Enhancing Xylanase and Cellulase Production by Two Locally Isolated Fungal Strains Under Solid-State Fermentation of Water Hyacinth and Sugarcane Bagasse
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Xylanase- and Cellulase-Producing Fungi
2.2. Sampling and Preparation of Water Hyacinth and Sugarcane Bagasse
2.3. Screening of Fungal Strains Producing Xylanases and Cellulases
2.4. Inoculum Preparation
2.5. Molecular Identification of the Isolated Fungal Strains
2.6. Determination of Radial Growth Rate in Aspergillus austwickii B6 and Trichoderma harzianum M7
2.7. Xylanase and Cellulase Production by Aspergillus austwickii B6 and Trichoderma harzianum M7 Under Solid-State Fermentation Using Different Culture Media
2.8. Analytical Assays
2.9. Xylanase and Cellulase Activity Assays
3. Results and Discussion
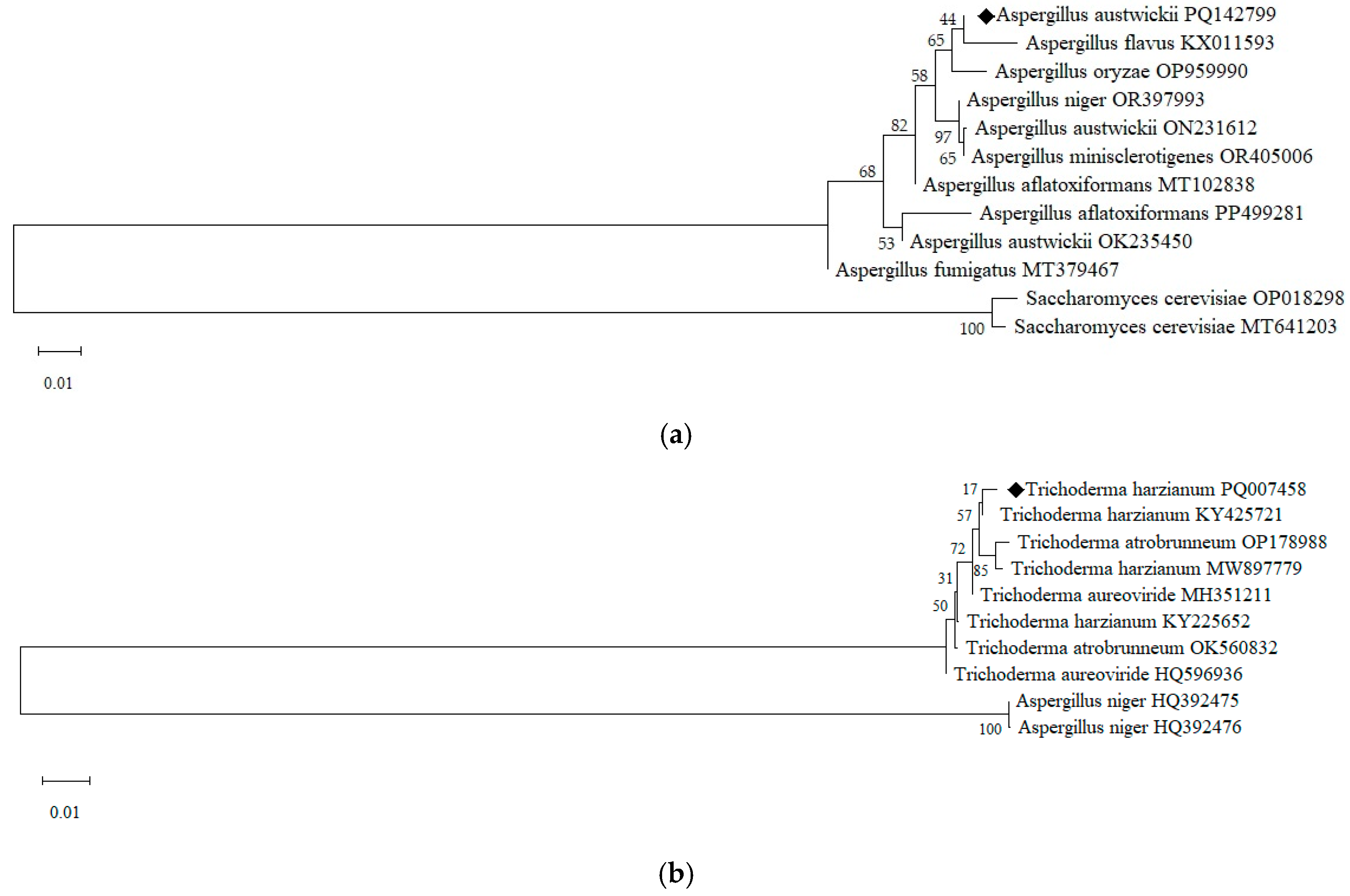
3.1. Isolation and Molecular Identification of Fungal Strains
3.2. Radial Growth Rate of A. austwickii B6 and T. harzianum M7
3.3. Effect of Culture Conditions on Xylanase and Cellulase Production by T. harzianum M7 and A. austwickii B6 Under Solid-State Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reena, R.; Alphy, M.P.; Reshmy, R.; Thomas, D.; Madhavan, A.; Chaturvedi, P.; Pugazhendhi, A.; Awasthi, M.K.; Ruiz, H.; Kumar, V.; et al. Sustainable Valorization of Sugarcane Residues: Efficient Deconstruction Strategies for Fuels and Chemicals Production. Bioresour. Technol. 2022, 361, 127759. [Google Scholar] [CrossRef]
- Wu, X.X.; Zhang, Z.Y.; Jin, Y.G. Physiological Mechanism of Eichhornia crassipes in Inhibiting the Growth of Microcytisaeruginosa. Russ. J. Plant Physiol. 2019, 66, 433–439. [Google Scholar] [CrossRef]
- Nompumelelo, S.; Oziniel, R.; Cuthbert, J.Z.; Arnold, B.M.; Chrispen, M. Exploring for the Possibility of Utilizing Pleurotus ostreatus to Manage Eichhornia crassipes in Zimbabwe. J. Yeast Fungal Res. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Díez, D.; Urueña, A.; Piñero, R.; Barrio, A.; Tamminen, T. Determination of Hemicellulose, Cellulose, and Lignin Content in Different Types of Biomasses by Thermogravimetric Analysis and Pseudocomponent Kinetic Model (TGA-PKM Method). Processes 2020, 8, 1048. [Google Scholar] [CrossRef]
