperature below 105 °C [224].
As a cross-linking agent, citric acid strengthens bonds by incorporating covalent bonds that complement intermolecular hydrogen bonds, improving the resistance of starch films to moisture. Strong hydrogen bonds between the carboxyl groups of citric acid and the hydroxyl groups of starch result in improved interactions between molecules and reduced solubility of the films in water [153].
When citric acid is added in the range of 1% to 10% to thermoplastic starch, it significantly reduces water vapor permeability. This effect is due to replacing hydrophilic groups with hydrophobic ester groups that impede the diffusion of water vapor molecules through the matrix. However, water vapor permeability increases when the citric acid concentration exceeds 10%. This can be attributed to the plasticizing effect caused by an excess of citric acid. Increased citric acid concentration leads to enhanced chain mobility and increased interchain spaces, resulting from the attachment of free citric acid to the polymer chain. As a result, the water vapor diffusion coefficient increases, accelerating water vapor penetration through the starch films [232,233,234].
Starch films cross-linked with citric acid have higher thermal resistance [226]. The reason for this is that the cross-links are responsible for the resistance of cross-linked films. Cross-linked starch films exhibit significantly improved thermal resistance at temperatures above 320 °C [230].
The three carboxyl groups and one hydroxyl group in citric acid also allow it to cross-link glycerol, cellulose, and sebacic acid through condensation reactions, forming ester copolymers capable of drug delivery [8]. Gentamicin incorporated into the polymer effectively kills bacteria. Citric acid delivers ketoconazole as a cross-linking agent for beta-cyclodextrins on hydrogel hydroxypropylmethylcellulose (HPMC) membranes. The formation of drug-cyclodextrin complexes contributes to increased solubility and bioavailability of poorly soluble drugs [235].
5.1.2. Citric Acid in the Synthesis of Deep Eutectic Solvents
Deep eutectic solvents (DES) are homogeneous mixtures of two or more components capable of interacting with each other as a hydrogen bond acceptor (HBA) and a hydrogen bond donor (HBD). Deep eutectic solvent mixtures are formed by mixing two or more components in the appropriate molar ratio in the presence of heat. Additionally, this process does not require an additional purification step [236,237]. Deep eutectic solvents are one of the most promising discoveries in “green chemistry”. They can serve as an alternative to conventional organic solvents and have numerous advantages, such as renewability, reusability, biodegradability, non-toxicity, widespread availability, shallow vapor pressure, low flammability, and ease of preparation. Moreover, the components that produce deep eutectic solvents are inexpensive and safe [238,239].
Deep eutectic solvents have been divided into four types depending on their composition. Types I, II, and IV contain metal salts and are considered toxic and less sustainable than type III deep eutectic solvents. Type III deep eutectic solvents are synthesized from readily biodegradable and regenerable raw materials such as feed additive (choline chloride, ChCl), fertilizer (urea), antifreeze (ethylene glycol), sweetener (glycerol), and plant metabolites (sugars, sugar alcohols, and organic acids) [240]. The most frequently studied eutectics in the literature are type III, based on the combination of quaternary ammonium salts and a compound serving as a hydrogen bond donor. Type III is the most commonly used deep eutectic solvent due to the strong interaction of hydrogen bonds between the hydrogen bond acceptor (HBA) and the hydrogen bond donor (HBD). Many compounds have been successfully utilized to create deep eutectic solvents. HBAs are mainly quaternary ammonium or phosphonium salts, while HBDs are most commonly amides, alcohols, and carboxylic acids. Citric acid is one of the most commonly employed HBDs among carboxylic acids [237]. The most popular systems for producing deep eutectic solvents using choline chloride and citric acid are 1:1, 1:2, and 2:1 [241,242]. The presence of hydroxyl and carboxyl groups allows for the formation of sufficiently strong hydrogen bonds. Deep eutectic solvents based on choline chloride and carboxylic acids demonstrate greater extraction efficiency than traditional solvents such as water and ethanol [243].
Various molar ratios of choline chloride and citric acid monohydrate significantly influence the physicochemical properties of deep eutectic solvents. Adding citric acid monohydrate increases viscosity, surface tension, and density. Deep eutectic solvents with a higher molar ratio of choline chloride exhibit a higher melting point. Citric acid-based deep eutectic solvents can find broad industrial applications, particularly in extracting hydrophilic components from plant or animal materials [243].
In the studies conducted by Kurtulbaş et al., deep eutectic solvents were intentionally designed, incorporating a hydrogen bond donor (HBD) (glycerol and ethylene glycol) and a hydrogen bond acceptor (HBA) (citric acid) in a specified molar ratio (1:4) for the extraction of bioactive compounds (phenols and anthocyanins). In the current investigation, Hibiscus sabdariffa was extracted using microwave-assisted extraction (MAEX). The most effective extract from Hibiscus sabdariffa was obtained from a mixture of citric acid and ethylene glycol through microwave-assisted extraction [239].
Hu et al., investigated the molecular mechanisms of isoliquiritigenin extraction using deep eutectic solvents of choline chloride and citric acid. The results indicated that deep eutectic solvents exhibited higher efficiency in isoliquiritigenin extraction as an extraction solvent than ethanol with water. Additionally, the increased efficiency in isoliquiritigenin extraction was primarily attributed to the strong interaction between isoliquiritigenin and the extraction solvent and the rapid diffusion of isoliquiritigenin [237].
5.1.3. Antibacterial Agent
Using organic acids to control bacterial flora in food, extend shelf life, and improve the safety of plant- and animal-derived products has become a common practice in the food industry. In Europe, the legal basis for using organic acids as agents contributing to the safety of animal-derived products is Regulation 853/2004 of the European Parliament and the Council [244,245,246].
Citric acid effectively combats pathogenic microflora in fresh and processed pork, beef, poultry, and fresh vegetables and fruits [247,248,249,250,251]. Its antibacterial activity involves penetrating through the cell membranes, where the pH is higher than in the surrounding environment. The mechanism of citric acid’s antibacterial action is related to acidifying the cytoplasm, disrupting metabolic processes, or accumulating the dissociated acid anion to a toxic level (Figure 12) [252,253]. Organic acids are weak acids, so they do not completely dissociate in an aqueous environment, and their microbiological activity depends on the degree of dissociation and the pH of the food product. Reducing the pH increases the concentration of the acid, reduces the polarity of the molecules, improves acid diffusion through microbial cell membranes into the cells, and consequently increases antibacterial activity [253,254]. The effectiveness of organic acids also depends on the acid concentration, acid properties, temperature, exposure time, and microbial susceptibility [251,255].
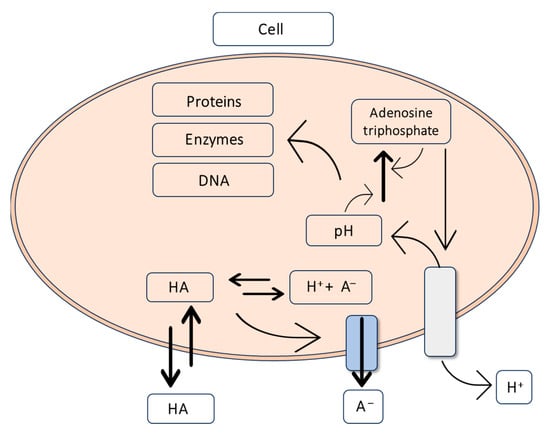
Figure 12.
The mechanism of citric acid’s action on a bacterial cell [253].
Citric acid in concentrations ranging from 0.1 to 3.0% restricts the growth of bacteria such as Listeria monocytogenes, Escherichia coli, Salmonella typhimurium, and Vibrio parahaemoliticus [244,247,249,251].
Citric acid exhibits synergistic effects when used in mixtures with other organic acids. A mixture of caprylic and citric acids significantly inhibits the growth of bacteria. The synergy between citric and caprylic acids is associated with the loss of cell membrane integrity and changes in its permeability. The mechanism of the synergistic action of both acids involves damaging or destabilizing the cell membrane, leading to increased permeability and, consequently, cell death. Damage to the bacterial membrane allows hydrogen ions to penetrate, resulting in a strong bactericidal effect [248,256,257].
Combining citric acid with other decontamination methods, such as ozonation, UV-C radiation, and ultrasonication, can significantly impact the inactivation of microorganisms in fresh food [41].
The effectiveness of citric acid’s antibacterial action varies and depends on many factors. Citric acid exhibits optimal antibacterial effects in a low pH environment, at low temperatures, and when used in high concentrations. Available literature sources report that using citric acid at concentrations above 2% may cause adverse sensory changes in food products [253,258]. The antibacterial effectiveness of citric acid also depends on the initial amount of microflora on the product’s surface. Depending on the initial bacterial count, citric acid reduces the number of microorganisms by 1 to 2 log cfu∙g−1 [244].
5.1.4. Deamidation of Gluten
Wheat gluten is widely used in the food industry, serving various purposes, such as emulsifiers and imparting cohesiveness and elasticity. However, its utility is limited due to its low solubility under neutral conditions. A practical method to enhance the properties of gluten is deamidation using carboxylic acids, including citric acid [259,260].
The deamidation reaction transforms amidic groups into carboxylic groups, mainly glutamine and asparagine residues. This transformation results in increased electrostatic repulsion, the disruption of hydrogen bonds, and the dissociation of polymers, ultimately improving the solubility of gluten. While treating gluten with citric acid, the availability of peptide bonds and hydrogen ions influences the competition between hydrolysis and deamidation. The degree of hydrolysis of deamidated gluten decreases with increased citric acid concentration and treatment time, contributing to an increase in soluble protein content after deamidation [215,261]. Deamidation by citric acid increases the solubility of gluten to around 70% at pH 7. The shift of the protein’s isoelectric point towards acidic pH confirms that deamidation increases the quantity of protein polyelectrolytes, resulting in improved solubility at neutral pH. Deamidation involves the cleavage of peptide bonds, indicating that it is primarily responsible for increasing protein solubility rather than hydrolysis [261,262].
Deamidation of gluten with citric acid significantly enhances its emulsifying, foaming, and elasticity properties. Emulsions stabilized with deamidated gluten feature smaller emulsion droplet sizes, indicating their ability to reduce surface tension. High emulsion stability results from the increased flexibility of the gliadin molecule or its molecular rearrangement [261]. The improvement in the foaming capacity of deamidated gluten is attributed to the increased molecule flexibility, leading to enhanced protein adsorption and anchoring at phase boundaries [263,264]. Deamidation also leads to changes in the secondary conformation of the protein, driven by increased electrostatic repulsion and a reduction in hydrogen bonds [262]. The secondary structure of gluten consists of 34.5% α-helices, 17.3% β-turns, and 44.8% β-sheets. Deamidation increases α-helices and β-turns while reducing β-sheets [260,263].
Citric acid exhibits a solid capability to break peptide bonds in gluten, which leads to a transformation in the tertiary structure of the protein. Protein fractions of gluten with a higher molecular weight are more susceptible to degradation than those with a lower molecular weight. After deamidation with citric acid, the presence of sulfhydryl groups in gluten has been confirmed, while the tertiary structure becomes less compact [263,264].
Deamidation with citric acid can contribute to the improvement of the nutritional properties of gluten. From a nutritional standpoint, gluten is not considered a good protein source due to its deficiency in lysine and threonine. Deamidation with citric acid increases the overall quantity of essential amino acids, including lysine [261].
