Abstract
Fermentation possesses intriguing and promising potential as a bioprocess for enhancing and/or transforming bioactive compounds derived from agricultural processing by-products. This study aimed to enhance the phenolic compounds and antioxidant properties of coffee cherry husks through the sustainable methodology of solid-state fermentation (SSF) using various Trichoderma fungi, specifically Trichoderma asperellum CB-Pin-01 and two Trichoderma isolates (NTY211 and PSUT001). The coffee cherry husks underwent fermentation at a controlled temperature of 28 ± 1 °C over a duration of 7 days. Both fermented and unfermented extracts, prepared using different solvents (water, ethanol, and acetone), were systematically evaluated concerning total phenolic content (TPC), total flavonoid content (TFC), and antioxidant capacities measured via DPPH and ABTS radical scavenging assays, as well as ferric reducing antioxidant power (FRAP). The findings indicated that SSF involving Trichoderma fungi significantly augmented the phenolic content and antioxidant activities in comparison to the unfermented samples (p < 0.05). Notably, the acetonic extract obtained from fermentation with the isolate NTY211 exhibited the highest contents of phenolic (191.48 ± 3.94 mg GAE/g extract) and flavonoid (106.61 ± 3.09 mg QE/g extract). The identification of phenolic compounds by UHPLC-QqQ-MS/MS analysis revealed a predominant increase in chlorogenic acid and quercetin through SSF. Consequently, SSF utilizing Trichoderma fungi may represent a viable strategy for enhancing the value of coffee cherry husks, rendering them into bioactive ingredients with potential applications in the cosmetic and food industries.
1. Introduction
Coffee (Coffea arabica L.) is one of the most widely consumed beverages globally. The coffee beans are prepared from coffee cherries using dry, semi-dry, or wet processing, with residues or by-products generated, including pulp, husk, silver skin, and outer skin []. These by-products are rich in valuable compounds, including carbohydrates, proteins, pectins, and bioactive molecules such as polyphenols, which comprise flavan-3-ols, hydroxycinnamic acids, flavonols, and anthocyanidins [,]. They are known for their potent antioxidant properties, offering various health benefits []. Coffee cherry husks, in particular, have been recognized as a promising underutilized by-product that contains these bioactive compounds []. Still, their potential is often limited by their recalcitrant nature and the difficulty in releasing bioactive components []. Given the increasing demand for natural antioxidants in various industries, utilizing coffee cherry husks as a source of bioactive compounds presents an exciting opportunity [].
Solid-state fermentation (SSF) is a biotechnological process that has gained increasing attention for its ability to enhance the bioactive properties of agricultural by-products []. The SSF involves the growth of microorganisms, particularly fungi, on solid substrates with minimal free water, which facilitates the degradation of complex macromolecules and enhances the release of bioactive compounds []. This process has been successfully applied to improve the antioxidant capacity and phenolic content of other agro-residues, including rice bran [], soybean [], and white maize grain []. However, its application to coffee husks has not been thoroughly explored, and its potential for enhancing the bioactive properties of this specific by-product is not well-documented [].
Among various microorganisms, Trichoderma species are well-known for their ability to degrade complex organic materials and enhance the bioactive properties of agricultural residues. Trichoderma fungi produce a range of extracellular enzymes, including cellulases, chitinases, glucanases, proteases, and polyphenol oxidases, which break down lignocellulosic materials, releasing bound phenolic compounds and other valuable metabolites [,]. Recent studies have demonstrated the effectiveness of Trichoderma fungi in improving the bioactive content of various substrates through SSF, leading to enhanced antioxidant activity and the production of bioactive compounds with potential benefits [,,]. Despite these successes, the use of Trichoderma to enhance the antioxidant and phenolic content of coffee cherry husks remains largely unexplored.
Fermentation processes, including lactic acid bacteria fermentation and fungal SSF, have been shown to improve the bioactive profile of coffee by-products significantly. For example, fermentation of coffee pulp using Lactobacillus plantarum resulted in enhanced antioxidant activity and modifications in the bioactive compound profile, including an increase in phenolic compounds []. Similarly, the infusion of submerged fermented green coffee beans via vacuum impregnation has been shown to enhance the bioactive components, demonstrating the potential of fermentation to improve the antioxidant properties of coffee []. Furthermore, fungal SSF has been demonstrated to enhance the phenolic content and antioxidant potential of other agricultural residues, such as grape pomace, by promoting the release of bound phenolic compounds during fermentation [].
The attraction of fermented extracts in the cosmetic and food industries has gained significant traction due to the growing demand for natural and sustainable ingredients. Fermented extracts are rich in bioactive compounds that can promote skin health, enhance gut microbiota, and support general well-being. Fermented plant extracts are highly valued for their ability to strengthen skin barrier function, offer antioxidant protection, and mitigate signs of aging. Studies have shown that fermented ingredients, such as those derived from plants, can enhance skin hydration and elasticity while also reducing inflammation [,]. In the food industry, these extracts serve as natural preservatives and functional ingredients that improve the nutritional profile of products. Fermentation enhances the bioavailability of antioxidants and other functional components, making them more accessible for human consumption []. Recent studies have highlighted the role of fermented ingredients in boosting immune function, supporting gut health, and contributing to cardiovascular well-being [,]. As the demand for clean-label products rises, the application of bioactive compounds from fermented agricultural residues presents a promising approach for sustainable product development in these industries [,].
Chlorogenic acid (CGA) and caffeine are naturally occurring bioactive compounds widely distributed in coffee, tea, and several plant-based materials. These compounds have biological properties, including antioxidant, anti-inflammatory, and metabolic regulatory effects []. In the cosmetic industry, these compounds play a key role in protecting skin from oxidative stress and photoaging, as well as contributing to skin-brightening and anti-aging effects through their melanogenesis-inhibitory activity []. Given their broad biological potential, chlorogenic acid and caffeine have become valuable ingredients in the development of health-promoting foods, nutraceuticals, and cosmeceutical formulations.
In the current investigation, we aimed to valorize coffee cherry husks through the application of SSF utilizing different Trichoderma fungi, with the intention of enhancing phenolic compounds and antioxidant properties, thereby establishing a sustainable methodology for the generation of valuable fermented extracts that remain relatively underexplored. The fermentation procedure was subsequently followed by the extraction of crude phenolic compounds utilizing a range of solvents, including water, ethanol, and acetone. These extracts were subjected to evaluation for total phenolic content and antioxidant activities employing colorimetric assays. Furthermore, the identification of phenolic compounds was conducted through ultra-high performance liquid chromatography combined with triple quadrupole mass spectrometry (HPLC-QqQ-MS/MS) analysis. The implications of these findings are anticipated to facilitate the application of fermented extracts derived from coffee cherry husks as bioactive ingredients in the cosmetic and food industries.
2. Materials and Methods
2.1. Microorganisms and Inoculum Preparation
Trichoderma species, Trichoderma asperellum CB-Pin-01 (formerly identified as Trichoderma harzianum), and two additional isolates (NTY211 and PSUT001) were used in this study []. The CB-Pin-01 is a commercial strain, while the isolates NTY211 and PSUT001 were collected from bamboo soil and agricultural plots, respectively (Figure S1). They were maintained on potato dextrose agar (PDA) medium after incubation at 28 ± 1 °C for 7 days. The inoculum was prepared by suspending Trichoderma spores from a 7-day culture in sterile water, and the concentration was adjusted to 1 × 108 spores/mL, which was counted via a hemocytometer (BOECO GmbH, Lauda-Königshofen, Germany).