- Abu Yazid, N.; Barrena, R.; Komilis, D.; Sánchez, A. Solid-State Fermentation as a Novel Paradigm for Organic Waste Valorization: A Review. Sustainability 2017, 9, 224. [Google Scholar] [CrossRef]
- Soccol, C.R.; Costa, E.S.F.D.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; Vandenberghe, L.P.D.S. Recent Developments and Innovations in Solid State Fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- Abdul Manan, M.; Webb, C. Modern Microbial Solid State Fermentation Technology for Future Biorefineries for the Production of Added-Value Products. Biofuel Res. J. 2017, 4, 730–740. [Google Scholar] [CrossRef]
- Espinoza-Abundis, C.; Soltero-Sánchez, C.; Romero-Borbón, E.; Córdova, J. Cellulase and Xylanase Production by a Newly Isolated Penicillium crustosum Strain under Solid-State Fermentation, Using Water Hyacinth Biomass as Support, Substrate, and Inducer. Fermentation 2023, 9, 660. [Google Scholar] [CrossRef]
- Acheampong, N.A.; Akanwariwiak, W.G.; Mensah, M.; Fei-Baffoe, B.; Offei, F.; Bentil, J.A.; Borquaye, L.S. Optimization of Hydrolases Production from Cassava Peels by Trametes polyzona BKW001. Sci. Afr. 2021, 12, e00835. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kumar, B.; Verma, P. A Detailed Overview of Xylanases: An Emerging Biomolecule for Current and Future Prospective. Bioresour. Bioprocess. 2019, 6, 40. [Google Scholar] [CrossRef]
- Nordberg Karlsson, E.; Schmitz, E.; Linares-Pastén, J.A.; Adlercreutz, P. Endo-Xylanases as Tools for Production of Substituted Xylooligosaccharides with Prebiotic Properties. Appl. Microbiol. Biotechnol. 2018, 102, 9081–9088. [Google Scholar] [CrossRef]
- Linares-Pasten, J.A.; Aronsson, A.; Karlsson, E.N. Structural Considerations on the Use of Endo-Xylanases for the Production of Prebiotic Xylooligosaccharides from Biomass. CPPS Curr. Protein Pept. Sci. 2017, 19, 48–67. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols; Academic Press: New York, NY, USA, 1990; pp. 315–322. ISBN 978-0-12-372180-8. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Ravichandra, K.; Yaswanth, V.V.N.; Nikhila, B.; Ahmad, J.; Srinivasa Rao, P.; Uma, A.; Ravindrababu, V.; Prakasham, R.S. Xylanase Production by Isolated Fungal Strain, Aspergillus fumigatus RSP-8 (MTCC 12039): Impact of Agro-Industrial Material as Substrate. Sugar Tech 2016, 18, 29–38. [Google Scholar] [CrossRef][Green Version]
