Abstract
Understanding drug release in the colon is fundamental to developing efficient treatments for colon-related diseases, while unraveling the relationship between the colonic microbiota and excipients is crucial to unveiling the effect of biomaterials on the release of drugs. In this contribution, the bio-release of ibuprofen (encapsulated in acetylated and palmitoylated agave fructans) was evaluated by fermentation with lactic acid bacteria in simulated physicochemical (pH and temperature) colon conditions. It was observed that the size of the acyl chain (1 in acetyl and 15 in palmitoyl) was critical both in the growth of the microorganisms and in the release of the drug. For example, both the bacterial growth and the release of ibuprofen were more favored with acetylated fructan microspheres. Among the microorganisms evaluated, Bifidobacterium adolescentis and Lactobacillus brevis showed great potential as probiotics useful to release drugs from modified fructans. The production of short-chain fatty acids (lactic, acetic, and propionic acids) in the course of fermentations was also determined, since such molecules have a positive effect both on colon-related diseases and on the regulation of the intestinal microbiota. It was found that a higher concentration of acetate is related to a lower growth of bacteria and less release of ibuprofen.
1. Introduction
Localized drug delivery to the colon is of great interest due to the need for efficient treatments for a variety of colon-related diseases, such as irritable bowel syndrome, ulcerous colitis, Crohn’s disease, and colorectal cancer []. Even though the intrinsic characteristics of the human colon have allowed the administration of drugs based on mechanisms that depend on pH and time [,], these parameters vary considerably among individuals and can also be affected by the same diseases that need to be treated []. Nowadays, the release of drugs targeted to the colon has been directed toward commercialized systems mediated by receptors, magnetically triggered, and activated by enzymes from the colonic microbiota []. However, challenges still remain for release systems in the colon, such as more stability in the physicochemical conditions of the upper gastrointestinal (GI) tract; acceptance of an ample gamut of drugs; and lowering the doses to mitigate systemic secondary effects [].
The use of carbohydrate polymers for sustained drug release has several advantages, including natural abundance, low immunogenicity, non-toxicity, and general biocompatibility [,]. However, their high solubility in aqueous systems causes early and undesirable drug release in the upper GI tract. In order to provide stability throughout the upper GI tract, chemical modifications are often carried out in carbohydrates to give them an amphiphilic character [].
Fructans from tequila agave (A. tequilana Weber var. azul) are idoneous oligosaccharides as colonic excipients because they are resistant to hydrolysis from human digestive enzymes and are not absorbed during the transit through the small intestine. These particularities are owed to the structural β 2–1 (inulin) and branched β 2–6 (levan) linkages []. Fructans are considered as prebiotics; i.e., they stimulate the growth of a limited number of microorganisms such as lactic acid bacteria that in turn act on the metabolism of lipids, producing short-chain fatty acids like acetic, propionic, butyric, and lactic acids. These compounds promote positive effects on human health by modifying cellular pH and controlling inflammation, absorption of minerals, proliferation of mucosa, and elimination of harmful nitrogenated compounds [,,,,]. Moreover, the use of agave fructans has been associated with positive effects on decreasing cardiovascular and gastrointestinal health risks, as well as the treatment of metabolic diseases through the restoration of dysbiosis seen in overweight patients [,,,].
The gastrointestinal tract hosts around 2000 species of bacteria, the majority residing in the colon, which have as their main source of nutrition the fermentation of carbohydrates []. Bifidobacterium and Lactobacillus, naturally found in the colon, have been used in symbiotic therapies along with linear fructan (inulin). It was found that these therapies exerted protective effects against tumor development in the colon, improved the integrity and function of the epithelial barrier, and reduced the risk of colorectal cancer []. Moreover, several of these strains have shown anti-inflammatory activity by modulating the production of cytokines in human dendritic cells, which may reduce the risk of producing cancer []. Weisella, a genus of lactic acid bacteria, has been studied as a probiotic for treatment of colonic diseases []. It has been found that the proper administration of probiotics has a strong positive effect on the recovery of equilibria of the intestinal microbiota, which in turn has a positive action on the immune modulation of the GI and on the inflammation of the intestinal mucosa [].
The purpose of this research was to evaluate the release of ibuprofen, as a model drug, in physicochemical colonic conditions by the fermentation of esterified agave fructans with lactic acid bacteria. Ibuprofen was encapsulated in acetylated or palmitoylated agave fructan microspheres and the effect of the modification of agave fructan was evaluated based on their ability to act as prebiotics (as seen in the growth of the strains) as well as the potential of these microorganisms as probiotic agents during the kinetics of the drug release.
2. Materials and Methods
2.1. Materials
Fructans were extracted from mature agave plants (A. tequilana Weber var. azul, 8 years of age) according to a method described elsewhere []. Briefly, the agave plants were milled, and the juice collected was subjected to protein flocculation, filtration, and clarification with activated carbon. Then, the fructan solution was ultra-filtered with cellulose membranes (Millipore, Burlington, MA, USA, Amicon 8400, MWCO 3 KDa) by applying pressure (5.3 kg/cm2) with nitrogen.
Reagents for the chemical modification of fructans were analytical grade and commercially purchased: acetic anhydride (Golden Bell, Guadalajara, Mexico), sodium acetate (Fermont, Ciudad de Mexico, Mexico), and N, N-dimethylformamide (Fermont, QC, Canada); ibuprofen, palmitoyl chloride, trimethylphosphite, Tween 80, N-methyl-2-pyrrolydinona (NMP), and sodium hydride were also an analytical grade and purchased from Aldrich (Toluca, Mexico). The fermentation of the fructan esters was carried out with the strain Bifidobacterium adolescentis ATCC 15703 from the American Type Culture Collection, Rockville, Md (ATCC), and Weisella paramesenteroides, Enterococcus mundtii, and Lactobacillus brevis; the latter three lactic acid strains were isolated from a tomato carposphere in the laboratory of molecular biology at the University Center of Exact Sciences and Engineering from the University of Guadalajara.
