Abstract
Intercropping systems and exogenous microorganism additives are recognized for their potential to influence silage fermentation and quality. This study aims to evaluate the impacts of maize–lablab bean intercropping and lactic acid bacteria (LAB) additives on silage yield, nutritional quality, and economic profitability. A randomized block design was employed with two cropping patterns—maize monocrop (M) and maize–lablab intercrop (ML)—and five additive treatments: No additives (CK), and varying ratios of Lactobacillus Plantarum (LP) and Lactobacillus Buchneri (LB), T1 (100% LP), T2 (9LP:LB), T3 (8LP:2LB), and T4 (100% LB). The silage was analyzed and evaluated for its nutritional quality, fermentation quality, and fermentation effect after 90 days of fermentation. ML intercropping significantly enhanced the fresh matter yields by 8.59% and crude protein content by 8.73% compared to M. From the point of view of inoculation with different lactobacilli, the pH, AA, and NH3-N/TN were lower in the T2 and T3 treatments than in the other treatments, while LA was significantly higher. The V-score, which reflects the overall fermentation quality, was excellent across all treatments, with scores exceeding 80 points; the T2 treatment in ML silage achieved the highest score of 99.58. In addition, intercropping can increase the net income of farmers by 21.67%. In conclusion, maize–lablab intercropping combined with LAB inoculation, particularly with the T2 and T3 treatments, significantly enhances the silage quality and economic returns by reducing pH, increasing the LA content, and improving the CP levels. This study is the first to comprehensively analyze the synergistic effects of altering cropping systems and adding functional microorganisms on forage yield and fermentation quality, offering strategic insights for farms, especially mixed farms, to produce high-quality feed. We recommend adopting these methods to improve feed quality and maximize the profitability of silage production systems.
1. Introduction
Driven by the expanding scale of livestock farming, the demand for high-quality silage has increased rapidly [1,2]. Maize is commonly used for silage production because of its high nutritional value, high yield and ease of cultivation and ensilaging [3,4]. However, the protein content of maize silage ranges from 70 to 90 g kg−1, which does not meet the protein requirements of ruminants [5,6]. In addition, evidence shows that continuous monoculture usually leads to a decline in crop yields due to the depletion of specific soil nutrients, increase in soil pests and diseases, and loss of biodiversity, leading to soil degradation and reduced fertility [7]. Diversified cropping (e.g., intercropping) maintains agricultural productivity [8].
Intercropping maize silage and legumes can take advantage of species complementarities to increase crop productivity and crop protein (CP) content [9]. This strategy of sustainable intensification improves productivity per unit area, offsetting common management and harvest complications. For example, intercropping maize and soybean increased the maize yield by 32.99%, while the soybean yield decreased by 13.92%, resulting in a total system output increase of 25.50% [4]. Similar diversified cropping systems have improved economic returns for smallholder farmers [10]. Lablab bean, a widely cultivated legume, is rich in crude protein and thrives in relatively infertile conditions due to its strong root system and nitrogen fixation capability [11,12]. Despite the challenge of twining lablab vines to complicate grain harvest, intercropping maize with lablab bean leads to increased grain production per unit area, with the potential to benefit farmer income and household nutrition [13]. Mixing silage maize with legume crops can address the challenges of single-crop silage production and facilitate the development of forage resources and silage quality [14]. Specifically, intercropping maize with lablab bean or climbing peas in silage production can improve fermentation characteristics, improve nutritional quality, and increase the crude protein and fiber content compared to sole maize silage [12,15].
The principle of silage is to create anaerobic conditions by compacting and sealing the feed, promoting the growth of LAB. This process depletes oxygen and accumulates lactic acid (LA), effectively inhibiting aerobic and harmful microorganisms [16]. However, typical silage contains less than 1% LA, making it essential to introduce additional LAB to enhance fermentation [17]. The addition of LP and other fermenting LAB to silage accelerates the fermentation process, leading to the production of LA and a decrease in pH [18]. This acidic environment inhibits the activity of plant enzymes and harmful bacteria, thereby preserving the nutrients associated with silage [19,20]. Additionally, aflatoxin production has been shown to decrease in silage inoculated with Lactobacillus [21]. Therefore, it is recommended that Lactobacillus is added during silage to effectively reduce dry matter (DM) loss and avoid clostridial fermentation. In various silages, LAB inoculants modulate the microbial community composition through different routes depending on the epiphytic microbiota of fresh forage. Essentially, these inoculants simplify the interactions among bacterial species to enhance the fermentation quality [22]. Lactobacillus strains used as silage inoculants not only improve the silage quality and aerobic stability, but also exhibit probiotic activity within the digestive tract of animals [23].
Although previous research has explored the impacts of intercropping systems on crop yield, silage quality, and economic efficiency, a holistic approach that integrates these factors remains underdeveloped [8,24,25]. Most existing studies have concentrated on enhancing specific elements rather than examining the combined effects of intercropping and additive use throughout the entire silage production chain. Furthermore, there is a notable lack of research on how intercropping not only maximizes total crop production but also improves the fermentation quality of silage and its economic outcomes. In order to fill this gap, our synthesis assessed the effects of intercropping on yield, silage quality and fermentation after additives, as well as their economic impact. Therefore, we conducted a two-year field trial of intercropping maize with lablab beans and mixed-silage fermentation trials. The effects of intercropping on the yield, silage quality, fermentation quality with additives and their economic returns were evaluated in an integrated manner. We hypothesize that the intercropping of maize with leguminous crops, combined with the application of lactic acid bacteria, will significantly improve the fermentation quality of silage and lead to higher economic returns compared to monoculture systems. The aim is to develop viable cropping and silage strategies for optimizing silage production and the livestock supply chain in the region and similar areas; in addition, the study aims to provide reliable options for enhancing the integration of agro-pastoral systems.
2. Materials and Methods
2.1. Experimental Site
The field experiment was conducted in the town of Sandaoqiao (40°86′ N, 106°92′ E), Hangjin Houqi, Bayannaoer City, Inner Mongolia. This region has a temperate continental climate, with an average annual temperature of 7.4 °C, annual precipitation of 138.2 mm, annual average sunshine of 3220 h, and a frost-free period of about 157 d throughout the year. In 2022, we analyzed the soil in the 0–20 cm soil layer before sowing. The soil had a pH of 9.09, organic matter content of 13.72 g kg−1, total N of 1.00 g kg−1, available P of 33.04 mg·kg−1, and available K of 98.33 mg kg−1. During the crop growth period, in addition to natural rainfall, artificial irrigation was applied three times, with a total irrigation amount of 600 mm. The weather data of the experiment are shown in Figure 1.
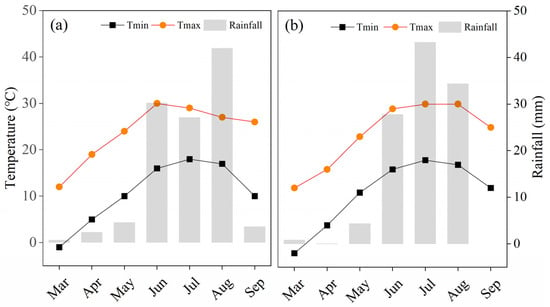
Figure 1.
