Abstract
This study investigates the efficient biogenic production of hydrogen via the thermophilic bacterium Thermotoga neapolitana, focusing on optimising process configurations to maximise yield and productivity. To determine optimal conditions, a 1 L anaerobic bioreactor with online gas analytics was designed and tested for batch, fed-batch and continuous fermentation. A maximum hydrogen production rate of 96.1 ± 1.7 Nml·L−1·h−1 was observed in the continuous reactor. The optimal dilution rate was 0.07 h−1. Each dilution rate was kept for ≥56 h fermentation time and resulted in yields of 2.7–3.0 molH2·molglucose−1. A consistently high cell viability (97%) was also observed across various dilution rates. A detailed carbon balance indicates acetate as the main by-product, closely linked to the hydrogen production pathway. Compared to fed batch and batch, the hydrogen production rate could be increased and remain constant over a longer time. In this way the continuous reactor design showed an additional method to produce hydrogen to the established ones. Fermentative hydrogen production is particularly promising when using carbohydrate containing biomass and biowaste, as it can be considered carbon dioxide neutral.
1. Introduction
In the context of climate change, research into alternatives to fossil fuels as energy sources has been ongoing for many years. These are not only finite and lead to resource depletion, but also continue to drive climate change due to greenhouse gas emissions [1]. In this context, hydrogen is a promising candidate to replace fossil fuels as the main energy source in many areas. With its high gravimetric energy density of 33.3 kWh·kg−1, it is already used in select industrial applications and is gaining recognition as a key candidate for a future sustainable energy economy [2,3]. The production of hydrogen in general offers the advantage that energy can be transported and utilised compared to electric energy. Hydrogen can be transported either in compressed or liquefied form, or via liquid organic carriers such as methanol or ammonia [4,5,6]. Yet the majority of hydrogen produced worldwide currently still comes from the process of steam reforming, which is based on fossil raw materials [1,7]. However, hydrogen can also be produced from renewable resources. One of the most widely used methods is electrolysis using renewable electricity [1,8]. On the other hand, there is the possibility of producing biogenic hydrogen using microorganisms, which is the focus of this work. There are some microorganisms that can produce hydrogen as part of their metabolism. This can also be referred to as renewable hydrogen, provided that the substrate resources used are renewable. Albuquerque et al. described that fermentation has the greatest potential for biohydrogen production [9]. This is particularly suitable as residual material streams can be utilised here.
Thermotoga neapolitana is a thermophilic, rod-shaped bacterium, which belongs to the Thermotoga genus and was first isolated and described by Belkin et al. in 1986 in the Bay of Naples [10,11]. This bacterium is able to produce H2 as part of dark fermentation. This metabolic pathway is an anaerobic metabolic pathway. For glucose as the starting substrate, the formula for the production of H2 is shown in Equation (1).
C6H12O6 + 2H2O → 4H2 + 2CO2+ 2CH3COO− + 2H+
Ideally, due to the microbial redox balance, dark fermentation can produce 4 moles of H2 per mole of glucose [12,13]. For T. neapolitana, yields close to the theoretical maximum have already been demonstrated in various approaches [14,15,16,17,18]. As an alternative to the H2 metabolic pathway, lactate is also possible as an end product. However, as this metabolic pathway competes with the H2 metabolic pathway, this is not the preferred end product in most cases. Previous studies have used 13C-based metabolic flux analysis to investigate T. neapolitana, demonstrating that the distribution of carbon through glycolysis and pyruvate branch points controls hydrogen production and by-product formation [19,20].
Other known by-products in noticeable quantities include ethanol and the amino acid L-alanine. The ability of T. neapolitana to produce ethanol was investigated in more detail by Sha et al. who described the metabolic pathway acetyl-CoA to ethanol [21]. Since acetyl-CoA also occurs in the same metabolic pathway as acetate and H2, ethanol production could also reduce the maximum hydrogen yield. The other possible by-product that has already been demonstrated for T. neapolitana is alanine [20] and here too, effects on the hydrogen yield are possible, as alanine is synthesised from the metabolic intermediate pyruvate.
The used thermophilic bacteria have an optimum temperature of 77 °C. This offers the advantage of minimising the risk of contamination, particularly with regard to the use of renewable raw materials and organic waste. It has already been shown for T. neapolitana that H2 production based on organic waste can be quite effective and achieve high yields [22,23,24,25]. For a close relative, T. maritima, an approach to H2 production consisting of seawater and date hydrolysate has already been demonstrated [26]. The use of seawater as a basis can simplify the process considerably and make it more cost effective, especially for scale up considerations.
The selection of the reactor system has been demonstrated to have a substantial impact on the efficacy of the process [27]. T. neapolitana as a thermophilic anaerobic bacterium must be taken into account when designing a reactor system. It is acknowledged that a variety of operating modes may be feasible. In terms of the batch approach, the reactor is set up with the medium and all the associated components. Then the bacteria are inoculated and the process is left to run until the substrate has been completely metabolised into products. This method is distinguished by its ease of implementation and, as a consequence, it is employed with great frequency [28,29]. Fed-batch fermentation, in which substrate is added constantly, period can be extended while minimising the risk of substrate inhibition. While in batch culture the depletion of substrates means that cell viability is expected to decline at a certain point, fed-batch fermentation offers the possibility of a longer-term supply of nutrients [30,31,32]. However, it has already been shown that bacterial cultures will inevitably enter a stationary phase despite the availability of nutrients and regardless of by-products, with a concomitant decrease in the proportion of proliferating cells [33]. Conversely, studies have also shown that the accumulation of metabolic products in the medium can inhibit further growth and metabolic activities [34]. The aim of this study is to test the differences between batch, fed-batch and continuous operation under otherwise identical conditions.
In numerous instances, the implementation of a continuous reactor has been demonstrated to be a viable strategy for attaining elevated production rates over an extended timeframe [35]. In this case, fresh medium is constantly fed in and used medium is removed. The permanent removal of used medium ensures that no inhibiting metabolic products can accumulate. Consequently, the production rate can be maintained at a consistent level that is permanently equivalent to the maximum production rate. In order to maintain the utilisation of the substrate at an optimal level, the dilution rate should be selected in such a manner that the growth rate of the cells is not exceeded.
In the context of hydrogen production with T. neapolitana, various working groups have already demonstrated success in the different fermentation operating modes. Trials have been carried out in batch operation. Research groups have reported H2 yields close to the theoretical maximum. These were in the range of 1.84 to 3.85 molH2·molglucose−1 depending on the batch fermentation parameters [16,18,20,36]. For fed-batch operation, i.e., the repeated supply of sugar, hydrogen production was observed over the duration of the experiment, which spanned 86 h [37]. Here, xylose was used as sugar. The xylose concentration was increased to 5 g·L−1 at four points in time using a feed. The H2 production rate fluctuated during the process between about 2 to 4 mmol·L−1·h−1 (equivalent to 45 to 90 Nml·L−1·h−1). One such factor is the consistent rise in the levels of acetate and lactate, which are by-products of the process. Secondly, the concentration of utilisable sugars varied throughout. The continuous mode of operation has already been demonstrated in some approaches. For example, Dipasquale et al. used immobilised T. neapolitana to prevent the cells from being flushed out during continuous operation [38]. Dreschke et al. investigated how glucose concentration in the feed can affect H2 production [15,38]. They found that an increase in the glucose concentration from 11.1 to 41.6 mM (corresponding to 2 g·L−1 to 7.5 g·L−1) resulted in a reduction in the yield from 3.6 ± 0.1 to 1.4 ± 0.1 molH2·molglucose−1. The highest H2 production rate was achieved with 27.8 mM (equivalent to 5 g·L−1) glucose in the feed, with a yield close to the maximum at 3.1 ± 0.1 molH2·molglucose−1. A direct comparison makes it possible to highlight the differences between the individual reactor operating modes more clearly. H2 production rates and long-lasting cell viability are particularly advantageous when it terms of scale-up proposals involving hydrogen-producing bacteria. This study presents the cell viability rates for the different reactor operating modes.
2. Materials and Methods
2.1. Bacterial Strain and Precultivation
T. neapolitana DSM 4359 was purchased from the DSMZ (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) and precultures were grown under anaerobic conditions in a modified TBGY media according to Childers [39] in 120 mL serum bottles. The media was containing glucose (5 g·L−1), NaCl (20 g·L−1), PIPES (6 g·L−1), KCl (2 g·L−1), L-Cysteine (1 g·L−1), MgSO4·7H2O (500 mg·L−1), yeast extract (500 mg·L−1), NH4Cl (250 mg·L−1), CaCl2·2H2O (50 mg·L−1), K2HPO4 (50 mg·L−1), FeSO4·7H2O (7 mg·L−1), resazurin (1 mg·L−1) and Biotin (20 µg·L−1). The pH was adjusted using 1 M NaOH to 7.5 at 77 °C. Precultures were incubated for 16 h at 77 °C in an IKA 4000 i control incubator (IKA-Werke GmbH & Co. KG, Staufen, Germany) with no shaking.