2.2. Plant Material
Cascaras, or dried coffee cherry husks, were obtained from Doi Lan, Chiang Rai, Thailand. They were then dried again in a tray dryer at 55 ± 1 °C until the weight remained constant. The residue size was coarsely reduced to approximately 3 × 3 cm using a blender (Figure 1), and the dried sample was stored at 4 °C until use.

Figure 1.
Appearance of dried coffee (Coffea arabica L.) cherry husks (A) before and (B) after size reduction.
2.3. Chemicals and Reagents
Folin–Ciocalteu reagent, epigallocatechin gallate, and catechin gallate were purchased from Merck (Darmstadt, Germany). 2,2-diphenyl-1-picryl hydroxyl (DPPH), potato dextrose agar (PDA), 2,4,6-tri(2-pyridyl)-5-triazine (TPTZ), caffeic acid, chlorogenic acid, gallic acid, protocatechuic acid, p-coumaric acid, quercetin, and caffeine were obtained from Sigma-Aldrich (Burlington, MA, USA). Formic acid and acetonitrile were purchased from Duksan Pure Chemicals (Ansan, Republic of Korea). Minimum Essential Medium (MEM), Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), L-glutamine, penicillin, and streptomycin were purchased from Thermo Fisher Scientific (Waltham, MA, USA).
2.4. Solid-State Fermentation (SSF) of Coffee Cherry Husk
SSF was conducted with each fungal isolate in a 250 mL Erlenmeyer flask. Ten grams of coffee cherry husk substrate were mixed and soaked with 17.5 mL of deionized (DI) water (solid to liquid ratio of 1:1.75 w/v). The mixture was then autoclaved at 121 °C for 15 min and cooled down to room temperature. A 0.5 mL aliquot of inoculum was added to the sterile solid medium, covered with a sterile cotton stopper, and the inoculated flask was incubated at 28 ± 1 °C for 7 days. Unfermented coffee cherry husks were also prepared without the addition of fungal inoculum. On the 7th day, the unfermented and fermented husks were harvested for further study. Each experiment was performed in triplicate.
2.5. Preparation of Crude Extracts from Coffee Cherry Husks
The unfermented and fermented samples were extracted with DI water, 95% ethanol, and acetone in a solid-to-liquid ratio of 1:10 w/v using a conventional shaking extraction method at 150 rpm for 3 h. All the mixtures were filtered through cheesecloth and then centrifuged at 8500 rpm and 4 °C for 30 min. A supernatant was collected and then evaporated using a rotary evaporator and/or dried with a freeze-dryer to remove the remaining solvent. The crude extracts were collected and kept at −20 °C until use.
2.6. Analyzes
2.6.1. Proximate Analysis
The proximate analysis of the coffee cherry husks was conducted to determine the moisture, ash, fiber, lipid, protein, and carbohydrate contents, and these values were calculated as a percentage of dry weight (% dw) using the standard methods of the Association of Official Analytical Chemists (AOAC) [].
2.6.2. Determination of Extraction Yield
The percentage yield of extracts was measured by calculating based on the equation below.
2.6.3. Determination of Total Phenolic Content
Total phenolic content was determined using a Folin–Ciocalteu assay [] with slight modification. Briefly, the sample extract was diluted with an appropriate solvent and mixed with 0.25 mL of Folin–Ciocalteu reagent. Then, 1.5 mL of sodium carbonate solution (7.5% w/v) was added to the mixture, which was allowed to stand for 30 min at ambient temperature. Absorbance was measured at 765 nm using a UV-VIS spectrophotometer (Biochrom Ltd., Cambridge, UK). A gallic acid calibration curve was plotted, with results expressed as milligram gallic acid equivalent per gram of extract (mg GAE/g extract).
2.6.4. Determination of Total Flavonoid Content
Total flavonoid content was determined by the aluminum colorimetric assay [] with slight modification. The sample extract solution 3.7 mL was mixed sequentially with 0.15 mL of sodium nitrite solution (5% w/v) and 0.15 mL of aluminum chloride solution (10% w/v). The mixture was allowed to stand for 5 min at ambient temperature. Then, 1.0 mL of sodium hydroxide solution (4% w/v) was added, and the mixture was incubated for 8 min. Absorbance was measured at 510 nm using a UV-VIS spectrophotometer. Quercetin was used as a standard. The result was expressed as milligrams of quercetin equivalents per gram of extract (mg QE/g extract).
2.6.5. Determination of 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Activity
DPPH radical scavenging activity assay was carried out according to Thaipong et al. [] with slight modification. The sample extract solution was first dissolved in ethanol (1.0 mL), then mixed with a 0.1 mM DPPH reagent (2.0 mL), and incubated in dark conditions for 30 min. Discoloration of the mixture was measured at 517 nm using a UV-VIS spectrophotometer. The percentage of DPPH radical inhibition was calculated as follows:
where Ac = absorbance of the control; and As = absorbance of the sample
The Trolox standard curve was prepared by plotting percentage radical inhibition against Trolox concentration. DPPH radical scavenging activity was expressed as milligram Trolox equivalent antioxidant capacity per gram of extract (mg TEAC/g extract).
2.6.6. Determination of ABTS Radical Scavenging Activity
The ABTS assay was performed according to Solaberrieta et al. [] with a slight modification. The ABTS radical cation was generated by mixing an ABTS solution (7.4 mM) with 2.45 mM potassium persulfate in a 1:1 ratio. The mixture was then allowed to stand in the dark at room temperature for 16 h. The sample extract solution (1.0 mL) was mixed with freshly prepared working ABTS solution (2.0 mL), and the mixture was then incubated in the dark at room temperature for 30 min. The absorbance was measured at 734 nm using a UV-VIS spectrophotometer, and the percentage of ABTS radical inhibition was calculated according to Equation (2). The inhibition of ABTS radicals calculated against a Trolox calibration curve was expressed as milligram Trolox equivalent antioxidant capacity per gram of extract (mg TEAC/g extract).
2.6.7. Determination of Ferric Reducing Antioxidant Power (FRAP)
Ferric reducing antioxidant power (FRAP) of the extract was determined by the Fe2+-TPTZ complex method []. The sample extract solution (1.5 mL) was mixed with 1.5 mL of FRAP reagent (10:1:1 by volume of 300 mM sodium acetate buffer pH 3.6, 10 mM TPTZ solution, and 20 mM FeCl3). The mixture was then incubated at 37 °C in a water bath for 30 min. The absorbance was measured at 593 nm by a UV-VIS spectrophotometer, and Trolox was used as a standard. Results were expressed as milligram Trolox equivalent antioxidant capacity per gram of extract (mg TEAC/g extract).