Monthly minimum temperature, maximum temperature and rainfall for the planting period 2022–2023.The letter (a) indicates the test year 2022 and (b) indicates 2023.
2.2. Field Management and Research Design
A randomized block experimental design was used to set up two planting patterns: maize–lablab intercropping and maize monoculture. Two different treatments were used in the experiment, each with 3 replications, resulting in a total of 6 plots. Each plot was 60 m2, measuring 6 m × 10 m. To avoid interference from nutrient flow, all plots were spaced within 2 m of each other. Maize was sown on 1 May 2022 at a seed rate of about 67,500 plants ha−1 (Figure 2). Two lablab seeds were planted in one planting spot between two maize plants. Then, 388 kg ha−1 pure nitrogen, 245 kg ha−1 P2O5, and 22 kg ha−1 K2O were applied to the maize. P2O5 and K2O were applied once as the base fertilizer, while pure nitrogen was applied two times as a base fertilizer and jointing fertilizer at a ratio of 4:6. The irrigation amount of each treatment was 600 mm, and 200 mm at the nodulation (15 June), silking (20 July) and grain filling (August) stages.
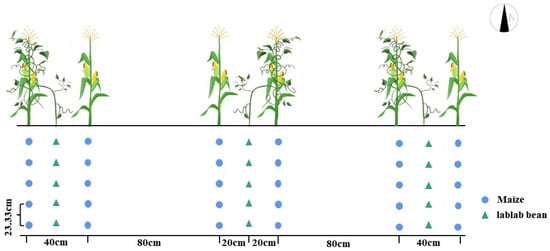
Figure 2.
Pattern of maize–lablab intercropping. The row ratio of maize–lablab bean intercropping was 2:1; the spacing between maize rows, soybean rows, and maize–soybean rows was 40 cm, 120 cm, and 80 cm, respectively. The maize plant spacing was 15.80 cm, and the soybean plant spacing was 7.80 cm.
We selected silage maize (Ximeng 6) and lablab bean (Prada). In terms of weed control, we used artificial weeding. The maize and lablab were harvested at the same time on 6 September 2022 (before the frost). At this time, the maize was in the early stage of milk ripening, and lablab was in the fruiting stage.
2.3. Field Sampling
In the maize harvest period, a 6 m2 quadrat (64 maize plants and 32 maize–lablab plants) was randomly selected in each plot, and biomass above the ground was harvested manually. Half of the plants obtained from the intercropping treatment were separated manually into a maize section and a lablabs section in order to calculate the yields independently. Then, the fresh weight of the maize monoculture plants and the rest of the intercropping plants was determined to calculate the fresh grass yield of the maize and lablab crops; these were then cut into 2–3 cm segments. One part was used to determine the chemical composition of fresh samples before silage, and the other part was used for ensiling.
2.4. Silage Preparation and Sampling
The remaining raw materials (maize plants and maize–lablab plants) were immediately taken to the laboratory and chopped to an approximate length of 2 cm using a handy cutter. Raw materials were separately subjected to ensiling treatments based on a 2 × 5 factorial arrangement in a completely randomized design, either with or without LAB additives: (1) distilled water control (CK); (2) 1.0 × 105 colony-forming units CFU g−1 of LP (T1); (3) 1.0 × 105 colony-forming units CFU g−1 of LP and LB with a ratio of 80:20 (T2); (4) 1.0 × 105 colony-forming units CFU g−1 of LP and LB with a ratio of 90:10 (T3); and (5) 1.0 × 105 colony-forming units CFU g−1 of LB (T4). LP and LB strains were isolated and purified from silages studied earlier, and the additives for silage preparation were made via lyophilization according to the reported procedures. The given solvent was sprayed with a disposable tiny sprayer onto minced leaves for every treatment. After mixing the ingredients thoroughly, four replicates (one for backup) of 400 g of each treated batch were packed into laboratory polyethylene bags (20 mm × 30 mm) and sealed with a vacuum sealing machine at a density of approximately 642 kg of fresh weight (FW) m−3. The silages were stored at ambient temperature conditions (25~28 °C) and opened after 90 days of ensiling for analysis.
2.5. Chemical Composition
We took 200 g of the fresh and silage materials, which were heated at 105 °C for 30 min and then dried at 65 °C to a constant weight. The dried samples were transferred to a desiccator, cooled to room temperature, weighed, and their DM contents were calculated. Subsequently, the dry samples were ground and sieved (40 mesh, pore size 0.425 mm). The Kjeldahl nitrogen method was used to determine the CP content of the samples. About 0.2 g of each dried sieved sample was weighed using a balance with a precision of 1.0 × 10−5 g (the weight of the samples was recorded) and then put into the Kjeldahl nitrogen digestion tube. One piece of catalyst and 12 mL of concentrated sulfuric acid were added in succession. The forage was boiled in a digestion furnace at 420 °C for 90 min and cooled to room temperature in a ventilator. The CP content of the forage was determined using a FOSS KJelTEC-8400 automatic nitrogen meter [26]. The contents of neutral detergent fiber (NDF) and acid detergent fiber (ADF) were determined by the van Soest’s fiber method [27]. The ether extract (EE) was determined by the Soxhlet ether extraction method using an ether extract analyzer (XT15, Ankom, America) [28]. Based on the above indices, the relative feeding value (RFV) of the forage was calculated [29]. The calculation formula was as follows:
where DMI is the random intake of dry matter of roughage, while DDM is the digestible dry matter. Table 1 shows the formulas and methods for V-score calculation.

Table 1.
Calculate method of V-Score.
2.6. Fermentation Indexes
A sample of 10 g was taken from each bag of silage after being manually and homogeneously mixed within the bag and mixed with 90 mL of sterilized water by vigorous shaking at 180 r min−1 for 2 h at 4 °C, and then filtered through a 0.45 μm membrane [30]. The pH of the samples was determined via a pH meter (Mettler Toledo CO., Ltd., Greifensee, Switzerland). The organic acid contents (lactic acid (LA), acetic acid (AA), propionic acid (PA) and butyric acid (BA)) were determined using high-performance liquid chromatography (Waters Alliance e2695, Waters, MA, USA). The ammoniacal nitrogen (NH3-N) concentration was determined using Berthelot colorimetry [31].
2.7. Economic Analysis
We used yield data from a two-year experimental study in our economic analysis. This analysis includes inputs (mulch costs, machine ploughing costs, fertilizer costs, seed costs, irrigation costs, labor costs) and outputs (the economic value is based on the actual local sales price of the forage) calculated from the cradle (crop planting) to the farm gate (harvesting). Thus, net income can be quantified by calculating the difference between output values and input values. Finally, the ratio of total outputs to total inputs was calculated to assess the economic benefits generated by each of the agricultural technologies under study.