- Yadav, P.; Maharjan, J.; Korpole, S.; Prasad, G.S.; Sahni, G.; Bhattarai, T.; Sreerama, L. Production, Purification, and Characterization of Thermostable Alkaline Xylanase From Anoxybacillus kamchatkensis NASTPD13. Front. Bioeng. Biotechnol. 2018, 6, 65. [Google Scholar] [CrossRef]
- Sinha, A.; Singh, R.; Rao, S.G.; Verma, A. Comprehensive Evaluation of Trichoderma harzianum and Trichoderma viride on Different Culture Media & at Different Temperature and pH. Pharma Innov. J. 2018, 7, 193–195. [Google Scholar]
- Jahan, N.; Sultana, S.; Adhikary, S.K. Sanzida Rahman Evaluation Of The Growth Performance Of Trichoderma harzianum (Rifai.) On Different Culture Media. IOSR-JAVS 2013, 3, 44–50. [Google Scholar] [CrossRef]
- Rabab, A.M.; Asmaa, S.T.; Asmaa, H.M.; Shereen, A.S. Adaptability Assessment of Aspergillus niger and Aspergillus terreus Isolated from Long-Term Municipal/Industrial Effluent-Irrigated Soils to Cadmium Stress. BMC Microbiol. 2025, 25, 297. [Google Scholar] [CrossRef]
- Belete, K.; Getu, E.; Mekonnen, A. Investigation on Suitability and Safeness of Water Hyacinth for Animal Feed, from Lake Ziway. SEJS 2022, 45, 283–295. [Google Scholar] [CrossRef]
- Romero-Borbón, E.; Oropeza-González, A.E.; González-García, Y.; Córdova, J. Thermochemical and Enzymatic Saccharification of Water Hyacinth Biomass into Fermentable Sugars. Processes 2022, 10, 210. [Google Scholar] [CrossRef]
- Arana-Cuenca, A.; Tovar-Jiménez, X.; Favela-Torres, E.; Perraud-Gaime, I.; González-Becerra, A.E.; Martínez, A.; Moss-Acosta, C.L.; Mercado-Flores, Y.; Téllez-Jurado, A. Use of Water Hyacinth as a Substrate for the Production of Filamentous Fungal Hydrolytic Enzymes in Solid-State Fermentation. 3 Biotech 2019, 9, 21. [Google Scholar] [CrossRef]
- Singh, A.; Bishnoi, N.R. Comparative Study of Various Pretreatment Techniques for Ethanol Production from Water Hyacinth. Ind. Crops Prod. 2013, 44, 283–289. [Google Scholar] [CrossRef]