2.2. Construction of Anaerobic Bioreactor
The anaerobic bioreactor was constructed using a borosilicate glass bottle with a volume of 1 L and is an improved version of the bioreactor described previously [14]. Figure 1 illustrates a process flow diagram of the bioreactor. The medium was the identical as previously described for the precultures. The oxygen was expelled by gassing with sterile N2. In order to reduce the possibility of gas leakage, all connections to the reactor were established using a cannula. 2 M NaOH was used to regulate the pH value. The addition of the NaOH solution was executed in a controlled dose using a peristaltic pump. Similarly, in the case of fed-batch and continuous operation, the feed and the effluent were conveyed in a targeted manner via peristaltic pumps. The pumps were precisely controlled using a BioFlo 120 control unit from Eppendorf. In addition, the system was intermittently sparged with N2 in order to expel the H2 produced and flush to the gas sensors.
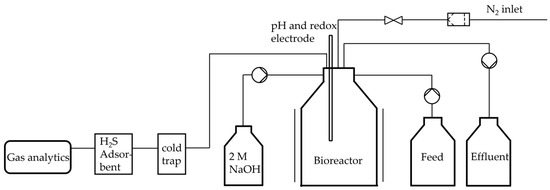
Figure 1.
Process flow diagram of the constructed anaerobic bioreactor for continuous fermentation system. The system can operate in batch, fed-batch or continuous mode, provided that the process conditions are otherwise identical. The reactor is equipped with temperature and pH control, as well as a gas outlet for monitoring the evolution of H2 and CO2.
The produced gas first passed through a cold trap in order to be cooled to room temperature to condense excess water. After that, the gas stream passed through a H2S adsorber, to prevent the gas analytics from coming in contact with the H2S. Afterward, the product gas stream first reached BlueVary sensors for humidity, temperature, pressure and relative gas share measurement. Secondly, the gas stream passed the gas counter. The pH value was regulated automatically during fermentation using the BioFlo 120 control unit. If the pH electrode installed in the reactor recorded a value <7.35, a pump automatically pumped 2 M NaOH solution into the reactor at a rate of 0.5 mL·min−1. The pump stopped automatically as soon as the pH value returned to ≥7.35. The pH fluctuations during all fermentations were ≤0.02.
2.3. Fermentation Conditions
The bioreactor was inoculated with 10% (v/v) preculture after incubation at 77 °C for 16 h. The working volume was 700 mL for all fermentations, except for the fermentation with increased cell concentration at the beginning of fermentation, which was 400 mL. The bioreactor was placed on a magnetic heating device and was insulated with thermal fibre materials. A thermometer for temperature control was placed between the reactor and isolation. Reactor mixing was performed using a magnetic stirrer at 350 rpm. Due to acid production as by-products, a pH correction during the fermentation with 2 M NaOH was implemented. In order to facilitate the transportation of the produced gases to the sensors and to prevent possible H2 inhibition, the bioreactor was subjected to gassing with sterile N2 for a period of 30 s at five-minute intervals during the fermentation process. The gassing rate was set at 40 mL·min−1 and was regulated by the BioFlo 120 Control unit. Liquid samples were taken at certain times. Samples were prepared for OD540 measurement, and cell free supernatant was frozen until HPLC analysis. All samples were taken in technical triplicates.
For the batch with high cell density inoculation, a precultivation of 2800 mL was performed. Cells were harvested using centrifugation at 2000 rcf for 30 min. The pellet was resuspended in 40 mL fresh media and used for inoculation of 400 mL main culture.
For fed-batch process, the feeding with modified TBGY media, containing 100 g·L−1 glucose, started after 24 h processing time. The continuous process was operated for the first 8 h as batch and then in continuous mode with modified TBGY media. Each adjusted dilution rate was kept as long as a steady state could be observed and lasted at least for 56 h at steady state before the dilution rate was altered.
2.4. Analytics
2.4.1. Cell Viability Test by Fluorescence Microscopy
In order to ascertain the viability of the cells during fermentation, staining was performed at regular intervals. For this purpose, fluorescence staining was conducted and analysed by microscopy. For each time point, four images were evaluated by counting the proportion of living and dead cells. The microscope used was a Zeiss axio Imager.M2m with a Plan-Apochromat 63x/1.4 Oil DIC objective (Carl Zeiss AG, Oberkochen, Germany). The corresponding images were taken with an Axiocam 712 mono installed on the microscope. The Bis-(1,3-Dibutylbarbituric Acid)Trimethine Oxonol (DiBAC4 (3)) dye used here to color cells, as it had already achieved promising results for this application [40]. First a stock solution with a concentration of 0.5 mM (dissolved in ethanol) was prepared. The final concentration of the dye in each sample was 0.5 µM DiBAC4 (3). In addition to the samples to be analysed, a dead and a living control were always stained and tested. DiBAC4 (3) is an anionic fluorescent dye that binds to intracellular proteins and membranes of dead cells. When excited at a wavelength of 493 nm, the maximum emission is at 516 nm. Accordingly, the exposure time was set on the microscope using the dead control. The living and dead control samples were taken from cultures, which were handled in an identical manner as the precultures above. The serum bottle for dead control was autoclaved for 15 min at 121 °C to ensure the cells were dead. To prevent cell lysis from falsifying the results, only fresh samples from the same day were used, or further samples were frozen in glycerol stocks and thawed when used.
2.4.2. Gas Analytics
The H2 and CO2 gases produced were analysed using a BlueVary device in combination with a BlueVCount online volumetric flow analyzer (both BlueSens gas sensor GmbH, Herten, Germany). The produced H2 and CO2 gases were analysed with a device equipped for continuous measurement at 5 s intervals, recording temperature, humidity, pressure, and gas concentrations. The volumetric flow was simultaneously measured, allowing for precise calculation of H2 and CO2 production volumes. The volume of gas produced is given in relation to the standard volume (Nml). This refers to the volume of gas at a temperature of 273.15 K and atmospheric pressure (equal to 101,325 Pa). Since the volume of gases is heavily dependent on pressure and temperature, this allows a comparable result to be presented. This combined gas and concentration analysis enabled the establishment of a comprehensive gas mass balance.
2.4.3. Biomass Analytics
Biomass as cell dry weight (CDW) was calculated using measurement of optical density at 540 nm wavelength using UltroSpec2100 photometer. To prevent adulteration by the coloring agent resazurin, the liquid samples were centrifuged for 15 min at 16,100 rcf. The supernatant was used for HPLC analysis and the pellet was resuspended in 1 mL 0.9% (w/w) NaCl solution. The subsequent formula (Equation (2)) allowed the biomass to be calculated based on the optical density.
2.4.4. High-Performance Liquid Chromatography Analytics
The supernatant of liquid samples was used for analysis using high-performance liquid chromatography (HPLC) from Agilent. The selected column was a Repromer H+ by Dr. Maisch 300·8 mm with a temperature of 30 °C during analysis and 5 µL injection volume. As detector a refractory index detector Infinity II was used. The eluent was 5 mM sulfuric acid with a flow rate of 0.6 mL·min−1. Using the already described method allowed to quantify concentrations of the substrate glucose and the possible by-products acetate, lactate and ethanol [25].
RP-HPLC was utilised for the analysis of amino acid concentration. C18 column from type Reprospher C18-Aqua 5 µM 125 · 4.6 mm was utilised for the separation of individual amino acids. The column temperature was set to 30 °C. A gradient method with two different running agents was used. Eluent A was a sodium buffer (Preparation: 0.05 M sodium acetate, 0.05 M NaH2PO·2H2O solved in H2O, then adjusted pH to 7.0 using 5 M NaOH and adding 2% v/v of each Methanol and Tetrahydrofuran). Eluent B was a 54% Methanol solution. The times for the gradient method are shown in Table 1. A xenon FLD was used as fluorescence detector. To detect amino acids this way, the samples were derivatised with ortho-phthaladehyde (OPA) dye just before injection. The OPA solution consists of 5.4 g·L−1 OPA and 4 mL·L−1 beta-mercaptoethanol solved in 0.4 M borate buffer pH 9.

Table 1.
Time profile of the gradient of the eluents of the described sodium buffer (eluent A) and 54% methanol (eluent B) for amino acid detection.
3. Results and Discussion
3.1. Cell Viability with Different Processing Types
Bacterial cell viability is an important factor in establishing long term processes. The expectation for each batch cultivation is a decreasing cell viability, as the consumption of all substrates and accumulation of by-products lead to increasingly unfavourable conditions. In fed-batch processes, where substrate limitations are mitigated, cell viability also tends to decline over time due to by-product accumulation, ultimately leading to cell lysis. The aforementioned effect is particularly critical in fed-batch systems with slow-growing organisms and in batch systems with cell retention, as long-term viability directly impacts process stability. Despite the accumulation of by-products, the cells begin to lyse at a certain point. Following the completion of the lysis process, the cells were undetectable in both the biomass and viability determinations.
As illustrated in Figure 2, dead (coloured) cells can be distinguished from living (uncoloured) cells. By counting the stained and unstained cells, the relative proportions of cells still alive could be determined. Samples were taken at different times for all fermentation approaches and compared with fresh dead or live controls. Figure 3 illustrates cell viability across different fermentation modes over time, highlighting the varying stability and resilience of each approach.
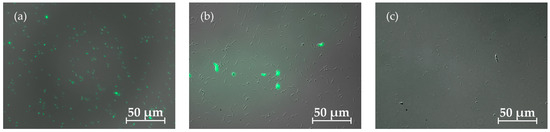
Figure 2.