5.1.5. Extractant
Citric acid can be an effective pectin extractor from fruit pomace instead of toxic mineral acids such as sulfuric or nitric acid. Pectin extraction using citric acid can reduce waste from fruit and vegetable processing and limit the harmful environmental impact of wastewater from conventional extraction methods [265,266].
The process of pectin extraction typically occurs at around 97 °C with a pH of 2.5 using water acidified with citric acid [193,265].
Extracting pectin from citrus fruit peels using citric acid allows for obtaining pectins with a high galacturonic acid content, essential for their application as a gelling agent. Pectins also have a high molecular weight, indicating a high content of neutral sugars. The viscosity of the extracted pectins increases with a higher concentration of citric acid. Although the extraction process may yield lower efficiency than traditional methods, it results in pectins of high purity [267].
The pectins extracted from cocoa husks were characterized by a high degree of acetylation, contained rhamnogalacturonan, and had side chains rich in galactose. Despite their high degree of acetylation, these pectins formed gels in a low pH environment and at a high glucose concentration, suggesting their potential use as an additive in acidic products [268].
5.1.6. Inhibition of Protein Adhesion
The issue of protein adhesion to steel surfaces poses numerous challenges in food production and processing. Proteins adhering to equipment surfaces can serve as a nutrient source for microorganisms, leading to product contamination. Removing allergens and preventing cross-contamination is a critical point in the food production process. One method to inhibit the adhesion of chicken egg white proteins to stainless steel surfaces is to use a citric acid solution [269].
The effect of inhibiting protein adhesion by citric acid involves changing the surface charge of steel from positive to negative in an environment with a pH of 7.4 due to the attachment of dissociated carboxyl groups from the acid. This leads to the repulsion of negatively charged protein molecules such as ovalbumin or ovomucoid [216,269].
6. Global Citric Acid Market
Acidity regulators are a significant part of the food additives industry, as they impact the taste of food and contribute to its shelf life. The acidity regulators market is estimated to reach USD 7.29 billion in 2023, with a CAGR of 7.09%. Citric acid dominates the acidity regulators market due to its wide applications in the non-alcoholic beverage industry and the increasing societal preference for safe food [270]. In 2016, approximately 67% of the citric acid produced worldwide was used in the food industry, 16% in the chemical industry, 8% in pharmaceuticals and cosmetics, and 7% in other sectors such as textiles and metallurgy [271].
In the 1930s, American companies Miles and Pfizer led citric acid producers with 4900 tons [27]. However, by 1978, the combined production of Pfizer and Miles had reached approximately 70,000 tons. In 1990, the annual production of citric acid had already reached 170,000 tons. Even though the largest citric acid producers in the 1990s were located in the United States, Europe produced around 255,000 tons annually. At that time, North America had 215,000 tons, Asia produced 66,000 tons, Africa produced 14,000 tons, Australia produced 8000 tons, and South America produced 7000 tons of citric acid per year [272].
The current citric acid market is considered the fastest-growing segment in the food additives industry, driven by its wide range of applications in various industries and the increasing use of citric acid as a cleaning agent. The global citric acid market increased from USD 3.87 billion in 2022 to USD 4.2 billion in 2023. The Russia-Ukraine war has ruined, at least in the short term, the chances of reviving the global economy after the COVID-19 pandemic. The war between the two countries has led to economic sanctions on many countries, rising commodity prices, and supply chain disruptions, causing inflation in goods and services and affecting many markets worldwide. The citric acid market is expected to grow to USD 5.52 billion in 2027 (Figure 13) [95]. The global citric acid market reached a volume of 2.8 million tons in 2022, and forecasts suggest that by 2028, the market will reach 3.3 million tons, indicating a CAGR of 2.78% for 2022–2028 [270].
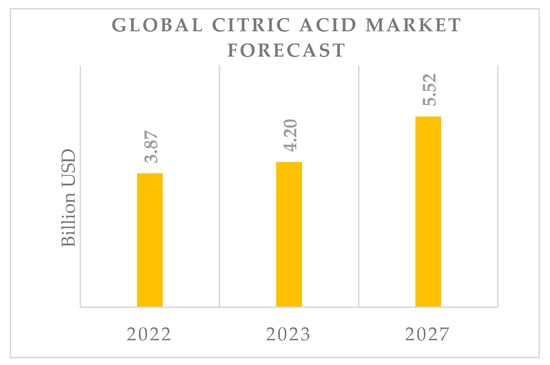
Figure 13.
Global citric acid market forecast.
The global citric acid market is divided among North America, Latin America, Western Europe, Eastern Europe, and Asia-Pacific, excluding Japan (APEJ). In 2016, the APEJ region dominated the citric acid market in regards to value. Regarding market size, Western Europe (including the United Kingdom, Germany, Italy, France, and Spain) led with production exceeding 500,000 tons. Following it were the APEJ region, North America (including the United States, Canada, and Mexico), and the rest of the world. The global COVID-19 pandemic significantly impacted the citric acid market in 2020. This resulted in a sharp increase in the sales of detergents and cleaning products and a substantial increase in the demand for citric acid [270]. The citric acid market is experiencing significant growth in Western Europe, driven by the established food and beverage sector, where citric acid is widely used as a preservative and flavor enhancer. The growing trend toward natural and organic products in countries such as Germany, France, and the UK creates opportunities for citric acid. Furthermore, the pharmaceutical sector in Western Europe is highly advanced and rigorously regulated, creating demand for trusted and high-quality ingredients, including citric acid, for use in medicines and dietary supplements [3]. On a global scale, the Asia-Pacific region is the largest consumer of citric acid, accounting for 28%. North American countries, with a consumption rate of 23%, come second, while Western Europe, with a share of 22%, ranks third [271].
Key players in the citric acid market include Archer Daniels Midland Company (Chicago, IL, USA), Cargill Inc. (Shanghai, China), Tate & Lyle PLC (London, UK), Jungbunzlauer Suisse AG (Des Plaines, IL, USA), Cofco Biochemical (Anhui) Co., Ltd. (Bengbu, China), Huangshi Xinghua Biochemical Co. Ltd. (Huangshi, China), RZBC Group Co. Ltd. (Rizhao, China), Weifang Ensign Industry Co., Ltd. (Weifang, China), Gadot Biochemical Industries Ltd. (Haifa, Israel), S.A. Citrique Belge N.V. (Tienen, Belgium) [273]. Manufacturers focus on diversifying and delivering high-quality products that meet customer requirements to maintain a competitive edge. Furthermore, companies compete by investing in developing next-generation products with high solubility and acceptable taste. An example is Gadot Biochemical Industries, which introduced a new product called Cal2Mg in 2022, a combination of calcium and magnesium citrate. In the same year, Jungbunzlauer introduced a new product called monomagnesium citrate, a one-to-one molar ratio magnesium salt mainly used as a mineral source in functional foods, beverages, and dietary supplements [274].
Currently, a challenge in the citric acid market is the pressure from imports originating in Asian countries, which has decreased selling prices. Additionally, European producers contend with high production costs. In 2021, the European Commission reaffirmed decisions made in 2016 and re-imposed anti-dumping duties on importing citric acid from the People’s Republic of China and Malaysia [275].
7. Conclusions
This literature review discusses the properties of citric acid, cultivation conditions primarily for Aspergillus niger, differences between filamentous fungi Aspergillus niger and yeast Yarrowia lipolytica, extensive applications of citric acid in the industry, and the global market for citric acid. The review indicates a growing interest in the microbiological production of citric acid and technological advancements in this area, aligned with market demands.
Additionally, the article emphasizes the use of waste substrates, reflecting the development of a closed-loop economy model. Such a model can reduce economic and environmental costs for producing enterprises. The aspect of exploring new, more cost-effective substrates from the agri-food industry could contribute to cost reduction and solutions for waste disposal issues.
A multifaceted view of citric acid arises from its wide-ranging applications in various industrial sectors and the exploration of new uses. The continuous increase in demand for citric acid is associated with the most economically viable industrial production process, characterized by high sensitivity, complexity, and dependence on parameters such as microorganisms used in substrates and fermentation techniques. In the bioproduction of citric acid, there is significant interest in improving process efficiency at relatively low costs. This serves as an incentive for the development of new technologies and innovations in the design of bioreactors, process scaling, and control. Metabolic engineering can also improve fermentation parameters, requiring substantial research efforts.
The review highlights the importance of Aspergillus niger as a versatile industrial microorganism, which, in biotechnological studies, will undergo further investigations into secondary metabolism, fermentation conditions in citric acid production, and enhancing efficiency through CRISPR-Cas9 genome editing.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest. The author alone is responsible for the content and writing of this article.
References
- Amato, A.; Becci, A.; Beolchini, F. Citric acid bioproduction: The technological innovation change. Crit. Rev. Biotechnol. 2020, 40, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Li, W.J.; Yang, H.Q.; Chen, J. Hong Current strategies and future prospects for enhancing microbial production of citric acid. Appl. Microbiol. Biotechnol. 2019, 103, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Citric Acid Market Size, Trends and Forecast 2023–2028. Available online: https://www.imarcgroup.com/citric-acid-manufacturing-plant (accessed on 3 October 2023).
- Max, B.; Salgado, J.M.; Rodríguez, N.; Cortés, S.; Converti, A.; Domínguez, J.M. Biotechnological production of citric acid. Braz. J. Microbiol. 2010, 41, 862–875. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.C.; Mishra, R.; Mohapatra, S. Microbial citric acid: Production, properties, application, and future perspectives. Food Front. 2021, 2, 62–76. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, S.; Tan, F.; Li, H.; Chu, R.; Wang, X.; Sun, H.; Zhang, M. A novel green production process of citric acid on the pilot scale by directly recycling its extraction effluent. J. Clean. Prod. 2020, 277, 124068. [Google Scholar] [CrossRef]
- Lende, S.V.; Karemore, H.; Umekar, M.J.; Lende, S.V.; Karemore, H.; Umekar, M.J. Review on production of citric acid by fermentation technology. GSC Biol. Pharm. Sci. 2021, 17, 85–93. [Google Scholar] [CrossRef]
- Lambros, M.; Tran, T.H.; Fei, Q.; Nicolaou, M. Citric Acid: A Multifunctional Pharmaceutical Excipient. Pharmaceutics 2022, 14, 972. [Google Scholar] [CrossRef]
- Mores, S.; de Vandenberghe, L.P.S.; Magalhães Júnior, A.I.; de Carvalho, J.C.; de Mello, A.F.M.; Pandey, A.; Soccol, C.R. Citric acid bioproduction and downstream processing: Status, opportunities, and challenges. Bioresour. Technol. 2021, 320, 124426. [Google Scholar] [CrossRef]
- Chemspider Search and Share Chemistry. Citric Acid |C6H8O7| ChemSpider. Royal Society of Chemistry. Available online: http://www.chemspider.com/Chemical-Structure.305.html?rid=4438fe13-8026-4f48-8dd8-a2625612fc65 (accessed on 22 June 2023).