- Hassan, H.M.; Keera, A.A.; Fadel, M. High-Yield Cellulases and Xylanase Production from Sugar-Cane Bagasse Pith by Aspergillus oryzae FK-923 Cultivated under Solid State Fermentation. Int. J. Curr. Res. Acad. Rev. 2016, 4, 1–13. [Google Scholar] [CrossRef]
- Rodríguez-Zúñiga, U.F.; Neto, V.B.; Couri, S.; Crestana, S.; Farinas, C.S. Use of Spectroscopic and Imaging Techniques to Evaluate Pretreated Sugarcane Bagasse as a Substrate for Cellulase Production Under Solid-State Fermentation. Appl. Biochem. Biotechnol. 2014, 172, 2348–2362. [Google Scholar] [CrossRef] [PubMed]
- Maciel, G.M.; Haminiuk, I.; Fendrich, R.C.; Bianca, B.E.D. Xylanase Production by Aspergillus niger LPB 326 in Solid-State Fermentation Using Statistical Experimental Designs. Food Technol. Biotechnol. 2008, 46, 183–189. [Google Scholar]
- Marques, N.P.; De Cassia Pereira, J.; Gomes, E.; Da Silva, R.; Araújo, A.R.; Ferreira, H.; Rodrigues, A.; Dussán, K.J.; Bocchini, D.A. Cellulases and Xylanases Production by Endophytic Fungi by Solid State Fermentation Using Lignocellulosic Substrates and Enzymatic Saccharification of Pretreated Sugarcane Bagasse. Ind. Crops Prod. 2018, 122, 66–75. [Google Scholar] [CrossRef]
- Yang, S.Q.; Yan, Q.J.; Jiang, Z.Q.; Li, L.T.; Tian, H.M.; Wang, Y.Z. High-Level of Xylanase Production by the Thermophilic Paecilomyces themophila J18 on Wheat Straw in Solid-State Fermentation. Bioresour. Technol. 2006, 97, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Moran-Aguilar, M.G.; Costa-Trigo, I.; Calderón-Santoyo, M.; Domínguez, J.M.; Aguilar-Uscanga, M.G. Production of Cellulases and Xylanases in Solid-State Fermentation by Different Strains of Aspergillus niger Using Sugarcane Bagasse and Brewery Spent Grain. Biochem. Eng. J. 2021, 172, 108060. [Google Scholar] [CrossRef]
- Leite, P.; Silva, C.; Salgado, J.M.; Belo, I. Simultaneous Production of Lignocellulolytic Enzymes and Extraction of Antioxidant Compounds by Solid-State Fermentation of Agro-Industrial Wastes. Ind. Crops Prod. 2019, 137, 315–322. [Google Scholar] [CrossRef]