Viability test for T. neapolitana with fluorescence microscopy using DiBAC4 (3). Dead cells were coloured by the dye and fluorescence and living cells did not light up. Image (a) shows a fresh autoclaved positive control of dead cells, (b) contains a mixture of living and dead cells as a batch sample after 24 h cultivation time was taken and (c) shows the positive control of a fresh preculture containing living cells.
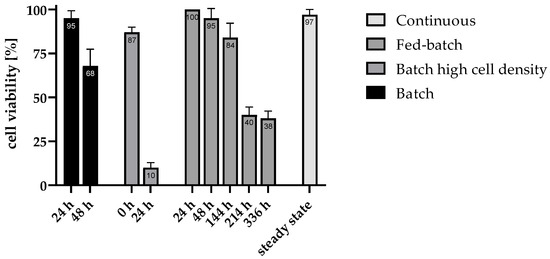
Figure 3.
Results of the viability tests in different fermentation approaches at different fermentation stages. Viability (% viable cells out of total number of cells) was determined using the dye DiBAC4 (3), as viable cells are not stained by the dye and non-viable cells are stained and exhibit a fluorescence signal. Four images were evaluated for each measurement. For the evaluation of the continuous process, only samples in the steady state of the tested dilution rates were considered.
The flattening curve of biomass (Figure 4a) combined with a high viability of 95% suggests that the batch is at the start of the stationary phase after 24 h [33]. Subsequently, a rapid decline in cell viability was observed, with a decrease to 68% at 48 h, indicating a limited capacity to sustain cells under conditions of nutrient depletion and by-product-accumulation. This observation is supported by the available glucose, which is shown in Figure 4a for the batch and Figure 4b for the batch with increased inoculum density. While viability declined more rapidly in the batch with increased inoculum density compared to the batch, this was also accompanied by a more rapid depletion of nutrients. The consumption of glucose in the first hours of fermentation is correspondingly higher in the increased inoculum density batch compared to the normal batch. This initially supports a correlation with decreasing glucose concentration, viability also decreases.
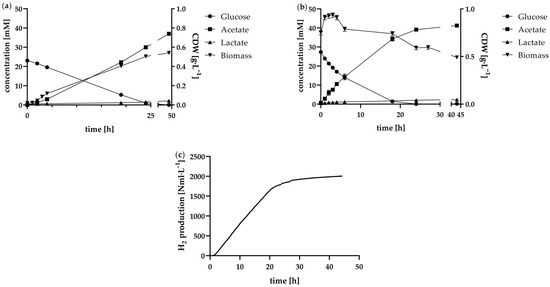
Figure 4.
Results of different batch approaches in 1 L anaerobic bioreactor concerning (a) glucose metabolism into the main dissolved by-products acetate in lactate in mM, as well as biomass evolution given as cell dry weight in g·L−1 of the normal batch, (b) the high-cell-density batch containing harvested cells of 2800 mL preculture at the beginning, and (c) the H2 standard volume in Nml·L−1 curve of the high cell density batch.
In contrast, the fed-batch approach demonstrated a more gradual decline in viability, starting from 100%, slight decreasing to 95% at 48 h and further decreasing to 84% at 144 h. Nevertheless, viability dropped to 38% by the end of fed-batch fermentation, even though the glucose concentration (Figure 5a) continued to rise after 72 h of fermentation, reaching a final concentration of 37.2 ± 0.2 mM. Thus, the glucose concentration at the end was even higher than the starting concentration of all fermentation batches. Since, in addition to glucose, the other nutrients of the modified TBGY medium were also added to the feed throughout the entire fermentation period, this suggests that the availability of nutrients alone does not guarantee high cell viability over a longer fermentation period.
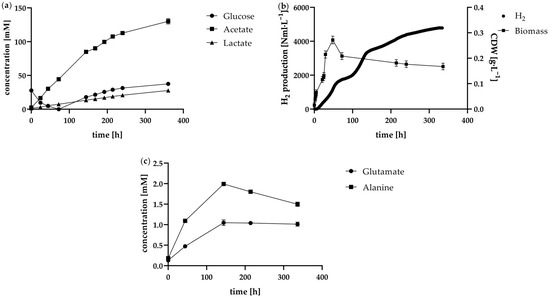
Figure 5.
Results of the fed-batch approach in the 1 L anaerobic bioreactor. The course of the glucose, acetate and lactate concentrations in mM over the fermentation time is shown in (a). Correspondingly, image (b) shows the biomass concentration as cell dry weight in g·L−1·and the cumulative H2 standard volume in Nml·L−1. Image (c) shows the course of the amino acid concentration in mM inside the bioreactor over the fermentation time.
High-density batch cultures exhibited high initial viability, which indicates that the process of centrifugation for preparing the fermentation approach did not damage most cells. Nonetheless the viability was reduced to 10% within the first 24 h displayed, indicates that even elevated cell concentrations cannot mitigate long-term viability loss in batch setups.
The consistently high viability of 97% observed in the continuous mode can be attributed to the absence of inhibitory by-product accumulation, as cells are continuously flushed out and replaced by freshly generated cells. This constant renewal maintains a stable and active cell population, supporting prolonged hydrogen production without the inhibitory effects seen in batch and fed-batch processes. This demonstrates that even at the lowest dilution rate tested (D = 0.03 h−1), there was a sufficiently high nutrient supply. Since the glucose in the feed was almost completely consumed, the glucose concentration in the reactor was mostly at the lower measurement limit of the HPLC method described, which was approximately 0.5 mM. Dreschke et al. also observed that, despite very low glucose concentrations in the reactor, continuous fermentation of T. neapolitana with active cells worked over a longer period of time [15]. There, a constant low glucose concentration (approximately 2 mM) was also observed at a residence time of 24 h (corresponding to D = 0.0417 h−1). The H2 production rate and yield, which remained constant over several days, suggest that the bacteria were supplied with sufficient nutrients.
3.2. Bacterial Cell Growth
Initially, the estimation of possible dilution rates for continuous processes and feeding rate for fed batch was conducted. This was achieved by performing normal batch cultivation and batch cultivation with high cell density at the beginning. In consideration of the results obtained, the highest growth rate and substrate consumption could be calculated.
In addition to the metabolisation of glucose, Figure 4a also provides a representation of the course of biomass formation in a normal batch mode fermentation. Based on biomass concentration measurements, the exponential growth phase between 1 h and 3 h was identified. The maximum growth rate µmax was 0.77 ± 0.06 h−1.
Despite this growth rate being marginally lower than the value of 0.94 h−1 reported by Yu [41] and Jannasch et al., who calculated a similar growth rate from a doubling time of 0.75 h (equivalent to 0.92 h−1) [11,41], it is notably higher than the rate reported by Frascari et al. for suspended cells with glucose as a substrate, which was 0.024 ± 0.005 h−1 [42]. Differences in media composition and preculture treatments likely account for these variations, highlighting how specific cultivation conditions can highly influence the maximum growth rate.
The maximum growth rate delineates the upper limit for feasible dilution rates in continuous operation, as higher dilution rates would lead to cell washout, exceeding the rate of cellular growth [43,44]. However, when utilising the maximum growth rate to formulate a fed-batch or continuous process, it is imperative to acknowledge that the conditions experienced by the cells during these processes are not uniform. In the context of fed-batch fermentation, by-product accumulation like acetate is limiting the maximum growth rate. For instance, it has previously been demonstrated that the biomass growth of T. neapolitana was reduced by 43% when 240 mM was added [15]. Another approach to determining optimal dilution rates is to consider the maximum substrate consumption rate, since the feed medium for continuous operation was identical to that used in batch fermentation. In this study, the maximum glucose consumption rate was found to be 0.97 ± 0.10 mmol·L−1·h−1. It is important to note, however, that cell density at the commencement of batch fermentation at least until 4 h, is significantly lower than after 24 h of fermentation (modified t-test according to Welch p < 0.05). Within the first 24 h, the substrate was almost entirely depleted, similar to findings in other batch studies using a pH-controlled bioreactor with glucose as the substrate [20,37]. Accordingly, the biomass concentration also almost reached its maximum after 24 h at 0.50 ± 0.02 g·L−1. It was observed that there was a paucity of metabolic activity in the following 24 h of fermentation. As demonstrated in Figure 3, this phenomenon is also reflected in the viability of the cells during the fermentation process. While the viability just decreased to 95% in the first 24 h of fermentation, it declined to 68% in the further course. From this point on it can be concluded that at later stages of the fermentation with higher cell densities in a batch approach, optimal growth conditions were no longer present. This can also be attributed to the fact that substrate was no longer available in excess and by-products accumulated in the reactor as a result of metabolism, which can have inhibiting effects. In order to be able to simulate how high the substrate consumption rate can be with cultures of increased biomass concentration, this was also determined for the batch approach with increased cell concentration. In the high-cell-concentration batch, a glucose consumption rate of 2.58 ± 0.16 mmol·L−1·h−1 was determined for the period between 0 and 4 h, during which exponential growth occurred in the normal batch. This value indicates how fast the substrate consumption rate can be under optimal conditions without inhibition by by-products. The feed rate for the fed-batch and the dilution rate for the chemostat were based on these results.