2.2. Methods
2.2.1. Identification of Lactic Acid Bacteria Strains
The taxonomic classification was carried out by using genomic DNA as a template to amplify the 16S ribosomal RNA gene using degenerated primers 16SFw: 5′-AGAGTTTGATCMTGGCTCAG-3′ and 16SRw: 5′-TACGGYTACCCTTGTTACGACTT-3′. Amplified PCR products (1500 bp) were sent for sequencing to MACROGEN Korea. Sequences (an amplicon of 1400 bp) were edited before the alignment and compared with deposited sequences found in the GenBank database (http://www.ncbi.nlm.nih.gov/BLAST, accessed on 10 September 2024) available at the National Center for Biotechnology Information website. The obtained sequences were compared with related sequences in the EzBioCloud database (https://www.ezbiocloud.net/, accessed on 10 September 2024) using the BLAST program. The phylogenetic analysis based on a neighbor-joining method [] was carried out using the MEGA version 5.2 program []. The resultant tree topology was evaluated by a bootstrap analysis [] of the neighbor-joining data based on 10,000 re-sampled datasets.
2.2.2. Esterification and NMR Characterization of Agave Fructans
Native agave fructan and agave fructan esters were characterized by 1H Nuclear Magnetic Resonance (NMR, Jeol SA600 600 MHz, Tokyo, Japan). In order to run NMR experiments, 20 mg of native agave fructans or 20 mg of a fructan derivative was dissolved in 600 µL of the appropriate deuterated solvent: deuterium oxide for unmodified samples, CDCl3 for palmitoylated fructans, and d6DMSO for acetylated fructans. Trimethyl silyl propionic acid sodium salt was added at 0.01% to the solvents as a reference. The samples were subjected to 32 scans, 64 K data, a relaxation time of 1 s, and a spectral width of 15 ppm.
The esterification of agave fructan was performed either with palmitoyl phosphonate or with acetic anhydride. The synthesis of palmitoyl phosphonate was carried out as follows: Trimethyl phosphite (86.77 mmol) was added dropwise to palmitoyl chloride (84.75 mmol) and was left to react at room temperature for 3 h []. Then, palmitoyl phosphonate was used to esterify agave fructans by dissolving 0.1 g of fructan (1.85 mmol available OH groups) in 0.4 mL NMP under a nitrogen blanket at 50 °C. Temperature was then raised to 60 °C and NaH was added (125 µmol) and the system was left under stirring until complete dissolution. Once NaH dissolved, the temperature was raised to 85 °C and palmitoyl phosphonate (84.75 mmol) was added under stirring and we left the reaction to proceed for 24 h. The esterified fructans were precipitated with acetone and centrifuged at 3000 rpm for 15 min. The precipitate was washed with dichloromethane and centrifuged as before, recovered, and dried under vacuum at 40 °C overnight. Excess NMP was removed by washing the fructan esters with DI water. Fructans were then precipitated with dioxane, and the precipitate was washed with acetone and finally vacuum oven-dried at 40 °C overnight [].
On the other hand, the acetylation of agave fructans was carried out by dissolving 1 g agave fructan (18.5 mmol available OH groups) in 7 mL dimethyl formamide (DMF) at 40 °C and then, acetic anhydride (111 mmol) was added dropwise. Sodium acetate was added as a catalyst and the reaction proceeded for 24 h. The product was precipitated in water and also profusely washed with water, centrifuged, and vacuum oven-dried at 40 °C overnight [,].
2.2.3. Microencapsulation of Ibuprofen
Microspheres were prepared with both acetylated and palmitoylated agave fructans as follows: palmitoyl agave fructans (75 mg) and ibuprofen (10 mg) were dissolved in methylene chloride (1 mL); the solution was added dropwise in an ethanol–acetone (50:50 v:v) solution that contained Tween 80 at 0.5% wt./vol. and kept under stirring during 2 h. The resulting suspension was concentrated in a rotaevaporator at 40 °C and microspheres were recovered by centrifugation.
Acetylated agave fructans (75 mg), on the other hand, were dissolved in 1 mL of acetone along with 10 mg of ibuprofen. This solution was poured in an aqueous solution of Tween 80 at 0.5% wt./vol. and kept under stirring for 2 h. The resulting suspension was concentrated in a rotaevaporator at 40 °C and microspheres were recovered by centrifugation [,].
The microspheres were characterized by Scanning Electron Microscopy (SEM, Jeol JSM-6400F).
2.2.4. Growth of Lactic Acid Bacteria on Esterified Fructan Microspheres and Release of Ibuprofen
Esterified agave fructan microspheres were fermented with the selected microorganisms in an enriched medium that contained as the only source of carbon the microspheres. The culture medium was supplemented as follows: casein peptone (5 g/L), ascorbic acid (10 g/L), sodium acetate (10 g/L), (NH4)2SO4 (5 g/L), urea (2 g/L), MgSO4 7H2O (0.2 g/L), MnSO4 3H2O (0.07 g/L), FeSO4 7H2O (0.01 g/L), NaCl (0.01 g/L), Tween 80 (1 g/L), hemina (0.05 g/L), Na2S (0.5 g/L), and microspheres as the source of carbon (5 g/L). Inocula were obtained from each strain by anaerobic cultivation in a solid medium (MRS) at 37 °C for 48 h. Then, the obtained biomass was resuspended in 5 mL of liquid media for each microorganism and stored in a sterile flask until use. Then, the microspheres underwent a pretreatment in which gastric conditions were simulated (pH 1.6 using HCl) at 37 °C for 2 h. Thereafter, the microspheres along with the simulated gastric juice were transferred to a vial and the volume was adjusted to 45 mL with the culture medium and then the volume was adjusted to 50 mL with the inoculum and the pH was adjusted to 6.8; 1 mL samples were periodically taken from time zero up to 48 h.