2.8. Statistical Analysis
Experimental data were collected, organized, and analyzed using Microsoft Excel 2019. The data were analyzed using a two-way ANOVA to assess the effects of cropping patterns and additive treatments on yield, nutritional, and fermentation quality, with interaction terms between factors included. Post-hoc analyses were performed using the Least Significant Difference (LSD) test for the pairwise comparisons of treatment means, controlling for multiple comparisons using a significance level of p < 0.05. All analyses were conducted in IBM SPSS 27.0 and all figures were generated using Origin 2022.
3. Results
3.1. Biomass Yield and Nutritional Quality of the Maize and Lablab
The yields of FM, DM and CP for maize silage and lablab bean in 2022 and 2023 are shown in Figure 3. The average FM yield of M over the two years was 64.34 t ha−1, while for ML, it was 69.87 t ha−1, representing an average yield increase of 8.59%. There was a significant increase of 15.24% in ML compared to M in 2022 (p < 0.05) and the difference was not significant in 2023 (p > 0.05). The average DM yield over both years was 26.03 t ha−1 for M and 27.81 t ha−1 for ML, representing an average DM yield increase of 6.82%. A significant increase of 12.19% was observed in ML over M in 2022 (p < 0.05) and the difference was not significant in 2023 (p > 0.05). The average CP yield over both years was 140.17 t ha−1 for M and 152.37 t ha−1 for ML, representing an average CP increase of 8.73%. There was a significant increase of 5.43% in ML over M in 2022 (p < 0.05) and a highly significant increase of 12.26% in 2023 (p < 0.01).
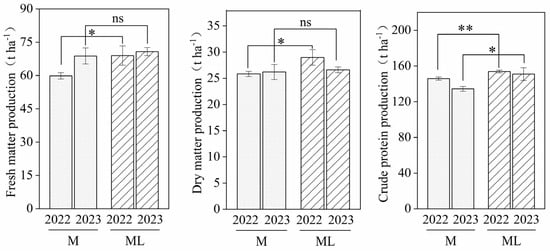
Figure 3.
Effects of maize–lablab intercropping on the biomass yield of fresh forage. M, maize plant; ML, intercropping of maize with lablab bean; “*” represents a significant difference at p < 0.05; “**” represents a highly significant difference at p < 0.01; “ns” represents a non-significant difference at p > 0.05.
The DM CP, EE, Ash, NDF, ADF of maize and lablab in 2022 and 2023 were as follows (Figure 4). The CP content of M and ML was 7.71% and 8.65%, respectively, in 2022 with a significant increase of 12.19% (p < 0.05) in ML compared to M. The CP was not significant (p > 0.05) in 2023. The rest of the treatments showed non-significant (p > 0.05) differences in nutritional indices. DM, CP, EE, ASH, NDF, and ADF were increased by −0.79%, 11.54%, −12.61%, 5.62%, 2.64%, and 5.49%, respectively, in the intercropping planting pattern compared to monocropping.
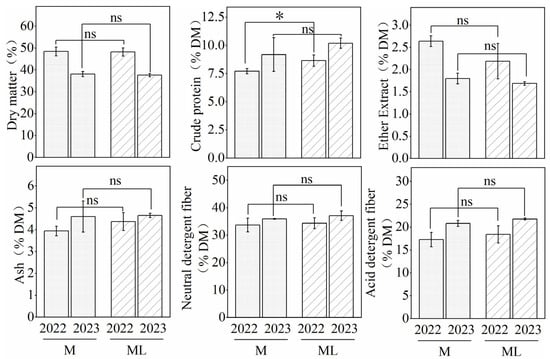
Figure 4.
Effects of maize–lablab intercropping on the Nutritional quality (DM-basis) of the whole plant crop. DM, dry matter. “*” represents a significant difference at p < 0.05; “ns” represents a non-significant difference at p > 0.05.
3.2. Nutritional Quality and Feed Value of Silage
The effects of cropping patterns and LAB additives on the silage nutritional quality are presented in Table 2. In terms of the cropping pattern, different cropping patterns had highly significant (p < 0.01) effects on the nutritional quality of silage. The DM content of ML was significantly reduced by 7.12% (p < 0.01) compared to the M silage, and the CP, EE, Ash, NDF and ADF contents of ML were significantly increased by 15.66%, 8.26%, 21.38%, 19.33% and 27.66%, respectively, compared to M (p < 0.01). The overall relative feeding value of ML silage was significantly reduced by 18.83% (p < 0.01) compared to M silage. In terms of LAB additives, different LAB additives did not significantly (p > 0.05) affect the DM, CP, EE and Ash contents of silage. Meanwhile, the effect of LAB additives on the NDF and ADF contents was highly significant (p < 0.01). The NDF and ADF contents of T3 did not differ significantly from those of CK, while the rest of the groups showed an increasing trend, with T1 having the highest NDF and ADF contents (38.64% and 19.52%). In addition, there was a significant interaction between NDF, ADF and RFV in C × A (p < 0.01). The RFV of silage ranged from 178.64 to 206.04, with T3 having the highest RFV, which was significantly increased by 4.02% (p < 0.01) compared to CK.

Table 2.
Effects of Lactobacillus additives on the nutritional quality (DM-basis) of silage in a maize–lablab intercropping system.
3.3. Silage Fermentation Quality
The nutrition of the silage fermentation by cropping pattern and Lactobacillus additive is shown in Table 3. From the planting pattern, the pH and NH3-N/TN of ML were significantly higher by 4.28% and 17.32% compared to M (p < 0.01). The LA content of ML was significantly lower by 15.04% and the AA content was highly significantly lower by 25.58% compared to M (p < 0.05). The PA and BA contents were not detected in this experiment. In terms of Lactobacillus additives, T3 had the lowest pH (3.79), followed by T2 group (3.80). The LA content of T1, T2 and T4 was significantly reduced by 12.19%, 5.85% and 14.52%, respectively, compared to CK. The highest LA content was found in T3 (8.26), which showed an increase of 3.77% compared to CK. The content of AA showed an increasing trend with the increase in LB. The levels of T1, T2, T3 and T4 increased significantly by 9.57%, 14.89%, 48.94% and 226.60%, respectively, over CK (p < 0.01). The NH3-N/TN content showed a decreasing trend with the addition of Lactobacillus, with T2 being the lowest (3.76), followed by T3 (3.77). The NH3-N/TN was significantly reduced by 7.15%, 20.93%, 20.82% and 16.09% at T1, T2, T3 and T4, respectively, compared to CK (p < 0.05).

Table 3.
Effects of Lactobacillus additives on the fermentation quality (DM-basis) of silage in the maize–lablab intercropping system.
The fermentation quality of the silage in each treatment after 90 d of silage was scored using the V-Score scoring system, and all treatments had scores above 80 points, which was a superior grade; the T2 treatment in the ML silage had the highest V-score (99.58 points). In conclusion, the ML silage pattern combined with T2 or T3 silage additives resulted in the best silage fermentation quality.