In contrast to the standard batch, no exponential growth of the bacteria was observed in the high cell density batch, as illustrated in Figure 4b. The biomass concentration exhibited an increase from 0.75 to 0.91 g·L−1, followed by a period of stagnation and subsequent decline after 4 h. With a cell viability of 87% at the start of fermentation, the concentrated cell inoculum demonstrated high viability, indicating that the initial cell concentration was suitable for the majority of cells. This high biomass concentration, along with an increased substrate consumption rate, contributed to correspondingly elevated H2 production rates. As illustrated in Figure 4c, H2 production between 1 h and 17 h exhibited a nearly linear trend. Calculated at 1 h intervals, the average H2 production rate during this period was 89.0 ± 6.5 Nml·L−1·h−1. A total of 2005 ± 44 Nml·L−1 H2 was produced over the fermentation period of 44 h. In a 2.4 L batch fermentation, the maximum H2 production rate reported by d’Ippolito et al. was 51 mL·L−1·h−1 [20]. In a batch fermentation bioaugmented with T. neapolitana for H2 production by dark fermentation, the maximum H2 production rate was 1.44 mmol·L−1·h−1, which is equivalent to approximately 32.3 Nml·L−1·h−1 [45]. The higher production rate measured here for almost the entire period during which glucose was present in the medium indicates that the higher cell density from the outset can contribute to a consistently high hydrogen production rate. The virtual halt in further H2 production after 17 h of fermentation is also accompanied by the depletion of glucose, as this drops from 27.4 mM to just 1.7 mM within the first 18 h, falling to 0 mM after 24 h. This highlights the connection between nutrient availability and the ability of bacteria to produce H2 efficiently.
In order to determine what was produced in the process of fermentation in addition to the products already mentioned, further by-product formation analysis was conducted. As already known from T. neapolitana, alanine was detected. However, another amino acid, namely glutamate, which has not been described as a by-product previously, was also detected. An increase in alanine (Figure S1) and glutamate (Figure S2) concentration could be observed. Both batch approaches ended up with comparable amino acid concentrations. The concentration of alanine attained its maximum at 0.84 ± 0.01 mM, while highest recorded glutamate concentration was 0.67 ± 0.08 mM. Alanine as by-product is known from T. neapolitana in low amounts. However, little is known so far about glutamate production. Nonetheless, a glutamate dehydrogenase has been described previously for a closely related species, T. maritima [46]. The concentrations measured here are comparatively low compared to the main by-products produced. Others with considerably higher production rates, such as Corynebacterium glutamicum, have already been established for the industrial production of amino acids [47]. Here, the analysis of the amino acids produced is primarily used to determine the products into which the glucose is metabolised, given that all by-products can affect the possible H2 yield.
3.3. Fed-Batch
The fed-batch was initially permitted to run for 26 h in batch mode. Subsequently, he feed was operated at a rate of 0.7 mL·h−1. At 20-fold glucose feed concentration, this corresponded to a feed of 0.56 mmolglucose·L−1·h−1. The maximum H2 production rate during fed-batch was 49.2 ± 1.4 Nml·L−1·h−1, which was maintained for 4 h during this first feed phase. Following a 35 h of feed supply, the feed was halted in order to ascertain that T. neapolitana was able to completely metabolise the glucose present (Figure 5a). As the glucose in the reactor was completely consumed, H2 production dropped rapidly, as evident from the decline in the H2 production curve (Figure 5b). At 73 h, following the complete depletion of glucose in the reactor, the feed rate was increased to 1 mL·h−1, corresponding to 0.793 mmol·L−1·h−1 of glucose. This adjustment initially led to an increase in hydrogen production, indicating that substrate availability directly influences H2 output. However, despite the increased glucose feed rate, there was no further increase in biomass concentration. Instead, it slightly decreased and subsequently turned stationary. This suggests that a threshold in cell growth was reached, possibly due to limitations from by-product accumulation (e.g., acetate and lactate) or other inhibitory effects specific to the metabolic environment.
One potential explanation for this phenomenon for this is the accumulation of by-products such as acetate and lactate. The concentrations of these two components were similar to the batch approach in the first 48 h but then increased accordingly (Figure 5a). The inhibitory effect of acetate had already been demonstrated by Dreschke et al. [15]. In addition to the aforementioned reduction in maximum growth rate due to increased acetate concentrations, this study also showed that H2 production decreases under these conditions. By increasing the acetate concentration to up to 240 mM at the start of fermentation, H2 production gradually decreased to a maximum reduction of 45% compared to a batch without added acetate. Another recent study focussed on the inhibition of acetate on hydrogen production by dark fermentation in a bacterial consortium. Here, increased acetate concentrations led to the lactate metabolic pathway being favoured and the composition of the dominant bacteria changed considerably [34]. After 144 h, the concentration of acetate already exceeded with 84.9 ± 0.5 mM more than twice that of the batch approach at the end of fermentation (37.0 ± 0.2 mM). This phenomenon was reflected in the productivity of the cells. As demonstrated in Figure 5a, there was an increase in glucose concentration, which was accompanied by a decrease in glucose consumption rate. Conversely, H2 production exhibited a marked decline, accompanied by a modest decrease in biomass within the reactor. As the cells were no longer able to consume all the glucose supplied from the feed, the feed rate was consequently reduced from 1 mL·h−1 to 0.7 mL·h−1 after 146 h of fermentation time, corresponding to a glucose supply of 0.556 mmolglucose·L−1·h−1. A similar trend emerged as the fermentation time progressed. The glucose concentration in the reactor continued to increase, indicating that the glucose consumption rate was still below the feed rate. The by-products acetate and lactate continued to increase, possibly leading to increasing inhibition effects. The H2 formation rate decreased to an average of 10.0 ± 3.0 Nml·L−1·h−1 between 146 h and 217 h. Given that the pH value of the reactor was consistently maintained at 7.35, the inhibitory effect due to the accumulation of the acids produced cannot be attributed to pH reduction. In this case, the increasing ionic strength in the reactor may potentially exert deleterious effects on the bacteria. During this period, a slight decrease in biomass was observed, and viability fell from around 84% to 40%. As this occurred despite continuous feeding and the associated nutrient supply, it could also be explained by the aforementioned effect of individual cells losing their ability to proliferate after a certain fermentation time, regardless of the nutrient supply available [33]. Due to the continuing increase in glucose concentration, the feed rate was further adjusted downwards from time 217 h, being changed to 0.5 mL·h−1, corresponding to 0.396 mmolglucose·L−1·h−1. During this period of fermentation, H2 was still produced, but at a lower production rate of 5.4 ± 3.1 Nml·L−1·h−1. Even at the lower feed rate, the rate of glucose metabolism was found to be less than the rate of glucose supply.
Interestingly, the concentrations of the by-products alanine and glutamate, which are depicted in Figure 5c, show different courses. The concentration of the analysed amino acids was found to be above 0 mM at the commencement of fermentation, mainly due to the addition of yeast extract to the media. In this instance, an increase in concentration was observed from the start of fermentation until after 144 h fermentation time. Alanine increased from 0.18 ± 0.03 mM to 1.99 ± 0.04 mM and glutamate from 0.13 ± 0.03 mM to 1.05 ± 0.07 mM. Unlike the continuous accumulation observed for acetate and lactate, glutamate concentration stabilised, remaining relatively constant until the end of the fermentation process. Conversely, alanine levels exhibited a decline, indicating a transition from synthesis to utilisation. Considering the initial amino acid concentrations (e.g., 0.18 mM alanine from yeast extract at 0 h) and the low amino acid input from the feed, which primarily contained glucose in concentrated form, it is likely that the ongoing addition of amino acids was minimal. Accordingly, it is conceivable that T. neapolitana consumed rather than produced amino acids in the later course of the fermentation, possibly as an adaptive response to prolonged fermentation conditions or limited nutrient availability.
An additional indication that the cells may have experienced inhibition in their metabolic pathway toward acetate and H2 is the accumulation of another by-product ethanol. While ethanol could not be quantified in all batch or continuous approaches (c < 0.1 g·L−1), the fed-batch showed an increasing ethanol concentration in the reactor over the course of 72 h fermentation time. The concentration profile is displayed in Table 2 and reached its maximum of 0.61 ± 0.04 g·L−1 at the end of fermentation. Although T. neapolitana possesses the necessary enzymatic system required for ethanol production, previous studies indicate that the activity of these enzymes is relatively low [21]. This finding indicates that ethanol accumulation may occur under specific conditions, such as those present in the fed-batch process, where inhibition of primary pathways (e.g., acetate and hydrogen production) prompts the cells to redirect metabolism toward ethanol as an alternative by-product. H2 and CO2 are unlikely to cause product inhibition, as they readily escape as gases and are continuously expelled through N2 sparging. However, dissolved by-products that accumulate in the liquid phase can reach inhibitory concentrations, potentially leading to a metabolic shift towards ethanol production. This accumulation of liquid-phase inhibitors may alter the metabolic balance, favouring ethanol as an alternative by-product under higher concentration conditions. As H2 is produced in the same metabolic pathway as acetate, inhibition of acetate production also results in lower H2 production. It would therefore be advisable to plan for in situ product removal of acetate if longer lasting H2 production in the fed-batch is intended.

Table 2.
Ethanol concentration in liquid phase of bioreactor in g·L−1 during the fed-batch for different time points. Liquid samples were taken in technical triplicate and analysed by using HPLC method described above.