- Apelblat, A. Physicochemical Properties of Inorganic Citrates. In Citric Acid; Springer International Publishing: Berlin/Heidelberg, Germany, 2014; pp. 267–357. [Google Scholar]
- Dziezak, J.D. Encyclopedia of Food Sciences and Nutrition; Academic Press: San Diego, CA, USA, 2003. [Google Scholar] [CrossRef]
- Behera, B.C. Citric acid from Aspergillus niger: A comprehensive overview. Crit. Rev. Microbiol. 2020, 46, 727–749. [Google Scholar] [CrossRef]
- Fiume, M.; Heldreth, B. On the Safety Assessment of Citric Acid, Inorganic Citrate Salts, and Alkyl Citrate Esters as Used in Cosmetics. 2012. Available online: http://www.cir-safety.org/sites/default/files/citric032012FR.pdf (accessed on 22 June 2014).
- Verhoff, F. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Leśniak, W. Biotechnologia Żywności: Procesy Fermentacji i Biosyntezy; Wydawnictwo Uniwersytetu Ekonomicznego we Wrocławiu: Wrocław, Poland, 2002. [Google Scholar]
- Poerwono, H.; Higashiyama, K.; Kubo, H.; Poernomo, A.T.; Suharjono; Sudiana, I.K.; Indrayanto, G. Citric Acid. Anal. Profiles Drug Subst. Excip. 2001, 28, 1–76. [Google Scholar] [CrossRef]
- The National Center for Biotechnology Information. Citric Acid. Pubchem Compound. Available online: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=311 (accessed on 22 June 2023).
- Oliveira, M.L.N.; Malagoni, R.A.; Franco, M.R. Solubility of citric acid in water, ethanol, n-propanol and in mixtures of ethanol+water. Fluid Phase Equilib. 2013, 352, 110–113. [Google Scholar] [CrossRef]
- Yang, H.; Wang, J. Solubilities of 3-Carboxy-3-hydroxypentanedioic Acid in Ethanol, Butan-1-ol, Water, Acetone, and Methylbenzene. J. Chem. Eng. Data 2011, 56, 1449–1451. [Google Scholar] [CrossRef]
- Daneshfar, A.; Baghlani, M.; Sarabi, R.S.; Sahraei, R.; Abassi, S.; Kaviyan, H.; Khezeli, T. Solubility of citric, malonic, and malic acids in different solvents from 303.2 to 333.2K. Fluid Phase Equilib. 2012, 313, 11–15. [Google Scholar] [CrossRef]
- Wyrzykowski, D.; Hebanowska, E.; Nowak-Wiczk, G.; Makowski, M.; Chmurzyński, L. Thermal behaviour of citric acid and isomeric aconitic acids. J. Therm. Anal. Calorim. 2010, 104, 731–735. [Google Scholar] [CrossRef]
- Leśniak, W. Selekcja wysokoaktywnych szczepów Aspergillus niger dla fermentacji. Pr. Nauk. Akad. Ekon. Wrocławiu. Technol. 1977, 118, 21–37. [Google Scholar]
- Organisation for Economic Co-Operation and Development. SIDS Initial Assessment Report for 11th SIAM. Citric Acid. Available online: http://www.inchem.org/documents/sids/sids/77929.pdf (accessed on 22 June 2022).
- Apelblat, A. Dissociation Equilibria in Solutions with Citrate Ions. In Citric Acid; Springer International Publishing: Berlin/Heidelberg, Germany, 2014; pp. 143–212. [Google Scholar]
- Pietkiewicz, J.J.; Biosynteza Kwasu Cytrynowego Przez Aspergillus niger w Warunkach Jedno-i Wielostopniowych Hodowli Ciągłych. Pr. Nauk. Akad. Ekon. Wrocławiu. Ser. Monogr. Opracowania. Available online: http://yadda.icm.edu.pl/yadda/element/bwmeta1.element.ekon-element-000000010784 (accessed on 29 September 2015).
- Berovic, M.; Legisa, M. Citric acid production. Biotechnol. Annu. Rev. 2007, 13, 303–343. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, K.; Collins, J. The roots—A short history of industrial microbiology and biotechnology. Appl. Microbiol. Biotechnol. 2013, 97, 3747–3762. [Google Scholar] [CrossRef]
- Grewal, H.S.; Kalra, K.L. Fungal production of citric acid. Biotechnol. Adv. 1995, 13, 209–234. [Google Scholar] [CrossRef]
- Vandenberghe, L.P.S.; Rodrigues, C.; de Carvalho, J.C.; Medeiros, A.B.P.; Soccol, C.R. 25—Production and Application of Citric Acid. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 557–575. ISBN 9780444636621. [Google Scholar]
- Apelblat, A. Introduction. In Citric Acid; Springer International Publishing: Berlin/Heidelberg, Germany, 2014; pp. 1–11. [Google Scholar]
- Singh Dhillon, G.; Kaur Brar, S.; Verma, M.; Tyagi, R.D. Recent Advances in Citric Acid Bio-production and Recovery. Food Bioprocess Technol. 2011, 4, 505–529. [Google Scholar] [CrossRef]
- Ozdal, M.; Kurbanoglu, E.B. Citric Acid Production by Aspergillus niger from Agro-Industrial By-Products: Molasses and Chicken Feather Peptone. Waste Biomass Valorization 2019, 10, 631–640. [Google Scholar] [CrossRef]
- Steiger, M.G.; Rassinger, A.; Mattanovich, D.; Sauer, M. Engineering of the citrate exporter protein enables high citric acid production in Aspergillus niger. Metab. Eng. 2019, 52, 224–231. [Google Scholar] [CrossRef]
- Roukas, T.; Kotzekidou, P. Pomegranate peel waste: A new substrate for citric acid production by Aspergillus niger in solid-state fermentation under non-aseptic conditions. Environ. Sci. Pollut. Res. 2020, 27, 13105–13113. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, L.P.; Soccol, C.R.; Pandey, A.; Lebeault, J.-M. Solid-state fermentation for the synthesis of citric acid by Aspergillus niger. Bioresour. Technol. 2000, 74, 175–178. [Google Scholar] [CrossRef]
- Soccol Carlos, R.; Vandenberghe; Luciana, P.S.; Rodrigues Cristine, P.A. New Perspectives for Citric Acid Production and Application. Food Technol Biotechnol. 2006, 44, 141–149. Available online: http://www.ftb.com.hr/index.php/archives/80-volume-44-issue-no-2/443 (accessed on 4 April 2017).
- Kumar, L.R.; Yellapu, S.K.; Yan, S.; Tyagi, R.D.; Drogui, P. Elucidating the effect of impurities present in different crude glycerol sources on lipid and citric acid production by Yarrowia lipolytica SKY7. J. Chem. Technol. Biotechnol. 2021, 96, 227–240. [Google Scholar] [CrossRef]
- Crolla, A.; Kennedy, K.J. In-line mixing for production of citric acid by Candida lipolytica grown on n-paraffins. J. Chem. Technol. Biotechnol. 2004, 79, 720–728. [Google Scholar] [CrossRef]
- Sauer, M.; Mattanovich, D.; Marx, H. 12—Microbial production of organic acids for use in food. In Microbial Production of Food Ingredients, Enzymes and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 288–320. ISBN 9780857093431. [Google Scholar]
- Chen, Y.; Nielsen, J. Biobased organic acids production by metabolically engineered microorganisms. Curr. Opin. Biotechnol. 2016, 37, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Vargas, D.A.; Medina, J.V. Citric Acid: Synthesis, Properties and Applications; Nova Science: New York, NY, USA, 2012; Available online: https://www.novapublishers.com/catalog/product_info.php?products_id=23842 (accessed on 4 April 2017).
- Schuster, E.; Dunn-Coleman, N.; Frisvad, J.; van Dijck, P. On the safety of Aspergillus niger—A review. Appl. Microbiol. Biotechnol. 2002, 59, 426–435. [Google Scholar] [CrossRef]
- Niu, J.; Arentshorst, M.; Nair, P.D.S.; Dai, Z.; Baker, S.E.; Frisvad, J.C.; Nielsen, K.F.; Punt, P.J.; Ram, A.F.J. Identification of a Classical Mutant in the Industrial Host Aspergillus niger by Systems Genetics: LaeA Is Required for Citric Acid Production and Regulates the Formation of Some Secondary Metabolites. G3 Genes Genomes Genet. 2016, 6, 193–204. [Google Scholar] [CrossRef]
- Goldberg, I.; Rokem, J.S.; Pines, O. Organic acids: Old metabolites, new themes. J. Chem. Technol. Biotechnol. 2006, 81, 1601–1611. [Google Scholar] [CrossRef]
- Tong, Z.; Zheng, X.; Tong, Y.; Shi, Y.C.; Sun, J. Systems metabolic engineering for citric acid production by Aspergillus niger in the post-genomic era. Microb. Cell Fact. 2019, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.J.; Wang, B.T.; Wang, Z.D.; Jin, L.; Han, P. CRISPR/Cas9-Based Genome Editing and Its Application in Aspergillus Species. J. Fungi 2022, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zheng, X.; Cairns, T.C.; Zhang, Z.; Wang, D.; Zheng, P.; Sun, J. Disruption or reduced expression of the orotidine-5’-decarboxylase gene pyrG increases citric acid production: A new discovery during recyclable genome editing in Aspergillus niger. Microb. Cell Fact. 2020, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Karaffa, L.; Kubicek, C.P. Aspergillus niger citric acid accumulation: Do we understand this well working black box? Appl. Microbiol. Biotechnol. 2003, 61, 189–196. [Google Scholar] [CrossRef]
- Papagianni, M. Advances in citric acid fermentation by Aspergillus niger: Biochemical aspects, membrane transport and modeling. Biotechnol. Adv. 2007, 25, 244–263. [Google Scholar] [CrossRef] [PubMed]
- Legiša, M.; Mattey, M. Changes in primary metabolism leading to citric acid overflow in Aspergillus niger. Biotechnol. Lett. 2007, 29, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Karaffa, L.; Sándor, E.; Fekete, E. The biochemistry of citric acid of accumulation by Aspergillus niger (A review). Acta Microbiol. Immunol. Hung. 2001, 48, 429–440. [Google Scholar] [CrossRef]
- Arisan-Atac, I.; Wolschek, M.F.; Kubicek, C.P. Trehalose-6-phosphate synthase A affects citrate accumulation by Aspergillus niger under conditions of high glycolytic flux. FEMS Microbiol. Lett. 1996, 140, 77–83. [Google Scholar] [CrossRef][Green Version]
- West, T.P. Citric Acid Production by Aspergillus niger Using Solid-State Fermentation of Agricultural Processing Coproducts. Appl. Biosci. 2023, 2, 1–13. [Google Scholar] [CrossRef]
- Berg, J.M.; Jeremy, M.; Tymoczko, J.L.; Stryer, L.; Stryer, L. Biochemistry. W.H. Freeman. 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK21154/ (accessed on 10 May 2017).