Bacterial growth was measured by optical density at 620 nm in a UV-vis spectrophotometer (BIO-RAD MacTM Microplate reader, Hercules, CA, USA). Ibuprofen release during fermentation was quantified by injecting 20 µL samples in an HPLC (Alliance Waters e2695, Waters Corporation, Milford, MA, USA) equipped with a PDA 2998 detector and a column (Intersil, Milpitas, CA, USA, 5 microns, ODS2, 150 × 040 Metachem). The mobile phase was acetonitrile (60%) and acidified water (40%) at 1 mL/min flow and 35 °C. On the other hand, short-chain fatty acids were quantified at 48 h of fermentation by injecting 20 μL samples in the HPLC using an Aminex HPX-87H column (300 mm × 7.8 mm, Biorad), with the oven set to 50 °C and using H2SO4 at 5 mM as the mobile phase at 0.6 mL/min. Calibration curves from 0.1 to 5.0 g/L for each acid were prepared for lactic, acetic, and propionic acids.
3. Results
3.1. Identification of Lactic Acid Bacteria Strains
Weissella paramesenteroides (strain 5, previously named as strain 1(J-1)-1, Jal1), Enterococcus mundtii (strain 56, previously identified as E. lactis strain BT5inv), and Lactobacillus brevis (strain 57, previously named as strain Col-1-10; Col18) isolated from a tomato carposphere have shown the ability to antagonize, by co-aggregation and inhibition, the foodborne pathogen Salmonella serotypes Typhimurium, Saintpaul, and Montevideo and E. coli O157:H7, which codify for Shiga-like toxins I and II []. The taxonomic classification resulted in the phylogenetic tree shown in Scheme 1. The submission IDs (Genebank) PQ268163, PQ268212, and PQ268234 were released on 4 September 2024.
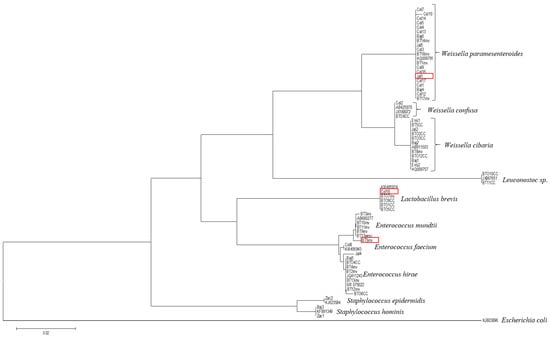
Scheme 1.
Phylogenetic tree for identification of Weissella paramesenteroides (Jal1), Enterococcus mundtii (BT5inv), and Lactobacillus brevis (Col18) isolated from carposphere of tomato. The red boxes indicate the identified strains in the phylogenetic tree.
3.2. NMR Characterization
A distinctive structural characteristic of fructans is the anomeric glycosidic proton belonging to the terminal glucose, which appears at 5.3 ppm in 1HNMR, isolated from other overlapping resonance signals []. This feature can be used to calculate the degree of polymerization by an end-group analysis. Figure 1A depicts a model of agave fructan []; at the top of the fructan model the glucose end group can be seen. Figure 1B(a) shows the 1H NMR spectrum of unmodified fructan in deuterium oxide. The bands located in the range 3.3–4.3 ppm have been assigned to non-exchangeable protons in glucose and fructose, while the signal at 5.3 is the anomeric proton in glucose. The integration of these signals resulted in a degree of polymerization (DP) of 23 or 3744 Da for the number average molecular weight.
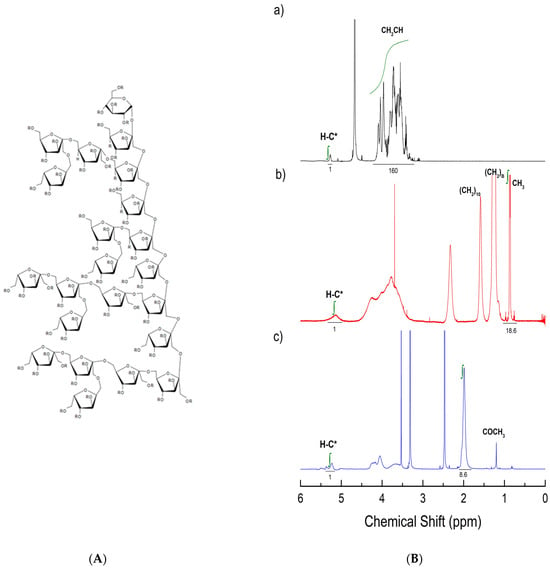
Figure 1.
(A) A model of agave fructan according to []; in native fructan, R is OH; in palmitoylated fructan, 4 Rs are substituted with palmitoyl moieties; for acetylated fructan, about 62 Rs should be acetyl groups. (B) 1H NMR spectra: (a) native fructan analyzed in D2O; H-C* denotes the proton at the anomeric carbon in glucose (b) palmitoylated fructan obtained in CDCl3; (c) acetylated fructans analyzed in d6DMSO.
The NMR spectra shown in Figure 1B(b,c) depict the results of the esterification with palmitoyl phosphonate (Figure 1B(b)) and acetic anhydride (Figure 1B(c)). Besides the signals for unmodified agave fructan, distinct signals at 0.86, 1.2, and 1.9 ppm in palmitoylated agave fructan (Figure 1B(b)), which belong to methyl, β-methylene, and methylene groups, respectively, allowed us to determine a degree of substitution (DS) of 0.282 (i.e., four palmitoyl chains per agave fructan molecule with a DP of 23). In the same manner, it was possible to determine the degree of acetylation of the agave fructan by integrating the signal at 1.2 ppm that belongs to methyl groups (Figure 1B(c)), which resulted in a DS of 2.8 (i.e., 2.8 hydroxyl groups, out of 3, were substituted in each sugar unit). To the left of Figure 1, a schematic of a fructan molecule is shown []. The main chain has β 1–2 linkages and is branched at C6; in the native fructan, R corresponds to hydrogen, which can be replaced by palmitoyl or acetyl groups, depending on the esterification reaction.