In addition to the expected results that in the course of a batch process, the viability decreased with time and the running out of the substrate, the same could also be determined for the fed-batch. In this instance, the consumption of the substrate was not the limiting factor that caused the viability to decrease over time (Figure 3). The inhibition caused by the accumulation of by-products inhibited the cells in their metabolism and growth. In this context, it is plausible to hypothesise that the ionic strength of the medium, rather than merely its by-products, was the pivotal factor. This naturally also increased due to the accumulation of by-products. Various experiments with different buffer concentrations have already shown that increased buffer concentrations may exert inhibitory effects on T. neapolitana [48]. Thus, by-product enrichment and the associated increase in ionic strength could also have a negative effect on T. neapolitana. In order to better define the influence of ionic strength, the osmolarity of the batch fermentation preparations would have to be compared with that of the fed-batch. Since it is not possible to analyse the extent to which the individual media components are completely or only partially bound in the production of biomass and metabolites, the osmolarity at the start of fermentation can be compared with the potential change due to glucose metabolism and NaOH addition. Neglecting yeast extract, which is not clearly defined, the osmolarity in the modified TBGY medium used here is 804 mOsmol·L−1. The metabolism of glucose to the dissolved products and the addition of NaOH resulted in a theoretical increase in osmolarity of 142 mOsmol·L−1 in the batch. The calculation took into account that the acids produced and the NaOH added dissociate in aqueous solutions and form the corresponding salts under fermentation conditions. This avoided double counting of the dissociating substances. In the fed-batch, the increase in ionic strength is greater. This phenomenon can be attributed, firstly, to the elevated glucose concentration in the feed, which is 20 times higher than in the initial medium. Secondly, it is due to the presence of higher concentrations of metabolic products in general, as well as a greater utilisation of NaOH for pH regulation. This resulted in a theoretical increase in osmolarity of 342 mOsmol·L−1 over the entire fermentation period in the fed-batch process. In any case, it can be deduced from the viability profile that a fed-batch can no longer maintain high viability rates over several weeks under the tested conditions. Whilst the viability remained relatively high at 84% after 144 h fermentation time, it decreased to only 40% after 214 h. After 336 h, viability was at its lowest at 38%. It is important to note that the staining method was only able to differentiate between living and dead cells. Accordingly, cells that died and were completely lysed during fermentation could not be detected. As the fermentation lasted a total of two weeks and low cell viabilities were present at later times, it can be assumed that several cells were already lysed during fermentation.
3.4. Continuous Fermentation
Due to the limitations observed in the fed-batch system, particularly with regard to extended fermentation times, a continuous bioreactor was implemented following the setup outlined in Figure 1. The continuous supply of fresh medium and removal of used medium ensured that no by-products could accumulate in the medium in higher concentrations. The dilution rates of 0.03 h−1, 0.05 h−1, 0.07 h−1 and 0.1 h−1 that were examined in this study were derived from the maximum observed glucose consumption rates of the batch runs. Since the feed had the same composition as the TBGY media described, the glucose feed rates were accordingly 0.833 mmolglucose·L−1·h−1, 1.388 mmolglucose·L−1·h−1, 1.943 mmolglucose·L−1·h−1 and 2.775 mmolglucose·L−1·h−1. The glucose concentration in the reactor increased from 0.61 ± 0.28 mM to 5.99 ± 0.17 mM at a dilution rate of 0.1 h−1. This indicates that the critical dilution rate was already exceeded. Accordingly, a steady state could not be achieved. Steady state was observed for all other tested dilution rates. The steady state was characterised by the fact that the glucose concentration in the reactor remained constantly low and fluctuated by less than 0.5 mM. Consequently, it can be deduced that the substrate provided was almost fully utilised. The highest glucose consumption rate in chemostat mode was 2.19 ± 0.05 mmolglucose·L−1·h−1. This observation is consistent with the results of the maximum glucose consumption rate, which could be determined in batch fermentations. Here, a glucose consumption rate of 2.58 ± 0.16 mmolglucose·L−1·h−1 could only be determined for the batch with an increased starting cell concentration under optimum conditions. This is marginally lower than the glucose supply rate at the 0.1 h−1 dilution rate in continuous operation. Theoretically, based on the maximum growth rate, which was µmax = 0.77 ± 0.06 h−1 during the batch fermentation in exponential growth phase, even considerably higher dilution rates would be conceivable. Several factors likely contribute to the observed discrepancy between the maximum growth rate and the achievable dilution rate in continuous operation. One primary factor is the significantly higher cell density in the steady state of the continuous reactor through all dilution rates compared to the exponential growth phase in batch mode (modified t-test according to Welch p < 0.05). The biomass grew between 0.023 ± 0.009 g·L−1 and 0.085 ± 0.011 g·L−1 during the exponential growth phase. The continuous reactor with a dilution rate of 0.03 h−1 had an average biomass concentration of 0.550 ± 0.065 g·L−1 during the steady state. The elevated biomass concentration could therefore prevent the cells from continuing to grow at the maximum possible growth rate, as the availability of nutrients might be limited, or the cell density itself inhibits further growth. This also indicates that, as already mentioned, the maximum growth rate can vary greatly under different conditions and is not consistent over extended periods. Accordingly, the specific growth rate can be lower for a longer period of time. With an experimentally determined maximum dilution rate D = 0.07 h−1, this is noticeably higher than the prognosed optimal dilution rate of D = 0.041 h−1 predicted by Frascari et al. for dissolved cells, which is based on substrate to product and biomass conversion rate and yield [42]. The results are displayed in Figure 6a–d.
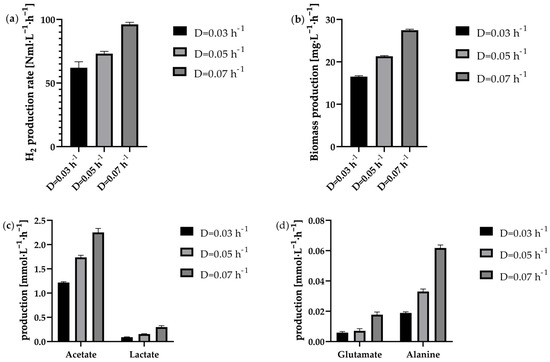
Figure 6.
Production rates in the continuous process in the 1 L anaerobic bioreactor at the tested dilution rates 0.03 h−1 (black bar), 0.05 h−1 (light grey bar) and 0.07 h−1 (dark grey bar). Depicted is the average of each steady state with regard to the following parameters: (a) the H2 production rate in Nml·L−1·h−1, (b) the biomass production rate in mg·L−1·h−1, (c) the acetate and lactate production rate in mmol·L−1·h−1 and (d) the amino acid production rate in mmol·L−1·h−1.
The H2 production rate was found to be consistent overall, indicating a stable steady state. Concurrently, the biomass concentration remained constant. So was the viability of the cells in continuous operation very high at steady state for all dilution rates tested and averaged 97 ± 3%. Thus, in contrast to the batch and fed-batch approaches, there was no recognisable downward trend over the entire steady state period. This indicates that suitable fermentation conditions were generally available for the cells at all different dilution rates. But as the cells are also permanently rinsed out during the continuous process, the cells are only in the bioreactor for a certain time depending on the respective dilution rate. In this case, the residence time was 33.3 h (D = 0.03 h−1), 20.0 h (D = 0.05 h−1) or 14.3 h (D = 0.07 h−1). For periods of time in this range, a comparably high viability was also observed at the start of fermentation in the batch or fed-batch. Nonetheless, it can be hypothesised that, during the fermentation process, a proportion of cells underwent lysis. However, upon their demise, they became undetectable.
Figure 6a–d illustrate that both biomass and metabolic production rates increased with increasing dilution rates, suggesting that the cells can utilise the higher nutrient supply. This is also reflected by the fact that the supplied glucose was almost entirely consumed in all cases. The increasing production rates of alanine and glutamate also suggest that nitrogen availability could increase with higher dilution rates, since amino acid production requires energy-rich carbohydrates, such as glucose, as well as usable nitrogen sources. In this case, alanine is synthesised from the glycolysis end product pyruvate via transamination [20]. Another indication is that the sustained high viability of the cells, which remained at a constant level close to 100% compared to batch and fed-batch approaches, resulted from a higher nutrient supply. As depicted in Figure 6a, the H2 production rate increased with increasing dilution rate. While at D = 0.03 h−1 it was 62.1 ± 4.7 Nml·L−1·h−1, at D = 0.07 h−1 it was even 96.1 ± 1.7 Nml·L−1·h−1 at steady state. An almost comparable increase in biomass production was also observed at higher dilution rates (Figure 6b). Reasons for this phenomenon pertains to the enhanced availability of glucose at elevated dilution rates. For the by-products analysed here, a trend of increasing production rate with increasing dilution rate could also be observed. However, the ratio of acetate to lactate production shifted slightly in favour of lactate as the dilution rate increased. While at the lowest dilution rate D = 0.03 h−1 the ratio was 13.4 acetate: 1 lactate, at D = 0.05 h−1 this ratio fell to 11.1 acetate: 1 lactate. At D = 0.07 h−1, the ratio was even decreased to 7.5 acetate: 1 lactate. While all of the dilution rates tested in a steady-state condition displayed high levels of viability, a consistent H2 production rate and nearly complete glucose consumption, a transition in the acetate-to-lactate ratio was observed as the dilution rate increased. In order to achieve a more comprehensive understanding of the underlying causes of this phenomenon, future research initiatives should incorporate metabolic flux analyses as a crucial component within their experimental designs. It is imperative to minimise the lactate production rate, given the competition between the metabolic pathways to lactate and H2 [14]. However, it has been demonstrated that the lactate production does not necessarily have to reduce the H2 yield, as T. neapolitana, or the strain T. neapolitana cf capnolactica (DSM33003) used in some cases, appears to be able to form lactate from acetate and CO2 without negatively affecting H2 production [16,49]. Activating this metabolic pathway could potentially be used to achieve higher H2 production rates. Dipasquale et al. report that gassing the reactor with CO2 instead of N2 increased the maximum H2 production rate from 34.6 ± 4.7 mL·L−1·h−1 to 67.0 ± 15.6 mL·L−1·h−1 [16]. At the same time, glucose was also consumed more quickly during this period. Higher glucose consumption rates would suggest that higher dilution rates can also be set in the continuous process. However, it must be noted that gassing with CO2 leads to an increased NaOH supply due to the drop in pH value. In fed-batch processes, this could lead to decreasing performance over a longer period of time, as the ionic strength increases even more with time. In continuous processes, on, however, the effect is expected to be less noticeable, as the medium is continuously renewed. However, the shift in the ratio of acetate to lactate produced towards lactate also suggests that the nutrient supply is noticeably higher at higher dilution rates. The breakdown of pyruvate to acetate enables the generation of an additional ATP molecule via acetate kinase [13,50]. However, if pyruvate is now reduced to lactate, no energy is generated in the form of ATP. Nevertheless, NAD+ can be regenerated in this way, which is otherwise provided by hydrogen synthesis. Since the yield of hydrogen remained very similar at all dilution rates, the increased ratio of lactate to acetate could indicate that the cells prioritise the regeneration of NAD+ at higher nutrient supply levels due to a possible excess of NADH. However, further experiments on metabolic analysis would be necessary to validate this hypothesis.