2.6.8. Identification of Bioactive Compounds by UHPLC-QqQ-MS/MS Analysis
Identification of phenolic compounds in the extracts was performed using UHPLC-QqQ-MS/MS. Phenolic compounds and caffeine were analyzed using a Shimadzu Nexera X2 UHPLC system (Shimadzu Corporation, Kyoto, Japan) equipped with a binary pump. The analytical column used was a Shimpack C18 (GIS 2.1 × 100 mm, 3 µm) with column oven temperature of 30 °C. The flow rate was set at 0.2 mL/min with an injection volume of 1 µL. Gradient solvents containing formic acid (0.2% v/v) (A) and acetonitrile (B) were used as mobile phases. The gradient elution program was set as follows: 5% B from 0 to 1 min, 13% B from 10 to 13 min, 100% B from 20 to 25 min, and 5% B from 27 to 30 min.
MS analysis was run using a Shimadzu LCMS-8060 (Shimadzu Corporation, Kyoto, Japan), a triple quadrupole mass spectrometer. The MS analytical conditions included the following: ionization, ESI source; nebulizing gas flow rate, 3 L/min; heating gas flow rate, 10 L/min; drying gas flow rate, 10 L/min; interface temperature, 300 °C; block heat temperature, 400 °C; and data acquisition in both positive and negative modes.
2.7. Cytotoxicity
2.7.1. Cell Line
The NIH/3T3 (ATCC CRL-1658) fibroblast cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% (v/v) penicillin–streptomycin, then maintained in a humidified atmosphere with 5% CO2 at 37 °C.
2.7.2. Cytotoxicity
The cytotoxicity of the extract was examined as described by Pramanik []. The NIH/3T3 cells were seeded in 96-well plates at a density of 2 × 105 cells/mL and incubated under the culture conditions for 24 h. Cells were then treated with the extract at concentrations ranging from 0.05 to 2 mg/mL for 24 h. MTT solution was added to each well at a concentration of 0.25 mg/mL, and the plates were further incubated at 37 °C for 4 h. The medium was discarded, and the purple MTT–formazan crystals were dissolved with 0.2 mL of DMSO. The culture plate was subjected to orbital shaking for 5 min, resulting in a reduction in soluble MTT to form a water-insoluble formazan. The absorbance was measured at 570 nm using a microplate reader (SPECTROstar Nano, BMG Labtech, Aylesbury, UK). The percentage of cell viability was calculated (Equation (3)) by comparing the absorbance of treated cells with that of untreated cells.
where Au = absorbance of the untreated cells as control: and At = absorbance of the treated sample.
2.8. Statistical Analysis
Means and standard deviations (SD) were calculated from data obtained from triplicate experiments. One-way analysis of variance (ANOVA), Tukey’s multiple comparison test, and Pearson’s correlation analysis were conducted. Different values of less than 0.05 (p < 0.05) were considered statistically significant differences.
3. Results and Discussion
3.1. Growth of Trichoderma Fungi on Coffee Cherry Husk Substrate
In this study, coffee cherry husk was employed as a solid medium for SSF with various Trichoderma species. The proximate analysis of the coffee cherry husk revealed the following composition: moisture content of 10.65 ± 1.12%, ash content of 10.27 ± 0.44%, fiber content of 20.23 ± 2.02%, lipid content of 0.92 ± 0.16%, protein content of 12.59 ± 0.73%, and carbohydrate content of 45.34 ± 2.03% (dw). These results indicate that coffee cherry husk is primarily composed of carbohydrates, making it a suitable substrate for Trichoderma spp., which thrive on lignocellulosic materials [].
Moisture content plays a crucial role in SSF, as it directly influences water activity (aw) and the overall environment for fungal growth. Our findings indicate that adding water to the solid medium at a ratio of 1.75:1 v/w, resulting in a moisture content of 58.5% (w/w), as measured by a moisture analyzer (model Ohaus MB45). This can support the optimal mycelial growth for all Trichoderma species. At this moisture level, the substrate provided sufficient water availability for metabolic processes, leading to vigorous mycelial expansion. However, no spore formation was observed for any of the Trichoderma isolates. This lack of sporulation may be attributed to the limited nutrient content in the coffee cherry husk, which, while rich in carbohydrates, may lack sufficient nitrogen and micronutrients needed for spore development. This finding is consistent with the results of Vitale et al. [], who reported that nutrient supplementation is essential for spore formation in Trichoderma cultures.
3.2. Extraction Yield
Coffee cherry husks were fermented with Trichoderma for 7 days and then promptly extracted using water, ethanol, and acetone as solvents. Solvent polarity and solubility play roles in extracting many plant compounds. The results, as depicted in Table 1, show that fermentation with different fungal strains significantly exhibited varying extraction efficiencies, depending on the solvent used (p < 0.05). Water provided the highest extraction yield (26.47 ± 1.37–32.13 ± 0.49%), followed by ethanol (9.67 ± 0.25–23.90 ± 1.31%) and acetone (8.27 ± 0.32–13.90 ± 0.17%). Coffee cherry husks contain different polar compounds, including water-soluble composites, as their major components. Hoseini et al. [] reported that coffee cherry husks are mainly composed of water-soluble compounds, including carbohydrates, proteins, and minerals, as well as minor components such as volatile oils and phenolic compounds.

Table 1.
Extraction yields of unfermented and fermented coffee cherry husks extracted using different solvents.
Yields of fermented coffee cherry husks significantly decreased compared with unfermented samples in all solvents (p < 0.05). The extract yield of isolate NTY211 was generally lower than that of the other two isolates, possibly due to the degradation of large molecules, such as those present in fungal cell walls, by Trichoderma enzymes, and to the production of low-molecular-weight compounds during fermentation [].
3.3. Total Phenolic Content and Total Flavonoid Content
Phenolic compounds serve as significant contributors to the antioxidant properties exhibited by coffee and its by-products []. Typically, these compounds can be quantified in terms of total phenolic content (TPC) using the Folin–Ciocalteu reagent. The TPC values, derived from the calibration curve (y = 0.0944x, R2 = 0.9952) of gallic acid and expressed as milligrams of gallic acid equivalent per gram of extract, are illustrated in Figure 2A. The findings demonstrated that phenolic compounds from coffee cherry husks can be extracted utilizing a variety of solvents, as evidenced by their diverse solubility and polarity characteristics. The highest TPC values were obtained using acetone. Conversely, water was identified as an ineffective solvent for the extraction of phenolic compounds. Likewise, acetone and a mixture of acetone and water (60% v/v acetone) were found to be highly effective solvents for the extraction of phenolic compounds from Robusta coffee cherry husks and brewer’s spent grains, respectively [,]. Nevertheless, elevated concentrations of phenolic compounds extracted from coffee husks using a combination of ethanol and water have also been documented [,]. These findings underscore that the choice of solvent is a pivotal factor in the extraction of various types of phenolic compounds from coffee cherry husks and other botanical sources.
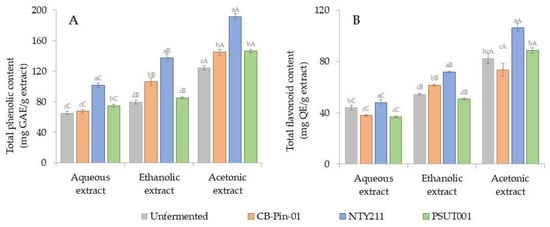
Figure 2.