3.4. Correlation Analysis between Silage Nutritional Quality and Fermentation Quality
Pearson correlation analyses were carried out to determine the relationship between the silage nutritional quality and fermentation quality, as shown in Figure 5. The silage DM was significantly and positively correlated with EE and significantly and negatively correlated with Ash, NDF and pH. The CP content of silage was significantly positively correlated with NDF, ADF and pH, and significantly negatively correlated with EE and LA. The EE content of silage showed a significant positive correlation with the LA content and negative correlation with Ash, NDF, ADF, pH and NH3-N/TN. The ash content of silage showed a positive correlation with NDF, ADF and pH. The NDF content of silage was significantly and positively correlated with ADF, NH3-N/TN and pH, and negatively correlated with LA. The ADF content was positively correlated with pH and NH3-N/TN, and negatively correlated with the LA and AA content. The silage pH was positively correlated with NH3-N/TN and negatively correlated with LA.
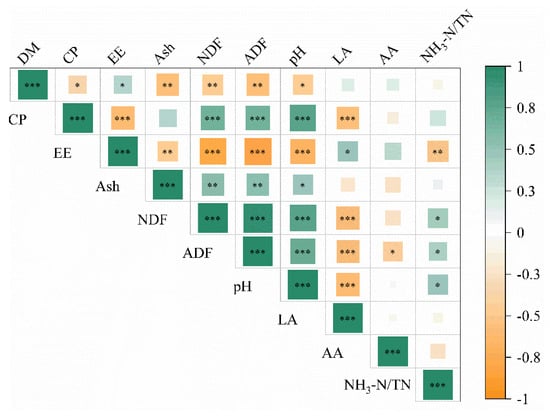
Figure 5.
Correlation between silage nutritional quality and fermentation quality. The corresponding values of the heatmap are the Pearson correlation coefficient r (−1 to 1), with a value below 0 representing a negative correlation (green) and a value over 0 representing a positive correlation (orange). *, ** and *** represent the relation between indices that are significant at the 5%, 1% and 0.1% level, respectively.
3.5. Gross Revenues, Net Returns and Output-to-Input Ratios for Different Treatments
This analysis included the inputs and outputs from the cradle (crop planting) to the farm gate (harvesting) (Table 4). The output costs include the actual local forage selling price. The total inputs were 1.32 and 1.37 million·hm−2 for M and ML, respectively; the total revenues were 1.92 and 2.09 million hm−2 for M and ML, respectively; and the net revenues averaged 0.60 and 0.73 million hm−2 for M and ML in both years, respectively. Compared with monocropping, due to the increase in intercropping, the overall cost of cultivation compared to monocropping increased due to the increased price inputs of lablab bean seed and the labor costs for manual weeding. However, based on local forage prices, the intercropping compensated for the high cost of planting inputs with increased yields, resulting in a balance where the net income of the intercropping was 21.67% higher than that of the monocropping, and the output/input ratio increased from 1.45 to 1.53.

Table 4.
Total income, net income and output/input ratios for different treatments.
4. Discussion
4.1. Effect of Maize–Lablab Intercropping on Yield and Nutritional Quality
This study highlights the potential of intercropping leguminous crops, such as lablab bean, with maize as a novel approach to improving the silage yield and nutritional quality. This is primarily due to the legume’s adaptability, high yield potential, and significant crude protein content [14]. Specifically, the ML system showed a 15.24% increase in FW yield, a 12.19% increase in DM, and a 5.43% increase in CP yield in 2022 compared to maize. In 2023, the CP yield saw a further increase of 12.26%. These results offer a fresh perspective on intercropping, showcasing how this system can enhance forage production without compromising the maize yield, a finding not always reflected in previous studies. For example, although Mthembu et al. [9] reported an increased forage yield and crude protein content with maize–lablab intercropping, they also observed a significant reduction in maize leaf yield. This discrepancy may be attributed to differences in the intercropping system or growing conditions. In our study, lablab bean did not impede maize growth, potentially due to its lower fiber content and the fact that it was in the flowering stage at the time of maize harvest.
Furthermore, while previous research has shown that intercropping maize with legumes can reduce the NDF and ADF content, thus improving feed digestibility [32,33], our findings indicate that there were no significant differences in the NDF and ADF content between the ML system and maize monoculture. This suggests that the benefits of intercropping may vary depending on specific environmental or management factors, highlighting the need for a further exploration of how these interactions affect feed quality. The slight decrease in the EE content observed in the ML system, although not significantly different from monoculture, suggests that the lipid profile of the forage could also be influenced by intercropping strategies, as indicated by Kintl et al. [34].
4.2. Effect of Mixed Maize/Lablab Silage and Lactobacillus Additives on the Nutritional and Fermentation Quality of Silage
Grasses are easy to silage but have an insufficient protein content, and legumes have a high nutritional value but are difficult to successfully silage alone [4]. The use of mixed silage combines the advantages of both, improving the silage success and nutritional quality of silage. In this study, the DM content of ML was significantly reduced by 7.12% compared to M silage, whereas the CP, NDF and ADF contents of ML were significantly increased by 15.66%, 19.33% and 27.66%, respectively. These findings highlight a novel approach to balancing silage fermentation and nutritional outcomes. The decline in DM may be due to the depletion of carbohydrates caused by the lactate metabolism [35]. This emphasizes the importance of understanding how intercropping and mixed-silage systems interact with fermentation dynamics to influence nutrient retention. In the present study, the effect of T3 on the ADF and NDF content of silage was not significant. However, in the other Lactobacillus treatment groups in this trial, ADF and NDF were higher than CK. A previous study concluded that both the ADF and NDF contents were increased in mixed silage compared to whole-plant maize single silage [12,36,37,38]. The results mentioned earlier suggested that different additives in the fermentation process had the ability to improve the fermentation quality, and the combined inoculation efficiently promoted the fermentation. Moreover, our study further supports the notion that the application of combined inoculants can improve the fermentation quality, with Lactobacillus plantarum (LP) promoting homo-fermentation and reducing nutrient losses by preventing the organic degradation associated with insufficient lactic acid production [39]. These results open new avenues for optimizing silage formulations and microbial inoculation strategies to achieve both a higher nutritional quality and better fermentation stability in mixed silages.
The increase in the crude protein content of mixed silage containing legumes has also been confirmed in studies by Ligoski et al. [40]. This demonstrates that the production of mixed silage with an increased protein content has significant benefits and relevance, as it can help reduce the cost of purchasing protein salts and/or concentrates to meet protein supply needs. Furthermore, exclusive maize silage has a high production cost [41], while maize silage mixed with legumes is a viable alternative that can reduce the cost of silage production. In this experiment, the CP mass content of mixed silage with whole maize and lablab bean increased significantly, in which the CP content of the compound bacterial agent ML-T2 treatment was the highest, followed by the ML-T3 treatment, indicating that the compound bacterial agent can improve the CP content of silage to a certain extent. The fermentation substrate of whole maize and soybean silage is sufficient, and the addition of Lactobacillus bacteria can promote the production of a large amount of LA at the early stage of silage fermentation, accelerating the acidification of the silage environment and thus preventing the multiplication of harmful microorganisms. In our experiments, the mixed silage comprising lablab beans and maize increased the CP concentration. Using a mixture of maize and lablab bean silage increased the apparent digestibility of nutrients and the milk production of dairy cows compared to silage from whole-plant maize [42].