An examination of the yields for all stable dilution rates showed hardly any differences in terms of product yields. As illustrated in Figure 7a, the H2 yield at steady state for the dilution rates tested was between 2.7 ± 0.1 molH2·molglucose−1 (D = 0.05 h−1) and 3.0 ± 0.1 molH2·molglucose−1 (D = 0.07 h−1). Furthermore, the yields demonstrated stability during the steady-state period, indicating consistent physiological responses. The yields for acetate and lactate also showed hardly any differences. Only the yields of the produced amino acids alanine and glutamate showed a slightly increased yield at the dilution rate of D = 0.07 h−1 (Figure 7b). The calculated yield of 0.044 ± 0.002 molalanine·molglucose−1 is comparable to the previously reported alanine yield [51]. However, among other things, production of alanine ensured the H2 yield was below the theoretical maximum. It has previously been demonstrated that both pyruvate and reduction equivalents are necessary for the production of alanine [16,20,52]. As a consequence, these components are not available to the maximum possible extent for H2 production. Conversely, it has been demonstrated that alanine, amongst other amino acids, can function as a nutrient source [53], a finding that is consistent with the results obtained from the fed batch process previously outlined. Accordingly, the use of pyruvate and reduction equivalents for alanine production may be counteracted by specifically adding small amounts of alanine to the fermentation.
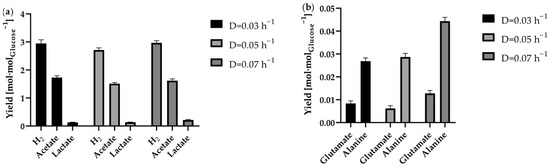
Figure 7.
Product yields in the continuous process in the 1 L anaerobic bioreactor at the tested dilution rates 0.03 h−1 (black bar), 0.05 h−1 (light grey bar) and 0.07 h−1 (dark grey bar). Depicted is the average yield based on glucose consumption of each steady state concerning (a) the H2 acetate and lactate yield in molproduct·molglucose−1 and (b) the yield of analysed amino acids in molamino acid·molglucose−1. The yield for products dissolved in liquid was calculated based on the difference in concentration of these products and glucose in the feed and effluent.
One potential approach to enhance the system would be to implement a membrane that facilitates the retention of cells within the bioreactor. It has already been shown that an increase in the biomass concentration can result in accelerated glucose metabolism [51]. Higher biomass concentration could also overcome possible inhibition because of higher glucose concentration in the feed. As shown by Dreschke et al. increased glucose concentration from 11.1 to 41.6 mM in the feed, reduced noticeably the hydrogen yield [15]. An enhanced glucose metabolism would confer numerous benefits, especially with regard to scale-up. In this way, higher throughputs could also be achieved with lower volumes, thereby rendering the entire process more cost-effective.
Another option for optimising the continuous system to enable an increase in the dilution rate would be to immobilise the cells in the reactor. Successful approaches for this have already been demonstrated [38,42]. This would serve to prevent a washout, even at higher dilution rates. However, it would have to be ensured that the substrate is as favourably accessible as possible for the cells and is metabolised expeditiously.
In addition to the individual production rates in a continuous process, the carbon balance is an important aspect to consider. This can provide information on whether all the main products produced have already been identified and quantified. Furthermore, an overview of the proportions of the products produced can provide information on whether contamination or a shift in metabolism has occurred over time.
The carbon balance, which is illustrates in Figure 8, refers to the substrate consumption and the resulting products in continuous operation at steady state. Empirical analysis of T. maritima revealed a carbon content of 45.9% of the total cell dry weight [54]. In this study, a C content of 38.7 ± 0.2% was determined experimentally for T. neapolitana. For this purpose, the dried cell biomass from the fermenter was analysed as part of a C-H-N analysis using a Vario EL cube elemental analyser. It is initially noticeable that the overall balance of 102.8% ± 7.4% exceeds the theoretical maximum. Compared to the carbon balance of Munro et al. [18] the value is quite similar, though. This could be explained by the assumption that the small amounts of yeast extract present in the media were also used to synthesise biomass. As the composition of yeast extract is not well defined, it is difficult to estimate the extent to which carbon from this source has been utilised. Nonetheless, given that the modified TBGY medium contains 0.5 g·L−1 of yeast extract and 5 g·L−1 of glucose, a maximum deviation of up to 10% cannot be ruled out in theory. However, the balance suggests that all carbon-containing products that are formed in noticeable quantities have been found and quantified here. In conclusion, the analysis revealed that acetate constituted the predominant carbon fraction, accounting for 54.0% of the total carbon content. This was followed by CO2 (27.3%) and biomass (11.1%). As acetate is produced in the same metabolic pathway as H2, a correspondingly high acetate production is also to be expected with a high H2 production [52,55]. Given that the carbon utilised at the inception of the process could be recovered in the form of various products, potential further utilisation options are quite calculable. For instance, the biomass can be used as a favourable substrate for further fermentation processes. The utilisation of CO2 gas in this manner paves the way for the subsequent production of methanol from CO2 and H2. Methanol has been considered a viable solution for the purpose of both capturing CO2 and enabling the H2 to be stored and transported more easily [56].
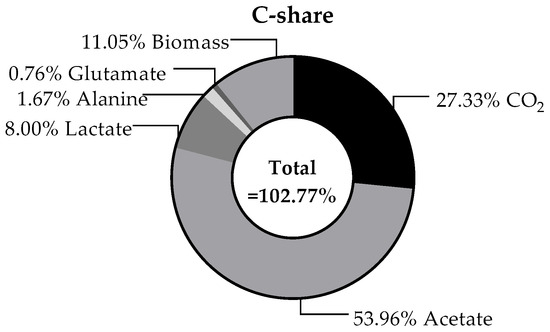
Figure 8.
Average calculated carbon balance of the continuous fermentation during steady state for tested dilution rates. Calculation was based on the glucose consumption as hexose containing 6 C-atoms. The production rate of all detected products was multiplied by the number of carbon atoms in order to identify the proportion of carbon. To calculate the biomass proportion, it was assumed that 38.7% of the mass was carbon, as determined by C-H-N analysis.
4. Conclusions
This study has demonstrated that in the fermentation of Thermotoga neapolitana for microbial hydrogen production, the employment of a continuous bioreactor is associated with a number of advantageous compared to the batch or fed-batch system. It has been demonstrated that by operating the continuous fermentation process with a dilution rate of D = 0.07 h−1, it is possible to achieve and maintain a constant H2 production rate of 96.1 ± 1.7 Nml·L−1·h−1. This rate is a higher compared to the highest production rate in the batch or fed-batch. These could also only maintain their highest H2 production rate for few hours. In the context of batch fermentation, the highest H2 production rate attained was 89.0 ± 6.5 Nml·L−1·h−1 in the batch with elevated inoculation density and could be maintained until the glucose was almost completely consumed. For the fed-batch fermentation, the highest H2 production rate could be only maintained for 4 h with 49.2 ± 1.4 Nml·L−1·h−1 in the first feed phase, while H2 production rate decreased in later stages due to inhibition effect. Inhibition could be avoided by continuous mode. At dilution rates of up to 0.07 h−1, no stationary low glucose concentrations were observed, which indicates that the supplied glucose was completely metabolised. In addition, the continuous reactor system could be upgraded to include a membrane that retains the biomass in the reactor. In this way, the dilution rate could possibly be set even higher, as the cells will not be washed out even above D = 0.07 h−1. The biomass concentration could also be increased in this way.