- Li, C.; Yang, X.; Gao, S.; Wang, H.; Lin, C.S.K. High efficiency succinic acid production from glycerol via in situ fibrous bed bioreactor with an engineered Yarrowia lipolytica. Bioresour. Technol. 2017, 225, 9–16. [Google Scholar] [CrossRef]
- Yang, L.; Lübeck, M.; Lübeck, P.S. Aspergillus as a versatile cell factory for organic acid production. Fungal Biol. Rev. 2017, 31, 33–49. [Google Scholar] [CrossRef]
- Cordes, T.; Michelucci, A.; Hiller, K. Itaconic Acid: The Surprising Role of an Industrial Compound as a Mammalian Antimicrobial Metabolite. Annu. Rev. Nutr. 2015, 35, 451–473. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Kim, D.S.; Choi, H.S.; Kim, C.K.; Thapa, L.P.; Park, C.; Kim, S.W. Repeated batch production of 1,3-propanediol from biodiesel derived waste glycerol by Klebsiella pneumoniae. Chem. Eng. J. 2017, 314, 660–669. [Google Scholar] [CrossRef]
- Akram, M. Citric Acid Cycle and Role of its Intermediates in Metabolism. Cell Biochem. Biophys. 2014, 68, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Torres, N.V.; Voit, E.O. A Model of Citric Acid Production in the Mold Aspergillus niger. Pathw. Anal. Optim. Metab. Eng. 2002, 75–133. [Google Scholar] [CrossRef]
- Carsanba, E.; Papanikolaou, S.; Fickers, P.; Agirman, B.; Erten, H. Citric acid production by yarrowia lipolytica. In Non-Conventional Yeasts: From Basic Research to Application; Springer: Berlin/Heidelberg, Germany, 2019; pp. 91–117. [Google Scholar]
- Tan, M.-J.; Chen, X.; Wang, Y.-K.; Liu, G.-L.; Chi, Z.-M. Enhanced citric acid production by a yeast Yarrowia lipolytica over-expressing a pyruvate carboxylase gene. Bioprocess Biosyst. Eng. 2016, 39, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Levinson, W.E.; Kurtzman, C.P.; Kuo, T.M. Characterization of Yarrowia lipolytica and related species for citric acid production from glycerol. Enzyme Microb. Technol. 2007, 41, 292–295. [Google Scholar] [CrossRef]
- Sivasankaran, C.; Ravichandran, V.; Mani, J. Comprehensive report on production of citric acid from crude glycerol. Int. J. Appl. Eng. Res. 2015, 10, 11777–11783. [Google Scholar]
- Żywicka, A.; Junka, A.; Ciecholewska-Juśko, D.; Migdał, P.; Czajkowska, J.; Fijałkowski, K. Significant enhancement of citric acid production by Yarrowia lipolytica immobilized in bacterial cellulose-based carrier. J. Biotechnol. 2020, 321, 13–22. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Chatzifragkou, A.; Fakas, S.; Galiotou-Panayotou, M.; Komaitis, M.; Nicaud, J.M.; Aggelis, G. Biosynthesis of lipids and organic acids by Yarrowia lipolytica strains cultivated on glucose. Eur. J. Lipid Sci. Technol. 2009, 111, 1221–1232. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Morgunov, I.G. Metabolic peculiarities of the citric acid overproduction from glucose in yeasts Yarrowia lipolytica. Bioresour. Technol. 2017, 243, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Sayın Börekçi, B.; Kaya, M.; Kaban, G. Citric Acid Production by Yarrowia lipolytica NRRL Y-1094: Optimization of pH, Fermentation Time and Glucose Concentration Using Response Surface Methodology. Fermentation 2022, 8, 731. [Google Scholar] [CrossRef]
- Morgunov, I.G.; Kamzolova, S.V.; Lunina, J.N. The citric acid production from raw glycerol by Yarrowia lipolytica yeast and its regulation. Appl. Microbiol. Biotechnol. 2013, 97, 7387–7397. [Google Scholar] [CrossRef] [PubMed]
- Rzechonek, D.A.; Dobrowolski, A.; Rymowicz, W.; Mirończuk, A.M. Aseptic production of citric and isocitric acid from crude glycerol by genetically modified Yarrowia lipolytica. Bioresour. Technol. 2019, 271, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Chi, Z.; Liu, G.L.; Madzak, C.; Chi, Z.M. Both decrease in ACL1 gene expression and increase in ICL1 gene expression in marine-derived yeast Yarrowia lipolytica expressing INU1 gene enhance citric acid production from inulin. Mar. Biotechnol. 2013, 15, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Rakicka, M.; Wolniak, J.; Lazar, Z.; Rymowicz, W. Production of high titer of citric acid from inulin. BMC Biotechnol. 2019, 19, 11. [Google Scholar] [CrossRef]
- Liu, X.; Lv, J.; Xu, J.; Zhang, T.; Deng, Y.; He, J. Citric acid production in Yarrowia lipolytica SWJ-1b yeast when grown on waste cooking oil. Appl. Biochem. Biotechnol. 2015, 175, 2347–2356. [Google Scholar] [CrossRef]
- Arslan, N.P.; Aydogan, M.N.; Taskin, M. Citric acid production from partly deproteinized whey under non-sterile culture conditions using immobilized cells of lactose—Positive and cold-adapted Yarrowia lipolytica B9. J. Biotechnol. 2016, 231, 32–39. [Google Scholar] [CrossRef]
- Sauer, M.; Porro, D.; Mattanovich, D.; Branduardi, P. Microbial production of organic acids: Expanding the markets. Trends Biotechnol. 2008, 26, 100–108. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Chi, Z.; Liu, G.-L.; Wang, F.; Madzak, C.; Chi, Z.-M. Inulin hydrolysis and citric acid production from inulin using the surface-engineered Yarrowia lipolytica displaying inulinase. Metab. Eng. 2010, 12, 469–476. [Google Scholar] [CrossRef]
- Carsanba, E.; Papanikolaou, S.; Fickers, P.; Erten, H. Screening various Yarrowia lipolytica strains for citric acid production. Yeast 2019, 36, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, E.; Charreau, H.; Cerrutti, P.; Foresti, M.L. Yarrowia lipolytica: A model yeast for citric acid production. FEMS Yeast Res. 2017, 17, fox084. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Kot, A.M.; Bzducha-Wróbel, A.; BŁażejak, S.; Gientka, I.; Kurcz, A. Biotechnological use of Candida yeasts in the food industry: A review. Fungal. Biol. Rev. 2017, 31, 185–198. [Google Scholar] [CrossRef]
- Börekçi, B.S.; Kaban, G.; Kaya, M. Citric acid production of yeasts: An overview. Eurobiotech. J. 2021, 5, 79–91. [Google Scholar] [CrossRef]
- Darouneh, E.; Alavi, A.; Vosoughi, M.; Arjmand, M.; Seifkordi, A.; Rajabi, R. Citric acid production: Surface culture versus submerged culture. Afr. J. Microbiol. Res. 2009, 3, 541–545. [Google Scholar]
- Couto, S.R.; Sanromán, M.Á. Application of solid-state fermentation to food industry—A review. J. Food Eng. 2006, 76, 291–302. [Google Scholar] [CrossRef]
- Kristiansen, B.; Linden, J.; Mattey, M. Citric Acid Biotechnology; Taylor & Francis: Oxfordshire, UK, 1999; ISBN 9780748405145. [Google Scholar]
- Tong, Z.; Tong, Y.; Wang, D.; Shi, Y.C. Whole maize flour and isolated maize starch for production of citric acid by Aspergillus niger: A review. Starch. 2023, 75, 2000014. [Google Scholar] [CrossRef]
- Papagianni, M.; Mattey, M.; Kristiansen, B. The influence of glucose concentration on citric acid production and morphology of Aspergillus niger in batch and culture. Enzyme Microb. Technol. 1999, 25, 710–717. [Google Scholar] [CrossRef]
- Mattedi, A.; Sabbi, E.; Farda, B.; Djebaili, R.; Mitra, D.; Ercole, C.; Cacchio, P.; Del Gallo, M.; Pellegrini, M. Solid-State Fermentation: Applications and Future Perspectives for Biostimulant and Biopesticides Production. Microorganisms 2023, 11, 1408. [Google Scholar] [CrossRef]
- Vandenberghe, L.P.S.; Pandey, A.; Carvalho, J.C.; Letti, L.A.J.; Woiciechowski, A.L.; Karp, S.G.; Thomaz-Soccol, V.; Martínez-Burgos, W.J.; Penha, R.O.; Herrmann, L.W.; et al. Solid-state fermentation technology and innovation for the production of agricultural and animal feed bioproducts. Syst. Microbiol. Biomanuf. 2021, 1, 142–165. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Soccol, C.R.; Pandey, A. Recent advances in solid-state fermentation. Biochem. Eng. J. 2009, 44, 13–18. [Google Scholar] [CrossRef]
- Anastassiadis, S.; Morgunov, I.G.; Kamzolova, S.V.; Finogenova, T.V. Citric acid production patent review. Recent Pat. Biotechnol. 2008, 2, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Kamzolova, S.V.; Fatykhova, A.R.; Dedyukhina, E.G.; Anastassiadis, S.G.; Golovchenko, N.P.; Morgunov, I.G. Citric Acid Production by Yeast Grown on Glycerol-Containing Waste from Biodiesel Industry. Food Technol. Biotechnol. 2011, 41, 65–74. [Google Scholar]
- Dhillon, G.S.; Brar, S.K.; Kaur, S.; Verma, M. Bioproduction and extraction optimization of citric acid from Aspergillus niger by rotating drum type solid-state bioreactor. Ind. Crops Prod. 2013, 41, 78–84. [Google Scholar] [CrossRef]
- Angumeenal, A.R.; Venkappayya, D. An overview of citric acid production. LWT-Food Sci. Technol. 2013, 50, 367–370. [Google Scholar] [CrossRef]
- Show, P.L.; Oladele, K.O.; Siew, Q.Y.; Aziz Zakry, F.A.; Lan, J.C.-W.; Ling, T.C. Overview of citric acid production from Aspergillus niger. Front. Life Sci. 2015, 8, 271–283. [Google Scholar] [CrossRef]
- Citric Acid Global Market Report 2023—Research and Markets. Available online: https://www.researchandmarkets.com/reports/5741687/citric-acid-global-market-report (accessed on 30 November 2023).