- Ajijolakewu, A.K.; Leh, C.P.; Wan Abdullah, W.N.; Lee, C.K. Optimization of Production Conditions for Xylanase Production by Newly Isolated Strain Aspergillus niger through Solid State Fermentation of Oil Palm Empty Fruit Bunches. Biocatal. Agric. Biotechnol. 2017, 11, 239–247. [Google Scholar] [CrossRef]
 ) and Aspergillus austwickii B6 (
) and Aspergillus austwickii B6 ( ) grown on PDA. Data represent the mean and standard deviation of three independent experiments.
) grown on PDA. Data represent the mean and standard deviation of three independent experiments.
 ) and Aspergillus austwickii B6 (
) and Aspergillus austwickii B6 ( ) grown on PDA. Data represent the mean and standard deviation of three independent experiments.
) grown on PDA. Data represent the mean and standard deviation of three independent experiments.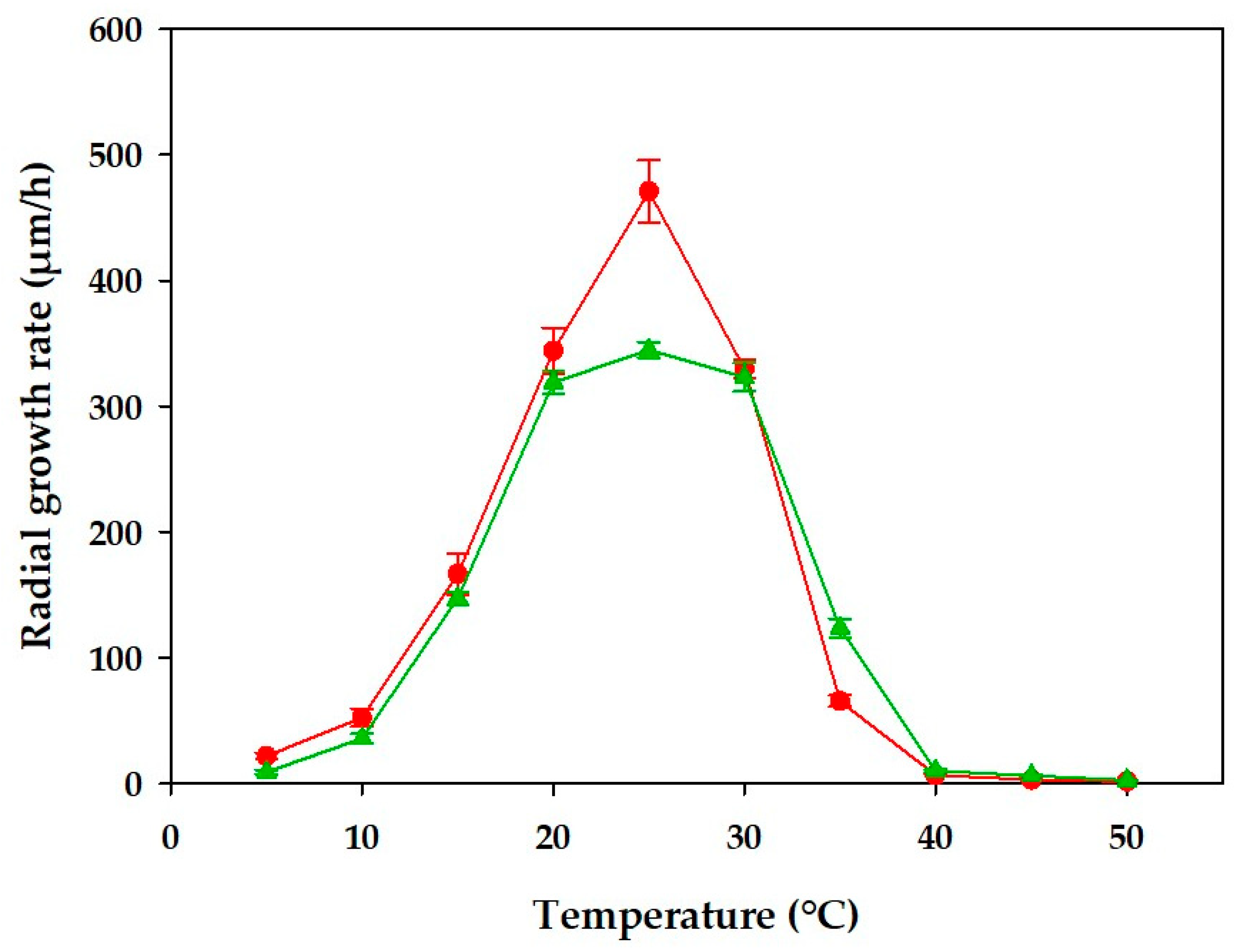
 ), (2) non-pretreated SCB + (NH4)2SO4 (
), (2) non-pretreated SCB + (NH4)2SO4 ( ), (3) pretreated SCB + NaNO3 (
), (3) pretreated SCB + NaNO3 ( ), (4) pretreated SCB + (NH4)2SO4 (
), (4) pretreated SCB + (NH4)2SO4 ( ), (5) non-pretreated WH + NaNO3 (
), (5) non-pretreated WH + NaNO3 ( ), (6) non-pretreated WH + (NH4)2SO4 (
), (6) non-pretreated WH + (NH4)2SO4 ( ), (7) pretreated WH + NaNO3 (
), (7) pretreated WH + NaNO3 ( ), and (8) pretreated WH + (NH4)2SO4 (
), and (8) pretreated WH + (NH4)2SO4 ( ). Error bars represent the standard deviation (n = 3) but are not shown when smaller than the symbols.
). Error bars represent the standard deviation (n = 3) but are not shown when smaller than the symbols.
 ), (2) non-pretreated SCB + (NH4)2SO4 (
), (2) non-pretreated SCB + (NH4)2SO4 ( ), (3) pretreated SCB + NaNO3 (
), (3) pretreated SCB + NaNO3 ( ), (4) pretreated SCB + (NH4)2SO4 (
), (4) pretreated SCB + (NH4)2SO4 ( ), (5) non-pretreated WH + NaNO3 (
), (5) non-pretreated WH + NaNO3 ( ), (6) non-pretreated WH + (NH4)2SO4 (
), (6) non-pretreated WH + (NH4)2SO4 ( ), (7) pretreated WH + NaNO3 (
), (7) pretreated WH + NaNO3 ( ), and (8) pretreated WH + (NH4)2SO4 (
), and (8) pretreated WH + (NH4)2SO4 ( ). Error bars represent the standard deviation (n = 3) but are not shown when smaller than the symbols.