In batch and fed-batch systems, cell viability decreased over time due to substrate depletion and by-product accumulation. While fed-batch mode mitigated substrate limitations, it faced challenges with by-product inhibition, particularly from acetate and lactate. This accumulation led to metabolic shifts, favouring ethanol production, and resulted in lower hydrogen yields over extended fermentation periods. The observed decline in cell viability and production rates under these conditions highlights the limitations of fed-batch systems for sustained hydrogen production. To achieve an enhanced process stability in fed-batch approaches an in situ product removal might be considered. In contrast, the continuous bioreactor maintained high cell viability (averaging 97%) and stable hydrogen production rates across various dilution rates, demonstrating the feasibility of long-term hydrogen production. The continuous mode enabled efficient removal of inhibitory by-products, thereby preventing the metabolic shifts observed in fed-batch mode. Notably, the maximum H2 production rate reached 96.1 ± 1.7 Nml·L−1·h−1 at a dilution rate of 0.07 h−1, with a steady-state H2 yield of up to 3.0 molH2·molglucose−1. This stability and efficiency position continuous fermentation as the optimal strategy for high-yield, long-term hydrogen production with T. neapolitana.
Across all fermentation modes, acetate was identified as the dominant carbon-containing by-product, reflecting its production via the same metabolic pathway as H2. The continuous process further allowed for consistent acetate and lactate production, although the ratio of these by-products shifted slightly with increased dilution rates, favouring lactate production. Amino acids, such as alanine and glutamate, were also detected, with alanine production partially diverting pyruvate and reduction equivalents, possibly impacting the maximum theoretical H2 yield.
The carbon balance analysis revealed that acetate and CO2 accounted for the largest carbon fractions, with acetate production closely linked to H2 output. The potential for downstream utilisation of both biomass and CO2 suggests future directions for integrating hydrogen production into bioeconomic frameworks. For example, CO2 can be converted into methanol using produced H2, enabling easier storage and transport and enhancing the sustainability of the hydrogen production process.
To overcome the observed limitations in batch and fed-batch systems, potential strategies for improving hydrogen production include cell retention methods, such as membrane integration and cell immobilisation, which could allow higher biomass densities and increased substrate utilisation without washout.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation11100579/s1, Figure S1: Alanine concentration at different times of the different batch fermentations. normal batch (black bars) and batch with increased cell concentration at the beginning (light grey bars) are shown, Figure S2: Glutamate concentration at different times of the different batch fermentations. normal batch (black bars) and batch with increased cell concentration at the beginning (light grey bars) are shown.
Author Contributions
Conceptualization, F.M. and L.H.; methodology, F.M. and J.T.; validation, F.M. and L.H.; formal analysis, F.M. and L.H.; investigation, F.M. and L.H.; resources, N.T.; data curation, L.H.; writing—original draft preparation, F.M.; writing—review and editing, N.T. and J.T.; visualization, F.M.; supervision, N.T.; project administration, N.T.; funding acquisition, N.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the state of North Rhine Westphalia via PtJ [grant number 005-2207 0036_0211]; the Ministry of Culture and Science of the state of North Rhine Westphalia [grant number 005-2105-0044] and the Aachen University of Applied Sciences library.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ishaq, H.; Dincer, I.; Crawford, C. A review on hydrogen production and utilization: Challenges and opportunities. Int. J. Hydrogen Energy 2022, 47, 26238–26264. [Google Scholar] [CrossRef]
- Zajic, J.E.; Kosaric, N.; Brosseau, J.D. Microbial production of hydrogen. Adv. Biochem. Eng. 1978, 9, 57–109. [Google Scholar] [CrossRef]
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen production from biomass using dark fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Teichmann, D.; Arlt, W.; Wasserscheid, P.; Freymann, R. A future energy supply based on Liquid Organic Hydrogen Carriers (LOHC). Energy Environ. Sci. 2011, 4, 2767. [Google Scholar] [CrossRef]
- Teichmann, D.; Arlt, W.; Wasserscheid, P. Liquid Organic Hydrogen Carriers as an efficient vector for the transport and storage of renewable energy. Int. J. Hydrogen Energy 2012, 37, 18118–18132. [Google Scholar] [CrossRef]
- Reuß, M.; Grube, T.; Robinius, M.; Preuster, P.; Wasserscheid, P.; Stolten, D. Seasonal storage and alternative carriers: A flexible hydrogen supply chain model. Appl. Energy 2017, 200, 290–302. [Google Scholar] [CrossRef]
- Panchenko, V.A.; Daus, Y.; Kovalev, A.A.; Yudaev, I.V.; Litti, Y. Prospects for the production of green hydrogen: Review of countries with high potential. Int. J. Hydrogen Energy 2023, 48, 4551–4571. [Google Scholar] [CrossRef]
- Cetinkaya, E.; Dincer, I.; Naterer, G.F. Life cycle assessment of various hydrogen production methods. Int. J. Hydrogen Energy 2012, 37, 2071–2080. [Google Scholar] [CrossRef]
- Albuquerque, M.M.; Sartor, G.d.B.; Martinez-Burgos, W.J.; Scapini, T.; Edwiges, T.; Soccol, C.R.; Medeiros, A.B.P. Biohydrogen Produced via Dark Fermentation: A Review. Methane 2024, 3, 500–532. [Google Scholar] [CrossRef]
- Belkin, S.; Wirsen, C.O.; Jannasch, H.W. A new sulfur-reducing, extremely thermophilic eubacterium from a submarine thermal vent. Appl. Environ. Microbiol. 1986, 51, 1180–1185. [Google Scholar] [CrossRef]
- Jannasch, H.W.; Huber, R.; Belkin, S.; Stetter, K.O. Thermotoga neapolitana sp. nov. of the extremely thermophilic, eubacterial genus Thermotoga. Arch. Microbiol. 1988, 150, 103–104. [Google Scholar] [CrossRef]
- Thauer, R.K.; Jungermann, K.; Decker, K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 1977, 41, 100–180. [Google Scholar] [CrossRef]
- Schroeder, C.; Selig, M.; Schoenheit, P. Glucose fermentation to acetate, CO2 and H2 in the anaerobic hyperthermophilic eubacterium Thermotoga maritima: Involvement of the Embden-Meyerhof pathway. Arch. Microbiol. 1994, 161, 460–470. [Google Scholar] [CrossRef]
- Pradhan, N.; Dipasquale, L.; d’Ippolito, G.; Panico, A.; Lens, P.N.L.; Esposito, G.; Fontana, A. Hydrogen Production by the Thermophilic Bacterium Thermotoga neapolitana. Int. J. Mol. Sci. 2015, 16, 12578–12600. [Google Scholar] [CrossRef]
- Dreschke, G.; Papirio, S.; Sisinni, D.M.G.; Lens, P.N.L.; Esposito, G. Effect of feed glucose and acetic acid on continuous biohydrogen production by Thermotoga neapolitana. Bioresour. Technol. 2019, 273, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Dipasquale, L.; d’Ippolito, G.; Fontana, A. Capnophilic lactic fermentation and hydrogen synthesis by Thermotoga neapolitana: An unexpected deviation from the dark fermentation model. Int. J. Hydrogen Energy 2014, 39, 4857–4862. [Google Scholar] [CrossRef]
- van Ooteghem, S.A.; Beer, S.K.; Yue, P.C. Hydrogen production by the thermophilic bacterium Thermotoga neapolitana. Appl. Biochem. Biotechnol. 2002, 98–100, 177–189. [Google Scholar] [CrossRef]
- Munro, S.A.; Zinder, S.H.; Walker, L.P. The fermentation stoichiometry of Thermotoga neapolitana and influence of temperature, oxygen, and pH on hydrogen production. Biotechnol. Prog. 2009, 25, 1035–1042. [Google Scholar] [CrossRef]
- Esercizio, N.; Lanzilli, M.; Vastano, M.; Xu, Z.; Landi, S.; Caso, L.; Gallo, C.; Nuzzo, G.; Manzo, E.; Fontana, A.; et al. Improvement of CO2 and Acetate Coupling into Lactic Acid by Genetic Manipulation of the Hyperthermophilic Bacterium Thermotoga neapolitana. Microorganisms 2021, 9, 1688. [Google Scholar] [CrossRef]
- d’Ippolito, G.; Dipasquale, L.; Vella, F.M.; Romano, I.; Gambacorta, A.; Cutignano, A.; Fontana, A. Hydrogen metabolism in the extreme thermophile Thermotoga neapolitana. Int. J. Hydrogen Energy 2010, 35, 2290–2295. [Google Scholar] [CrossRef]