- Alben, E.; Erkmen, O. Production of Citric Acid from a New Substrate, Undersized Semolina, by Aspergillus niger. Food Technol. Biotechnol. 2004, 42, 19–22. [Google Scholar]
- Dashen, M.M.; Ado, S.A.; Ameh, J.B.; Mawak, J.D. Effect of different nitrogen sources on citric acid production by Aspergillus niger. Int. J. Biosci. 2008, 3, 102–106. [Google Scholar]
- Haq, I.; Ali, S.; Ashraf, H.; Butt, W.A.; Shafiq, K.Q.; Iqbal, J. Effect of mineral nutrient of the biosynthesis of citric acid by Aspergillus niger UV-6, using sucrose salt media. Pak. J. Bot. 2001, 33, 535–540. [Google Scholar]
- Ikram-Ul-Haq Ali, S.; Qadeer, M.A.; Iqbal, J. Optimization of Nitrogen for Enhanced Citric Acid Productivity by a 2-Deoxy d-Glucose Resistant Culture of Aspergillus niger NGd-280. Bioresour. Technol. 2005, 96, 645–648. [Google Scholar] [CrossRef]
- Papagianni, M.; Wayman, F.; Mattey, M. Fate and role of ammonium ions during fermentation of citric acid by Aspergillus niger. Appl. Environ. Microbiol. 2005, 71, 7178–7186. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, L.P.S.; Soccol, C.R.; Pandey, A.; Lebeault, J.M. Microbial Production of Citric Acid. Braz. Arch. Biol. 761 Technol. 1998, 42, 263–276. [Google Scholar] [CrossRef]
- Milsom, P.E. Organic Acids by Fermentation, especially Citric Acid. In Food Biotechnology—1; Springer: Dordrecht, The Netherlands, 1987; pp. 273–307. [Google Scholar]
- Yigitoglu, M. Production of citric acid by fungi biotechnology. J. Islam. Acad. Sci. 1992, 52, 100–106. [Google Scholar]
- Kubicek, C.P.; Hampel, W.; Rohr, M. Manganese deficiency leads to elevated amino acid pools in citric acid accumulating Aspergillus niger. Arch. Microbiol. 1979, 123, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, M.; Mattey, M. Morphological development of Aspergillus niger in submerged citric acid fermentation as a function of the spore inoculum level. Application of neural network and cluster analysis for characterization of mycelial morphology. Microb. Cell Fact. 2006, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.-U.; Ali, S.; Qadeer, M.; Iqbal, J. Stimulatory effect of alcohols (methanol and ethanol) on citric acid productivity by a 2-deoxy D-glucose resistant culture of Aspergillus niger GCB-47. Bioresour. Technol. 2003, 86, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Dymarska, E.; Pietkiewicz, J.J. Wpływ metanolu na proces biosyntezy kwasu cytrynowego z sacharozy przez Aspergillus niger. Zesz. Probl. Postępów Nauk Rol. 2016, 584, 23–31. [Google Scholar]
- Pazouki, M.; Felse, P.A.; Sinha, J.; Panda, T. Comparative studies on citric acid production by Aspergillus niger and Candida lipolytica using molasses and glucose. Bioprocess Eng. 2000, 22, 353–361. [Google Scholar] [CrossRef]
- Leśniak, W. Przydatność niektórych substancji jako stymulatorów w procesie fermentacji wgłębnej kwasu cytrynowego. Pr. Nauk. Wyższej Szk. Ekon. Wrocławiu. 1974, 52, 49–61. [Google Scholar]
- Barrington, S.; Kim, J.-W. Response surface optimization of medium components for citric acid production by Aspergillus niger NRRL 567 grown in peat moss. Bioresour. Technol. 2008, 99, 368–377. [Google Scholar] [CrossRef]
- Nadeem, A.; Syed, Q.; Baig, S.; Irfan, M.; Nadeem, M. Enhanced Production of Citric Acid by Aspergillus niger M-101 Using Lower Alcohols. Turk. J. Biochem. 2010, 35, 7–13. [Google Scholar]
- Rugasaseel, S.; Morikawa, S.; Kirimura, K.; Usami, S. Stimulation of citric acid production in Aspergillus niger by addition of viscous substances in shake culture. Appl. Microbiol. Biotechnol. 1995, 42, 839–843. [Google Scholar] [CrossRef]
- Podgórski, W. Kształtowanie aktywności oddechowej i kwasotwórczej Aspergillus niger podczas produkcji kwasu cytrynowego w podłożach z melasą trzcinową. Pr. Nauk. Akad. Ekon. Wrocławiu. Ser. Monogr. Opracowania 2002, 914, 138. [Google Scholar]
- Benuzzi, D.A.; Segovia, R.F. Effect of the copper concentration on citric acid productivity by an Aspergillus niger strain. Appl. Biochem. Biotechnol. 1996, 61, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Maddox, I.S.; Hossain, M.; Brooks, J.D. The effect of methanol on citric acid production from galactose by Aspergillus niger. Appl. Microbiol. Biotechnol. 1986, 23, 203–205. [Google Scholar] [CrossRef]
- Yaykaşlı, K.O.; Demirel, G.; Yaşar, A. Influence of alcohols on citric acid production by Aspergillus niger A-9 entrapped in polyacrylamide gels. J. Food Eng. 2005, 70, 518–522. [Google Scholar] [CrossRef]
- MOYER, A.J. Effect of alcohols on the mycological production of citric acid in surface and submerged culture. II. Fermentation of crude carbohydrates. Appl. Microbiol. 1953, 1, 7–13. [Google Scholar] [CrossRef]
- Roukas, T.; Kotzekidou, P. Pretreatment of date syrup to increase citric acid production. Enzyme Microb. Technol. 1997, 21, 273–276. [Google Scholar] [CrossRef]
- Ashraf, H.; Rehman, A.; Haq, I. Effect of alcohols on the production of citric acid by Aspergillus niger using solid state fermentation. J. Food Technol. 2004, 2, 1–3. [Google Scholar]
- Kareem, S.O.; Akpan, I.; Alebiowu, O.O. Production of citric acid by Aspergillus niger using pineapple waste. Malays. J. Microbiol. 2010, 6, 161–166. [Google Scholar]
- Shetty, V.G. Production and optimization of citric acid by Aspergillus niger using molasses and corncob. Int. J. Pharm. Pharm. Sci. 2015, 7, 152–157. [Google Scholar]
- Ramesh, T.; Kalaiselvam, M. An Experimental Study on Citric Acid Production by Aspergillus niger Using Gelidiella acerosa as a Substrate. Indian J. Microbiol. 2011, 51, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Auta, H.S.; Abidoye, K.T.; Tahir, H.; Ibrahim, A.D.; Aransiola, S.A. Citric Acid Production by Aspergillus niger Cultivated on Parkia biglobosa Fruit Pulp. Int. Sch. Res. Not. 2014, 2014, 762021. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.R.; Lehmann, L.; Nielsen, J. Systemic analysis of the response of Aspergillus niger to ambient pH. Genome Biol. 2009, 10, R47. [Google Scholar] [CrossRef] [PubMed]
- Walaszczyk, E.; Podgórski, W.; Janczar-Smuga, M.; Dymarska, E. Effect of medium pH on chemical selectivity of oxalic acid biosynthesis by Aspergillus niger W78C in submerged batch cultures with sucrose as a carbon source. Chem. Zvesti 2018, 72, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Dawson, M.W.; Maddox, I.S.; Brooks, J.D. Effect of interruptions to the air supply on citric acid production by Aspergillus niger. Enzyme Microb. Technol. 1986, 8, 37–40. [Google Scholar] [CrossRef]
- Shaikh, Y.; Jagtap, M.R. Organic Acid and Solvent Production from Microbial Fermentation. In Microbial Products for Future Industrialization. Interdisciplinary Biotechnological Advances; Sarkar, A., Ahmed, I.A., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Fan, X.; Burton, R.; Zhou, Y. Glycerol (Byproduct of Biodiesel Production) as a Source for Fuels and Chemicals—Mini Review. Open Fuels Energy Sci. J. 2010, 3, 17–22. [Google Scholar] [CrossRef]
- Shankar, T.; Sivakumar, T. Optimization of Citric Acid Production Using Aspergillus niger Isolated from the Leaf Litter Soil of Sathuragiri Hills. Univ. J. Microbiol. Res. 2016, 4, 79–87. [Google Scholar] [CrossRef]
- Hussein, E.L.A.; Tawfig, S.M.; Siddig, M.; Siddig, M. Citric acid production from kenana cane molasses by Aspergillus niger in submerged fermentation. J. Genet. Eng. Biotechnol. 2009, 7, 51–57. [Google Scholar]
- Rehman, A.; Ali, S.; Haq, I. Temperature Optima for Citric Acid Accumulation by Aspergillus niger. Biotechnology 2002, 1, 108–110. [Google Scholar] [CrossRef]
- Papadaki, E.; Mantzouridou, F.T. Citric acid production from the integration of Spanish-style green olive processing wastewaters with white grape pomace by Aspergillus niger. Bioresour. Technol. 2019, 280, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Chergui, D.; Akretche-Kelfat, S.; Lamoudi, L.; Al-Rshaidat, M.; Boudjelal, F.; Ait-Amar, H. Optimization of citric acid production by Aspergillus niger using two downgraded Algerian date varieties. Saudi J. Biol. Sci. 2021, 28, 7134–7141. [Google Scholar] [CrossRef] [PubMed]
- Addo, M.G.; Kusi, A.; Andoh, L.A.; Obiri-Danso, K. Citric Acid Production by Aspergillus Niger on a Corn Cob Solid Substrate using One factor at a time Optimisation Method. Int. Adv. Res. J. Sci. Eng. Technol. 2016, 3. [Google Scholar] [CrossRef]
- Adeoye, A.O.; Lateef, A. Improving the Yield of Citric Acid Through Valorization of Cashew Apple Juice by Aspergillus niger: Mutation, Nanoparticles Supplementation and Taguchi Technique. Waste Biomass Valorization 2022, 13, 2195–2206. [Google Scholar] [CrossRef]
- Adeoye, A.O.; Lateef, A.; Gueguim-Kana, E.B. Optimization of citric acid production using a mutant strain of Aspergillus niger on cassava peel substrate. Biocatal. Agric. Biotechnol. 2015, 4, 568–574. [Google Scholar] [CrossRef]
- Alam, M.Z.; Bari, M.N.; Muyibi, S.A.; Jamal, P. Development of Culture Inoculum for Scale-Up Production of Citric Acid from Oil Palm Empty Fruit Bunches by Aspergillus niger. Procedia Environ. Sci. 2011, 8, 396–402. [Google Scholar] [CrossRef]
- Bibi, N.; Ali, S.; Tabassum, R. Statistical Optimization of Pectinase Biosynthesis from Orange Peel by Bacillus licheniformis Using Submerged Fermentation. Waste Biomass Valorization 2016, 7, 467–481. [Google Scholar] [CrossRef]
- Amenaghawon, N.; Osazuwa, O.; Okieimen, C. Dynamic Modelling and Simulation of Citric Acid Production from Dilute Acid Hydrolysed Corn Starch Using Aspergillus Niger. Niger. J. Technol. 2014, 33, 222. [Google Scholar] [CrossRef][Green Version]
- Amenaghawon, N.; Oronsaye, J.; Ogbeide, S. Statistical Optimisation of Fermentation Conditions for Citric Acid Production from Pineapple Peels. Niger. J. Technol. Res. 2014, 9, 20. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Mahboubi, A.; Lennartsson, P.R.; Taherzadeh, M.J. Waste biorefineries using filamentous ascomycetes fungi: Present status and future prospects. Bioresour. Technol. 2016, 215, 334–345. [Google Scholar] [CrossRef]
- Xu, D.-B.; Madrid, C.; Rohr, M.; Kubicek, C. The influence of type and concentration of the carbon source on production of citric acid by Aspergillus niger. Appl. Microbiol. Biotechnol. 1989, 30, 553–558. [Google Scholar] [CrossRef]
- Ghanbartabar, S.A.; Najafpour, G.D.; Mohammadi, M. Comparative studies on citric acid production from cheese why by submerged and immobilized aspergillus niger. J. Biotechnol. 2016, 13, 79–85. [Google Scholar]
- Lotfy, W.; Ghanem, K.; Elhelow, E. Citric acid production by a novel Aspergillus niger isolate: II. Optimization of process parameters through statistical experimental designs. Bioresour. Technol. 2007, 98, 3470–3477. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, W.A.; Ghanem, K.M.; El-Helow, E.R. Citric acid production by a novel Aspergillus niger isolate: I. Mutagenesis and cost reduction studies. Bioresour. Technol. 2007, 98, 3464–3469. [Google Scholar] [CrossRef] [PubMed]
- Jianlong, W.; Xianghua, W.; Ding, Z. Production of Citric Acid from Molasses Integrated with In-Situ Product Separation by Ion-Exchange Resin Adsorption. Bioresour. Technol. 2000, 75, 231–234. [Google Scholar] [CrossRef]
- Al-Mahin, A.; Hasan, S.M.; Khan, M.H.; Begum, R. Citric Acid Production by Aspergillus niger through Solid-State Fermentation on Sugarcane Bagasse. Bangladesh J. Microbiol. 2010, 25, 9–12. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, H.; Yang, J.; Qi, H. Preparation of levoglucosan by pyrolysis of cellulose and its citric acid fermentation. Bioresour. Technol. 2001, 79, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Bari, M.N.; Alam, M.Z.; Muyibi, S.A.; Jamal, P. Abdullah-Al-Mamun Improvement of production of citric acid from oil palm empty fruit bunches: Optimization of media by statistical experimental designs. Bioresour. Technol. 2009, 100, 3113–3120. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Brar, S.K.; Verma, M.; Tyagi, R.D. Apple pomace ultrafiltration sludge—A novel substrate for fungal bioproduction of citric acid: Optimisation studies. Food Chem. 2011, 128, 864–871. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Brar, S.K.; Verma, M.; Tyagi, R.D. Utilization of different agro-industrial wastes for sustainable bioproduction of citric acid by Aspergillus niger. Biochem. Eng. J. 2011, 54, 83–92. [Google Scholar] [CrossRef]
- Drysdale, C.R.; McKay, A.M. Citric acid production by Aspergillus niger in surface culture on inulin. Lett. Appl. Microbiol. 1995, 20, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Improving the barrier and mechanical properties of corn starch-based edible films: Effect of citric acid and carboxymethyl cellulose. Ind. Crops Prod. 2011, 33, 229–235. [Google Scholar] [CrossRef]
- Rao, P.R.; Reddy, M.K. Production of Citric Acid by Aspergillus Niger Using Oat Bran as Substrate. Int. J. Chem. Chem. Eng. 2013, 3, 2248–9924. [Google Scholar]
- Betiku, E.; Adesina, O.A. Statistical approach to the optimization of citric acid production using filamentous fungus Aspergillus niger grown on sweet potato starch hydrolyzate. Biomass Bioenergy 2013, 55, 350–354. [Google Scholar] [CrossRef]
- Surendra Babu, A.; Parimalavalli, R.; Rudra, S.G. Effect of citric acid concentration and hydrolysis time on physicochemical properties of sweet potato starches. Int. J. Biol. Macromol. 2015, 80, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Bauweleers, H.M.K.; Groeseneken, D.R. Process for the Preparation of Citric Acid Employing Filamentous Fungi in a Culture 802 Medium Comprising Glycerol. Published Online 13 September 2008. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2008107472 (accessed on 7 January 2016).