3.3. Morphological Characterization of Microspheres
Microspheres were prepared from esterified agave fructans. Figure 2 shows SEM micrographs from both types of fructan derivatives. The size of acetylated fructan microspheres ranged from 0.2 µm to 10 µm, while palmitoylated fructans resulted in rough microspheres with sizes from 5 to 18 µm. These results are in agreement with previous reports of microspheres synthesized with acetylated/palmitoylated inulin and fructan [,,].
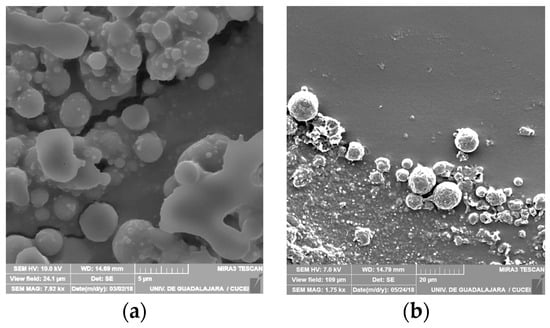
Figure 2.
Scanning Electron Micrographs of (a) acetylated (7920X) and (b) palmitoylated fructan (1750X).
3.4. Bacterial Growth in Acetylated or Palmitoylated Fructan Microspheres
Bacterial growth was evaluated in a culture medium in which microspheres prepared with acetyl-/palmitoyl-fructan loaded with ibuprofen were the only carbon source. Figure 3 shows the growth of W. paramesenteroides, E. mundtii, L. brevis, B. adolescentis, and their admixtures (as a bacterial consortium) on ibuprofen-loaded microspheres. In Figure 3a (acetylated fructan), it can be seen that L. brevis reached the highest growth (OD = 0.54) at 16 h, followed by the bacterial consortium (OD = 0.42). However, the consortium reached the stationary phase at half the time required by L. brevis. On the other hand, although the growth of E. mundtii was lower (OD = 0.36), it reached the stationary phase at 4 h. The growth of B. adolescentis was similar to the growth of E. mundtii, but took 24 h to reach the stationary phase.
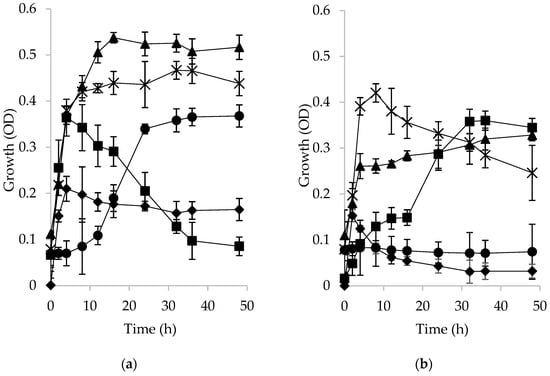
Figure 3.
Growth profile of strains and their consortium obtained by optical density of (a) acetylated fructan microspheres and (b) palmitoylated fructan microspheres: B. adolescentis (●); Weisella paramesenteroides (◆); Enterococcus mundtii (◼); Lactobacillus brevis (▲); consortium (∗).
Figure 3b shows the growth of the strains using as a carbon source palmitoylated fructan microspheres. It can be seen that the growth was lower as compared to their growth on acetylated fructan microspheres. The higher growth was obtained by the consortium (OD = 0.42 in 8 h), similar to the growth in acetylated fructan. E. mundtii took 32 h to achieve the stationary phase on palmitoylated fructan microspheres (OD = 0.35), while L. brevis took only 4 h to reach the stationary phase at OD = 0.26; the growth of W. mesenteroides was rather poor and reached the maximum at 2 h and then decreased, while B. adolescentis was unable to grow on palmitoylated fructan. E. mundtii reached the same growth in both types of substrates (acetylated or palmitoylated fructans), although the time to reach the stationary phase was longer with the palmitoylated fructan microspheres.
3.5. Release of Ibuprofen from Derivatized-Fructan Microspheres
The in vitro release of ibuprofen was evaluated by the fermentation of acetylated/palmitoylated fructan microspheres in colonic conditions using the four strains under study and their consortium. It is noteworthy that before carrying out the release study, both types of microspheres were subjected to gastric conditions using HCl at pH 1.2 and 37 °C for 2 h, which resulted in a 5–15% loss of the loaded drug for both substrates. A sustained release of ibuprofen was observed for acetylated fructan microspheres (Figure 4a). The highest release of the drug (41%) was achieved with L. brevis at 48 h, in agreement with the higher growth of this strain in acetylated fructan microspheres. On the other hand, the released ibuprofen using B. adolescentis or the consortium was 36% and 39%, respectively, while E. mundtii released 26% of the drug and W. paramesenteroides was able to release only 17.6% of the encapsulated ibuprofen.
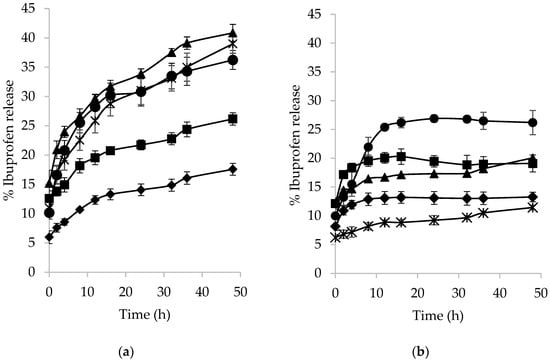
Figure 4.