(A) Total phenolic content (TPC) and (B) total flavonoid content (TFC) of unfermented and fermented coffee cherry husks extracted using different solvents. Different lowercase letters indicate significant differences (p < 0.05) among inocula within the same solvent, while different uppercase letters indicate significant differences (p < 0.05) among solvents within the same inoculum.
The phenolic composition of coffee cherry husks exhibited an enhancement during solid-state fermentation (SSF) with Trichoderma fungi. As illustrated in Figure 2A, an increment in total phenolic content (TPC) values was observed subsequent to a 7-day fermentation with Trichoderma. The application of SSF with the isolate NTY211 led to a significant modification in TPC values from 65.08 ± 2.43, 79.40 ± 2.99, and 124.34 ± 2.68 to 101.75 ± 2.91, 137.57 ± 4.38, and 191.48 ± 3.94 mg GAE/g extract in aqueous, ethanolic, and acetonic extracts (p < 0.05), which corresponded to approximate increases of 1.56, 1.73, and 1.54-fold, respectively. Phenolic compounds increased during SSF with fungi because secretion of the fungal enzymes degraded the bonds of large compounds []. Palomino García et al. [] revealed that coffee residues fermented with Penicillium purpurogenum gave higher phenolic compounds after SSF. Major compounds identified included phenolic acids, such as chlorogenic acid, caffeic acid, and rutin.
The quantification of flavonoids present in coffee cherry husks was conducted utilizing the aluminum chloride assay, which enabled the determination of the total flavonoid content (TFC) by establishing a quercetin standard curve (y = 11.034x, R2 = 0.9979). As illustrated in Figure 2B, the TFC values exhibited significant variation following a period of 7 days of solid-state fermentation (SSF) with Trichoderma fungi (p < 0.05). Likewise, the TPC results showed that acetonic extracts had higher TFC values compared to aqueous and ethanolic extracts under the same conditions. This finding suggests that acetonic extraction serves as the most effective solvent for the recovery of flavonoids from coffee cherry husks. Among the solid-state fermentation processes involving various Trichoderma isolates, the isolate NTY211 was primarily responsible for the notable enhancement of flavonoid content, yielding values of 48.06 ± 2.34, 71.87 ± 0.82, and 106.61 ± 3.09 mg QE/g extract in the aqueous, ethanolic, and acetonic extracts, respectively. The observed increase in flavonoid concentrations during SSF is likely attributable to the enzymatic actions of fungi, which mediate the degradation of intricate plant cell wall structures, thereby facilitating the liberation of bound flavonoids [].
3.4. Antioxidant Activities
The antioxidant capacity of the extracts was assessed through their DPPH radical scavenging capabilities. The DPPH radical is neutralized via hydrogen donation upon interaction with antioxidant entities. The DPPH radical scavenging activity of the extracts was determined based on a calibration curve (y = 12.217x, R2 = 0.9991) derived from Trolox and expressed as mg TEAC/g extract (Figure 3A). The findings indicated that variations in antioxidant efficacy were influenced by both the fermentation process and the nature of the extraction solvent. The polarity of the extraction solvent significantly impacted the scavenging efficacy (p < 0.05), indicating that acetonic extracts exhibited superior activity relative to aqueous and ethanolic extracts. Notably, SSF, particularly with NTY211, markedly enhanced the DPPH-based antioxidant activity from 52.14 ± 1.75, 54.66 ± 1.55, and 80.84 ± 1.51 to 79.61 ± 2.26, 99.05 ± 4.80, and 150.51 ± 3.47 mg TEAC/g extract, as evidenced in aqueous, ethanolic, and acetonic extracts, respectively. Torino et al. [] elucidated that phenolic compounds are subject to degradation into simpler, more biologically active forms. In research conducted by Torres-Mancera et al. [], it was reported that hydroxycinnamic acids, encompassing ferulic, caffeic, p-coumaric, and chlorogenic acids, exhibited substantial antioxidant properties and remained present following SSF of coffee pulp utilizing fungi. Therefore, fermentation with Trichoderma spp. exerted significant influences on the manifestation of antioxidant activity. The antioxidant activity increased during the fermentation process could be due to breaking down the structure of the cell wall and/or changing the structure to increase the release of phenolic and flavonoid compounds [].
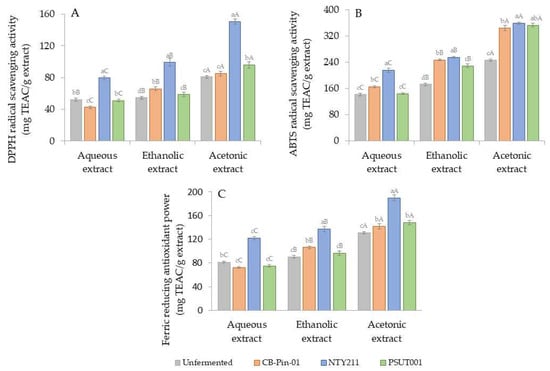
Figure 3.
(A) DPPH radical scavenging activity, (B) ABTS radical scavenging activity, and (C) FRAP of unfermented and fermented coffee cherry husks extracted using different solvents. Different lowercase letters indicate significant differences (p < 0.05) among inocula within the same solvent, while different uppercase letters indicate significant differences (p < 0.05) among solvents within the same inoculum.
The additional two antioxidant activity assays were also conducted in this study. The ABTS radical scavenging activity assay was employed to measure the antioxidant capacity of a sample by quantifying its ability to scavenge or reduce ABTS radical cations. The ABTS-based antioxidant activity of the extracts was determined based on a calibration curve (y = 13.609x, R2 = 0.9968) derived from Trolox and expressed as mg TEAC/g extract (Figure 3B). Concurrently, the FRAP assay was performed to measure the reducing potential of antioxidants reacting with ferric tripyridyltriazine (Fe3+-TPTZ) complexes, producing colored ferrous tripyridyltriazine (Fe2+-TPTZ). The calibration curve of Trolox standard (y = 0.2246x, R2 = 0.9997) was prepared, and antioxidant activity was expressed in terms of mg TEAC/g extract (Figure 3C). The patterns of antioxidant activity profiles, as evaluated by these two assays, exhibited similarity to the results of the DPPH-based antioxidant activity assay. Minor variations in antioxidant activity were observed after fermentation with the strain CB-Pin-01 and isolate PSUT001. In comparison to each unfermented extract, a notable enhancement in antioxidant activity was significantly observed following fermentation with the isolate NTY221 (p < 0.01). The antioxidant activities of the acetonic extract derived from coffee cherry husks (246.92 ± 3.15 and 130.91 ± 2.10 mg TEAC/g extract) were significantly augmented to 359.59 ± 3.38 and 189.97 ± 5.30 mg TEAC/g extract, as determined by ABTS and FRAP, respectively, after a 7-day fermentation with NTY211.
A study of the antioxidant capacity of the extract using various analytical assays for antioxidant activity demonstrates that the antioxidant activities of the coffee cherry husk can be enhanced through solid-state fermentation with Trichoderma fungi, particularly the isolate NTY211. These results concurred with those of the total phenolic content and total flavonoid content, as mentioned previously.