). Error bars represent the standard deviation (n = 3) but are not shown when smaller than the symbols.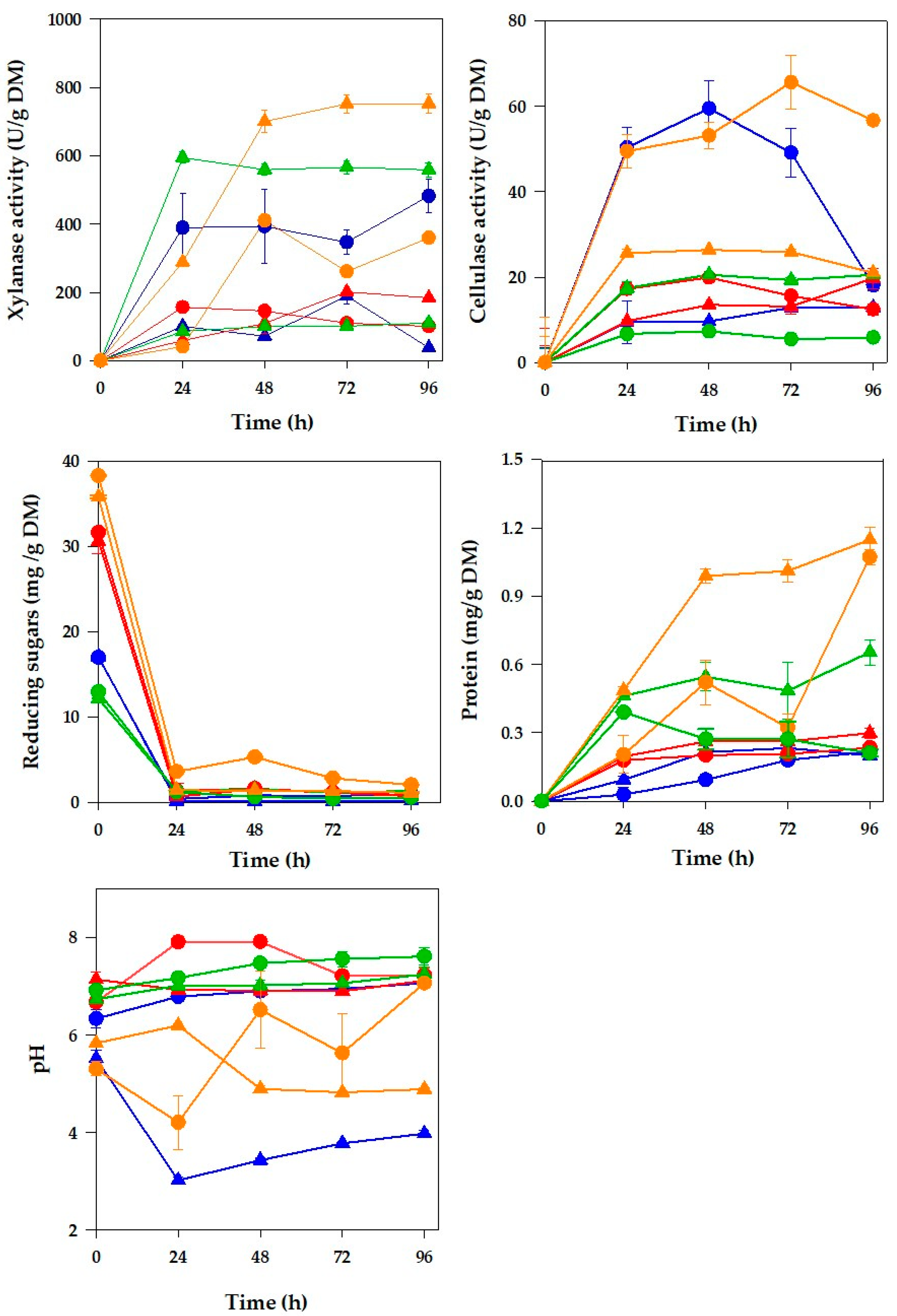
 ), (2) non-pretreated SCB + (NH4)2SO4 (
), (2) non-pretreated SCB + (NH4)2SO4 ( ), (3) pretreated SCB + NaNO3 (
), (3) pretreated SCB + NaNO3 ( ), (4) pretreated SCB + (NH4)2SO4 (
), (4) pretreated SCB + (NH4)2SO4 ( ), (5) non-pretreated WH + NaNO3 (
), (5) non-pretreated WH + NaNO3 ( ), (6) non-pretreated WH + (NH4)2SO4 (
), (6) non-pretreated WH + (NH4)2SO4 ( ), (7) pretreated WH + NaNO3 (
), (7) pretreated WH + NaNO3 ( ), and (8) pretreated WH + (NH4)2SO4 (
), and (8) pretreated WH + (NH4)2SO4 ( ). Error bars represent the standard deviation (n = 3) but are not shown when smaller than the symbols.