Percentage of ibuprofen release as function of fermentation time from (a) acetylated and (b) palmitoylated fructan microspheres with B. adolescentis (●); Weisella paramesenteroides (◆); Enterococcus mundtii (◼); Lactobacillus brevis (▲); and consortium (∗).
Figure 4b shows the release of ibuprofen from palmitoylated fructan microspheres; it can be seen that the release of ibuprofen was lower as compared to acetylated fructan microspheres. The higher release of the drug was achieved with B. adolescentis (26.9%), while E. mundtii and L. brevis released around 20% of the drug; the consortium and W. paramesenteroides released only 15.5% and 13.2%, respectively, after 48 h.
3.6. Production of Short-Chain Fatty Acids (SCFAs)
The production profile of short-chain fatty acids (SCFAs) from acetylated or palmitoylated fructan microspheres was also determined (Figure 5). It can be seen that the bacteria under study produced mainly lactic acid (above 1 g/L). The four bacteria and the consortium also produced, besides lactic acid, acetic acid and propionic acid. L. brevis and B. adolescentis produced more propionic acid than acetic acid and also released a higher proportion of ibuprofen. W. paramesenteroides and the consortium produced more acetate (as compared to the other bacteria on both acetylated and palmitoylated fructans), had lower growth, and released a smaller amount of ibuprofen.
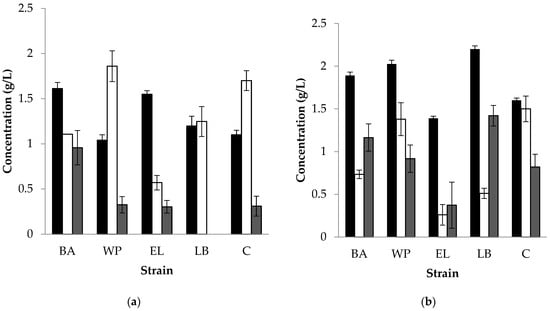
Figure 5.
Production of SCFA (g/L) by lactic acid bacteria at 48 h of fermentation: (a) acetylated fructan microspheres and (b) palmitoylated fructan microspheres, (■) lactic acid, (□) acetic acid, and ( ) propionic acid. BA: B. adolescentis; WP: Weisella paramesenteroides; EL: Enterococcus mundtii; LB: Lactobacillus brevis; C: consortium.
) propionic acid. BA: B. adolescentis; WP: Weisella paramesenteroides; EL: Enterococcus mundtii; LB: Lactobacillus brevis; C: consortium.
4. Discussion
Agave fructans are prebiotics that have shown positive effects on human health [,,,,,,,,]; while several studies have been carried out to encapsulate drugs in fructans, the release of the cargo has been evaluated mainly by enzymatic means [,,,]. The encapsulation of drugs is related to their affinity with excipients and, since many drugs are hydrophobic, hydrophilic carbohydrates are often esterified to turn them hydrophobic []. In this contribution, esterified agave fructans were used to prepare microspheres, by coacervation, in order to encapsulate ibuprofen. Unmodified agave fructans have been used to prepare microspheres to encapsulate ibuprofen and their encapsulation was rather poor and lacked stability []. By adding hydrophobic groups to hydrophilic fructans, the stability of microspheres is improved by reducing their solubility and allowing a controlled release of drugs [].
Acetyl groups represent the shortest acyl chain (only a methyl group) and therefore a high degree of acetylation of agave fructans was sought and achieved (DS: 2.8). On the other hand, since the chain length of palmitoyl groups consists of 14 methylene groups and 1 methyl group, a low degree of substitution (0.282) was sufficient and permitted accessibility of the microorganisms to the carbohydrate substrate. Both modified fructans are expected to have a similar hydrophobic effect, since a degree of substitution of 0.28 for palmitoyl fructans contains a similar amount of methylene/methyl groups (approximately 60 groups per fructan molecule) to a degree of substitution of 2.8 for acetylated fructans. As expected, both agave fructan derivatives were soluble only in organic solvents, therefore allowing us to prepare microspheres by coacervation. The morphology and size of microspheres depended on the length of the acyl chain, since palmitoylated fructan microspheres were larger and had a rough surface as compared to acetylated microspheres. The capacity for drug encapsulation was also different, since acetylated fructan microspheres were able to encapsulate up to 17% of the drug, while palmitoylated fructan microspheres were able to encapsulate up to 21.5% of the drug. Nonetheless, these results are much higher as compared to other systems, such as nanostructured-dihydro-peptide-modified inulin, specifically designed with a hydrophobic core, that were able to encapsulate only 2.1% of a hydrophobic drug (ornidazol) []. On the other hand, the results obtained here are similar to hybrid materials such as chitosan and silver, in which the encapsulation of ibuprofen reached values from 10.9% to 24.8% [].
The fermentation of agave fructan esters was carried out with four lactic acid bacteria, each on their own, or as a consortium. The growth of bacteria, the release of the drug, and the production of metabolites as SCFA were evaluated. It can be seen that the growth of the strains was higher in the acetylated agave fructans, which had a high degree of substitution. Such a behavior has also been observed with B. animalis on highly acetylated fructan microspheres (DS = 2.7) loaded with ibuprofen, in which the growth, similar to the one reported in this research with B. adolescentis, was obtained in less time [].