- Schneider, M.; Zimmer, G.F.; Cremonese, E.B.; de C de S Schneider, R.; Corbellini, V.A. By-products from the biodiesel chain as a substrate to citric acid production by solid-state fermentation. Waste Manag. Res. 2014, 32, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Haq, I.; Qadeer, M.A.; Iqbal, J. Production of citric acid by Aspergillus niger using cane molasses in a stirred fermentor. Electron. J. Biotechnol. 2002, 5, 19–20. [Google Scholar] [CrossRef]
- Dymarska, E.; Janczar–Smuga, M. Application of the central composite rotatable design in the optimization of medium constituents for the production of citric acid from anhydrous glycerol. Przem. Chem. 2018, 97, 1276–1282. [Google Scholar] [CrossRef]
- Ikram-ul, H.; Ali, S.; Qadeer, M.A.; Iqbal, J. Citric acid production by selected mutants of Aspergillus niger from cane molasses. Bioresour. Technol. 2004, 93, 125–130. [Google Scholar] [CrossRef]
- Kishore, K.; Anand, K.M.; Praveen, K.V.; Ravi, R.G.V. Optimization of Process Variables of Citric Acid Production Using Aspergillus Niger In A Batch Fermentor. Eng. Lett. 2008, 16, 572–577. Available online: http://www.engineeringletters.com/issues_v16/issue_4/EL_16_4_17.pdf (accessed on 24 March 2017).
- Mourya, S.; Jauhri, K.S. Production of citric acid from starch-hydrolysate by Aspergillus niger. Microbiol. Res. 2000, 155, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.-U.; Ali, S.; Iqbal, J. Direct production of citric acid from raw starch by Aspergillus niger. Process Biochem. 2003, 38, 921–924. [Google Scholar] [CrossRef]
- Foryś, E.; Podgórski, W.; Kaczyńska, M. Wpływ makroelementów na proces biosyntezy kwasu cytrynowego z glicerolu przez 826 Aspergillus niger W78B 1. Acta Sci. Pol. Biotechnol. 2007, 6, 31–37. [Google Scholar]
- Książek, E.; Janczar-Smuga, M.; Pietkiewicz, J.J. Bioconversion of glycerol into citric acid. Przem. Chem. 2020, 99, 141–150. [Google Scholar] [CrossRef]
- Książek, E.; Janczar-Smuga, M. Efektywność biosyntezy kwasu cytrynowego w zasilanych okresowych hodowlach wgłębnych. Zesz. Probl. Postępów Nauk Rol. 2019, 595, 77–91. [Google Scholar] [CrossRef]
- Yadegary, M.; Hamidi, A.; Alavi, S.A.; Khodaverdi, E.; Yahaghi, H.; Sattari, S.; Bagherpour, G.; Yahaghi, E. Citric Acid Production from Sugarcane Bagasse through Solid State Fermentation Method Using Aspergillus niger Mold and Optimization of Citric Acid Production by Taguchi Method. Jundishapur J. Microbiol. 2013, 6, 595. [Google Scholar] [CrossRef]
- Kumar, D.; Jain, V.K.; Shanker, G.; Srivastava, A. Citric acid production by solid state fermentation using sugarcane bagasse. Process Biochem. 2003, 38, 1731–1738. [Google Scholar] [CrossRef]
- Chaturvedi, M. Citric acid production from cane molasses using submerged fermentation by Aspergillus niger ATCC9142. J. Pharm. Res. 2010, 3, 1215–1222. [Google Scholar]
- Javed, S.; Asgher, M.; Sheikh, M.A.; Nawaz, H.; Jamil, A. Enhanced citric acid production by Aspergillus niger EB-3 mutant using an inert solid support in molasses medium. Afr. J. Biotechnol. 2011, 10, 11784–11791. [Google Scholar]
- Adham, N. Attempts at improving citric acid fermentation by Aspergillus niger in beet-molasses medium. Bioresour. Technol. 2002, 84, 97–100. [Google Scholar] [CrossRef]
- Podgórski, W.; Gąsiorek, E.; Leśniak, W. Produkty uboczne z przerobu buraków cukrowych jako substraty do biosyntezy kwasu cytrynowego. Inżynieria Rol. 2006, 79, 103–109. [Google Scholar]
- Kumar, D.; Jain, V.K.; Shanker, G.; Srivastava, A. Utilisation of fruits waste for citric acid production by solid state fermentation. Process Biochem. 2003, 38, 1725–1729. [Google Scholar] [CrossRef]
- El-Holi, M.A.; Al-Delaimy, S. Citric acid production from whey with sugars and additives by Aspergillus niger. Afr. J. Biotechnol. 2004, 2, 356–359. [Google Scholar]
- Mostafa, Y.S.; Alamri, S.A. Optimization of date syrup for enhancement of the production of citric acid using immobilized cells of Aspergillus niger. Saudi J. Biol. Sci. 2012, 19, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Barrington, S.; Sheppard, J.; Lee, B. Nutrient optimization for the production of citric acid by Aspergillus niger NRRL 567 grown on peat moss enriched with glucose. Process Biochem. 2006, 41, 1253–1260. [Google Scholar] [CrossRef]
- Xie, G.; West, T.P. Citric acid production by Aspergillus niger ATCC 9142 from a treated ethanol fermentation co-product using solid-state fermentation. Lett. Appl. Microbiol. 2009, 48, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; West, T.P. Citric Acid Production by Aspergillus niger on the Ethanol Dry Milling Coproduct Thin Stillage. Res. J. Microbiol. 2007, 2, 678–683. [Google Scholar] [CrossRef]
- Mehyar, G.F.; Delaimy, K.S.; Ibrahim, S.A. Citric Acid Production by Aspergillus niger Using Date-Based Medium Fortified with Whey and Additives. Food Biotechnol. 2005, 19, 137–144. [Google Scholar] [CrossRef]
- Hamdy, H.S. Citric acid production by Aspergillus niger grown on orange peel medium fortified with cane molasses. Ann. Microbiol. 2013, 63, 267–278. [Google Scholar] [CrossRef]
- Goud, K.H.; Srilakshmi, A.; Kumar, A.P.G. Narasimha Citric acid production by Aspergillus niger through solid state fermentation using fruit wastes. Biotechnol. Indian J. 2012, 6, 93–96. [Google Scholar]
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.; Teixeira, J.A. Use of agro-industrial wastes in solid-state fermentation processes. In 866 Industrial Waste; Yeow, S.K., Xinxin, G., Eds.; In Tech: Rijeka, Croatia, 2012. [Google Scholar]
- Mussatto, S.I.; Teixeira, J.A. Lignocellulose as raw material in fermentation processes. In Current Research, Technology and 868 Education Topics in Applied Microbiology and Microbial Biotechnology; Méndez-Vilas, A., Ed.; Formatex Research: Badajoz, Spain, 2010. [Google Scholar]
- Książek, E.E.; Janczar-Smuga, M.; Pietkiewicz, J.J.; Walaszczyk, E. Optimization of Medium Constituents for the Production of Citric Acid from Waste Glycerol Using the Central Composite Rotatable Design of Experiments. Molecules 2023, 28, 3268. [Google Scholar] [CrossRef] [PubMed]
- Salazar Peña, M. Systems Biology of Glucose Sensing and Repression in Aspergillus niger: Lessons from Genomics and 874 Transcriptomics. Ph.D Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2010. Available online: http://publications.lib.chalmers.se/records/fulltext/127099.pdf (accessed on 19 May 2017).