Although the synergistic effect of microbial interaction may not be immediately apparent, the combination of LP and LB can lead to a more stable fermentation environment. LP, primarily a homofermentative bacterium, promotes lactic acid production. LA is the main fermentation product of silage, converting water-soluble carbohydrates into LA by LAB under anaerobic conditions. LA rapidly lowers pH and prevents the growth of undesirable microorganisms, whereas PA usually prevents the growth of fungi [12,36]. In contrast, LB, a heterofermentative bacterium, produces acetic acid, which enhances aerobic stability during storage. This interaction between LP and LB not only accelerates the fermentation process but also improves the overall silage quality by reducing spoilage losses and preserving more nutrients over time. The fermentation quality of silage usually reflects the silage nutrient storage: the better the fermentation, the more nutrients that are preserved [43]. In general, legume silage tends to have a higher pH because of the high buffering capacity of organic acids [44]. While pH directly reflects the total acid content produced during whole-plant maize silage, the lower the pH, the more acidic substances such as LA and the better the silage effect; the pH required for conventional successful silage should be lower than 4.2 [45]. This study showed that the pH (4.7) was much higher than our standard for high-quality maize silage, but the CP, DM and LA contents were higher and the fermentation quality was better overall after mixing whole maize with soybean in the ratio of 25:75 [46]. The observed acceleration in acid production and subsequent reduction in pH in silage containing a combination of two LAB species highlight the potential synergistic effects of microbial interactions on fermentation.
The NH3-N/TN ratio reflects the protein degradation caused by undesirable microorganisms [47]. In this experiment, all treatments had NH3-N/TN ratios below 10%, indicating a favorable fermentation quality. Notably, the maize–lablab bean mixture demonstrated a lower NH3-N/TN ratio compared to maize alone, suggesting reduced protein degradation attributable to legume inclusion. These findings corroborate previous study observations [48], emphasizing the impact of mixing ratios on protein hydrolysis and the NH3-N/TN levels. The difference in the ratio of NH3-N/TN may be due to the difference in the CP content of the feedstock, and the ammonia nitrogen content may be high in forages with a high CP concentration [30]. It may also be due to the fact that maize has a high concentration of soluble carbohydrates that can be utilized for fermentation [49]. BA is usually produced by Clostridium perfringens, which leads to poor fermentation and produces unpleasant odors [50,51]. Although BA levels were undetected in our experiment, it is crucial to acknowledge the potential role of Clostridium perfringens in silage deterioration. The absence of detectable BA may be attributed to the inhibitory effect of a low pH (<4.2) on BA-producing bacteria [52]. Several studies have demonstrated that LP and LB can synergistically enhance silage quality. They do this by accelerating Lactobacillus growth, metabolizing acids, inhibiting spoilage microorganisms, and speeding up fermentation, which reduces feed dry matter loss [53,54]. Yang et al. [55] found that adding LP to alfalfa silage increased the LA content, lowered pH, and improved the fermentation quality. LP supplementation increased the LA content and improved the fermentation quality, while LB enhanced the aerobic stability and mitigated nutrient oxidation during aerobic exposure [56,57]. These findings suggest that using LP and LB can significantly optimize the fermentation process and nutrient preservation in silage, enhancing overall quality. In this experiment, ML reduced the LA content, probably due to the lower number of soluble carbohydrates in lablab beans, which reduced the fermentation substrate in mixed-silage fermentation and allowed the multiplication of LAB to produce LA in smaller quantities.
The V-score results indicated that all treatments had a good fermentation quality, with ML-T2 being the best. Mixed maize–lablab bean silage increased the protein content without compromising the fermentation quality, potentially improving the quality of animal products such as milk and meat [58]. To maximize fermentation and the nutritional benefits, further research should focus on the mixed storage of maize and lablab beans with lactobacilli inoculation at the rates of T2 or T3.
4.3. Relationship between Nutritional Quality and Fermentation Quality
In silage research, a high pH is typically a sign of inadequate fermentation, often leading to a reduced silage quality [59]. In the current study, CP was degraded by undesirable microorganisms during silage to produce NH3-N and AA [60]. Similarly, in an analytical report on silage fermentation, it was stated that lower mean NH3-N values in silage were due to the fact that adequate lactic fermentation helped to reduce CP hydrolysis and inhibit undesirable microorganisms [61]. Gao et al. [62] found that a low pH in silage ensured better fermentation, which resulted in the preservation of more CP, which is in agreement with our results in the present study. Reducing silage losses and inhibiting secondary fermentation are important objectives in silage research; therefore, some researchers, such as Raza et al. [63], have suggested controlling the DM content to around 30% when the silage pH is even lower. Although the present experiment resulted in a higher dry matter content at harvest due to climatic and geographical reasons, it is still consistent with the results of this study that pH is negatively correlated with DM. Taken together, the above study shows that the silage DM content directly affects the quality of late silage.
5. Conclusions
This study aimed to evaluate the effects of maize–lablab intercropping and LAB inoculation on the forage yield, silage nutritional quality, and economic profitability. The findings confirm that intercropping maize with lablab significantly improve both the fresh grass yield and dry matter yield, with notable increases in the crude protein content, aligning with the study’s objective of improving forage quality. Additionally, LAB inoculation successfully enhanced the fermentation process, reducing the silage pH and increasing lactic acid production, further supporting improved silage quality. In terms of economic benefits, maize–lablab intercropping paired with LAB inoculation resulted in higher economic returns, demonstrating its potential as a sustainable strategy for improving both the silage yield and profitability of livestock systems. We recommend the adoption of maize–lablab intercropping and LAB inoculation in silage production for farms aiming to enhance forage quality and economic efficiency. Further research should explore varying intercropping ratios and microbial inoculation combinations to optimize silage production across different environmental conditions.
Author Contributions
Conceptualization, H.W.; methodology, H.R.; formal analysis, H.R. and D.L.; investigation, H.R, L.Z. and Y.H.; data curation, D.L and Y.H.; writing—original draft preparation, D.L. and H.R.; writing—review and editing, D.L., H.R. and H.W.; visualization, D.L. and H.R.; supervision, H.W.; funding acquisition, H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key R&D program of China funded by the Ministry of Science and Technology of the People’s Republic of China (MOST, grant no. 2022YFD1900300).