In spite of the fact that both types of microspheres had similar proportions of methyl and methylene groups, the growth of bacteria was favored in the fructan microspheres modified with the shorter acyl chain. Nonetheless, it is plausible that the strains might be able to use acetate and palmitate as a source of carbon for growth. Fructose released from agave fructans can enter into glycolysis by means of hexokinase to produce lactate or to produce biomass [,]. Also, it has been found that Saccharomyces boulardii is able to metabolize both unmodified and acetylated inulin []. On the other hand, the culture media (i.e., MRS) for lactic acid bacteria (LAB) such as Lactococcus, Lactobacillus, Enterococcus, Streptococcus, Leuconostoc, and Pediococcus have acetate as an ingredient, meaning that these strains are able to use it. Moreover, acetate is obtained from carbohydrates by LAB []. These facts may explain the higher growth of the strains on acetylated fructan microspheres as compared to palmitoylated fructan microspheres. Carbohydrate metabolism in LAB is amply known. However, the information about lipid metabolism is scarce and is more focused on the presence of LAB in food processing [] or on the effect of LAB on lipid reduction in humans []. Nonetheless, esterase activity has been detected in various lactobacilli, leading to the release of free fatty acids, glycerol, mono-acylglycerides, and diacylglycerides []. Free fatty acids may be produced by lipases that are at relatively high levels in Lactococccus and Lactobacillus species. The oxidation of unsaturated fatty acids is carried out in these bacteria in the presence of free radicals that form hydroperoxides by β-oxidation []. Hence, bacteria may use acetate more readily as compared to palmitate.
It is noteworthy that before carrying out the fermentations, both types of microspheres were subjected to gastric conditions using HCl at pH 1.2 and 37 °C for 2 h, which resulted in a 5–15% loss of the loaded drug for both substrates. Nonetheless, both types of microspheres retained most of their cargo in such conditions, as seen elsewhere []; in contrast, guar gum microspheres crosslinked with glutaraldehyde released almost all the cargo (90%, budesonide) at gastric conditions in 2 h. Afterward, only 8% of the remaining drug was released in the first 2 h in colonic conditions [].
The highest release of the drug (41%) was achieved with L. brevis at 48 h on acetylated fructan microspheres. This is in agreement with other studies where albendazole was released from guar gum (as excipient) in an anaerobic colonic condition with cecal content from rats [] Guar gum based microcapsules for colonic delivery of albendazole: Development and in-vitro evaluation 2010, Research Journal of Pharmaceutical Biological and Chemical Sciences 1(4):373–382]. In other studies [], guar gum and xanthan gum were used as coatings for releasing metronidazole in human cecal media for controlled release in the colon, at a ratio of 3.5:1 polysaccharide coatings/drug, resulting in a drug release of 35.7 ± 1.29% at 24 h, similar to the release of ibuprofen with L. brevis. Despite the fact that both derivatized fructans were expected to have a similar hydrophobic character, it has been shown that the strains B. adolescentis and L. brevis and the consortium of microorganisms had a better interaction with the fructans derivatized with short acyl chains, while long C16 acyl chains may have steric constraints that limited the release of the drug.
Obligated homolactic LAB produce only lactic acid from fermentation; however, LAB may also produce acetate, ethanol, and carbon dioxide. It was found that the four strains produced, besides lactic acid, acetic and propionic acids. The metabolic pathways that some microorganisms carry out to degrade hexoses (fructose mainly and glucose as the terminal group) are achieved by converting pyruvate (1 mol) and acetyl phosphate (1.5 mol) to generate lactic acid, acetic acid, and formic acid []. In general terms, bacteria obtain ATP by oxidizing the sugar to pyruvate, which in turn is reduced to lactic acid, and acetic and propionic acids. L. brevis and B. adolescentis produced more propionic acid than acetic acid and also released a higher proportion of ibuprofen. Alternative electron acceptors allow pyruvate to obtain ATP by decarboxylation and conversion into acetate [].
W. paramesenteroides and the consortium produced more acetate (as compared to the other bacteria on both acetylated and palmitoylated fructans), had lower growth, and released a smaller amount of ibuprofen. These facts may be related to a higher use of energy for maintenance that results in a higher production of acetate to obtain ATP.
It should be pointed out that all the strains produced more lactic and propionic acid on acetylated fructan microspheres as compared to palmitoylated fructans, while more acetic acid was produced on palmitoylated fructan microspheres, suggesting a correlation between the growth and production of acids; interestingly, L. brevis lacked the production of propionic acid in palmitoylated fructan. A high acetate concentration in palmitoylated fructan may be explained by the need of bacteria to obtain ATP for the utilization of palmitate.
Fermentation pathways of lactic acid bacteria are inefficient in terms of energy gains (one or two ATPs per sugar molecule), which decrease by high conversion rates, resulting in low biomass gain as observed in this contribution. Moreover, the central carbon metabolism and the biosynthetic pathways necessary for cell growth are decoupled in lactic acid bacteria, owed to their adaptation to niches rich in nutrients that allow these bacteria to require many cellular components, instead of their synthesis from the carbon source [].
On the other hand, recent findings have revealed that SCFAs have a physiological role in colon diseases, and they can also regulate the human intestinal microbiota []. The production of propionate promotes satiety, prevents hepatic lipogenesis, reduces cholesterol, and has anticancer activity []. Therefore, when lactic acid bacteria release simple carbohydrates from fructan, these are utilized for growth, and then SCFAs are produced and concomitantly the drug is released.
5. Conclusions
In this contribution, it was found that hydrophobic agave fructans are susceptible to being fermented by lactic acid bacteria, which were able to grow and produce SCFA and release encapsulated drugs in appropriate amounts and time (approximately 37% in 24 h). The biomass growth, production of SCFA, and release of ibuprofen were higher in acetylated agave fructan as compared to palmitoylated fructan, due to both the accessibility to the carbohydrate and the ability of such microorganisms to metabolize acetate.