- Kośmider, A.; Czaczyk, K. Perspektywy wykorzystania glicerolu w procesach biotechnologicznych. Postępy Mikrobiol. 2009, 48, 277–287. [Google Scholar]
- Honecker, S.; Bisping, B.; Yang, Z.; Rehm, H.-J. Influence of sucrose concentration and phosphate limitation on citric acid production by immobilized cells of Aspergillus niger. Appl. Microbiol. Biotechnol. 1989, 31, 17–24. [Google Scholar] [CrossRef]
- Hossain, M.; Brooks, J.D.; Maddox, I.S. The effect of the sugar source on citric acid production by Aspergillus niger. Appl. Microbiol. Biotechnol. 1984, 19, 393–397. [Google Scholar] [CrossRef]
- Thorat, S.S.; Patil, G. Standardization of Process Parameters for Production of Citric Acid from Mahua Flowers (Madhuca indica) by Surface Fermentation using Aspergillus niger NCIM-545 and NCIM-595. Intl. J. Food. Ferment. Technol 2016, 6, 111–120. [Google Scholar] [CrossRef]
- Omar, S.; Honecker, S.; Rehm, H.-J. A comparative study on the formation of citric acid and polyols and on morphological changes of three strains of free and immobilized Aspergillus niger. Appl. Microbiol. Biotechnol. 1992, 36, 518–524. [Google Scholar] [CrossRef]
- Tric acid and salts Handling/Processing. Citric Acid and salts Handling/Processing. Published Online 2015. Available online: https://www.ams.usda.gov/sites/default/files/media/CitricAcid892TR2015.pdf (accessed on 16 April 2017).
- Ciriminna, R.; Chavarría-Hernández, N.; Inés Rodríguez Hernández, A.; Pagliaro, M. Pectin: A new perspective from the biorefinery standpoint. Biofuels Bioprod. Biorefining 2015, 9, 368–377. [Google Scholar] [CrossRef]
- Ke, S.; Huang, Y.; Decker, E.A.; Hultin, H.O. Impact of citric acid on the tenderness, microstructure and oxidative stability of beef muscle. Meat Sci. 2009, 82, 113–118. [Google Scholar] [CrossRef]
- Sammel, L.M.; Claus, J.R. Citric acid and sodium citrate effects on pink color development of cooked ground turkey irradiated pre- and post-cooking. Meat Sci. 2006, 72, 567–573. [Google Scholar] [CrossRef]
- Sammel, L.M.; Claus, J.R.; Greaser, M.L.; Richards, M.P. Investigation of mechanisms by which sodium citrate reduces the pink color defect in cooked ground turkey. Meat Sci. 2006, 72, 585–595. [Google Scholar] [CrossRef]
- Kappes, S.M.; Schmidt, S.J.; Lee, S.Y. Relationship between Physical Properties and Sensory Attributes of Carbonated Beverages. J. Food Sci. 2007, 72, S001–S011. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zou, L.; Liu, J.; Zhang, Z.; Liu, C.; Liang, R. The effect of citric acid on the activity, thermodynamics and conformation of mushroom polyphenoloxidase. Food Chem. 2013, 140, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, C.; da Silva, A.J.R.; Lopes, M.L.M.; Fialho, E.; Valente-Mesquita, V.L. Polyphenol oxidase activity, phenolic acid composition and browning in cashew apple (Anacardium occidentale L.) after processing. Food Chem. 2011, 125, 128–132. [Google Scholar] [CrossRef]
- Goyeneche, R.; Agüero, M.V.; Roura, S.; Di Scala, K. Application of citric acid and mild heat shock to minimally processed sliced radish: Color evaluation. Postharvest Biol. Technol. 2014, 93, 106–113. [Google Scholar] [CrossRef]
- Lücke, F.-K. Encyclopedia of Food Safety; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780123786135. [Google Scholar]
- Manolopoulou, E. Effect of Storage Conditions on the Sensory Quality, Colour and Texture of Fresh-Cut Minimally Processed Cabbage with the Addition of Ascorbic Acid, Citric Acid and Calcium Chloride. Food Nutr. Sci. 2011, 2, 956–963. [Google Scholar] [CrossRef]
- Goyeneche, R.; Di Scala, K.; Roura, S. Biochemical characterization and thermal inactivation of polyphenol oxidase from radish (Raphanus sativus var. sativus). LWT-Food Sci. Technol. 2013, 54, 57–62. [Google Scholar] [CrossRef]
- Sotoyama, M.; Uchida, S.; Tanaka, S.; Hakamata, A.; Odagiri, K.; Inui, N.; Watanabe, H.; Namiki, N. Citric Acid Suppresses the Bitter Taste of Olopatadine Hydrochloride Orally Disintegrating Tablets. Biol. Pharm. Bull. 2017, 40, 451–457. [Google Scholar] [CrossRef]
- Yıldız, S.; Aytekin, E.; Yavuz, B.; Bozdağ Pehlivan, S.; Vural, İ.; Ünlü, N. Development and evaluation of orally disintegrating tablets comprising taste-masked mirtazapine granules. Pharm. Dev. Technol. 2018, 23, 488–495. [Google Scholar] [CrossRef]
- Shalaev, E.Y.; Johnson-Elton, T.D.; Chang, L.; Pikal, M.J. Thermophysical properties of pharmaceutically compatible buffers at sub-zero temperatures: Implications for freeze-drying. Pharm. Res. 2002, 19, 195–201. [Google Scholar] [CrossRef]
- Islam, K.M.S.; Schaeublin, H.; Wenk, C.; Wanner, M.; Liesegang, A. Effect of dietary citric acid on the performance and mineral metabolism of broiler. J. Anim. Physiol. Anim. Nutr. 2012, 96, 808–817. [Google Scholar] [CrossRef]
- Paleckiene, R.; Sviklas, A.; Slinksiene, R. Reaction of Urea with Citric Acid. Russ. J. Appl. Chem. 2005, 78, 1651–1655. [Google Scholar] [CrossRef]
- Naeini, A.T.; Adeli, M.; Vossoughi, M.; Sobhani, Z.; Atyabi, F.; Safinya, C.R. Poly(citric acid)-block-poly(ethylene glycol) copolymers--new biocompatible hybrid materials for nanomedicine. Nanomedicine 2010, 6, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Nath, B.; Sarkar, S.; Islam, S.M.; Bundschuh, J.; Chatterjee, D.; Hidalgo, M. Application of natural citric acid sources and their role on arsenic removal from drinking water: A green chemistry approach. J. Hazard. Mater. 2013, 262, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Rastegar, H.; Shoeibi, S.; Yazdanpanah, H.; Amirahmadi, M.; Khaneghah, A.M.; Campagnollo, F.B.S.; Sant’Ana, A. Removal of aflatoxin B1 by roasting with lemon juice and/or citric acid in contaminated pistachio nuts. Food Control 2017, 71, 279–284. [Google Scholar] [CrossRef]
- Ghorpade, V.S.; Yadav, A.V.; Dias, R.J. Citric acid crosslinked β-cyclodextrin/carboxymethylcellulose hydrogel films for controlled delivery of poorly soluble drugs. Carbohydr. Polym. 2017, 164, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Olaimat, A.N.; Al-Nabulsi, A.A.; Osaili, T.M.; Al-Holy, M.; Ayyash, M.M.; Mehyar, G.F.; Jaradat, Z.W.; Ghoush, M.A. Survival and inhibition of Staphylococcus aureus in commercial and hydrated tahini using acetic and citric acids. Food Control 2017, 77, 179–186. [Google Scholar] [CrossRef]
- Mielcarek, A.; Rodziewicz, J.; Janczukowicz, W.; Dabrowska, D.; Ciesielski, S.; Thornton, A.; Struk-Sokołowska, J. Citric acid application for denitrification process support in biofilm reactor. Chemosphere 2017, 171, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Han, X.; Chen, L.; Ni, L.; Liu, Z.; Zhang, W.; Chen, Q. Comparative characterization of the deamidation of carboxylic acid deamidated wheat gluten by altering the processing conditions. Food Chem. 2016, 210, 520–529. [Google Scholar] [CrossRef]
- Hagiwara, T.; Hagihara, S.; Handa, A.; Sasagawa, N.; Kawashima, R.; Sakiyama, T. Pretreatment with citric acid or a mixture of nitric acid and citric acid to suppress egg white protein deposit formation on stainless steel surfaces and to ease its removal during cleaning. Food Control 2015, 53, 35–40. [Google Scholar] [CrossRef]
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M. Citric acid: Emerging applications of key biotechnology industrial product. Chem. Cent. J. 2017, 11, 22. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef]
- Seligra, P.G.; Medina Jaramillo, C.; Famá, L.; Goyanes, S. Biodegradable and non-retrogradable eco-films based on starch–glycerol with citric acid as crosslinking agent. Carbohydr. Polym. 2016, 138, 66–74. [Google Scholar] [CrossRef]
- Sun, D.; Chen, Y.; Tran, R.T.; Xu, S.; Xie, D.; Jia, C.; Wang, Y.; Guo, Y.; Zhang, Z.; Guo, J.; et al. Citric Acid-based Hydroxyapatite Composite Scaffolds Enhance Calvarial Regeneration. Sci. Rep. 2014, 4, 6912. [Google Scholar] [CrossRef] [PubMed]
- Alberts, A.H.; Rothenberg, G. Process for Preparing Foamed Polymer. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2012052385&recNum=239&docAn=EP2011068076&queryString948=IC/C08G-63/00&maxRec=3738 (accessed on 8 April 2017).