Data Availability Statement
The data presented in this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ni, K.; Zhao, J.; Zhu, B.; Su, R.; Pan, Y.; Ma, J.; Zhou, G.; Tao, Y.; Liu, X.; Zhong, J. Assessing the Fermentation Quality and Microbial Community of the Mixed Silage of Forage Soybean with Crop Corn or Sorghum. Bioresour. Technol. 2018, 265, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Luo, Y.; Zhang, Y.; Wang, H.; Shen, Y.; Liu, Y.; Shang, S. Comparison on Environmental Impacts of Cereal and Forage Production in the Loess Plateau of China: Using Life Cycle Assessment with Uncertainty and Variability Analysis. J. Clean. Prod. 2022, 380, 135094. [Google Scholar] [CrossRef]
- Guan, H.; Shuai, Y.; Yan, Y.; Cai, Y.; Zhang, X. Microbial Community and Fermentation Dynamics of Corn Silage Prepared with Heat-Resistant Lactic Acid Bacteria in Hot Environment. J. Dairy Sci. 2020, 103, 228. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, D.; Ge, Q.; Yang, B.; Li, S. Effects of Harvest Period and Mixed Ratio on the Characteristic and Quality of Mixed Silage of Alfalfa and Maize. Anim. Feed. Sci. Technol. 2023, 306, 115796. [Google Scholar] [CrossRef]
- Paludo, F.; de Pinho Costa, K.A.; de Castro Dias, M.B.; Santos e Silva, F.A.; Gomes Silva, A.C.; Rodrigues, L.G.; Almeida Silva, S.A.; Souza, W.F.; Bilego, U.O.; Muniz, M.P. Fermentative Profile and Nutritive Value of Corn Silage with Tamani Guinea Grass. Semin. Cienc. Agrar. 2020, 41, 2733–2746. [Google Scholar] [CrossRef]
- Xin, Y.; Chen, C.; Zhong, Y.; Bu, X.; Huang, S.; Tahir, M.; Du, Z.; Liu, W.; Yang, W.; Li, J.; et al. Effect of Storage Time on the Silage Quality and Microbial Community of Mixed Maize and Faba Bean in the Qinghai-Tibet Plateau. Front. Microbiol. 2023, 13, 1090401. [Google Scholar] [CrossRef]
- Strom, N.; Hu, W.; Haarith, D.; Chen, S.; Bushley, K. Interactions between Soil Properties, Fungal Communities, the Soybean Cyst Nematode, and Crop Yield under Continuous Corn and Soybean Monoculture. Appl. Soil Ecol. 2020, 147, 103388. [Google Scholar] [CrossRef]
- Raza, M.A.; Zhiqi, W.; Yasin, H.S.; Gul, H.; Qin, R.; Rehman, S.U.; Mahmood, A.; Iqbal, Z.; Ahmed, Z.; Luo, S.; et al. Effect of Crop Combination on Yield Performance, Nutrient Uptake, and Land Use Advantage of Cereal/Legume Intercropping Systems. Field Crops Res. 2023, 304, 109144. [Google Scholar] [CrossRef]
- Mthembu, B.E.; Everson, T.M.; Everson, C.S. Intercropping Maize (Zea mays L.) with Lablab (Lablab purpureus L.) for Sustainable Fodder Production and Quality in Smallholder Rural Farming Systems in South Africa. Agroecol. Sustain. Food Syst. 2018, 42, 362–382. [Google Scholar] [CrossRef]
- Mourtzinis, S.; Marburger, D.; Gaska, J.; Diallo, T.; Lauer, J.; Conley, S. Corn and Soybean Yield Response to Tillage, Rotation, and Nematicide Seed Treatment. Crop Sci. 2017, 57, 1704–1712. [Google Scholar] [CrossRef]
- Luo, M.; Jiang, Y. First Report of Anthracnose Caused by Colletotrichum Karsti in Lentil (Lablab purpureus). Crop Prot. 2022, 155, 105903. [Google Scholar] [CrossRef]
- Umesh, M.R.; Angadi, S.; Begna, S.; Gowda, P. Planting Density and Geometry Effect on Canopy Development, Forage Yield and Nutritive Value of Sorghum and Annual Legumes Intercropping. Sustainability 2022, 14, 4517. [Google Scholar] [CrossRef]
- Gott, J.; Massawe, P.; Miller, N.R.; Goerndt, M.; Streubel, J.; Burton, M.G. Little or No Maize (Zea mays) Grain Yield Loss Occurred in Intercrop with Mid-Maturity Lablab (Lablab purpureus) in Northeastern Tanzania. Crop Sci. 2024, 64, 413–421. [Google Scholar] [CrossRef]
- da Silva, L.M.; de Pinho Costa, K.A.; Goncalves, E.; Silva, J.A.; Campos Pinho Costa, J.V.; Costa, A.C.; da Costa Severiano, E.; Fernandes, P.B.; Oliveira, K.J.; Magalhaes Mendonca, K.T.; et al. Fermentative Profile and Nutritive Value of Maize, Legume and Mixed Silage. Semin. Cienc. Agrar. 2023, 44, 1909–1926. [Google Scholar] [CrossRef]
- Lai, X.; Wang, H.; Yan, J.; Zhang, Y.; Yan, L. Exploring the Differences between Sole Silages of Gramineous Forages and Mixed Silages with Forage Legumes Using 16S/ITS Full-Length Sequencing. Front. Microbiol. 2023, 14, 1120027. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Jiang, Y.; Wang, L.; Wang, S.; Zhang, Z.; Tong, X.; Wang, S. Effects of Different Soybean and Maize Mixed Proportions in a Strip Intercropping System on Silage Fermentation Quality. Fermentation 2022, 8, 696. [Google Scholar] [CrossRef]
- Okoye, C.O.; Wang, Y.; Gao, L.; Wu, Y.; Li, X.; Sun, J.; Jiang, J. The Performance of Lactic Acid Bacteria in Silage Production: A Review of Modern Biotechnology for Silage Improvement. Microbiol. Res. 2023, 266, 127212. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.D.; Choi, K.C.; Kim, D.; Lee, K.D.; Choi, K.C. Role of LAB in Silage Fermentation: Effect on Nutritional Quality and Organic Acid Production—An Overview. Agric. Mark. Inf. Syst. 2021, 6, 216–234. [Google Scholar] [CrossRef]
- Avila, C.L.S.; Carvalho, B.F. Silage Fermentation-Updates Focusing on the Performance of Micro-Organisms. J. Appl. Microbiol. 2020, 128, 966–984. [Google Scholar] [CrossRef]