3.5. Correlation Analysis
Correlation analysis is a statistical method for measuring the relationship between two quantitative variables, primarily determined using Pearson’s correlation coefficient (r), which ranges from 0 to 1. An r-value close to 1 indicates a stronger relationship between the variables. Schober et al. [] verified that a correlation coefficient from 0.10 to 0.39 indicated weak correlation, 0.40 to 0.69 moderate correlation, 0.70 to 0.89 strong correlation and 0.9 to 1.0 very strong correlation. In the present study, to verify the relationship among the parameters tested, including TPC, TFC, DPPH radical scavenging activity, ABTS radical scavenging activity, and FRAP, a correlation analysis was performed (Table 2). The results revealed that all values are positively correlated with a strong (>0.8) to very strong (>0.9) level. The TPC showed a very strong correlation with all values: TFC (r = 0.933), DPPH radical scavenging activity (r = 0.966), ABTS radical scavenging activity (r = 0.918), and FRAP (r = 0.979). These findings emphasized that the antioxidant activities of coffee cherry husks could be mainly driven by the presence of phenolic compounds, including flavonoids and other phenolic types. Similarly to the reports of Tan et al. [] and Patial et al. [], the presence of phenolic and flavonoid compounds primarily contributed to the antioxidant capacity.

Table 2.
Pearson’s correlation coefficients among total phenolic content (TPC), total flavonoid content (TFC), and antioxidant activities (DPPH, ABTS, and FRAP).
3.6. Identification of Phenolic Compounds and Caffeine
The unfermented and fermented extracts derived from coffee cherry husks were identified and quantified using the UHPLC-QqQ-MS/MS analysis. A total of seven reference standards, encompassing phenolic acids (including gallic acid, protocatechuic acid, chlorogenic acid, caffeic acid, and p-coumaric acid), quercetin, and caffeine, were used to establish calibration curves based on linear regression equations and corresponding R2 coefficients. The findings delineated in Table 3 elucidated that phenolic compounds and caffeine in coffee cherry husks changed after SSF with different Trichoderma isolates, compared with unfermented extracts. The contents of chlorogenic acid and quercetin predominantly increased in the fermented extracts, particularly in the ethanolic and acetonic extracts. Remarkably, the highest increment of these two compounds was observed in the coffee cherry husks fermented with the isolate NTY211. This phenomenon may serve as empirical evidence to support the enhancement of TPC and antioxidant activities. Furthermore, the caffeine concentration also exhibited an increase after SSF involving all Trichoderma isolates. T. harzianum primarily targets and degrades the cell walls during fermentation, mainly when it produces enzymes like cellulases and laccases, often increasing the overall antioxidant capacity of the substrate by releasing bound phenolics []. It could be possible that the increase in caffeine content observed during fermentation may be attributed to enzymatic hydrolysis of caffeine precursors or methylxanthines by Trichoderma enzymes, while limited demethylase activity could also contribute to caffeine accumulation [,]. Moreover, alterations in substrate pH and moisture content induced by fermentation may increase the solubility and extraction efficiency of caffeine, thereby resulting in elevated quantifiable levels of caffeine []. Higher chlorogenic acid content exhibited greater antioxidant activity efficiency to scavenge DPPH radicals and reduce Fe3+ to Fe2+ []. Consequently, solid-state fermentation utilizing Trichoderma fungi has the potential to enhance and modify bioactive compounds, particularly phenolic acids, thereby directly augmenting the biological activities inherent to coffee cherry husks.

Table 3.
Composition and concentration of phenolic compounds and caffeine in unfermented and fermented coffee cherry husk extracts determined by UHPLC-QqQ-MS/MS analysis.
From our investigation, the augmentation of phenolic compounds may have emerged through various mechanisms: (1) bound phenolic compounds were released during the enzymatic degradation of plant cell walls, and (2) Trichoderma itself secreted phenolic compounds as secondary metabolites during SSF [,]. Moreover, (3) the biotransformation of phenolic compounds by numerous enzymes also resulted in the modification and degradation of phenolic acids [,,]. Razak et al. [] detected caffeic acid after fermentation with fungi. This phenomenon can be elucidated by the fact that the phenolic compounds were altered during fermentation by the metabolic activity of microorganisms, while p-coumaric acid was transformed into caffeic acid []. The enzymatic activity during SSF also facilitated the biotransformation of catechin and its derivatives [], whereas the phenolic content diminished during fermentation through the oxidation of phenolic compounds during fungal metabolism and degradation [].
3.7. Cytotoxicity and Cell Viability
In this study, safety information on the use of unfermented and fermented extracts derived from coffee cherry husks was assessed. The cytotoxicity was evaluated based on the toxic concentration of the extract, and the results were expressed as the safety concentration at which cell viability was maintained using NIH/3T3 fibroblast cells. Cell viability was calculated by comparing the absorbance of the control cells without treatment to 100%, while higher absorbance values indicated greater cell proliferation. The cytotoxic effects of unfermented and fermented extracts of coffee cherry husks obtained by different extraction solvents were determined at a concentration range of 0.05–2 mg/mL (Table S1). Based on the evidence of phenolic content and antioxidant capacity as mentioned previously, the crude extracts from coffee cherry husks fermented with the Trichoderma fungus isolate NTY211 were selected to measure their cytotoxicity in comparison with the unfermented extracts.
The results shown in Figure 4 demonstrate that, after treatment with the extracts, cell viability declined in a manner proportional to the concentration of the extract. Nevertheless, the percentages of cell viability of unfermented and fermented extracts were more than 70% throughout the concentration range tested. Conversely, the percentages of cell viability below 70% were found in the aqueous (61.25 ± 0.64%) and acetonic (68.41 ± 5.99%) extracts derived from unfermented coffee cherry husks, and the aqueous extract (67.01 ± 2.26%) of fermented coffee cherry husks at the maximum concentration of 2 mg/mL, which was the highest concentration tested in this study. In accordance with ISO 10993-5, a cell viability of ≥70% confirms that the substance is classified as non- cytotoxic. This finding suggests their safe application for subsequent commercial utilization. Notably, the cell proliferation of NIH/3T3 fibroblast cells was observed at a low concentration of 0.05–0.01 mg/mL, especially the ethanolic extract of fermented coffee cherry husk. According to a previous study, caffeic acid has been shown to enhance fibroblast survival []. Moreover, quercetin has been reported to enhance fibroblast proliferation at low concentrations and to protect cells against oxidative stress []. Additionally, gallic acid gave antioxidant and cytoprotective action at low doses, while demonstrating cytotoxicity at elevated doses [].
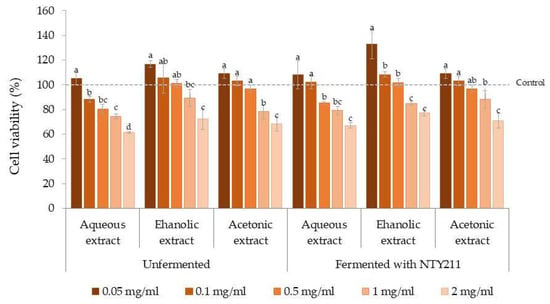
Figure 4.