Author Contributions
Conceptualization, R.I.C.-G. and G.T.; Methodology, C.M.-C., A.C.-C., M.E.M.-R. and A.E.; Formal analysis, C.M.-C., M.E.M.-R., R.I.C.-G. and G.T.; Investigation, R.I.C.-G. and G.T.; Writing—original draft, C.M.-C.; Writing—review & editing, A.E., R.I.C.-G. and G.T.; Supervision, M.E.M.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nadeem, M.S.; Kumar, V.; Al-Abbasi, F.A.; Kamal, M.A.; Anwar, F. Risk of colorectal cancer in inflammatory bowel diseases. Semin. Cancer Biol. 2020, 64, 51–60. [Google Scholar] [CrossRef]
- Amidon, S.; Brown, J.E.; Dave, V.S. Colon-targeted oral drug delivery systems: Design trends and approaches. AAPS Pharmscitech 2015, 16, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Hua, S. Advances in Oral Drug Delivery for Regional Targeting in the Gastrointestinal Tract-Influence of Physiological, Pathophysiological and Pharmaceutical Factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Željko Prebeg, Ž.; Kurjaković, N. A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers: I. Manipulation of drug release using Eudragit® L100-55 and Eudragit® S100 combinations. J. Control. Release 1999, 58, 215–222. [Google Scholar] [CrossRef]
- Kotla, N.G.; Rana, S.; Sivaraman, G.; Sunnapu, O.; Vemula, P.K.; Pandit, A.; Rochev, Y. Bioresponsive drug delivery systems in intestinal inflammation: State-of-the-art and future perspectives. Adv. Drug Deliv. Rev. 2019, 146, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Bajracharya, R.; Min, J.Y.; Han, J.W.; Park, B.J.; Han, H.K. Strategic Approaches for Colon Targeted Drug Delivery: An Overview of Recent Advancements. Pharmaceutics 2020, 12, 68. [Google Scholar] [CrossRef]
- Arévalo-Pérez, R.; Maderuelo, C.; Lanao, J.M. Recent advances in colon drug delivery systems. J. Control. Release 2020, 327, 703–724. [Google Scholar] [CrossRef]
- Barclay, T.G.; Day, C.M.; Petrovsky, N.; Garg, S. Review of polysaccharide particle-based functional drug delivery. Carbohydr. Polym. 2019, 221, 94–112. [Google Scholar] [CrossRef]
- Miramontes-Corona, C.; Escalante, A.; Delgado, E.; Corona-González, R.I.; Vázquez-Torres, H.; Toriz, G. Hydrophobic agave fructans for sustained drug delivery to the human colon. React. Funct. Polym. 2020, 146, 104396. [Google Scholar] [CrossRef]
- Amaretti, A.; Bernardi, T.; Tamburini, E.; Zanoni, S.; Lomma, M.; Matteuzzi, D.; Rossi, M. Kinetics and Metabolism of Bifidobacterium adolescentis MB 239 Growing on Glucose, Galactose, Lactose, and Galactooligosaccharides. Appl. Environ. Microbiol. 2007, 73, 3637–3644. [Google Scholar] [CrossRef]
- Trevisi, P.; De Filippi, S.; Modesto, M.; Mazzoni, M.; Casini, L.; Biavati, B.; Bosi, P. Investigation on the ability of different strains and doses of exogenous Bifidobacteria, to translocate in the liver of weaning pigs. Livest. Sci. 2007, 108, 109–112. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.; Ashaolu, J.; Adeyeye, S. Fermentation of prebiotics by human colonic microbiota in vitro and short-chain fatty acids production: A critical review. J. Appl. Microbiol. 2021, 130, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Williams, C.M. Prebiotics and lipid metabolism. Curr. Opin. Lipidol. 2002, 13, 61–67. [Google Scholar] [CrossRef]
- Urías-Silvas, J.E.; López, M.G. Efecto Prebiótico de los Fructanos de Agave. 1er Encuentro Participación de La Mujer En La Ciencia. 2004. Available online: https://congresos.cio.mx/1_enc_mujer/files/Extensos/Posters/B-03.pdf (accessed on 22 July 2024).
- Macfarlane, S. Chapter 10—Prebiotics in the Gastrointestinal Tract. In Bioactive Foods in Promoting Health; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Boston, MA, USA, 2010; pp. 145–156. [Google Scholar] [CrossRef]
- Catry, E.; Bindels, L.B.; Tailleux, A.; Lestavel, S.; Neyrinck, A.M.; Goossens, J.F.; Lobysheva, I.; Plovier, H.; Essaghir, A.; Demoulin, J.B.; et al. Targeting the gut microbiota with inulin-type fructans: Preclinical demonstration of a novel approach in the management of endothelial dysfunction. Gut 2018, 67, 271–283. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.J.; Zhang, W. Role of Dietary Nutrients in the Modulation of Gut Microbiota: A Narrative Review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1117–1132. [Google Scholar] [CrossRef]
- Rafter, J.; Bennett, M.; Caderni, G.; Clune, Y.; Hughes, R.; Karlsson, P.C.; Klinder, A.; O’Riordan, M.; O’Sullivan, G.C.; Pool-Zobel, B.; et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am. J. Clin. Nutr. 2007, 85, 488–496. [Google Scholar] [CrossRef]
- Drago, L. Probiotics and Colon Cancer. Microorganisms 2019, 7, 66. [Google Scholar] [CrossRef]
- Le, B.; Ngoc, A.; Yang, S. Synbiotic Fermented Soymilk with Weissella cibaria FB069 and Xylooligosaccharides Prevents Proliferation in Human Colon Cancer Cells. J. Appl. Microbiol. 2020, 128, 1486–1496. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Partida, V.Z.; Lopez, A.C.; Gomez, A.d.J.M. Method of Producing Fructose Syrup from Agave Plants. U.S. Patent 5,846,333, 8 December 1998. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Phylogenies and the Comparative Method. Am. Nat. 1985, 125, 1–15. [Google Scholar] [CrossRef]