- Dong, J.; Li, S.; Chen, X.; Sun, Z.; Sun, Y.; Zhen, Y.; Qin, G.; Wang, T.; Demelash, N.; Zhang, X. Effects of Lactiplantibacillus Plantarum Inoculation on the Quality and Bacterial Community of Whole-Crop Corn Silage at Different Harvest Stages. Chem. Biol. Technol. Agric. 2022, 9, 57. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Chen, S.; Shao, T.; Tao, X.; Yuan, X. Effect of Lactic Acid Bacteria on the Fermentation Quality and Mycotoxins Concentrations of Corn Silage Infested with Mycotoxigenic Fungi. Toxins 2021, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xu, D.; Li, F.; Bai, J.; Su, R. Current Approaches on the Roles of Lactic Acid Bacteria in Crop Silage. Microb. Biotechnol. 2023, 16, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Kumari, N.; Mani, V.; Pradhan, D.; Gowane, G.R.; Kumar, S.; Tyagi, N. Effects of Lactiplantibacillus plantarum, Limosilactobacillus fermentum, and Propionic Acid on the Fermentation Process of Sugarcane Tops Silages Along with Variations in pH, Yeast and Mould Count After Aerobic Exposure. Waste Biomass Valorization 2023, 15, 2215–2230. [Google Scholar] [CrossRef]
- Qian, X.; Zang, H.; Xu, H.; Hu, Y.; Ren, C.; Guo, L.; Wang, C.; Zeng, Z. Relay Strip Intercropping of Oat with Maize, Sunflower and Mung Bean in Semi-Arid Regions of Northeast China: Yield Advantages and Economic Benefits. Field Crops Res. 2018, 223, 33–40. [Google Scholar] [CrossRef]
- Edson, C.; Takarwirwa, N.N.; Kuziwa, N.L.; Stella, N.; Maasdorp, B. Effect of Mixed Maize-Legume Silages on Milk Quality and Quantity from Lactating Smallholder Dairy Cows. Trop. Anim. Health Prod. 2018, 50, 1255–1260. [Google Scholar] [CrossRef]
- Holzer, M.; Mayrhuber, E.; Danner, H.; Braun, R. The Role of Lactobacillus Buchneri in Forage Preservation. Trends Biotechnol. 2003, 21, 282–287. [Google Scholar] [CrossRef]
- Vansoest, P.; Robertson, J.; Lewis, B. Methods for Dietary Fiber, Neutral Detergent Fiber, And Nonstarch Polysaccharides In Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Tucak, M.; Ravlic, M.; Horvat, D.; Cupic, T. Improvement of Forage Nutritive Quality of Alfalfa and Red Clover through Plant Breeding. Agronomy 2021, 11, 2176. [Google Scholar] [CrossRef]
- Arif, M.; Kumar, A.; Pourouchottamane, R.; Gupta, D.L.; Singh, M.K.; Rai, B. Effect of Intercropping Row Ratios on Yield and Nutritive Value of Maize and Cowpea Fodder. Range Manag. Agrofor. 2022, 43, 292–298. [Google Scholar]
- Yan, Y.; Li, X.; Guan, H.; Huang, L.; Ma, X.; Peng, Y.; Li, Z.; Nie, G.; Zhou, J.; Yang, W.; et al. Microbial Community and Fermentation Characteristic of Italian Ryegrass Silage Prepared with Corn Stover and Lactic Acid Bacteria. Bioresour. Technol. 2019, 279, 166–173. [Google Scholar] [CrossRef]
- Zeng, T.; Li, X.; Guan, H.; Yang, W.; Liu, W.; Liu, J.; Du, Z.; Li, X.; Xiao, Q.; Wang, X.; et al. Dynamic Microbial Diversity and Fermentation Quality of the Mixed Silage of Corn and Soybean Grown in Strip Intercropping System. Bioresour. Technol. 2020, 313, 123655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shi, W.; Ali, S.; Chang, S.; Jia, Q.; Hou, F. Legume/Maize Intercropping and N Application for Improved Yield, Quality, Water and N Utilization for Forage Production. Agronomy 2022, 12, 1777. [Google Scholar] [CrossRef]
- Zaeem, M.; Nadeem, M.; Pham, T.H.; Ashiq, W.; Ali, W.; Gillani, S.S.M.; Moise, E.; Elavarthi, S.; Kavanagh, V.; Cheema, M.; et al. Corn-Soybean Intercropping Improved the Nutritional Quality of Forage Cultivated on Podzols in Boreal Climate. Plants 2021, 10, 1015. [Google Scholar] [CrossRef]
- Kintl, A.; Smeringai, J.; Sobotkova, J.; Hunady, I.; Brtnicky, M.; Hammerschmiedt, T.; Elbl, J. Mixed Cropping System of Maize and Bean as a Local Source of N-Substances for the Nutrition of Farm Animals. Eur. J. Agron. 2024, 154, 127059. [Google Scholar] [CrossRef]
- Winters, A.; Fychan, R.; Jones, R. Effect of Formic Acid and a Bacterial Inoculant on the Amino Acid Composition of Grass Silage and on Animal Performance. Grass Forage Sci. 2001, 56, 181–192. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, X.-j.; Li, J.-f.; Wang, S.-r.; Dong, Z.-h.; Shao, T. Effect of Lactic Acid Bacteria and Propionic Acid on Conservation Characteristics, Aerobic Stability and in Vitro Gas Production Kinetics and Digestibility of Whole-Crop Corn Based Total Mixed Ration Silage. J. Integr. Agric. 2017, 16, 1592–1600. [Google Scholar] [CrossRef]
- Contreras-Govea, F.; Marsalis, M.; Angadi, S.; Smith, G.; Lauriault, L.M.; VanLeeuwen, D. Fermentability and Nutritive Value of Corn and Forage Sorghum Silage When in Mixture with Lablab Bean. Crop Sci. 2011, 51, 1307–1313. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, R.; Wang, C.; Dong, W.; Zhang, Z.; Zhao, L.; Zhang, X. Effects of Cellulase and Lactobacillus Plantarum on Fermentation Quality, Chemical Composition, and Microbial Community of Mixed Silage of Whole-Plant Corn and Peanut Vines. Appl. Biochem. Biotechnol. 2022, 194, 2465–2480. [Google Scholar] [CrossRef]
- Sifeeldein, A.; Yuan, X.; Dong, Z.; Li, J.; Shao, T. Effect of Applying Lactobacillus Plantarum and Pediococcus Acidilactici Isolated on Fermentation Dynamics, Microbial Community and Aerobic Stability of Napier Grass (Pennisetum Purpureum) Silage. Kafkas Univ. Vet. Fak. Derg. 2018, 24, 371–378. [Google Scholar]
- Ligoski, B.; Gonçalves, L.F.; Claudio, F.L.; Alves, E.M.; Krüger, A.M.; Bizzuti, B.E.; Lima, P.d.M.T.; Abdalla, A.L.; Paim, T.d.P. Silage of Intercropping Corn, Palisade Grass, and Pigeon Pea Increases Protein Content and Reduces In Vitro Methane Production. Agronomy 2020, 10, 1784. [Google Scholar] [CrossRef]
- Mekuriaw, S.; Tsunekawa, A.; Ichinohe, T.; Tegegne, F.; Haregeweyn, N.; Kobayashi, N.; Tassew, A.; Mekuriaw, Y.; Walie, M.; Tsubo, M.; et al. Effect of Feeding Improved Grass Hays and Eragrostis Tef Straw Silage on Milk Yield, Nitrogen Utilization, and Methane Emission of Lactating Fogera Dairy Cows in Ethiopia. Animals 2020, 10, 1021. [Google Scholar] [CrossRef] [PubMed]