Cell viability of unfermented and NTY211-fermented coffee cherry husks extracted using different solvents. Different lowercase letters indicate significant differences (p < 0.05) within the same extract.
4. Conclusions
Coffee cherry husks obtained from coffee processing have demonstrated considerable potential as an innovative alternative substrate for cultivating Trichoderma through SSF. The concentrations of phenolic and flavonoid compounds, as well as the antioxidant capacity, of coffee cherry husks can be modified and enhanced by fermenting for seven days. The Trichoderma isolate NTY211 significantly contributed to the augmentation of phenolic and flavonoid contents, along with the enhancement of antioxidant activities. The coffee cherry husks subjected to fermentation with the isolate NTY211 and subsequently extracted using acetone exhibited the highest recorded values for total phenolic content (TPC) (191.48 ± 3.94 mg GAE/g extract), total flavonoid content (TFC) (106.61 ± 3.09 mg QE/g extract), DPPH radical scavenging activity (150.51 ± 3.47 mg TEAC/g extract), ABTS radical scavenging activity (359.59 ± 3.38 mg TEAC/g extract), and ferric reducing antioxidant power (FRAP) (189.97 ± 5.30 mg TEAC/g extract). Correlation analysis indicated that elevated antioxidant activity was positively correlated with increased phenolic and flavonoid contents. The results from the UHPLC-QqQ-MS/MS analysis corroborated the enhancement of chlorogenic acid and quercetin concentrations following solid-state fermentation.
Consequently, the application of SSF utilizing Trichoderma fungi may be proposed as a strategic approach to increase the value of coffee cherry husks as a fermented extract for prospective utilization as an active ingredient in the cosmetic and food sectors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation11110625/s1, Figure S1: Morphological appearance of Trichoderma fungi (A) Trichoderma asperellum CB-Pin-01, (B) Trichoderma sp. isolate NTY211, and (C) Trichoderma sp. isolate PSUT001. Table S1: Cytotoxic effects of unfermented and fermented extracts of coffee cherry husks obtained by different extraction solvents on fibroblast cell lines (NIH/3T3) at various concentrations (0.001–10 mg/mL).
Author Contributions
Conceptualization, P.P.; methodology, P.P. and N.T.; software, B.S.; validation, P.C. and S.S.; formal analysis, P.P.; investigation, P.C.; resources, P.P.; data curation, P.P. and B.S.; writing—original draft preparation, P.P. and B.S.; writing—review and editing, P.P. and P.C.; visualization, S.S.; supervision, N.T.; project administration, P.P.; funding acquisition, P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Mae Fah Luang University, grant number F24-681G-02-010.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- De Melo Pereira, G.V.; de Carvalho Neto, D.P.; Magalhães Júnior, A.I.; do Prado, F.G.; Pagnoncelli, M.G.B.; Karp, S.G.; Soccol, C.R. Chemical composition and health properties of coffee and coffee by-products. Adv. Food Nutr. Res. 2020, 91, 65–96. [Google Scholar]
- Murthy, P.S.; Naidu, M.M. Sustainable Management of Coffee Industry By-Products and Value Addition-A Review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Ramirez-Coronel, M.A.; Marnet, N.; Kolli, V.K.; Roussos, S.; Guyot, S.; Augur, C. Characterization and Estimation of Proanthocyanidins and Other Phenolics in Coffee Pulp (Coffea arabica) by Thiolysis−High-Performance Liquid Chromatography. J. Agric. Food Chem. 2004, 52, 1344–1349. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Ali, A.; Bhowmik, S. Bioactive Compounds in Coffee Husk: Extraction, Functional Properties, Applications, and Sustainable Approach in Circular Economy. RSC Sustain. 2025, 3, 4410–4425. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, C.; Zhang, Y.; Zeng, W.; Cesarino, I. Coffee Cell Walls—Composition, Influence on Cup Quality and Opportunities for Coffee Improvements. Food Qual. Saf. 2021, 5, fyab012. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Aguilera, Y.; Gil-Ramírez, A.; Benitez, V.; Cañas, S.; Braojos, C.; Martin-Cabrejas, M.A. Biorefinery and Stepwise Strategies for Valorizing Coffee By-Products as Bioactive Food Ingredients and Nutraceuticals. Appl. Sci. 2023, 13, 8326. [Google Scholar] [CrossRef]
- Álvarez, A.; Rodríguez, A.; Chaparro, S.; Borrás, L.M.; Rache, L.Y.; Brijaldo, M.H.; Martínez, J.J. Solid-State Fermentation as a Biotechnological Tool to Reduce Antinutrients and Increase Nutritional Content in Legumes and Cereals for Animal Feed. Fermentation 2025, 11, 359. [Google Scholar] [CrossRef]
- Soccol, C.R.; Da Costa, E.S.F.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; de Souza Vandenberghe, L.P. Recent Developments and Innovations in Solid State Fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- Janarny, G.; Gunathilake, K.D.P.P. Changes in Rice Bran Bioactives, Their Bioactivity, Bioaccessibility and Bioavailability with Solid-State Fermentation by Rhizopus oryzae. Biocatal. Agric. Biotechnol. 2020, 23, 101510. [Google Scholar] [CrossRef]
- Singh, H.B.; Singh, B.N.; Singh, S.P.; Nautiyal, C.S. Solid-State Cultivation of Trichoderma harzianum NBRI-1055 for Modulating Natural Antioxidants in Soybean Seed Matrix. Bioresour. Technol. 2010, 101, 6444–6645. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Villela-Castrejón, J.; Perez-Carrillo, E.; Gómez-Sánchez, C.E.; Gutiérrez-Uribe, J.A. Effects of Solid-State Fungi Fermentation on Phenolic Content, Antioxidant Properties and Fiber Composition of Lime Cooked Maize by-Product (Nejayote). J. Cereal Sci. 2019, 90, 102837. [Google Scholar] [CrossRef]
- Cangussu, L.B.; Melo, J.C.; Franca, A.S.; Oliveira, L.S. Chemical Characterization of Coffee Husks, a by-Product of Coffea arabica Production. Foods 2021, 10, 3125. [Google Scholar] [CrossRef]
- Verduzco-Oliva, R.; Gutierrez-Uribe, J.A. Beyond Enzyme Production: Solid State Fermentation (SSF) as an Alternative Approach to Produce Antioxidant Polysaccharides. Sustainability 2020, 12, 495. [Google Scholar] [CrossRef]