). Error bars represent the standard deviation (n = 3) but are not shown when smaller than the symbols.
 ), (2) non-pretreated SCB + (NH4)2SO4 (
), (2) non-pretreated SCB + (NH4)2SO4 ( ), (3) pretreated SCB + NaNO3 (
), (3) pretreated SCB + NaNO3 ( ), (4) pretreated SCB + (NH4)2SO4 (
), (4) pretreated SCB + (NH4)2SO4 ( ), (5) non-pretreated WH + NaNO3 (
), (5) non-pretreated WH + NaNO3 ( ), (6) non-pretreated WH + (NH4)2SO4 (
), (6) non-pretreated WH + (NH4)2SO4 ( ), (7) pretreated WH + NaNO3 (
), (7) pretreated WH + NaNO3 ( ), and (8) pretreated WH + (NH4)2SO4 (
), and (8) pretreated WH + (NH4)2SO4 ( ). Error bars represent the standard deviation (n = 3) but are not shown when smaller than the symbols.
). Error bars represent the standard deviation (n = 3) but are not shown when smaller than the symbols.
| Combination | Support and Substrate | Thermal–Acid Pretreatment | Nitrogen Source |
|---|---|---|---|
| 1 | SCB | No | NaNO3 |
| 2 | SCB | No | (NH4)2SO4 |
| 3 | SCB | Yes | NaNO3 |
| 4 | SCB | Yes | (NH4)2SO4 |
| 5 | WH | No | NaNO3 |
| 6 | WH | No | (NH4)2SO4 |
| 7 | WH | Yes | NaNO3 |
| 8 | WH | Yes | (NH4)2SO4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soltero-Sánchez, C.; Romero-Borbón, E.; Ortega-de la Rosa, N.D.; Camacho-Ruiz, M.A.; Córdova, J. Enhancing Xylanase and Cellulase Production by Two Locally Isolated Fungal Strains Under Solid-State Fermentation of Water Hyacinth and Sugarcane Bagasse. Fermentation 2025, 11, 578. https://doi.org/10.3390/fermentation11100578
Soltero-Sánchez C, Romero-Borbón E, Ortega-de la Rosa ND, Camacho-Ruiz MA, Córdova J. Enhancing Xylanase and Cellulase Production by Two Locally Isolated Fungal Strains Under Solid-State Fermentation of Water Hyacinth and Sugarcane Bagasse. Fermentation. 2025; 11(10):578. https://doi.org/10.3390/fermentation11100578
Chicago/Turabian StyleSoltero-Sánchez, Carlos, Evelyn Romero-Borbón, Nestor David Ortega-de la Rosa, María Angeles Camacho-Ruiz, and Jesús Córdova. 2025. "Enhancing Xylanase and Cellulase Production by Two Locally Isolated Fungal Strains Under Solid-State Fermentation of Water Hyacinth and Sugarcane Bagasse" Fermentation 11, no. 10: 578. https://doi.org/10.3390/fermentation11100578
APA StyleSoltero-Sánchez, C., Romero-Borbón, E., Ortega-de la Rosa, N. D., Camacho-Ruiz, M. A., & Córdova, J. (2025). Enhancing Xylanase and Cellulase Production by Two Locally Isolated Fungal Strains Under Solid-State Fermentation of Water Hyacinth and Sugarcane Bagasse. Fermentation, 11(10), 578. https://doi.org/10.3390/fermentation11100578