- Chirinda, N.; Murungweni, C.; Waniwa, A.; Nyamangara, J.; Tangi, A.; Peters, M.; Notenbaert, A.; Burkart, S. Perspectives on Reducing the National Milk Deficit and Accelerating the Transition to a Sustainable Dairy Value Chain in Zimbabwe. Front. Sustain. Food Syst. 2021, 5, 726482. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage Review: Interpretation of Chemical, Microbial, and Organoleptic Components of Silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Drouin, P.; Tremblay, J.; da Silva, E.B.; Apper, E. Changes to the Microbiome of Alfalfa during the Growing Season and after Ensiling with Lentilactobacillus buchneri and Lentilactobacillus hilgardii Inoculant. J. Appl. Microbiol. 2022, 133, 2331–2347. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage Review: Factors Affecting Dry Matter and Quality Losses in Silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, G.Q.; Wei, S.N.; Kim, H.J.; Li, Y.F.; Kim, J.G. Changes in Fermentation Pattern and Quality of Italian Ryegrass (Lolium Multiflorum Lam.) Silage by Wilting and Inoculant Treatments. Anim. Biosci. 2020, 34, 48–55. [Google Scholar] [CrossRef]
- Denek, N.; Aydin, S.S.; Can, A. The Effects of Dried Pistachio (Pistachio Vera L.) by-Product Addition on Corn Silage Fermentation and in Vitro Methane Production. J. Appl. Anim. Res. 2017, 45, 185–189. [Google Scholar] [CrossRef]
- Zhu, Y.; Bai, C.S.; Guo, X.S.; Xue, Y.L.; Ataku, K. Nutritive Value of Corn Silage in Mixture with Vine Peas. Anim. Prod. Sci. 2011, 51, 1117–1122. [Google Scholar] [CrossRef]
- de Souza, W.F.; de Pinho Costa, K.A.; Guarnieri, A.; da Costa Severiano, E.; da Silva, J.T.; Alves Teixeira, D.A.; Oliveira, S.S.; de Castro Dias, M.B. Production and Quality of the Silage of Corn Intercropped with Paiaguas Palisadegrass in Different Forage Systems and Maturity Stages. Rev. Bras. Zootec. Braz. J. Anim. Sci. 2019, 48, e20180222. [Google Scholar] [CrossRef]
- Melaku, M.; Zhong, R.; Han, H.; Wan, F.; Yi, B.; Zhang, H. Butyric and Citric Acids and Their Salts in Poultry Nutrition: Effects on Gut Health and Intestinal Microbiota. Int. J. Mol. Sci. 2021, 22, 10392. [Google Scholar] [CrossRef]
- Muck, R.E. Recent Advances in Silage Microbiology. Agric. Food Sci. 2013, 22, 3–15. [Google Scholar] [CrossRef]
- Chiou, P.; Chang, S.; Yu, B. The Effects of Wet Sorghum Distillers’ Grains Inclusion on Napiergrass Silage Quality. J. Sci. Food Agric. 2000, 80, 1199–1205. [Google Scholar] [CrossRef]
- Filya, I. The Effect of Lactobacillus buchneri and Lactobacillus plantarum on the Fermentation, Aerobic Stability, and Ruminal Degradability of Low Dry Matter Corn and Sorghum Silages. J. Dairy Sci. 2003, 86, 3575–3581. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, J.; Zhang, Q.; Jiang, Y.; Wu, Z.; Yu, Z. Effects of Mixing Red Clover with Alfalfa at Different Ratios on Dynamics of Proteolysis and Protease Activities during Ensiling. J. Dairy Sci. 2018, 101, 8954–8964. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Y.; Zhao, S.; Wang, Y. Lactobacillus plantarum Inoculants Delay Spoilage of High Moisture Alfalfa Silages by Regulating Bacterial Community Composition. Front. Microbiol. 2020, 11, 1989. [Google Scholar] [CrossRef]
- Li, T. Planting Structure Adjustment and Layout Optimization of Feed Grain and Food Grain in China Based on Productive Potentials. Land 2023, 12, 45. [Google Scholar] [CrossRef]
- Xu, D.; Ding, W.; Ke, W.; Li, F.; Zhang, P.; Guo, X. Modulation of Metabolome and Bacterial Community in Whole Crop Corn Silage by Inoculating Homofermentative Lactobacillus plantarum and Heterofermentative Lactobacillus buchneri. Front. Microbiol. 2019, 9, 3299. [Google Scholar] [CrossRef]
- Ghizzi, L.G.; Del Valle, T.A.; Zilio, E.M.; Sakamoto, L.Y.; Marques, J.A.; Dias, M.S.; Nunes, A.T.; Gheller, L.S.; de P. Silva, T.B.; Grigoletto, N.T.S.; et al. Partial Replacement of Corn Silage with Soybean Silage on Nutrient Digestibility, Ruminal Fermentation, and Milk Fatty Acid Profile of Dairy Cows. Anim. Feed. Sci. Technol. 2020, 266, 114526. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Wang, C.; He, L.; Zhou, W.; Yang, F.; Zhang, Q. The Bacterial Community and Fermentation Quality of Mulberry (Morus Alba) Leaf Silage with or without Lactobacillus casei and Sucrose. Bioresour. Technol. 2019, 293, 122059. [Google Scholar] [CrossRef]
- Alvarado-Ramírez, E.R.; Maggiolino, A.; Elghandour, M.M.M.Y.; Rivas-Jacobo, M.A.; Ballesteros-Rodea, G.; Palo, P.D.; Salem, A.Z.M. Impact of Co-Ensiling of Maize with Moringa Oleifera on the Production of Greenhouse Gases and the Characteristics of Fermentation in Ruminants. Animals 2023, 13, 764. [Google Scholar] [CrossRef]
- Liu, X.; Rahman, T.; Song, C.; Su, B.; Yang, F.; Yong, T.; Wu, Y.; Zhang, C.; Yang, W. Changes in Light Environment, Morphology, Growth and Yield of Soybean in Maize-Soybean Intercropping Systems. Field Crops Res. 2017, 200, 38–46. [Google Scholar] [CrossRef]
- Gao, R.; Wang, B.; Jia, T.; Luo, Y.; Yu, Z. Effects of Different Carbohydrate Sources on Alfalfa Silage Quality at Different Ensiling Days. Agriculture 2021, 11, 58. [Google Scholar] [CrossRef]
- Raza, M.A.; Gul, H.; Wang, J.; Yasin, H.S.; Qin, R.; Bin Khalid, M.H.; Naeem, M.; Feng, L.Y.; Iqbal, N.; Gitari, H.; et al. Land Productivity and Water Use Efficiency of Maize-Soybean Strip Intercropping Systems in Semi-Arid Areas: A Case Study in Punjab Province, Pakistan. J. Clean. Prod. 2021, 308, 127282. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).