- Yao, X.; Guo, H.; Zhang, K.; Zhao, M.; Ruan, J.; Chen, J. Trichoderma and Its Role in Biological Control of Plant Fungal and Nematode Disease. Front. Microbiol. 2023, 14, 1160551. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.; Kabli, S.; Al-Garni, S.; Al-Ghamdi, M.; Abdel-Aty, A.; Mohamed, S. Solid-state Fermentation by Trichoderma viride for Enhancing Phenolic Content, Antioxidant and Antimicrobial Activities in Ginger. Lett. Appl. Microbiol. 2018, 67, 161–167. [Google Scholar] [CrossRef]
- Serna-Díaz, M.; Mercado-Flores, Y.; Jiménez-González, A.; Anducho-Reyes, M.; Medina-Marín, J.; Tuoh-Mora, J.S.; Téllez-Jurado, A. Use of Barley Straw as a Support for the Production of Conidiospores of Trichoderma harzianum. Biotechnol. Rep. 2020, 26, e00445. [Google Scholar] [CrossRef] [PubMed]
- Kritsadaruangchai, U.; Chaiwut, P.; Chomnunti, P.; Thaochan, N.; Saikeur, A.; Pintathong, P. Effect of Solid State Fermentation with Trichoderma spp. on Phenolic Content and Antioxidant Capacities of Mature Assam Tea Leaves. J. Food Sci. Agric. Technol. 2019, 5, 106–113. [Google Scholar]
- Tangjaidee, P.; Braspaiboon, S.; Singhadechachai, N.; Phongthai, S.; Therdtatha, P.; Rachtanapun, P.; Sommano, S.R.; Seesuriyachan, P. Enhanced Bioactive Coffee Cherry: Infusion of Submerged-Fermented Green Coffee Beans via Vacuum Impregnation. Foods 2025, 14, 1165. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, D.; Zhang, J.; Shen, J.; Cao, J.; Gu, H.; Cui, M.; He, L.; Chen, G.; Liu, S. Improving Soluble Phenolic Profile and Antioxidant Activity of Grape Pomace Seeds through Fungal Solid-State Fermentation. Foods 2024, 13, 1158. [Google Scholar] [CrossRef]
- Yang, F.; Hu, Y.; Wu, M.; Guo, M.; Wang, H. Biologically Active Components and Skincare Benefits of Rice Fermentation Products: A Review. Cosmetics 2025, 12, 29. [Google Scholar] [CrossRef]
- Majchrzak, W.; Motyl, I.; Śmigielski, K. Biological and Cosmetical Importance of Fermented Raw Materials: An Overview. Molecules 2022, 27, 4845. [Google Scholar] [CrossRef]
- Zhao, Y.-S.; Eweys, A.S.; Zhang, J.-Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.-B.; Xiao, X. Fermentation Affects the Antioxidant Activity of Plant-Based Food Material through the Release and Production of Bioactive Components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef]
- Yu, H.; Khanum, S.; Ali, M.M.; Rahman, A.; Hayat, Z.; Waqas, M.; Riaz, R.; Sajid, M.; Khan, M.; Zafar, M.S. Research Note: Effects of Fermented Vegetable Extract Supplementation via Drinking Water on Growth Performance, Immune Function, and Antioxidant Status in Broiler Chickens. Poult. Sci. 2025, 104, 105583. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Stanton, C.; O’toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Vivekanand, V.; Mohanakrishna, G.; Pattnaik, B.; Muddapur, U.M.; Aminabhavi, T.M. Production of Bioactive Phenolic Compounds from Agricultural By-Products towards Bioeconomic Perspectives. J. Clean. Prod. 2023, 414, 137460. [Google Scholar] [CrossRef]
- Messinese, E.; Pitirollo, O.; Grimaldi, M.; Milanese, D.; Sciancalepore, C.; Cavazza, A. By-Products as Sustainable Source of Bioactive Compounds for Potential Application in the Field of Food and New Materials for Packaging Development. Food Bioprocess Technol. 2024, 17, 606–627. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The Potential Effects of Chlorogenic Acid, the Main Phenolic Components in Coffee, on Health: A Comprehensive Review of the Literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.; Oliveira, M.B.P.P.; Alves, R.C. Chlorogenic Acids and Caffeine from Coffee By-Products: A Review on Skincare Applications. Cosmetics 2023, 10, 12. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Fattahi, S.; Zabihi, E.; Abedian, Z.; Pourbagher, R.; Ardekani, A.M.; Mostafazadeh, A.; Akhavan-Niaki, H. Total Phenolic and Flavonoid Contents of Aqueous Extract of Stinging Nettle and in Vitro Antiproliferative Effect on Hela and BT-474 Cell Lines. Int. J. Mol. Cell. Med. 2014, 3, 102. [Google Scholar] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Solaberrieta, I.; Jiménez, A.; Cacciotti, I.; Garrigós, M.C. Encapsulation of Bioactive Compounds from Aloe Vera Agrowastes in Electrospun Poly (Ethylene Oxide) Nanofibers. Polymers 2020, 12, 1323. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Pramanik, F.; Satari, M.H.; Azhari, A. Cytotoxic Activity of Gambier Leave (Uncaria gambir) Ethyl Acetate Extract on Mouse Embryonic Fibroblast Cell (NIH-3T3) Using MTT Assay. Open Dent. J. 2023, 17, e187421062212300. [Google Scholar] [CrossRef]
- Bulgari, D.; Alias, C.; Peron, G.; Ribaudo, G.; Gianoncelli, A.; Savino, S.; Boureghda, H.; Bouznad, Z.; Monti, E.; Gobbi, E. Solid-State Fermentation of Trichoderma spp.: A New Way to Valorize the Agricultural Digestate and Produce Value-Added Bioproducts. J. Agric. Food Chem. 2023, 71, 3994–4004. [Google Scholar] [CrossRef]
- Vitale, S.; Salzano, F.; Staropoli, A.; Marra, R.; Turrà, D.; Lorito, M.; Vinale, F. Nitrogen Source Orchestrates pH Modulation and Secondary Metabolism in Trichoderma harzianum. Chem. Biol. Technol. Agric. 2025, 12, 19. [Google Scholar] [CrossRef]
- Hoseini, M.; Cocco, S.; Casucci, C.; Cardelli, V.; Corti, G. Coffee By-Products Derived Resources. A Review. Biomass Bioenergy 2021, 148, 106009. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma Species—Opportunistic, Avirulent Plant Symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Komes, D. Extraction and formulation of bioactive compounds. In Handbook of Coffee Processing By-Products: Sustainable Applications, 1st ed.; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 93–140. [Google Scholar]
- García, L.R.P.; Del Bianchi, V.L. The Effect of Fungal Fermentation in Phenolics Content in Robusta Coffee Husk. Semin. Ciências Agrárias 2015, 36, 777–785. [Google Scholar] [CrossRef]
- Meneses, N.G.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of Extraction Solvents on the Recovery of Antioxidant Phenolic Compounds from Brewer’s Spent Grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef]