- Olsson, E.; Hedenqvist, M.S.; Johansson, C.; Järnström, L. Influence of citric acid and curing on moisture sorption, diffusion and permeability of starch films. Carbohydr. Polym. 2013, 94, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.S.; Grossmann, M.V.E.; Yamashita, F.; Mali, S.; Dall’Antonia, L.H.; Barreto, W.J. Citric acid as multifunctional agent in blowing films of starch/PBAT. Quim. Nova 2011, 34, 1507–1510. [Google Scholar] [CrossRef]
- Olsson, E.; Menzel, C.; Johansson, C.; Andersson, R.; Koch, K.; Järnström, L. The effect of pH on hydrolysis, cross-linking and barrier properties of starch barriers containing citric acid. Carbohydr. Polym. 2013, 98, 1505–1513. [Google Scholar] [CrossRef]
- Menzel, C.; Olsson, E.; Plivelic, T.S.; Andersson, R.; Johansson, C.; Kuktaite, R.; Järnström, L.; Koch, K. Molecular structure of citric acid cross-linked starch films. Carbohydr. Polym. 2013, 96, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Zhang, Z.; Liu, Q.; Han, Y.; Zhang, L.; Chen, D.; Tian, W. Characterization of citric acid/glycerol co-plasticized thermoplastic starch prepared by melt blending. Carbohydr. Polym. 2007, 69, 748–755. [Google Scholar] [CrossRef]
- Ma, X.; Chang, P.R.; Yu, J.; Stumborg, M. Properties of biodegradable citric acid-modified granular starch/thermoplastic pea starch composites. Carbohydr. Polym. 2009, 75, 1–8. [Google Scholar] [CrossRef]
- Fajd, E.; Marton, G. Starch Citrate as an Ion Exchange Material–Preparation and Investigation. Hung. J. Ind. Chem. 2004, 32. [Google Scholar]
- Wing, R.E. Starch Citrate: Preparation and Ion Exchange Properties. Starch-Starke 1996, 48, 275–279. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]
- Shi, R.; Bi, J.; Zhang, Z.; Zhu, A.; Chen, D.; Zhou, X.; Zhang, L.; Tian, W. The effect of citric acid on the structural properties and cytotoxicity of the polyvinyl alcohol/starch films when molding at high temperature. Carbohydr. Polym. 2008, 74, 763–770. [Google Scholar] [CrossRef]
- Garcia, P.S.; Grossmann, M.V.E.; Shirai, M.A.; Lazaretti, M.M.; Yamashita, F.; Muller, C.M.O.; Mali, S. Improving action of citric acid as compatibiliser in starch/polyester blown films. Ind. Crops Prod. 2014, 52, 305–312. [Google Scholar] [CrossRef]
- Abdillahi, H.; Chabrat, E.; Rouilly, A.; Rigal, L. Influence of citric acid on thermoplastic wheat flour/poly(lactic acid) blends. II. Barrier properties and water vapor sorption isotherms. Ind. Crops Prod. 2013, 50, 104–111. [Google Scholar] [CrossRef]
- Chabrat, E.; Abdillahi, H.; Rouilly, A.; Rigal, L. Influence of citric acid and water on thermoplastic wheat flour/poly(lactic acid) blends. I: Thermal, mechanical and morphological properties. Ind. Crops Prod. 2012, 37, 238–246. [Google Scholar] [CrossRef]
- Ghorpade, V.S.; Yadav, A.V.; Dias, R.J. Citric acid crosslinked cyclodextrin/hydroxypropylmethylcellulose hydrogel films for hydrophobic drug delivery. Int. J. Biol. Macromol. 2016, 93, 75–86. [Google Scholar] [CrossRef]
- Hu, Y.; Liang, P.; Wang, Z.; Zhu, H.; Liu, Q. Developing amino acid-citric acid-based deep eutectic solvent for food applications: Preparation, characterization, antibacterial activity, biosafety, and formation mechanism exploration. Sustain. Chem. Pharm. 2023, 36, 101317. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Liang, P.; Zhu, H.; Liu, Q. Exploring the molecular mechanisms of isoliquiritin extraction using choline chloride-citric acid deep eutectic solvents. Sustain. Chem. Pharm. 2023, 33, 101099. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Qin, H.; Hu, X.; Wang, J.; Cheng, H.; Chen, L.; Qi, Z. Overview of acidic deep eutectic solvents on synthesis, properties and applications. Green Energy Environ. 2020, 5, 8–21. [Google Scholar] [CrossRef]
- Zaib, Q.; Eckelman, M.J.; Yang, Y.; Kyung, D. Are deep eutectic solvents really green?: A life-cycle perspective. Green Chem. 2022, 24, 7924–7930. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Omar, K.A.; Sadeghi, R. Physicochemical properties of deep eutectic solvents: A review. J. Mol. Liq. 2022, 360, 119524. [Google Scholar] [CrossRef]
- Koigerova, A.; Gosteva, A.; Samarov, A.; Tsvetov, N. Deep Eutectic Solvents Based on Carboxylic Acids and Glycerol or Propylene Glycol as Green Media for Extraction of Bioactive Substances from Chamaenerion angustifolium (L.) Scop. Molecules 2023, 28, 6978. [Google Scholar] [CrossRef] [PubMed]
- González-Fandos, E.; Herrera, B.; Maya, N. Efficacy of citric acid against Listeria monocytogenes attached to poultry skin during refrigerated storage. Int. J. Food Sci. Technol. 2009, 44, 262–268. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Skandamis, P. 18—New research on organic acids and pathogen behaviour. In Advances in Microbial Food Safety; Elsevier: Amsterdam, The Netherlands, 2013; pp. 355–384. ISBN 9780857094384. [Google Scholar]
- Schmidt, S.E.; Taylor, T.M.; Davidson, P.M. Chemical Preservatives and Natural Antimicrobial Compounds. In Food Microbiology; American Society of Microbiology: Washington, DC, USA, 2013; pp. 765–801. [Google Scholar]
- Al-Nabulsi, A.A.; Olaimat, A.N.; Osaili, T.M.; Shaker, R.R.; Zein Elabedeen, N.; Jaradat, Z.W.; Abushelaibi, A.; Holley, R.A. Use of acetic and citric acids to control Salmonella Typhimurium in tahini (sesame paste). Food Microbiol. 2014, 42, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Kim, S.A.; Lee, N.Y.; Rhee, M.S. New decontamination method based on caprylic acid in combination with citric acid or vanillin for eliminating Cronobacter sakazakii and Salmonella enterica serovar Typhimurium in reconstituted infant formula. Int. J. Food Microbiol. 2013, 166, 499–507. [Google Scholar] [CrossRef]
- Mahmoud, B.S.M. The efficacy of grape seed extract, citric acid and lactic acid on the inactivation of Vibrio parahaemolyticus in shucked oysters. Food Control 2014, 41, 13–16. [Google Scholar] [CrossRef]
- García-Soto, B.; Fernández-No, I.C.; Barros-Velázquez, J.; Aubourg, S.P. Use of citric and lactic acids in ice to enhance quality of two fish species during on-board chilled storage. Int. J. Refrig. 2014, 40, 390–397. [Google Scholar] [CrossRef]
- Bermúdez-Aguirre, D.; Barbosa-Cánovas, G.V. Disinfection of selected vegetables under nonthermal treatments: Chlorine, acid citric, ultraviolet light and ozone. Food Control 2013, 29, 82–90. [Google Scholar] [CrossRef]
- Alakomi, H.-L.; Puupponen-Pimiä, R.; Aura, A.-M.; Helander, I.M.; Nohynek, L.; Oksman-Caldentey, K.-M.; Saarela, M. Weakening of salmonella with selected microbial metabolites of berry-derived phenolic compounds and organic acids. J. Agric. Food Chem. 2007, 55, 3905–3912. [Google Scholar] [CrossRef] [PubMed]
- Mani-López, E.; García, H.S.; López-Malo, A. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res. Int. 2012, 45, 713–721. [Google Scholar] [CrossRef]
- Doores, S. Organic acid. In Antimicrobials in Foods, 3rd ed.; Davidson, P.M.B.A.L., Ed.; Marcel Dekker Inc: New York, NY, USA, 2005; pp. 95–136. [Google Scholar]
- Radkowski, M.; Mikołajczyk, A. Wplyw kwasu cytrynowego na unieszkodliwianie paleczek Salmonella w miesie drobiowym. Med. Weter 2009, 65, 840–843. [Google Scholar]
- Seo, S.; Jung, D.; Wang, X.; Seo, D.J.; Lee, M.H.; Lee, B.-H.; Choi, C. Combined effect of lactic acid bacteria and citric acid on Escherichia coli O157:H7 and Salmonella Typhimurium. Food Sci. Biotechnol. 2013, 22, 1171–1174. [Google Scholar] [CrossRef]
- Rey, M.S.; García-Soto, B.; Fuertes-Gamundi, J.R.; Aubourg, S.; Barros-Velázquez, J. Effect of a natural organic acid-icing system on the microbiological quality of commercially relevant chilled fish species. LWT-Food Sci. Technol. 2012, 46, 217–223. [Google Scholar] [CrossRef]
- Tamblyn, K.C.; Conner, D.E. Bactericidal Activity of Organic Acids against Salmonella typhimurium Attached to Broiler Chicken Skin. J. Food Prot. 1997, 6, 610–737. [Google Scholar]
- Qiu, C.; Sun, W.; Cui, C.; Zhao, M. Effect of citric acid deamidation on in vitro digestibility and antioxidant properties of wheat gluten. Food Chem. 2013, 141, 2772–2778. [Google Scholar] [CrossRef]
- He, W.; Zhao, W.; Yang, R. Effects of wheat gluten modified by deamidation-heating with three different acids on the microstructure of model oil-in-water emulsion and rheological–physical property of ice cream. Food Hydrocoll. 2019, 87, 679–690. [Google Scholar] [CrossRef]
- Qiu, C.; Sun, W.; Zhao, Q.; Cui, C.; Zhao, M. Emulsifying and surface properties of citric acid deamidated wheat gliadin. J. Cereal Sci. 2013, 58, 68–75. [Google Scholar] [CrossRef]
- Liao, L.; Liu, T.; Zhao, M.; Zhao, H.; Cui, C. Aggregation behavior of wheat gluten during carboxylic acid deamidation upon hydrothermal treatment. J. Cereal Sci. 2011, 54, 129–136. [Google Scholar] [CrossRef]
- Liao, L.; Luo, Y.; Zhao, M.; Wang, Q. Preparation and characterization of succinic acid deamidated wheat gluten microspheres for encapsulation of fish oil. Colloids Surf. B. Biointerfaces 2012, 92, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhong, W.; Hu, X.; Ji, X.; Sun, Q.; Wang, R.; Yu, D.; Wu, F.; Wang, L. Conformational and functional changes from deamidation of wheat gluten with electrochemical treatment. J. Sci. Food Agric. 2023, 103, 5677–5686. [Google Scholar] [CrossRef] [PubMed]
- Canteri-Schemin, M.H.; Fertonani, H.C.R.; Waszczynskyj, N.; Wosiacki, G. Extraction of pectin from apple pomace. Braz. Arch. Biol. Technol. 2005, 48, 259–266. [Google Scholar] [CrossRef]
- Yapo, B.M. Lemon juice improves the extractability and quality characteristics of pectin from yellow passion fruit by-product as compared with commercial citric acid extractant. Bioresour. Technol. 2009, 100, 3147–3151. [Google Scholar] [CrossRef] [PubMed]
- Kurita, O.; Fujiwara, T.; Yamazaki, E. Characterization of the pectin extracted from citrus peel in the presence of citric acid. Carbohydr. Polym. 2008, 74, 725–730. [Google Scholar] [CrossRef]
- Vriesmann, L.C.; Teófilo, R.F.; Lúcia de Oliveira Petkowicz, C. Extraction and characterization of pectin from cacao pod husks (Theobroma cacao L.) with citric acid. LWT-Food Sci. Technol. 2012, 49, 108–116. [Google Scholar] [CrossRef]
- Sakiyama, T.; Sato, K.; Tsuda, S.; Sugiyama, H.; Hagiwara, T. Citric acid pretreatment for suppressing adhesion of major egg allergens to a stainless steel surface. Food Control 2013, 32, 702–706. [Google Scholar] [CrossRef]
- Acidity Regulators Market Size, Share, Growth, Trends and Outlook Report 2032. Available online: https://www.thebusinessresearchcompany.com/report/acidity-regulators-global-market-report (accessed on 3 October 2023).
- Citric Acid—Chemical Economics Handbook (CEH)|IHS Markit. 2015. Available online: https://www.ihs.com/products/citric-acid-chemical-economics-handbook.html (accessed on 17 February 2017).
- John, M. Connor. Global Price Fixing. In Studies in Industrial Organization, 2nd ed.; Springer: Boston, MA, USA, 2008. [Google Scholar]
- Citric Acid Market Analysis: Industry Market Size, Plant Capacity, Production, Operating, Efficiency, Demand and Supply, Edn-User Industries, Type, Sales Channel, Regional Demand, Company Share, Manufacturing Process, 2015–2030. Available online: https://www.chemanalyst.com/industry-report/citric-acid-market-695 (accessed on 5 October 2023).
- Citric Acid Market—Size, Share & Trends. Available online: https://www.mordorintelligence.com/industry-reports/citric-acid-market (accessed on 6 October 2023).
- Union PO of the E. C/2021/2431, Regulation (EU) 2016/1036 of the European Parliament and the Council. Available online: https://op.europa.eu/en/publication-detail/-/publication/a4c7f050-9dda-11eb-b85c-01aa75ed71a1 (accessed on 6 October 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).