- Sha, C.; Wang, Q.; Wang, H.; Duan, Y.; Xu, C.; Wu, L.; Ma, K.; Shao, W.; Jiang, Y. Characterization of Thermotoga neapolitana Alcohol Dehydrogenases in the Ethanol Fermentation Pathway. Biology 2022, 11, 1318. [Google Scholar] [CrossRef]
- Alibardi, L.; Cossu, R. Effects of carbohydrate, protein and lipid content of organic waste on hydrogen production and fermentation products. Waste Manag. 2016, 47, 69–77. [Google Scholar] [CrossRef]
- Shao, W.; Wang, Q.; Rupani, P.F.; Krishnan, S.; Ahmad, F.; Rezania, S.; Rashid, M.A.; Sha, C.; Md Din, M.F. Biohydrogen production via thermophilic fermentation: A prospective application of Thermotoga species. Energy 2020, 197, 117199. [Google Scholar] [CrossRef]
- Lanzilli, M.; Esercizio, N.; Vastano, M.; Xu, Z.; Nuzzo, G.; Gallo, C.; Manzo, E.; Fontana, A.; d’Ippolito, G. Effect of Cultivation Parameters on Fermentation and Hydrogen Production in the Phylum Thermotogae. Int. J. Mol. Sci. 2020, 22, 341. [Google Scholar] [CrossRef]
- Tix, J.; Moll, F.; Krafft, S.; Betsch, M.; Tippkötter, N. Hydrogen Production from Enzymatic Pretreated Organic Waste with Thermotoga neapolitana. Energies 2024, 17, 2938. [Google Scholar] [CrossRef]
- Saidi, R.; Hamdi, M.; Bouallagui, H. Improvement of Biohydrogen Production from Date Wastes by Thermotoga maritima Using a Continuous Anaerobic Membrane Bioreactor. Waste Biomass Valorization 2023, 14, 1859–1868. [Google Scholar] [CrossRef]
- Wang, S.-J.; Zhong, J.-J. Bioreactor Engineering. In Bioprocessing for Value-Added Products from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2007; pp. 131–161. ISBN 9780444521149. [Google Scholar]
- Cinar, A.; Parulekar, S.J.; Undey, C.; Birol, G. Batch Fermentation, 1st ed.; CRC Press: Boca Raton, FL, USA, 2003; ISBN 9780203911358. [Google Scholar]
- Liu, Z.; Antolli, P.G. Bioreactors: Design, Properties, and Applications (Biochemistry Research Trends Series); Nova Science Publishers Incorporated: Hauppauge, NY, USA, 2012; ISBN 978-1-62100-164-5. [Google Scholar]
- Luli, G.W.; Strohl, W.R. Comparison of growth, acetate production, and acetate inhibition of Escherichia coli strains in batch and fed-batch fermentations. Appl. Environ. Microbiol. 1990, 56, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Modak, J.M.; Lim, H.C.; Tayeb, Y.J. General characteristics of optimal feed rate profiles for various fed-batch fermentation processes. Biotechnol. Bioeng. 1986, 28, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.-J. Recent advances in bioreactor engineering. Korean J. Chem. Eng. 2010, 27, 1035–1041. [Google Scholar] [CrossRef]
- Ughy, B.; Nagyapati, S.; Lajko, D.B.; Letoha, T.; Prohaszka, A.; Deeb, D.; Der, A.; Pettko-Szandtner, A.; Szilak, L. Reconsidering Dogmas about the Growth of Bacterial Populations. Cells 2023, 12, 1430. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Song, W.; Wang, J. Inhibitory effect of acetic acid on dark-fermentative hydrogen production. Bioresour. Technol. 2022, 364, 128074. [Google Scholar] [CrossRef]
- Jung, K.-W.; Kim, D.-H.; Kim, S.-H.; Shin, H.-S. Bioreactor design for continuous dark fermentative hydrogen production. Bioresour. Technol. 2011, 102, 8612–8620. [Google Scholar] [CrossRef]
- Nguyen, T.; Pyokim, J.; Sunkim, M.; Kwanoh, Y.; Sim, S. Optimization of hydrogen production by hyperthermophilic eubacteria, Thermotoga maritima and Thermotoga neapolitana in batch fermentation. Int. J. Hydrogen Energy 2008, 33, 1483–1488. [Google Scholar] [CrossRef]
- Ngo, T.A.; Kim, M.-S.; Sim, S.J. Thermophilic hydrogen fermentation using Thermotoga neapolitana DSM 4359 by fed-batch culture. Int. J. Hydrogen Energy 2011, 36, 14014–14023. [Google Scholar] [CrossRef]
- Basile, M.A.; Carfagna, C.; Cerruti, P.; Gomez d’Ayala, G.; Fontana, A.; Gambacorta, A.; Malinconico, M.; Dipasquale, L. Continuous hydrogen production by immobilized cultures of Thermotoga neapolitana on an acrylic hydrogel with pH-buffering properties. RSC Adv. 2012, 2, 3611. [Google Scholar] [CrossRef]
- Childers, S.E.; Vargas, M.; Noll, K.M. Improved Methods for Cultivation of the Extremely Thermophilic Bacterium Thermotoga neapolitana. Appl. Environ. Microbiol. 1992, 58, 3949–3953. [Google Scholar] [CrossRef] [PubMed]
- Beck, P.; Huber, R. Detection of cell viability in cultures of hyperthermophiles. FEMS Microbiol. Lett. 2006, 148, 11–14. [Google Scholar] [CrossRef][Green Version]
- Yu, X. Biohydrogen Production by the Hyperthermophilic Bacterium Thermotoga neapolitana. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2007. [Google Scholar][Green Version]
- Frascari, D.; Cappelletti, M.; Mendes, J.D.S.; Alberini, A.; Scimonelli, F.; Manfreda, C.; Longanesi, L.; Zannoni, D.; Pinelli, D.; Fedi, S. A kinetic study of biohydrogen production from glucose, molasses and cheese whey by suspended and attached cells of Thermotoga neapolitana. Bioresour. Technol. 2013, 147, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Arya, S.K. Hydrogen from algal biomass: A review of production process. Biotechnol. Rep. 2017, 15, 63–69. [Google Scholar] [CrossRef]
- Esener, A.A.; Roels, J.A.; Kossen, N.W.F.; Roozenburg, J.W.H. Description of microbial growth behaviour during the wash-out phase; determination of the maximum specific growth rate. Eur. J. Appl. Microbiol. Biotechnol. 1981, 13, 141–144. [Google Scholar] [CrossRef]
- Okonkwo, O.; Papirio, S.; Trably, E.; Escudie, R.; Lakaniemi, A.-M.; Esposito, G. Enhancing thermophilic dark fermentative hydrogen production at high glucose concentrations via bioaugmentation with Thermotoga neapolitana. Int. J. Hydrogen Energy 2020, 45, 17241–17249. [Google Scholar] [CrossRef]
- Kort, R.; Liebl, W.; Labedan, B.; Forterre, P.; Eggen, R.I.; de Vos, W.M. Glutamate dehydrogenase from the hyperthermophilic bacterium Thermotoga maritima: Molecular characterization and phylogenetic implications. Extremophiles 1997, 1, 52–60. [Google Scholar] [CrossRef]
- Wendisch, V.F. Microbial production of amino acids and derived chemicals: Synthetic biology approaches to strain development. Curr. Opin. Biotechnol. 2014, 30, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, M.; Bucchi, G.; de Sousa Mendes, J.; Alberini, A.; Fedi, S.; Bertin, L.; Frascari, D. Biohydrogen production from glucose, molasses and cheese whey by suspended and attached cells of four hyperthermophilic Thermotoga strains. J. Chem. Technol. Biotechnol. 2012, 87, 1291–1301. [Google Scholar] [CrossRef]
- Pradhan, N.; d’Ippolito, G.; Dipasquale, L.; Esposito, G.; Panico, A.; Lens, P.N.; Fontana, A. Simultaneous synthesis of lactic acid and hydrogen from sugars via capnophilic lactic fermentation by Thermotoga neapolitana cf capnolactica. Biomass Bioenergy 2019, 125, 17–22. [Google Scholar] [CrossRef]
- Esercizio, N.; Lanzilli, M.; Landi, S.; Caso, L.; Xu, Z.; Nuzzo, G.; Gallo, C.; Manzo, E.; Esposito, S.; Fontana, A.; et al. Occurrence of Capnophilic Lactic Fermentation in the Hyperthermophilic Anaerobic Bacterium Thermotoga sp. Strain RQ7. Int. J. Mol. Sci. 2022, 23, 12049. [Google Scholar] [CrossRef] [PubMed]
- Dreschke, G.; d’Ippolito, G.; Panico, A.; Lens, P.N.; Esposito, G.; Fontana, A. Enhancement of hydrogen production rate by high biomass concentrations of Thermotoga neapolitana. Int. J. Hydrogen Energy 2018, 43, 13072–13080. [Google Scholar] [CrossRef]
- Hallenbeck, P. Biological hydrogen production; fundamentals and limiting processes. Int. J. Hydrogen Energy 2002, 27, 1185–1193. [Google Scholar] [CrossRef]
- van Ooteghem, S.A.; Jones, A.; van der Lelie, D.; Dong, B.; Mahajan, D. H2 production and carbon utilization by Thermotoga neapolitana under anaerobic and microaerobic growth conditions. Biotechnol. Lett. 2004, 26, 1223–1232. [Google Scholar] [CrossRef]
- Rinker, K.D.; Kelly, R.M. Effect of carbon and nitrogen sources on growth dynamics and exopolysaccharide production for the hyperthermophilic archaeon Thermococcus litoralis and bacterium Thermotoga maritima. Biotechnol. Bioeng. 2000, 69, 537–547. [Google Scholar] [CrossRef]
- Nath, K.; Das, D. Improvement of fermentative hydrogen production: Various approaches. Appl. Microbiol. Biotechnol. 2004, 65, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Dalena, F.; Senatore, A.; Marino, A.; Gordano, A.; Basile, M.; Basile, A. Methanol Production and Applications: An Overview. In Methanol; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–28. ISBN 9780444639035. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).