- Ribeiro, E.F.; Luzia, D.M.M.; Jorge, N. Antioxidant Compounds Extraction from Coffee Husks: The Influence of Solvent Type and Ultrasound Exposure Time. Acta Sci. Technol. 2019, 41, e36451. [Google Scholar] [CrossRef]
- De Silva, M.O.; Honfoga, J.N.B.; de Medeiros, L.L.; Madruga, M.S.; Bezerra, T.K.A. Obtaining Bioactive Compounds from the Coffee Husk (Coffea arabica L.) Using Different Extraction Methods. Molecules 2020, 26, 46. [Google Scholar] [CrossRef]
- Londoño-Hernandez, L.; Ruiz, H.A.; Ramírez, T.C.; Ascacio, J.A.; Rodríguez-Herrera, R.; Aguilar, C.N. Fungal Detoxification of Coffee Pulp by Solid-State Fermentation. Biocatal. Agric. Biotechnol. 2020, 23, 101467. [Google Scholar] [CrossRef]
- Palomino Garcia, L.R.; Biasetto, C.R.; Araujo, A.R.; Bianchi, V.L.D. Enhanced Extraction of Phenolic Compounds from Coffee Industry’s Residues through Solid State Fermentation by Penicillium purpurogenum. Food Sci. Technol. 2015, 35, 704–711. [Google Scholar] [CrossRef]
- Nguyen Thai, H.; Van Camp, J.; Smagghe, G.; Raes, K. Improved Release and Metabolism of Flavonoids by Steered Fermentation Processes: A Review. Int. J. Mol. Sci. 2014, 15, 19369–19388. [Google Scholar] [CrossRef] [PubMed]
- Torino, M.I.; Limón, R.I.; Martínez-Villaluenga, C.; Mäkinen, S.; Pihlanto, A.; Vidal-Valverde, C.; Frias, J. Antioxidant and Antihypertensive Properties of Liquid and Solid State Fermented Lentils. Food Chem. 2013, 136, 1030–1037. [Google Scholar] [CrossRef]
- Torres-Mancera, M.T.; Cordova-López, J.; Rodríguez-Serrano, G.; Roussos, S.; Ramírez-Coronel, M.A.; Favela-Torres, E.; Saucedo-Castañeda, G. Enzymatic Extraction of Hydroxycinnamic Acids from Coffee Pulp. Food Technol. Biotechnol. 2011, 49, 369–373. [Google Scholar]
- Erskine, E.; Ozkan, G.; Lu, B.; Capanoglu, E. Effects of Fermentation Process on the Antioxidant Capacity of Fruit Byproducts. ACS Omega 2023, 8, 4543–4553. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Tan, Y.; Wu, H.; Shi, L.; Barrow, C.; Dunshea, F.R.; Suleria, H.A. Impacts of Fermentation on the Phenolic Composition, Antioxidant Potential, and Volatile Compounds Profile of Commercially Roasted Coffee Beans. Fermentation 2023, 9, 918. [Google Scholar] [CrossRef]
- Patial, P.K.; Sharma, A.; Kaur, I.; Cannoo, D.S. Correlation Study among the Extraction Techniques, Phytochemicals, and Antioxidant Activity of Nepeta Spicata Aerial Part. Biocatal. Agric. Biotechnol. 2019, 20, 101275. [Google Scholar] [CrossRef]
- Khasanah, H.; Widianingrum, D.C.; Purnamasari, L.; Wafa, A.; Hwang, S.-G. Evaluation of Coffee Bean Husk Fermented by a Combination of Aspergillus niger, Trichoderma harzianum, and Saccharomyces cerevisiae as Animal Feed. J. Ilmu-Ilmu Peternak. 2022, 32, 416–426. [Google Scholar] [CrossRef]
- Lin, Z.; Wei, J.; Hu, Y.; Pi, D.; Jiang, M.; Lang, T. Caffeine Synthesis and Its Mechanism and Application by Microbial Degradation: A Review. Foods 2023, 12, 2721. [Google Scholar] [CrossRef]
- Mock, M.B.; Summers, R.M. Microbial Metabolism of Caffeine and Potential Applications in Bioremediation. J. Appl. Microbiol. 2024, 135, lxae080. [Google Scholar] [CrossRef]
- Vandeponseele, A.; Draye, M.; Piot, C.; Chatel, G. Study of Influential Parameters of the Caffeine Extraction from Spent Coffee Grounds: From Brewing Coffee Method to the Waste Treatment Conditions. Clean Technol. 2021, 3, 335–350. [Google Scholar] [CrossRef]
- Wu, L. Effect of Chlorogenic Acid on Antioxidant Activity of Flos lonicerae Extracts. J. Zhejiang Univ. Sci. B 2007, 8, 673–679. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, G.; Pan, H.; Fan, L.; Soccol, C.R.; Pandey, A. Production of Powerful Antioxidant Supplements via Solid-State Fermentation of Wheat (Triticum aestivum L.) by Cordyceps militaris. Food Technol. Biotechnol. 2012, 50, 32–39. [Google Scholar]
- Razak, D.L.A.; Rashid, N.Y.A.; Jamaluddin, A.; Sharifudin, S.A.; Long, K. Enhancement of Phenolic Acid Content and Antioxidant Activity of Rice Bran Fermented with Rhizopus oligosporus and Monascus purpureus. Biocatal. Agric. Biotechnol. 2015, 4, 33–38. [Google Scholar] [CrossRef]
- Buenrostro-Figueroa, J.J.; Velázquez, M.; Flores-Ortega, O.; Ascacio-Valdés, J.A.; Huerta-Ochoa, S.; Aguilar, C.N.; Prado-Barragán, L.A. Solid State Fermentation of Fig (Ficus carica L.) by-Products Using Fungi to Obtain Phenolic Compounds with Antioxidant Activity and Qualitative Evaluation of Phenolics Obtained. Process Biochem. 2017, 62, 16–23. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Chen, J.; Tang, H.; Wang, C.; Li, Z.; Xiao, Y. Bioprocessing of Soybeans (Glycine max L.) by Solid-State Fermentation with Eurotium cristatum YL-1 Improves Total Phenolic Content, Isoflavone Aglycones, and Antioxidant Activity. RSC Adv. 2020, 10, 16928–16941. [Google Scholar] [CrossRef]
- Arnous, A.; Meyer, A.S. Grape Skins (Vitis vinifera L.) Catalyze the in Vitro Enzymatic Hydroxylation of p-Coumaric Acid to Caffeic Acid. Biotechnol. Lett. 2009, 31, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Chen, F.; Jiang, Z.D.; Cai, M.Y.; Yang, Y.F.; Xiao, A.F.; Cai, H.N. Biotransformation of tea catechins using Aspergillus niger tannase prepared by solid state fermentation on tea byproduct. LWT–Food Sci. Technol. 2015, 60, 1206–1213. [Google Scholar] [CrossRef]
- Ritthibut, N.; Oh, S.-J.; Lim, S.-T. Enhancement of Bioactivity of Rice Bran by Solid-State Fermentation with Aspergillus Strains. LWT 2021, 135, 110273. [Google Scholar] [CrossRef]
- Haq, H.M.S.U.; Ashfaq, R.; Mehmood, A.; Shahid, W.; Azam, H.G.; Azam, M.; Tasneem, S.; Akram, S.J.; Malik, K.; Riazuddin, S. Priming with Caffeic Acid Enhances the Potential and Survival Ability of Human Adipose-Derived Stem Cells to Counteract Hypoxia. Regen. Ther. 2023, 22, 115–127. [Google Scholar] [CrossRef]
- Kant, V.; Jangir, B.L.; Kumar, V.; Nigam, A.; Sharma, V. Quercetin Accelerated Cutaneous Wound Healing in Rats by Modulation of Different Cytokines and Growth Factors. Growth Factors 2020, 38, 105–119. [Google Scholar] [CrossRef]
- Zhao, B.; Hu, M. Gallic Acid Reduces Cell Viability, Proliferation, Invasion and Angiogenesis in Human Cervical Cancer Cells. Oncol. Lett. 2013, 6, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).