- Rogge, T.M.; Stevens, C.V.; Colpaert, A.; Levecke, B.; Booten, K. Use of Acyl Phosphonates for the Synthesis of Inulin Esters and Their Use as Emulsion Stabilizing Agents. Biomacromolecules 2007, 8, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Poulain, N.; Dez, I.; Perrio, C.; Lasne, M.C.; Prud’homme, M.P.; Nakache, E. Microspheres based on inulin for the controlled release of serine protease inhibitors: Preparation, characterization and in vitro release. J. Control. Release 2003, 92, 27–38. [Google Scholar] [CrossRef]
- Starbird, R.; Zuñiga, V.; Delgado, E.; Saake, B.; Toriz, G. Design of Microspheres from Blue Agave Fructans for Drug Delivery to the Colon. Part 1. Esterification of Agave Fructans. J. Biobased Mater. Bioenergy 2007, 1, 238–244. [Google Scholar] [CrossRef]
- Arellano-Ayala, K.; Ascencio-Valle, F.; Gutiérrez-González, P.; Estrada-Girón, Y.; Torres-Vitela, M.; Macías-Rodríguez, M. Hydrophobic and adhesive patterns of lactic acid bacteria and their antagonism against foodborne pathogens on tomato surface (Solanum lycopersicum L.). J. Appl. Microbiol. 2020, 129, 876–891. [Google Scholar] [CrossRef]
- Delgadillo, E.; Corona, R.I.; Toriz, G.; Contreras, H.J.; Sadeghifar, H.; Baobing, W.; Yang, G.; Lucia, L.A.; Delgado, E. Coacervated liposoluble fructan-based host–guest microspheres as unique drug delivery materials. RSC Adv. 2015, 5, 67759–67766. [Google Scholar] [CrossRef]
- Toriz, G.; Delgado, E.; Zúñiga, V. A proposed chemical structure for fructans from blue agave plant (Tequilana weber var. azul). e-Gnosis 2007, 5, 1. [Google Scholar]
- Kesharwani, S.S.; Dachineni, R.; Bhat, G.J.; Tummala, H. Hydrophobically modified inulin-based micelles: Transport mechanisms and drug delivery applications for breast cancer. J. Drug Deliv. Sci. Technol. 2019, 54, 101254. [Google Scholar] [CrossRef]
- Walz, M.; Hirth, T.; Weber, A. Investigation of chemically modified inulin as encapsulation material for pharmaceutical substances by spray-drying. Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 47–52. [Google Scholar] [CrossRef]
- Shivhare, K.; Garg, C.; Priyam, A.; Gupta, A.; Sharma, A.K.; Kumar, P. Enzyme sensitive smart inulin-dehydropeptide conjugate self-assembles into nanostructures useful for targeted delivery of ornidazole. Int. J. Biol. Macromol. 2018, 106, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.K.S.; Reis, D.T.; Barbosa, K.M.; Scheidt, G.N.; da Costa, L.S.; Santos, L.S.S. Antibacterial effects and ibuprofen release potential using chitosan microspheres loaded with silver nanoparticles. Carbohydr. Res. 2020, 488, 107891. [Google Scholar] [CrossRef]
- Sauer, M.; Russmayer, H.; Grabherr, R.; Peterbauer, C.K.; Marx, H. The efficient clade: Lactic acid bacteria for industrial chemical production. Trends Biotechnol. 2017, 35, 756–769. [Google Scholar] [CrossRef]
- Nelson David, L.; Cox Michael, M.; Nelson David, L. Lehninger Principles of Biochemistry; WH Freeman: New York, NY, USA, 2005. [Google Scholar]
- Buitrago-Arias, C.; Londoño-Moreno, A.; Avila-Reyes, S.; Arenas-Ocampo, M.; Alamilla-Beltran, L.; Jimenez-Aparicio, A.; Camacho-Diaz, B. Evaluation of the fermentation of acetylated agave fructans (agavins), with Saccharomyces boulardii as a probiotic. Rev. Mex. Ing. Química 2021, 20. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Vermeulen, N.; Vogel, R.F. Carbohydrate, peptide and lipid metabolism of lactic acid bacteria in sourdough. Food Microbiol. 2007, 24, 128–138. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Wen, R.; Chen, Q.; Kong, B. Role of lactic acid bacteria in flavor development in traditional Chinese fermented foods: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2741–2755. [Google Scholar] [CrossRef]
- Toshimitsu, T. Development of a lactic acid bacteria strain that suppresses chronic inflammation and improves glucose and lipid metabolism. Biosci. Microbiota Food Health 2023, 42, 3–7. [Google Scholar] [CrossRef]
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018, 4, 665. [Google Scholar] [CrossRef]
- Kaushik, D.; Sardana, S.; Mishra, D. 5-fluorouracil loaded guar gum microspheres for colon delivery: Preparation, characterization and in vitro release. Yao Xue Xue Bao = Acta Pharm. Sin. 2009, 44, 1278–1284. [Google Scholar]
- Liu, Y.; Zhou, H. Budesonide-Loaded Guar Gum Microspheres for Colon Delivery: Preparation, Characterization and in Vitro/in Vivo Evaluation. Int. J. Mol. Sci. 2015, 16, 2693–2704. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.M. Advances in polysaccharide-based oral colon-targeted delivery systems: The journey so far and the road ahead. Cureus 2023, 15, e33636. [Google Scholar] [CrossRef] [PubMed]
- Kotla, N.; Shivapooja, A.; Muthyala, J.; Pinakin, P. Effect of guar gum and xanthan gum compression coating on release studies of metronidazole in human faecal media for colon targeted drug delivery system. Asian J. Pharm. Clin. Res. 2013, 6, 310–313. [Google Scholar]
- Usman, M.; Zhang, C.; Patil, P.J.; Mehmood, A.; Li, X.; Bilal, M.; Haider, J.; Ahmad, S. Potential applications of hydrophobically modified inulin as an active ingredient in functional foods and drugs—A review. Carbohydr. Polym. 2021, 252, 117176. [Google Scholar] [CrossRef]
- Sharon, G.; Garg, N.; Debelius, J.; Knight, R.; Dorrestein, P.C.; Mazmanian, S.K. Specialized metabolites from the microbiome in health and disease. Cell Metab. 2014, 20, 719–730. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).